Abstract
Background
Recently, it has been reported that the A930G and C242T polymorphisms within p22phox (CYBA) gene are involved in the pathogenesis of hypertension. However, the results remain controversial. Furthermore, no previous meta-analysis has been conducted to evaluate the relationship between the A930G and C242T polymorphisms and hypertension. Therefore, we performed this meta-analysis to clarify these controversies.
Objective and Methods
All of the included articles were retrieved from the PubMed and Embase databases, as well as the CNKI, CBM, Chongqing VIP and Wan Fang databases according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Odds ratios (OR) with corresponding 95% confidence intervals (CI) were used to assess the strength of the association. Accounting for heterogeneity, a fixed or random effects model was respectively adopted. Heterogeneity was checked using the Q test and the I2 statistic. A cumulative meta-analysis was conducted to estimate the tendency of pooled OR. Funnel plots and Egger’s tests were performed to test for possible publication bias.
Results
Five articles on A930G with 2003 cases/2434 controls and eight articles on C242T with 2644 cases/1967 controls were identified. A significant association of A930G polymorphisms with the risk of hypertension was found in the dominant model (OR=0.59, 95% CI: 0.38–0.92, p=0.021) and allelic model (OR=0.66, 95% CI: 0.46–0.95, p=0.024). In the stratified analysis, a significant association could be found among the hospital-based and population-based studies. However, no evidence of a significant association of the C242T polymorphism with hypertension was found in the overall analysis and subgroup analysis.
Conclusions
This meta-analysis indicates that the A930G polymorphism, but not the C242T variation, might be a protective factor for hypertension.
Introduction
Hypertension is now considered a major public health issue [1] that affects nearly one billion people worldwide [2]. It is recognized as the leading contributor to death and disability globally [3], and the prevalence is dramatically increasing [4,5]. According to the World Health Organization (WHO), hypertension can be attributed to the loss of 7.6 million lives annually (13.5% of all deaths globally) and the loss of 57 million disability-adjusted life years (DALYs) worldwide [6]. Hypertension is one of the primary risk factors for cardiovascular disease [7], such as stroke [8-10] and coronary heart disease [11]. Nearly 54% of stroke and 47% of coronary heart disease cases can be attributed to hypertension [7]. Hypertension is widely accepted as a multifactorial disease, resulting from the interaction of many risk genes together with environmental factors [12]. Approximately 30% to 50% of the variation of blood pressure in the general population is genetically determined [13].
The p22phox (CYBA) gene is located on the long arm of chromosome 16q24 [14-16]. It encodes for p22phox, a major component of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases and plays a crucial role in the activation of NADPH oxidase [17-20]. The NADPH oxidase system, which constitutes the most important source of reactive oxygen species (ROS) in the vessel wall, is mostly expressed in phagocytes, endothelial cells, smooth muscle cells and fibroblasts [21,22]. ROS induce oxidative stress, and have been implicated in the pathogenesis of hypertension [23-25]. An animal study showed that functional polymorphisms in the p22phox gene promoter are associated with hypertension [26]. Several functional polymorphisms in the p22phox gene have also been explored in association with hypertension. The p22phox gene A675T polymorphism plays a functional role in NADPH oxidase-mediated oxidative stress in patients that suffer from hypertension [27]. However, the CYBA C852G and C536T polymorphisms have not been reported in association with hypertension [27].
Recently, more studies have focused on the association of the CYBA A930G and C242T polymorphisms with hypertension. One study conducted by Sales et al. [28] indicated that the A930G polymorphism is not related to hypertension. However, Kokubo et al. [29], Moreno et al. [30], Pang et al. [31] and Ha et al. [32] support the association between the A930G polymorphism and hypertension. Similarly, a statistically significant association between the C242T polymorphism and hypertension has been reported by Ji et al. [33]. Subsequent studies [34-40] have been conducted to investigate the association between the C242T polymorphism and hypertension. However, the results have generated considerable controversy. To the best of our knowledge, no meta-analyses on the association between the A930G and C242T polymorphisms and hypertension have been conducted. Therefore, we performed this meta-analysis to better clarify the association between the A930G and C242T polymorphisms and hypertension in view of the abovementioned inconsistent results. The association was also evaluated by further subgroup analysis according to ethnicity, the sources of controls and Hardy-Weinberg equilibrium (HWE).
Materials and Methods
Search strategy
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [41], we searched electronic databases including PubMed, Embase, Chinese National Knowledge Infrastructure (CNKI), Chinese Biological Medical Literature database (CBM), Wan Fang and Chongqing VIP database with no language restrictions. The literature search was updated on July 1, 2013 using the following keywords: ("A930G" OR "C242T" OR “p22phox” OR “CYBA” OR “NADPH oxidase” OR “nicotinamide adenine dinucleotide phosphate oxidase”) AND (“polymorphism” OR “mut*” OR “varia*”) AND ("high blood pressure" OR "hypertension" OR "arterial hypertension" OR "hypertensive disorder"). In order to acquire the relevant publications, reference lists of all retrieved publications were also scanned. We contacted the authors by email to request detailed information if necessary.
Selection criteria
All the included original studies were selected according to the following inclusion criteria: a) studies evaluating the association between CYBA A930G or C242T polymorphisms and hypertension; b) case-control or cohort studies; c) sufficient data provided with information on the genotypes and allele frequencies. The exclusion criteria were: a) pedigree and family-based studies, b) duplicate publications.
Data extraction
The data were carefully extracted from all the included publications independently by two investigators (Liang and Peng) according to the selection criteria listed above. If there was any disagreement, it was discussed among the authors or by consulting another reviewer (Su) to reach a consensus. Data extracted from the studies included the name of the first author, publication year, country, ethnicity, study design, source of controls, number of cases and controls, diagnostic criteria and selection criteria of cases and controls, genotyping methods, genotype or allele distribution, and the matching method. Incomplete data regarding the genotypes and allele frequencies were calculated using the available information.
Statistical analysis
All statistical analyses were implemented in Stata statistical software version 11.1. The Hardy-Weinberg equilibrium (HWE) for the genotype distributions in controls was assessed by the chi-square test (p>0.05). We used the following genetic models to pool the data: dominant model (GG + GA versus AA/CC + CT versus TT), recessive model (GG versus GA + AA/CC versus CT +TT), codominant model 1 (GG versus GA/CC versus CT), codominant model 2 (GA versus AA/CT versus TT), and the allelic model (G allele versus A allele/C allele versus T allele). The strength of the association between the CYBA A930G or C242T polymorphism and hypertension was estimated by calculating ORs with corresponding 95% CIs. Heterogeneity was analyzed using the Q test and the I2 statistic. If significant heterogeneity existed (P<0.10, or I2 >50%), the random effects model was used to calculate the pooled OR [42]; otherwise, a fixed effects model was adopted [43,44]. Sensitivity analysis was performed to assess the stability of the results. Begg's test and Egger's test were performed to estimate the possible publication bias. The asymmetrical funnel plot and P<0.05 were considered representative of publication bias. To further detect heterogeneity, subgroup analyses were performed regarding ethnicity, the source of controls, and HWE. We also conducted a cumulative meta-analysis of the association between the CYBA C242T polymorphism and hypertension to assess the trends in the pooled OR over time under the dominant contrast in the random effect model. Studies included in the cumulative meta-analysis were sorted by the year of publication.
Results
Search results
We identified 315 potentially relevant articles by our predefined search strategy in the database of PubMed (n=48), Embase (n=67), CNKI (n=72), CBM (n=7), Chinese Wan Fang (n=62), and Chongqing VIP (n=22). After reviewing these titles and abstracts, we obtained 47 potential eligible studies. By scanning the full texts, 34 articles were excluded according to the selection criteria. Finally, thirteen qualified articles (five articles for A930G and eight articles for C242T) were included in the meta-analysis (Figure 1).
Figure 1. Flow chart of the articles selection for associations of the A930G/C242T polymorphism with hypertension.
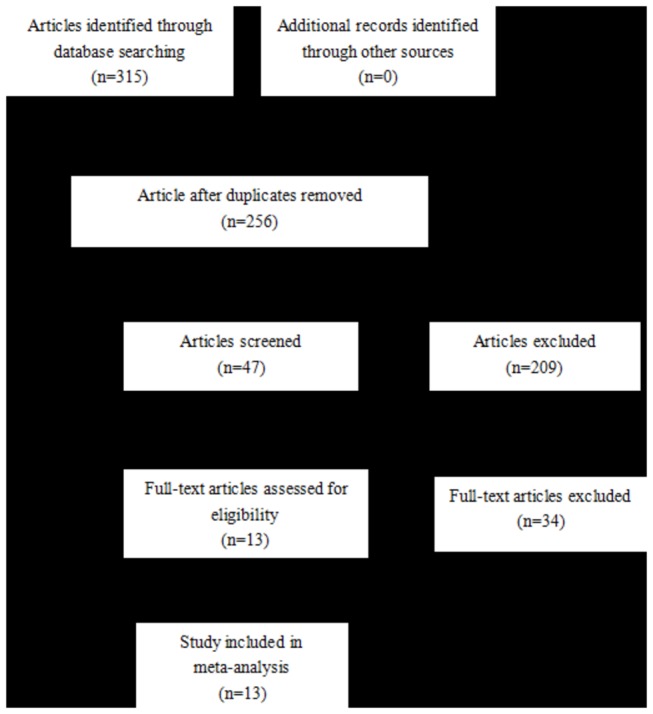
(Follow the PRISMA guidelines).
Study characteristics
Five included studies investigating A930G polymorphisms in hypertension were examined, including 2003 cases and 2434 controls. All of the included studies were published between 2003 and 2007. The ethnicities of the research populations were as follows: three studies involved Asians and two involved in Caucasians. There were two population-based studies [28,29] and three hospital-based studies [30-32]. Polymerase chain reaction (PCR) was used for genotyping in all the included studies. The diagnostic criteria for hypertension were different among the studies: patients in three studies were diagnosed according to systolic blood pressure(SBP) and/or diastolic blood pressure (DBP) greater than 140 and 90 mmHg, patients in one study were diagnosed according to SBP and/or DBP greater than 139 and 89 mmHg, and the diagnostic criteria were not mentioned in one study. Controls were matched for gender, age, smokers, and other indices reported in three studies [30-32], but this was not mentioned in the other two studies [28,29]. The distribution of the genotypes in all the control groups obeyed HWE. The characteristics, genotypes, and allele frequencies are listed in Table 1.
Table 1. The main characteristics of the eligible studies regarding associations between the CYBA A930G polymorphism and hypertension.
| Authos | Ethnicity | Sample size |
Diagnostic criteria |
Matched | Genotyping methods | Source of controls | case |
control |
P value for HWE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | GG | AG | AA | G | A | GG | AG | AA | G | A | ||||||
| Moreno et al./2003 | Caucasian | 88 | 68 | SBP>139 mmHg/ DBP>89 mm Hg | normal blood pressure | gender, smokers, diabetes, GLU, TC, HDL-C, LDL-C | PCR | HB | 36 | 41 | 11 | 113 | 63 | 23 | 29 | 16 | 75 | 61 | 0.3252 |
| Ha et al./2004 | Asian | 83 | 66 | SBP≥ 140 mmHg/ DBP≥ 90 mmHg | normal blood pressure | age, BMI, blood lipid levels | PCR | HB | 35 | 38 | 10 | 108 | 58 | 19 | 26 | 21 | 64 | 68 | 0.0884 |
| Pang et al./2004 | Asian | 123 | 105 | SBP≥ 140 mmHg/ DBP≥ 90 mmHg | normal blood pressure | gender, age, GLU, HDL-C, LDL-C | PCR | HB | 45 | 57 | 21 | 147 | 99 | 12 | 51 | 42 | 75 | 135 | 0.672 |
| Kokubo et al./2005 | Asian | 1515 | 2125 | SBP≥ 140 mmHg/ DBP≥ 90 mmHg | normal blood pressure | NA | PCR | PB | 481 | 749 | 285 | 1711 | 1319 | 615 | 1050 | 460 | 2280 | 1970 | 0.7603 |
| Sales et al./2007 | Caucasian | 194 | 70 | NA | normal blood pressure | NA | PCR | PB | 73 | 83 | 38 | 229 | 159 | 20 | 42 | 8 | 82 | 58 | 0.083 |
HWE: Hardy-Weinberg equilibrium; NA: not available; PCR: polymerase chain reaction; HB: hospital based; PB: population based; SBP: systolic blood pressure; DBP: diastolic blood pressure
GLU: glucose; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TC: total cholesterol
As shown in Table 2, eight studies (1842 cases/1967 controls) on the association of the C242T polymorphism and hypertension were included. Studies were conducted from 2003 to 2013 among various ethnicities (four studies involved in Caucasians and four involved Asians). Three studies were population-based [34,35,37] and five were hospital-based studies [33,36,38-40]. Genotyping was consistently performed in these studies by PCR. Patients with hypertension in five studies were diagnosed based on systolic and/or diastolic blood pressure over 140 and 90 mmHg. Among the other three studies, patients in one study were diagnosed according to systolic and/or diastolic blood pressure over 139 and 89 mmHg, patients in one study were diagnosed according to systolic and/or diastolic blood pressure over 130 and 80mmHg, and the diagnostic criteria were not mentioned in one study. Among the eight eligible studies, five studies [34,36,38-40] stated that the controls were matched for age, gender and clinical index; however, matching was not mentioned in the other three studies [33,35,37]. The distribution of genotypes and allele in the control group deviated from HWE in two studies. The general characteristics and the distribution of C242T genotypes and alleles in this meta-analysis are shown in Table 2.
Table 2. The main characteristics of the eligible studies regarding associations between the CYBA C242T polymorphism and hypertension.
| Authors | Ethnicity | Sample size |
Diagnostic criteria |
Matched | Genotyping methods | Source of controls | case |
control |
P value for HWE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | CC | CT | TT | C | T | CC | CT | TT | C | T | ||||||
| Ji et al./2003 | Asian | 57 | 106 | SBP≥ 140 mmHg/ DBP≥ 90 mmHg | normal blood pressure | NA | PCR | HB | 41 | 15 | 1 | 97 | 17 | 91 | 13 | 2 | 195 | 17 | 0.1251 |
| Pang et al./2005 | Asian | 123 | 105 | SBP≥ 140 mmHg/ DBP≥ 90 mmHg | normal blood pressure | age, gender, GLU, HDL-C, LDL-C | PCR | HB | 89 | 29 | 5 | 207 | 39 | 91 | 13 | 1 | 195 | 15 | 0.4158 |
| Hsueh et al./2005 | Asian | 79 | 213 | SBP≥ 140 mmHg/ DBP≥ 90 mmHg | normal blood pressure | NA | PCR | PB | 68 | 9 | 2 | 145 | 13 | 193 | 17 | 3 | 403 | 23 | 0.0156 |
| Moreno et al./2006 | Caucasian | 326 | 297 | SBP>139 mmHg/ DBP>89 mm Hg | normal blood pressure | gender, DM, TC, HDL-C, LDL-C | PCR | HB | 133 | 143 | 50 | 409 | 243 | 93 | 156 | 48 | 342 | 252 | 0.2348 |
| Wang et al./2007 | Asian | 135 | 135 | SBP≥140mmHg/ DBP≥90 mmHg | SBP< 140/DBP< 90mmHg | age, gender | PCR | HB | 113 | 22 | 0 | 248 | 22 | 100 | 35 | 0 | 235 | 35 | 0.1267 |
| Kuznetsova et al./2008 | Caucasian | 272 | 97 | NA | NA | NA | PCR | PB | 131 | 122 | 19 | 384 | 160 | 41 | 52 | 4 | 134 | 60 | 0.0167 |
| Schreiber et al./2012 | Caucasian | 1030 | 826 | SBP≥ 140 mmHg/ DBP≥ 90 mmHg | normal blood pressure | age, gender, smokers, BMI, GLU, DM, LDL-C,HDL-C, triglycerides, uric acid | PCR | PB | 452 | 459 | 119 | 1363 | 697 | 367 | 369 | 90 | 1103 | 549 | 0.8757 |
| Petrovic et al./2013 | Caucasian | 622 | 188 | Subjects with type 2 diabetes with SBP≥130mmHg or DBP ≥80mmHg | WHO Classification of Diabetes Mellitus | gender, DM, smokers, HbaIc, GLU, TC, LDL-C, HDL-C, triglycerides | PCR | HB | 274 | 257 | 91 | 805 | 439 | 64 | 99 | 25 | 227 | 149 | 0.2221 |
HWE: Hardy-Weinberg equilibrium; NA: not available; PCR: polymerase chain reaction;HB: hospital based; PB: population based;SBP: systolic blood pressure; DBP: diastolic blood pressure
GLU: glucose; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TC: total cholesterol
DM: diabetes mellitus; BMI: Body Mass Index
WHO: World Health Organization
The A930G polymorphism associated with hypertension
Significant associations between the A930G polymorphism and the risk of hypertension were identified in the dominant model (OR=0.59, 95%CI: 0.38-0.92, p=0.021) and the allelic model (OR=0.66, 95% CI: 0.46-0.95, p=0.024) (Figure 2). However, there was not a significant association in the other genetic models, i.e. the recessive model (OR=0.59, 95%CI: 0.32-1.07, p=0.083), co-dominant model 1 (OR=0.70, 95%CI: 0.40-1.21, p=0.197), or co-dominant model 2 (OR=0.68, 95%CI: 0.46-1.01, p=0.054) (Table 3).
Figure 2. Forest plots of hypertension associated with the CYBA A930G polymorphism.
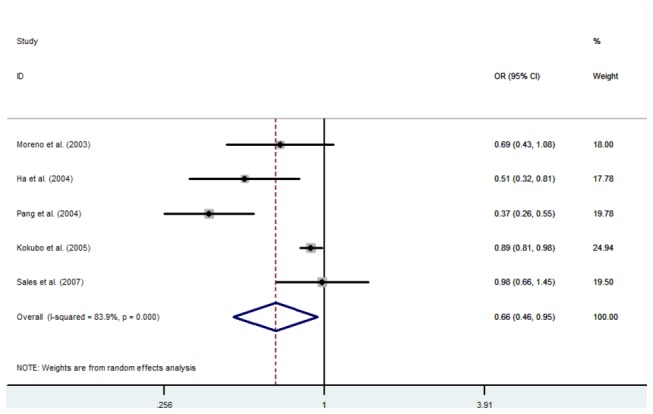
Table 3. Main Results of ORs with 95%CI of CYBA A930G polymorphism and hypertension.
|
Variables
|
No. |
Dominant model
|
Recessive model
|
Codominant model 1
|
Codominant model 2
|
Allele model
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |||
| Total | 5 | 0.59(0.38-0.92) | 0.021 | 0.59(0.32-1.07) | 0.083 | 0.70(0.40-1.21) | 0.197 | 0.68(0.46-1.01) | 0.054 | 0.66(0.46-0.95) | 0.024 | |
| Ethnicity | Caucasian | 2 | 0.70(0.45-1.08) | 0.107 | 0.94(0.24-3.72) | 0.930 | 1.09(0.23-5.22) | 0.914 | 0.68(0.41-1.12) | 0.127 | 0.84(0.59-1.19) | 0.318 |
| Asian | 3 | 0.50(0.22-1.13) | 0.098 | 0.45(0.20-1.02) | 0.056 | 0.56(0.30-1.05) | 0.071 | 0.64(0.33-1.23) | 0.178 | 0.57(0.31-1.04) | 0.067 | |
| Source of controls | HB | 3 | 0.45(0.23-0.91) | 0.027 | 0.34(0.22-0.52) | 0.000 | 0.42(0.27-0.66) | 0.000 | 0.60(0.30-1.19) | 0.144 | 0.50(0.35-0.71) | 0.000 |
| PB | 2 | 0.86(0.75-0.99) | 0.037 | 1.14(0.53-2.45) | 0.746 | 1.32(0.50-3.53) | 0.576 | 0.77(0.48-1.24) | 0.283 | 0.90(0.82-0.98) | 0.019 | |
In the further subgroup analysis according to the source of controls, a statistically significant association of the A930G polymorphism with hypertension was found in the dominant model in the hospital-based studies (OR: 0.45, 95%CI: 0.23-0.91, p=0.027) and the population-based studies (OR: 0.86, 95%CI: 0.75-0.99, p=0.037). Similar results were found in the allelic model among the hospital-based studies (OR: 0.50, 95%CI: 0.35-0.71, p=0.000) and the population-based studies (OR: 0.90, 95%CI: 0.82-0.98, p=0.019) (Figure 3). Statistical significance was also found in the recessive model (OR: 0.34, 95%CI: 0.22-0.52, p=0.000) and co-dominant model 1 (OR: 0.42 95%CI: 0.27-0.66, p=0.000) in the hospital-based studies, but not in the population-based studies (recessive model: OR=1.14, 95%CI: 0.53-2.45, p=0.746; OR=1.32 95%CI:0.50-3.53, p=0.576). However, no significance was observed in the co-dominant model 2 in either the hospital-based studies (OR: 0.60 95%CI: 0.30-1.19, p=0.144) or in the population-based studies (OR: 0.77 95%CI: 0.48-1.24, p=0.283). In the stratified analysis for ethnicity, no significant association was detected in any of the genetic models (Table 3).
Figure 3. Forest plots for the association of the CYBA A930G polymorphism in the stratified analysis for source of controls.
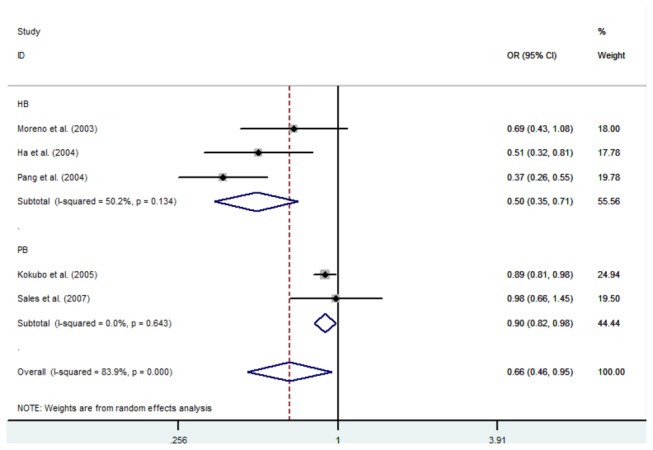
The C242T polymorphism associated with hypertension
There was no statistically significant association between the C242T polymorphism and hypertension in any of the genetic models: dominant model (OR=0.97, 95% CI: 0.72-1.32, p=0.870), recessive model (OR=1.08, 95%CI: 0.88-1.34, p=0.443), co-dominant model 1 (OR=1.17, 95%CI: 0.94-1.46, p=0.162), co-dominant model 2 (OR=0.94, 95%CI: 0.68-1.29, p=0.692), and allelic model (OR=1.02, 95%CI:0.82-1.26, p=0.846) (Figure 4). Similarly, no significant association was detected between the C242T polymorphism and hypertension in any of the genetic models in the subgroup analysis. The results of this meta-analysis are listed in Table 4.
Figure 4. Forest plots of hypertension associated with the CYBA C242T polymorphism.
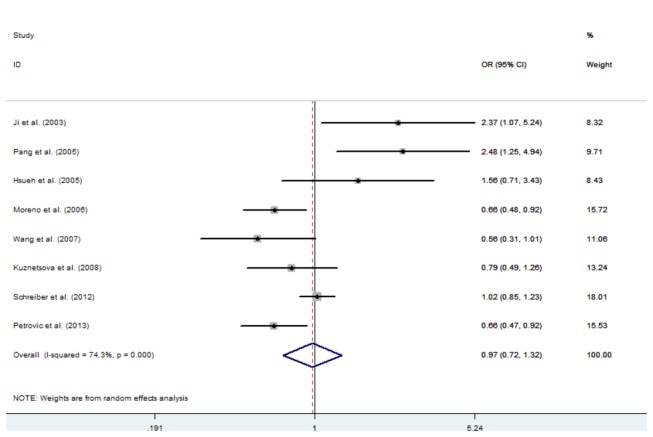
Table 4. Main results of ORs with 95%CI of CYBA C242T polymorphism and hypertension.
|
Variables
|
No. |
Dominant model
|
Recessive model
|
Codominant model 1
|
Codominant model 2
|
Allele model
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |||
| 8 | 0.97(0.72-1.32) | 0.870 | 1.08(0.88-1.34) | 0.443 | 1.17(0.94-1.46) | 0.162 | 0.94(0.68-1.29) | 0.692 | 1.02(0.82-1.26) | 0.846 | ||
| Ethnicity | Asian | 4 | 1.47(0.69-3.13) | 0.317 | 2.03(0.61-6.75) | 0.250 | 1.15(0.32-4.12) | 0.827 | 1.44(0.67-3.10) | 0.346 | 1.44(0.73-2.83) | 0.288 |
| Caucasian | 4 | 0.79(0.61-1.02) | 0.076 | 1.06(0.86-1.31) | 0.564 | 1.17(0.94-1.46) | 0.167 | 0.75(0.56-1.00) | 0.053 | 0.91(0.80-1.04) | 0.187 | |
| Source of controls | HB | 5 | 0.99(0.59-1.66) | 0.974 | 1.05(0.76-1.43) | 0.777 | 1.24(0.89-1.73) | 0.196 | 0.96(0.57-1.62) | 0.877 | 1.05(0.72-1.52) | 0.808 |
| PB | 3 | 1.00(0.81-1.24) | 0.970 | 1.12(0.85-1.47) | 0.439 | 1.11(0.83-1.49) | 0.468 | 0.97(0.76-1.24) | 0.818 | 1.03(0.91-1.17) | 0.661 | |
| HWE | YES | 6 | 0.97(0.67-1.40) | 0.874 | 1.06(0.85-1.31) | 0.604 | 1.14(0.91-1.43) | 0.244 | 0.94(0.64-1.38) | 0.764 | 1.01(0.78-1.31) | 0.935 |
| NO | 2 | 1.03(0.53-1.98) | 0.934 | 1.77(0.69-4.53) | 0.237 | 1.80(0.68-4.78) | 0.238 | 0.96(0.49-1.89) | 0.904 | 1.10(0.68-1.78) | 0.691 | |
Sensitivity Analysis
Sensitivity analysis was performed to strengthen the confidence in the results by limiting the included studies according to HWE. After two studies [35,37] without HWE were excluded, the corresponding pooled ORs were not significantly altered. The results were accordance with those of the initial analysis.
Cumulative meta-analysis
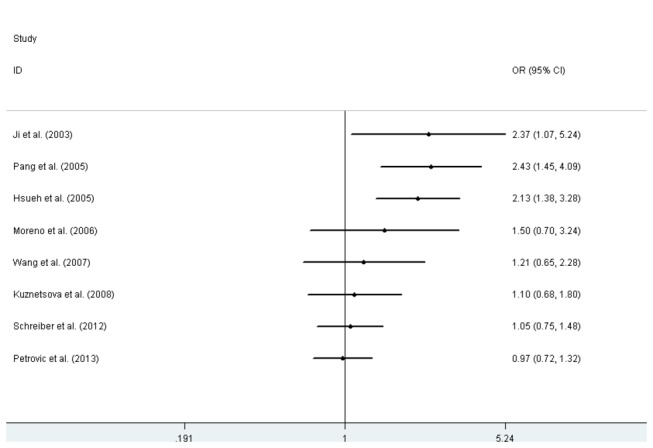
Cumulative analysis of the C242T polymorphism with hypertension was performed according to the publication date. A significant association was confirmed in the dominant contrast among the studies from 2003 to 2005. However, subsequent publications from 2006 to 2013 failed to repeat the initial results: Moreno et al. (OR=1.50, 95%CI: 0.70-3.24), Wang et al. (OR=1.21, 95%CI: 0.65–2.28), Kuznetsova et al. (OR=1.10, 95%CI: 0.68–1.80), Schreiber et al. (OR=1.05, 95%CI: 0.75–1.48), Petrovicet al. (OR=0.97, 95%CI: 0.72–1.32) (Figure 5).
Figure 5. Cumulative plot of the association between the CYBA C242T polymorphism and hypertension for the dominate model chronologically.
Publication Bias
Funnel plots, Begg’s test and Egger's test were performed to assess the publication bias of the studies. The shapes of the funnel plots were visually symmetrical. No publication bias was found regarding the A930G and C242T polymorphism and its association with hypertension by using Egger’s test under the allelic model (t=-1.62, p=0.204/t=0.90, p=0.401), dominant model (t=-2.08, p=0.129/t=0.61, p=0.565), recessive model (t=-0.95, p=0.410/t=2.11, p=0.089), codominant model 1 (t=-0.63, p=0.574/t=0.72, p=0.501), or codominant model 2 (t=-1.71, p=0.186/t=0.56, p=0.597).
Discussion
In this meta-analysis, thirteen qualified articles (five articles with 2003 cases/2434 controls for A930G and eight articles with 2644 cases/1967 controls for C242T) were included. To the best of our knowledge, this is the first meta-analysis to assess the relationship of the A930G and C242T polymorphisms with hypertension. Our results suggest that the A930G polymorphism is associated with hypertension under both the dominant and allelic model, which was in accordance with the results of the studies by Kokubo et al. [29], Moreno et al. [30], and Pang et al. [31]. Large-scale studies are needed to further evaluate the association between the A930G polymorphism and hypertension. However, no significant of association between the C242T polymorphism and hypertension was observed in any genetic model, which was consistent with the findings of the studies by Schreiber et al. [34], Kuznetsova et al. [35] and Wang et al. [38]. In order to assess the robustness of our results, two studies were excluded for deviating from HWE, but the results of our meta-analysis were not altered, suggesting that our initial results were reliable. Even so, additional studies with larger sample sizes are required to further confirm the identified association.
In the stratified analysis by ethnicity, no significant association of the A930G polymorphism with hypertension was detected in any of the genetic models. Similarly, no significant association between the C242T polymorphism and hypertension could be found in any of the genetic models in the further subgroup analysis by ethnicity. According to the data from the International HapMap project, the frequency of the minor G allele in the A930G polymorphism (rs9932581) among the CEU population (northern and western European ancestry) is 0.350; the minor allele frequency (MAF) of the A930G polymorphism is similar among Japanese in Tokyo (JPT) (MAF=0.477), whereas it is A with a frequency of 0.360 among Han Chinese in Beijing (CHB). The MAF of the A930G polymorphism was found to be different between Caucasian populations and Asian Chinese populations according to the HapMap data. However, a sample size of 1515 cases and 2125 controls, which was used to evaluate the genetic susceptibility of hypertension in the Japanese population was larger than that in the Chinese population (206 cases/171 controls). The MAF of the A930G polymorphism is similar in Caucasian and Asian Japanese populations, which is in accordance to our findings that the effect of the A930G polymorphism on hypertension is similar across different ethnic populations. Furthermore, the C242T MAF varies between European and Asian populations, as the frequency of the T allele is higher in Europeans than in Asians (HapMap-CEU: 0.314, HapMap-CHB/JPT: 0.070). Our result is not consistent with the data from the International HapMap project, probably because of the limited eligible studies and sample sizes. Thus, large-scale studies are required to further evaluate our findings.
A significant association between the A930G polymorphism and hypertension was found for the dominant and allelic models in both the hospital-based studies and the population-based studies. However, a significant association was found only in the hospital-based studies for the recessive model and co-dominant model 1, but not in the population-based studies, which might be attributed to differences in the sources of controls. The controls in hospital-based studies might suffer from other diseases that could possibly involve in the same genetic pathogenesis of hypertension. They may not be representative of the general population and result in a false positive. Thus, selective bias, which is likely to affect the quality and reliability of our findings, could not be ignored in the hospital-based studies. Therefore, this positive result should be interpreted with caution. As to the subgroup analysis of studies based on population, only two articles were included in the analysis; therefore, studies with larger sample sizes are required to further confirm our findings. Moreover, no significant association between the C242T polymorphism and hypertension could be found in any of the genetic models in the subgroup analysis according to the source of controls.
The biological mechanism of the A930G and C242T polymorphisms in the physiopathogenesis of hypertension is unclear. ROS have been suggested to play a major role in oxidative stress, and contribute significantly to the development of hypertension [24,25]. Higher production of ROS not only causes the inactivation of NO by an oxidative reaction [45,46], but also produces peroxynitrite [47]. Peroxynitrite mediates the oxidative inactivation of proteins, DNA, and lipids in the vascular endothelium [48], and leads to tissue injury. For this reason, ROS impair endothelial function [49] and significantly contribute to the development of hypertension [25].
According to the publication date, a cumulative analysis of the C242T polymorphism in hypertension was performed. A significant association between the C242T polymorphism and hypertension was confirmed with an increasingly narrow 95% confidence interval (CI) from 2003 to 2005. Nevertheless, no trend for the association between the C242T polymorphism and hypertension was found in subsequent studies from 2006 to 2013, which is in accordance with the results of the current meta-analysis. However, only eight studies were included in the cumulative analysis. To be sure of these results, further studies re required to confirm our findings.
An exploration in the source of heterogeneity was conducted by subgroup analysis. Strong heterogeneity was still observed in studies assessing the relationship between the A930G polymorphism and hypertension when stratified by ethnicity. Thus, Asian and Caucasian ethnicity was likely to be one of the causes of heterogeneity. In addition, the environment that people lived in and genetic variations should be considered as sources of heterogeneity. Similarly, significant heterogeneity still persisted in the subgroup analysis according to the source of controls, which suggested that the source of controls, whether studies were hospital-based or population-based, also had an influence on heterogeneity. Additionally, no obvious changes in heterogeneity could be observed among the studies of Asian populations in the association between the C242T polymorphism and hypertension when stratified by ethnicity. Nevertheless, heterogeneity decreased in studies on Caucasian populations, which indicated that the source of heterogeneity might originate from the Asian ethnicity. Heterogeneity was still significant in hospital-based studies, but obviously reduced in the population-based studies, which suggested that heterogeneity might be attributed to the hospital-based studies. There was considerable variation in the sample sizes of the studies included in this meta-analysis; of the qualified studies on the A930G polymorphism in hypertension, the largest sample size was 1515 cases/2125 controls and the smallest sample size was 83 cases/66 controls. Similarly, of the included studies used to evaluate the association between the C242T polymorphism and genetic susceptibility to hypertension, the largest sample size was 1030 cases/826 controls and the smallest sample size was 57 cases/106 controls. These different sample sizes might also contribute to the source of heterogeneity. Furthermore, other confounding factors, such as matching methods, study design, and individual biological characteristics were identified as a potential source of heterogeneity. Furthermore, there was no publication bias in the present meta-analysis, as a relatively comprehensive search strategy was conducted.
Finally, our results should be considered with some limitations in the present meta-analysis. First, the sample size in this meta-analysis was not large, which limited the statistical power. As to the subgroup analysis by ethnicity, only two Caucasian studies and three Asian studies were included for the A930G polymorphism in hypertension. Similarly, in the subgroup analysis according to the source of controls, only two population-based studies were included for the A930G polymorphism in hypertension, in contrast to three hospital-based studies. In order to better decipher our results, more studies with larger sample sizes are needed in the future. Second, this meta-analysis only focused on the articles from English and Chinese databases without language restrictions, which might lead to a potential language bias. Third, information on confounding factors, such as age, sex, smoking, and drinking, could not be obtained from all the original articles; these are considered effective influencing factors in the pathogenesis of hypertension. In addition, we did not perform an evaluation of potential interactions such as gene-gene or gene-environment, which might be involved in susceptibility to hypertension.
In summary, in the overall analysis, the present meta-analysis shows that the A930G polymorphism contributes to susceptibility to hypertension. Similarly, a statistically significant association was found in the subgroup analysis according to ethnicity. However, there is a lack of evidence to support the association of C242T with hypertension. Further studies considering gene-gene and gene-environment interactions, larger sample sizes, and well-matched controls are needed to identify the association between the A930G and C242T polymorphisms and hypertension.
Supporting Information
PRISMA checklist.
(DOC)
PRISMA 2009 Flow Diagram.
(DOC)
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81260594); the Guangxi National Natural Science Foundation (Grant No. 2013GXNSFAA019145); and the significant scientific research foundation of the Guangxi health department (Grant No.2012047). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Picon RV, Fuchs FD, Moreira LB, Riegel G, Fuchs SC (2012) Trends in prevalence of hypertension in Brazil: a systematic review with meta-analysis. PLOS ONE 7: e48255. doi: 10.1371/journal.pone.0048255. PubMed: 23118964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ma C, Chen S, You L, Luo Z, Xing C (2012) Development and psychometric evaluation of the Treatment Adherence Questionnaire for Patients with Hypertension. J Adv Nurs 68: 1402-1413. doi: 10.1111/j.1365-2648.2011.05835.x. PubMed: 21954893. [DOI] [PubMed] [Google Scholar]
- 3. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K et al. (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224-2260. doi: 10.1016/S0140-6736(12)61766-8. PubMed: 23245609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD et al. (2012) Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation 125: e2-e220. doi: 10.1161/CIR.0b013e31823ac046. PubMed: 22179539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention (CDC) (2012) Vital signs: awareness and treatment of uncontrolled hypertension among adults--United States, 2003-2010. MMWR Morb Mortal Wkly Rep 61: 703-709. PubMed: 22951452. [PubMed] [Google Scholar]
- 6. Arima H, Barzi F, Chalmers J (2011) Mortality patterns in hypertension. J Hypertens 29 Suppl 1: S3-S7. doi: 10.1097/00004872-201106001-00009. PubMed: 22157565. [DOI] [PubMed] [Google Scholar]
- 7. Lawes CM, Vander Hoorn S, Rodgers A (2008) Global burden of blood-pressure-related disease, 2001. Lancet 371: 1513-1518. doi: 10.1016/S0140-6736(08)60655-8. PubMed: 18456100. [DOI] [PubMed] [Google Scholar]
- 8. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R (2002) Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903-1913. doi: 10.1016/S0140-6736(02)11911-8. PubMed: 12493255. [DOI] [PubMed] [Google Scholar]
- 9. Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL (2012) Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet 379: 905-914. doi: 10.1016/S0140-6736(11)61710-8. PubMed: 22293369. [DOI] [PubMed] [Google Scholar]
- 10. Iseki K, Kimura Y, Wakugami K, Okumura K, Muratani H et al. (2000) Comparison of the effect of blood pressure on the development of stroke, acute myocardial infarction, and end-stage renal disease. Hypertens Res 23: 143-149. doi: 10.1291/hypres.23.143. PubMed: 10770261. [DOI] [PubMed] [Google Scholar]
- 11. Gu Q, Dillon CF, Burt VL, Gillum RF (2010) Association of hypertension treatment and control with all-cause and cardiovascular disease mortality among US adults with hypertension. Am J Hypertens 23: 38-45. doi: 10.1038/ajh.2009.191. PubMed: 19851295. [DOI] [PubMed] [Google Scholar]
- 12. Krzesinski JM, Saint-Remy A (2012) Essential hypertension, a complex trait. Rev Med Liege 67: 279-285. PubMed: 22891479. [PubMed] [Google Scholar]
- 13. Ehret GB, Caulfield MJ (2013) Genes for blood pressure: an opportunity to understand hypertension. Eur Heart J 34: 951-961. doi: 10.1093/eurheartj/ehs455. PubMed: 23303660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dinauer MC, Pierce EA, Bruns GA, Curnutte JT, Orkin SH (1990) Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest 86: 1729-1737. doi: 10.1172/JCI114898. PubMed: 2243141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soccio M, Toniato E, Evangelista V, Carluccio M, De Caterina R (2005) Oxidative stress and cardiovascular risk: the role of vascular NAD(P)H oxidase and its genetic variants. Eur J Clin Invest 35: 305-314. doi: 10.1111/j.1365-2362.2005.01500.x. PubMed: 15860042. [DOI] [PubMed] [Google Scholar]
- 16. Rae J, Noack D, Heyworth PG, Ellis BA, Curnutte JT et al. (2000) Molecular analysis of 9 new families with chronic granulomatous disease caused by mutations in CYBA, the gene encoding p22(phox). Blood 96: 1106-1112. PubMed: 10910929. [PubMed] [Google Scholar]
- 17. Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK (1996) p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem 271: 23317-23321. doi: 10.1074/jbc.271.38.23317. PubMed: 8798532. [DOI] [PubMed] [Google Scholar]
- 18. Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R et al. (2004) Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279: 45935-45941. doi: 10.1074/jbc.M406486200. PubMed: 15322091. [DOI] [PubMed] [Google Scholar]
- 19. Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y et al. (2002) Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res 91: 1160-1167. doi: 10.1161/01.RES.0000046227.65158.F8. PubMed: 12480817. [DOI] [PubMed] [Google Scholar]
- 20. Lassègue B, Clempus RE (2003) Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277-R297. PubMed: 12855411. [DOI] [PubMed] [Google Scholar]
- 21. Griendling KK, Sorescu D, Ushio-Fukai M (2000) NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494-501. doi: 10.1161/01.RES.86.5.494. PubMed: 10720409. [DOI] [PubMed] [Google Scholar]
- 22. Katsuyama M (2010) NOX/NADPH oxidase, the superoxide-generating enzyme: its transcriptional regulation and physiological roles. J Pharmacol Sci 114: 134-146. doi: 10.1254/jphs.10R01CR. PubMed: 20838023. [DOI] [PubMed] [Google Scholar]
- 23. Paravicini TM, Touyz RM (2008) NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care 31 Suppl 2: S170-S180. doi: 10.2337/dc08-s247. PubMed: 18227481. [DOI] [PubMed] [Google Scholar]
- 24. Taniyama Y, Griendling KK (2003) Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension 42: 1075-1081. doi: 10.1161/01.HYP.0000100443.09293.4F. PubMed: 14581295. [DOI] [PubMed] [Google Scholar]
- 25. Redón J, Oliva MR, Tormos C, Giner V, Chaves J et al. (2003) Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 41: 1096-1101. doi: 10.1161/01.HYP.0000068370.21009.38. PubMed: 12707286. [DOI] [PubMed] [Google Scholar]
- 26. Zalba G, San José G, Beaumont FJ, Fortuño MA, Fortuño A et al. (2001) Polymorphisms and promoter overactivity of the p22(phox) gene in vascular smooth muscle cells from spontaneously hypertensive rats. Circ Res 88: 217-222. doi: 10.1161/01.RES.88.2.217. PubMed: 11157675. [DOI] [PubMed] [Google Scholar]
- 27. Moreno MU, San José G, Fortuño A, Beloqui O, Redón J et al. (2007) A novel CYBA variant, the -675A/T polymorphism, is associated with essential hypertension. J Hypertens 25: 1620-1626. doi: 10.1097/HJH.0b013e3281ac211d. PubMed: 17620958. [DOI] [PubMed] [Google Scholar]
- 28. Sales ML, Ferreira MC, Leme CA Jr., Velloso LA, Gallani MC et al. (2007) Non-effect of p22-phox -930A/G polymorphism on end-organ damage in Brazilian hypertensive patients. J Hum Hypertens 21: 504-506. PubMed: 17314996. [DOI] [PubMed] [Google Scholar]
- 29. Kokubo Y, Iwai N, Tago N, Inamoto N, Okayama A et al. (2005) Association analysis between hypertension and CYBA, CLCNKB, and KCNMB1 functional polymorphisms in the Japanese population--the Suita Study. Circ J 69: 138-142. doi: 10.1253/circj.69.138. PubMed: 15671602. [DOI] [PubMed] [Google Scholar]
- 30. Moreno MU, San José G, Orbe J, Páramo JA, Beloqui O et al. (2003) Preliminary characterisation of the promoter of the human p22(phox) gene: identification of a new polymorphism associated with hypertension. FEBS Lett 542: 27-31. doi: 10.1016/S0014-5793(03)00331-4. PubMed: 12729892. [DOI] [PubMed] [Google Scholar]
- 31. Pang J HD (2004) Relationship of the Promoter of NAD(P)H Oxidase p22phox Gene Polymorphism to Hypertension. Chin J Hyper 12: 502-504
- 32. Ha DW PJ, (2004) Relationship of P22phox gene 930A/G polymorphism to hypertension in elderly patients. Chin J of Hypertens 24: 1122-1124 [Google Scholar]
- 33. Ji Z LB, Pan SY (2003) Relationship of the p22phox C242T gene polymorphimn to hypertension. Chin J Geriatr Cardiovasc Cerebrovasc Dis 5: 103-105 [Google Scholar]
- 34. Schreiber R, Ferreira-Sae MC, Tucunduva AC, Mill JG, Costa FO et al. (2012) CYBA C242T polymorphism is associated with obesity and diabetes mellitus in Brazilian hypertensive patients. Diabet Med 29: e55-e61. doi: 10.1111/j.1464-5491.2012.03594.x. PubMed: 22268370. [DOI] [PubMed] [Google Scholar]
- 35. Kuznetsova T, Gavrilov DV, Dudanov IP, Makarevich PI, Balatskii AV et al. (2008) Influence of polymorphism's of endothelial nitric oxide synthase gene and polymorphism of NADPH oxidase gene on development of complications of arterial hypertension. Kardiologiia 48: 27-33. [PubMed] [Google Scholar]
- 36. Moreno MU, San José G, Fortuño A, Beloqui O, Díez J et al. (2006) The C242T CYBA polymorphism of NADPH oxidase is associated with essential hypertension. J Hypertens 24: 1299-1306. doi: 10.1097/01.hjh.0000234110.54110.56. PubMed: 16794479. [DOI] [PubMed] [Google Scholar]
- 37. Hsueh YM, Lin P, Chen HW, Shiue HS, Chung CJ et al. (2005) Genetic polymorphisms of oxidative and antioxidant enzymes and arsenic-related hypertension. J Toxicol Environ Health A 68: 1471-1484. doi: 10.1080/15287390590967414. PubMed: 16076760. [DOI] [PubMed] [Google Scholar]
- 38. Wang B WX, (2007) Association of the WNK4 gene and CYBA gene polymorphisms with essential hypertension. Central South Univ.. [Google Scholar]
- 39. Pang J HD, (2005) Association bewteen NAD(p)H oxidase subunit P22phox gene Polymorphysims and Primary hypertension in Wuhan city of Hubei Province. Wuhan Univ.. [Google Scholar]
- 40. Petrovič D (2013) Association of the -262C/T polymorphism in the catalase gene promoter and the C242T polymorphism of the NADPH oxidase P22phox gene with essential arterial hypertension in patients with diabetes mellitus type 2. Clin Exp Hypertens: ([MedlinePgn:]) PubMed: 23701472. [DOI] [PubMed] [Google Scholar]
- 41. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719-748. PubMed: 13655060. [PubMed] [Google Scholar]
- 43. DerSimonian R, Kacker R (2007) Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 28: 105-114. doi: 10.1016/j.cct.2006.04.004. PubMed: 16807131. [DOI] [PubMed] [Google Scholar]
- 44. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177-188. doi: 10.1016/0197-2456(86)90046-2. PubMed: 3802833. [DOI] [PubMed] [Google Scholar]
- 45. Gryglewski RJ, Palmer RM, Moncada S (1986) Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454-456. doi: 10.1038/320454a0. PubMed: 3007998. [DOI] [PubMed] [Google Scholar]
- 46. Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C et al. (2002) Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 105: 1656-1662. doi: 10.1161/01.CIR.0000012748.58444.08. PubMed: 11940543. [DOI] [PubMed] [Google Scholar]
- 47. Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A 87: 1620-1624. doi: 10.1073/pnas.87.4.1620. PubMed: 2154753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL et al. (2000) Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res 86: 960-966. doi: 10.1161/01.RES.86.9.960. PubMed: 10807868. [DOI] [PubMed] [Google Scholar]
- 49. Panza JA, Quyyumi AA, Brush JE Jr., Epstein SE (1990) Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 323: 22-27. doi: 10.1056/NEJM199007053230105. PubMed: 2355955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)
PRISMA 2009 Flow Diagram.
(DOC)


