Abstract
The aim of this study was to describe the incidence of metabolic syndrome and to identify five components as metabolic syndrome predictors. The final study included 1,095 subjects enrolled in a rural part of Daegu Metropolitan City, Korea for a cohort study in 2003. Of these, 762 (69.6%) subjects had participated in the repeat survey. During the five-year follow-up, incidence density was significantly higher for women than for men (men, 30.0/1,000 person-years; women, 46.4/1,000 person-years). In both men and women, incidence of metabolic syndrome showed a significant increase with increasing number of metabolic syndrome components at baseline. Compared with individuals presenting none of components at baseline, relative risks were increased 1.22 (men; 95% CI, 0.43-3.51), 2.21 (women; 95% CI, 0.98-4.97) times more for individuals with one component of metabolic syndrome and 5.30 (men; 95% CI, 2.31-12.13), 5.53 (women; 95% CI, 2.78-11.01) times more for those who had two components. In multivariate analysis, the most powerful risk factor for metabolic syndrome was abdominal obesity in men and low HDL-cholesterol in women (adjusted relative risk, 3.28, 2.53, respectively). Consequently, finding a high risk group for metabolic syndrome according to gender and prevention of metabolic syndrome through lifestyle modification are essential.
Keywords: Cohort Studies, Incidence, Metabolic Syndrome, Risk Factors
INTRODUCTION
Metabolic syndrome (MetS), clustering of glucose intolerance, obesity, hypertension, and dyslipidemia, is showing a rapid increase worldwide (1). This increase is associated with the global epidemic of obesity and diabetes (2). Each component of MetS is a well-known risk factor for cardiovascular disease (3). These components do not occur independently by chance, but co-occur in an individual. When these components occur simultaneously in a person, they are synergistically associated with increased risk of cardiovascular disease (4, 5).
Several previous cross-sectional studies have suggested an increase in the prevalence of MetS. In the United States, the prevalence of MetS showed a marked increase over the period from 1988 to 2006 (27.9% in the Third National Health and Nutrition Examination Survey [1988-1994 NHANES] and 34.1% in the 1999-2006 NHANES) (6). In Korea, the prevalence of MetS also showed a marked increase over the period from 1998 to 2007 (24.9% in the 1998 Korea National Health and Nutrition Examination Survey [1998 KNHANES] and 31.3% in the 2007 KNHANES) (7). Although several cross-sectional studies in the general population like the one described above have been reported, there were no longitudinal data in general population, especially in Korea.
Therefore, the purposes of the this study were 1) to estimate the incidence of MetS in a rural area of Korea and 2) to evaluate five components as a predictor of MetS.
MATERIALS AND METHODS
Study population
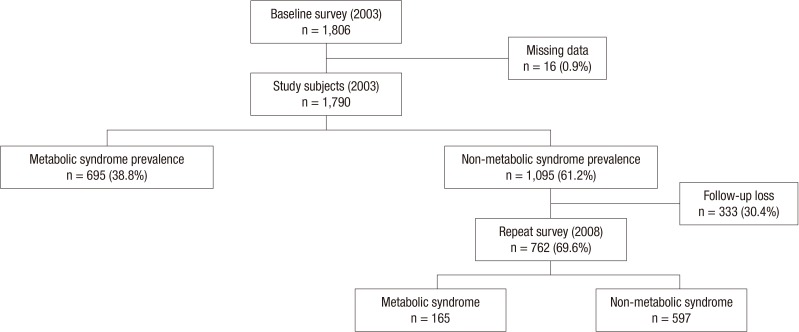
Dalseong-gun is a rural part located near Daegu Metropolitan City in Korea. A baseline survey using a cluster sampling method was conducted in Dalseong-gun in August 2003. We selected nine primary health posts of community (basic healthcare unit in rural area). In each territory of nine posts, two or three villages were randomly selected so that a total of 26 villages was chosen as a survey unit. All voluntary subjects over the age of 20 yr in each sampled village were enrolled. At the time of the baseline survey, a total of 1,806 people was recruited. Of these, 16 (0.9%) subjects were excluded because of missing data. Of the 1,790 remaining subjects, 695 (38.8%) were identified as having MetS at the baseline survey. The MetS-free cohort, comprised of 1,095 (61.2%) subjects, was reexamined in August 2008. Meanwhile, 333 (30.4%) of the 1,095 subjects did not participate in the repeat survey; thus, the follow-up rate was 69.6% (Fig. 1). Subjects who were lost to follow-up were regarded as censored cases when statistical analysis was performed.
Fig. 1.
Frame of the study design and numbers of subjects.
Methods
Medical history taking, physical examination, anthropometric measurement, a questionnaire survey on health-related behavior, and biochemical measurements were included in the health examination. Blood pressure was taken twice in a seating position by trained nurses using a standard mercury sphygmomanometer. The first and fifth Korotkoff sounds were used for estimation of systolic and diastolic blood pressure. Body weight was obtained with the participant dressed in light clothing and barefoot. Waist circumference was measured midway between the lower limit of the rib cage and the iliac crest. The questionnaire asked about alcohol intake (non-, ex-, or current drinker), smoking habits (non-, ex-, or current smoker), and regular physical exercise (no, yes). Blood samples were collected from an antecubital vein after 12 hr of fasting. The levels of fasting blood sugar, total cholesterol, triglyceride, and high-density lipoprotein cholesterol (HDL-cholesterol) were measured using an automatic analyzer (Dimension AR, Dade Behring, Deerfield, IL, USA). The definition of MetS was based on the the Third Report of the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) criteria; however, only the criteria for central obesity were based on the International Diabetes Federation (IDF) definition of South Asian cut-points (8-10). When at least three of the following characteristics were observed, we considered the presence of MetS: waist circumference ≥90 cm in men and ≥80 cm in women; blood pressure ≥130/85 mmHg or antihypertensive drug medication; triglyceride ≥150 mg/dL; HDL-cholesterol <40 mg/dL in men and <50 mg/dL in women; and fasting glucose ≥100 mg/dL or antidiabetic drug medication.
Statistical analysis
Descriptive summary statistics from the study sample were generated using mean and standard deviation for continuous variables and proportions for categorical variables. In this study, age, health-related behavior, and each component of MetS were considered possible risk factors. Analysis of differences in independent variables between subjects who were lost to follow-up and repeated subjects was performed using t-test and chi-square test. As there are differences in diagnostic criteria for MetS and general characteristics between men and women, incidence and risk factors of MetS were analyzed separately by gender. Incidence density and 95% confidence intervals (CI) were calculated by dividing the number of cases by person-years at risk. Time at risk was considered as the number of years between the first and repeat survey in those who did not develop MetS. In individuals who developed MetS or were lost to follow-up, time at risk was estimated as the mid-point of the period between the two surveys (11). Cumulative incidence rate was presented additionally in order to help in better understanding of the incidence of MetS. Cox proportional hazards model was used to assess the risk factors for MetS so that crude and adjusted relative risks were calculated. Available risk factors included age, each component of MetS, alcohol intake, smoking habits, and regular physical activity. The five components of MetS were divided into two categories according to the diagnostic criteria for MetS. Statistical significance was defined as a P value of less than 0.05. Statistical analysis was performed using SAS (9.1 version, Cary, NC, USA).
Ethics statement
The study protocol was approved by the institutional review board of Kyungpook National University Hospital (IRB No. KNUHBIO_08-1004). Written informed consent was obtained from all participants.
RESULTS
Baseline characteristics after stratification according to follow-up status are shown in Table 1. Repeated subjects were significantly older than the subjects who were lost to follow-up. However, no significant differences were observed in blood pressure, fasting glucose, waist circumference, triglyceride, HDL-cholesterol, the proportion of gender, alcohol intake, smoking, and regular physical exercise.
Table 1.
Comparison of baseline characteristics between follow-up loss and repeated subjects
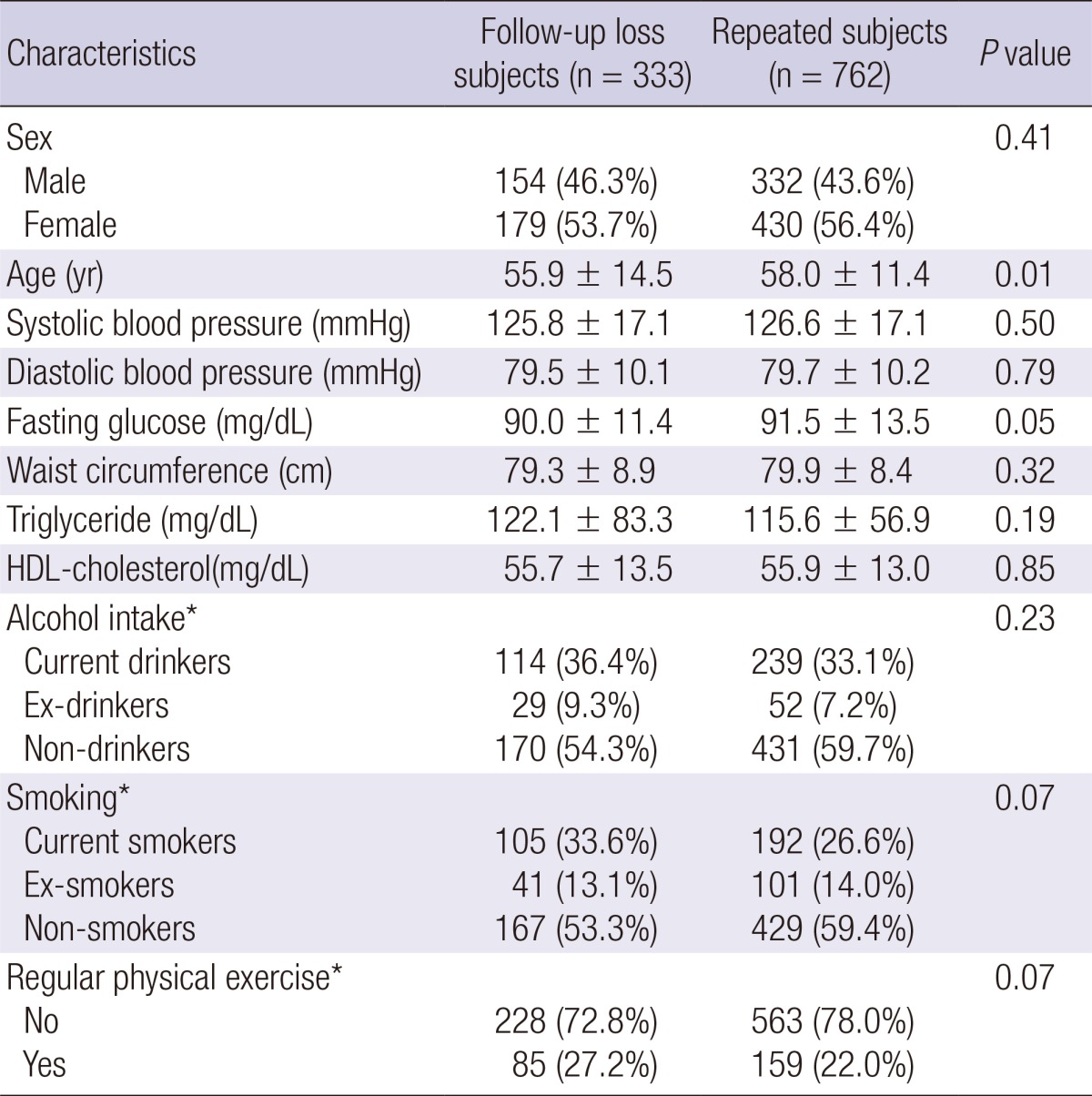
*Only 313 follow-up loss subjects and 722 repeated subjects had information on alcohol intake, smoking, and regular physical exercise, respectively.
During the five-year follow-up period, 13.9% of men and 20.8% of women were newly diagnosed as MetS. When the annual probability of progression to MetS was calculated based on the assumption of the constant risks, the annual incidence rate was 2.8% (men) and 4.2% (women) (Table 2).
Table 2.
Cumulative incidence rate of metabolic syndrome
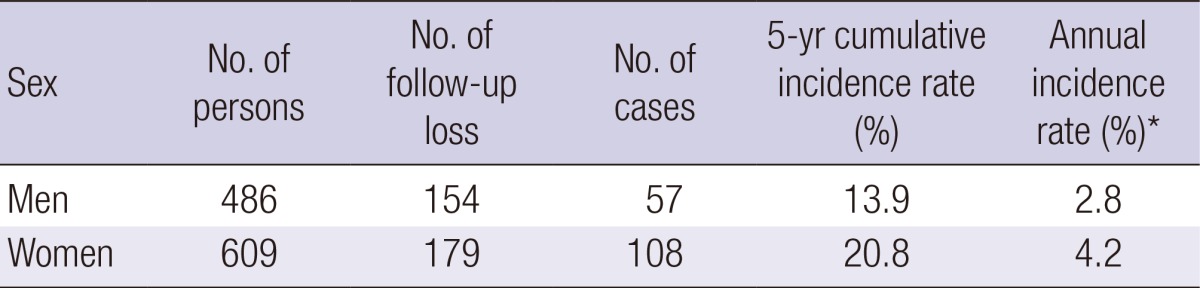
*Annual incidence rate was calculated based on assumption that annual probability of progression to metabolic syndrome had constant risks.
As shown in Table 3, incidence density of MetS in women was significantly higher than in men (men, 30.0/1,000 person-years; women, 46.4/1,000 person-years). In addition, a difference in the incidence density was observed between men and women according to age. Incidence of MetS showed a significant increase with age in women, while it tended to show a non-significant decrease with age in men. In both men and women, incidence of MetS showed a significant increase with increasing number of MetS components at baseline. Compared with individuals presenting none of the components of metabolic syndrome at baseline, relative risks were increased 1.22 (men; 95% CI, 0.43-3.51), 2.21 (women; 95% CI, 0.98-4.97) times more for individuals who had one metabolic component, and 5.30 (men; 95% CI, 2.31-12.13), 5.53 (women; 95% CI, 2.78-11.01) times more for those who had two components.
Table 3.
Incidence density of metabolic syndrome according to age and the number of baseline metabolic syndrome components
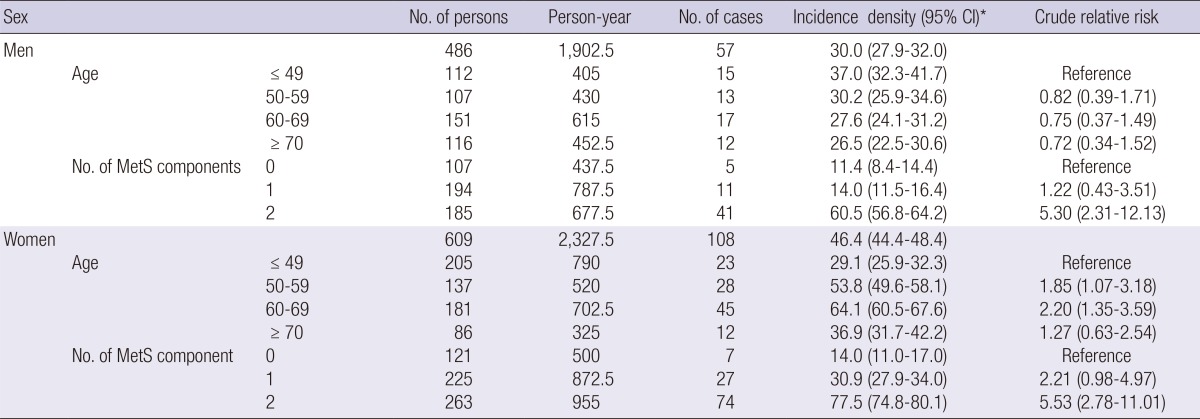
*Per 1,000 person-years.
Table 4 shows relative risks for MetS by each component of MetS. In men, components of abdominal obesity and hypertriglycemia showed a significant association with incidence of MetS. After adjustment for potential risk factors for MetS, components of hypertension and diabetes were added for risk factors, and abdominal obesity was the most powerful risk factor for MetS (adjusted relative risk, 3.28). On the other hand, components of abdominal obesity and low HDL-cholesterol showed a significant association with incidence of MetS in women. However, after adjustment for covariates, incidence of MetS showed a significant association with low HDL-cholesterol, abdominal obesity, diabetes, and hypertension, except hypertriglycemia (adjusted relative risk, 2.53, 2.51, 2.47, and 1.92, respectively).
Table 4.
Relative risks (95% confidence interval) of metabolic syndrome according to baseline components
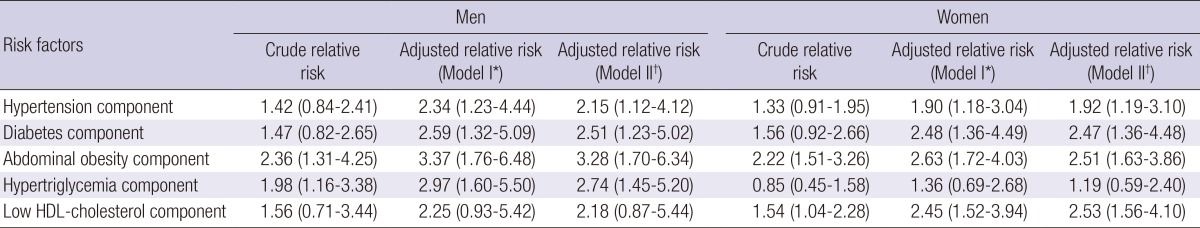
*Adjusted for age and five components of metabolic syndrome; †Only 465 male and 570 female had information on alcohol intake, smoking, and regular physical exercise, respectively. Adjusted for alcohol intake, smoking, and regular physical regular exercise adding to Model I variables.
DISCUSSION
Several previous epidemiological studies have evaluated the incidence rate or incidence density of MetS. In this cohort study, the incidence density of MetS was 30.0/1,000 person-years in men and 46.4/1,000 person-years in women. In a study conducted in Portugal, incidence density of MetS (47.2/1,000 person-year) was similar to that of our study (12). In addition, the annual cumulative incidence rate in the current study was 2.8% in men and 4.2% in women, similar to that of the French study (men, 3.5%; women, 2.7% ) (13). Although our results were higher than that of the Coronary Artery Risk Development in Young Adults (CARDIA) study (10/1,000 person-year), this might be explained by age distribution of the study population (population in the CARDIA study, 18-30 yr) (14). Some differences between these results could be explained by differences of the study population, methodologies, and the definition of MetS among studies. In other words, incidence of MetS in the rural Korean population is similar to that of western countries. This tendency has been explained by increasing prevalence of life-related disease (e.g., hypertension) in developing countries, contrary to stable or decreasing prevalence of those in developed countries (15). In addition, high economic development, westernization in lifestyle and diet, and an increase in the prevalence of obesity may explain a similar trend in incidence of MetS between Korea and western countries.
In this study, incidence of MetS showed an increase with age in women, except for a slight decline in the incidence density of subjects over the age of 70. On the other hand, the group of men under age 50 had the highest incidence density. The incidence of MetS in women was lower than that of men until age 50; however, incidence of MetS in their 50's, period of menopausal transition in women, showed crossover between that of men and women. These associations were also presented in previous cross-sectional studies (16, 17). The causes of the crossover can be explained as follows. First, abdominal obesity plays a major role in development of MetS. The pattern of lipid accumulation differs in men and women. Women mainly develop peripheral adiposity with gluteal fat accumulation, whereas men are more prone to development of central or android obesity. However, concentrations of lipoproteins as well as body fat distribution in women shift to a male pattern after menopause (18, 19). Therefore, men have higher incidence of MetS than women at a younger age, but have lower incidence than women after menopause, like the current study. Second, according to the fourth Korea National Health and Nutrition Examination Survey, there are gender differences in the components of MetS. Components of MetS, such as hypertriglycemia, abdominal obesity, and lower HDL-cholesterol tend to show a sharp increase with age in women. Otherwise, the rate of increase for central obesity and lower HDL-cholesterol with age of men is lower than that of women. In addition, hypertriglycemia decreases with age in men (20). In our study, unlike women, abdominal obesity and hypertriglycemia, the two most powerful risk factors, also showed a decrease with age in men (data not shown). Therefore, it was considered that the incidence of MetS differed between men and women. These differences in incidence between men and women according to age were supported by findings of a previous study. In a study conducted in Taiwan, the mean age of MetS in men (51.3 yr) was earlier than that in women (56.2 yr) by 4.9 yr (21). In addition, the period from the appearance of any isolated component to MetS was 12.8 yr in women and 5.7 yr in men, regardless of which component was presenting as a case of MetS. In other words, MetS occurs earlier and more rapidly in men than in women.
MetS is a heterogenous cluster of five components in men and women, and the exact mechanism of interaction between components is not well-known. Therefore, it is difficult to clarify which component is the most powerful predictor of MetS. In men, results of this study showing that abdominal obesity was the most powerful predictor among five components were consistent with previous findings (12, 22, 23). In the study conducted in Portugal, the strength of association was remarkably similar to that of our study, in the following order: abdominal obesity, hypertriglycemia, diabetes, and hypertension (12). In addition, low HDL-cholesterol component was not statistically significant. In contrast with men, low HDL-cholesterol component was the most powerful predictor in women, although the differences in the strength of association among three components except hypertension and hypertriglycemia were small. In addition, hypertriglycemia, which was the second most important predictor of MetS in men did not show a significant association with incidence of MetS in women. These gender differences of components as predictors of MetS were observed in other studies. In a Hong Kong Chinese study using same criteria for MetS with our study, for example, central obesity was the most powerful predictor in both men and women; other significant components included hypertriglycemia and hyperglycemia in men and low HDL-cholesterol in women (23). A previous study of a non-diabetes population also revealed that abdominal obesity was the best predictor of MetS in both men and women; however, the second predictor of MetS was glucose intolerance and HDL-cholesterol, respectively (22). According to previous studies, obesity and insulin resistance are closely linked, and obesity may precede development of insulin resistance (22, 24, 25). Insulin resistance subsequently leads to elevation in triglyceride, glucose level and blood pressure, and reduction of HDL-cholesterol levels (26, 27). However, temporal relationship of five components were not well-known and there were gender differences in components of MetS (19). These studies supported our results indicating that there might be differences in the mechanism and pathogenesis of five components as a predictor of MetS between men and women.
Due to the small sample size, we did not determine incidence of MetS according to the combination of five components; however, incidence of MetS showed a significant increase with increasing number of MetS components. Regardless of the type of combination of components in previous studies, the more the number of components increased, the greater the increase in incidence of MetS (12, 23, 28). Therefore, those who have two components are in a higher risk group for MetS than those who have none or one component, thus, more intensive lifestyle modification was needed in the higher risk group.
There are several limitations in the current study. First, in this study, loss to follow-up can be a source of bias. Of the participants in the baseline survey, 30.4% were not examined in repeat survey. This may have some impact on the results. However, in comparison between subjects who were lost to follow-up and repeated subjects, we observed no significant differences, except age. Accordingly, it could be suggested that there was little impact on our findings due to the loss to follow-up. Second, we could not include some important confounding variables in this study, such as dietary habits, C-reactive protein, and γ-glutamyltranspeptidase (28, 29). Therefore, these risk factors should be included in future study. Finally, study population of the current study was confined to residents in a rural part of Daegu City; therefore, our results may be applied to other research or polices with caution. Despite these limitations, the major strength of our study includes prospective cohort study designed with general population in a Korean rural area. To our knowledge, this study describes incidence of MetS and relationship between five components in Korean general population for the first time.
In conclusion, this study provides information on incidence of MetS in a rural area of Korea as 30.0/1,000 person-years in men and 46.4/1,000 person-years in women, and relative importance of five components as a predictor of MetS. Differences in the incidence rate of MetS are observed according to age and gender. In addition, differences in components as a predictor of MetS, such as abdominal obesity in men and low HDL-cholesterol in women, are observed according to gender. Moreover, those who have two components belong to a particularly high risk group for MetS. Accordingly, finding a high risk group for MetS and prevention of MetS through lifestyle modification is essential.
ACKNOWLEDGMENTS
We would like to thank the study investigators of the Hypertension-Diabetes Daegu Initiative (HYDDI).
HYDDI Study Investigators: Moon-Young An, MD, Ji-Yong Choi, MD, Sung-Woo Ha, MD, Nam-Soo Hong, MD, Seung-Ho Hur, MD, Soo-Hee Jin, PhD, Eui-Dal Jung, MD, Bo-Wan Kim, MD, Hye-Soon Kim, MD, Jung-Guk Kim, MD, Keon-Yeop Kim, MD, Ki-Su Kim, MD, Duk-Hee Lee, MD, Hyoung Woo Lee, MD, In-Kyu Lee, MD, Jung-Jeung Lee, MD, Mi-Young Lee, MD, Sang-Hee Lee, MD, Bu-Dol Lim, MD, Ji-Seun Lim, MD, Chang-Wook Nam, MD, Keun-Gyu Park, MD, Sun-Kyun Park, MD, Jae Kean Ryu, MD, Dong-Gu Shin, MD, Ho-Sang Shon, MD, Hyo-Kyung Son, MD, Kyu Chang Won, MD, Jin-Hoon Yang, MD, Gyeong-Im Yu, PhD. Jang Hoon Lee, MD, Yongkeun Cho, MD, Jae Eun Jun, MD, Wee Hyun Park, MD, Dong Hoon Shin, MD, Kyeong Soo Lee, MD, Kee-Sik Kim, MD, Kwon-Bae Kim, MD, Young Jo Kim, MD
Footnotes
This sudy was supported in part by a grant from Daegu Metropolitan City (2008, 200807340000).
Authors of this paper have no conflicts of interest to disclose.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 3.Lee MG, Jeong MH, Ahn Y, Chae SC, Hur SH, Hong TJ, Kim YJ, Seong IW, Chae JK, Rhew JY, et al. Impact of the metabolic syndrome on the clinical outcome of patients with acute ST-elevation myocardial infarction. J Korean Med Sci. 2010;25:1456–1461. doi: 10.3346/jkms.2010.25.10.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 5.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 6.Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999-2006. Diabetes Care. 2011;34:216–219. doi: 10.2337/dc10-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, Choi SH, Cho SI, Park KS, Lee HK, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care. 2011;34:1323–1328. doi: 10.2337/dc10-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 9.International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. [accessed on 29 November 2012]. Available at http://www.idf.org/webdata/docs/Metac_syndrome_def.pdf.
- 10.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new world-wide definition: a Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 11.Szklo M, Nieto FJ. Epidemiology beyond the basics. 2nd ed. Sudbury: Jones & Bartlett Pub; 2005. pp. 68–70. [Google Scholar]
- 12.Santos AC, Severo M, Barros H. Incidence and risk factors for the metabolic syndrome in an urban South European population. Prev Med. 2010;50:99–105. doi: 10.1016/j.ypmed.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Balkau B, Vernay M, Mhamdi L, Novak M, Arondel D, Vol S, Tichet J, Eschwège E D.E.S.I.R. Study Group. The incidence and persistence of the NCEP (National Cholesterol Education Program) metabolic syndrome: the French D.E.S.I.R. Study. Diabetes Metab. 2003;29:526–532. doi: 10.1016/s1262-3636(07)70067-8. [DOI] [PubMed] [Google Scholar]
- 14.Carnethon MR, Loria CM, Hill JO, Sidney S, Savage PJ, Liu K Coronary Artery Risk Development in Young Adults Study. Risk factors for the metabolic syndrome: the Coronary Artery Risk Development in Young Adults (CARDIA) Study, 1985-2001. Diabetes Care. 2004;27:2707–2715. doi: 10.2337/diacare.27.11.2707. [DOI] [PubMed] [Google Scholar]
- 15.Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 2004;22:11–19. doi: 10.1097/00004872-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Bener A, Mohammad AG, Ismail AN, Zirie M, Abdullatef WK, Al-Hamaq AO. Gender and age-related differences in patients with the metabolic syndrome in a highly endogamous population. Bosn J Basic Med Sci. 2010;10:210–217. doi: 10.17305/bjbms.2010.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weng C, Yuan H, Tang X, Huang Z, Yang K, Chen W, Yang P, Chen Z, Chen F. Age- and gender dependent association between components of metabolic syndrome and subclinical arterial stiffness in a Chinese population. Int J Med Sci. 2012;9:730–737. doi: 10.7150/ijms.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams CM. Lipid metabolism in women. Proc Nutr Soc. 2004;63:153–160. doi: 10.1079/PNS2003314. [DOI] [PubMed] [Google Scholar]
- 19.Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. 2006;95:136–147. doi: 10.1007/s00392-006-0351-5. [DOI] [PubMed] [Google Scholar]
- 20.Korea National Health and Nutrition Examination Survey. KNHANES statistical information. [accessed on 29 November 2012]. Available at http://knhanes.cdc.go.kr.
- 21.Hwang LC, Bai CH, Chen CJ, Chien KL. Gender difference on the development of metabolic syndrome: a population-based study in Taiwan. Eur J Epidemiol. 2007;22:899–906. doi: 10.1007/s10654-007-9183-5. [DOI] [PubMed] [Google Scholar]
- 22.Palaniappan L, Carnethon MR, Wang Y, Hanley AJ, Fortmann SP, Haffner SM, Wagenknecht L Insulin Resistance Atherosclerosis Study. Predictors of the incident metabolic syndrome in adults: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004;27:788–793. doi: 10.2337/diacare.27.3.788. [DOI] [PubMed] [Google Scholar]
- 23.Cheung BM, Wat NM, Tam S, Thomas GN, Leung GM, Cheng CH, Woo J, Janus ED, Lau CP, Lam TH, et al. Components of the metabolic syndrome predictive of its development: a 6-year longitudinal study in Hong Kong Chinese. Clin Endocrinol (Oxf) 2008;68:730–737. doi: 10.1111/j.1365-2265.2007.03110.x. [DOI] [PubMed] [Google Scholar]
- 24.DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 25.Anderson PJ, Critchley JA, Chan JC, Cockram CS, Lee ZS, Thomas GN, Tomlinson B. Factor analysis of the metabolic syndrome: obesity vs insulin resistance as the central abnormality. Int J Obes Relat Metab Disord. 2001;25:1782–1788. doi: 10.1038/sj.ijo.0801837. [DOI] [PubMed] [Google Scholar]
- 26.Goff DC, Jr, Zaccaro DJ, Haffner SM, Saad MF Insulin Resistance Atherosclerosis Study. Insulin sensitivity and the risk of incident hypertension: insights from the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2003;26:805–809. doi: 10.2337/diacare.26.3.805. [DOI] [PubMed] [Google Scholar]
- 27.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X) Diabetes. 1992;41:715–722. doi: 10.2337/diab.41.6.715. [DOI] [PubMed] [Google Scholar]
- 28.Ryu S, Song J, Choi BY, Lee SJ, Kim WS, Chang Y, Kim DI, Suh BS, Sung KC. Incidence and risk factors for metabolic syndrome in Korean male workers, ages 30 to 39. Ann Epidemiol. 2007;17:245–252. doi: 10.1016/j.annepidem.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: executive summary. Crit Pathw Cardiol. 2005;4:198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]