Abstract
Importance
The use of advanced treatment technologies (ie, intensity-modulated radiotherapy [IMRT] and robotic prostatectomy) for prostate cancer is increasing. The extent to which these advanced treatment technologies have disseminated among patients at low risk of dying from prostate cancer is uncertain.
Objective
To assess the use of advanced treatment technologies, compared with prior standards (ie, traditional external beam radiation treatment [EBRT] and open radical prostatectomy) and observation, among men with a low risk of dying from prostate cancer.
Design, Setting, and Patients
Using Surveillance, Epidemiology, and End Results (SEER)-Medicare data, we identified a retrospective cohort of men diagnosed with prostate cancer between 2004 and 2009 who underwent IMRT (n=23 633), EBRT (n=3926), robotic prostatectomy (n=5881), open radical prostatectomy (n=6123), or observation (n=16 384). Follow-up data were available through December 31, 2010.
Main Outcomes and Measures
The use of advanced treatment technologies among men unlikely to die from prostate cancer, as assessed by low-risk disease (clinical stage ≤T2a, biopsy Gleason score ≤6, and prostate-specific antigen level ≤10 ng/mL), high risk of noncancer mortality (based on the predicted probability of death within 10 years in the absence of a cancer diagnosis), or both.
Results
In our cohort, the use of advanced treatment technologies increased from 32% (95% CI, 30%–33%) to 44% (95% CI, 43%–46%) among men with low-risk disease (P<.001) and from 36% (95% CI, 35%–38%) to 57% (95% CI, 55%–59%) among men with high risk of noncancer mortality (P<.001). The use of these advanced treatment technologies among men with both low-risk disease and high risk of noncancer mortality increased from 25% (95% CI, 23%–28%) to 34% (95% CI, 31%–37%) (P<.001). Among all patients diagnosed in SEER, the use of advanced treatment technologies for men unlikely to die from prostate cancer increased from 13% (95% CI, 12%–14%), or 129.2 per 1000 patients diagnosed with prostate cancer, to 24% (95% CI, 24%–25%), or 244.2 per 1000 patients diagnosed with prostate cancer (P<.001).
Conclusion and Relevance
Among men diagnosed with prostate cancer between 2004 and 2009 who had low-risk disease, high risk of noncancer mortality, or both, the use of advanced treatment technologies has increased.
Prostate cancer is a common and expensive disease in the United States.1,2 In part because of the untoward morbidity of traditional radiation and surgical therapies, advances in the treatment of localized disease have evolved over the last decade. Chief among these are the development of intensity-modulated radiotherapy (IMRT) and robotic prostatectomy. Although the evidence underlying these technologies is mixed,3,4 both are generally perceived as being more targeted and less toxic than prior therapies. During a period of increasing population-based rates of prostate cancer treatment,5,6 both of these advanced treatment technologies have disseminated rapidly.
However, the rapid growth of IMRT and robotic prostatectomy may have occurred among men with a low risk of dying from prostate cancer.7,8 Recognizing the protracted clinical course for most of these cancers, clinical guidelines recommend local treatment only for men with at least a 10-year life expectancy.9,10 Men with low-risk cancer have a particularly favorable prognosis, with a much greater likelihood of dying from other causes even 20 years after diagnosis.11 Because many older men will die with rather than from prostate cancer, observation (with delayed intervention if needed) has been promoted by some experts,12 although the extent to which this has gained traction in the community is uncertain. Aggressive direct-to-consumer marketing and incentives associated with fee-for-service payment may promote the use of these advanced treatment technologies.
For these reasons, we performed a study to better understand relationships among the use of advanced treatment technologies (ie, IMRT and robotic prostatectomy), prior standards (ie, traditional external beam radiation treatment [EBRT] and open radical prostatectomy), and observation for men with a low risk of dying from prostate cancer. Understanding patterns of new technology use in this population is particularly important given the growing concerns about overtreatment.
METHODS
We used Surveillance, Epidemiology, and End Results (SEER)-Medicare data to identify patients with newly diagnosed prostate cancer between 2004 and 2009, with follow-up data available through December 31, 2010. SEER represents approximately 26% of the US population.13 Using the Medicare Provider Analysis and Review (MEDPAR), outpatient, and carrier files, we further identified men aged 66 years or older who were primarily treated with IMRT (Healthcare Common Procedure Coding System [HCPCS] codes 77418, G0174, and 0073T), EBRT (HCPCS codes 77401-77416), robotic prostatectomy (HCPCS code 55866), open radical prostatectomy (HCPCS codes 55840, 55842, 55845), and observation (ie, no treatment) within the first 12 months of diagnosis. We included only fee-for-service beneficiaries who were eligible for both Medicare Parts A and B from 12 months prior to diagnosis to 12 months after diagnosis. Men aged 65 years were excluded to ensure accurate comorbidity estimation using Medicare claims for the 12-month period prior to diagnosis.14
Outcomes
The objective of our study was to compare the use of advanced treatment technologies to treat prostate cancer (ie, IMRT or robotic prostatectomy) with prior standards (ie, EBRT or open radical prostatectomy) and observation. Because we were primarily interested in the use of these treatments among men who would least likely benefit from any treatment, we focused on 3 groups of patients: men with low-risk prostate cancer, those with a high risk of non-cancer mortality, and those with both low-risk disease and a high risk of non-cancer mortality.
We defined low-risk prostate cancer (clinical stage ≤T2a, biopsy Gleason score ≤6, and prostate-specific antigen [PSA] level ≤10 ng/mL), intermediate-risk prostate cancer (clinical stage T2b, biopsy Gleason score=7, or PSA level between 10.1 and 20 ng/mL), and high-risk prostate cancer (clinical stage ≥T2c, biopsy Gleason score ≥8, or PSA level >20 ng/mL) according to the well-established D’Amico classification.15 Generally speaking, patients with low-risk disease are the least likely to die from prostate cancer.16 For example, men older than 65 years with low-risk disease have about a 25% chance of dying from prostate cancer 20 years after diagnosis but more than a 60% chance of dying of other causes.11
Thus, we also chose to examine treatment among men at high risk of non-cancer mortality. Specifically, we assessed the probability of noncancer mortality within 10 years, largely because clinical guidelines recommend active treatment only in men with a life expectancy of at least a decade.9,10
We estimated each patient’s probability of dying within 10 years in the absence of a cancer diagnosis based on methods established by Gross et al.17 Briefly, using the 5% random sample of Medicare beneficiaries without cancer, we modeled mortality as a function of demographic data available in the Medicare Surveillance Summarized Denominator (SUMDENOM) file as well as comorbidity measurements extracted from the MEDPAR, outpatient, and carrier files. Specifically, we generated a random sample of patients within the SUMDENOM file (n=5000). Because these patients do not have a cancer diagnosis date, an index date was chosen based on the following 2 criteria: (1) the date had to be prior to December 31, 1999, allowing for the possibility of 10 years of follow-up; (2) patients had to be enrolled in both Medicare Parts A and B for 12 months prior to the index date to ensure accurate comorbidity estimation.14 Of note, patients who died within this follow-up window were observed and censored at death.
Using this sample, we modeled the probability of dying within 10 years based on age, race, comorbidity, socioeconomic class, area of residence (eg, rural vs urban), and SEER region. This model was internally cross-validated using a bootstrap aggregating technique.18 The discrimination of the prediction model was high (C index= 0.90). We applied this model to men diagnosed with prostate cancer as their first and only cancer to estimate their probability of dying within 10 years. We sorted patients into terciles based on this mortality probability.
In addition, we estimated the use of prostate cancer treatments for men least likely and most likely to benefit. When we calculated the percentage of men treated, the numerator was determined by the sum of the number of men least likely to benefit (ie, those with low-risk disease or a high risk of noncancer mortality) or the number of men most likely to benefit (ie, those with high-risk disease or a low risk of non-cancer mortality). The denominator was the total number of Medicare beneficiaries diagnosed with prostate cancer during the study period.
Statistical Analysis
We first compared demographics and clinical characteristics of patients treated with IMRT, EBRT, robotic prostatectomy, open radical prostatectomy, and observation using χ2 tests. For each of the 3 groups of patients (those with low-risk disease, high risk of noncancer mortality, and both), we fit multinomial logistic regression models.19 To assess the fit of these models, we used a likelihood ratio test for the null hypothesis that independent variables have no effect on treatment type (P <.001). We assessed the use of advanced treatment technologies, prior standards, and observation among men least likely to benefit. We then looked more critically at advanced treatment technologies by examining IMRT and robotic prostatectomy individually. All models were adjusted for age, race, socioeconomic class, comorbidity, tumor grade, year of diagnosis, SEER region, and disease risk, where appropriate.
Race was self-determined by patients and was examined because it has been shown to influence cancer treatment.20 Socioeconomic class was ascertained at the zip code level using the approach of Diez Roux et al.21 Because it is difficult to interpret the marginal effect of an independent variable on treatment probabilities directly from a multinomial regression model’s coefficient estimates, we back-transformed the model estimates into predicted probabilities. We derived 95% CIs using bootstrapping (n=1000).
We performed several sensitivity analyses to assess the strength of our results. First, to examine the robustness of our predicted model of 10-year mortality, we sorted patients into quartiles, which generated fairly similar mortality probabilities for the lowest and highest groups. Second, because some case records lack the specific staging information to precisely classify disease risk (eg, differentiate T2a from T2b from T2c), we repeated our analyses after excluding these patients. Third, because of the concern that older patients would generally not be considered for surgery, we reran our analyses stratified by age. These additional analyses did not alter our findings, so we present results from our primary analyses only.
All analyses were performed using SAS version 9.2 and R version 2.11.22 The probability of a type I error was set at .05 and all testing was 2-sided. Because patients cannot be identified, the institutional review board of the University of Michigan exempted this study from review.
RESULTS
Our study population included men who had prostate cancer as their first and only cancer (n=83 191). We then excluded those with metastatic disease (n=2621) and those not receiving one of the designated treatments (n=21 898). Among these men, we further excluded those with missing information needed to classify disease risk (ie, missing stage, Gleason score, or PSA levels; n=1647) or with missing demographic information (n=1078). Using these criteria, our study population consisted of 23 633 IMRT, 3926 EBRT, 5881 robotic prostatectomy, 6123 open radical prostatectomy, and 16 384 observation patients. When we applied our predictive model to our cohort of prostate cancer patients, the predicted mortality rates at 10 years were 19%, 61%, and 91% for patients in low, intermediate, and high terciles, respectively.
As shown in the Table, the use of IMRT and observation was more common than EBRT, robotic prostatectomy, and open radical prostatectomy among Medicare beneficiaries with prostate cancer. The use of advanced treatment technologies increased over time, particularly for robotic prostatectomy, while the use of prior standards significantly decreased. Throughout the study period, the overall treatment of prostate cancer was relatively stable. For example, in 2004 and 2009, there were 6556 and 6169 patients, respectively, treated with either surgery or some form of external beam radiotherapy (ie, IMRT or EBRT). Intensity-modulated radiotherapy was a common method of treatment regardless of socioeconomic class and disease risk. Robotic prostatectomy use was more common among younger men, while those undergoing observation tended to have low-risk disease. Many SEER regions that contained a large number of patients treated with advanced treatment technologies also had a relatively large number of patients treated with prior standards or observation (eg, Greater California, New Jersey, Seattle, and Detroit).
Table.
Demographics and Clinical Characteristics of the Study Population
| Characteristics | No. (%)a | P Valueb | |||||
|---|---|---|---|---|---|---|---|
| IMRT (n = 23 633) | EBRT (n = 3926) | Robotic Prostatectomy (n = 5881) | Open Radical Prostatectomy (n = 6123) | Observation (n = 16 384) | |||
| Age, y 66–69 |
5536 (23) |
978 (25) |
3334 (57) |
3479 (57) |
3766 (23) |
|
<.001 |
| 70–74 | 8540 (36) | 1409 (36) | 2071 (35) | 2174 (36) | 4557 (28) | ||
| 75–79 | 6670 (28) | 1063 (27) | 438 (7) | 437 (7) | 4086 (25) | ||
| >80 | 2887 (12) | 476 (12) | 38 (<1) | 33 (<1) | 3975 (24) | ||
| Race/ethnicity White |
19 232 (81) |
3207 (82) |
5056 (86) |
5238 (86) |
13 236 (81) |
|
<.001 |
| Black | 2508 (11) | 454 (12) | 349 (6) | 461 (8) | 2044 (12) | ||
| Hispanic | 498 (2) | 96 (2) | 66 (1) | 127 (2) | 306 (2) | ||
| Asian | 700 (3) | 100 (3) | 152 (3) | 114 (2) | 412 (3) | ||
| Other | 695 (3) | 69 (2) | 258 (4) | 183 (3) | 386 (2) | ||
| Socioeconomic class Low |
6151 (26) |
1390 (35) |
1289 (22) |
1703 (28) |
4889 (30) |
|
<.001 |
| Medium | 10 305 (44) | 1372 (35) | 1843 (31) | 1930 (32) | 5836 (36) | ||
| High | 7177 (30) | 1164 (30) | 2749 (47) | 2490 (41) | 5659 (35) | ||
| Comorbidity score 0 |
14 612 (62) |
2415 (62) |
4501 (77) |
4537 (74) |
10 830 (66) |
|
<.001 |
| 1 | 5697 (24) | 992 (25) | 1048 (18) | 1214 (20) | 3241 (20) | ||
| 2 | 2019 (9) | 324 (8) | 243 (4) | 265 (4) | 1313 (8) | ||
| ≥3 | 1305 (6) | 195 (5) | 89 (2) | 107 (2) | 1000 (6) | ||
| Tumor grade Well or moderately differentiated |
8482 (36) |
1260 (32) |
1971 (34) |
2276 (37) |
10 565 (64) |
|
<.001 |
| Poorly differentiated or undifferentiated | 15 151 (64) | 2666 (68) | 3910 (66) | 3847 (63) | 5819 (36) | ||
| Clinical stage T1 |
13 080 (55) |
1923 (49) |
3551 (60) |
3099 (51) |
9611 (59) |
|
<.001 |
| T2 | 9614 (41) | 1809 (46) | 2237 (38) | 2867 (47) | 6571 (40) | ||
| T3/T4 | 939 (4) | 194 (5) | 93 (2) | 157 (3) | 202 (1) | ||
| Disease risk classification Low |
6266 (27) |
785 (20) |
1663 (28) |
1730 (28) |
8871 (54) |
|
<.001 |
| Intermediate | 8870 (38) | 1427 (36) | 3022 (51) | 2765 (45) | 4618 (28) | ||
| High | 8497 (36) | 1714 (44) | 1196 (20) | 1628 (27) | 2895 (18) | ||
| Risk of noncancer mortality Low |
6644 (28) |
1015 (26) |
3169 (54) |
3078 (50) |
4113 (25) |
|
<.001 |
| Intermediate | 8307 (35) | 1417 (36) | 2058 (35) | 2276 (37) | 4977 (30) | ||
| High | 8682 (37) | 1494 (38) | 654 (11) | 769 (13) | 7294 (45) | ||
| Year of diagnosis 2004 |
2990 (13) |
1618 (41) |
432 (7) |
1516 (25) |
2624 (16) |
|
<.001 |
| 2005 | 3609 (15) | 927 (24) | 614 (10) | 1219 (20) | 2661 (16) | ||
| 2006 | 4237 (18) | 613 (16) | 906 (15) | 1056 (17) | 2889 (18) | ||
| 2007 | 4569 (19) | 415 (11) | 1191 (20) | 976 (16) | 2893 (18) | ||
| 2008 | 4195 (18) | 191 (5) | 1345 (23) | 775 (13) | 2737 (17) | ||
| 2009 | 4033 (17) | 162 (4) | 1393 (24) | 581 (9) | 2580 (16) | ||
| SEER regionc San Francisco |
715 (3) |
216 (6) |
216 (4) |
190 (3) |
748 (5) |
|
<.001 |
| Connecticut | 1766 (7) | 196 (5) | 303 (5) | 187 (3) | 1011 (6) | ||
| Detroit (Metropolitan) | 2046 (9) | 369 (9) | 479 (8) | 257 (4) | 1243 (8) | ||
| Hawaii | 470 (2) | 35 (1) | 137 (2) | 47 (1) | 186 (1) | ||
| Iowa | 1168 (5) | 337 (9) | 286 (5) | 513 (8) | 750 (5) | ||
| New Mexico | 627 (3) | 44 (1) | 87 (1) | 268 (4) | 588 (4) | ||
| Seattle (Puget Sound) | 1110 (5) | 271 (7) | 406 (7) | 625 (10) | 1338 (8) | ||
| Utah | 214 (<1) | 250 (6) | 121 (2) | 421 (7) | 653 (4) | ||
| Atlanta (Metropolitan) | 1002 (4) | 128 (3) | 69 (1) | 94 (2) | 535 (3) | ||
| San Jose | 629 (3) | 96 (2) | 110 (2) | 111 (2) | 457 (3) | ||
| Los Angeles | 1529 (6) | 141 (4) | 719 (12) | 600 (10) | 1185 (7) | ||
| Greater California | 3733 (16) | 630 (16) | 1468 (25) | 1536 (25) | 3423 (21) | ||
| Kentucky | 1276 (5) | 489 (12) | 518 (9) | 353 (6) | 1187 (7) | ||
| Louisiana | 1810 (8) | 220 (6) | 296 (5) | 497 (8) | 1094 (7) | ||
| New Jersey | 5466 (23) | 475 (12) | 658 (11) | 418 (7) | 1948 (12) | ||
Abbreviations: EBRT, traditional external beam radiation treatment; IMRT, intensity-modulated radiotherapy; SEER, Surveillance, Epidemiology, and End Results.
Percentages might not sum to 100 because of rounding.
P values generated from χ2 tests.
Data not shown for 153 patients in the SEER Rural Georgia registry according to SEER-Medicare guidelines.
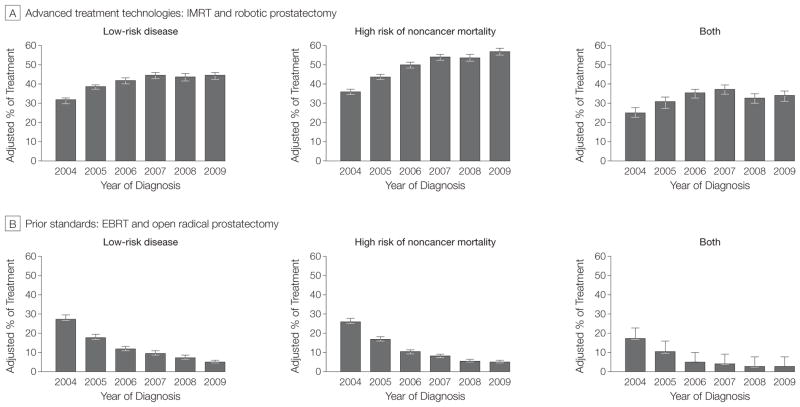
We next assessed the treatment of prostate cancer among patients least likely to benefit from treatment because of either nonaggressive cancer biology or competing causes of death (Figure 1). The use of advanced treatment technologies was common among men with low-risk disease (an increase from 32% [95% CI, 30%–33%] in 2004 to 44% [95% CI, 43%–46%] in 2009), those with a high risk of non-cancer mortality (from 36% [95% CI, 35%–38%] in 2004 to 57% [95% CI, 55%–59%] in 2009), and those with both low-risk disease and a high risk of noncancer mortality (from 25% [95% CI, 23%–28%] in 2004 to 34% [95% CI, 31%–37%] in 2009). For each of these 3 groups of patients, there was greater use of advanced treatments over time, particularly among those with an increased probability of noncancer mortality (P <.001 for trend). Conversely, there was a decreased use of prior standards across these 3 groups of patients (P <.001 for trend), making the overall use of treatment among men least likely to benefit relatively stable over time.
Figure 1.
Treatment of Prostate Cancer Among Men Who Are the Least Likely to Benefit
The percentage of treatment was adjusted for age, race, socioeconomic class, comorbidity, tumor grade, year of diagnosis, and Surveillance, Epidemiology, and End Results region. Use of advanced treatment technologies significantly increased among prostate cancer patients with low-risk disease, high risk of noncancer mortality, and both low-risk disease and high risk of noncancer mortality (P<.001 for trend). Conversely, there was a decreased use of prior standards among these patients (P<.001 for trend), making the overall use of treatment relatively stable over time. Error bars indicate 95% CIs; EBRT, traditional external beam radiation treatment; IMRT, intensity-modulated radiotherapy.
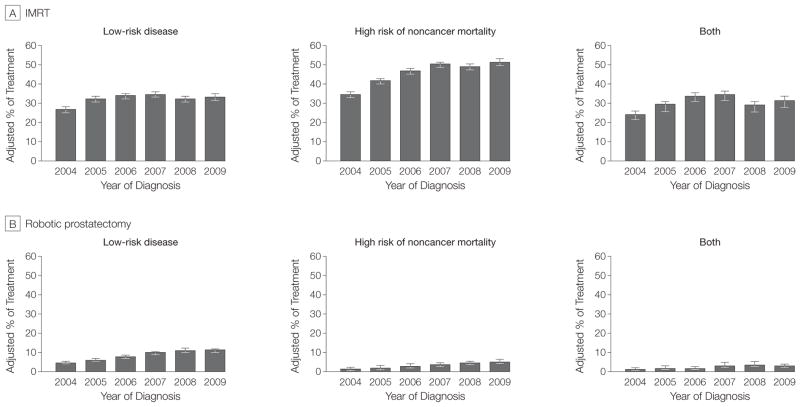
We next examined the use of IMRT and robotic prostatectomy separately among men who are the least likely to benefit (Figure 2). Use of IMRT increased among men with low-risk disease (from 27% [95% CI, 25%–28%] in 2004 to 33% [95% CI, 32%–35%] in 2009), those with a high risk of non-cancer mortality (from 35% [95% CI, 33%–36%] in 2004 to 52% [95% CI, 50%–53%] in 2009), and those with both low-risk disease and high risk of noncancer mortality (from 24% [95% CI, 22%–26%] in 2004 to 32% [95% CI, 28%–34%] in 2009; P <.001 for trend). Although less common than IMRT, robotic prostatectomy use increased over time among men with low-risk disease (from 5% [95% CI, 4%–6%] in 2004 to 11% [95% CI, 10%–12%] in 2009), those with a high risk of non-cancer mortality (from 2% [95% CI, 1%–2%] in 2004 to 5% [95% CI, 5%–6%] in 2009), and those with both low-risk disease and a high risk of noncancer mortality (from 1% [95% CI, <1%–2%] in 2004 to 3% [95% CI, 2%–4%] in 2009; P <.001 for trend).
Figure 2.
Use of Advanced Treatment Technologies Among Prostate Cancer Patients Who Are the Least Likely to Benefit
The percentage of treatment was adjusted for age, race, socioeconomic class, comorbidity, tumor grade, year of diagnosis, and Surveillance, Epidemiology, and End Results region. Use of intensity-modulated radiotherapy (IMRT) and robotic prostatectomy both increased among men who stand the least to gain in terms of survival (both P<.001 for trend). The majority of these patients were treated with IMRT. Error bars indicate 95% CI.
In addition, we estimated the use of prostate cancer treatments for men least likely (Figure 3) and most likely to benefit (Figure 4). Among Medicare beneficiaries diagnosed with prostate cancer, the use of advanced treatment technologies for men unlikely to die of prostate cancer increased from 13% (95% CI, 12%–14%) in 2004 to 24% (95% CI, 24%–25%) in 2009, a relative increase of 85% (P <.001, Pearson χ2 test). That is, rates of IMRT and robotic prostatectomy use increased from 129.2 per 1000 patients in 2004 to 244.2 per 1000 patients diagnosed with prostate cancer in 2009. At the same time, the use of prior standard treatments for men least likely to benefit decreased from 11% (95% CI, 10%–11%) in 2004 to 3% (95% CI, 2%–3%) in 2009 (P <.001, Pearson χ2 test). For these men, rates of EBRT and open radical prostatectomy use decreased from 106.9 per 1000 patients diagnosed with prostate cancer in 2004 to 27.2 per 1000 patients diagnosed with prostate cancer in 2009. The estimated use of advanced treatment technologies for men most likely to benefit (ie, those with high-risk disease or a low risk of non-cancer mortality) increased 11%, while the use of prior standards decreased 10% over the study period (both P <.001, Pearson χ2 test).
Figure 3.
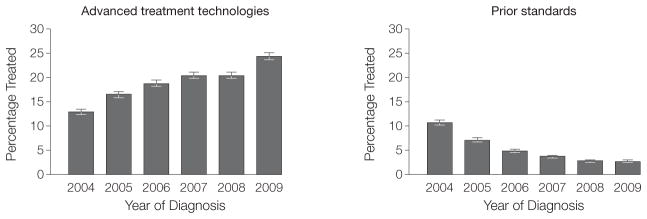
Estimating the Use of Prostate Cancer Treatments for Men Least Likely to Benefit
Among Medicare beneficiaries diagnosed with prostate cancer, the use of advanced treatment technologies increased from 13% (95% CI, 12%–14%) in 2004 to 24% (95% CI, 24%–25%) in 2009 for men unlikely to die of prostate cancer (P <.001, Pearson χ2 test). At the same time, the use of prior standard treatments for these patients decreased 8% (P <.001, Pearson χ2 test).
Figure 4.
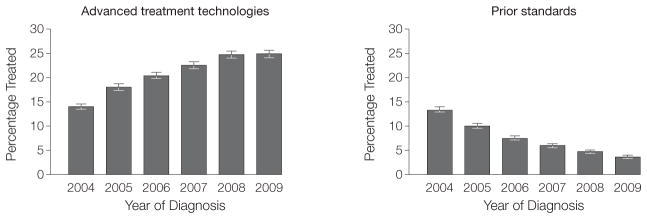
Estimating the Use of Prostate Cancer Treatments for Men Most Likely to Benefit
Among Medicare beneficiaries diagnosed with prostate cancer, the use of advanced treatment technologies for men most likely to benefit (ie, those with high-risk disease or a low risk of noncancer mortality) increased 11%, while the use of prior standards decreased 10% over the study period (both P <.001, Pearson χ2 test).
DISCUSSION
The use of advanced treatment technologies for prostate cancer was common among patients with low-risk disease and among those with a high risk of noncancer mortality. The most common treatment modality was IMRT, which accounted for the greatest use among men who stand the least to gain in terms of survival. The increasing use of both IMRT and robotic prostatectomy in populations unlikely to benefit from treatment was largely explained by their substitution for the treatments they aim to replace, namely EBRT and open radical prostatectomy.
The absolute magnitude of the use of advanced treatment technologies in these populations has 2 important implications. First, both treatments are considerably more expensive than the prior standards. Start-up costs for both approach $2 million.5,7 Further, IMRT is associated with higher total episode payments, which translate into an additional $1.4 billion in spending annually.23 Thus, the implications of any potential overtreatment with these advanced treatment technologies are amplified in financial terms. Second, and perhaps more important, the implementation of these technologies in populations unlikely to benefit from treatment occurred during a time of increasing awareness about the indolent nature of some prostate cancers and of growing dialogue about limiting treatment in these patients.11,12 Our findings suggest that, even during this period of enhanced stewardship, incentives favoring the diffusion of these technologies outweighed those related to implementing a more conservative management strategy.
There are several potential explanations as to why this might be the case. First, there is uncertainty about the natural history of prostate cancer. Even for low-risk cancer, patients or physicians may be reluctant to observe when there is a high chance of cure with treatment. Patients who choose observation may live with a high level of anxiety knowing that they have cancer. Further, there is potential to underestimate the severity of disease based on the prostate biopsy.24 These concerns aside, the outcomes of men with low-risk disease following conservative management (ie, observation) are fairly well established,16 causing some to even push for removing the label of cancer from low-risk tumors.25 Second, a perceived improvement in outcomes compared with prior alternatives may make these advanced treatment technologies seem more palatable. However, comparative studies have shown that the advantages of these newer treatments are marginal at best.3,4 Third, financial incentives—through ownership opportunities,7 growing market share,26 and fee-for-service reimbursement23—may be too strong to overcome.
In quantifying the use of advanced treatment technologies among men who are unlikely to benefit from treatment, we rely on 2 measures of patient risk. The first is the risk of death from prostate cancer, which is determined entirely by the biology of the disease itself. It is well established that men with low-risk disease—as assessed by low grade, stage, and PSA level (the criteria used in this study)—have an extremely low chance of dying from prostate cancer, particularly in the elderly population.16 Because of its protracted course, clinical guidelines recommend treating prostate cancer only in men expected to live at least 10 years,9,10 which informs our second measure of patient risk, noncancer mortality. For this competing measure of mortality, we calculated the 10-year probability of death in a cohort of patients without prostate cancer. Although our model lacked certain information (eg, baseline functional status), we were able to generate a robust model with high discrimination (C index=0.90) by incorporating several conditions closely associated with mortality.17,27 Although neither risk assessment is absolute, both are well informed by the expansive knowledge about the natural history of prostate cancer and provide an objective approach for quantifying the potential overtreatment burden.
Another limitation of our study is the inclusion of only Medicare beneficiaries 66 years or older. Younger men are frequently diagnosed with prostate cancer. However, nearly two-thirds of men with prostate cancer are older than 65 years.28 In addition, we are unaware of a biological rationale for why the use of advanced treatment technologies would be different across ages. For these reasons, our findings are informative to the vast majority of men with prostate cancer.
These limitations notwithstanding, the increasing use of advanced treatment technologies among men unlikely to die of prostate cancer carries significant policy implications, particularly in light of the recent US Preventive Services Task Force recommendations. The task force recently recommended against screening asymptomatic men for prostate cancer, largely based on the evidence that PSA screening results in the detection of many cases of indolent cancer that remain insignificant over a person’s lifespan.29 However, by using treatments more selectively, the benefits of screening may become clearer. For instance, in the European Randomized Study of Screening for Prostate Cancer trial, higher-grade cancer was less common in the screened group (as compared with the control group), with a 40% lower incidence of locally advanced and meta-static disease.30 From our findings, the use of advanced treatment technologies is not much higher in men who would most likely benefit (ie, those with high-risk disease and low risk of non-cancer mortality).
There are 2 potential reasons for this observation. First, the ability to risk-stratify patients is imperfect. However, the development of molecular markers and identification of gene signatures are examples of ongoing research that may make it easier to differentiate indolent disease from “bad actors” in the near future. Second, it is difficult for clinicians to evaluate a patient’s risk of noncancer mortality.31,32 For example, clinicians inaccurately predict 10-year life expectancy nearly 20% of the time.32 With this in mind, clinicians and policy makers could better focus efforts on identifying men who will benefit from screening and subsequent treatment, rather than on eliminating screening efforts altogether.
At the same time, more diligence is needed to reduce the potentially unnecessary treatment of men with a low risk of dying from prostate cancer. Patients with medical conditions that portend poor prognoses are still aggressively treated with radiation or surgery for their prostate cancer.31 A Swedish study demonstrated that patients diagnosed with indolent prostate cancer account for a substantial and growing part of the increase in radical prostatectomies.8 Although the majority of physicians consider active surveillance an effective strategy, less than one-third of urologists and radiation oncologists recommend surveillance over treatment for patients with low-risk disease.33 Understanding the underpinnings of the discrepancy between these physician beliefs and what is occurring in practice will be fundamental to broadening the use of observation among appropriate patients. Furthermore, garnering support for surveillance in an environment that encourages the adoption of novel technologies will remain a challenge.
Research and policy change represent 2 avenues that could affect the current treatment patterns for prostate cancer. The Surveillance Therapy Against Radical Treatment (START) trial is a randomized controlled trial that will provide valuable information regarding the effectiveness of radiation, surgery, and active surveillance for low-risk prostate cancer.34 However, because of the protracted natural history of prostate cancer, results from this trial are not expected for another 5 to 10 years. In the interim, well-designed observational studies can inform this debate. More immediately, policy change may help curtail the excessive use of advanced treatment technologies among patients least likely to benefit. For example, value-based insurance design encourages the use of services when clinical benefits outweigh the cost and discourages their use when the benefits do not justify the cost.35 Taken together, efforts in these domains can help eliminate the overuse of advanced treatment technologies when patients stand little to gain.
In conclusion, we found that the use of advanced treatment technologies increased among men diagnosed with prostate cancer between 2004 and 2009 who had low-risk disease, high risk of noncancer mortality, or both. Continued efforts to differentiate indolent from aggressive disease and to improve the prediction of patient life expectancy may help reduce the use of advanced treatment technologies in this patient population.
Acknowledgments
Funding/Support: Dr Jacobs is supported in part by an American Cancer Society Postdoctoral Fellowship Grant (121805-PF-12-008-01-CPHPS). Dr Schroeck is supported in part by an American Cancer Society Postdoctoral Fellowship Grant (PF-12-118-01-CPPB) and by a National Institutes of Health Training Grant (NIH 5 T32 DK007782-12). Dr Miller is supported in part by Blue Cross Blue Shield of Michigan for efforts as director of the Michigan Urological Surgery Improvement Collaborative. Dr Hollenbeck is supported in part by the National Cancer Institute (R01-CA-168691-01).
Role of the Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Schroeck reported having received an honorarium for writing a CME review article from the American Urological Association. Dr Skolarus reported being a consultant for ArborMetrix. Dr Montie reported having owned stock or stock options in HistoSonics. Dr Miller reported being a consultant for United Healthcare. Dr Hollenbeck is an Associate Editor of Urology. No other disclosures were reported.
Disclaimer: The views expressed in this article do not reflect the views of the federal government.
Online-Only Material: The Author Video Interview is available at http://www.jama.com.
Author Contributions: Dr Jacobs had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Jacobs, Zhang, Montie, Miller, Hollenbeck.
Acquisition of data: Jacobs, Zhang, Skolarus.
Analysis and interpretation of data: Jacobs, Zhang, Schroeck, Skolarus, Wei, Gilbert, Strope, Dunn, Miller, Hollenbeck.
Drafting of the manuscript: Jacobs, Zhang, Skolarus, Dunn, Hollenbeck.
Critical revision of the manuscript for important intellectual content: Jacobs, Zhang, Schroeck, Skolarus, Wei, Montie, Gilbert, Strope, Miller, Hollenbeck.
Statistical analysis: Jacobs, Zhang, Schroeck, Dunn. Obtained funding: Jacobs, Schroeck, Miller, Hollenbeck.
Administrative, technical, or material support: Skolarus.
Study supervision: Skolarus, Montie, Gilbert, Miller, Hollenbeck.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Roehrig C, Miller G, Lake C, Bryant J. National health spending by medical condition, 1996–2005. Health Aff (Millwood) 2009;28(2):w358–w367. doi: 10.1377/hlthaff.28.2.w358. [DOI] [PubMed] [Google Scholar]
- 3.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302(14):1557–1564. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs BL, Zhang Y, Skolarus TA, et al. Comparative effectiveness of external-beam radiation approaches for prostate cancer. Eur Urol. doi: 10.1016/j.eururo.2012.06.055. [published online July 6, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbash GI, Glied SA. New technology and health care costs: the case of robot-assisted surgery. N Engl J Med. 2010;363(8):701–704. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs BL, Zhang Y, Skolarus TA, Hollenbeck BK. Growth of high-cost intensity-modulated radio-therapy for prostate cancer raises concerns about overuse. Health Aff (Millwood) 2012;31(4):750–759. doi: 10.1377/hlthaff.2011.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carreyrou J, Tamman M. A device to kill cancer, lift revenue. [Accessed May 1, 2013];Wall Street Journal. 2010 Dec 7; http://online.wsj.com/article/SB10001424052748703904804575631222900534954.html?KEYWORDS=device+to+kill+cancer+lift+revenue+john+carreyrou+maurice+tamman+december+7+2010.
- 8.Etzioni R, Mucci L, Chen S, Johansson JE, Fall K, Adami HO. Increasing use of radical prostatectomy for nonlethal prostate cancer in Sweden. Clin Cancer Res. 2012;18(24):6742–6747. doi: 10.1158/1078-0432.CCR-12-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8(2):162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 10.Heidenreich A, Bellmunt J, Bolla M, et al. European Association of Urology. EAU guidelines on prostate cancer: part 1, screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 11.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293 (17):2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 12.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126–131. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 13.Altekruse SF, Kosary CL, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 14.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 15.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 16.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11):1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross CP, McAvay GJ, Krumholz HM, Paltiel AD, Bhasin D, Tinetti ME. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Ann Intern Med. 2006;145(9):646–653. doi: 10.7326/0003-4819-145-9-200611070-00006. [DOI] [PubMed] [Google Scholar]
- 18.Buhlmann P, Yu B. Analyzing bagging. Ann Stat. 2002;30(4):927–961. [Google Scholar]
- 19.Allison PD. Logistic Regression Using SAS: Theory and Application. Cary, NC: SAS Institute; 1999. [Google Scholar]
- 20.Smedley BD, Stitch AY, Nelson AR, editors. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 21.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 22.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 23.Konski A, Watkins-Bruner D, Feigenberg S, et al. Using decision analysis to determine the cost-effectiveness of intensity-modulated radiation therapy in the treatment of intermediate risk prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66(2):408–415. doi: 10.1016/j.ijrobp.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 24.Grossfeld GD, Chang JJ, Broering JM, et al. Under staging and under grading in a contemporary series of patients undergoing radical prostatectomy: results from the Cancer of the Prostate Strategic Urologic Research Endeavor database. J Urol. 2001;165 (3):851–856. [PubMed] [Google Scholar]
- 25.Carter HB, Partin AW, Walsh PC, et al. Gleason score 6 adenocarcinoma: should it be labeled as cancer? J Clin Oncol. 2012;30(35):4294–4296. doi: 10.1200/JCO.2012.44.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the US, 2004. Cancer. 2005;104(6):1296–1303. doi: 10.1002/cncr.21284. [DOI] [PubMed] [Google Scholar]
- 27.Litwin MS, Greenfield S, Elkin EP, Lubeck DP, Broering JM, Kaplan SH. Assessment of prognosis with the total illness burden index for prostate cancer: aiding clinicians in treatment choice. Cancer. 2007;109(9):1777–1783. doi: 10.1002/cncr.22615. [DOI] [PubMed] [Google Scholar]
- 28.Ries LAG, Krapcho M, Stinchcomb DG, et al., editors. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 29.Moyer VA US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 30.Schröder FH, Hugosson J, Roobol MJ, et al. ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 31.Chamie K, Daskivich TJ, Kwan L, et al. Comorbidities, treatment and ensuing survival in men with prostate cancer. J Gen Intern Med. 2012;27(5):492–499. doi: 10.1007/s11606-011-1869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krahn MD, Bremner KE, Asaria J, et al. The ten-year rule revisited: accuracy of clinicians’ estimates of life expectancy in patients with localized prostate cancer. Urology. 2002;60(2):258–263. doi: 10.1016/s0090-4295(02)01712-0. [DOI] [PubMed] [Google Scholar]
- 33.Kim SP, Karnes RJ, Nguyen PL, et al. A national survey of radiation oncologists and urologists on active surveillance for low-risk prostate cancer. J Clin Oncol. 2012;30(suppl):4657. [Google Scholar]
- 34.Observation or radical treatment in patients with prostate cancer. [Accessed May 31, 2013];ClinicalTrials.gov. http://clinicaltrials.gov/ct2/show/NCT00499174.
- 35.Fendrick AM, Martin JJ, Weiss AE. Value-based insurance design: more health at any price. Health Serv Res. 2012;47(1 pt 2):404–413. doi: 10.1111/j.1475-6773.2011.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]