Abstract
Significance
Abnormal wound repair results from disorders in granulation tissue remodeling, and can lead to hypertrophic scarring and fibrosis. Excessive scarring can compromise tissue function and decrease tissue resistance to additional injuries. The development of potential therapies to minimize scarring is, thus, necessary to address an important clinical problem.
Recent Advances
It has been clearly established that multiple cytokines and growth factors participate in the regulation of cutaneous wound healing. More recently, it has become apparent that these factors do not necessarily activate isolated signaling pathways. Rather, in some cases, there is cross-modulation of several cellular pathways involved in this process. Two of the key pathways that modulate each other during wound healing are activated by transforming growth factor-β and by extracellular matrix proteins acting through integrins.
Critical Issues
The pathogenesis of excessive scarring upon wound healing is not fully understood, as a result of the complexity of this process. However, the fact that many pathways combine to produce fibrosis provides multiple potential therapeutic targets. Some of them have been identified, such as focal adhesion kinase and integrin-linked kinase. Currently, a major challenge is to develop pharmacological inhibitors of these proteins with therapeutic value to promote efficient wound repair.
Future Directions
The ability to better understand how different pathways crosstalk during wound repair and to identify and pharmacologically modulate key factors that contribute to the regulation of multiple wound-healing pathways could potentially provide effective therapeutic targets to decrease or prevent excessive scar formation and/or development of fibrosis.

Lina Dagnino, PhD
Scope and Significance
During cutaneous wound healing, extensive interactions between cells of the epidermis, the dermis, and the bone marrow take place. These interactions are important to appropriately coordinate the different phases of wound healing, starting with inflammation and continuing with deposition of new connective tissue, keratinocyte migration over the granulation tissue, fibroblast activation, and scar formation (reviewed by Martin).1 Although skin wound healing occurs efficiently in most individuals, the regenerated tissue is generally not completely recapitulated because scarring takes place. In some individuals, excessive scarring can compromise tissue function, whereas in others, defects in repair result in the development of chronic wounds.2 At the center of these processes are the various types of cells that constitute the dermis. In particular, dermal fibroblasts are key contributors to scarring and may also be affected during impaired wound healing. Targeting responses to wounding in these cells shows promise in the prevention and/or treatment of abnormal skin repair.
Translational Relevance
The development of fibroblast cultures from genetically altered mice provides an excellent system to elucidate at the cellular and molecular level the mechanisms involved in excessive extracellular matrix (ECM) formation, as well as in the generation and survival of myofibroblasts and the activation of fibrotic responses. The in vivo significance of these models using cultured cells can be appropriately assessed through the analysis of responses to wounding in genetically altered mice. Although wound healing in mice and humans does not follow identical mechanisms, model organisms are valuable in the investigation of pathways that can be targeted for their potential therapeutic value in a clinical setting.
Clinical Relevance
Wound healing results in the formation of scars composed of excess ECM. The consequences of scarring include undesirable appearance of the regenerated tissue, lack of hair follicles, sebaceous glands, and sensory nerves, and greater susceptibility to reinjury of the skin surrounding the scar due to reduced tensile strength.1,3 Scarring can be aggravated by genetic predisposition, wound depth, location, and infection.2 Current treatments to promote efficient wound repair are limited to the standard principles of wound management. Modulation of the responses of dermal fibroblasts during skin regeneration and scar formation provides a therapeutic avenue with significant potential.
Relevant Basic Science Context
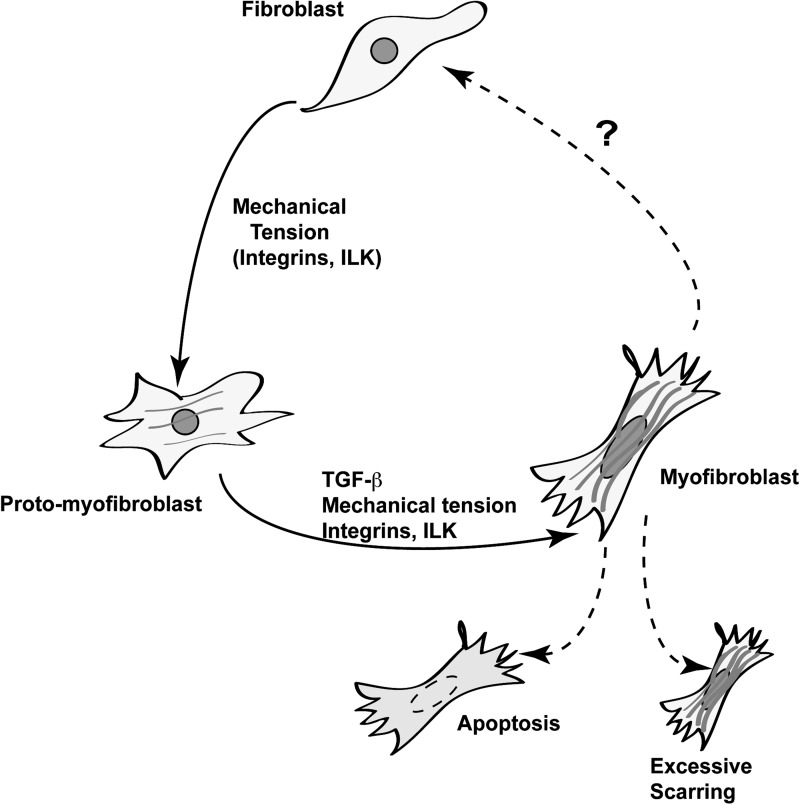
A few days after cutaneous injury, and after the initial inflammatory responses, new stroma composed of macrophages, fibroblasts, and blood vessels, termed granulation tissue, forms.3 Within the granulation tissue, a large number of growth factors and cytokines are produced which, collectively, promote regeneration of the injured skin. Two major pathways activated by proteins produced at the wound result from the activation of transforming growth factor-β (TGF-β) receptors and of integrins. Within the granulation tissue, fibroblasts become activated in response to various factors present in the wound. For example, TGF-β1 induces fibroblast proliferation and migration into the site of injury.4 This cytokine also triggers the production of a collagen-rich matrix, which contributes to induce differentiation of fibroblasts into myofibroblasts. Myofibroblasts are characterized by expression of alpha–smooth muscle actin and acquisition of contractile capacity, which promotes wound closure (Fig. 1).4,5
Figure 1.
Model of myofibroblast differentiation. Stromal and granulation tissue in vivo contains several cell types, including fibroblasts, characterized by the presence of cortical, but not stress actin, fibers. Upon mechanical tension, transduced by integrins and their associated proteins, such as integrin-linked kinase (ILK), fibroblasts change into protomyofibroblasts, which exhibit actin-containing stress fibers. The joint presence of mechanical stress and transforming growth factor beta (TGF-β) induces transition of protomyofibroblasts into differentiated myofibroblasts. The latter possess contractile properties due, in part, to the formation of robust stress fibers containing α-smooth muscle actin. “?” indicates poorly understood molecular pathways.
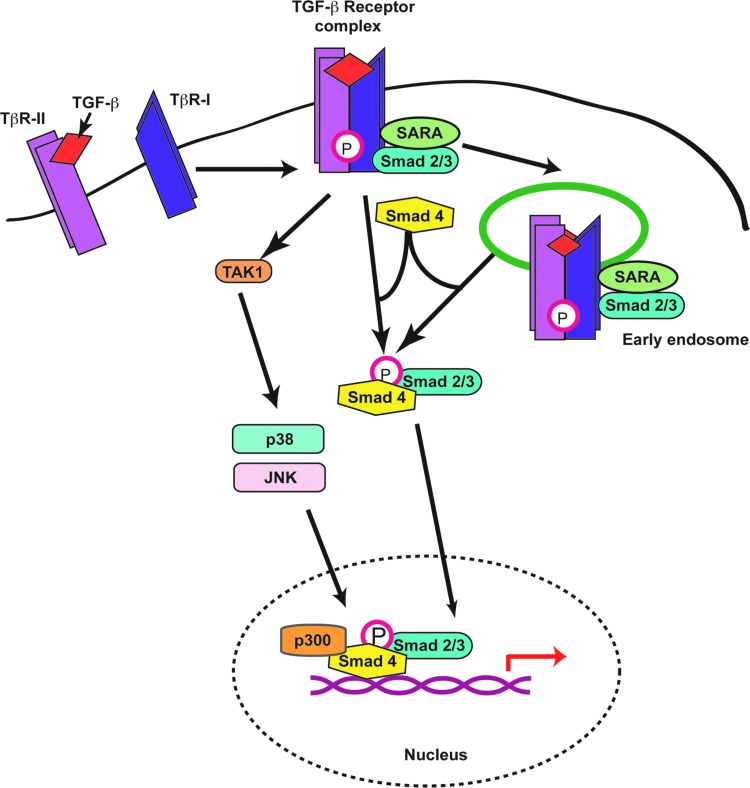
TGF-β1 signals through specific receptor serine/threonine kinases and activates several pathways (Fig. 2). The canonical pathway begins with TGF-β binding to the type-II TGF-β receptor (TβR-II), which leads to its association with TβR-I. This activated heteromeric complex phosphorylates Smad2 and/or Smad3 proteins in the cytosol, allowing them to recruit Smad4. The Smad2/4 or Smad3/4 species then translocate to the nucleus, where they activate transcription of a variety of genes involved in matrix remodeling and cytoskeletal organization.4 TGF-β1 also induces signaling through noncanonical pathways. For example, TGF-β treatment leads to activation of p38 and ERK1/2 mitogen-activated protein kinase, which promote, respectively, the production of type I collagen and fibroblast proliferation.6 TGF-β–activated kinase 1 (TAK1) is another major kinase involved in expression of collagen and fibronectin in response to TGF-β1 stimulation, acting through activation of p38 and JNK. TAK1 is also an important mediator of the profibrotic effects of TGF-β1.7 TGF-β is one of the main cytokines that mediates fibrosis in the skin and other organs. These effects are due to its ability to promote fibroblast proliferation, survival, and ECM synthesis, and to downregulate matrix metalloproteinases that degrade the ECM components.8
Figure 2.
TGF-β-signaling pathways. Binding of TGF-β to TβR-II induces association with and phosphorylation of TβR-I. The kinase properties of the latter are thus activated, which results in phosphorylation of Smad2 and/or Smad3 and their association with Smad4. Although this step occurs at the plasma membrane, active, ligand-bound TGF-β receptor complexes can also be endocytosed, thus triggering Smad2/3 phosphorylation from endosomal vesicles. The active phospho-Smad complexes then shuttle from the cytoplasm to the nucleus, where they associate with transcriptional cofactors, such as p300, to modulate transcription of target genes. TGF-β receptor complexes also initiate other noncanonical signaling pathways, including activation of the TAK1 kinase, which in turn induces phosphorylation of mitogen-activated kinases, such as p38 and JNK. Ultimately, the activation of these kinases also contributes to the transcriptional regulation of target genes. TAK1, TGF-β–activated kinase 1; TβR, TGF-β receptor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
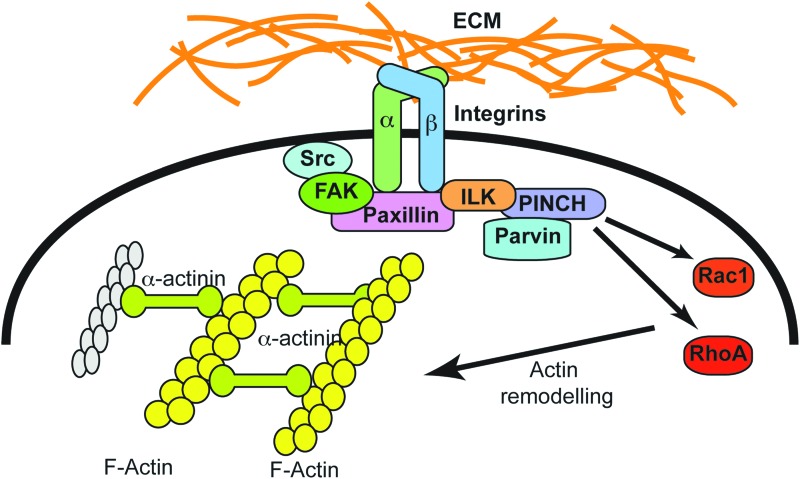
The ECM proteins that participate in defining the microenvironment surrounding the cells in granulation tissues play important roles in regulating the behavior of these cells. A major group of ubiquitous transmembrane proteins that mediate cell adhesion to and transduce stimuli from the ECM are the integrins (Fig. 3).9 Integrins are assembled as heterodimers that contain one alpha- and one beta-subunit, and play important roles in re-epithelialization, regeneration of the basement membrane and dermal ECM, cell migration, and intercellular communication at the wound site. Integrins also link the ECM with the actin cytoskeleton, mediating transduction of mechanical stimuli key for wound contraction and closure.10
Figure 3.
Regulation of integrin-mediated responses by ECM substrates. Binding of ECM proteins to the alpha-subunit of integrins triggers a variety of processes that involve formation of multiprotein complexes. Integrins that are activated by the ECM substrates bind to a variety of cytosolic scaffold and signaling proteins, including ILK, FAK, and vinculin. ILK can bind to paxillin, to PINCH and/or parvins, which then modulate F-actin polymerization and remodeling, regulating cell adhesion to the ECM and/or migration. ECM, extracellular matrix; FAK, focal adhesion kinase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
In dermal fibroblasts, integrins α1β1 and α2β1 are the main collagen receptors. The former mediate collagen synthesis and cell proliferation, whereas the latter are important for differentiation into myofibroblasts and acquisition of contractile properties.11 Integrins lack intrinsic enzymatic activity and transduce ECM signals through their interactions with multiple scaffold and signaling proteins.12 Upon binding to ECM substrates, integrins cluster and associate with their cytosolic partners, as well as generate crosstalk signals with growth factor receptors, including TβRs.13 Thus, integrins and TGF-β signaling, which constitute two pathways that are key for cutaneous regeneration after injury, not only activate individual signaling cascades, but also cross-modulate each other during this process.
Experimental Models
Embryonic wound healing occurs without scarring and is associated with differences in the characteristics of embryonic dermal fibroblasts, relative to the adult counterparts.14 Specifically, embryonic skin regeneration occurs with little inflammation in an environment in which TGF-β3 is the most abundant isoform of this growth factor. In contrast, adult wounds are characterized by pronounced inflammation and the predominance of TGF-β1 at the wound site. The key role of TGF-β signaling in adult scarring has been demonstrated in mice with inducible, conditional inactivation of the Tgfbr2 gene in dermal fibroblasts.15 In these animals, the loss of TβRII expression has profound effects in full-thickness excisional wound repair. Specifically, the loss of dermal TGF-β signaling accelerated re-epithelialization and wound closure. In addition, the regenerated dermis in these animals showed significantly reduced collagen deposition and remodeling, as well as decreased wound contraction, but without any detectable alterations in the abundance of myofibroblasts. Notably, the loss of TGF-β signaling in fibroblasts was accompanied by decreased expression of α1, α2, and β1 integrins and reduced contractile properties in myofibroblasts.
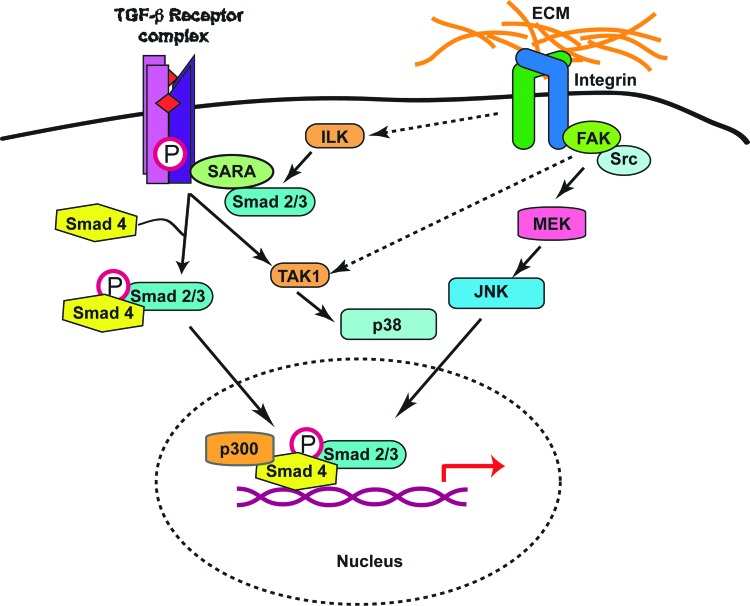
Embryonic fibroblasts isolated from mice with targeted Tak1 inactivation showed impaired responses to TGF-β1 treatment.16 These cells were not capable of inducing collagen matrix contraction and did not exhibit upregulation of fibrotic genes such as alpha–smooth muscle actin after stimulation by TGF-β1. Significantly, the ability of this cytokine to induce activation of TAK1 in normal fibroblasts was also abolished in the presence of focal adhesion kinase (FAK) inhibitors, indicating an additional level of crosstalk between the TGF-β pathways and integrin-associated proteins (Fig. 4).
Figure 4.
Model for the modulation of TGF-β signaling pathways by integrin-associated proteins. ECM substrate binding to integrins results in activation of FAK, which in turn can modulate the activity of TAK1 jointly with TβR complexes. In addition, the presence of ILK in dermal fibroblasts is necessary for normal phosphorylation, and thus activation of Smad proteins in response to stimulation by TGF-β. Several pools of ILK appear to exist in cells. Whether the ILK pool that modulates Smad phosphorylation is also associated with integrins still remains to be determined. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Stimulation of dermal fibroblasts and other mesenchymal cells by TGF-β1 also induces expression of connective tissue growth factor (CCN)2, which in turn is required for normal stimulation of collagen expression.17 Depletion of endogenous CCN2 in dermal fibroblasts abrogated the activation of Smad1 and ERK1/2 in response to TGF-β1. In addition to CCN2, a complete response to TGF-β1 required involvement of the αvβ3 integrin and Src, indicating that a full fibrotic response to this growth factor is mediated by the coordinated action of CCN2, αvβ3 integrin, Src, and Smad1.18
Given the crosstalk between the TGF-β and integrin pathways, the effects of alterations in integrin-binding proteins have been studied and shown to modulate fibrotic responses. In particular, the analysis of FAK-deficient mouse embryonic fibroblasts showed an absence of actin stress fiber formation, collagen matrix contraction, and expression of profibrotic genes when these cells were cultured in the presence of TGF-β1.19
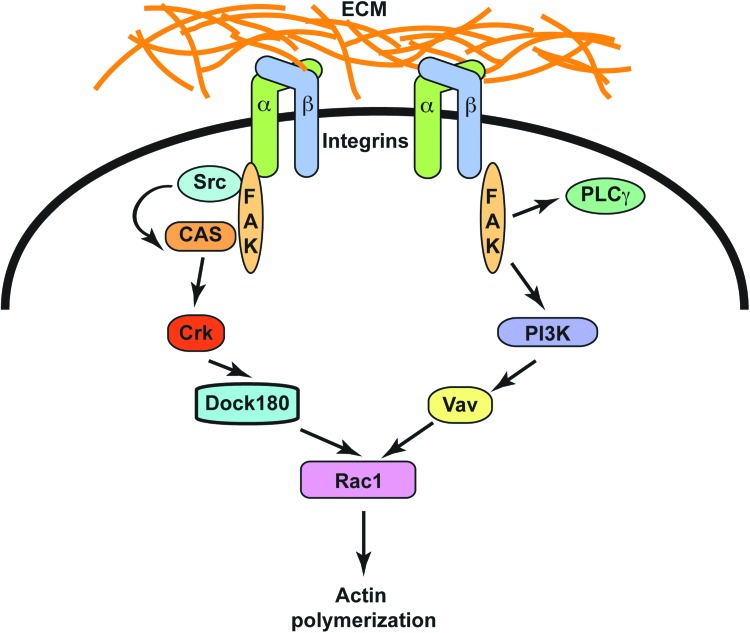
Integrins not only participate in the responses to TGF-β1 during wound healing, they also transduce mechanical signals that are central for wound closure. The role of FAK in these processes has been difficult to evaluate in vivo, as inactivation of the gene that encodes this protein (Ptk2) results in embryonic lethality. Recently, in a mouse model with dermal fibroblast-restricted inactivation of the Ptk2 gene was generated, and the responses of these animals to wounding were evaluated. These mice exhibit substantial decreases in inflammation and fibrosis under conditions that promote hypertrophic scar formation.20 These studies also showed that FAK-dependent activation of ERK1/2 caused secretion of monocyte chemoattractant protein-1, a cytokine linked to human fibrotic disorders. Significantly, pharmacological inhibition of FAK also blocked secretion of inflammatory cytokines and reduced scar formation in vivo by attenuating inflammatory cell recruitment to the wound site (Fig. 5).
Figure 5.
Stimulation of FAK signaling pathways by integrins. Binding of ECM substrates to integrins triggers phosphorylation and activation of FAK. The active kinase subsequently associates with and activates Src, another tyrosine kinase, thus initiating a signaling cascade that involves further stimulation of the Crk and Dock 180. The latter stimulates activation of the small GTPase Rac1, which regulates the actin cytoskeleton and promotes cell migration, a key step in wound regeneration. Active FAK also induces stimulation of PI3K, which promotes activation of Vav, another protein that induces Rac 1 activation and cell migration. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
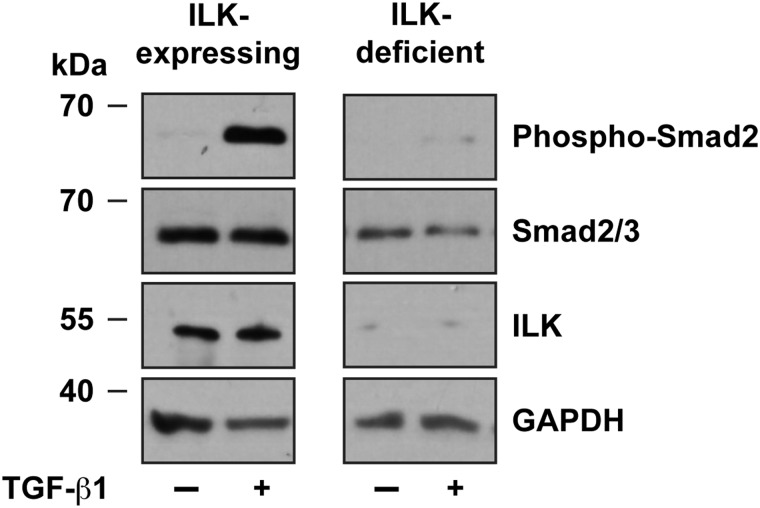
Integrin-linked kinase is a scaffold protein that associates with β1 and β3 integrins and mediates a variety of responses to stimulation by ECM substrates.21 Inactivation of the Ilk gene in the epidermal stem cells of the hair follicle bulge delays wound healing in full-thickness excision wounds.22 This effect likely involves impairment of keratinocyte motility during re-epithelialization.22,23 Cultured integrin-linked kinase-deficient dermal fibroblasts isolated from newborn mice fail to express alpha–smooth muscle actin and to show Smad2 and Smad3 phosphorylation upon stimulation by exogenous TGF-β1 (Fig. 6). These cells also exhibit impaired cell migration and differentiation of fibroblasts into myofibroblasts.24 In these cells, collagen matrix remodeling and contraction are also severely affected. In contrast, using a different model consisting of cultured dermal fibroblasts isolated from older animals, reduced baseline TGF-β1 responses were associated with decreased production of, rather than response to, this cytokine. Normal fibroblast responses were restored upon addition of exogenous TGF-β1.25 In vivo, adult (3-week old) mice with fibroblast-restricted inactivation of the Ilk gene displayed severely impaired wound repair due to a pronounced reduction in the abundance of myofibroblasts within the granulation tissue, without detectable effects on inflammatory cells or vascularization.25
Figure 6.
Abnormal stimulation of TGF-β signaling pathways in ILK-deficient dermal fibroblasts. Primary dermal fibroblasts isolated from Ilkf/f mice24 were infected with adenovirus encoding β-galactosidase (ILK-expressing) or Cre recombinase (ILK-deficient), and, 72 h later, were treated with TGF-β1 (10 ng/mL). Cell lysates were prepared after 1 h, resolved by denaturing polyacrylamide gel electrophoresis, and analyzed by immunoblot with the indicated antibodies. The levels of GAPDH were used to normalize for loading. GAPDH, glyceraldehyde 3-phosphodehydrogenase.
Discussion of Findings and Relevant Literature
Integrin-linked kinase is an important scaffold protein that binds to β1 and β3 integrins, mediating several responses to ECM stimulation.21 Integrin-linked kinase is ubiquitously expressed and can associate with multiple proteins in addition to integrins, forming complexes important for a wide variety of processes relevant to wound healing. These processes include cell adhesion to the ECM, migration in response to chemoattractants, fibroblast proliferation, and phagocytosis.23,24,26,27
One of the first proteins that was identified downstream of integrin stimulation is FAK. This is a ubiquitous, nonreceptor tyrosine kinase that localizes to the sites of integrin-mediated adhesion to the ECM.28 FAK is maintained in an inactive state, until integrin binding to the ECM causes its autophosphorylation and consequent activation. This leads to binding and activation of the Src tyrosine kinase. The FAK/Src complex subsequently stimulates the phosphorylation and activation of multiple downstream substrates. Activation of FAK results in increased cell motility and survival and other cell responses involved in wound healing and the development of fibrosis.20
The crosstalk between integrins and TGF-β occurs at various levels in fibroblasts and other cell types. Latent TGF-β is an important ECM constituent that can bind and stimulate integrins. In a reciprocal manner, αvβ5 integrin can activate latent TGF-β, allowing it to then activate TβR-II, promoting expression of various integrins and ECM proteins.13 In fibroblasts found in fibrotic tissues, αvβ5 integrin is upregulated, which further increases TGF-β activation and reinforces autocrine TGF-β signaling, promoting myofibroblast formation.29 The development of models in which important integrin downstream mediators, such as integrin-linked kinase and FAK, are manipulated has indicated that individual or combined components of the pathways activated by wounding may be useful targets for cutaneous healing therapies. Given the crosstalk between integrins and the TGF-β signaling pathways, the modulation of proteins that transduce signaling from the ECM or regulate TGF-β bioavailability offers a tremendous potential value in the treatment or prevention of excessive scarring. In particular, different drugs have been or are being developed that target various components of the TGF-β or integrin pathways, such as FAK inhibitors.
Innovation
The consequences of abnormal wound healing have been recognized for years. Efforts to either improve wound healing through the administration of growth factors or to reduce scarring by limiting the availability of certain growth factors have met limited success. This is likely due to the complexity of the regeneration process itself, and the myriad components that modulate cell behavior at wound sites. As an alternative approach, we propose that targeting key factors, such as TAK1, FAK, or integrin-linked kinase, which function just downstream from growth factor receptor or integrin activation during wound healing, may provide an effective means to reduce abnormal healing conducive to excessive scarring.
Caution, Critical Remarks, and Recommendations
The identification of drugs that modulate intracellular factors involved in wound-healing responses is at various stages of development. Recently, intense efforts to identify FAK inhibitors have yielded several drugs with promising therapeutic effects in early-phase clinical trials.30 Although these drugs have been primarily developed to target carcinomas, their applicability to other disorders, such as abnormal wound healing and fibrosis, is potentially important. A key limiting factor to use these drugs is their potential interference with normal biological processes, yielding unacceptable undesired effects. However, the possibility of using topical delivery of these drugs, thus maximizing their effects at target sites without substantial systemic exposure, warrants considering and examining their usefulness in the prevention of excessive scarring.
Small-molecule inhibitors of integrin-linked kinase have been reported, although their specificity is unclear, and hence, they should be tested with caution. Further efforts to develop this class of drugs are needed, as they could provide synergistic therapeutic effects in combination with other pharmacological modulators.
Take-Home Messages.
Basic science advances
Activation of TGF-β receptors and integrins is key for cutaneous wound healing. In addition, these two pathways modulate each other during this process.
Two important cellular proteins activated by integrin stimulation and involved in survival and generation of dermal fibroblasts and myofibroblasts are integrin-linked kinase and FAK.
These two proteins jointly modulate several pathways that are activated during fibrotic wound healing, including integrins and the TGF-β pathway.
Clinical science advance
Integrin-linked kinase and FAK are necessary for the generation of myofibroblasts and excessive ECM deposition.
Relevance to clinical care
Pharmacological inhibition of integrin-linked kinase or FAK could serve as a target to alleviate fibrosis and excessive cutaneous wound scarring. This strategy, if successful, opens potential avenues for the treatment of fibrotic disorders in other organs, in addition to the skin.
Future Development of Interest
Excessive scarring and fibrotic disorders are the consequence of disruption of normal wound-healing processes. Significantly, many processes that lead to fibrosis appear to be shared by multiple organs, including skin, the kidneys, heart, and brain. Therefore, any therapeutic intervention that ameliorates excessive scarring in the skin has the additional potential benefit of addressing excessive scarring in other tissues. Excessive deposition of the ECM and hypercellularity result from the sum of multiple stimuli. Many molecular and cellular factors are involved in fibrosis, complicating our understanding of the pathogenesis of this disorder. However, this same complexity also provides multiple potential targets for therapy, including the fibroblasts and myofibroblasts. The TGF-β and integrin pathways are two major contributors to excessive scarring. Given the extensive crosstalk between these two pathways, it is conceivable that, by targeting a common factor, such as FAK or integrin-linked kinase, multiple pathways involved in excessive scarring may be downregulated.
Abbreviations and Acronyms
- CCN2
connective tissue growth factor
- GAPDH
glyceraldehyde-3-phosphodehydrogenase
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- MAPK
mitogen-activated protein kinase
- TAK1
TGF-β–activated kinase 1
- TGF-β
transforming growth factor beta
- TβR
TGF-β receptor
Acknowledgments and Funding Sources
S.B. is the recipient of an Ontario Graduate Studentship award. L.D. is funded with grants from the Canadian Institutes of Health Research.
Author Disclosure and Ghostwriting
The authors declare no conflicts of interest. Ghostwriters were not used in production of this article.
About the Authors
Stellar Boo is a student in the Graduate Program in Physiology at the University of Western Ontario. Lina Dagnino is a Professor in the Department of Physiology and Pharmacology at the University of Western Ontario. Dr. Dagnino's research program focuses on understanding the mechanisms implicated in epidermal morphogenesis and neoplastic transformation, as well as in understanding the pathways involved in regeneration of the skin.
References
- 1.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 2.Heng MC. Wound healing in adult skin: aiming for perfect regeneration. Int J Dermatol. 2011;50:1058. doi: 10.1111/j.1365-4632.2011.04940.x. [DOI] [PubMed] [Google Scholar]
- 3.Singer AJ. Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 4.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 5.Desmouliere A. Chaponnier C. Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 6.Sato M. Shegogue D. Gore EA. Smith EA. McDermott PJ. Trojanowska M. Role of p38 MAPK in transforming growth factor beta stimulation of collagen production by scleroderma and healthy dermal fibroblasts. J Invest Dermatol. 2002;118:704. doi: 10.1046/j.1523-1747.2002.01719.x. [DOI] [PubMed] [Google Scholar]
- 7.Choi ME. Ding Y. Kim SI. TGF-beta signaling via TAK1 pathway: role in kidney fibrosis. Semin Nephrol. 2012;32:244. doi: 10.1016/j.semnephrol.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarrazy V. Billet F. Micallef L. Coulomb B. Desmouliere A. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen. 2011;19(Suppl 1):s10. doi: 10.1111/j.1524-475X.2011.00708.x. [DOI] [PubMed] [Google Scholar]
- 9.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geiger B. Spatz JP. Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 11.Widgerow AD. Chronic wounds—is cellular ‘reception’ at fault? Examining integrins and intracellular signalling. Int Wound J. 2013;10:185. doi: 10.1111/j.1742-481X.2012.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delon I. Brown NH. Integrins and the actin cytoskeleton. Curr Opin Cell Biol. 2007;19:43. doi: 10.1016/j.ceb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Munger JS. Sheppard D. Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol. 2011;3:a005017. doi: 10.1101/cshperspect.a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullard KM. Longaker MT. Lorenz HP. Fetal wound healing: current biology. World J Surg. 2003;27:54. doi: 10.1007/s00268-002-6737-2. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Ferrer M. Afshar-Sherif AR. Uwamariya C. de Crombrugghe B. Davidson JM. Bhowmick NA. Dermal transforming growth factor-beta responsiveness mediates wound contraction and epithelial closure. Am J Pathol. 2010;176:98. doi: 10.2353/ajpath.2010.090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi-wen X. Parapuram SK. Pala D. Chen Y. Carter DE. Eastwood M. Denton CP. Abraham DJ. Leask A. Requirement of transforming growth factor beta-activated kinase 1 for transforming growth factor beta-induced alpha-smooth muscle actin expression and extracellular matrix contraction in fibroblasts. Arthritis Rheum. 2009;60:234. doi: 10.1002/art.24223. [DOI] [PubMed] [Google Scholar]
- 17.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 18.Nakerakanti SS. Bujor AM. Trojanowska M. CCN2 is required for the TGF-beta induced activation of Smad1-Erk1/2 signaling network. PLoS One. 2011;6:e21911. doi: 10.1371/journal.pone.0021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S. Xu SW. Kennedy L. Pala D. Chen Y. Eastwood M. Carter DE. Black CM. Abraham DJ. Leask A. FAK is required for TGFbeta-induced JNK phosphorylation in fibroblasts: implications for acquisition of a matrix-remodeling phenotype. Mol Biol Cell. 2007;18:2169. doi: 10.1091/mbc.E06-12-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong VW. Rustad KC. Akaishi S. Sorkin M. Glotzbach JP. Januszyk M. Nelson ER. Levi K. Paterno J. Vial IN. Kuang AA. Longaker MT. Gurtner GC. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2012;18:148. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin J. Wu C. ILK: a pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr Opin Cell Biol. 2012;24:607. doi: 10.1016/j.ceb.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakrieko KA. Rudkouskaya A. Irvine TS. D'Souza SJ. Dagnino L. Targeted inactivation of integrin-linked kinase in hair follicle stem cells reveals an important modulatory role in skin repair after injury. Mol Biol Cell. 2011;22:2532. doi: 10.1091/mbc.E11-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakrieko KA. Welch I. Dupuis H. Bryce DM. Pajak A. St-Arnaud RS. Dedhar S. D'Souza SJ. Dagnino L. Impaired hair follicle morphogenesis and polarized keratinocyte movement upon conditional inactivation of integrin-linked kinase in the epidermis. Mol Biol Cell. 2008;19:1462. doi: 10.1091/mbc.E07-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vi L. de Lasa C. DiGuglielmo GM. Dagnino L. Integrin-linked kinase is required for TGF-beta1 induction of dermal myofibroblast differentiation. J Invest Dermatol. 2011;131:586. doi: 10.1038/jid.2010.362. [DOI] [PubMed] [Google Scholar]
- 25.Blumbach K. Zweers MC. Brunner G. Peters AS. Schmitz M. Schulz JN. Schild A. Denton CP. Sakai T. Fassler R. Krieg T. Eckes B. Defective granulation tissue formation in mice with specific ablation of integrin-linked kinase in fibroblasts—role of TGFbeta1 levels and RhoA activity. J Cell Sci. 2010;123:3872. doi: 10.1242/jcs.063024. [DOI] [PubMed] [Google Scholar]
- 26.Ho E. Dagnino L. Epidermal growth factor induction of front-rear polarity and migration in keratinocytes is mediated by integrin-linked kinase and ELMO2. Mol Biol Cell. 2012;23:492. doi: 10.1091/mbc.E11-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sayedyahossein S. Nini L. Irvine TS. Dagnino L. Essential role of integrin-linked kinase in regulation of phagocytosis in keratinocytes. FASEB J. 2012;26:4218. doi: 10.1096/fj.12-207852. [DOI] [PubMed] [Google Scholar]
- 28.Lechertier T. Hodivala-Dilke K. Focal adhesion kinase and tumour angiogenesis. J Pathol. 2012;226:404. doi: 10.1002/path.3018. [DOI] [PubMed] [Google Scholar]
- 29.Asano Y. Ihn H. Yamane K. Jinnin M. Tamaki K. Increased expression of integrin alpha(v)beta(5) induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol. 2006;168:499. doi: 10.2353/ajpath.2006.041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultze A. Fiedler W. Therapeutic potential and limitations of new FAK inhibitors in the treatment of cancer. Expert Opin Investig Drugs. 2010;19:777. doi: 10.1517/13543784.2010.489548. [DOI] [PubMed] [Google Scholar]