Abstract
Significance
This review highlights the critical role of transforming growth factor beta (TGF-β)1–3 within different phases of wound healing, in particular, late-stage wound healing. It is also very important to identify the TGF-β1–controlling factors involved in slowing down the healing process upon wound epithelialization.
Recent Advances
TGF-β1, as a growth factor, is a known proponent of dermal fibrosis. Several strategies to modulate or regulate TGF's actions have been thoroughly investigated in an effort to create successful therapies. This study reviews current discourse regarding the many roles of TGF-β1 in wound healing by modulating infiltrated immune cells and the extracellular matrix.
Critical Issues
It is well established that TGF-β1 functions as a wound-healing promoting factor, and thereby if in excess it may lead to overhealing outcomes, such as hypertrophic scarring and keloid. Thus, the regulation of TGF-β1 in the later stages of the healing process remains as critical issue of which to better understand.
Future Directions
One hypothesis is that cell communication is the key to regulate later stages of wound healing. To elucidate the role of keratinocyte/fibroblast cross talk in controlling the later stages of wound healing we need to: (1) identify those keratinocyte-released factors which would function as wound-healing stop signals, (2) evaluate the functionality of these factors in controlling the outcome of the healing process, and (3) formulate topical vehicles for these antifibrogenic factors to improve or even prevent the development of hypertrophic scarring and keloids as a result of deep trauma, burn injuries, and any type of surgical incision.

Aziz Ghahary, PhD
Scope and Significance
The main scope of this review is (1) to clarify the role of transforming growth factor beta (TGF-β) in the wound-healing process and (2) to highlight the recent clinically significant advances in treatment of non-healing wounds and reduction of scar formation. We define the different phases of wound healing and discuss the role of various molecules and cells in these phases, with a particular emphasis on TGF-β. The main members of TGF-β family, including TGF-β1–3, are discussed in more detail; especially, their effect on inflammatory cells and their role in scar formation is clarified. We discuss clinical applications of therapeutics based on TGF-β, and major findings of our group in reducing scar formation are explained. This review provides a broad view of our recent knowledge about the role of TGF-β in wound healing, helping young researchers to understand the existing pitfalls and equipping them with a comprehensive vision for their future studies.
Translational Relevance
The major basic issues with significant translational importance in the wound-healing process are discussed. In this review, we discuss (1) the effect of TGF-β on recruitment of inflammatory cells into the wound area, (2) fibroblast and keratinocyte migration, (3) extracellular matrix (ECM) production and remodeling phase, and finally (4) the cross-talk between fibroblasts and keratinocytes. Deeper understanding of the wound-healing process and the specific roles of different cells and molecules such as TGF-β has enabled scientists to develop more sophisticated therapies for nonhealing wounds and prevention and treatment of scar formation. In this regard, by focusing on the cross-talk between keratinocytes and fibroblasts, our group has found the importance of keratinocyte-secreted stratifin in reducing the fibrogenic effects of TGF-β.
Clinical Relevance
Based on the basic knowledge regarding the role of TGF-β in wound healing, several clinical trials have been designed for prevention and treatment of hypertrophic scaring. For instance, antibodies against TGF-β1 and 2 as well as recombinant TGF-β3 have been used in clinical trials to prevent hypertrophic scaring. Furthermore, this knowledge will be used in treating other diseases caused by fibrosis, such as systemic sclerosis and diabetic kidney fibrosis.
Background
Wound healing process
Wound healing is a complex and dynamic interplay between various cell types, the ECM, cytokines, and growth factors. Hemostasis, inflammation, cell migration and proliferation, wound contraction, and remodeling are distinct but overlapping phases of the wound healing process.
Hemostasis is an essential step for the initiation and continuation of the healing process. It is well established that the major factors in hemostasis are vasoconstriction, platelet degranulation and aggregation, and fibrin deposition. Together these events result in the formation of a clot and bleeding cessation. The first subset of cells that enter the injury site are platelets. As reviewed by Guo and Dipietro,1 platelets release many different growth factors and inflammatory cytokines, such as platelet-derived growth factor (PDGF), TGF-β1, epidermal growth factor (EGF), and fibroblast growth factor (FGF), all of which promote the inflammatory phase and some of which function as chemoattractants. Interestingly, soon after the release of these initiation factors, epithelial cells migrate under the newly formed granulation tissue. This process is activated by several cytokines and growth factors; specifically, interleukin (IL)-1α appears to be expressed within the epidermis and released upon the dermal injury, which in turn stimulates more than 90 genes, including adhesion molecules, chemokines, cytokines, proteolytic enzymes, and matrix proteins in different types of skin cells.2 In addition, fibroblasts release another important growth factor called keratinocyte growth factor, which stimulates proliferation, migration, and differentiation of keratinocytes.
Inflammatory phase
The inflammatory phase is characterized by increased capillary permeability and cell migration into the wound site. Vasodilation that follows the early vasoconstriction is the result of capillary leaks that are mediated by histamine, leukotrienes, and prostaglandins.3 Neutrophils are the first cells infiltrating the wound site, followed by monocytes and lymphocytes.4 Neutrophils have innate antimicrobial functions, in addition to releasing proteases to eliminate the denatured ECM components. Leukocyte migration into the injured tissue is mediated by factors such as TGF-β, PDGF, IL-1, and many other cytokines and growth factors. Monocytes transform into macrophages as they enter the wounded area under the influence of TGF-β, ECM, complement particles, and serum factors. Macrophages clear the area of debris and microbes and release many important cytokines and factors, such as FGF, TGF-β, PDGF, and EGF, which in turn initiate the formation of granulation tissue.5 Macrophages also act as antigen-presenting cells.4 IL-8 functions as a chemoattractant for fibroblasts and neutrophils, promoting mitosis of keratinocytes.6
Proliferative phase
The proliferative phase begins after the primary inflammatory responses to the injury. The cells and cytokines in this phase provide necessary stimulatory factors for further production of functional skin.7 The main and leading events that happen during this phase include re-epithelialization, angiogenesis, and fibroplasia. Epidermal restoration begins with keratinocyte migration and proliferation stimulated by TGF-α, followed by neoepithelium differentiation and basement membrane restoration. As a ligand for EGF family receptors, heparin-binding EGF-like growth factor modulates keratinocyte migration and skin wound healing.8 Angiogenesis is primarily promoted by macrophage-released cytokines, such as TGF-β, FGF, and vascular EGF (VEGF). These factors modulate endothelial cell proliferation and promote angiogenesis.7 During fibroplasia, fibroblasts migrate, proliferate, and produce ECM components, which result in formation of granulation tissue within the wound site. Fibroblast migration is mediated by TGF-β1 and PDGF in this phase.
Wound contraction phase
Wound contraction starts soon after dermal tissue injury and peaks in the 2 weeks after the initial insult.7 As an event during granulation tissue formation, fibroblasts begin to transform into myofibroblast phenotypes enriched in alpha–smooth muscle actin bundles similar to those found in smooth muscle cells. These cells play the main role in wound contraction. Wound contraction markedly promotes wound closure. IL-4 induces matrix synthesis after promoting fibroblast differentiation.9
Tissue remodeling in wound healing
Tissue remodeling is the final phase of wound healing and continues for 6–24 months after the initial injury. This involves vascular regression and granulation tissue remodeling, in addition to new formation of ECM components. During remodeling, type III collagen is replaced with newly synthesized type I collagen.10 ECM formation begins with replacement of granulation tissue collagens and continues with production of newly synthesized collagens. The production of most of these key ECM components, such as collagens and fibronectin, is promoted in part through PDGF and TGF-β1.11,12 Matrix metalloproteinases (MMPs) released from fibroblasts, macrophages, and neutrophils cause collagen breakdown. TGF-β1 inhibits the synthesis of MMPs and results in greater accumulation of collagen fibers. EGF released by macrophages and platelets stimulates MMP secretion by fibroblasts, mainly during the remodeling phase.
Discussion of Findings and Relevant Literature
Roles of TGF-β in wound healing
The TGF-β superfamily and signaling pathway
The TGF-β family consists of TGF-β types 1, 2, and 3; bone morphogenic proteins; activin A, B, and C; growth differential factors; and anti-Müllerian hormone. Macrophages, fibroblasts, keratinocytes, and platelets are the primary sources of synthesis and production of these factors.13 TGF-β superfamily ligands bind to a type II TGF-β receptor, a serine/threonine receptor kinase, and that in turn phosphorylates a type I TGF-β receptor.14 This interaction results in activation of the SMAD pathway through which the R-Smads are phosphorylated, and through binding to a common Smad mediator (SMAD4), they form R-Smad/coSmad complexes. These complexes are translocated into the nucleus, where it regulates the expression of a variety of genes.15
Although there is a high affinity of type II receptors for TGF-β, their interaction is controlled by availability of the active form of TGF-β. There are several known mechanisms through which TGF-β becomes activated. One of those is integrin αvβ6, which interacts with inactive complexes of TGF-β1 with its latency-associated protein (LAP) and causes the activation of TGF-β1. This integrin can activate TGF-β1 by binding to the RGD motif present in LAP.16 It has been suggested that integrins activate latent TGF-β1 (L-TGF-β1) through two different mechanisms. One of these mechanisms is through conformational change to the L-TGF-β1 complex. This causes the release of active TGF-β1, which becomes available to interact with its receptor. The other mechanism is through a protease-dependent activation. In this case, integrins simultaneously bind the L-TGF-β1 complex and proteinases (like MMP-2 and MMP-9), and thereby the MMPs cleave the active TGF-β from its inactive form (reviewed by Wipff and Hinz).17 On the other hand, there are some factors, such as CD109, that function as coreceptors for TGF-β.18 It has been shown that CD109, which is a 180 kDa glycosylphosphatidylinositol-anchored protein, serves as an inhibitor of TGF-β signaling in human keratinocytes.19
The role of TGF-β1 during the wound healing process
Numerous studies have underscored the role of TGF-β1 in cutaneous wound healing. Denton et al.20 demonstrated that the lack of TGF-β receptor II in a transgenic mouse resulted in impairment of wound healing, though epidermal proliferation was increased. This study concluded that the functionality of TGF-β1 and its receptors are critical in the wound healing process. The release of TGF-β1 at an early stage of the healing process prompts recruitment of inflammatory cells into the injury site, which are later involved in a negative feedback via release of superoxide from macrophages. During this interim stage, granulation tissues are gradually formed and TGF-β1 prompts the expression of key components of ECM proteins, such as fibronectin, collagen types I and III, and VEGF.13 Further, TGF-β1 improves the angiogenic properties of endothelial progenitor cells to facilitate blood supply to the injured site21 and stimulates contraction of fibroblasts to enable wound closure.22 Keratinocyte migration is also promoted by TGF-β1 via regulation of cell migration–associated integrins, such as β1, α5, αv, and β5.23 TGF-β1 is one the main collagen-stimulating factors, especially type I in fibroblasts. It also inhibits different MMPs, which further promotes the accumulation of collagen fibers.24
The role of TGF-β2 and -β3 during the wound healing process
Similar to TGF-β1, TGF-β2 is involved in the recruitment of both fibroblasts and immune cells from circulation and the wound edges into the wounded area. These events lead to granular tissue formation, angiogenesis, and collagen synthesis and production.13 The role of TGF-β2 in scar formation has been well-investigated. Findings have demonstrated that TGF-β2 is needed for expression and organization of collagen and other key ECM components during the healing process.25 In a study conducted by Shah et al., wounds of an animal model were treated with antibodies against TGF-β1 and -β2, and this study found a marked reduction in the numbers of infiltrated immune cells in general and of monocytes and macrophages in particular. They also found markedly less deposition of collagen types I and III and fibronectin, which resulted in an improvement in scar formation. However, further studies showed that a combination of these two antibodies is needed to see this effect and that neither of these antibodies is independently effective in reducing scar formation.26
Although the third member of the TGF-β superfamily has many different roles in wound healing, in contrast to TGF-β1 and -β2, this isoform was shown to have a TGF-β1–antagonistic effect in scar formation.27 In wounds with minimal or no scar formation, such as in oral mucosa, the level of TGF-β1 decreases along with a significant upsurge in the ratio of TGF-β3 to TGF-β1.28 It has been shown that exogenous TGF-β3 injection in wounds reduces collagen type I deposition by restricting myofibroblast differentiation and promoting collagen degradation by MMP-9. All of these lead to decreased scar formation.29
The effect of TGF-β1 on inflammatory cells
As a general rule, TGF-β1 is known for its immunosuppressive features. However, it is well-established that TGF-β1 is a double-edged sword. For example, it activates some elements of the inflammatory response, while inhibiting others. It has been reported that a lack of TGF-β1 signaling in Smad3 knockout mice leads to a significant reduction in monocyte infiltration into the wound site.30 On the other hand, it has long been demonstrated that there is an increase in inflammation in multiple organs of TGF-β1 knockout mice. Knockout mice not only exhibited an increase in peripheral lymphocytes and immature neutrophils, but also in proliferating cells within the spleen and the lymph nodes. It is evident from these studies that TGF-β1 is an intricate player in modulating inflammation and immunity.31 Nonetheless, organ-specific TGF-β1 may not play an anti-inflammatory role in the skin. This is because TGF-β1 knockout mice did not develop any inflammatory response in skin.31 Surprisingly, overexpression of TGF-β1 in keratinocytes causes chronic inflammation in the wound, which leads to delayed healing.32 The inconsistent effect of TGF-β1 on inflammation between tissues may be due in part to a variance in its concentration in particular tissues. For instance, it has been shown that fibroblasts infiltrating the wound site produce much more TGF-β1 on day 7 compared to day 1 post–dermal insult. This finding indicates that the amount of TGF-β1 may determine the transition from an inflammatory to an immunoregulatory phase during the course of the healing process.33 It has been reported that TGF-β inhibits the production of IL-2, an essential cytokine for thymocyte proliferation. In addition, TGF-β1 reduces the capability of naïve T cells to become effector T cells.34 This immunosuppressive effect of TGF-β1 is one of the main mechanisms by which the tumor cells escape from immune attack.
Crowe et al. examined the importance of TGF-β1 and lymphocytes in wound healing in transgenic mouse models lacking B and T lymphocytes (Scid−/−) and also in TGF-β1 (Tgfb−/−) knockout mice. These investigations found that neither the absence of just TGF-β1 (Tgfb−/−), nor the absence of lymphocytes alone (Scid−/−), led to an initial delay in wound healing. However, the wound healing was markedly delayed in a mouse model lacking both lymphocytes and TGF-β1 (Tgfb−/− Scid−/−). Crowe et al. then suggested that TGF-β1 and lymphocytes have compensatory effects on different cells in the wound repair process.35
The role of TGF-β1 in development of fibrosis
It is well established that TGF-β1 contributes to the development of scar formation through its stimulatory effects on expression of the key ECM components and its inhibitory effects on expression of MMPs in fibroblasts. For example, TGF-β1 is a potent collagen (types I/III)–stimulating factor in fibroblasts. On the other hand, it inhibits the expression of MMPs, resulting in the accumulation of collagen fibers within the wound sites.24
Our research group previously showed that TGF-β1 accelerates wound closure in partial-thickness wounds, while delaying this process in full-thickness wounds. Based on our results, the scar-forming effect of TGF-β1 in partial-thickness wounds can be attributed to its stimulatory effect on fibroblast chemotaxis and ECM deposition rather than to its inhibitory effect on keratinocyte migration. However, in full-thickness wounds, TGF-β1 delays wound closure by its inhibitory effect on keratinocyte migration, which in turn prolongs the inflammatory phase of wound healing. This mechanism emphasizes the potential role of TGF-β1 in hypertrophic scar (HSc) formation in full thickness wounds.36
The role of TGF-β1 in the later stages of wound healing
As stated before, TGF-β1 is one the main growth factor involved in all aspects of the healing process. In particular, it has a profound contribution to dermal fibrosis in patients after thermal injury, surgical incision, and deep trauma. Naturally, TGF-β is not the only key growth factor responsible for regulating wound healing. For example, it has been shown that insulin-like growth factor (IGF-1) is another factor whose fibrogenic effect on dermal fibroblasts is similar to that of TGF-β1.37 Similar to TGF-β1, IGF-1 is expressed locally in response to tissue injury and its expression increases in parallel to the formation of granulation tissue.38 In fact, we found a greater expression of TGF-β1 and IGF-1 in post-burn HSc tissues as compared with those of normal dermis from the same patients.39 Similar results have also been reported in other fibrotic conditions, including scleroderma and hepatic, intraocular, and pulmonary fibrosis (reviewed by Border and Noble).40
Although the role of these two wound-healing promoting factors during the course of the healing process has extensively been studied, the mechanism through which the expression of these two fibrogenic factors is slowed down and/or abrogated in the later stages of the healing process is not known. To explore this mechanism, we have hypothesized that upon wound epithelialization, keratinocyte-released factors counteract the fibrogenic role of both IGF-1 and TGF-β1 in fibroblasts. To test this hypothesis, Gharary et al. evaluated the levels of collagenase (MMP-1), as an index for ECM degradation, in dermal fibroblasts in response to either keratinocyte-conditioned medium (KCM) or the recently-identified keratinocyte-released stratifin in the presence and absence of IGF-1, TGF-β1, or both. The results demonstrated that KCM contains high levels of stratifin, also known as 14-3-3 sigma, and that the recombinant stratifin increases the expression of MMP-1 in fibroblasts.41 The 14-3-3 proteins are a ubiquitous family of acidic eukaryotic proteins that function as a class of highly conserved molecular chaperones. Since the discovery of the first 14-3-3 protein, the members of this protein family are routinely found to be associated with numerous biological activities (primarily in signal transduction pathways), such as interaction with protein kinase C and RAF family members.42 In fact, 14-3-3 sigma is known to serve as a p53-regulated inhibitor of G2/M progression.43 Although certain isoforms of these proteins are found in extracellular environments, both in vivo (the cerebrospinal fluid of patients with Creutzfeldt–Jakob disease)44 and in vitro (KCM),45 no physiological function for extracellular stratifin had been reported before our findings. For this reason, the MMP-1–stimulating activity of keratinocyte-released stratifin on fibroblasts was surprising, given that members of the protein 14-3-3 family are known solely for their intracellular activities.
Experimental Analysis
These findings encouraged our research group to conduct a series of experiments whose result showed that differentiated keratinocytes express and release a higher level of stratifin relative to that of basal keratinocytes.46 We then asked the question of whether keratinocyte-released stratifin influences the expression of IGF-1 and TGF-β1 in fibroblasts. The statistical significance of differences in MMP-1 mRNA or protein expression between treated and untreated dermal fibroblasts was tested by using a Wilcoxon's test. p-Values <0.05 were considered to be significant.
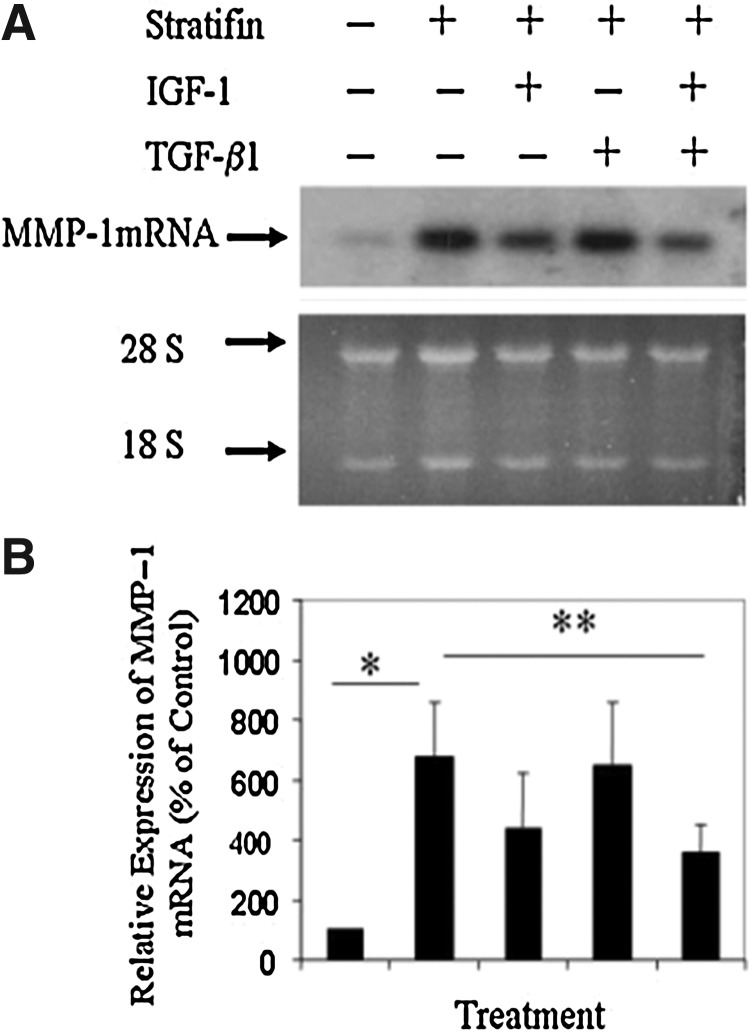
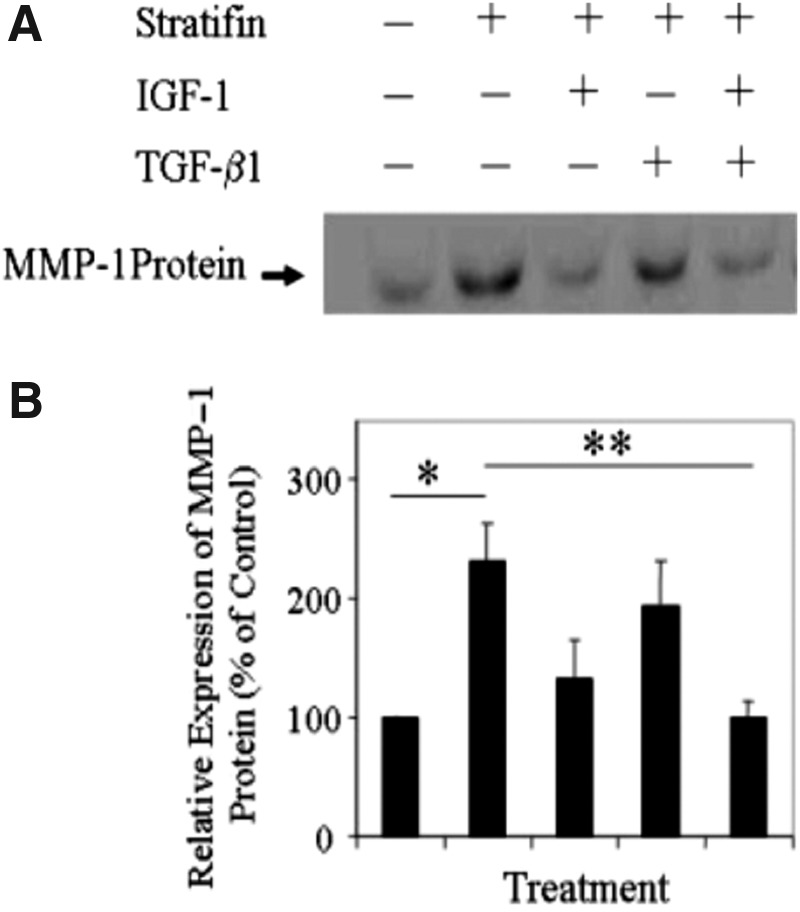
As shown in Fig. 1, the results of Northern analysis revealed a significant increase in the levels of MMP-1 mRNA expression in three different dermal primary cell strains isolated from three individuals treated with stratifin, and that was markedly reduced in response to IGF-1 but not TGF-β1 treatment. Addition of both IGF-1 and TGF-β1 to these stratifin-treated cells had a significant (p<0.05) inhibitory effect on the expression of MMP-1 mRNA. These findings were also confirmed by the result obtained from Western blot analysis. As shown in Fig. 2, the level of MMP-1 protein was significantly (p<0.05) increased in stratifin-treated fibroblasts relative to untreated controls and that was markedly reduced by treating cells with IGF-1. This effect was more pronounced when a mixture of IGF-1 and TGF-β1 was used. As shown in Fig. 2, at the concentration used, even though TGF-β1 did not reduce the increased level of MMP-1 mRNA after stratifin treatment, it had an additive effect when it was used in the presence of IGF-1.
Figure 1.
Quantitative analysis of the stratifin-stimulated matrix metalloproteinase (MMP)–1 mRNA in fibroblasts in the presence and absence of insulin-like growth factor (IGF)–1 and transforming growth factor (TGF)–β1. Three different strains of primary dermal fibroblasts isolated from 3 different individuals were either left untreated or treated for 24 h with stratifin—alone, or in the presence of IGF-1 (100 ng/mL), TGF-β1 (100 pg/mL), or both. Cells were then harvested, total RNA was extracted, and the expression of MMP-1 mRNA was evaluated by Northern blot analysis. (A) The representative pattern of MMP-1 mRNA, as well as that of 18S ribosomal RNA, which was used as loading control. (B) The expression of MMP-1 mRNA was then quantified by densitometry, and the mean±SD obtained from three separate experiments was then expressed as percentage of their corresponding controls. Significant difference was found in comparing *between untreated and stratifin-treated samples (data on lane 1 vs. 2) and **between the stratifin-treated and stratifin+TGF-β1+IGF-1–treated cells (data on lane 2 vs. 5).
Figure 2.
Quantitative analysis of the stratifin-stimulated MMP-1 protein in fibroblasts in the presence and absence of IGF-1 and TGF-β1. As in Figure 1, three different strains of primary dermal fibroblasts isolated from 3 different individuals were left untreated or treated for 48 h with stratifin—alone, or in the presence of IGF-1 (100 ng/mL), TGF-β1 (100 pg/mL), or both. Total cellular protein was then extracted and lysate (40 μg/lane) from each treatment was subjected to SDS-PAGE analysis. (A) The levels of MMP-1 protein were then evaluated by Western blot analysis. The blot represents three separate experiments. (B) The levels of MMP-1 protein were then quantified by densitometry, and the mean±SD obtained from three separate experiments were then expressed as percentage of their corresponding controls. Significant difference was found in comparing *between untreated and stratifin-treated samples (data on lane 1 vs. 2) and **between the stratifin-treated and stratifin+TGF-β1+IGF-1–treated cells (data on lane 2 vs. 5). SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
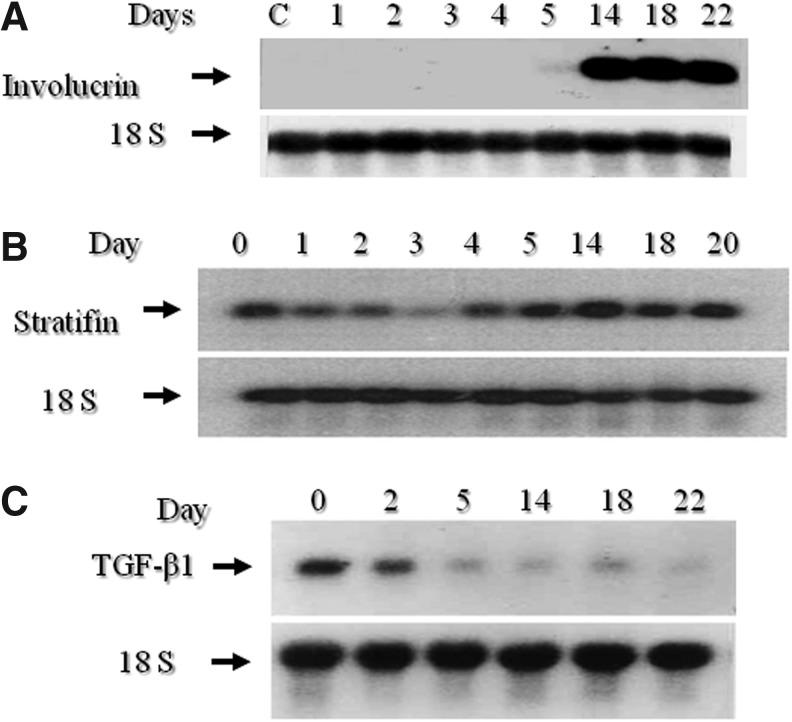
These findings collectively suggest that the wound-healing promoting effect of both TGF-β1 and IGF-1, at least on one key collagen-degrading enzyme, MMP-1, would be counteracted by keratinocyte-released factors such as stratifin. Interestingly, as shown in Fig. 3, we found that the expression of TGF-β1 is inversely related to keratinocyte differentiation, and the level of stratifin proportionately increases with keratinocyte differentiation. This inverse regulation between TGF-β1 and stratifin with keratinocyte differentiation might be a strong mechanism by which the wound-healing promoting factors, such as TGF-β1 and IGF-1, regulate the transition from epithelialization through to remodeling in the final stage of wound healing.
Figure 3.
Differentiation of keratinocytes is associated with an increase in the mRNA expression of stratifin while decreasing the expression TGF-β1 mRNA. Keratinocytes were cultured in test medium consisting of 49% keratinocytes serum free medium, 49% Dulbecco's modified Eagle's medium, and 2% fetal bovine serum for different durations. Keratinocytes were then harvested and evaluated for the mRNA expression for (A) involucrin as an index for keratinocyte differentiation, (B) stratifin, and (C) TGF-β1. Each blot was then evaluated for the expression of 18S RNA, which was used as a loading control.
Clinical Application of TGF-βs and Their Antibodies In Wound Healing
The well-characterized role of TGF-β1 and -β2 on promoting wound healing has provided the basis for the use of TGF-β1 or -β2 as potential therapeutic agents to treat chronic wounds, such as ulcers in diabetic patients. Topical application of TGF-β improved the rate of healing and wound strength in animal models.47 A clinical trial was performed using TGF-β2 for treatment of venous stasis, and the findings revealed an improvement in healing.48 Similarly, in phase I/II and II clinical trials, exogenous application of TGF-β2 demonstrated the safety and improvement in wound closure in diabetic foot ulcers.49 However, the information on whether these drugs have passed a phase III clinical trial is not available.
As the role of TGF-β1 and -β2 in stimulating fibroblast proliferation and ECM modulation has been suggested, several TGF-β inhibiting peptides and antibodies have been developed to be used as antiscarring factors in the clinical setting. A peptide called P144, an antagonist for TGF-β type III receptor, has been used to treat dermal fibrosis in systemic sclerosis. The findings showed its safety and efficacy in a phase II clinical trial.50 In another study, several humanized antibodies were raised against either TGF-β2 and -β1, and their use in both phase I/II or phase III clinical trials showed them to be safe, but ineffective in reducing scarring or fibrosis.51,52 In another study, a humanized antibody against TGF-β1 was raised and used to treat diabetic kidney fibrosis; the results of an ongoing phase II clinical trial showed safety and efficacy in protecting kidney function in these patients. Furthermore, a recombinant TGF-β3 protein as an antiscarring factor has also been developed by the Renova company and used to treat scar formation. However, the phase II clinical trial was stopped in February 2011, as it had not reached its primary or secondary efficacy end points.
Summary and Future Directions
From the TGF-β superfamily, TGF-β types 1, 2, and 3 are involved in almost every stage of wound healing. The presence and concentration of these factors as well as other wound-healing promoting factors, such as IGF-1, EGF, PDGF, ILs, and their ratios determine to a great extent the outcome of the wound healing process. This study discussed, the critical roles of each of these factors in the context of the healing process, as well as their interactions with one another, immune cells, and nonimmune cells such as keratinocytes and fibroblasts present in the wound environment. Although TGF-β1 is considered to be one of the main wound-healing promoting factors,53 the important roles of other wound-healing–related factors in wound healing, such as IGF-1, EGF, PDGF, and FGF, has to be appreciated.
There is now supporting evidence that IGF-1 also functions as a fibrogenic factor as it stimulates collagen production in human lung fibroblasts37 and human dermal fibroblasts.54 It is present in many tissues and coexpressed by many different cell types. However, it is not clear how IGF-1 functions in a system in which other growth factors are present. For this reason, we have conducted a keratinocyte/fibroblast coculture study and, as shown in Figs. 1 and 2, we found that KCM remarkably stimulates the expression of MMP-1 mRNA in fibroblasts and could not be counteracted by either IGF-1, TGF-β1, or a combination of both. Nonetheless, these growth factors reduced the expression of MMP-1 in both KCM-treated and untreated cells. This finding raised another question: how much of the KCM-derived MMP-1 stimulatory effect is actually due to the presence of stratifin in this conditioned medium? We have previously demonstrated that keratinocytes not only have the capacity to express stratifin at levels of mRNA and protein, but they also release a large amount of this protein into conditioned medium.55 To address this question, we immunodepleted stratifin from KCM and used it to treat cells. The finding demonstrated that, at least in part, the presence of stratifin is responsible for the MMP-1 stimulatory effects of KCM. This is mainly because the levels of MMP-1 expression in stratifin immunodepleted cells was reduced to 40%–50% of that of KCM-treated cells in three different repeated experiments.55 This was not surprising, as KCM also contains some other MMP-1 stimulatory factors such as IL-1.56 These studies need to be further investigated by conducting the following series of projects:
(1) Identify the other potential keratinocyte released factors which may function as wound healing stop signals by contracting the wound healing promoting factors, such as TGF-β1, PDGF, and IGF-1.
(2) Evaluate the functionality of these factors in controlling the outcome of healing process.
(3) Formulate topical applications of these antifibrogenic factors to improve or even prevent the development of hypertrophic scarring and keloids frequently seen post–deep trauma, burn injuries, and any type of surgical incision.
Conclusion
Although the aforementioned studies indicate that the expression of wound-healing promoting factors, such as immune-cell releasable TGF-β1, PDGF, and IGF-1, is the key player in initiation and continuation of healing process, there is a need to investigate how these factors are regulated at later stages of the healing process. Our works presented here indicate that the expression of these factors seems to be slowed down as keratinocytes become differentiated and at the same time the release of antifibrogenic factors such as stratifin is progressively increased. The release of stratifin then may, in part, be one of the mechanisms whereby the epidermal cells signal to the dermis to slow down matrix production by fibroblasts in healing wounds. Expanding upon the evidence depicted herein, further studies examining the cross-talk orchestrating cell–cell interplay in wound healing will identify other factors which may function as stop signals for the healing process and thereby reduce formation of fibrosis.
Abbreviations and Acronyms
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- HSc
hypertrophic scar
- IGF
insulin-like growth factor
- IL
interleukin
- KCM
keratinocyte-conditioned medium
- LAP
latency-associated protein
- L-TGF-β1
latent TGF-β1
- MMP
matrix metalloproteinase
- PDGF
platelet derived growth factor
- TGF-β
transforming growth factor beta
- VEGF
vascular EGF
Acknowledgments and funding Sources
This research was supported by a grant from the Canadian Institute for Health Research (A.G.).
Author Disclosure and Ghostwriting
The authors declare no conflicts of interest. Ghostwriters were not used in production of this article.
About the Authors
Mohammadreza Pakyari is a graduate of Shiraz University of Medical Sciences and is a graduate student in the Experimental Medicine program at the University of British Columbia (UBC). In medical school, his work included projects on burn injury and wound healing. He is also interested in reconstructive surgery and nonrejectable skin allograft studies. Ali Farrokhi is a PhD candidate in UBC's Experimental Medicine program under supervision of Dr. Aziz Ghahary. He completed his BSc in Genetics in the University of Ahwaz and his MSc in Cellular and Molecular Biology at the University of Tehran. His current research interest is studying the interaction of fibroblasts and keratinocytes during wound healing. Mohsen Khosravi Maharlooei is a graduate of Shiraz University of Medical Sciences and is now a graduate student in Experimental Medicine program at UBC. Aziz Ghahary is a Professor in the Department of Surgery and Division of Plastic Surgery at UBC. He also has a joint appointment as an Associate Member of Dermatology and Skin Sciences at UBC. He is a Principal Investigator at the International Collaboration on Repair Discoveries (ICORD), the Director of the British Columbia Professional Fire Fighters' Burn and Wound Healing Research Group, and a member of the active staff at Vancouver General Hospital. Dr. Ghahary attended the University of Ahwaz for his BSc and the University of Tehran for his MSc in Public Health. He completed both his PhD in Physiology and his Post-Doctoral Fellowship at the University of Manitoba. Dr. Ghahary works on the biology of wound healing. One of his main focuses is on burns and other nonhealing wounds. He works on making a biological skin substitute which can replace skin for burn patients and finding ways to treat patients with scarring after a burn wound. He is also working to develop a liquid skin material for use on nonhealing wounds. Such a material would provide a scaffold for the patient's skin to close the wound and allow it to heal. Another focus of his research is type I diabetes; he aims to develop insulin-producing cells which cannot be rejected by the receiving patient.
References
- 1.Guo S. Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy JE. Robert C. Kupper TS. Interleukin-1 and cutaneous inflammation: a crucial link between innate and acquired immunity. J Invest Dermatol. 2000;114:602. doi: 10.1046/j.1523-1747.2000.00917.x. [DOI] [PubMed] [Google Scholar]
- 3.Richardson M. Acute wounds: an overview of the physiological healing process. Nurs Times. 2004;100:50. [PubMed] [Google Scholar]
- 4.Zaja-Milatovic S. Richmond A. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol. 2008;23:1399. doi: 10.14670/hh-23.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behm B. Babilas P. Landthaler M. Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol. 2012;26:812. doi: 10.1111/j.1468-3083.2011.04415.x. [DOI] [PubMed] [Google Scholar]
- 6.Rennekampff HO. Hansbrough JF. Kiessig V. Dore C. Sticherling M. Schroder JM. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J Surg Res. 2000;93:41. doi: 10.1006/jsre.2000.5892. [DOI] [PubMed] [Google Scholar]
- 7.Li J. Chen J. Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Shirakata Y. Kimura R. Nanba D. Iwamoto R. Tokumaru S. Morimoto C. Yokota K. Nakamura M. Sayama K. Mekada E. Higashiyama S. Hashimoto K. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci. 2005;118:2363. doi: 10.1242/jcs.02346. [DOI] [PubMed] [Google Scholar]
- 9.Fertin C. Nicolas JF. Gillery P. Kalis B. Banchereau J. Maquart FX. Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol Biol. 1991;37:823. [PubMed] [Google Scholar]
- 10.Ramasastry SS. Acute wounds. Clin Plast Surg. 2005;32:195. doi: 10.1016/j.cps.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Papakonstantinou E. Aletras AJ. Roth M. Tamm M. Karakiulakis G. Hypoxia modulates the effects of transforming growth factor-beta isoforms on matrix-formation by primary human lung fibroblasts. Cytokine. 2003;24:25. doi: 10.1016/s1043-4666(03)00253-9. [DOI] [PubMed] [Google Scholar]
- 12.Jinnin M. Ihn H. Mimura Y. Asano Y. Yamane K. Tamaki K. Regulation of fibrogenic/fibrolytic genes by platelet-derived growth factor C, a novel growth factor, in human dermal fibroblasts. J Cell Physiol. 2005;202:510. doi: 10.1002/jcp.20154. [DOI] [PubMed] [Google Scholar]
- 13.Barrientos S. Stojadinovic O. Golinko MS. Brem H. Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 14.Wrana JL. Attisano L. Carcamo J. Zentella A. Doody J. Laiho M. Wang XF. Massague J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y. Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 16.Munger JS. Huang X. Kawakatsu H. Griffiths MJ. Dalton SL. Wu J. Pittet JF. Kaminski N. Garat C. Matthay MA. Rifkin DB. Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 17.Wipff PJ. Hinz B. Integrins and the activation of latent transforming growth factor beta1—an intimate relationship. Eur J Cell Biol. 2008;87:601. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Bizet AA. Liu K. Tran-Khanh N. Saksena A. Vorstenbosch J. Finnson KW. Buschmann MD. Philip A. The TGF-beta co-receptor, CD109, promotes internalization and degradation of TGF-beta receptors. Biochim Biophys Acta. 2011;1813:742. doi: 10.1016/j.bbamcr.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Finnson KW. Tam BY. Liu K. Marcoux A. Lepage P. Roy S. Bizet AA. Philip A. Identification of CD109 as part of the TGF-beta receptor system in human keratinocytes. FASEB J. 2006;20:1525. doi: 10.1096/fj.05-5229fje. [DOI] [PubMed] [Google Scholar]
- 20.Denton CP. Khan K. Hoyles RK. Shiwen X. Leoni P. Chen Y. Eastwood M. Abraham DJ. Inducible lineage-specific deletion of TbetaRII in fibroblasts defines a pivotal regulatory role during adult skin wound healing. J Invest Dermatol. 2009;129:194. doi: 10.1038/jid.2008.171. [DOI] [PubMed] [Google Scholar]
- 21.Evrard SM. d'Audigier C. Mauge L. Israel-Biet D. Guerin CL. Bieche I. Kovacic JC. Fischer AM. Gaussem P. Smadja DM. The profibrotic cytokine transforming growth factor-beta1 increases endothelial progenitor cell angiogenic properties. J Thromb Haemost. 2012;10:670. doi: 10.1111/j.1538-7836.2012.04644.x. [DOI] [PubMed] [Google Scholar]
- 22.Desmouliere A. Geinoz A. Gabbiani F. Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gailit J. Welch MP. Clark RA. TGF-beta 1 stimulates expression of keratinocyte integrins during re-epithelialization of cutaneous wounds. J Invest Dermatol. 1994;103:221. doi: 10.1111/1523-1747.ep12393176. [DOI] [PubMed] [Google Scholar]
- 24.White LA. Mitchell TI. Brinckerhoff CE. Transforming growth factor beta inhibitory element in the rabbit matrix metalloproteinase-1 (collagenase-1) gene functions as a repressor of constitutive transcription. Biochim Biophys Acta. 2000;1490:259. doi: 10.1016/s0167-4781(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 25.Thompson HG. Mih JD. Krasieva TB. Tromberg BJ. George SC. Epithelial-derived TGF-beta2 modulates basal and wound-healing subepithelial matrix homeostasis. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1277. doi: 10.1152/ajplung.00057.2006. [DOI] [PubMed] [Google Scholar]
- 26.Shah M. Foreman DM. Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108(Pt 3):985. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 27.Waddington SN. Crossley R. Sheard V. Howe SJ. Buckley SM. Coughlan L. Gilham DE. Hawkins RE. McKay TR. Gene delivery of a mutant TGFbeta3 reduces markers of scar tissue formation after cutaneous wounding. Mol Ther. 2010;18:2104. doi: 10.1038/mt.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrementi ME. Ferreira AM. Zender C. DiPietro LA. Site-specific production of TGF-beta in oral mucosal and cutaneous wounds. Wound Repair Regen. 2008;16:80. doi: 10.1111/j.1524-475X.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 29.Hosokawa R. Nonaka K. Morifuji M. Shum L. Ohishi M. TGF-beta 3 decreases type I collagen and scarring after labioplasty. J Dent Res. 2003;82:558. doi: 10.1177/154405910308200714. [DOI] [PubMed] [Google Scholar]
- 30.Ashcroft GS. Yang X. Glick AB. Weinstein M. Letterio JL. Mizel DE. Anzano M. Greenwell-Wild T. Wahl SM. Deng C. Roberts AB. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 31.Kulkarni AB. Huh CG. Becker D. Geiser A. Lyght M. Flanders KC. Roberts AB. Sporn MB. Ward JM. Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li AG. Wang D. Feng XH. Wang XJ. Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J. 2004;23:1770. doi: 10.1038/sj.emboj.7600183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daley JM. Brancato SK. Thomay AA. Reichner JS. Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim IY. Kim MM. Kim SJ. Transforming growth factor-beta: biology and clinical relevance. J Biochem Mol Biol. 2005;38:1. doi: 10.5483/bmbrep.2005.38.1.001. [DOI] [PubMed] [Google Scholar]
- 35.Crowe MJ. Doetschman T. Greenhalgh DG. Delayed wound healing in immunodeficient TGF-beta 1 knockout mice. J Invest Dermatol. 2000;115:3. doi: 10.1046/j.1523-1747.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 36.Tredget EB. Demare J. Chandran G. Tredget EE. Yang L. Ghahary A. Transforming growth factor-beta and its effect on reepithelialization of partial-thickness ear wounds in transgenic mice. Wound Repair Regen. 2005;13:61. doi: 10.1111/j.1067-1927.2005.130108.x. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein RH. Poliks CF. Pilch PF. Smith BD. Fine A. Stimulation of collagen formation by insulin and insulin-like growth factor I in cultures of human lung fibroblasts. Endocrinology. 1989;124:964. doi: 10.1210/endo-124-2-964. [DOI] [PubMed] [Google Scholar]
- 38.Steenfos HH. Jansson JO. Gene expression of insulin-like growth factor-I and IGF-I receptor during wound healing in rats. Acta Chir-Eur J Surg. 1992;158:327. [PubMed] [Google Scholar]
- 39.Ghahary A. Shen YJ. Nedelec B. Scott PG. Tredget EE. Enhanced expression of mRNA for insulin-like growth factor-1 in post-burn hypertrophic scar tissue and its fibrogenic role by dermal fibroblasts. Mol Cell Biochem. 1995;148:25. doi: 10.1007/BF00929499. [DOI] [PubMed] [Google Scholar]
- 40.Border WA. Noble NA. Transforming growth factor beta in tissue fibrosis. New Engl J Med. 1994;331:1286. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 41.Ghahary A. Karimi-Busheri F. Marcoux Y. Li Y. Tredget EE. Taghi Kilani R. Li L. Zheng J. Karami A. Keller BO. Weinfeld M. Keratinocyte-releasable stratifin functions as a potent collagenase-stimulating factor in fibroblasts. J Invest Dermatol. 2004;122:1188. doi: 10.1111/j.0022-202X.2004.22519.x. [DOI] [PubMed] [Google Scholar]
- 42.Fu H. Subramanian RR. Masters SC. 14–3-3 Proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- 43.Hermeking H. Lengauer C. Polyak K. He TC. Zhang L. Thiagalingam S. Kinzler KW. Vogelstein B. 14-3-3 Sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- 44.Hsich G. Kenney K. Gibbs CJ. Lee KH. Harrington MG. The 14-3-3 brain protein in cerebrospinal fluid as a marker for transmissible spongiform encephalopathies. New Engl J Med. 1996;335:924. doi: 10.1056/NEJM199609263351303. [DOI] [PubMed] [Google Scholar]
- 45.Katz AB. Taichman LB. A partial catalog of proteins secreted by epidermal keratinocytes in culture. J Invest Dermatol. 1999;112:818. doi: 10.1046/j.1523-1747.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 46.Ghahary A. Marcoux Y. Karimi-Busheri F. Li Y. Tredget EE. Kilani RT. Lam E. Weinfeld M. Differentiated keratinocyte-releasable stratifin (14-3-3 sigma) stimulates MMP-1 expression in dermal fibroblasts. J Invest Dermatol. 2005;124:170. doi: 10.1111/j.0022-202X.2004.23521.x. [DOI] [PubMed] [Google Scholar]
- 47.Flanders KC. Burmester JK. Medical applications of transforming growth factor-beta. Clin Med Res. 2003;1:13. doi: 10.3121/cmr.1.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robson MC. Phillip LG. Cooper DM. Lyle WG. Robson LE. Odom L. Hill DP. Hanham AF. Ksander GA. Safety and effect of transforming growth factor-beta(2) for treatment of venous stasis ulcers. Wound Repair Regen. 1995;3:157. doi: 10.1046/j.1524-475X.1995.30207.x. [DOI] [PubMed] [Google Scholar]
- 49.Robson MC. Steed DL. McPherson JM. Pratt BM. The TGFβ2 Study Group: Effects of transforming growth factor β2 on wound healing in diabetic foot ulcers: a randomized controlled safety and dose-ranging trial. J Appl Res. 2002;2:S18. [Google Scholar]
- 50.Santiago B. Gutierrez-Canas I. Dotor J. Palao G. Lasarte JJ. Ruiz J. Prieto J. Borras-Cuesta F. Pablos JL. Topical application of a peptide inhibitor of transforming growth factor-beta1 ameliorates bleomycin-induced skin fibrosis. J Invest Dermatol. 2005;125:450. doi: 10.1111/j.0022-202X.2005.23859.x. [DOI] [PubMed] [Google Scholar]
- 51.Mead AL. Wong TT. Cordeiro MF. Anderson IK. Khaw PT. Evaluation of anti-TGF-beta2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Ophthalmol Vis Sci. 2003;44:3394. doi: 10.1167/iovs.02-0978. [DOI] [PubMed] [Google Scholar]
- 52.Denton CP. Merkel PA. Furst DE. Khanna D. Emery P. Hsu VM. Silliman N. Streisand J. Powell J. Akesson A. Coppock J. Hoogen F. Herrick A. Mayes MD. Veale D. Haas J. Ledbetter S. Korn JH. Black CM. Seibold JR. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2007;56:323. doi: 10.1002/art.22289. [DOI] [PubMed] [Google Scholar]
- 53.Varga J. Rosenbloom J. Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597. doi: 10.1042/bj2470597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bird JL. Tyler JA. Dexamethasone potentiates the stimulatory effect of insulin-like growth factor-I on collagen production in cultured human fibroblasts. J Endocrinol. 1994;142:571. doi: 10.1677/joe.0.1420571. [DOI] [PubMed] [Google Scholar]
- 55.Kilani RT. Guilbert L. Lin X. Ghahary A. Keratinocyte conditioned medium abrogates the modulatory effects of IGF-1 and TGF-beta1 on collagenase expression in dermal fibroblasts. Wound Repair Regen. 2007;15:236. doi: 10.1111/j.1524-475X.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- 56.Maas-Szabowski N. Stark HJ. Fusenig NE. Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in resting fibroblasts. J Invest Dermatol. 2000;114:1075. doi: 10.1046/j.1523-1747.2000.00987.x. [DOI] [PubMed] [Google Scholar]