Abstract
Treatment with interferon beta (IFN-β) induces the production of binding antibodies (BAbs) and neutralizing antibodies (NAbs) in patients with multiple sclerosis (MS). NAbs against IFN-β are associated with a loss of IFN-β bioactivity and decreased clinical efficacy of the drug. The objective of this study was to evaluate the incidence and the prevalence of binding antibodies (BAbs) and neutralizing antibodies (NAbs) to IFN-β in MS patients receiving CinnoVex, Rebif, or Betaferon. The presence of BAbs was studied in serum samples from 124 MS patients using one of these IFN-β medications by ELISA. The NAbs against IFN-β were measured in BAb-positive MS patients receiving IFN-β using an MxA gene expression assay (real-time RT-PCR). Of the 124 patients, 36 (29.03%) had BAbs after at least 12 months of IFN-β treatment. The proportion of BAb+ was 38.1% for Betaferon, 21.9% for Rebif, and 26.8% for CinnoVex. Five BAb-positive MS patients were lost to follow-up; thus 31 BAb-positive MS patients were studied for NAbs. NAbs were present in 25 (80.6%) of BAb-positive MS patients receiving IFN-β. In conclusion, the three IFN-β preparations have different degrees of immunogenicity.
Keywords: Binding Antibodies; Bioactivity; Interferon-beta; Multiple Sclerosis; Myxovirus Resistance Rrotein A (MxA); Antibodies, Neutralizing
INTRODUCTION
Interferons (IFNs) were the first agents to show clinical efficacy in relapsing-remitting multiple sclerosis (RRMS). Interferon beta (IFN-β) decreases clinical relapses, reduces brain disease activity, and possibly slows down progression of disability (1).
The treatment may induce IFN-β antibody production in some patients. Such antibodies bind to the IFN-β, and a subgroup of these binding antibodies (BAbs) is neutralizing antibodies (NAbs), which bind to domains of IFN-β essential for receptor binding. NAbs interfere with receptor-mediated cell signaling and transcription of IFN-β inducible genes and reduce the bioactivity of injected IFN-β. The loss of IFN-β bioactivity is associated with decreased clinical efficacy of the drug and subsequent worsening of the disease (2-4).
In the last several years, accumulating evidence has indicated that anti-IFN-β antibodies decrease IFN-β bioactivity (2, 5, 6) which is associated with a reduction in the therapeutic benefit of IFN-β therapy as measured by clinical and radiologic parameters (7). The bioactivity of IFN-β is determined by biomarkers of the IFN-β response, such as neopterin, β2-microglobulin, and myxovirus resistance protein A (MxA) (2-4).
MxA has proven to be a sensitive measure of IFN-β bioactivity in multiple sclerosis (MS) (8). Human MxA is an interferon-induced dynamin-like GTPase, which has the highest specificity for detection of IFNAR stimulation induced by class I IFNs (i.e. IFN-α, IFN-β) in a dose-dependent manner. MxA can be measured by in vitro or in vivo assay (2-4). The most important advantage of the in vivo assay is that it determines the in vivo biologic response of a specific patient to his or her IFN-β therapy (9).
Differences in NAbs development and persistence are influenced by numerous factors, including formulation, dosage, frequency, and route of administration (7). Two types of recombinant human IFN-β are IFN-β-1a and IFN-β-1b, made by different companies in the world (10). According to several trials, NAbs were present in 30%-40% of patients receiving Betaferon (IFN-β-1b; Schering, Berlin, Germany), 2%-6% receiving Avonex (Biogen, Cambridge, USA), and 12%-25% receiving Rebif (Serono, Genevè, Switzerland) (11).
Currently, CinnoVex, a biosimilar product to Avonex produced by CinnaGen Company, Iran, is used to treat RRMS patients in Iran. However, to date, there have been no studies demonstrating NAbs development in CinnoVex-treated MS patients. In the present study, we aimed to determine the frequency of BAbs and Nabs positive RRMS patients receiving CinnoVex, Rebif, and Betaferon.
MATERIALS AND METHODS
Study patients
Patients were randomly selected from the clinic of the MS Centre Kashani Hospital in Isfahan, Iran during 2010-2011. Patients had clinically definite RRMS according to the McDonald criteria. Serum samples were collected from 40 healthy individuals (mean±SD of age=29.8±7.16; age range, 19-44 yr; 14 males and 26 females) and 124 RRMS patients receiving IFN-β (mean±SD of age=33.25±9.5; age range, 12-60 yr; 19 males and 105 females) for at least 3 months. Serum samples were obtained from patients who had not received any corticosteroids in the month preceding blood sampling and had not been treated with any immunosuppressive drugs associated with IFN-β.
All patients signed written informed consent and donated samples of blood. Their demographic and clinical data were recorded. Table 1 shows the baseline demographic and clinical characteristics of the patients.
Table 1.
Baseline characteristics of the patients grouped by the IFN-β formulation
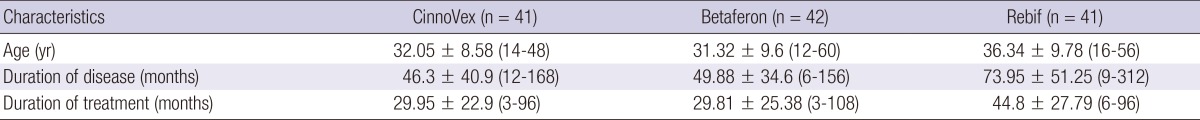
All values are mean±SD (range). n, number of individuals; SD, Standard deviation.
ELISA for detecting BAbs
Blood samples were allowed to clot at room temperature for an hour prior to centrifugation. Serum samples were collected and stored at -20℃ in small aliquots until analysis. An indirect ELISA technique was used as previously described by Fernandez et al. (12).
Each test well of a microtiter plate (Nunc, Kamstrup, DK-4000 Roskilde, Denmark) was coated with 1 µg of one of the three types or all three types of IFN-β in 100 µL phosphate buffered saline (PBS) and was incubated overnight at 4℃. The control wells were filled with PBS, BSA, and the IFN-β types, and the corresponding blank wells were incubated overnight at 4℃.
The coated plates were taken from the refrigerator and were washed six times with 1×PBS 0.05% Tween 20, pH 7.4 solution (wash buffer). Then each well of the plate was blocked with 5% bovine serum albumin (BSA) in PBS overnight at 4℃. Then, the serum samples were diluted in PBS with various dilutions of 1:10, 1:50, 1:100, 1:1,000, and 1:10,000 to determine the appropriate dilution. The most appropriate comparable dilution was found to be 1:50. Thus 100 µL of serum samples were incubated in doubling dilutions from a baseline of 1:50 in test wells for 1 hr at 37℃.
Next, the plates were washed six times with wash buffer and were incubated with 100 µL horseradish peroxidase-conjugated anti-human IgG (AbD Serotec, Düsseldorf, Germany) diluted 1:8,000 in 0.15% BSA in PBS. After washing, the color was developed by adding 100 µL tetramethyl benzidine (TMB, Cyto Matin Gene, Isfahan, Iran) to each well for 10 min at 18-28℃. The reaction was stopped with 100 µL 1 M H2SO4 3% after the 10 min. The absorbance at 450 nm was read in an ELISA reader.
To analyze variability and reproducibility, the ELISA assays for all samples were done in duplicate within each plate and for some samples were repeated on different days.
NAbs testing by real-time PCR
Of the 36 recruited patients, 5 subjects were lost to follow up: 2 subjects due to pregnancy, 3 patients due to switching or discontinuing therapy. Therefore, samples were obtained from 31 BAb-positive MS patients receiving IFN-β for at least 12 months, consisting of 10 patients (mean±SD of age=30.40±8; age range [12-43]; 2 males and 8 females) in the CinnoVex group, 12 patients (mean±SD of age=33.42±9.4; age range [21-48]; 1 male and 11 females) in the Betaferon group and 9 patients (mean±SD of age=38.4±9.79; age range [24-48]; 1 male and 8 females) in the Rebif group.
Thirteen individuals served as a control group for the MxA assay. The control group was divided into two groups: group 1 consisted of 8 healthy individuals without medical illness (mean±SD of age=35.75±11; age range [25-48 yr]; 1 male and 7 female) and group 2 consisted of five MS patients not on IFN-β therapy (mean±SD of age=23.2±5.2; age range [16-30 yr]; 5 females).
RNA isolation and cDNA synthesis
Peripheral venous blood (8 mL) was drawn into CBC tubes. Peripheral blood mononuclear cells (PBMCs) were separated on a Ficoll-Hypaque gradient, and then total RNA was extracted using a total RNA purification kit (Jena Bioscience, GmbH, Jena, Germany). RNA quantity and quality were assessed using a spectrophotometer and gel electrophoresis. Then, RNA was subsequently reverse-transcribed to cDNA using an AccuPower® RocketScript™ RT PreMix kit (Bioneer, Seoul, Korea) according to the manufacturer's protocol.
TaqMan® real-time PCR for MxA: The gene expression assay
A TaqMan® real-time PCR system was used to determine MxA and GAPDH mRNA expression levels. We used primers and probes for MxA and GAPDH as described by Pachner et al. (2). The sequences of the forward and reverse primers were as follows:
GAPDH 5': CCA GTG GAC TCC ACG ACG TA
GAPDH 3': GCG AGA TCC CTC CAA AAT CA
MxA 5': AAG CTG ATC CGC CTC CAC TT
MxA 3': TGC AAT GCA CCC CTG TAT ACC.
The amplified product was detected using a Hex oligonucleotide probe specific for the GAPDH and MxA products. The sequence of the GAPDH and MxA probes was as follows:
GAPDH probe: Hex-AGC GCC AGC ATC GCC CCA C-BHQ-1
MxA probe: Hex-CCA GAT GGA ACA GAT TGT CTA CTG CCA G-BHQ-1
All real-time PCR reactions were conducted in 25 µL volumes using a qPCR ProbesMaster (Jena Bioscience, GmbH) and a Rotor-Gene 6000 (Corbett Life Science, Sydney, Australia). The following thermal cycling conditions were applied: one hold at 50℃ for 2 min, one hold at 95℃ for 2 min, and 50 cycles at 95℃ for 15 sec and 62℃ for 1 min.
The transcriptional expression of MxA was normalized with an internal control of choice to avoid differences due to possible RNA degradation/contamination or different reverse transcription efficiency. The relative quantification of MxA mRNA was calculated using the 2-ΔΔct method and according to the formula normalization ratio (N.R.)=2-ΔΔct, where the ΔΔCt value was obtained by the difference between the ΔCt of the sample and the ΔCt of the calibrator. The ΔCt values were calculated as the Ct of MxA for each sample minus the Ct of GAPDH. According to the formula, the normalization ratio (N.R.) of the calibrator in each run is 1. As a calibrator in each sample run, we used the same RNA extracted from a single healthy control. The individual chosen for the normalization standard for MxA had Ct levels near the median for healthy individuals for both genes (3).
Statistical analysis
Data analysis was carried out using the statistical software SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Multivariate analysis of covariance (MANCOVA) was used to assess post-test differences across outcome variables. The multiple comparisons of data were conducted using the least significant difference (LSD) post hoc test between the different treated groups. Values are given as mean±standard deviation (SD). A P value less than 0.05 was considered to be statistically significant.
Ethics statements
The study protocol was approved by the institutional review board of Isfahan University of Medical Sciences (IRB registration number-189112). All patients from the clinic of the MS Centre Kashani Hospital in Isfahan, Iran signed a written informed consent and donated samples of blood.
RESULTS
Binding antibody
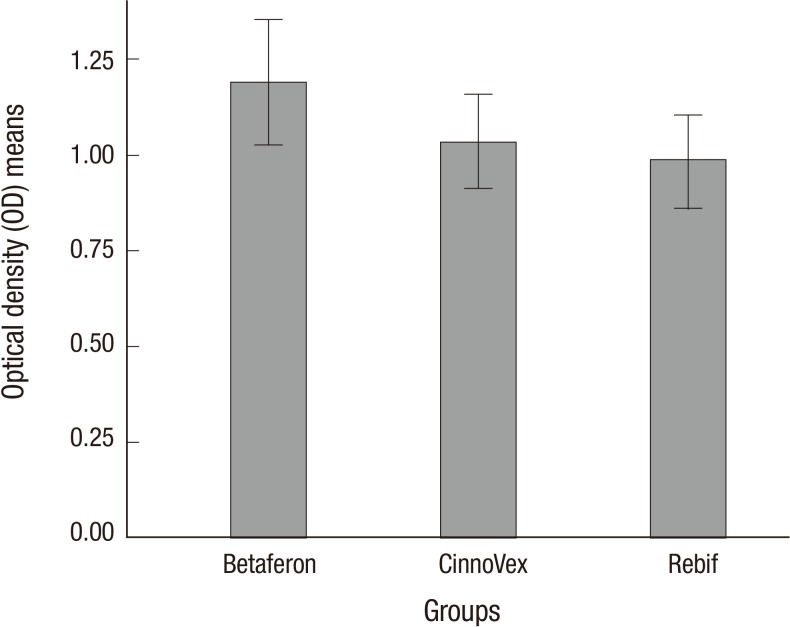
The optical densities (OD) of BAbs were determined in the control and patient groups. The duplicate values were averaged and ODs were calculated by subtracting the ODs of the wells lacking IFN-β from the ODs of the IFN-β-treated wells. The cut-off value separating positive from negative serum samples was calculated as 3 standard deviations above the mean of values obtained from 40 healthy controls (0.7 + 3 [0.17]=1.21). Therefore, patients with a positive sample with an OD>1.2 were considered positive for BAbs. The mean of the absorbance was 1.03±0.4 for antibodies to CinnoVex, 1.18±0.53 for antibodies to Betaferon, and 0.98±0.38 for antibodies to Rebif. The mean optical density is shown in Fig. 1.
Fig. 1.
The mean optical density (OD) in the three groups. The mean absorbance values are 1.19, 1.03, and 0.98 for Betaferon, CinnoVex, and Rebif, respectively. Betaferon has shown the highest mean OD values for BAbs. The mean OD value in CinnoVex was between the Betaferon and Rebif groups. Error bars indicate (+/-2) standard error.
BAbs were detected in 36 (29.0%) of 124 RRMS patients. Among the samples, 16 (38.1%) were BAb-positive in the Betaferon group, 11 (26.8%) in the CinnoVex group, and 9 (21.9%) in the Rebif group. In the pairwise comparisons of proportional differences, the incidence of BAbs in the Betaferon group was greater than the frequencies of BAbs detection in the CinnoVex and Rebif groups, and also the frequency of BAbs detection in the CinnoVex group fell between those of the Betaferon and Rebif groups. According to the MANCOVA test, there were no statistically significant differences between the treatment groups in the average of the optical density (P=0.135). The LSD post hoc test revealed that there were significant differences between the Betaferon and Rebif groups (P=0.018).
Cross-reactivity
Our ELISA results indicate that there is cross-reactivity between antibodies that develop during therapy with IFN-β-1a and 1b. It also showed that the antibodies were able to react to the IFN-β not used in the treatment, indicating a strong cross-reactivity. This is possibly due to the great similarity in their chemical structures, among which the antibodies that were induced are unable to distinguish.
Neutralizing antibodies
The in vivo bioactivity of IFN-β was studied by MxA mRNA in the RRMS patient and control groups. The average timing after the last IFN-β injection was 9.2±0.4 hr (8-12 hr). The healthy controls, who had the baseline MxA mRNA expression (mean 1.002, median 0.93, SD 0.46), and the group of individuals not receiving IFN-β (mean 0.46, median 0.22, SD 0.49) showed the lowest levels of relative MxA expression.
Of the 31 RRMS patients, 6 were considered responders (NAbs-negative), defined as having a response above the mean plus 3 standard deviations of the normal controls. The mean MxA N.R. of this group was 4.05±2.43, with a range of 2.45-8.39. Twenty five of 31 RRMS patients were considered non-responders (NAbs-positive) because their mean MxA expression was below the MxA cut-off (2.38), the mean plus 3 standard deviations of the normal controls, 0.79±3 (0.53). The mean MxA NR of the NAbs-positive group was 1.05±0.54.
NAbs were detected in 25 patients (80.6%) of the 31 RRMS patients. Table 2 presents the findings for IFN-β BAbs and NAbs results. The incidence of NAbs in the CinnoVex group was less than in the Betaferon and Rebif groups. Therefore CinnoVex is less immunogenic than the Betaferon and Rebif groups.
Table 2.
Results summary for samples tested for IFN-β antibodies: overall, 29.03% of serum samples were positive for BAbs, and 80.6% of BAbs-positive samples were NAbs-positive
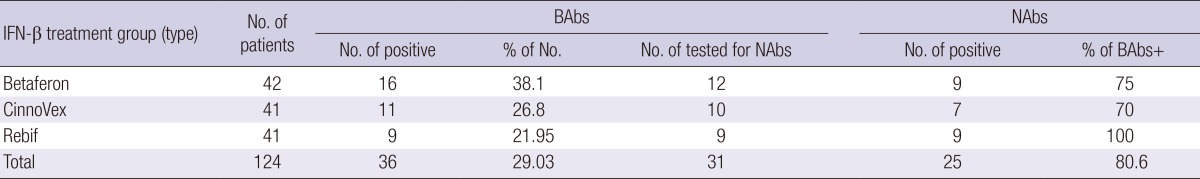
IFN-β, interferon beta; N, the numbers of patients in each treatment group; BAbs, binding antibodies; NAbs, neutralizing antibodies.
According to the MANCOVA test, there were no statistically significant differences between the treatment groups in the average of the optical density (P=0.47). There was a significant difference between the MxA mRNA levels in both the NAb-negative and NAb-positive MS patients compared to the control group (ANOVA, P<0.001). The post-hoc LSD test showed that there was no significant inhibition of MxA mRNA expression in NAb-positive MS patients compared to controls (P=0.4), indicating a loss of IFN-β bioactivity in these patients. The MxA mRNA expression was higher in the NAb-negative MS patients than the control subjects and NAb-positive MS patients (P<0.001).
It seems that the longer duration of IFN-β treatment is associated with a higher probability of NAb-positivity; however, there were no statistically significant differences in the duration of IFN-β treatment between the treatment groups (MANCOVA, P=0.27) or between the NAb-positive and NAb-negative patients (ANOVA, P=0.07). Nor were there any significant difference in EDSS (ANOVA, P=0.12) or the number of relapses (P=0.06) between the NAb-positive and NAb-negative patients. In addition, no significant differences were found in EDSS (MANCOVA, P=0.7) or the number of relapses (MANCOVA, P=0.69) between the treatment groups.
DISCUSSION
Anti-IFN-β antibodies interfere with the normal interaction of IFN-β and the IFN alpha/beta receptor (IFNAR) and prevent subsequent signal transduction of the IFN receptor, which is a necessary step for the clinical efficacy of IFN-β in MS (13). Previous studies showed that the incidence of anti-IFN-β antibody formation varies in MS patients depending on the type of IFN-β product used for treatment (14). In the current study, indirect ELISA was used for BAbs screening in serum samples from MS patients. Then, relative MxA mRNA expression was determined in peripheral blood mononuclear cells from BAbs-positive patients using real-time PCR.
ELISA is the most commonly used method for determining BAbs and this can be performed in two ways: with and without a capture antibody. In many studies, capture ELISA described by pachner et al., 2003 was used for BAbs screening (2). In this study, BAbs were detected by an indirect ELISA method, based on the procedure of Fernandez et al., 2001 with some modification (12).
It has been shown that the incidence of antibody formation varies depending on the type of IFN-β product used for treatment (14). Fernandez et al. reported that binding antibodies were positive in 32% and of 52% of cases treated by IFN-β1a and IFN-β1b respectively. The published frequencies of BAbs detection were in the general ranges of 5%-30% for patients receiving Avonex, 25%-45% for those receiving Rebif, and 50%-80% for those receiving Betaferon (15-17). Our findings demonstrated that 21.9% of patients in Rebif group and 38.1% in Betaferon group were Bab positive, which these frequencies were lower than those reported in previous studies. This might be due to the difference of our study with those previously published articles in patients' genetic background, duration of treatment, or the arbitrary cut off in ELISA assay. Our result also revealed for the first time that the frequency of BAbs positive patents was 26.8% in the CinnoVex group, which was similar to Avonex in previous studies.
NAbs can be detected by measuring the inhibition of MxA induction by therapeutic IFN-β. Our data indicate that MxA gene induction was reduced in all NAb-positive patients compared to NAb-negative subjects, and was reduced to the level of the MxA expression in the control groups. This confirms previous work demonstrating that NAbs can completely abrogate cellular responses to IFN-β in MS patients (18).
The prevalence of NAbs treated with Avonex, Rebif, and Betaferon has been previously reported as 2%-6%, 12%-28%, and 28%-47%, respectively (11). In the current study, the incidence of NAbs in 31 BAbs-positive RRMS patients receiving CinnoVex, Rebif, and Betaferon was 70%, 100%, and 75%, respectively. It was artificially high because in most of previous studies prevalence of NAbs has been reported in all RRMS patients, not just in BAbs-positive RRMS patients.
Previous studies have shown that Betaferon was more immunogenic than Rebif, and Rebif was more immunogenic than Avonex (19). In our study, because the sample size was small and there was no significant difference among the treatment groups, we were unable to compare the immunogenicity among various IFN-β products. Moreover, since none of our MS patients were treated by Avonex because of its high cost and unavailability in Iran, we failed to compare the incidence of BAbs and NAbs in Avonex and CinnoVex treated patients.
To the best of our knowledge, this study showed for the first time the immunogenicity of CinnoVex, a biosimilar product to Avonex, in MS patients by determining BAbs and NAbs. Since biosimilar products may have differences in their physicochemical characteristics, biological activity, clinical efficacy and safety, the immunogenicity of CinnoVex needs to be studied in large sample size with more MS patients receiving various IFN-β products particularly Avonex.
ACKNOWLEDGMENT
My most cordial thanks go to all those MS patients and control subjects who participated in this study.
Footnotes
This study was financially supported by grant 189112 from Isfahan University of Medical Sciences.
The authors have no conflicts of interest to disclose.
References
- 1.Van Baarsen LG, Vosslamber S, Tijssen M, Baggen JM, van der Voort LF, Killestein J, van der Pouw Kraan TC, Polman CH, Verweij CL. Pharmacogenomics of interferon-beta therapy in multiple sclerosis: baseline IFN signature determines pharmacological differences between patients. PLoS One. 2008;3:e1927. doi: 10.1371/journal.pone.0001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pachner A, Narayan K, Price N, Hurd M, Dail D. MxA gene expression analysis as an interferon-beta bioactivity measurement in patients with multiple sclerosis and the identification of antibody-mediated decreased bioactivity. Mol Diagn. 2003;7:17–25. doi: 10.1007/BF03260016. [DOI] [PubMed] [Google Scholar]
- 3.Hartung HP, Munschauer F, 3rd, Schellekens H. Significance of neutralizing antibodies to interferon beta during treatment of multiple sclerosis: expert opinions based on the Proceedings of an International Consensus Conference. Eur J Neurol. 2005;12:588–601. doi: 10.1111/j.1468-1331.2005.01104.x. [DOI] [PubMed] [Google Scholar]
- 4.Bermel RA, Rudick RA. Interferon-beta treatment for multiple sclerosis. Neurotherapeutics. 2007;4:633–646. doi: 10.1016/j.nurt.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolotto A, Gilli F, Sala A, Audano L, Castello A, Magliola U, Melis F, Giordana MT. Evaluation of bioavailability of three types of IFNbeta in multiple sclerosis patients by a new quantitative-competitive-PCR method for MxA quantification. J Immunol Methods. 2001;256:141–152. doi: 10.1016/s0022-1759(01)00434-3. [DOI] [PubMed] [Google Scholar]
- 6.Bertolotto A, Gilli F, Sala A, Capobianco M, Malucchi S, Milano E, Melis F, Marnetto F, Lindberg RL, Bottero R, et al. Persistent neutralizing antibodies abolish the interferon beta bioavailability in MS patients. Neurology. 2003;60:634–639. doi: 10.1212/01.wnl.0000046662.03894.c5. [DOI] [PubMed] [Google Scholar]
- 7.Cohen BA, Oger J, Gagnon A, Giovannoni G. The implications of immunogenicity for protein-based multiple sclerosis therapies. J Neurol Sci. 2008;275:7–17. doi: 10.1016/j.jns.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 8.McKay F, Schibeci S, Heard R, Stewart G, Booth D. Analysis of neutralizing antibodies to therapeutic interferon-beta in multiple sclerosis patients: a comparison of three methods in a large Australasian cohort. J Immunol Methods. 2006;310:20–29. doi: 10.1016/j.jim.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Deisenhammer F, Schellekens H, Bertolotto A. Measurement of neutralizing antibodies to interferon beta in patients with multiple sclerosis. J Neurol. 2004;251:II31–II39. doi: 10.1007/s00415-004-1206-5. [DOI] [PubMed] [Google Scholar]
- 10.Sominanda A, Hillert J, Fogdell-Hahn A. In vivo bioactivity of interferon-beta in multiple sclerosis patients with neutralising antibodies is titre-dependent. J Neurol Neurosurg Psychiatry. 2008;79:57–62. doi: 10.1136/jnnp.2007.122549. [DOI] [PubMed] [Google Scholar]
- 11.Namaka M, Pollitt-Smith M, Gupta A, Klowak M, Vasconcelos M, Turcotte D, Gong Y, Melanson M. The clinical importance of neutralizing antibodies in relapsing-remitting multiple sclerosis. Curr Med Res Opin. 2006;22:223–239. doi: 10.1185/030079906X80413. [DOI] [PubMed] [Google Scholar]
- 12.Fernández O, Mayorga C, Luque G, Guerrero M, Guerrero R, Leyva L, León A, Blanca M. Study of binding and neutralising antibodies to interferon-beta in two groups of relapsing-remitting multiple sclerosis patients. J Neurol. 2001;248:383–388. doi: 10.1007/s004150170178. [DOI] [PubMed] [Google Scholar]
- 13.Cutrone EC, Langer JA. Identification of critical residues in bovine IFNAR-1 responsible for interferon binding. J Biol Chem. 2001;276:17140–17148. doi: 10.1074/jbc.M009663200. [DOI] [PubMed] [Google Scholar]
- 14.Meager A, Dolman C, Dilger P, Bird C, Giovannoni G, Schellekens H, Thorpe R, Wadhwa M. An assessment of biological potency and molecular characteristics of different innovator and noninnovator interferon-beta products. J Interferon Cytokine Res. 2011;31:383–392. doi: 10.1089/jir.2010.0113. [DOI] [PubMed] [Google Scholar]
- 15.Kivisäkk P, Alm GV, Fredrikson S, Link H. Neutralizing and binding anti-interferon-beta (IFN-beta) antibodies: a comparison between IFN-beta-1a and IFN-beta-1b treatment in multiple sclerosis. Eur J Neurol. 2000;7:27–34. doi: 10.1046/j.1468-1331.2000.00002.x. [DOI] [PubMed] [Google Scholar]
- 16.Monzani F, Meucci G, Caraccio N, Saviozzi M, Casolaro A, Moscato G, Lombardo F, Mosti S, Scagnolari C, Bruschi F, et al. Discordant effect of IFN-beta1a therapy on anti-IFN antibodies and thyroid disease development in patients with multiple sclerosis. J Interferon Cytokine Res. 2002;22:773–781. doi: 10.1089/107999002320271369. [DOI] [PubMed] [Google Scholar]
- 17.Perini P, Calabrese M, Biasi G, Gallo P. The clinical impact of interferon beta antibodies in relapsing-remitting MS. J Neurol. 2004;251:305–309. doi: 10.1007/s00415-004-0312-8. [DOI] [PubMed] [Google Scholar]
- 18.Gilli F, Marnetto F, Caldano M, Sala A, Malucchi S, Capobianco M, Bertolotto A. Biological markers of interferon-beta therapy: comparison among interferon-stimulated genes MxA, TRAIL and XAF-1. Mult Scler. 2006;12:47–57. doi: 10.1191/135248506ms1245oa. [DOI] [PubMed] [Google Scholar]
- 19.Sominanda A, Rot U, Suoniemi M, Deisenhammer F, Hillert J, Fogdell-Hahn A. Interferon beta preparations for the treatment of multiple sclerosis patients differ in neutralizing antibody seroprevalence and immunogenicity. Mult Scler. 2007;13:208–214. doi: 10.1177/1352458506070762. [DOI] [PubMed] [Google Scholar]