Abstract
Selected reaction monitoring (SRM) is an accurate quantitative technique, typically used for small-molecule mass spectrometry (MS). SRM has emerged as an important technique for targeted and hypothesis-driven proteomic research, and is becoming the reference method for protein quantification in complex biological samples. SRM offers high selectivity, a lower limit of detection and improved reproducibility, compared to conventional shot-gun based tandem MS (LC-MS/MS) methods. Unlike LC-MS/MS, which requires computationally intensive informatic post-analysis, SRM requires pre-acquisition bioinformatic analysis to determine proteotypic peptides and optimal transitions to uniquely identify and to accurately quantitate proteins of interest. Extensive arrays of bioinformatics software tools, both web-based and stand-alone, have been published to assist researchers to determine optimal peptides and transition sets. The transitions are oftentimes selected based on preferred precursor charge state, peptide molecular weight, hydrophobicity, fragmentation pattern at a given collision energy (CE), and instrumentation chosen. Validation of the selected transitions for each peptide is critical since peptide performance varies depending on the mass spectrometer used. In this review, we provide an overview of open source and commercial bioinformatic tools for analyzing LC-MS data acquired by SRM.
Keywords: Bioinformatics, Mass Spectrometry, Selected Reaction Monitoring, Transition
1. Introduction to the selected reaction monitoring transition process
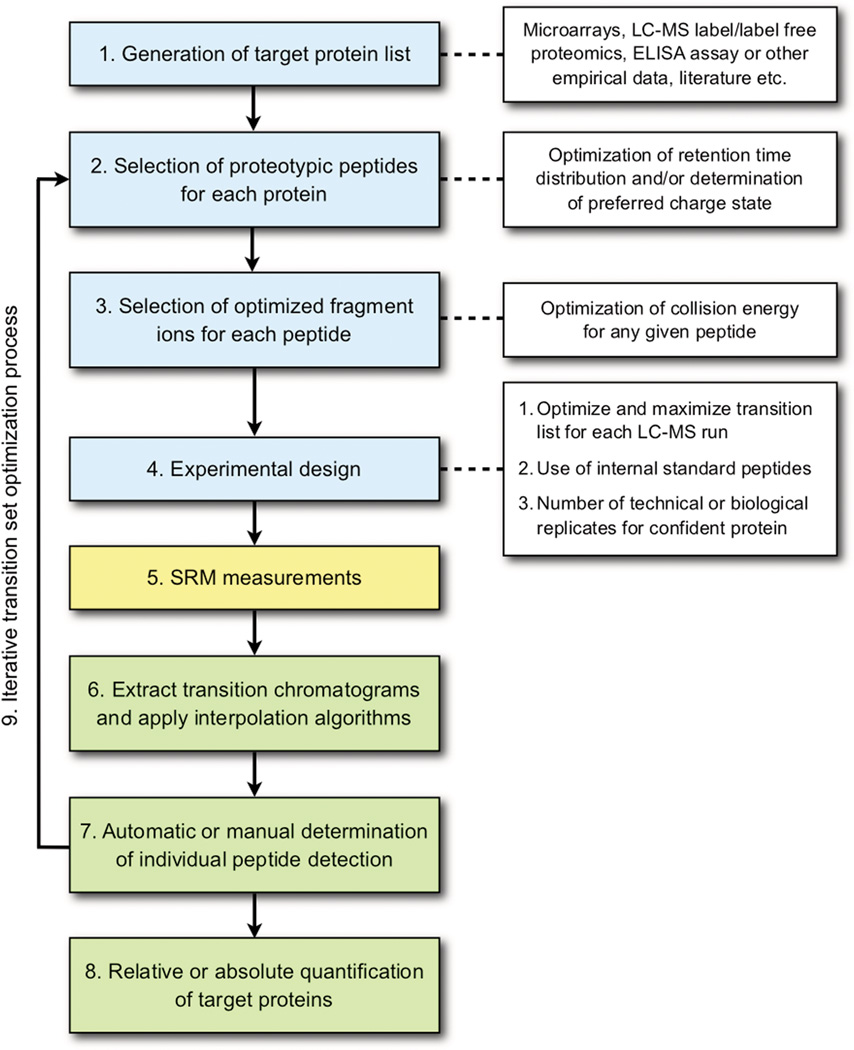
Selected reaction monitoring (SRM) is a mass spectrometric technique that has employed triple-quadrupole instruments (QQQ) since their development primarily for detecting low amounts of small molecules in a sample [1, 2]. Recently, the technique has been adapted for quantitative targeted proteomics [3] (for additional reviews see [4, 5, 6]) requiring software tools for the large volumes of data produced with peptide-based SRM, which is the subject of this review. In a traditional shot-gun LC-MS/MS experiments, the resultant peptide ion fragments from collision-induced dissociation (CID) MS/MS spectra are searched against protein databases using pattern-matching algorithms to assign spectral matches to a given peptide sequence to identify a protein [7]. For accurate determination of the peptide sequence deduced from the often sporadic and incomplete fragmentation profile, additional statistical analyses must also be performed to determine the accuracy of the match and to estimate a false discovery rate (FDR) [8]. Other discovery-based LC-MS/MS experiments, such as label and label-free quantification, also require intensive post data acquisition analysis involving identification of MS/MS spectra, along with determining the relative amount of the proteins present in the sample by spectral count or MS1 profile [9, 10]. Unlike the intensive post data acquisition analysis of discovery-driven LC-MS/MS proteomics experiments, hypothesis-driven SRM experiments require a comprehensive analysis of the peptide fragmentation characteristics given the large variation in fragmentation abundance with QQQ CID. Software tools are available for both pre data acquisition informatics analysis for optimal transition selections and post data acquisition [10]. Figure 1 summarizes a typical bioinformatic processing pipeline required for a typical SRM experiment (blue boxes indicate pre data acquisition processing, green boxes are post data acquisition processing, and white boxes are additional factors to be considered that are associated with the processing steps as indicated by a dotted line).
Figure 1.
Overall bioinformatic processing steps in SRM-based proteomics studies.
For a successful SRM experiment for a set of proteins of interest, (Fig 1, box 1), one first selects proteotypic peptides (PTPs) for each protein accounting for the charge state (Fig 1, box 2) and the optimal collision energy for each peptide (Fig 1, box 3), both being essential to yield optimal fragment ions. In general, an optimal transition set can be defined as one that generates a limit of detection (LOD) and limit of quantification (LOQ) calibration curve to determine reproducible quantification with high sensitivity in the lower femtomole or upper attomole range by selecting peptide ions that display high efficiency of ionization and fragmentation. Peptides and their fragment ions are selected using rule-based prediction models or from observed spectra libraries from similar MS instrument types.
To maximize the number of transitions in a single SRM LC-MS run, a dynamic SRM experiment is an optimal approach where retention time optimization (Fig 1, box 3) is a critical factor since the transitions are to be scanned only within a discrete retention time window derived from either prediction models [11] or empirical methods [12]. When SRM measurements are composed of both isotopically labeled (e.g, N15 or C13) and endogenous peptides, the transition list should account for either heavy or light matched pairs of Q1 (precursor m/z) and Q3 (fragment m/z) for each labeled and endogenous peptide pair. In this case, the increased number of transitions should be considered when optimizing dynamic SRM transition lists. Published SRM studies have demonstrated the reproducibility of optimized transitions for analysis of plasma samples [13] as well as successful implementation in a large biomarker verification study [14], for example. In addition to its use in biomarker verification studies, the SRM technique can be used for general proteomic studies. In a comprehensive SRM-based study of total yeast lysate tryptic digests to understand central carbon metabolism of yeast, Picotti et al. demonstrated the ability to quantify proteins in the range of 40 to 1X 106 protein copies per cell [15].
Software tools (both free and open source) for selecting optimal SRM transitions have been recently reviewed by Cham et al. [16], and thus are not covered in this review. Instead, in this article, we survey both publicly available and commercial software for analyzing SRM data results to address the processes indicated in the green boxes in Figure 1.
2. Open source tools for SRM post-acquisition analysis
Post-analysis of SRM acquired data has three conceptual analytical modules (Fig 1, boxes 6–8). The first step (Fig 1, box 6) is the extraction of chromatograms from measured data, using m/z and retention time with respective tolerances from transition input Q1 m/z, Q3 m/z, and retention time values. Most of the software tools reviewed have one or more smoothing algorithms that can be applied to the extracted data. The second step (Fig 1, box 7) is the detection of confidently identified transitions. The third step (Fig 1, box 8) is to select quantifiable transitions and determine either absolute or relative quantification of each protein from all the peptides and transitions belonging to the target protein under investigation. The software programs reviewed in this paper perform one or more of the processes shown in Figure 1 green boxes in either a fully automated or semi-automated manner. We use the term “fully automated” for software that does not allow manual transition verification/quantification adjustment. For analytical platform that provide a rough estimation of verification/quantification for transition sets, allowing users to reclassify verification status or quantification value, we annotated the software as “semi-automated”.
Table 1 provides a general overview of open source software tools available for SRM post-acquisition analysis, along with input formats (Fig 1, box 6). For the identification of the transitions (Fig 1, box 7), MRMer [17] and Skyline [18] allow users to determine the acceptance and rejection of transitions by manual selection of transitions and inspection of each transition. Additionally, both MRMer and Skyline allow users to interactively select the start and stop retention times of the curve that is used for quantification for a given transition, and to manually select/unselect verified transitions for a given peptide ion. To compute accurate error rates, mProphet, a semi-supervised learning algorithm is used for the identification of target peptides in SRM data in a fully automated manner algorithm [19]. mProphet uses the “decoy transition concept” to maximize the separation of targets and decoys, thereby improving the confidence of the identifications. This is performed by selecting and weighting a user-given parameter set in an iterative manner. mQuest [19] and ATAQS [20] generate parameters for transition properties (e.g., retention time deviation, dot product of transition intensity between light and heavy) as a preprocessor to mProphet for the SRM dataset under analysis. A unique feature to ATAQS, provides an interface for the user not only to select optimum transitions of given peptides, but also to select biologically relevant proteins using PIPE2 [21].
Table 1.
General Overview of Open source Tools for SRM Post Acquisition.
 |
 |
 |
 |
||||
|---|---|---|---|---|---|---|---|
| License | Open Source (Apache 2.0) |
Open Source (Apache 2.0) |
Free | Open Source (Apache 2.0) |
Open Source (Apache 2.0) |
Open Source (Apache 2.0) |
Open Source (Apache 2.0) |
| Input files and format (such as transition list in txt and .raw for SRM data measurement) |
mzXML | .raw, .d, .wiff and mzXML |
.cvs file containing sample classification, replicate, peptide, transition and standard and indigenous quantification |
mzXML | mzXML and mzML | tsv file containing peptide group, transitions and parameters for semi- supervised learning algorithm |
tsv containg protein, peptide, transition intensity, sample classification |
| Multi Input file Processing Capability |
No (Single mzXML) | Yes | Single .csv | Yes | Yes | one file containing data from many SRM runs |
one file containing quantification data from many SRM runs |
| Operating System | Windows, Linux and Mac |
Windows | Windows, Linux and Mac by web browser |
Windows VM | Linux | Windows & Linux | Windows, Linux and Mac |
| Web based or stand- alone |
stand-alone | stand-alone | Web service by GenePattern website |
stand-alone | stand-alone | stand-alone | stand-alone |
| Reference | http://proteomics.fhcrc.org/CPL/MRMer.html | https://skyline.gs.washington.edu/labkey/project/home/software/Skyline/begin.view | http://www.broadinstitute.org/cancer/software/genepattern/modules/AuDIT.html | http://tools.proteomecenter.org/ATAQS/ATAQS.html | http://www.mprophet.org/ | http://www.stat.purdue.edu/~ovitek/Software.html | |
| Applications | Yeast | Plasma | Plasma | Spiked in heavy/light peptides in C. elegans, H. sapiens cell, interrogans. |
Yeast and Human Cancer Cell Line |
Human Cell Line | Yeast and Human Plasma |
| Internal Standard Peptide Measurement Required |
No | No | Yes | No | Yes | No | No |
| Software Language | Java | C/C# | R | perl | Java | R | R |
| Automation | Manual | semi-automated | fully automated | fully automated | fully automated | fully automated | fully automated |
While mProphet uses all transitions to determine the validation of peptides in the sample, AuDIT [22] automatically detects imprecise transitions for each peptide using the t-test and coefficient of variation (CV) between endogenous analyte and internal standard peptide transitions, if such have been used. mProphet and AuDIT are automated modules that can be used to generate probability estimates for observed peptides and transition level accuracy.
For inferring protein quantification (Fig 1, box 8), some published SRM measurements of biological samples have used only one peptide and one transition for protein quantification by highly optimizing transition selection using limit of detection (LOD) and limit of quantification (LOQ) data. However, there is still some debate regarding the minimum number of transitions and peptides necessary per protein [23]. Some SRM measurements have used 3 to 5 or more transitions and peptide quantifications to estimate changes in individual protein levels [24]. SRMStat employs user-filtered transitions, and takes the transition quantification values to infer protein-level abundance changes by comparing the protein level of quantification among classes of samples (e.g., case vs. control) [25].
3. Commercial tools for SRM post-acquisition analysis
All mass spectrometers offer vendor-specific software tools to analyze SRM data acquired using their instruments. MultiQuant™ is a software tool from ABSciex for processing SRM data generated on the QTRAP® LC-MS/MS systems. MultiQuant™ processes the ABSciex .wiff file formatted LC-MS/MS data to provide quantitative peak information from targeted m/z values eluted from a chromatographic column. MultiQuant™ supports relative and absolute quantitation experiments as well as the use of unlabeled or stable isotope-labeled internal standards and offers a customizable user interface for data review, which includes an option to overlay transitions. Acquired peaks can be automatically integrated using either the default integration values or user-defined integration settings such as Gaussian smooth width and noise percentage. Peak integration can be manually adjusted and data queries can be established to shorten the manual peak review process. Using a defined name convention (e.g. {Protein Name}.{Peptide Sequence}.{Transition}.{Light or Heavy}) in ABSciex’s instrument operation software, Analyst 1.5, enables automatic calculation of peak area ratios and creation of quantitation methods. MultiQuant™ supports the generation of standard concentration curves for absolute quantification and provides a statistical summary, including standard deviation, percent CV and accuracy to evaluate the quality of the standard concentration curve. For absolute quantification, the software also allows the removal of outliers and calculates concentration and accuracy of the endogenous biological analyte. In addition, the software provides an audit trail with electronic signature functionality. MultiQuant™ has been widely used in a number of different SRM studies [13, 15, 26–32] and also supports processing MIDAS™ (MRM triggered MS/MS) workflow datasets as well as MRM3 data [33] as well as TOF MS and MRMHR workflows on the TripleTOF 5600 system.
Thermo Scientific instruments come with Pinpoint™ software that supports the iSRM workflow on the TSQ line of triple quadrupole mass spectrometers (e.g., TSQ Quantum, and TSQ Vantage QQQ) and orbitrap LC-MS/MS series (e.g., Q-Exactive) mass spectrometers. Pinpoint™ allows the user to perform pre-SRM data acquisition assay development as well as post acquisition data analysis in a stepwise manner, allowing the user to develop and analyze an experiment within a single software program. The features for data analysis of Pinpoint™ are similar to those of Skyline, where the transitions are rated, and graphical plots such as retention time reproducibility and collision energy optimization can be generated to visualize the data. Pinpoint™ supports .raw file formats and provides a user-friendly interface for each protein, peptide and transition level of the quantification method. The user also has the ability to manually validate transitions and peptides and quantify proteins. Pinpoint™ has been successfully used to study human proteins [35, 36], the yeast proteome [37] and identify 16 protein biomarkers in urine samples [38].
MassHunter™ Workstation Quantitative and Qualitative Analysis (Agilent Technologies, Inc) is a commercial program with two separate software platforms for the quantitation and analysis of SRM data from their 6400 series triple quadrupoles and has been shown to be successful for human proteomic studies. Domanski et al. analyzed 26 plasma proteins using both the 6490 QQQ (ifunnel) with MassHunter™ and the 4000 QTrap with MultiQuant™ and provided illustrations of the chromatograms of the individual integrated SRM traces for all the targeted peptides across a gradient and determined the protein quantitation using the MassHunter™ Workstation [28]. One of the highlights of the MassHunter™ Workstation’s Qualitative Analysis is the software’s ability to generate composite MS2 spectra based on the transitions included in the SRM traces. Table 2 summarizes the commercial software tools for SRM post-acquisition analysis.
Table 2.
General Overview of Commercial Software Tools for SRM Post Acquisition.
 |
 |
 |
 |
|
|---|---|---|---|---|
| Name | MultiQuant™ | Pinpoint™ | MassHunter™ | TargetLynx™ |
| License | Commercial | Commercial | Commercial | Commercial |
| Input files and its format (such as transition list in txt and .raw for SRM data measurement) |
.wiff files (created by Analyst® instrument control software), transition list can be imported in Analyst® as Microsoft Excel csv file |
.raw | .d | .raw directory |
| Multi Input file Processing Capability |
Yes | Yes | Yes | Yes |
| Operating System | Windows | Windows | Windows | Windows |
| Reference paper or website of the software |
stand-alone | stand-alone | stand-alone | stand-alone |
| Reference paper or website of the software |
http://www.absciex.com/Products/Software/MultiQuant-Software | http://www.thermoscientific.com/ecomm/servlet/productsdetail?productId=12784473&&storeId=11152 | http://www.chem.agilent.com/en-US/products/software/chromatography/ms/masshunterworkstation/Pages/default.aspx | http://www.waters.com/waters/nav.htm?cid=513164 |
| Applications | C. elegans, Yeast, Human Cell line |
Human Plasma and Urine, yeast |
Human Plasma | Human blood, serum, CSF |
| Interner Standard Peptide Measurement Required |
No | No | No | Yes |
| Automation | semi-automated | semi-automated | semi-automated | fully automated |
Waters® Corporation offers both QuanLynx™ and TargetLynx™ as post-acquisition quantitative analysis applications as components within their MassLynx® software suite, and these applications are able to measure analyte concentrations from SRM, single-ion recording (SIR), and full-scan acquisition data acquired from a variety of Waters GC/MS and LC/MS instruments. Pertaining to peptide MRM, TargetLynx™ offers additional features beyond QuanLynx™, and accepts quantitation methods imported from Waters Verifye™, a pre-acquisition SRM development software, as well as methods imported from Waters Quanpedia™, a database tool that stores chromatography, mass spectral, and quantitation information. QuanLynx™ software has been used to measure ceruloplasmin in blood [39], prostate-specific antigen in serum [40], and angiotensin IV in rat brain dialysates [41], while TargetLynx™ has been used to measure amyloid beta peptides in cerebrospinal fluid [41] and hydroxyproline-containing peptides in blood [43].
4. Discussion
As a guide to the current availability of software programs for processing SRM data, we review here the functionality of several open source and commercially available programs. While all of the software presented here allow for manual integration and validation, some tools do offer fully automated data analysis modes, using predefined parameters for simple procedures such as auto detection of the transitions, peak integration, signal-to-noise calculations, and transition validation. Although successful in performing these routines, manual correction and validation of transitions is still necessary due to the inherent limitations of SRM, namely misidentification of peaks and incorporation of contaminating transitions due to precursor and fragment ion pairs with similar masses. However, many of these assessments can vary since the interpretations are manual entries and are user-dependent. There are several publications demonstrating the application of SRM for proteomics where much of the data analysis was performed through manual inspection as part of the validation process. This is especially important for those peptides that have low signal-to-noise. For peptides with low signal-to-noise without alternative peptides, this can be a difficult task, due to the limited number of data points measured for each transition. Therefore, the choice of smoothing algorithms can result in significant changes in quantitation at the peptide and protein level. The start and end of a trace can also pose a problem if the analyte itself has chromatographic characteristics such as splitting into multiple peaks. For example, midsized to long peptides can containing one or more proline residues which can produce more than one 3D confirmation per peptide. This results in the peptide eluting as multiple peaks from a RP-HPLC column for the same peptide composition. Such analytes can be avoided in the SRM assay development process for a given protein by redirecting the processing step indicated as step 9 in Figure 1. However, the chromatographic behavior of each analyte has to be well understood before manual integration of the transition trace curve. Another consideration is how many transitions and peptides ions are needed to infer protein quantification.
Since there is variability with manual inspection and validation, the concepts of qualifier and quantifier have been introduced. More specifically, several transitions for a given peptide ion are measured to validate the identification of a peptide, otherwise known as qualifiers. However, only subsets of the transitions, oftentimes the transition(s) with the highest intensities, are used for quantification of the peptide, also known as the quantifier. Some of the quantifications of proteins given in the papers cited here were performed with only one quantifier transition, and proteins quantified in this manner will not produce accurate CV values, whereas CVs generated from multiple proteotypic peptides for a given protein will provide more confidence in protein quantifications (with low CV) or can provide additional biological insights for proteins of interest (with high CV pattern among the set of peptides) such as proteolytic variants. Currently, there is limited evidence and only a few rigorous studies that have defined acceptable values for the number of transitions or the CV requirement [44]. There are no consensus guidelines regarding the number of transitions, CV requirement, or validation by SRM technology. However, guidelines do exist for bioanalytical method validation in small molecule analysis that could be used as a starting point for the proteomics field [45]. Ludwig et al. presents parameters for estimating absolute protein quantities in unlabeled samples by SRM using the two most abundant transitions of the top three ionizing peptides per protein in Leptospira interrogans lysate [46]. Therefore careful consideration must be made in selecting the PTPs and fragment ions for any given protein (boxes 2 and 3, Figure 1).
Moreover, retention time optimization of the peptide ion is also a critical aspect to consider in SRM assay development (box 2 association, Figure 1). Furthermore, the retention time window for acquisition, along with choice of a smoothing algorithm, also affects the transition validation process. Data supplied through a central resource at the ISB through the SRMAtlas (www.srmatlas.org) will address this issue of providing substantive information on peptide fragmentation across a broad CE range, accurate retention time, and chromatographic performance over a wide population of ~200,000 proteotypic peptides. Another consideration is the potential for use of internal standards, such as stable isotope labeled peptides, in an experiment where quantitation is desired (association box for box 4, Figure 1). Internal standards add confidence in scheduled SRM experiments and depending on the use of labeled peptides or labeled proteins, lower CV’s can be obtained as each addresses a different level of integration in the assay. Isotopically labeled proteins provide the greatest control of the SRM experiment and address the issues of digestion, etc., and they can be incorporated into automated approaches in SRM data analysis, but their use is limited by both the cost and availability of equivalent protein standards.
5. Concluding remarks and outlook
With the emergence of SRM as the method of choice for quantitating specific targeted proteins in biological samples, there has been extensive parallel development of software tools by both commercial instrumental vendors as well as the academic sector to assist scientists with the post data acquisition end of the pipeline. Thus, there is now a fairly wide selection of tools available for both the experienced users, as well as those researchers interested in adopting SRM methods as part of their own research workflow, which we have outlined and discussed here. However, post acquisition methodologies for automatically selecting qualifiers and quantifiers, assigning statistical probability to SRM and automation of SRM data processing remain works in progress. We expect that continued development of high-throughput and robust SRM assays in parallel with development of software tools to analyze such high-throughput SRM data will be essential to both proteomics and systems biology, as they pertain to furthering the goals of measuring responses to specific perturbations within simple systems such as enzymatic reactions, cells and higher animal models, and diagnosing and treating human health and disease.
Acknowledgements
We thank Julie Bletz for editorial assistance. R.M. acknowledges the generous support of National Institutes of Health, National Human Genome Research Institute (grant HG005805), the National Institute for General Medical Sciences (grant P50 GM076547/Center for Systems Biology) and the Grand Duchy of Luxembourg Systems Biology initiative.
Abbreviations
- CE
collision energy
- FDR
false discovery rate
- PTP
proteotypic peptides
- SIR
single-ion recording
- QQQ
triple quadrupole
Footnotes
The authors declare no financial/commercial conflicts of interest with the work presented here.
References
- 1.Baty JD, Robinson PR. Single and multiple ion recording techniques for the analysis of diphenylhydantoin and its major metabolite in plasma. Biomed. Mass spectrom. 1977;4:36–41. doi: 10.1002/bms.1200040104. [DOI] [PubMed] [Google Scholar]
- 2.Kovarik P, Grivet C, Bourgogne E, Hopfgartner G. Method development aspects for the quantitation of pharmaceutical compounds in human plasma with a matrixassisted aser desorption/ionization source in the multiple reaction monitoring mode. Rapid Commun. Mass Spectrom. 2007;21:911–919. doi: 10.1002/rcm.2912. [DOI] [PubMed] [Google Scholar]
- 3.Yocum AK, Chinnaiyan AM. Current affairs in quantitative targeted proteomics: multiple reaction monitoring-mass spectrometry. Briefings Funct. Genomics Proteomics. 2009;8(2):145–157. doi: 10.1093/bfgp/eln056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lange V, Picotti P, Domon B, Aebersold R. Review selected reaction monitoring for quantitative proteomics. Mol. Syst. Biol. 2008;4:222. doi: 10.1038/msb.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallien S, Duriez E, Domon B. Selected reaction monitoring applied to proteomics. J. Mass Spectrom. 2011;46(3):298–312. doi: 10.1002/jms.1895. [DOI] [PubMed] [Google Scholar]
- 6.James A, Jorgensen C. Basic design of MRM assays for peptide quantification. Methods Mol. Biol. 2010;658:167–185. doi: 10.1007/978-1-60761-780-8_10. [DOI] [PubMed] [Google Scholar]
- 7.Domon B, Aebersold R. Options and considerations when selecting a quantitative proteomics strategy. Nat. Biotechnol. 2010;28(7):710–721. doi: 10.1038/nbt.1661. [DOI] [PubMed] [Google Scholar]
- 8.Nesvizhski A. A survey of computational methods and error rate estimation procedures for peptide and protein identification in shotgun proteomics. J. Proteomics. 2010;71(11):2092–2123. doi: 10.1016/j.jprot.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller L, Brusniak M-Y, Mani DR, Aebersold R. An assessment of software solutions and applied LC-MS data types for quantitative LC-MS based proteomics studies. J. Proteome Res. 2008;7(01):51–61. doi: 10.1021/pr700758r. [DOI] [PubMed] [Google Scholar]
- 10.Christin C, Bischoff R, Hovatovich P. Data processing pipelines for comprehensive profiling of proteomics sample by label-free LC-MS for biomarker discovery. Talanta. 2011:1209–1224. doi: 10.1016/j.talanta.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Krokhin OV, Spicer V. Chapter 13:Predicting Peptide Retention Times for Proteomics. Current Protocols in Bioinformatics. 2010 doi: 10.1002/0471250953.bi1314s31. [DOI] [PubMed] [Google Scholar]
- 12.Mirzaei H, Brusniak M-Y, Mueller L, LeTarte S, Watts JD, Aebersold R. Halogenated-peptides as internal standards (H-PINS) Introduction of a MS-based internal standard set of liquid chromatography mass spectrometry. Mol. Cell. Proteomics. 2009;8:1934–1946. doi: 10.1074/mcp.M800569-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzyk MA, Smith D, Yang J, Cross TJ, Jackson AM, Hardie DB, Anderson L, Borchers CH. Multiple Reaction Monitoring-based multiplexed, absolute quantitation of 45 proteins in human plasma. Mol. Cell. Proteomics. 2009;8(8):1860–1877. doi: 10.1074/mcp.M800540-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoenherr RM, Kelly-Spratt KS, Lin C, Whiteaker JR, Liu T, Holzman T, et al. Proteome and transcriptome profiles of a Her2/Neudriven mouse model of breast cancer. Proteomics Clin. 2011;5:179–188. doi: 10.1002/prca.201000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picotti P, Bodenmiller B, Mueller LN, Domon B, Aebersold R. Full Dynamic Range Proteome Analysis of S. cerevisiae by Targeted Proteomics. Cell. 2009;138:795–806. doi: 10.1016/j.cell.2009.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cham JA, Bianco L, Bessant C. Free computational resources for designing selected reaction monitoring transitions. Proteomics. 2010;10:1106–1126. doi: 10.1002/pmic.200900396. [DOI] [PubMed] [Google Scholar]
- 17.Martin DB, Holzman T, May D, Peterson A, Eastham A, et al. MRMer: An interactive open-source and cross-platform system for data extraction and visualization of Multiple Reaction Monitoring experiments. Mol. Cell. Proteomics. 2008;7(1):2270–2278. doi: 10.1074/mcp.M700504-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26(7):966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiter L, Rinner O, Picotti P, Huttenhain R, et al. mProphet: automated data processing and statistical validation for large-scale SRM experiments. Nature Methods. 2011;8:430–435. doi: 10.1038/nmeth.1584. [DOI] [PubMed] [Google Scholar]
- 20.Brusniak M-Y, Kwok S-T, Christiansen M, Campbell D, Reiter L, et al. ATAQS: A computational software tool for high throughput transition optimization and validation for selected reaction monitoring mass spectrometry. BCM Bioinformatics. 2011;12:78. doi: 10.1186/1471-2105-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramos H, Shannon P, Brusniak M-Y, Kusebauch U, Moritz RL, et al. The Protein Information and Property Explorer 2: Gaggle-like exploration of biological proteomic data within one webpage. proteomics. 2011;11:154–158. doi: 10.1002/pmic.201000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbatiello SE, Mani DR, Keshishian H, Carr SA. Automated detection of inaccurate and imprecise transitions in peptide quantification by multiple reaction monitoring mass spectrometry. Clin. Chem. 2010;56(2):291–305. doi: 10.1373/clinchem.2009.138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinsinger CR, Apffel J, Baker M, Bian X, Borchers C, et al. Recommendations for Mass Spectrometry Data Quality Metrics for Open Access Data (Corollary to the Amsterdam Principles) Proteomics. 2011 doi: 10.1002/pmic.201100562. [DOI] [PubMed] [Google Scholar]
- 24.Lange V, Malmstrom JA, Didion J, King NL, Johansson BP, Schafer J, Rameseder J, Wong C-H, Deutsch EW, Brusniak M-Y, Buhlmann P, Bjorck L, Bommon B, Aebersold R. Targeted quantitative analysis of Streptococcus pyogenes virulence factors by multiple reaction monitoring. Mol. Cell. Proteomics. 2008;7(8):1489. doi: 10.1074/mcp.M800032-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang C-Y, Picotti P, Huttenhain R, Heinzelmann-schwarz V, Jovanovic M, Aebersold R, Vitek O. Protein significance analysis in selected reaction monitoring (SRM) measurements. Mol. Cell. Proteomics. doi: 10.1074/mcp.M111.014662. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jovanovic M, Reiter L, Picotti P, Lange V, et al. A quantitative targeted proteomics approach to validate predicted microRNA targets in C. elegans. Nat. Meth. 2010;7:837–842. doi: 10.1038/nmeth.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costenoble R, Picotti P, Reiter L, Stallmach R, et al. Comprehensive quantitative analysis of central carbon and amino-acid metabolism in Saccharomyces cerevisiae under multiple conditions by targeted proteomics. Mol. Syst. Biol. 2011;7:464. doi: 10.1038/msb.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domanski D, Murphy LC, Borchers CH. Assay Development for the Determination of Phosphorylation Stoichiometry Using Multiple Reaction Monitoring Methods with and without Phosphatase Treatment: Application to Breast Cancer Signaling Pathways. Anal. Chem. 2010;82:5610–5620. doi: 10.1021/ac1005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keshishian H, Addona T, Burgess M, Mani DR, et al. Quantification of Cardiovascular Biomarkers in Patient Plasma by Targeted Mass Spectrometry and Stable Isotope Dilution. Mol. Cell. Proteomics. 2009;8:2339–2349. doi: 10.1074/mcp.M900140-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whiteaker JR, Zhao L, Abbatiello SE, Burgess M, et al. Evaluation of Large Scale Quantitative Proteomic Assay Development Using Peptide Affinity-based Mass Spectrometry. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M110.005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy PJ, Pinto DM. Targeted Proteomic Analysis of Glycolysis in Cancer Cells. J. Proteome Res. 2010;10:604–613. doi: 10.1021/pr100774f. [DOI] [PubMed] [Google Scholar]
- 32.Anderson L, Hunter CL. Quantitative Mass Spectrometric Multiple Reaction Monitoring Assays for Major Plasma Proteins. Mol. Cell. Proteomics. 2006;5:573–588. doi: 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Wolf-Yadlin A, Hautaniemi S, Lauffenburger DA, White M. Multiple reaction monitoring for robust quantitative proteomic analysis of cellular signaling networks. PNAS. 2007;104(14):5860–5865. doi: 10.1073/pnas.0608638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fortin T, Salvador A, Charrier JP, Lenz C, et al. Multiple Reaction Monitoring Cubed for Protein Quantification at the Low Nanogram/Milliliter Level in Nondepleted Human Serum. Anal. Chem. 2009;81:9343–9352. doi: 10.1021/ac901447h. [DOI] [PubMed] [Google Scholar]
- 35.Campbell J, Rezai T, Prakash A, Krastins B, Dayon L, et al. Evaluation of absolute peptide quantitation strategies using selected reaction monitoring. Proteomics. 2011;11(6):1148–1152. doi: 10.1002/pmic.201000511. [DOI] [PubMed] [Google Scholar]
- 36.Prakash A, Rezai T, Krastins B, Sarracino D, et al. Platform for establishing interlaboratory reproducibility of selected reaction monitoring-based mass spectrometry peptide assays. J. Proteome. Res. 2010;9(12):6678–6688. doi: 10.1021/pr100821m. [DOI] [PubMed] [Google Scholar]
- 37.Kiyonami R, Schoen A, Prakash A, Peterman S, Zabrouskov V, Picotti P, Aebersold R, Huhmer A, Domon B. Increased selectivity, analytical precision, and throughput in targeted proteomics. Mol. Cell. Proteomics. 2011;10(2):M110 002931. doi: 10.1074/mcp.M110.002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selevsek N, Matondo M, Sanchez Carbayo M, Aebersold R, Domon B. Systematic quantification of peptides/proteins in urine using selected reaction monitoring. Proteomics. 2011;11(6):1135–1147. doi: 10.1002/pmic.201000599. [DOI] [PubMed] [Google Scholar]
- 39.DeWilde A, Sadilkova K, Sadilek M, Vasta V, Hahn S. Tryptic peptide analysis of ceruloplasmin in dried blood spots using liquid chromatography-tandem mass spectrometry: application to newborn screening. Clin Chem. 2008;54(12):1961–1968. doi: 10.1373/clinchem.2008.111989. [DOI] [PubMed] [Google Scholar]
- 40.Barnidge DR, Goodmanson MK, Klee GG, Muddiman DC. Absolute quantification of the model biomarker prostate-specific antigen in serum by LC-Ms/MS using protein cleavage and isotope dilution mass spectrometry. J. Proteome. Res. 2004;3(3):644–652. doi: 10.1021/pr049963d. [DOI] [PubMed] [Google Scholar]
- 41.Lanckmans K, Sarre S, Smolders I, Michotte Y. Use of a structural analogue versus a stable isotope labeled internal standard for the quantification of angiotensin IV in rat brain dialysates using nano-liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2007;21(7):1187–1195. doi: 10.1002/rcm.2950. [DOI] [PubMed] [Google Scholar]
- 42.Lame ME, Chambers EE, Blatnik M. Quantitation of amyloid beta peptides Abeta(1–38), Abeta(1–40), and Abeta(1–42) in human cerebrospinal fluid by ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2011;419(2):133–139. doi: 10.1016/j.ab.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Ichikawa S, Morifuji M, Ohara H, Matsumoto H, Takeuchi Y, Sato K. Hydroxyproline-containing dipeptides and tripeptides quantified at high concentration in human blood after oral administration of gelatin hydrolysate. Int. J. Food. Sci. Nutr. 2010;61(1):52–60. doi: 10.3109/09637480903257711. [DOI] [PubMed] [Google Scholar]
- 44.Addona T, Abbatiello SE, Schiling B, Skates SJ, et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring–based measurements of proteins in plasma. Nature Biotechnology. 2009;27:633–641. doi: 10.1038/nbt.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guidance for Industry Bioanalytical Method Validation. 2001 http://www.mendeley.com/research/guidance-industry-bioanalytical-method-validation-guidance-industry-bioanalytical-method-validation/
- 46.Ludwig C, Claassen M, Schmidt A, Aebersold R. Estimation of Absolute Protein Quantities of Unlabeled Samples by Selected Reaction Monitoring Mass Spectrometry. Mol. Cell. Proteomics. 2011 Nov; doi: 10.1074/mcp.M111.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]