Abstract
Significance
Wound healing is an intricate biological process in which the skin, or any other tissue, repairs itself after injury. Normal wound healing relies on the appropriate levels of cytokines and growth factors to ensure that cellular responses are mediated in a coordinated manner. Among the many growth factors studied in the context of wound healing, transforming growth factor beta (TGF-β) is thought to have the broadest spectrum of effects.
Recent Advances
Many of the molecular mechanisms underlying the TGF-β/Smad signaling pathway have been elucidated, and the role of TGF-β in wound healing has been well characterized. Targeting the TGF-β signaling pathway using therapeutic agents to improve wound healing and/or reduce scarring has been successful in pre-clinical studies.
Critical Issues
Although TGF-β isoforms (β1, β2, β3) signal through the same cell surface receptors, they display distinct functions during wound healing in vivo through mechanisms that have not been fully elucidated. The challenge of translating preclinical studies targeting the TGF-β signaling pathway to a clinical setting may require more extensive preclinical research using animal models that more closely mimic wound healing and scarring in humans, and taking into account the spatial, temporal, and cell-type–specific aspects of TGF-β isoform expression and function.
Future Directions
Understanding the differences in TGF-β isoform signaling at the molecular level and identification of novel components of the TGF-β signaling pathway that critically regulate wound healing may lead to the discovery of potential therapeutic targets for treatment of impaired wound healing and pathological scarring.

Anie Philip, PhD
Scope and Significance
Wound healing is a complex physiological response to injury and involves three main overlapping phases: inflammation, proliferation, and maturation.1 Among the many cytokines and growth factors involved in wound healing, transforming growth factor beta (TGF-β) has the broadest spectrum of effects.1 TGF-β plays an essential role in wound healing through its pleiotropic effects on cell proliferation and differentiation, extracellular matrix (ECM) production, and immune modulation.1 The present review focuses on the current state of knowledge on the TGF-β signaling pathway and the approaches that have been used to manipulate this pathway to improve wound healing and reduce scarring.
Translational Relevance
Research on understanding the molecular mechanisms by which TGF-β signaling regulates wound healing have led to the development and use of therapeutic agents that modulate TGF-β signaling. Therapeutic agents tested in wound healing and scarring models include small-molecule inhibitors of the type I TGF-β receptor (activin-like receptor kinase 5 [ALK-5]), anti–TGF-β neutralizing antibodies, and recombinant TGF-β3 protein. These molecules have shown promise in pre-clinical studies, but none have yet been approved by the U.S. Food & Drug Administration for clinical use.
Clinical Relevance
TGF-β regulates almost all aspects of wound healing, and aberrant TGF-β signaling has been implicated in pathological skin disorders, including chronic wounds and excessive scarring. Targeting the TGF-β signaling pathway represents a viable strategy for the development of novel therapeutic agents that improve wound healing and reduce pathological scarring.
Discussion of Findings and Relevant Literature
Overview of TGF-β signaling
The TGF-β signaling pathway is essential for numerous cell functions and was thought to arise with the development of metazoans. In development, TGF-β plays numerous roles, including induction of epithelial-to-mesenchymal transition (EMT) in endocardial cells, which is necessary for normal heart development.1,2 TGF-β also has several roles in normal tissue homeostasis, regulating diverse functions such as cellular differentiation, apoptosis, cell-cycle arrest, ECM production, and cellular migration. Partly owing to its pleiotropic effects in numerous cell types, TGF-β has also been implicated in several pathologies, including fibrosis. In wound healing, TGF-β promotes wound closure and resolution through the production of ECM proteins and the inhibition of matrix metalloproteinases (MMPs). However, in fibrotic diseases, excessive TGF-β production and signaling promotes extensive tissue fibrosis, which can compromise normal tissue function.3 Understanding the molecular mechanisms involved in regulating TGF-β signaling during wound healing and scarring may provide important insights into how its dysregulation may contribute to impaired wound healing or abnormal scarring.
TGF-β superfamily members
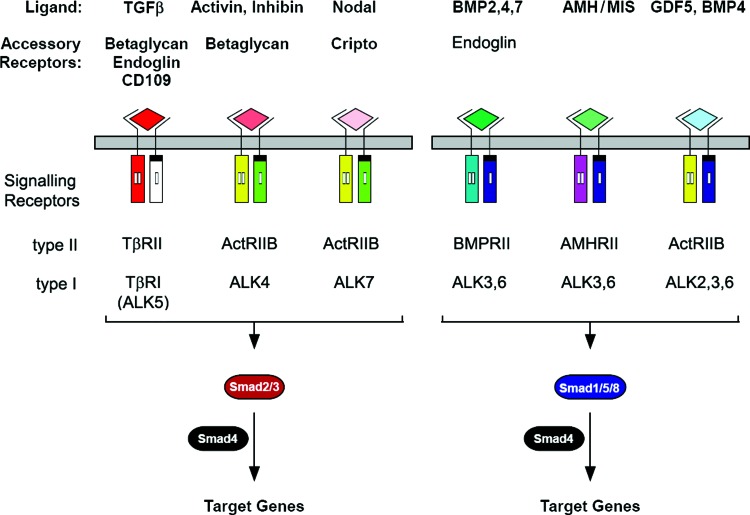
The TGF-β superfamily consists of structurally and functionally related cytokines that signal through a pair of transmembrane serine-threonine kinase receptors known as the type I and type II receptors (TβRI [also known as ALK] and TβRII, respectively), which, in turn, activate intracellular Smad transcription factors to mediate downstream signaling events. The TGF-β superfamily contains >30 ligands, which are structurally characterized by the presence of a cysteine-knot motif.4 This superfamily is divided into two subfamilies, the bone morphogenetic protein (BMP)/growth and differentiation factor (GDF)/müllerian-inhibiting substance (MIS) subfamily and the TGF-β/activin/Nodal subfamily (Fig. 1).5 These two subfamilies are classified partially on the basis of which ALK they bind as well as the subset of Smad transcription factors they activate. The BMP/GDF/MIS subfamily generally binds to ALK1, -2, -3, or -6, and activates the receptor-regulated Smads (rSmads) Smad-1, -5, and -8; whereas the TGF-β/activin/Nodal subfamily binds to ALK4, -5, and -7, and activates the rSmads Smad-2 and -3.6 In addition to the rSmads, another class of Smads known as the inhibitory Smads (I-Smads), Smad-6 and -7 act as adaptor proteins that bind Smad ubiquitin regulatory factor (Smurfs)-1 and Smurf-2, which ubiquitinate the receptor complex, targeting it for degradation via proteosomal and lysosomal pathways.
Figure 1.
Members of the TGF-β superfamily and their signaling components. TGF-β superfamily members include TGF-β (-β1, -β2, and -β3), activin, nodal, BMPs (-2, -4, and -7), AMH/MIS, and GDF-5. TGF-β superfamily members signal through a unique pair of transmembrane serine-threonine kinases known as the type I and type II receptors to mediate intracellular Smad signaling. The TGF-β/activin/Nodal subfamily binds to ALK 4, 5, and 7 and activates Smads 2 and 3; whereas the BMP/GDF/MIS subfamily generally binds to ALK 1, 2, 3, or 6 and activates Smads 1, 5, and 8. Activated Smad2/3 and Smad1/5/8 form a complex with Smad4 and enter the nucleus, where they regulate target gene expression. Accessory or co-receptors (betaglycan, endoglin, CD109, and cripto) are potent modulators of signaling by TGF-β superfamily members. TGF-β, transforming growth factor beta; BMP, bone morphogenetic protein; AMH, anti-müllerian hormone; MIS, Müllerian inhibiting substance; GDF, growth and differentiation factor; ALK, activin-like receptor kinase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The BMP/GDF/MIS subfamily is best known for its role in bone development and regeneration and consists of ∼20 cytokines.4 BMPs are so called due to their ability to induce osteoblast differentiation and promote bone formation.4 While the majority of BMP ligand gene knockout mice are non-viable and die during embryogenesis, mice lacking BMP type I receptor A (BMPR-IA) are viable and have appendicular skeletal defects.7 Although BMPs are known to inhibit myogenic (muscle cell) differentiation and promote osteogenic differentiation during embryonic development, emerging evidence indicates that BMPs have pro-proliferative effects on muscle progenitor (satellite) cells during adult skeletal muscle regeneration after injury.8 For example, administering BMP4 to mice that have undergone muscle injury reduces differentiation of satellite cells and sustains their proliferative potential.9 Therefore, it seems that BMPs are not only implicated in fracture healing, but can also act as a signal to provide precursor cells during muscle repair.
GDFs are also known for their roles in tissue development. For example, GDF-8 (also known as myostatin) is a negative regulator of skeletal muscle growth and has been implicated in the maintenance of whole body homeostasis.10 Finally MIS, also known as anti-müllerian hormone, inhibits development of the müllerian ducts during embryogenesis and appears to have a negative regulatory role in follicular development in the adult ovary.11
The TGF-β/activin/Nodal subfamily has been studied in-depth due to its roles in tissue morphogenesis, cancer, and wound healing. It consists of five activins/inhibins, Nodal, and three TGF-βs (TGF-β1, TGF-β2, and TGF-β3).6 While the activins were initially discovered due to their ability to induce follicle stimulating hormone release, it has now been appreciated that activins, particularly activin A, play a role in wound healing.12 Activin is up-regulated during cutaneous wound healing in humans.13 Keratinocytes, fibroblasts, and inflammatory cells appear to be sources of activin in the wound microenvironment.12,13 Further, transgenic mice overexpressing activin A in the epidermis display enhanced wound healing with increased granulation tissue formation as compared with wild-type (WT) littermates;14 whereas epidermal overexpression of the activin antagonist follistatin is associated with impaired wound healing and reduced granulation tissue formation.15 A critical role for endogenous activin in wound healing was also shown in another study where blocking follistatin expression in the epidermis led to enhanced re-epithelialization without affecting the quality of the healed wound.16
Similar to other TGF-β superfamily members, Nodal is critical for proper early development and is best known for its role in inducing mesoderm and endoderm formation.17 Nodal is also essential for maintaining human embryonic stem cell pluripotency, which may explain its frequent overexpression in a number of human cancers.18 However, Nodal is generally not found in adult tissues, and its potential role in wound repair is not known.18
While the role of other TGF-β superfamily members in wound healing is still being investigated, the TGF-β subfamily, consisting of TGF-β1, 2, and 3, has long been appreciated as having critical roles in wound healing, and will be the focus of the remainder of this review.
The TGF-β isoforms (-β1, -β2, and -β3) have been conserved throughout evolution, and orthologs to human TGF-β can be found in Drosophila melanogaster and Xenopus laevis (reviewed by Wu and Hill).2 The TGF-β ligands share a significant sequence homology. Together, they have >76% identity in their active domains.19,20 Despite structural similarities, TGF-β ligands have distinct affinities for TGF-β receptors. The three TGF-β ligands are produced by a number of different cell types, and the production of all three occurs during development, although TGF-β1 is the predominant type in adults.21,22 Each TGF-β ligand has relatively specific, non-overlapping functions in vivo. Mice containing deletions of these genes illustrate that these ligands have distinct functional roles. For example, Tgfb1−/− mice develop significant problems in utero, including vasculogenic and hematopoietic defects.23 Mice that survive gestation develop a severe wasting inflammatory syndrome.23 Tgfb2−/− mice have a myriad of developmental defects, including skeletal, cardiovascular, pulmonary, and visual problems.24 Interestingly, Tgfb3−/− null mice have the least defects and die after birth due to an inability to suckle caused by cleft palate.25 Similar to their non-redundant roles in development, the TGF-β ligands have different effects during wound healing and scarring. For example, TGF-β1 is found at very high levels in the wound microenvironment and promotes myofibroblast differentiation, production of ECM components, and fibroblast chemotaxis (reviewed by Ferguson and O'Kane).26 Overall, TGF-β1 promotes the formation of a scar during adult wound healing. On the other hand, the embryonic wound microenvironment contains high levels of TGF-β3 and low levels of TGF-β1.26 Further, adding exogenous TGF-β3 to an adult wound promotes scar-free healing in rats,27 and injuries obtained in utero heal scar free possibly due to the relatively high levels of TGF-β3 compared with TGF-β1.26
Activation of latent TGF-β
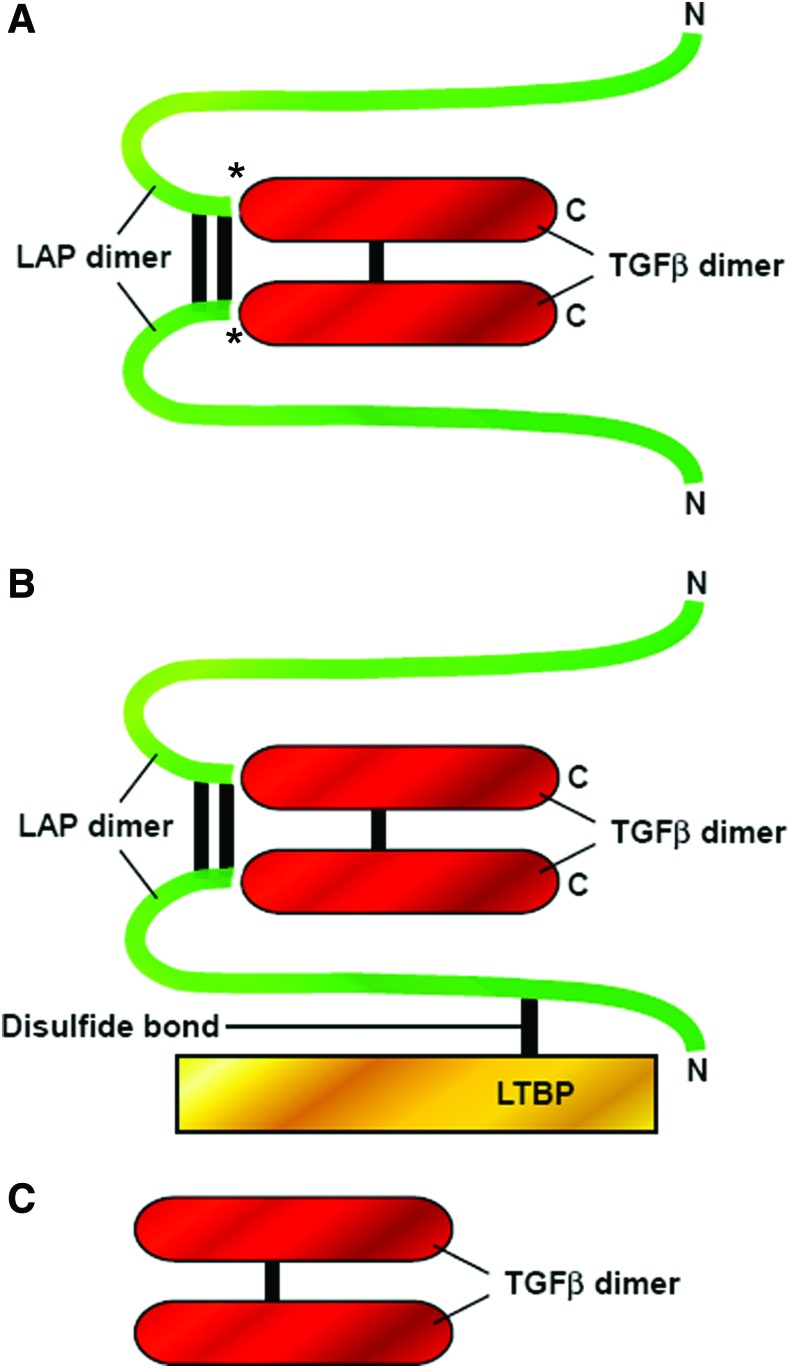
TGF-β is synthesized as a homo-dimeric proprotein (pro-TGF-β) and undergoes proteolytic cleavage in the trans-Golgi network by furin-like enzymes, giving rise to a C-terminal mature TGF-β dimer and N-terminal pro-peptide known as latency-associated peptide (LAP). LAP remains non-covalently associated with mature TGF-β, rendering TGF-β inactive in a so-called small latent complex (SLC) (Fig. 2). In most cases, the SLC forms a complex with another protein called latent TGF-β binding protein (LTBP) via intermolecular disulfide bonds, giving rise to the large latent complex (LLC), the most abundant secreted form. The LLC can associate with the ECM by covalent cross-linking of LTBP with ECM proteins (Fig. 2).28,29 Latent TGF-β can be activated in vivo by molecules such as thrombospondin 1 (TSP-1), integrins, MMPs, and plasmin, and in vitro by acidic or alkali conditions, heat denaturation, or shear stress.30–33 All three TGF-β isoforms exist in latent complexes34,35 and, in their active forms, exist as homodimers that are stabilized by disulfide bridges and hydrophobic interactions.
Figure 2.
Latent and active forms of TGF-β ligand. TGF-β is synthesized as a homo-dimeric proprotein (pro–TGF-β) and undergoes proteolytic cleavage in the trans-Golgi network by furin-like enzymes, giving rise to the mature TGF-β dimer and its pro-peptide, also known as LAP. TGF-β is secreted either as an SLC, which comprises the mature TGF-β dimer in association with LAP, or as an LLC in which the LAP portion of SLC is covalently linked to a protein known as latent TGF-β binding protein (LTBP). (A) SLC: The mature TGF-β dimer (red) is non-covalently associated with its LAP (green). *Asterisks indicate the regions that have undergone proteolytic processing by furin-like enzymes in the trans-Golgi before secretion. (B) LLC: The SLC is covalently linked to latent TGF-β binding protein (LTBP, yellow) by disulfide bonds to form the LLC. (C) Mature TGF-β dimer: The mature TGF-β dimer is released from the latent complex by different mechanisms, giving rise to the active form of TGF-β that can bind its receptors and elicit biological responses. LAP, latency associated peptide; SLC, small latent complex; LLC, large latent complex. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
It has been suggested that activation of latent TGF-β occurs at two time points during wound healing: immediately after wounding, and during re-epithelialization.32 Platelets are thought to be the primary source of activated TGF-β immediately after injury. Of the total TGF-β that is released by platelets, only a small amount is activated, and it is thought that the rest of it may remain in its SLC form.32 Platelet-derived latent TGF-β can be activated by TSP-1, which is also contained in the platelet secretory granules,36 as well as by the fibrinolytic enzyme plasmin during dissolution of the blood clot.37 The activated TGF-β then acts as a potent chemoattractant for inflammatory cells that invade the wound microenvironment, leading to further activation of latent TGF-β during the early re-epithelialization phase. Macrophages recruited to the wound microenvironment secrete plasminogen activators, which can also activate latent TGF-β.38 Overall, the spatiotemporal control of latent TGF-β activation may represent an important regulatory mechanism that controls TGF-β bioavailability and action during wound healing.
TGF-β/Smad signaling pathway
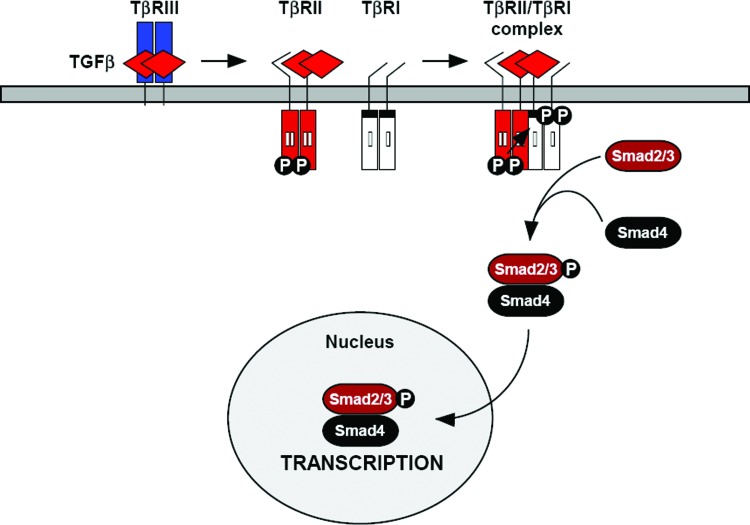
As mentioned earlier, TGF-β signaling is mediated by a pair of transmembrane serine-threonine kinase receptors known as TβRI (or ALK) and TβRII. TβRII is a 62 kDa protein containing a short cysteine-rich, N-glycosylated extracellular domain, a single transmembrane domain, and a serine-threonine kinase intracellular domain.39 The cytoplasmic domain of TβRII is also serine-threonine rich, which is lacking in TβRI.40 At the cell surface, TβRII exists as a homodimer in the absence and presence of ligand.41 TβRII binds TGF-β1 and TGF-β3 with a relatively high affinity,42,43 but is unable to bind TGF-β2, which requires the presence of a TGF-β coreceptor known as betaglycan (TβRIII).39 In the absence of ligands, TβRII undergoes autophosphorylation on serine residues Ser549, Ser551, Ser223, Ser226, and Ser227.43,44 In response to TGF-β binding, TβRII forms a heterotetrameric complex comprising two pairs of TβRII and TβRI. TβRII then phosphorylates TβRI at serine-threonine residues in its glycine/serine (GS) domain, leading to the activation of TβRI, which, in turn, phosphorylates and activates intracellular Smad2 and Smad3 proteins, which are central mediators of TGF-β signaling.45 Activated Smad-2 and -3 then interact with the co-Smad (Smad4) and enter the nucleus to regulate gene transcription (Fig. 3).
Figure 3.
TGF-β/Smad signal transduction pathway. TGF-β signaling is initiated when the TGF-β ligand binds to the extracellular domain of TβRII. TGF-β1 and TGF-β3 bind TβRII with a high affinity, whereas TGF-β2 requires the TGF-β co-receptor betaglycan (TβRIII) to “present” it to TβRII. TGF-β-associated TβRII then recruits TβRI, resulting in the formation of a heterotetrameric receptor signaling complex comprising one TGF-β ligand, one homo-dimeric TβRII, and one homo-dimeric TβRI. TβRII is a constitutively active kinase that phosphorylates TβRI, resulting in activation of TβRI kinase activity. TβRI then phosphorylates intracellular Smad2 and Smad3 proteins, which, in turn, form a complex with Smad4 and enter the nucleus to regulate gene transcription. TβRII, type II TGF-β receptor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
TβRI and TβRII are structurally similar, although TβRI contains a shorter extracellular domain and cannot bind ligands in the absence of TβRII.46 Akin to TβRII, TβRI also exists as a homodimer at the cell surface and contains a serine/threonine kinase intracellular domain.39 However, TβRI contains a unique intracellular GS-rich region that is phosphorylated by TβRII.19 Once phosphorylated, the GS domain of TβRI acts as a docking platform for the so-called receptor-regulated Smad proteins, Smad-2 and -3 (Fig. 3).47 Mutations of the GS domain have highlighted the importance of this region to TGF-β signal transduction; mutations of two or more glycine or serine residues in the GS domain impair TGF-β signaling activity.48 Mutation of threonine 204 to aspartic acid increases TGF-β signal transduction in the absence of ligands, as it generates a constitutively active TβRI.48 These mutational studies confirm that TβRI is the key player in Smad signal transduction.
Smad proteins typically consist of two domains that are separated by a variable linker region. The amino Mad homology (MH) 1 domain has DNA binding capabilities in some Smad sub-types, while the carboxy MH2 domain has been shown to mediate interactions with a variety of proteins.49 The activated GS domain of TβRI serves as a docking site for Smad2 and Smad3 via their MH1 domains.47 TβRI phosphorylates rSmads on the conserved SSXS motif located at the C-termini of Smads 2 and 3 (e.g., serine residues 465 and 467 in the MH2 domain of Smad2).50–52 The phosphorylated serine residues of Smad2/3 serve as a docking site for Smad4, promoting the dissociation of Smad2/3 from TβRI and the formation of a heteromeric complex with Smad4.50,53 Smad2/3 are generally located cytoplasmically in the absence of ligands, but on ligand stimulation, they translocate to the nucleus through its interaction with Smad4,5 which is able to bind with nucleoporins; the interaction of Smad4 with the nucleoporin importin-1α is thought to mediate the translocation of the Smad heteromeric complex to the nucleus.54 In the nucleus, the heteromeric Smad complex binds to promoters or enhancers of TGF-β target genes,such as the Smad binding element via its MH1 domain, and interacts with transcriptional co-activators and co-repressors in order to induce cell-specific transcriptional programs.5,19
TGF-β coreceptors
In addition to type I and type II TGF-β signaling receptors, there are three TGF-β coreceptors: betaglycan (TβRIII), endoglin, and the recently discovered CD109. They play important roles in modulating TGF-β signaling and are considered accessory receptors, as they have no signaling or enzymatic activity. Endoglin and betaglycan are structurally related, with large, heavily glycosylated extracellular domains, and a short cytoplasmic region with high sequence similarity.55–57 Both receptors can be phosphorylated on serine/threonine residues in their cytoplasmic domain.58–60 At the cell surface, endoglin and betaglycan form homodimers,61,62 as well as form complexes with TβRI and TβRII.59,63 Though structurally similar, these co-receptors differ in their ligand-binding ability and expression. Betaglycan can bind all three TGF-β isoforms with a high affinity,62 whereas endoglin binds TGF-β1 and TGF-β3 in the presence of TβRII, but does not bind TGF-β2.58,59 Betaglycan is widely expressed in adult and fetal tissues,39 whereas endoglin is thought to be primarily expressed in proliferating endothelial cells,64 although recent studies show its expression in other cell types such as chondrocytes65–67 and skin fibroblasts.68,69 Betaglycan is thought to facilitate TGF-β signaling by “presenting” the TGF-β ligand to TβRII, whereas endoglin inhibits TGF-β/ALK5/Smad signaling, although other mechanisms also exist.61
Another TGF-β coreceptor, CD109, has been shown to bind TGF-β ligand and inhibit TGF-β signal transduction.70–74 CD109 is a glycophosphatidylinositol-anchored protein that binds TGF-β1 with a high affinity and also forms a complex with TβRI, TβRII, and betaglycan.70,74 Work by Bizet et al. demonstrated that the association of CD109 with the TGF-β receptor complex increases the internalization of the receptors via caveolae and enhances receptor degradation.75 In a follow-up article, Bizet et al. illustrated that CD109 promotes TβRI degradation in a Smad7/Smurf2-dependent manner.76
Endocytosis and TGF-β signaling
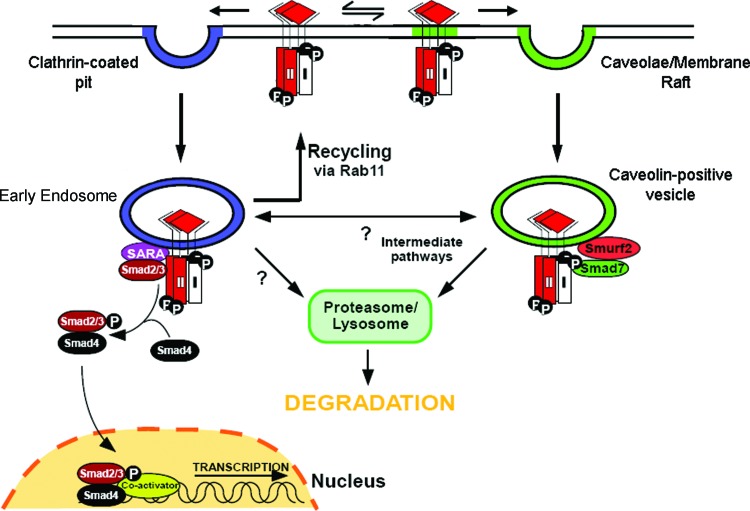
Endocytosis refers to the process in which cell-surface associated molecules enter the cell without passing through the plasma membrane. Essentially, the plasma membrane invaginates, budding off and forming a vesicle containing the internalized cargo. There are several methods of endocytosis of cell-surface receptors, including clathrin-mediated endocytosis and membrane-raft dependent endocytosis.77 Current evidence indicates that distinct endocytic pathways regulate TGF-β receptor signaling and turnover. At the cell surface, TGF-β receptor complexes can access both clathrin-coated pits and membrane rafts (Fig. 4).78 Inhibition of clathrin-coated pit internalization through the use of a dominant-negative epidermal growth factor substrate 15 (Eps15) mutant shifts receptors into membrane raft fractions; similarly, inhibition of membrane raft formation through cholesterol depletion shifts receptors back into non-membrane raft fractions.78 TGF-β receptors internalized via clathrin-mediated endocytosis access the early endosome, a signaling endosome, which propagates TGF-β signal transduction through the recruitment of Smad2 and Smad3.78,79 Membrane raft endocytosis of TGF-β receptors, however, results in receptors being targeted to the caveolin-1 positive vesicle.80 Unlike the early endosome, the caveolin-1 positive vesicle promotes association of Smad7, not Smad2/3, with the receptor complex,78 with Smad7 acting as an adaptor that binds Smurf-2, which ubiquitinates the receptor complex while targeting it for degradation78,81,82 (Fig. 4).
Figure 4.
Regulation of TGF-β signaling by clathrin-dependent and -independent endocytosis. TGF-β receptors can be internalized by clathrin-dependent and clathrin-independent, membrane-raft dependent mechanisms. TGF-β receptors internalized via clathrin-coated pit mediated endocytosis traffic to Smad anchor for receptor activation (SARA)-containing early endosome and propagate signal transduction. TGF-β receptors in the early endosome can be recycled back to the plasma membrane in a Rab11-dependent manner. TGF-β receptors internalized by membrane-raft dependent endocytosis traffic to caveolin-1 positive vesicles, where they are targeted for Smad7/Smurf2-mediated ubiquitination and proteosomal/lysosomal degradation. Other potential intracellular trafficking pathways for TGF-β receptors, including bi-directional trafficking between the early endosome and caveolin-1 positive vesicles (intermediate pathways) or direct trafficking of TGF-β receptors from the early endosome to proteosomal/lysosomal degradation pathways, are current topics of investigation. Smurf, Smad ubiquitination regulatory factor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
TGF-β and wound healing
Role of TGF-β in cutaneous wound healing: an overview
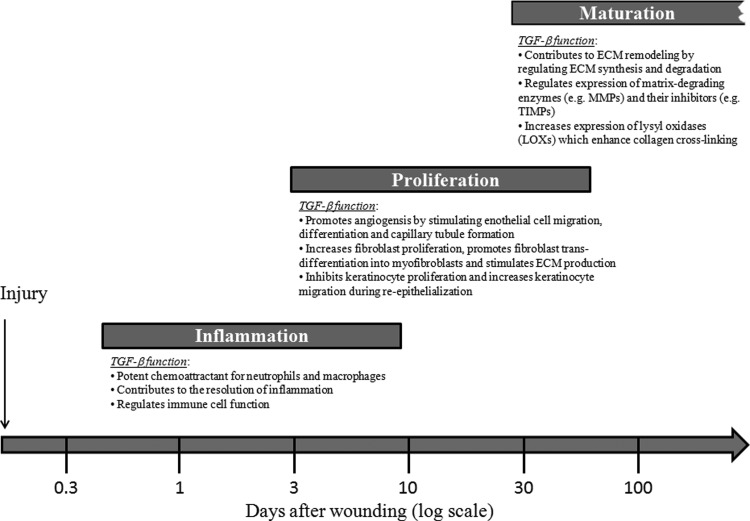
Wound healing is a complex and dynamic physiological process that involves numerous secreted cytokines and growth factors and the interaction of a variety of different cell types.1,83 The three phases of wound healing are known as inflammation, tissue formation (proliferation), and maturation (tissue remodeling), which overlap in time. TGF-β plays a critical role in regulating multiple cellular responses that occur in all three phases of wound healing (Fig. 5). Hemostasis may be defined as the stoppage of bleeding after an injury and involves vasoconstriction, platelet aggregation, and blood coagulation.84 Inflammation ensues shortly thereafter and lasts for about 2–4 days. Inflammation is characterized by the recruitment of immune cells such as neutrophils and macrophages to the injured site in response to chemotactic cytokines such as TGF-β.85 Neutrophils cleanse the wounded area of foreign particles, secrete chemicals to kill bacteria, and are then extruded with the eschar (scab) or phagocytosed by macrophages.86 Monocytes also infiltrate the wound site in response to TGF-β, differentiate into activated macrophages (also in response to TGF-β) that engulf and digest foreign particles and necrotic debris, and release TGF-β and other growth factors to stimulate capillary growth and initiate granulation tissue formation. Once immune cells become activated, they are susceptible to TGF-β1-mediated suppression to reverse the inflammatory process.87 Thus, TGF-β plays a dual role in the inflammation phase of wound healing by exerting pro-inflammatory effects during the early stages and later contributing to the resolution of inflammation.
Figure 5.
Schematic diagram showing the role of TGF-β in regulating all three phases of the wound healing process. Inflammation: TGF-β acts as a potent chemoattractant for immune cells (neutrophils and macrophages) during the early stages of inflammation, regulates immune cell function, and contributes to resolution of inflammation. Proliferation: TGF-β promotes angiogenesis by stimulating endothelial cell migration, differentiation, and capillary tubule formation. TGF-β also stimulates fibroblast proliferation, promotes fibroblast trans-differentiation into myofibroblasts, and stimulates ECM production. In addition, TGF-β inhibits keratinocyte proliferation and enhances keratinocyte migration, promoting re-epithelialization. Maturation: TGF-β regulates the balance of ECM synthesis and degradation by tightly controlling the production of ECM components and regulating their rate of degradation by modulating synthesis of MMPs and production of protease inhibitors such as TIMPs. TGF-β also regulates ECM remodeling by stimulating production of LOXs, which play an important role in collagen cross-linking. ECM, extracellular matrix; MMPs, matrix metalloproteinases; TIMPs, tissue inhibitors of matrix metalloproteinases; LOXs, lysyl oxidases.
The second phase of wound healing is known as “tissue formation” or the proliferative phase. During this phase, TGF-β orchestrates many cellular responses, including re-epithelialization as well as formation of new blood vessels (angiogenesis), fibroblast proliferation, and production of ECM components, leading to granulation tissue formation and wound contraction.88 TGF-β regulates wound angiogenesis by stimulating endothelial cell migration, differentiation, and capillary tubule formation.89 TGF-β also promotes fibroblast trans-differentiation into myofibroblasts (Fig. 6), which play a critical role in wound contraction.90 TGF-β1 has been shown to increase the expression ECM components, including fibronectin, the fibronectin receptor, and collagens, and to reduce their degradation by down-regulating the expression and activity of matrix-degrading enzymes such as MMPs and increasing the expression of protease inhibitors such as tissue inhibitors of MMPs (TIMPs).91 Although TGF-β is a potent inhibitor of epithelial (keratinocyte) cell proliferation in vitro, TGF-β may have both pro- and antiproliferative effects in keratinocytes in vivo, depending on the differentiation state and/or other factors such as TGF-β concentration or the timing of its administration.91 In addition, TGF-β stimulates keratinocyte migration in vitro, possibly by regulating integrin expression,92 which is thought to be important for the migratory component of re-epithelialization.87 Thus, TGF-β affects multiple cell types during the proliferative phase of wound healing, contributing to the formation of granulation tissue consisting of newly formed blood vessels, proliferating fibroblasts, and ECM components, which sets the stage for normal scar formation.
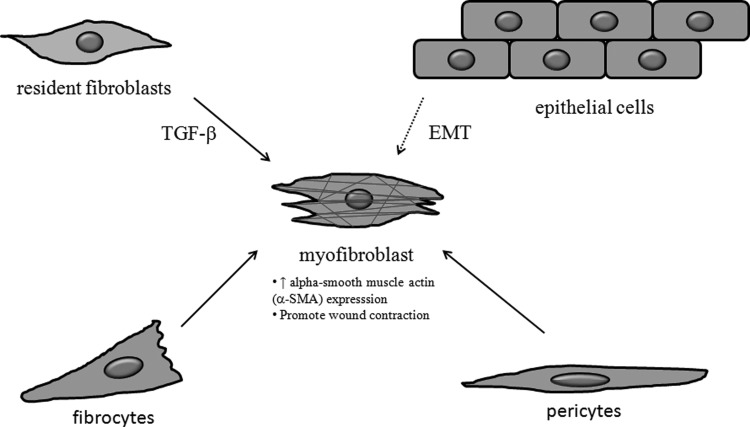
Figure 6.
Myofibroblasts originate from different cell types and play a critical role in wound healing and scarring. Myofibroblasts are specialized cells that express alpha smooth muscle actin (α-SMA, red) and display a contractile phenotype. During wound healing, resident fibroblasts trans-differentiate into myofibroblasts in response to TGF-β and promote wound contraction. Fibrocytes are circulating bone marrow-derived cells that can enter tissues and differentiate into myofibroblasts in response to TGF-β. Myofibroblasts also originate from other cell types such as epithelial cells through epithelial-to-mesenchymal transition (EMT) and perivascular cells (pericytes) by trans-differentiation, and these processes have been implicated in the pathogenesis of hypertrophic scarring.
The final stage of wound healing, known as “maturation” or the remodeling phase, can last for several years. Although the proliferation phase of wound healing is characterized by an increase in collagen production, which is tightly regulated by TGF-β, the transition from the proliferative phase to maturation phase is dependent on the ongoing synthesis and degradation of collagen at a low rate.86 Collagen degradation is mediated by MMPs and other serine proteases that are also regulated by TGF-β.93 Further, collagen degradation by MMPs is counterbalanced by the presence of endogenous TIMPs whose expression is also regulated by TGF-β.94 Although the rate of collagen production and degradation are balanced during the maturation phase, there continues to be an extensive remodeling process in which disorganized collagen fibers are re-organized, cross-linked, and aligned along tension lines.95 Cross-linking of collagen is mediated by enzymes known as lysyl oxidases (LOXs), which have been shown to increase collagen cross-link dependent contraction in vitro96 and enhance the wound's tensile strength in vivo.97,98 TGF-β has been shown to increase LOX expression in many different cell types, including skin fibroblasts in vitro,99,100 but its role in regulating LOX expression during wound healing in vivo remains to be explored.
Analysis of the functional role of TGF-β in wound healing using animal models
Much of what has been learned about the role of TGF-β in wound healing has come from animal studies. These studies have used different approaches, including incisional and excisional wounds generated in various animal species followed by measurement of TGF-β signaling pathway components, and treating wounds with recombinant TGF-β (-β1, -β2, and -β3) proteins or anti–TGF-β neutralizing antibodies and measuring various wound healing and/or scarring parameters. Another approach used was to perform wound healing studies on mice with genetic modifications of specific components of the TGF-β signaling pathway. This section highlights the current knowledge on the role of TGF-β signaling in wound healing in non–genetically modified animal models. For information on the role of TGF-β signaling in wound healing obtained using mice with genetic modification in the TGF-β signaling pathway, the reader is referred to the “Critical Review” by Finnson et al.,101 published in the current issue of Advances in Wound Care.
The relationship between endogenous TGF-β and wound healing first became evident in studies that measured TGF-β isoform levels in experimental wound healing models. For example, an early study investigating TGF-β1 expression and activity in vivo using two different models of cutaneous injury (human suction blister and partial thickness excisional wounds in porcine skin) showed that TGF-β1 expression/activity was increased immediately (within 5 min) after injury and progressed outward from the site of injury, particularly at the leading edge of the migrating epithelial sheet.102 Another study showed that TGF-β isoforms (-β1, -β2, and -β3) display marked differences in spatiotemporal expression during excisional wound repair in pigs, with TGF-β2 and TGF-β3 expression becoming prevalent 24 h after wounding, particularly in the migrating epidermis, and TGF-β1 expression increasing later at 5 days post-wounding, when re-epithelialization was complete.103 All three TGF-β isoforms were detected in the mesenchymal cells (dermis) and basal lamina, suggesting their involvement in dermal–epidermal interaction during wound healing.103 An intriguing aspect of TGF-β isoform expression comes from studies comparing their expression profiles in embryonic (scar-free) and adult (scar forming) wounds. Embryonic wounds have been shown to express high levels of TGF-β3, produced mainly by skin cells (keratinocytes and fibroblasts), and low levels of TGF-β1 and TGF-β2; whereas adults wounds contain mostly TGF-β1 and TGF-β2 derived from degranulating platelets and immune cells.26 These findings prompted the hypothesis that the ratio of TGF-β3 to TGF-β1 (or TGF-β2) is an important factor in scar-free wound healing and led to studies aimed at increasing TGF-β3 levels by exogenous application of recombinant TGF-β3 protein or reducing TGF-β1 and TGF-β2 levels using neutralizing antibodies (see below).
The potential for TGF-β to regulate wound healing was first implicated in a study showing that a subcutaneous injection of TGF-β into normal (non-wounded) skin of new born mice caused granulation tissue formation with the induction of angiogenesis and collagen production.104 A subsequent study showed that TGF-β administered to rat incisional wounds accelerated wound healing and increased wound strength.105 These studies used TGF-β purified from human platelets and, therefore, could not distinguish which TGF-β isoform(s) was responsible for these effects. Subsequent studies showed that neutralization of TGF-β1 and TGF-β2 using anti-TGF-β1/2 neutralizing antibody, or exogenous addition of TGF-β3, reduces cutaneous scarring in rat incisional wounds without reducing the wound tensile strength.27,106,107 These studies were important in establishing the pro-scarring effects of TGF-β1 and TGF-β2 and the antiscarring potential of TGF-β3.
The role of TGF-β in wound healing and scarring was further examined by Thomas Mustoe's group, who investigated the effects of an anti-TGF-β1, 2, and 3 monoclonal antibody on wound healing and hypertrophic scar (HTS) formation in rabbit ear wounds.108 They found that early treatment of the wounds (days 0, 2, and 4 post-wounding) with neutralizing antibody impaired wound healing without decreasing scar hypertrophy, whereas as middle (days 7, 9, and 11) and late (days 11, 12, and 13) treatment of wounds significantly reduced scar hypertrophy.108 Although this study could not determine which TGF-β isoform(s) are involved, a follow-up study investigating the temporal expression of TGF-β isoforms in the rabbit ear model showed that elevated levels of TGF-β1, and possibly TGF-β2, are associated with HTS formation.109 Another study using the rabbit ear model showed that viral delivery of a dominant negative (truncated) mutant of TβRII (to block endogenous TGF-β signaling) 8–12 days post-wounding decreased hypertrophic scarring.110 The rabbit ear model has also provided insight on the role of TGF-β signaling during the earlier wound healing events. For example, one study showed that an injection of adenovirus-containing Smad3 48 h before wound healing led to enhanced re-epithelialization and granulation tissue formation in rabbit ear wounds.111 In addition, using the rabbit ear model under ischemic conditions, it was shown that adenoviral delivery of Smad3 enhanced re-epithelialization but that granulation tissue parameters were not affected by Smad3 under ischemic conditions.112 Further studies using TGF-β isoform–specific neutralizing antibodies and adenoviral delivery of Smad2, Smad3, and/or Smad4 at the early (days 0–4) and late (after day 7) stages of wound healing in the rabbit ear model are possible avenues of future research to further elucidate the role of TGF-β/Smad signaling in this valuable animal model.
Further evidence that decreasing TGF-β signaling improves scarring outcome without compromising wound healing is provided by a recent study using transgenic mice overexpressing CD109, a TGF-β co-receptor and a potent TGF-β antagonist, in the skin. These mice display decreased inflammation and granulation tissue formation and improved collagen architecture without a compromise in wound tensile strength as compared with WT littermates, in a manner consistent with inhibition of TGF-β signaling.113 Furthermore, these mice also show a diminished fibrotic response in a bleomycin-induced skin fibrosis model.114 Together, these studies support the notion that dampening TGF-β signaling during cutaneous wound healing is beneficial for improving scarring outcome.
Aberrant TGF-β signaling in pathological scarring: HTSs and keloids
Hypertrophic scarring
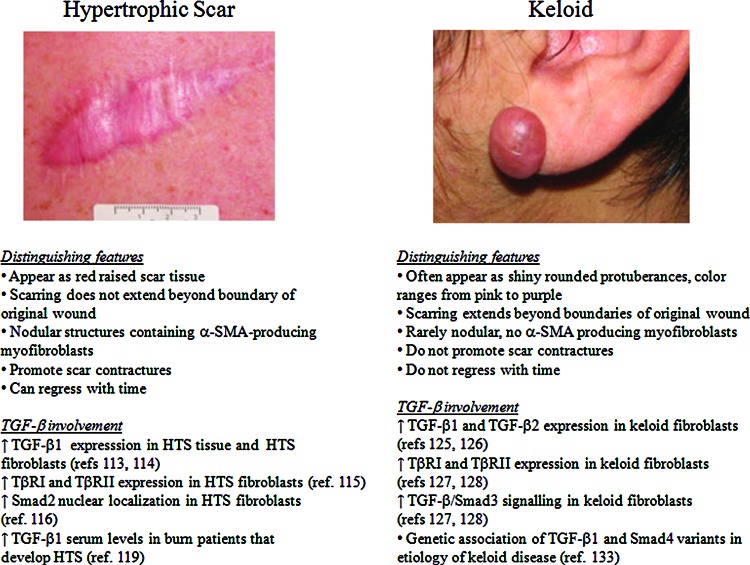
Hypertrophic scarring often occurs after deep burn injury or trauma and is characterized by excessive ECM deposition.115 HTSs appear as elevated red scar tissue that remains within the boundary of the original injury (Fig. 7A).115 Although the pathophysiological mechanism(s) involved in hypertrophic scarring are not fully understood, many studies indicate that aberrant TGF-β signaling plays a key role in its etiology. For example, Scott and colleagues showed that TGF-β1 protein was present in HTSs, particularly in the deep dermis, as well as in the dermis of mature “normal” scars but was not detected in normal (non-scarred) dermis.116 In addition, Wang and colleagues showed that TGF-β1 mRNA levels are higher in HTS tissue and in cultured HTS fibroblasts as compared with normal skin tissue and cells, and that cultured HTS fibroblasts secrete more TGF-β1 protein than normal skin fibroblasts.117 HTS skin tissue has been shown to display persistent ALK5 and TβRII expression compared with normal wound tissue, where ALK5 and TβRII expression declines during the tissue remodeling phase.118 In addition, downstream TGF-β/Smad signaling appears to be activated in HTS fibroblasts, as evidenced by a predominantly nuclear localization of Smad2 in HTS skin fibroblasts as compared with normal fibroblasts.119 The pleiotropic effects of TGF-β1 in fibroblasts, including the induction of their differentiation into myofibroblasts, increasing ECM production,90 and stimulating synthesis of TIMPs that inhibit ECM degradation by MMPs,120 contribute to the formation of HTS.
Figure 7.
Characteristics of human hypertrophic scars and keloids. (A) Hypertrophic scar: This appears as a red, raised scar that does not extend beyond the boundaries of the original injury. They have nodular collagen deposits containing α-SMA producing myofibroblasts that are involved in scar contracture. Hypertrophic scars can regress with time. The main findings from studies on the role of TGF-β signaling and hypertrophic scarring are indicated. (B) Keloid: This appears as a shiny and smooth protuberance ranging from pink to purple in color and extends beyond the boundaries of the original wound. Unlike hypertrophic scars, keloids do not have nodular collagen deposits, α-SMA-producing myofibroblast, do not undergo scar contracture, and do not regress with time. The main findings from studies on the role of TGF-β signaling and keloid formation are indicated. Images were obtained with permission from the DermNet NZ Web site (www.dermnetnz.org). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
In addition to the effects of local TGF-β production by resident fibroblasts, alterations in systemic TGF-β levels have also been implicated in the pathogenesis of HTS. For example, Tredget et al. showed that patients with severe HTS display increased TGF-β1 serum levels after thermal injury and that treatment with interferon (IFN)-α 2b significantly reduced TGF-β1 serum levels and enhanced resolution of HTS.121 IFN-α 2b has been shown to inhibit TGF-β1 protein production by HTS fibroblasts,122 and other studies have shown that IFNs increase expression of the I-Smad7,123 which has been shown to inhibit TGF-β signaling by different mechanisms (see the section Endocytosis and TGF-β signaling). In addition, IFN-α 2b has also been shown to inhibit TGF-β-induced alpha smooth muscle actin expression in a unique subpopulation of leukocytes known as fibrocytes,124 which are circulating bone marrow derived cells that can leave the blood, enter tissues, and differentiate into myofibroblasts in response to TGF-β.125 Interestingly, dermal fibroblasts treated with conditioned medium (CM) from burn patient fibrocytes (but not by CM from normal fibrocytes) showed an increase in proliferation, migration, and contractility, which was abrogated by application of a TGF-β1 neutralizing antibody.126 These findings suggest that fibrocytes in burn patients may act as a source of systemic TGF-β1 as well as a target for TGF-β1-induced myofibroblast differentiation action in tissues, thereby creating a vicious cycle of TGF-β1 production and action in pathogenesis of HTS. In addition, myofibroblasts originating from other cell types, such as epithelial cells by EMT and perivascular cells (pericytes) via trans-differentiation, have also been implicated in pathological scarring (Fig. 6).127 A recent study showing that a major proportion of collagen-overproducing cells generated by scarring are derived from profibrotic progenitors residing in the perivascular space128 suggests that non-resident fibroblasts may play a more important role in scarring that previously thought.
Although TGF-β1 serum levels have been shown to be increased in patients with severe HTS resulting from burn injury, these findings have not been universally reproduced. For example, a recent study on post-burn scarring in children showed that although plasma TGF-β1 levels significantly increased during the first 2 weeks post-injury and then declined in patients who healed with good quality post-burn scars, the early increase in plasma TGF-β1 levels was not detected in patients who developed HTS.129 Further longitudinal studies characterizing TGF-β1 serum levels in a larger cohort of burn patients will be needed to determine whether TGF-β1 serum levels can be used as an indicator for predicting clinical scar outcomes or whether they might serve as a clinical tool for selecting patient groups to be targeted for anti-scarring therapy.
Keloids
Keloids are another type of pathological scar for which aberrant TGF-β signaling is thought to play a pathophysiological role. Unlike HTS, keloids often appear as shiny rounded protuberances with colors ranging from pink to purple, and scarring extends beyond the boundaries of the original injury (Fig. 7B).115 Studies using cultured keloid fibroblasts have shown that these cells produce higher amounts of “pro-scarring” TGF-β1 and TGF-β2 as compared with normal fibroblasts.130,131 Another study demonstrated that keloid fibroblasts exhibit increased expression of ALK5 and TβRII as well as increased phosphorylation of Smad3 relative to normal fibroblasts.132 Interestingly, increased TGF-β/Smad3 signaling has been implicated in keloid pathogenesis via epithelial–mesenchymal interactions, where keloid keratinocytes act through a paracrine mechanism to increase ALK5 and TβRII expression and Smad3 signaling in keloid fibroblasts.133 Genetic studies have not revealed an association between keloid disease and the occurrence of common polymorphisms or mutations in genes encoding the three TGF-β isoforms (-β1, -β2, and -β3) in Caucasians134–136 or Smads (-3, -6, and -7) in a Jamaican population.137 However, a recent study has revealed an association of TGF-β1 and Smad4 variants in the etiology of keloid scar in the Malay population.138 Further genetic studies investigating the potential link between keloid disease and polymorphisms/mutations in components of the TGF-β signaling pathway using larger cohorts of patients, particularly individuals with darker skin pigmentation who are more susceptible to keloid formation, are warranted.
Strategies used for targeting the TGF-β signaling pathway to improve wound healing outcome
The TGF-β signaling pathway is considered a promising target for the treatment of many pathological skin conditions, ranging from chronic (non-healing) wounds to hypertrophic scarring and keloid formation. Manipulation of the TGF-β signaling pathway is also thought to be a suitable strategy for improving the cosmetic appearance of non-pathological “normal” scars that result from surgery or minor injury. This section highlights some of the therapeutic avenues used to target the TGF-β signaling pathway for improving clinical scar outcomes.
Neutralizing antibodies and ligand traps
The concept of blocking TGF-β signaling using anti-TGF-β neutralizing antibodies has been around since the early 1990s with the pioneering work of Ferguson and colleagues, who showed that neutralizing antibodies to TGF-β1/β2 reduces cutaneous scarring in adult rodents.27,106 This work was expanded on by Mustoe's group, who investigated the effects of an anti-TGF-β1, 2, and 3 monoclonal antibody on wound healing and HTS formation in rabbit ear wounds.108 They found that early treatment of the wounds (days 0, 2, and 4 post-wounding) with a neutralizing antibody impaired wound healing without decreasing scar hypertrophy; whereas middle (days 7, 9, and 11) and late (days 11, 12, and 13) treatment of wounds significantly reduced scar hypertrophy.108 CAT-192 is a human monoclonal antibody that neutralizes TGF-β1 and was shown to improve corneal wound healing in bovine organ cultures by promoting re-epithelialization.139
Scleroderma, or systemic sclerosis (SSc), is a rare connective tissue disease that is characterized by autoimmunity, vasculopathy, and fibrosis (scarring) of the skin and internal organs.140 Due to the potentially critical role of TGF-β in the pathogenesis of SSc, it was thought that CAT-192 might provide therapeutic benefit to SSc patients. Although a Phase I/II clinical trial showed that CAT-192 was safe and well tolerated across all dose levels, no conclusions regarding its efficacy in SSc could be made.141 Future preclinical studies showing that CAT-192 reduces cutaneous scarring would be expected to translate well to a clinical setting, as safety and tolerability studies on CAT-192 have already been performed.141
In addition to TGF-β neutralizing antibodies, TGF-β ligand traps represent another class of molecules in development for neutralizing excess TGF-β produced in pathological conditions. One example is the soluble TβRII (sTβRII) containing the extracellular domain of TβRII fused to the Fc region of IgG1, which was developed as a TGF-β–sequestering agent and has shown efficacy in various animal models of human disease.142 A recent study has shown that adenoviral delivery of sTβRII accelerates lymphatic regeneration and decreases inflammation and fibrosis in a mouse tail model of lymphedema,143 suggesting that sTβRII may be applicable to other models of wound healing, scarring, and fibrosis. LAP is another molecule that has potential for development as a TGF-β trap for the treatment of TGF-β-driven pathologies. As an example, one study has shown that treatment with human recombinant LAP (TGF-β1) prevents skin fibrosis in a mouse model of scleroderma (murine sclerodermatous graft-versus-host disease).144 Other molecules with the potential to act as TGF-β ligand traps include decorin and fibromodulin, which are members of the small leucine-rich protein (SLRP) family. Decorin is thought to inhibit all TGF-β isoforms,145 which may pose some limitations for its potential as an antiscarring agent, as it would be expected to block not only the pro-scarring effects of TGF-β1 and TGF-β2, but also the anti-scarring effects of TGF-β3. A recent study demonstrated that a recombinant decorin protein fused to a wound-homing peptide (CARSKNKDC; CAR peptide) displays enhanced TGF-β1 and TGF-β2 neutralization activity and reduced TGF-β3 neutralization activity as compared with non-targeted decorin in vitro, and also enhances wound healing and suppresses scar formation in mice at doses where non-targeted decorin is inactive.146 Decorin has also been shown to block the activity of connective tissue growth factor (CCN2), a downstream mediator of pro-fibrotic TGF-β action,147 suggesting that its antiscarring activity may extend beyond its effects on TGF-β neutralization. Fibromodulin has also been shown to promote wound healing and to reduce scarring in animal models148,149 and may represent a therapeutic target for the treatment of HTS.150,151
TGF-β co-receptors represent a unique class of molecules that are starting to receive attention for their potential as TGF-β ligand neutralizing agents. The ability of TGF-β co-receptors, particularly betaglycan and CD109, to bind TGF-β isoforms with high affinity and specificity make them attractive targets for the development of TGF-β isoform-specific traps. As an example, peptide 144 (P144) is a 14-mer peptide from human betaglycan that was designed as a TGF-β1 inhibitor and has shown efficacy in reducing fibrosis in different animal models.152,153 P144 is currently being tested in a Phase II clinical trial for the treatment of skin fibrosis in SSc.154,155
ALK5 kinase inhibitors
Since excessive TGF-β has been shown to lead to fibrotic scarring, blocking TGF-β action by inhibiting TGF-β receptor kinase activity using small-molecule inhibitors is expected to be beneficial in promoting an antiscarring effect. Several small molecule inhibitors that block ALK5 kinase activity have been tested for their efficacy to improve scarring in preclinical models. The small molecule SB431542, which was developed as a potent inhibitor of ALK5,156 has been shown to reduce scar formation in the eye after glaucoma filtration surgery in rabbits as evidenced by a decrease in collagen deposition in the subconjunctival space in the experimental groups.157 SB431542 used in combination with recombinant human granulocyte colony-stimulating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) was shown to improve wound breaking strength in full-thickness incisional wounds in the rat skin.158 Topical application of a novel ALK5 inhibitor (CP-639180) was shown to reduce collagen deposition in a rat dermal incision wound healing model.159 The latter two studies demonstrate the feasibility of using topically applied small-molecule ALK5 kinase inhibitors to improve cutaneous scarring after wound healing. A recent study reported the discovery of a series of small molecules known as 2-(1H-pyrazol-1-yl)pyridines that act as potent ALK5 kinase inhibitors and have demonstrated their potential utility in the prevention of dermal scarring.160 Topical application of one of these compounds (PF-03671148) in a rat incisional wound repair model led to a reduction in fibrotic gene expression without altering the normal wound healing process.160 Although these studies are promising, it remains to be seen whether any of these ALK5 inhibitors will be of clinical use, as none of them have yet been screened in Phase I clinical trials for safety and tolerability.
TGF-β3
As mentioned earlier, research on the expression and function of TGF-β isoforms (-β1, -β2, and -β3) in scar-free (embryonic) and scar-forming (adult) wound healing models has shown that the three TGF-β isoforms play very different roles in scarring, with TGF-β1 and -β2 showing pro-scarring effects and TGF-β3 displaying antiscarring effects.161 The discovery of the antiscarring properties of TGF-β3 led to a clinical development program for evaluating recombinant human TGF-β3 (avotermin) as a therapeutic intervention (prophylatic) to reduce scarring in human surgical wounds. Prophylactic administration of avotermin was successful for improvement of skin scarring in several double-blind, placebo-controlled, phase I/II studies.162–165 Avotermin was shown to significantly improve the visual appearance of scars,162–165 decrease scar surface area, and promote a collagen organization that more closely resembled normal skin in 14 of 19 cases.162 Unfortunately, avotermin failed to show efficacy in Phase III trials, possibly due to the use of a different TGF-β3 standard, which led to a twofold overestimation of TGF-β3 concentration, and, therefore, a 50% lower dose of TGF-β3 used in the Phase III clinical trial as compared with the Phase I/II clinical trials.166
Antisense oligonucleotides
Antisense oligonucleotides are single-stranded DNA or RNA that are complementary to a specific mRNA sequence which blocks target gene expression either by inhibiting RNA translation or by promoting enzymatic degradation of the mRNA target.167 Several studies demonstrate that antisense oligonucleotides targeting components of the TGF-β signaling pathway have the potential to modulate wound healing and scarring outcomes. In one study, topical application of antisense TGF-β1 oligonucleotides reduced scarring of incisional wounds in mice as compared with sense control oligonucleotides.168 In addition, TGF-β1 antisense oligonucleotides reduced scarring and improved surgical outcome in animal models in which surgical procedures performed resemble those done in glaucoma patients.169 In addition, Smad3 antisense oligonucleotides accelerated wound healing and reduced scarring in a mouse excisional wound model.170 More advanced methods of gene silencing such as RNA interference have also been successfully employed in wound healing and scarring studies. Accordingly, transcutaneous delivery of Smad3 siRNA decreases radiation-induced skin fibrosis as compared with control siRNA.171
Take-Home Messages.
Basic science advances
TGF-β plays important roles in all three phases (inflammation, proliferation, and maturation) of wound healing.
TGF-β isoforms (-β1, -β2, and -β3) play distinct roles in wound healing with TGF-β1/2 having predominantly pro-scarring roles and TGF-β3 having mainly anti-scarring effects.
Recent advances in our understanding of TGF-β signaling, including TGF-β synthesis and activation, TGF-β receptor activity, Smad pathways, and modulation by co-receptors, provide new opportunities to delineate the mechanisms by which TGF-β signaling regulates specific wound healing events.
Clinical science advances
Several therapeutic agents, including anti–TGF-β neutralizing antibodies, TGF-β ligand traps, small-molecule inhibitors of TGF-β signaling receptors, and TGF-β3, have shown promising results in improving scarring in preclinical studies. TGF-β3 was successful in reducing scarring in Phase I/II clinical trials but failed to show efficacy in a Phase III clinical trial.
The potential beneficial effects of TGF-β isoforms during the early stages of wound healing and the different effects of TGF-β1/β2 (pro-scarring) and TGF-β3 (anti-scarring) at the later stages of wound healing suggest that modulation of TGF-β signaling to promote wound healing and/or reduce scarring may require agents that modulate TGF-β signaling in a temporal and isoform-specific manner.
Relevance to clinical care
The development of therapeutic agents that target the TGF-β signaling pathway to reduce scarring is relevant to clinicians and surgeons who treat conditions such as burn injury or other trauma that lead to hypertrophic scarring, keloid formation, and/or tissue fibrosis.
Future studies targeting other components of the TGF-β signaling pathway to modulate wound healing and scarring outcome are eagerly awaited. Figure 8 depicts strategies for targeting the TGF-β pathway to improve wound healing outcome as described earlier.
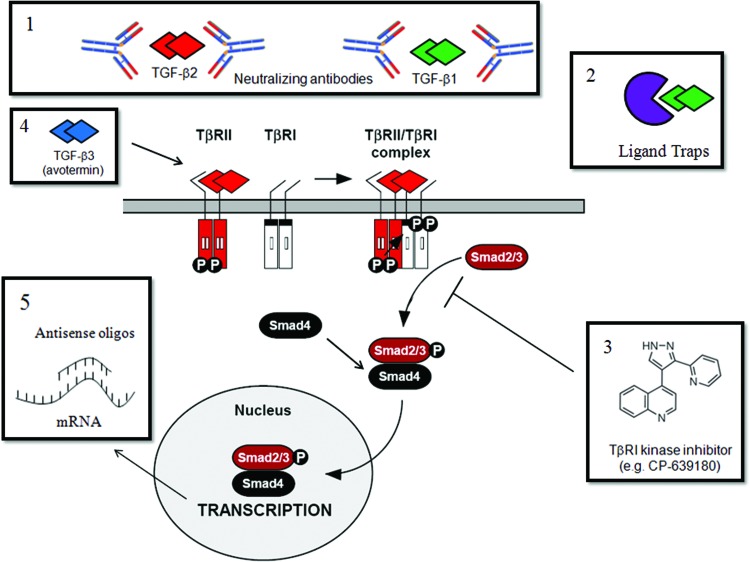
Figure 8.
Therapeutic strategies that have been used to target the TGF-β signaling pathway to reduce scarring. (1) Neutralizing TGF-β antibodies and (2) ligand traps bind TGF-β and neutralize TGF-β activity by preventing its binding to the TGF-β signaling receptors (TβRII and TβRI). (3) Small molecules that inhibit ALK5 kinase activity, including CP-639180 (depicted), prevent ALK5-induced phosphorylation of Smad2 and Smad3 and downstream signaling events. (4) Recombinant TGF-β3 protein (avotermin) binds to TGF-β signaling receptors and elicits Smad2/3-dependent signaling. Unlike TGF-β1 and TGF-β2 isoforms that have pro-scarring effects, TGF-β3 has anti-scarring properties. The molecular mechanisms underlying the different responses of the TGF-β isoforms have not been elucidated. (5) Antisense oligonucleotides are single-stranded DNA or RNA sequences that are complementary to a specific mRNA sequence. They bind to their target mRNA sequence and silence gene expression by blocking protein translation or promoting degradation of the mRNA transcript. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Limitations and Future Directions
Although the TGF-β signaling pathway is considered a promising therapeutic target for the treatment of impaired wound healing and excessive scarring (fibrosis), currently, no therapies are available that target the TGF-β signaling pathway to improve wound healing outcome. One potential explanation for the lack of “translatability” of TGF-β research is that the animal models used in preclinical research may not mimic the wound healing and scarring responses in humans. Accordingly, there has been recent progress in the development of animal models such as the nude mouse model of hypertrophic scarring, which displays many characteristics of human hypertrophic scarring172,173 and chemically induced animal models of systemic sclerosis (scleroderma) that reproduce the entire spectrum of the human disease, including inflammation, autoimmunity, vasculopathy, and fibrosis.174 Such models provide powerful tools for future preclinical studies, exploring the utility of agents that target the TGF-β signaling pathway to improve wound healing or scarring outcomes. In addition, identifying the cellular source of TGF-β that causes scarring and fibrosis and devising strategies using specific modes of delivery such as topical treatment and intralesional delivery or systemic administration may be important. For example, emerging evidence indicates that fibroblasts from the deeper dermal layers produce more of the pro-fibrotic TGF-β1 and less anti-fibrotic decorin, fibromodulin, and TGF-β3 than fibroblasts from superficial layers and contribute to the development of HTS after injuries involving the deep dermis.150,151,175 The latter findings have important implications for selecting an appropriate strategy for delivering an anti-TGF-β therapy specifically to the deep dermis to ameliorate fibrosis in HTS patients.
Abbreviations and Acronyms
- ALK
activin-like receptor kinase
- AMH
anti-müllerian hormone
- BMP
bone morphogenetic protein
- CM
conditioned medium
- ECM
extracellular matrix
- EMT
epithelial-to-mesenchymal transition
- GDF
growth and differentiation factor
- GS
glycine/serine
- HTS
hypertrophic scar
- IFN
interferon
- I-Smad
inhibitory Smad
- LAP
latency-associated peptide
- LLC
large latent complex
- LOX
lysyl oxidase
- LTBP
latent TGF-β binding protein
- MH
Mad homology
- MIS
müllerian inhibiting substance
- MMP
matrix metalloproteinase
- P144
peptide 144
- rSmad
receptor regulated Smads
- SLC
small latent complex
- Smurf
Smad ubiquitination regulatory factor
- SSc
systemic sclerosis
- sTβRII
soluble TβRII
- TβRI, TβRII
TGF-β receptor types I and II
- TIMP
tissue inhibitor of MMPs
- TGF-β
transforming growth factor beta
- TSP-1
thrombospondin 1
- WT
wild-type
Acknowledgments and Funding Source
The authors thank Phililp lab members Anshuman Saksena and Christopher Chiavatti for help with the figure preparation. The work presented in this article was supported by a Canadian Institute of Health Research Grant to A.P.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Dr. Kenneth Finnson is a Research Associate with Dr. Anie Philip in the Department of Surgery, McGill University, Montreal, Quebec, Canada. Dr. Gianni Di Guglielmo is an Associate Professor in the Department of Physiology and Pharmacology, Western University, London, Ontario, Canada. Dr. Di Guglielmo's research focuses on understanding the role of TGF-β receptor endocytosis in the regulation of TGF-β signaling. Dr. Sarah McLean is a recent PhD graduate from Dr. Di Guglielmo's lab. Dr. McLean's PhD work focused on the role of the TGF-β co-receptor betaglycan in regulating TGF-β signaling. Dr. Anie Philip is a Professor in the Department of Surgery, McGill University. Dr. Philip's research program focuses on understanding the molecular mechanisms of TGF-β signaling in wound healing, scarring, and fibrosis.
References
- 1.Penn JW. Grobbelaar AO. Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2:18. [PMC free article] [PubMed] [Google Scholar]
- 2.Wu MY. Hill CS. TGF-β superfamily signaling in embryonic development and homeostasis. Dev Cell. 2009;16:329. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Biernacka A. Dobaczewski M. Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. 2011;29:196. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rider CC. Mulloy B. Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. Biochem J. 2010;429:1. doi: 10.1042/BJ20100305. [DOI] [PubMed] [Google Scholar]
- 5.Schmierer B. Hill CS. TGF-β/Smad signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 6.Horbelt D. Denkis A. Knaus P. A portrait of transforming growth factor-β superfamily signalling: background matters. Int J Biochem Cell Biol. 2012;44:469. doi: 10.1016/j.biocel.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Chen D. Zhao M. Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 8.Ruschke K. Hiepen C. Becker J. Knaus P. BMPs are mediators in tissue crosstalk of the regenerating musculoskeletal system. Cell Tissue Res. 2012;347:521. doi: 10.1007/s00441-011-1283-6. [DOI] [PubMed] [Google Scholar]
- 9.Ono Y. Calhabeu F. Morgan JE. Katagiri T. Amthor H. Zammit PS. BMP signalling permits population expansion by preventing premature myogenic differentiation in muscle satellite cells. Cell Death Differ. 2011;18:222. doi: 10.1038/cdd.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott B. Renshaw D. Getting S. Mackenzie R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol (Oxf) 2012;205:324. doi: 10.1111/j.1748-1716.2012.02423.x. [DOI] [PubMed] [Google Scholar]
- 11.Knight PG. Glister C. TGF-β superfamily members and ovarian follicle development. Reproduction. 2006;132:191. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 12.Werner S. Alzheimer C. Roles of activin in tissue repair, fibrosis, and inflammatory disease. Cytokine Growth Factor Rev. 2006;17:157. doi: 10.1016/j.cytogfr.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Hubner G. Hu Q. Smola H. Werner S. Strong induction of activin expression after injury suggests an important role of activin in wound repair. Dev Biol. 1996;173:490. doi: 10.1006/dbio.1996.0042. [DOI] [PubMed] [Google Scholar]
- 14.Munz B. Smola H. Engelhardt F. Bleuel K. Brauchle M. Lein I. Evans LW. Huylebroeck D. Balling R. Werner S. Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. EMBO J. 1999;18:5205. doi: 10.1093/emboj/18.19.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wankell M. Munz B. Hübner G. Hans W. Wolf E. Goppelt A. Werner S. Impaired wound healing in transgenic mice overexpressing the activin antagonist follistatin in the epidermis. EMBO J. 2001;20:5361. doi: 10.1093/emboj/20.19.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antsiferova M. Klatte JE. Bodó E. Paus R. Jorcano JL. Matzuk MM. Werner S. Kögel H. Keratinocyte-derived follistatin regulates epidermal homeostasis and wound repair. Lab Invest. 2009;89:131. doi: 10.1038/labinvest.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schier AF. Nodal morphogens. Cold Spring Harb Perspect Biol. 2009;1:a003459. doi: 10.1101/cshperspect.a003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strizzi L. Hardy KM. Kirschmann DA. Ahrlund-Richter L. Hendrix MJ. Nodal expression and detection in cancer: experience and challenges. Cancer Res. 2012;72:1915. doi: 10.1158/0008-5472.CAN-11-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y. Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 20.Laverty HG. Wakefield LM. Occleston NL. O'Kane S. Ferguson MW. TGF-β3 and cancer: a review. Cytokine Growth Factor Rev. 2009;20:305. doi: 10.1016/j.cytogfr.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlingensiepen KH. Schlingensiepen R. Steinbrecher A. Hau P. Bogdahn U. Fischer-Blass B. Jachimczak P. Targeted tumor therapy with the TGF-β2 antisense compound AP 12009. Cytokine Growth Factor Rev. 2006;17:129. doi: 10.1016/j.cytogfr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Dunker N. Krieglstein K. Targeted mutations of transforming growth factor-β genes reveal important roles in mouse development and adult homeostasis. Eur J Biochem. 2000;267:6982. doi: 10.1046/j.1432-1327.2000.01825.x. [DOI] [PubMed] [Google Scholar]
- 23.Shull MM. Ormsby I. Kier AB. Pawlowski S. Diebold RJ. Yin M. Allen R. Sidman C. Proetzel G. Calvin D. Annunziata N. Doetschman T. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanford LP. Ormsby I. Gittenberger-de Groot AC. Sariola H. Friedman R. Boivin GP. Cardell EL. Doetschman T. TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development. 1997;124:2659. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proetzel G. Pawlowski SA. Wiles MV. Yin M. Boivin GP. Howles PN. Ding J. Ferguson MW. Doetschman T. Transforming growth factor-β3 is required for secondary palate fusion. Nat Genet. 1995;11:409. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson MW. O'Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci. 2004;359:839. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah M. Foreman DM. Ferguson MW. Neutralisation of TGF-β1 and TGF-β2 or exogenous addition of TGF-β3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108(Pt 3):985. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 28.Horiguchi M. Ota M. Rifkin DB. Matrix control of transforming growth factor-β function. J Biochem. 2012;152:321. doi: 10.1093/jb/mvs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annes JP. Munger JS. Rifkin DB. Making sense of latent TGF-β activation. J Cell Sci. 2003;116:217. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 30.Sato Y. Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-β1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pircher R. Jullien P. Lawrence DA. Beta-transforming growth factor is stored in human blood platelets as a latent high molecular weight complex. Biochem Biophys Res Commun. 1986;136:30. doi: 10.1016/0006-291x(86)90872-7. [DOI] [PubMed] [Google Scholar]
- 32.Brunner G. Blakytny R. Extracellular regulation of TGF-β activity in wound repair: growth factor latency as a sensor mechanism for injury. Thromb Haemost. 2004;92:253. doi: 10.1160/TH04-05-0324. [DOI] [PubMed] [Google Scholar]
- 33.Yu Q. Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163. [PMC free article] [PubMed] [Google Scholar]
- 34.Annes JP. Rifkin DB. Munger JS. The integrin αVβ6 binds and activates latent TGFβ3. FEBS Lett. 2002;511:65. doi: 10.1016/s0014-5793(01)03280-x. [DOI] [PubMed] [Google Scholar]
- 35.Dallas SL. Zhao S. Cramer SD. Chen Z. Peehl DM. Bonewald LF. Preferential production of latent transforming growth factor β-2 by primary prostatic epithelial cells and its activation by prostate-specific antigen. J Cell Physiol. 2005;202:361. doi: 10.1002/jcp.20147. [DOI] [PubMed] [Google Scholar]
- 36.Ahamed J. Janczak CA. Wittkowski KM. Coller BS. In vitro and in vivo evidence that thrombospondin-1 (TSP-1) contributes to stirring- and shear-dependent activation of platelet-derived TGF-β1. PLoS One. 2009;4:e6608. doi: 10.1371/journal.pone.0006608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grainger DJ. Wakefield L. Bethell HW. Farndale RW. Metcalfe JC. Release and activation of platelet latent TGF-β in blood clots during dissolution with plasmin. Nat Med. 1995;1:932. doi: 10.1038/nm0995-932. [DOI] [PubMed] [Google Scholar]
- 38.Nunes I. Shapiro RL. Rifkin DB. Characterization of latent TGF-β activation by murine peritoneal macrophages. J Immunol. 1995;155:1450. [Google Scholar]
- 39.Derynck R. Feng XH. TGF-β receptor signaling. Biochim Biophys Acta. 1997;1333:F105. doi: 10.1016/s0304-419x(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 40.Attisano L. Wrana JL. Signal transduction by members of the transforming growth factor-β superfamily. Cytokine Growth Factor Rev. 1996;7:327. doi: 10.1016/s1359-6101(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 41.Henis YI. Moustakas A. Lin HY. Lodish HF. The types II and III transforming growth factor-β receptors form homo-oligomers. J Cell Biol. 1994;126:139. doi: 10.1083/jcb.126.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin HY. Moustakas A. Knaus P. Wells RG. Henis YI. Lodish HF. The soluble exoplasmic domain of the type II transforming growth factor (TGF)-β receptor. A heterogeneously glycosylated protein with high affinity and selectivity for TGF-β ligands. J Biol Chem. 1995;270:2747. doi: 10.1074/jbc.270.6.2747. [DOI] [PubMed] [Google Scholar]
- 43.Lin HY. Wang XF. Ng-Eaton E. Weinberg RA. Lodish HF. Expression cloning of the TGF-β type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992;68:775. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- 44.Luo K. Zhou P. Lodish HF. The specificity of the transforming growth factor β receptor kinases determined by a spatially addressable peptide library. Proc Natl Acad Sci USA. 1995;92:11761. doi: 10.1073/pnas.92.25.11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wrana JL. Attisano L. Wieser R. Ventura F. Massague J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 46.Bierie B. Moses HL. Tumour microenvironment: TGF-β: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 47.Huse M. Muir TW. Xu L. Chen YG. Kuriyan J. Massagué J. The TGF-β receptor activation process: an inhibitor- to substrate-binding switch. Mol Cell. 2001;8:671. doi: 10.1016/s1097-2765(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 48.Wieser R. Wrana JL. Massague J. GS domain mutations that constitutively activate TβR-I, the downstream signaling component in the TGF-β receptor complex. EMBO J. 1995;14:2199. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Attisano L. Lee-Hoeflich ST. The Smads. Genome Biol. 2001;2:REVIEWS3010. doi: 10.1186/gb-2001-2-8-reviews3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakao A. Imamura T. Souchelnytskyi S. Kawabata M. Ishisaki A. Oeda E. Tamaki K. Hanai J. Heldin CH. Miyazono K. ten Dijke P. TGF-β receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997;16:5353. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakao A. Röijer E. Imamura T. Souchelnytskyi S. Stenman G. Heldin CH. ten Dijke P. Identification of Smad2, a human Mad-related protein in the transforming growth factor-β signaling pathway. J Biol Chem. 1997;272:2896. doi: 10.1074/jbc.272.5.2896. [DOI] [PubMed] [Google Scholar]
- 52.Macías-Silva M. Abdollah S. Hoodless PA. Pirone R. Attisano L. Wrana JL. MADR2 is a substrate of the TGFβ receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- 53.Massague J. Gomis RR. The logic of TGF-β signaling. FEBS Lett. 2006;580:2811. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 54.Xiao Z. Latek R. Lodish HF. An extended bipartite nuclear localization signal in Smad4 is required for its nuclear import and transcriptional activity. Oncogene. 2003;22:1057. doi: 10.1038/sj.onc.1206212. [DOI] [PubMed] [Google Scholar]
- 55.López-Casillas F. Cheifetz S. Doody J. Andres JL. Lane WS. Massagué J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991;67:785. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- 56.Moren A. Ichijo H. Miyazono K. Molecular cloning and characterization of the human and porcine transforming growth factor-β type III receptors. Biochem Biophys Res Commun. 1992;189:356. doi: 10.1016/0006-291x(92)91566-9. [DOI] [PubMed] [Google Scholar]
- 57.Gougos A. Letarte M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem. 1990;265:8361. [PubMed] [Google Scholar]
- 58.Yamashita H. Ichijo H. Grimsby S. Morén A. ten Dijke P. Miyazono K. Endoglin forms a heteromeric complex with the signaling receptors for transforming growth factor-β. J Biol Chem. 1994;269:1995. [PubMed] [Google Scholar]
- 59.Guerrero-Esteo M. Sanchez-Elsner T. Letamendia A. Bernabeu C. Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-β receptors I and II. J Biol Chem. 2002;277:29197. doi: 10.1074/jbc.M111991200. [DOI] [PubMed] [Google Scholar]
- 60.Chen W. Kirkbride KC. How T. Nelson CD. Mo J. Frederick JP. Wang XF. Lefkowitz RJ. Blobe GC. β-Arrestin 2 mediates endocytosis of type III TGF-β receptor and down-regulation of its signaling. Science. 2003;301:1394. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 61.Bernabeu C. Lopez-Novoa JM. Quintanilla M. The emerging role of TGF-β superfamily co-receptors in cancer. Biochim Biophys Acta. 2009;1792:954. doi: 10.1016/j.bbadis.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Wong SH. Hamel L. Chevalier S. Philip A. Endoglin expression on human microvascular endothelial cells: association with betaglycan and formation of higher order complexes with TGF-β signalling receptors. Eur J Biochem. 2000;267:5550. doi: 10.1046/j.1432-1327.2000.01621.x. [DOI] [PubMed] [Google Scholar]
- 63.McLean S. Di Guglielmo GM. TβRIII directs clathrin-mediated endocytosis of TGFβ type I and II receptors. Biochem J. 2010;429:137. doi: 10.1042/BJ20091598. [DOI] [PubMed] [Google Scholar]
- 64.ten Dijke P. Goumans MJ. Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis. 2008;11:79. doi: 10.1007/s10456-008-9101-9. [DOI] [PubMed] [Google Scholar]
- 65.Finnson KW. Parker WL. Chi Y. Hoemann CD. Goldring MB. Antoniou J. Philip A. Endoglin differentially regulates TGF-β-induced Smad2/3 and Smad1/5 signalling and its expression correlates with extracellular matrix production and cellular differentiation state in human chondrocytes. Osteoarthritis Cartilage. 2010;18:1518. doi: 10.1016/j.joca.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Finnson KW. Parker WL. ten Dijke P. Thorikay M. Philip A. ALK1 opposes ALK5/Smad3 signaling and expression of extracellular matrix components in human chondrocytes. J Bone Miner Res. 2008;23:896. doi: 10.1359/jbmr.080209. [DOI] [PubMed] [Google Scholar]
- 67.Parker WL. Goldring MB. Philip A. Endoglin is expressed on human chondrocytes and forms a heteromeric complex with betaglycan in a ligand and type II TGFβ receptor independent manner. J Bone Miner Res. 2003;18:289. doi: 10.1359/jbmr.2003.18.2.289. [DOI] [PubMed] [Google Scholar]
- 68.Morris E. Chrobak I. Bujor A. Hant F. Mummery C. Ten Dijke P. Trojanowska M. Endoglin promotes TGF-β/Smad1 signaling in scleroderma fibroblasts. J Cell Physiol. 2011;226:3340. doi: 10.1002/jcp.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leask A. Abraham DJ. Finlay DR. Holmes A. Pennington D. Shi-Wen X. Chen Y. Venstrom K. Dou X. Ponticos M. Black C. Bernabeu C. Jackman JK. Findell PR. Connolly MK. Dysregulation of transforming growth factor β signaling in scleroderma: overexpression of endoglin in cutaneous scleroderma fibroblasts. Arthritis Rheum. 2002;46:1857. doi: 10.1002/art.10333. [DOI] [PubMed] [Google Scholar]
- 70.Tam B. Germain L. Philip A. TGF-β receptor expression on human keratinocytes: a 150 kDa GPI-anchored TGF-β1 binding protein forms a heteromeric complex with type I and type II receptors. J Cell Biochem. 1998;70:573. doi: 10.1002/(sici)1097-4644(19980915)70:4<573::aid-jcb13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 71.Tam B. Philip A. Transforming growth factor-β receptor expression on human skin fibroblasts: dimeric complex formation of type I and type II receptors and identification of glycosyl phosphatidylinositol-anchored transforming growth factor-β binding proteins. J Cell Physiol. 1998;176:553. doi: 10.1002/(SICI)1097-4652(199809)176:3<553::AID-JCP12>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 72.Tam B. Larouche D. Germain L. Hooper N. Philip A. Characterization of a 150 kDa accessory receptor for TGF-β1 on keratinocytes: direct evidence for a GPI anchor and ligand binding of the released form. J Cell Biochem. 2001;83:494. doi: 10.1002/jcb.1074. [DOI] [PubMed] [Google Scholar]
- 73.Tam BYY. Finnson KW. Philip A. Glycosylphosphatidylinositol-anchored proteins regulate transforming growth factor-β signaling in human keratinocytes. J Biol Chem. 2003;278:49610. doi: 10.1074/jbc.M308492200. [DOI] [PubMed] [Google Scholar]
- 74.Finnson KW. Tam BY. Liu K. Marcoux A. Lepage P. Roy S. Bizet AA. Philip A. Identification of CD109 as part of the TGF-β receptor system in human keratinocytes. FASEB J. 2006;20:1525. doi: 10.1096/fj.05-5229fje. [DOI] [PubMed] [Google Scholar]
- 75.Bizet AA. Liu K. Tran-Khanh N. Saksena A. Vorstenbosch J. Finnson KW. Buschmann MD. Philip A. The TGF-β co-receptor, CD109, promotes internalization and degradation of TGF-β receptors. Biochim Biophys Acta: Molecular Cell Research. 2011;1813:742. doi: 10.1016/j.bbamcr.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 76.Bizet AA. Tran-Khanh N. Saksena A. Liu K. Buschmann MD. Philip A. CD109-mediated degradation of the TGF-β receptors and inhibition of TGF-β responses involve regulation of Smad7 and Smurf2 localization and function. J Cell Biochem. 2012;113:238. doi: 10.1002/jcb.23349. [DOI] [PubMed] [Google Scholar]
- 77.Doherty GJ. McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 78.Di Guglielmo GM. Le Roy C. Goodfellow AF. Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol. 2003;5:410. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 79.Mitchell H. Choudhury A. Pagano RE. Leof EB. Ligand-dependent and -independent transforming growth factor-β receptor recycling regulated by clathrin-mediated endocytosis and Rab11. Mol Biol Cell. 2004;15:4166. doi: 10.1091/mbc.E04-03-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Razani B. Zhang XL. Bitzer M. von Gersdorff G. Böttinger EP. Lisanti MP. Caveolin-1 regulates transforming growth factor (TGF)-β/Smad signaling through an interaction with the TGF-β type I receptor. J Biol Chem. 2001;276:6727. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- 81.Kavsak P. Rasmussen RK. Causing CG. Bonni S. Zhu H. Thomsen GH. Wrana JL. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF-β receptor for degradation. Mol Cell. 2000;6:1365. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 82.Ogunjimi AA. Briant DJ. Pece-Barbara N. Le Roy C. Di Guglielmo GM. Kavsak P. Rasmussen RK. Seet BT. Sicheri F. Wrana JL. Regulation of Smurf2 ubiquitin ligase activity by anchoring the E2 to the HECT domain. Mol Cells. 2005;19:297. doi: 10.1016/j.molcel.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 83.Schultz GS. Davidson JM. Kirsner RS. Bornstein P. Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19:134. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clemetson KJ. Platelets and primary haemostasis. Thromb Res. 2012;129:220. doi: 10.1016/j.thromres.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 85.Wang XJ. Han G. Owens P. Siddiqui Y. Li AG. Role of TGF β-mediated inflammation in cutaneous wound healing. J Investig Dermatol Symp Proc. 2006;11:112. doi: 10.1038/sj.jidsymp.5650004. [DOI] [PubMed] [Google Scholar]
- 86.Singer AJ. Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 87.Wahl S. R&D Systems, Inc.; 2002. [May 7;2013 ]. Cytokines in wound healing. [Google Scholar]
- 88.Midwood KS. Williams LV. Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36:1031. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 89.Li J. Zhang YP. Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- 90.Hinz B. Phan SH. Thannickal VJ. Prunotto M. Desmoulière A. Varga J. De Wever O. Mareel M. Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barrientos S. Stojadinovic O. Golinko MS. Brem H. Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 92.Gailit J. Welch MP. Clark RA. TGF-β1 stimulates expression of keratinocyte integrins during re-epithelialization of cutaneous wounds. J Invest Dermatol. 1994;103:221. doi: 10.1111/1523-1747.ep12393176. [DOI] [PubMed] [Google Scholar]
- 93.Xue M. Le NT. Jackson CJ. Targeting matrix metalloproteases to improve cutaneous wound healing. Expert Opin Ther Targets. 2006;10:143. doi: 10.1517/14728222.10.1.143. [DOI] [PubMed] [Google Scholar]
- 94.Brew K. Dinakarpandian D. Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 95.Lorenz H. Longaker M. Wounds: Biology, Pathology, Management. New York: Springer; 2003. 2003. pp. 77–88. [Google Scholar]
- 96.Redden RA. Doolin EJ. Collagen crosslinking and cell density have distinct effects on fibroblast-mediated contraction of collagen gels. Skin Res Technol. 2003;9:290. doi: 10.1034/j.1600-0846.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 97.Udupa SL. Inhibition of lysyl oxidase by isoniazid and its effect on wound healing. Indian J Exp Biol. 1995;33:278. [PubMed] [Google Scholar]
- 98.Lau YK. Gobin AM. West JL. Overexpression of lysyl oxidase to increase matrix crosslinking and improve tissue strength in dermal wound healing. Ann Biomed Eng. 2006;34:1239. doi: 10.1007/s10439-006-9130-8. [DOI] [PubMed] [Google Scholar]
- 99.Colwell AS. Krummel TM. Longaker MT. Lorenz HP. Early-gestation fetal scarless wounds have less lysyl oxidase expression. Plast Reconstr Surg. 2006;118:1125. doi: 10.1097/01.prs.0000221056.27536.db. discussion 1130–1131. [DOI] [PubMed] [Google Scholar]
- 100.Szauter KM. Cao T. Boyd CD. Csiszar K. Lysyl oxidase in development, aging and pathologies of the skin. Pathol Biol (Paris) 2005;53:448. doi: 10.1016/j.patbio.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 101.Finnson KW. Arany P. Philip A. Transforming growth factor-beta signaling in cutaneous wound healing: lessons learned from animal studies. Adv Wound Care. 2013;2:XXX. doi: 10.1089/wound.2012.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kane CJ. Hebda PA. Mansbridge JN. Hanawalt PC. Direct evidence for spatial and temporal regulation of transforming growth factor β1 expression during cutaneous wound healing. J Cell Physiol. 1991;148:157. doi: 10.1002/jcp.1041480119. [DOI] [PubMed] [Google Scholar]
- 103.Levine JH. Moses HL. Gold LI. Nanney LB. Spatial and temporal patterns of immunoreactive transforming growth factor β1, β2, and β3 during excisional wound repair. Am J Pathol. 1993;143:368. [PMC free article] [PubMed] [Google Scholar]
- 104.Roberts AB. Sporn MB. Assoian RK. Smith JM. Roche NS. Wakefield LM. Heine UI. Liotta LA. Falanga V. Kehrl JH. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mustoe TA. Pierce GF. Thomason A. Gramates P. Sporn MB. Deuel TF. Accelerated healing of incisional wounds in rats induced by transforming growth factor-β. Science. 1987;237:1333. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- 106.Shah M. Foreman DM. Ferguson MW. Neutralising antibody to TGF-β 1, 2 reduces cutaneous scarring in adult rodents. J Cell Sci. 1994;107(Pt 5):1137. doi: 10.1242/jcs.107.5.1137. [DOI] [PubMed] [Google Scholar]
- 107.Shah M. Foreman DM. Ferguson MW. Control of scarring in adult wounds by neutralising antibody to transforming growth factor β. Lancet. 1992;339:213. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- 108.Lu L. Saulis AS. Liu WR. Roy NK. Chao JD. Ledbetter S. Mustoe TA. The temporal effects of anti-TGF-β1, 2, and 3 monoclonal antibody on wound healing and hypertrophic scar formation. J Am Coll Surg. 2005;201:391. doi: 10.1016/j.jamcollsurg.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 109.Kryger ZB. Sisco M. Roy NK. Lu L. Rosenberg D. Mustoe TA. Temporal expression of the transforming growth factor-β pathway in the rabbit ear model of wound healing and scarring. J Am Coll Surg. 2007;205:78. doi: 10.1016/j.jamcollsurg.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 110.Reid RR. Roy N. Mogford JE. Zimmerman H. Lee C. Mustoe TA. Reduction of hypertrophic scar via retroviral delivery of a dominant negative TGF-β receptor II. J Plast Reconstr Aesthet Surg. 2007;60:64. doi: 10.1016/j.bjps.2005.12.026. discussion 73–74. [DOI] [PubMed] [Google Scholar]
- 111.Sumiyoshi K. Nakao A. Setoguchi Y. Okumura K. Ogawa H. Exogenous Smad3 accelerates wound healing in a rabbit dermal ulcer model. J Invest Dermatol. 2004;123:229. doi: 10.1111/j.0022-202X.2004.22730.x. [DOI] [PubMed] [Google Scholar]
- 112.Kloeters O. Jia SX. Roy N. Schultz GS. Leinfellner G. Mustoe TA. Alteration of Smad3 signaling in ischemic rabbit dermal ulcer wounds. Wound Repair Regen. 2007;15:341. doi: 10.1111/j.1524-475X.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 113.Vorstenbosch J. Gallant-Behm C. Trzeciak A. Roy S. Mustoe T. Philip A. Transgenic mice overexpressing CD109 in the epidermis display decreased inflammation and granulation tissue and improved collagen architecture during wound healing. Wound Repair Regen. 2013;21:235. doi: 10.1111/wrr.12023. [DOI] [PubMed] [Google Scholar]
- 114.Vorstenbosch J. Al-Ajmi H. Winocour S. Trzeciak A. Lessard L. Philip A. CD109 overexpression ameliorates skin fibrosis in a bleomycin-induced mouse model of scleroderma. Arthritis Rheum. 2013;65:1378. doi: 10.1002/art.37907. [DOI] [PubMed] [Google Scholar]
- 115.Gauglitz GGM. Korting HCM. Pavicic TM. Ruzicka TM. Jeschke MGMP. Hypertrophic scarring and keloids: pathomechanisms, current and emerging treatment strategies. Mol Med. 2011;17:113. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scott PG. Dodd CM. Tredget EE. Ghahary A. Rahemtulla F. Immunohistochemical localization of the proteoglycans decorin, biglycan and versican and transforming growth factor-β in human post-burn hypertrophic and mature scars. Histopathology. 1995;26:423. doi: 10.1111/j.1365-2559.1995.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 117.Wang R. Ghahary A. Shen Q. Scott PG. Roy K. Tredget EE. Hypertrophic scar tissues and fibroblasts produce more transforming growth factor-β1 mRNA and protein than normal skin and cells. Wound Repair Regen. 2000;8:128. doi: 10.1046/j.1524-475x.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- 118.Schmid P. Itin P. Cherry G. Bi C. Cox DA. Enhanced expression of transforming growth factor-β type I and type II receptors in wound granulation tissue and hypertrophic scar. Am J Pathol. 1998;152:485. [PMC free article] [PubMed] [Google Scholar]
- 119.Xie JL. Qi SH. Pan S. Xu YB. Li TZ. Liu XS. Liu P. Expression of Smad protein by normal skin fibroblasts and hypertrophic scar fibroblasts in response to TGF-β1. Dermatol Surg. 2008;34:1216. doi: 10.1111/j.1524-4725.2008.34261.x. discussion 1224–1225. [DOI] [PubMed] [Google Scholar]
- 120.Eickelberg O. Köhler E. Reichenberger F. Bertschin S. Woodtli T. Erne P. Perruchoud AP. Roth M. Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-β1 and TGF-β3. Am J Physiol. 1999;276:L814. doi: 10.1152/ajplung.1999.276.5.L814. [DOI] [PubMed] [Google Scholar]
- 121.Tredget EE. Shankowsky HA. Pannu R. Nedelec B. Iwashina T. Ghahary A. Taerum TV. Scott PG. Transforming growth factor-β in thermally injured patients with hypertrophic scars: effects of interferon alpha-2b. Plast Reconstr Surg. 1998;102:1317. doi: 10.1097/00006534-199810000-00001. discussion 1329–1330. [DOI] [PubMed] [Google Scholar]
- 122.Tredget EE. Wang R. Shen Q. Scott PG. Ghahary A. Transforming growth factor-β mRNA and protein in hypertrophic scar tissues and fibroblasts: antagonism by IFN-α and IFN-γ in vitro and in vivo. J Interferon Cytokine Res. 2000;20:143. doi: 10.1089/107999000312540. [DOI] [PubMed] [Google Scholar]
- 123.Ulloa L. Doody J. Massague J. Inhibition of transforming growth factor-β/Smad signalling by the interferon-γ/STAT pathway. Nature. 1999;397:710. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 124.Wang J. Jiao H. Stewart TL. Shankowsky HA. Scott PG. Tredget EE. Improvement in postburn hypertrophic scar after treatment with IFN-alpha2b is associated with decreased fibrocytes. J Interferon Cytokine Res. 2007;27:921. doi: 10.1089/jir.2007.0008. [DOI] [PubMed] [Google Scholar]
- 125.Grieb G. Steffens G. Pallua N. Bernhagen J. Bucala R. Circulating fibrocytes: biology and mechanisms in wound healing and scar formation. Int Rev Cell Mol Biol. 2011;291:1. doi: 10.1016/B978-0-12-386035-4.00001-X. [DOI] [PubMed] [Google Scholar]
- 126.Wang JF. Jiao H. Stewart TL. Shankowsky HA. Scott PG. Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15:113. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 127.Sarrazy V. Billet F. Micallef L. Coulomb B. Desmouliere A. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen. 2011;19(Suppl 1):s10. doi: 10.1111/j.1524-475X.2011.00708.x. [DOI] [PubMed] [Google Scholar]
- 128.Dulauroy S. Di Carlo SE. Langa F. Eberl G. Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 129.Rorison P. Thomlinson A. Hassan Z. Roberts SA. Ferguson MW. Shah M. Longitudinal changes in plasma transforming growth factor β-1 and post-burn scarring in children. Burns. 2010;36:89. doi: 10.1016/j.burns.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 130.Lee TY. Chin GS. Kim WJ. Chau D. Gittes GK. Longaker MT. Expression of transforming growth factor β 1, 2, and 3 proteins in keloids. Ann Plast Surg. 1999;43:179. [PubMed] [Google Scholar]
- 131.Xia W. Phan TT. Lim IJ. Longaker MT. Yang GP. Complex epithelial-mesenchymal interactions modulate transforming growth factor-β expression in keloid-derived cells. Wound Repair Regen. 2004;12:546. doi: 10.1111/j.1067-1927.2004.012507.x. [DOI] [PubMed] [Google Scholar]
- 132.Chin GS. Liu W. Peled Z. Lee TY. Steinbrech DS. Hsu M. Longaker MT. Differential expression of transforming growth factor-β receptors I and II and activation of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 2001;108:423. doi: 10.1097/00006534-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 133.Phan TT. Lim IJ. Aalami O. Lorget F. Khoo A. Tan EK. Mukhopadhyay A. Longaker MT. Smad3 signalling plays an important role in keloid pathogenesis via epithelial-mesenchymal interactions. J Pathol. 2005;207:232. doi: 10.1002/path.1826. [DOI] [PubMed] [Google Scholar]
- 134.Bayat A. Bock O. Mrowietz U. Ollier WE. Ferguson MW. Genetic susceptibility to keloid disease and transforming growth factor β2 polymorphisms. Br J Plast Surg. 2002;55:283. doi: 10.1054/bjps.2002.3853. [DOI] [PubMed] [Google Scholar]
- 135.Bayat A. Bock O. Mrowietz U. Ollier WE. Ferguson MW. Genetic susceptibility to keloid disease and hypertrophic scarring: transforming growth factor β1 common polymorphisms and plasma levels. Plast Reconstr Surg. 2003;111:535. doi: 10.1097/01.PRS.0000041536.02524.A3. discussion 544–546. [DOI] [PubMed] [Google Scholar]
- 136.Bayat A. Walter JM. Bock O. Mrowietz U. Ollier WE. Ferguson MW. Genetic susceptibility to keloid disease: mutation screening of the TGFβ3 gene. Br J Plast Surg. 2005;58:914. doi: 10.1016/j.bjps.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 137.Brown JJ. Ollier W. Arscott G. Ke X. Lamb J. Day P. Bayat A. Genetic susceptibility to keloid scarring: Smad gene SNP frequencies in Afro-Caribbeans. Exp Dermatol. 2008;17:610. doi: 10.1111/j.1600-0625.2007.00654.x. [DOI] [PubMed] [Google Scholar]
- 138.Emami A. Halim AS. Salahshourifar I. Yussof SJ. Khoo TL. Kannan TP. Association of TGF-β1 and Smad4 variants in the etiology of keloid scar in the Malay population. Arch Dermatol Res. 2012;304:541. doi: 10.1007/s00403-012-1262-0. [DOI] [PubMed] [Google Scholar]
- 139.Carrington LM. Albon J. Anderson I. Kamma C. Boulton M. Differential regulation of key stages in early corneal wound healing by TGF-β isoforms and their inhibitors. Invest Ophthalmol Vis Sci. 2006;47:1886. doi: 10.1167/iovs.05-0635. [DOI] [PubMed] [Google Scholar]
- 140.Leask A. Emerging targets for the treatment of scleroderma. Expert Opin Emerg Drugs. 2012;17:173. doi: 10.1517/14728214.2012.678833. [DOI] [PubMed] [Google Scholar]
- 141.Denton CP. Merkel PA. Furst DE. Khanna D. Emery P. Hsu VM. Silliman N. Streisand J. Powell J. Akesson A. Coppock J. Hoogen FV. Herrick A. Mayes MD. Veale D. Haas J. Ledbetter S. Korn JH. Black CM. Seibold JR Cat-192 Study Group. Scleroderma Clinical Trials Consortium: Recombinant human anti-transforming growth factor β1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2007;56:323. doi: 10.1002/art.22289. [DOI] [PubMed] [Google Scholar]
- 142.Russo LM. Brown D. Lin HY. The soluble transforming growth factor-beta receptor: advantages and applications. Int J Biochem Cell Biol. 2009;41:472. doi: 10.1016/j.biocel.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 143.Avraham T. Daluvoy S. Zampell J. Yan A. Haviv YS. Rockson SG. Mehrara BJ. Blockade of transforming growth factor-β1 accelerates lymphatic regeneration during wound repair. Am J Pathol. 2010;77:3202. doi: 10.2353/ajpath.2010.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang Y. McCormick LL. Gilliam AC. Latency-associated peptide prevents skin fibrosis in murine sclerodermatous graft-versus-host disease, a model for human scleroderma. J Invest Dermatol. 2003;121:713. doi: 10.1046/j.1523-1747.2003.12517.x. [DOI] [PubMed] [Google Scholar]
- 145.Hildebrand A. Romarís M. Rasmussen LM. Heinegård D. Twardzik DR. Border WA. Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem J. 1994;302(Pt 2):527. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jarvinen TA. Ruoslahti E. Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice. Proc Natl Acad Sci USA. 2010;107:21671. doi: 10.1073/pnas.1016233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Neill T. Schaefer L. Iozzo RV. Decorin: a guardian from the matrix. Am J Pathol. 2012;181:380. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stoff A. Rivera AA. Mathis JM. Moore ST. Banerjee NS. Everts M. Espinosa-de-los-Monteros A. Novak Z. Vasconez LO. Broker TR. Richter DF. Feldman D. Siegal GP. Stoff-Khalili MA. Curiel DT. Effect of adenoviral mediated overexpression of fibromodulin on human dermal fibroblasts and scar formation in full-thickness incisional wounds. J Mol Med (Berl) 2007;85:481. doi: 10.1007/s00109-006-0148-z. [DOI] [PubMed] [Google Scholar]
- 149.Zheng Z. Nguyen C. Zhang X. Khorasani H. Wang JZ. Zara JN. Chu F. Yin W. Pang S. Le A. Ting K. Soo C. Delayed wound closure in fibromodulin-deficient mice is associated with increased TGF-β3 signaling. J Invest Dermatol. 2010;131:769. doi: 10.1038/jid.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Honardoust D. Varkey M. Hori K. Ding J. Shankowsky HA. Tredget EE. Small leucine-rich proteoglycans, decorin and fibromodulin, are reduced in postburn hypertrophic scar. Wound Repair Regen. 2011;19:368. doi: 10.1111/j.1524-475X.2011.00677.x. [DOI] [PubMed] [Google Scholar]
- 151.Honardoust D. Varkey M. Marcoux Y. Shankowsky HA. Tredget EE. Reduced decorin, fibromodulin, and transforming growth factor-β3 in deep dermis leads to hypertrophic scarring. J Burn Care Res. 2012;33:218. doi: 10.1097/BCR.0b013e3182335980. [DOI] [PubMed] [Google Scholar]
- 152.Ezquerro IJ. Lasarte JJ. Dotor J. Castilla-Cortázar I. Bustos M. Peñuelas I. Blanco G. Rodríguez C. Lechuga Mdel C. Greenwel P. Rojkind M. Prieto J. Borrás-Cuesta F. A synthetic peptide from transforming growth factor-β type III receptor inhibits liver fibrogenesis in rats with carbon tetrachloride liver injury. Cytokine. 2003;22:12. doi: 10.1016/s1043-4666(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 153.Santiago B. Gutierrez-Cañas I. Dotor J. Palao G. Lasarte JJ. Ruiz J. Prieto J. Borrás-Cuesta F. Pablos JL. Topical application of a peptide inhibitor of transforming growth factor-β1 ameliorates bleomycin-induced skin fibrosis. J Invest Dermatol. 2005;125:450. doi: 10.1111/j.0022-202X.2005.23859.x. [DOI] [PubMed] [Google Scholar]
- 154.Matucci M. Krieg T. Müller-Ladner U. Czirják L. Denton C. Pablos JL. Phase II, multicenter, randomized, double-blind, intraindividually placebo controlled clinical trial, to evaluate efficacy, safety of P144 topical administration for skin fibrosis in patients with systemic sclerosis. ClinicalTrials.gov. 2013. Feb 8, http://clinicaltrials.gov/ct2/show/NCT00574613. [May 7;2013 ]. ClinicalTrials.govhttp://clinicaltrials.gov/ct2/show/NCT00574613 [Identifier: NCT00574613]
- 155.Matucci M. Krieg T. Müller-Ladner U. Czirják L. Denton C. Pablos JL. Open label extension (OLE) for the patients treated in the ISD002-P144-07 study with P144 topical adminsitration for skin fibrosis in patients with systemic sclerosis. ClinicalTrials.gov. 2013. Feb 8, http://clinicaltrials.gov/show/NCT00781053. [May 7;2013 ]. ClinicalTrials.govhttp://clinicaltrials.gov/show/NCT00781053 [Identifier: NCT00781053]
- 156.Inman GJ. Nicolás FJ. Callahan JF. Harling JD. Gaster LM. Reith AD. Laping NJ. Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 157.Xiao YQ. Liu K. Shen JF. Xu GT. Ye W. SB-431542 inhibition of scar formation after filtration surgery and its potential mechanism. Invest Ophthalmol Vis Sci. 2009;50:1698. doi: 10.1167/iovs.08-1675. [DOI] [PubMed] [Google Scholar]
- 158.Sugiyama K. Ishii G. Ochiai A. Esumi H. Improvement of the breaking strength of wound by combined treatment with recombinant human G-CSF, recombinant human M-CSF, and a TGF-β1 receptor kinase inhibitor in rat skin. Cancer Sci. 2008;99:1021. doi: 10.1111/j.1349-7006.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bian F. Render J. Ren XD. Chio C. Chan K. Boys M. Lala DS. Pocalyko D. An activin receptor-like kinase 5 inhibitor reduces collagen deposition in a rat dermal incision wound healing model. Plast Reconstr Surg. 2011;128:451e. doi: 10.1097/PRS.0b013e31822b65c7. [DOI] [PubMed] [Google Scholar]
- 160.Boys ML. Bian F. Kramer JB. Chio CL. Ren XD. Chen H. Barrett SD. Sexton KE. Iula DM. Filzen GF. Nguyen MN. Angell P. Downs VL. Wang Z. Raheja N. Ellsworth EL. Fakhoury S. Bratton LD. Keller PR. Gowan R. Drummond EM. Maiti SN. Hena MA. Lu L. McConnell P. Knafels JD. Thanabal V. Sun F. Alessi D. McCarthy A. Zhang E. Finzel BC. Patel S. Ciotti SM. Eisma R. Payne NA. Gilbertsen RB. Kostlan CR. Pocalyko DJ. Lala DS. Discovery of a series of 2-(1H-pyrazol-1-yl)pyridines as ALK5 inhibitors with potential utility in the prevention of dermal scarring. Bioorg Med Chem Lett. 2012;22:3392. doi: 10.1016/j.bmcl.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 161.Durani P. Occleston N. O'Kane S. Ferguson MW. Avotermin: a novel antiscarring agent. Int J Low Extrem Wounds. 2008;7:160. doi: 10.1177/1534734608322983. [DOI] [PubMed] [Google Scholar]
- 162.So K. McGrouther DA. Bush JA. Durani P. Taylor L. Skotny G. Mason T. Metcalfe A. O'Kane S. Ferguson MW. Avotermin for scar improvement following scar revision surgery: a randomized, double-blind, within-patient, placebo-controlled, phase II clinical trial. Plast Reconstr Surg. 2011;128:163. doi: 10.1097/PRS.0b013e318217429b. [DOI] [PubMed] [Google Scholar]
- 163.McCollum PT. Bush JA. James G. Mason T. O'Kane S. McCollum C. Krievins D. Shiralkar S. Ferguson MW. Randomized phase II clinical trial of avotermin versus placebo for scar improvement. Br J Surg. 2011;98:925. doi: 10.1002/bjs.7438. [DOI] [PubMed] [Google Scholar]
- 164.Bush J. Duncan JA. Bond JS. Durani P. So K. Mason T. O'Kane S. Ferguson MW. Scar-improving efficacy of avotermin administered into the wound margins of skin incisions as evaluated by a randomized, double-blind, placebo-controlled, phase II clinical trial. Plast Reconstr Surg. 2010;126:1604. doi: 10.1097/PRS.0b013e3181ef8e66. [DOI] [PubMed] [Google Scholar]
- 165.Ferguson MW. Duncan J. Bond J. Bush J. Durani P. So K. Taylor L. Chantrey J. Mason T. James G. Laverty H. Occleston NL. Sattar A. Ludlow A. O'Kane S. Prophylactic administration of avotermin for improvement of skin scarring: three double-blind, placebo-controlled, phase I/II studies. Lancet. 2009;373:1264. doi: 10.1016/S0140-6736(09)60322-6. [DOI] [PubMed] [Google Scholar]
- 166.Little JA. Murdy R. Cossar N. Getliffe KM. Hanak J. Ferguson MW. TGF β3 immunoassay standardization: comparison of NIBSC reference preparation code 98/608 with avotermin lot 205-0505-005. J Immunoassay Immunochem. 2012;33:66. doi: 10.1080/15321819.2011.600402. [DOI] [PubMed] [Google Scholar]
- 167.Deleavey GF. Damha MJ. Designing chemically modified oligonucleotides for targeted gene silencing. Chem Biol. 2012;19:937. doi: 10.1016/j.chembiol.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 168.Choi BM. Kwak HJ. Jun CD. Park SD. Kim KY. Kim HR. Chung HT. Control of scarring in adult wounds using antisense transforming growth factor-β1 oligodeoxynucleotides. Immunol Cell Biol. 1996;74:144. doi: 10.1038/icb.1996.19. [DOI] [PubMed] [Google Scholar]
- 169.Cordeiro MF. Mead A. Ali RR. Alexander RA. Murray S. Chen C. York-Defalco C. Dean NM. Schultz GS. Khaw PT. Novel antisense oligonucleotides targeting TGF-β inhibit in vivo scarring and improve surgical outcome. Gene Ther. 2003;10:59. doi: 10.1038/sj.gt.3301865. [DOI] [PubMed] [Google Scholar]
- 170.Hong HJ. Jin SE. Park JS. Ahn WS. Kim CK. Accelerated wound healing by Smad3 antisense oligonucleotides-impregnated chitosan/alginate polyelectrolyte complex. Biomaterials. 2008;29:4831. doi: 10.1016/j.biomaterials.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 171.Lee JW. Tutela JP. Zoumalan RA. Thanik VD. Nguyen PD. Varjabedian L. Warren SM. Saadeh PB. Inhibition of Smad3 expression in radiation-induced fibrosis using a novel method for topical transcutaneous gene therapy. Arch Otolaryngol Head Neck Surg. 2010;136:714. doi: 10.1001/archoto.2010.107. [DOI] [PubMed] [Google Scholar]
- 172.Momtazi M. Kwan P. Ding J. Anderson CC. Honardoust D. Goekjian S. Tredget EE. A nude mouse model of hypertrophic scar shows morphologic and histologic characteristics of human hypertrophic scar. Wound Repair Regen. 2013;21:77. doi: 10.1111/j.1524-475X.2012.00856.x. [DOI] [PubMed] [Google Scholar]
- 173.Wang J. Ding J. Jiao H. Honardoust D. Momtazi M. Shankowsky HA. Tredget EE. Human hypertrophic scar-like nude mouse model: characterization of the molecular and cellular biology of the scar process. Wound Repair Regen. 2011;19:274. doi: 10.1111/j.1524-475X.2011.00672.x. [DOI] [PubMed] [Google Scholar]
- 174.Batteux F. Kavian N. Servettaz A. New insights on chemically induced animal models of systemic sclerosis. Curr Opin Rheumatol. 2011;23:511. doi: 10.1097/BOR.0b013e32834b1606. [DOI] [PubMed] [Google Scholar]
- 175.Wang J. Dodd C. Shankowsky HA. Scott PG. Tredget EE. Deep dermal fibroblasts contribute to hypertrophic scarring. Lab Invest. 2008;88:1278. doi: 10.1038/labinvest.2008.101. [DOI] [PubMed] [Google Scholar]