Abstract
Significance
There is no effective drug therapy for scarring and fibrotic disease. The cytokine transforming growth factor beta (TGF-β) promotes tissue repair, but its excessive action can lead to over exuberant scarring and fibrotic disease. However, owing to the multifunctional nature of TGF-β, broad targeting of the canonical Smad–TGF-β signaling pathway in vivo is likely to have unintended, deleterious consequences.
Recent Advances
(1) The myofibroblast is the essential cell type that mediates tissue repair and fibrosis. (2) TGF-β is an essential contributor to myofibroblast differentiation and activity. (3) TGF-β selectively promotes tissue repair and fibrosis via the noncanonical focal adhesion kinase (FAK) pathway; FAK mediates myofibroblast differentiation, and hence may represent a novel intervention point for drugs treating fibrotic disease.
Critical Issues
Excessive scarring (e.g., in hypertrophic scars, keloids, and scleroderma) is characterized by enhanced TGF-β signaling and is a major clinical problem. Drugs that selectively and effectively control the profibrotic action of TGF-β is therefore of clinical relevance.
Future Directions
FAK inhibition may represent a novel therapy for scarring disorders.

Andrew Leask, BSc, PhD
Scope
Skin is comprised of an outer epithelial layer under which lies the dermis, which is comprised of fibroblasts and a highly organized meshwork of the extracellular matrix (ECM). If damaged, new connective tissue must form. During this process, fibroblasts migrate into the damaged tissue where they synthesize and remodel a new ECM. This response involves the action of growth factors such as transforming growth factor beta (TGF-β), which promotes the appearance of specialized fibroblasts termed myofibroblasts, which are characterized by the expression of the highly contractile protein α–smooth muscle actin (α-SMA), which appears in the cell as cables connected to the ECM through cell surface structures called focal adhesions (FAs).1 FAs contain clusters of integrins, which are the cell surface ECM receptors. Myofibroblasts are not only essential for executing connective tissue repair but they also are responsible for scarring and fibrotic disease.1 It is now appreciated that substantial crosstalk exists between growth factor and adhesive signaling pathways. In particular, adhesion-mediated activation of focal adhesion kinase (FAK), which is recruited to FA postintegrin clustering, appears to be essential for myofibroblast differentiation and activity.2
Clinical Relevance
Scarring and fibrotic disorders such as keloids, hypertrophic scars, or scleroderma (systemic sclerosis [SSc]) are characterized by the abnormal persistence of myofibroblasts, which are characterized by excessive adhesive signaling.
Translational Relevance
TGF-β induces ECM production and remodeling via a canonical Smad signaling pathway.3 The critical cell type responsible for scarring and chronic fibrotic disease is the myofibroblast, a cell type characterized by excessive adhesion to and contraction of ECM. FAK appears to be a central mediator not only of cell attachment to ECM but also of TGF-β–mediated myofibroblast differentiation (Figure 1).4,5 Thus, FAK inhibitors suppress fibrogenic responses by blocking TGF-β signaling. Drugs suppressing adhesive signaling by targeting FAK are therefore likely to be of clinical benefit.
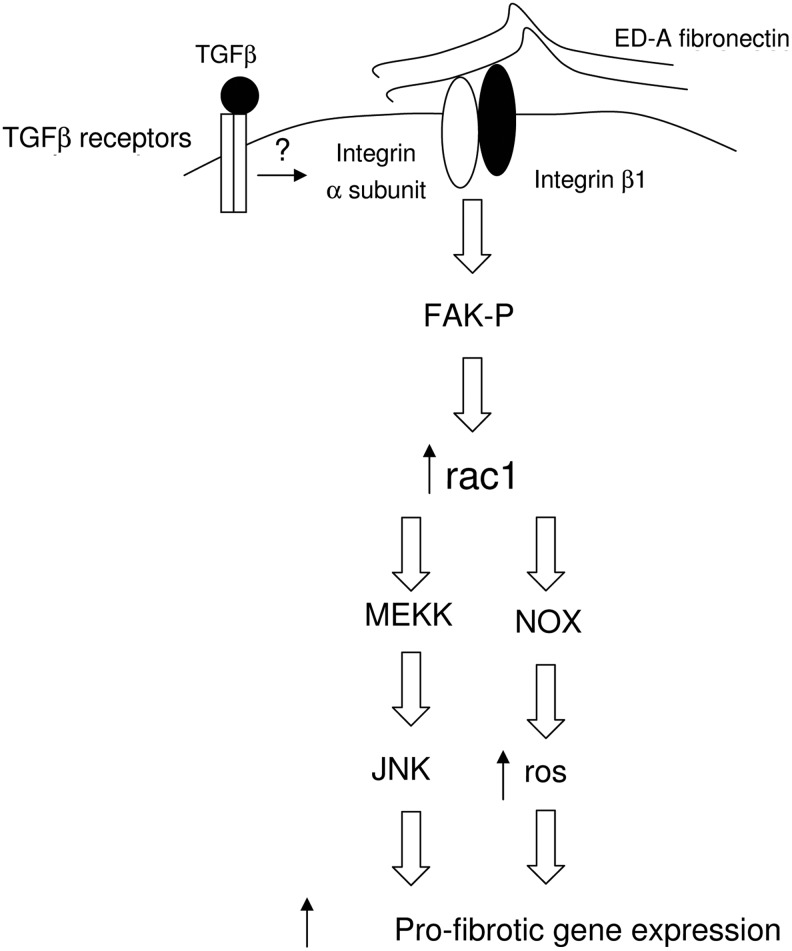
Figure 1.
Control of profibrotic gene expression by FAK. FAK plays a central role in mediating cellular responses to adhesion and TGF-β, resulting in profibrotic gene expression. ED-A, extradomain A; FAK, focal adhesion kinase; JNK, c-Jun N-terminal kinase; MEKK, mitogen-activated protein kinase kinase kinase; NOX, nicotinamide adenine dinucleotide phosphate oxidase; ROS, reactive oxygen species; TGF-β, transforming growth factor beta.
Discussion of Findings
Experimental model and material—advantages and limitations
The bleomycin-induced model of fibrosis, which is well established and is mediated by excessive canonical TGF-β signaling,6,7 is considered a good predictive tool to assess the mechanism underlying fibrogenesis. Moreover, data have been accumulated using fibroblasts derived from healthy and diseased humans. However, results derived from these models need to be confirmed directly in humans.
Experimental observations
The receptors for active TGF-β are a heterodimer of type I and type II TGF-β receptors (TβRI and TβRII). TβRI has kinase activity that results in the phosphorylation of Smad2 and Smad3. Phosphorylated Smad2/3 associate with their common partner Smad4, translocate into the nucleus, and trigger target gene transcription. This canonical pathway mediates essentially all functions of TGF-β.3
Noncanonical signaling pathways also exist, and these play a role in cell type- or process-specific events. In response to TGF-β, extradomain A (ED-A) fibronectin is produced and assembled into fibers that are connected with the terminal portion of α-SMA-positive stress fibers through FA. As this assembly progresses, FA size is increased while both tensin and FAK are neoexpressed in FA, and vinculin and paxillin are recruited from the cytoplasmic pool into the FA.8 TGF-β1–induced myofibroblast differentiation of lung fibroblasts does not occur in nonadherent cells in spite of the fact that activation of the canonical Smad pathway is retained.4 TGF-β1 induces FAK phosphorylation in a cell adhesion-dependent fashion; FAK inhibition inhibits TGF-β1-induced myofibroblast differentiation, smooth muscle actin expression, stress fiber formation, and cellular hypertrophy.4 Downstream of FAK, myofibroblast differentiation occurs via TGF-β–activated kinase 1 (TAK1)/mitogen-activated protein kinase kinase kinase 1 (MEKK1)/c-Jun N-terminal kinase (JNK) (Figure 1).5,9
FAK inhibition may represent a novel antifibrotic therapeutic approach. FAK is constitutively phosphorylated in fibrotic fibroblasts in patients with the autoimmune disease scleroderma (SSc), and FAK inhibition reverses the fibrotic phenotype of these cells.10,11 In these cells, FAK acts downstream of integrin β1, but upstream of Rac/reactive oxygen species (ROS) (Figure 1).11 Rac can promote both JNK and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which produces ROS, suggesting that the JNK and NADPH oxidase pathways may act in parallel.12,13 Recently, it was shown that pharmacologic or siRNA-mediated targeting of FAK resulted in marked abrogation of bleomycin-induced lung fibrosis, and fibroblast-specific FAK knockout mice have substantially less inflammation and fibrosis than control mice in a model of hypertrophic scar formation.2,14
Take-Home Messages.
FAK mediates profibrotic TGF-β signaling in fibroblasts.
FAK inhibitors have been shown in vitro and in vivo to be effective at impairing fibrotic responses.
FAK inhibitors are currently being considered as treatment options in cancer15 and may also represent a key approach for antifibrotic drug intervention.
In the future, drugs targeting FAK may prove to be of clinical benefit in controlling scarring.
Abbreviations and Acronyms
- α-SMA
alpha–smooth muscle actin
- ECM
extracellular matrix
- ED-A
extradomain A
- FA
focal adhesion
- FAK
focal adhesion kinase
- JNK
c-Jun N-terminal kinase
- MEKK
mitogen-activated protein kinase kinase kinase
- NADPH
nicotinamide adenine dinucleotide phosphate
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- ROS
reactive oxygen species
- TAK
TGF-β–activated kinase
- TGF-β
transforming growth factor beta
- TβRI and TβRII
type I and type II TGF-β receptors
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author listed. No ghostwriters were used to write this article.
About the Author
Andrew Leask is an Associate Professor at the University of Western Ontario.
References
- 1.Vedrenne N. Coulomb B. Danigo A. Bonté F. Desmoulière A. The complex dialogue between (myo)fibroblasts and the extracellular matrix during skin repair processes and ageing. Pathol Biol (Paris) 2012;60:20. doi: 10.1016/j.patbio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Lagares D. Busnadiego O. García-Fernández RA. Kapoor M. Liu S. Carter DE. Abraham D. Shi-Wen X. Carreira P. Fontaine BA. Shea BS. Tager AM. Leask A. Lamas S. Rodríguez-Pascual F. Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum. 2012;64:1653. doi: 10.1002/art.33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 4.Thannickal VJ. Lee DY. White ES. Cui Z. Larios JM. Chacon R. Horowitz JC. Day RM. Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 5.Liu S. Xu SW. Kennedy L. Pala D. Chen Y. Eastwood M. Carter DE. Black CM. Abraham DJ. Leask A. FAK is required for TGFbeta-induced JNK phosphorylation in fibroblasts: implications for acquisition of a matrix-remodeling phenotype. Mol Biol Cell. 2007;18:2169. doi: 10.1091/mbc.E06-12-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takagawa S. Lakos G. Mori Y. Yamamoto T. Nishioka K. Varga J. Sustained activation of fibroblast transforming growth factor-beta/Smad signaling in a murine model of scleroderma. J Invest Dermatol. 2003;121:41. doi: 10.1046/j.1523-1747.2003.12308.x. [DOI] [PubMed] [Google Scholar]
- 7.Cutroneo KR. Phan SH. TGF-beta1-induced Smad 3 binding to the Smad 7 gene: knockout of Smad 7 gene transcription by sense phosphorothioate oligos, autoregulation, and effect on TGF-beta1 secretion: bleomycin acts through TGF-beta1. J Cell Biochem. 2003;89:474. doi: 10.1002/jcb.10528. [DOI] [PubMed] [Google Scholar]
- 8.Dugina V. Fontao L. Chaponnier C. Vasiliev J. Gabbiani G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci. 2001;114(Pt 18):3285. doi: 10.1242/jcs.114.18.3285. [DOI] [PubMed] [Google Scholar]
- 9.Shi-wen X. Parapuram SK. Pala D. Chen Y. Carter DE. Eastwood M. Denton CP. Abraham DJ. Leask A. Requirement of transforming growth factor beta-activated kinase 1 for transforming growth factor beta-induced alpha-smooth muscle actin expression and extracellular matrix contraction in fibroblasts. Arthritis Rheum. 2009;60:234. doi: 10.1002/art.24223. [DOI] [PubMed] [Google Scholar]
- 10.Mimura Y. Ihn H. Jinnin M. Asano Y. Yamane K. Tamaki K. Constitutive phosphorylation of focal adhesion kinase is involved in the myofibroblast differentiation of scleroderma fibroblasts. J Invest Dermatol. 2005;124:886. doi: 10.1111/j.0022-202X.2005.23701.x. [DOI] [PubMed] [Google Scholar]
- 11.Shi-wen X. Thompson K. Khan K. Liu S. Murphy-Marshman M. Baron M. Denton CP. Leask A. Abraham DJ. Focal adhesion kinase and reactive oxygen species contribute to the persistent fibrotic phenotype of lesional scleroderma fibroblasts. Rheumatology. 2012;51:2146. doi: 10.1093/rheumatology/kes234. [DOI] [PubMed] [Google Scholar]
- 12.Chan A. Akhtar M. Brenner M. Zheng Y. Gulko PS. Symons M. The GTPase Rac regulates the proliferation and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Mol Med. 2007;13:297. doi: 10.2119/2007-00025.Chan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boureux A. Furstoss O. Simon V. Roche S. Abl tyrosine kinase regulates a Rac/JNK and a Rac/Nox pathway for DNA synthesis and Myc expression induced by growth factors. J Cell Sci. 2005;118(Pt 16):3717. doi: 10.1242/jcs.02491. [DOI] [PubMed] [Google Scholar]
- 14.Wong VW. Rustad KC. Akaishi S. Sorkin M. Glotzbach JP. Januszyk M. Nelson ER. Levi K. Paterno J. Vial IN. Kuang AA. Longaker MT. Gurtner GC. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2011;18:148. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultze A. Fiedler W. Therapeutic potential and limitations of new FAK inhibitors in the treatment of cancer. Expert Opin Investig Drugs. 2010;19:777. doi: 10.1517/13543784.2010.489548. [DOI] [PubMed] [Google Scholar]