Abstract
Previous studies reported that oxaliplatin is associated with sinusoidal obstruction syndrome. However few reports on oxaliplatin induced liver fibrosis are found in the literature. Furthermore pathogenesis of liver fibrosis is not well known. We report a case of 45-yr-old Korean man in whom liver fibrosis with splenomegaly developed after 12 cycles of oxaliplatin based adjuvant chemotherapy for colon cancer (T4N2M0). Thorough history taking and serological examination revealed no evidence of chronic liver disease. Restaging CT scans demonstrated a good response to chemotherapy. Five month after chemotherapy, he underwent right hepatectomy due to isolated metastatic lesion. The liver parenchyma showed diffuse sinusoidal dilatation and centrilobular vein fibrosis with necrosis without steatosis. We could conclude that splenomegaly was due to perisinusoidal liver fibrosis and liver cell necrosis induced portal hypertension by oxaliplatin. In addition, to investigate the pathogenesis of liver fibrosis, immunohistochemical stains such as CD31 and α-smooth muscle actin (α-SMA) were conducted with control group. The immunohistochemical stains for CD31 and α-SMA were positive along the sinusoidal space in the patient, while negative in the control group. Chemotherapy with oxaliplatin induces liver fibrosis which should be kept in mind as a serious complication.
Keywords: Liver Cirrhosis, Splenomegaly, Oxaliplatin
INTRODUCTION
Oxaliplatin-based chemotherapy regimens are currently a standard of care for the treatment of colorectal cancer in both the adjuvant treatment and metastatic disease setting. Significant improvement in outcomes have been achieved with oxaliplatin-based combination in these setting when compared with administration of 5-fluorouracil alone. The most popular regimens are those that combine fluoropyrimidines with irinotecan or oxaliplatin, including the 5-fluorouracil (5-FU) and oxaliplatin (FOLFOX) and 5-FU, oxaliplatin and irinotecan (FOLFIRI) regimens.
Increased use of chemotherapy for colorectal cancer has led to a growing awareness of potential hepatotoxicity caused by such treatment. For 5-FU, an associated with hepatic steatosis has been shown (1, 2), and sinusoidal obstruction syndrome or "blue liver syndrome" are reported to be oxaliplatin specific (3, 4). However, few reports on oxaliplatin induced liver fibrosis are found in the literature. We herein report a case of 45-yr-old Korean man in whom liver fibrosis with splenomegaly developed after 12 cycles of oxaliplatin based adjuvant chemotherapy for colon cancer. In addition, to investigate the pathogenesis of liver fibrosis immunohistochemical stains such as CD31 and α-SMA were conducted.
CASE DESCRIPTION
A 45-yr-old Korean man presented with lower abdominal pain in June, 2011. Workup revealed obstructing descending colon mass with pericolic infiltration. He had no significant past medical history. The patient also denied any previous use of alcohol and was not taking any prescription medication or herb. Result of his initial laboratory physical examination was unremarkable, and all initial laboratory values were within normal limits. Evaluation for evidence of viral infection with either hepatitis B or C was negative. The patient underwent left hemicolectomy of the primary T4N2M0 (Stage III) tumor. Surgical pathology revealed moderately differentiated adenocarcinoma of colon. He was then treated with 12 cycle of FOLFOX4 (5-flurouracil [Day 1, 2: 400 mg/m2 + 600 mg/m2]+ oxapliplatin [Day 1: 85 mg/m2] over a 6-month period. The latter part of this treatment was complicated by persistent thrombocytopenia (lowest platelet count of 52,700/µL during chemotherapy). Restaging CT scans demonstrated a good response to treatment. But 4 month after treatment, splenomegaly was noted radiographically (Fig. 1). Gastro-esophageal varix was not observed in endoscopy, but we postulated portal hypertension from splenomegaly with enlarged splenic artery and portal vein. Five month after chemotherapy, he underwent right hepatectomy due to isolated metastatic lesion in S6 and S8 lobe. Platelet count ranged 100,000/µL when hepatectomy was peformed. The liver parenchyma showed diffuse sinusoidal dilatation and centrilobular vein fibrosis with liver cell necrosis without steatosis. To investigate the pathogenesis of liver fibrosis, immunohistochemical stains such as CD31 and α-SMA were conducted with control group which was obtained from grossly normal autopsy liver. The immunohistochemical stains for CD31 and α-SMA were positive along the sinusoidal spaces in the patient, while negative in the control group (Fig. 2). After operation, the patient is currently being treated with systemic chemotherapy.
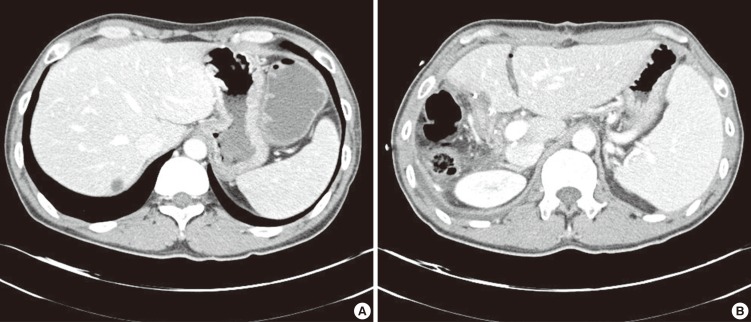
Fig. 1.
Abdominal CT finding of spleen. (A) Before operation and chemotherapy the maximal spleen diameter was 105 mm, (B) After operation and chemotherapy (5 month after last chemotherapy) the maximal spleen diameter was 131 mm.
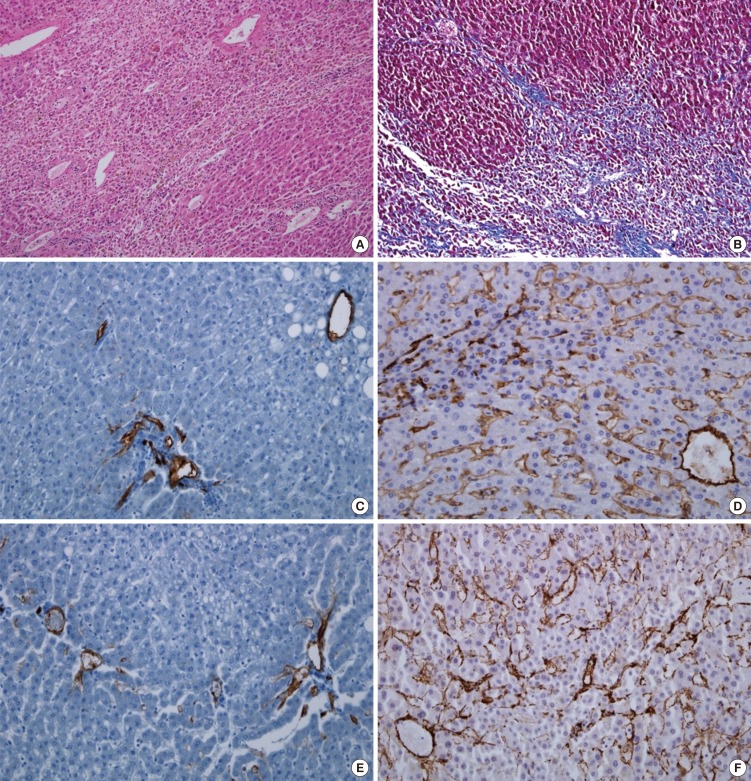
Fig. 2.
Histologic finding of the liver parenchyma. (A) It revealed liver cell necrosis and perisinusoidal fibrosis (H&E, ×100). (B) Masson's trichrome stain. Diffuse fibrosis in portal area and perisinusoidal space (MT, ×100). (C) Negative stain of CD31 in the control group. (D) CD31 immunohistochemical stain. Diffuse positive in sinusoidal wall, central vein and portal vein branch wall (CD31, ×200). (E) Negative stain of α-SMA in the control group. (F) α-SMA immunohistochemical stain. Diffuse positive in sinusoidal wall, central vein and portal vein or hepatic artery branch wall (α-SMA, ×200).
DISCUSSION
The cytotoxic drugs used for colorectal liver metastasis, fluorouracil, irinotecan, and oxaliplatin, are usually given as combination therapy and can exert synergistic toxicities on the liver. The molecular changes caused by these drugs are not well understood; it has been suggests an important role for oxidative stress caused by reactive oxygen species from mitochondria, as well as extramitochondial oxidative pathways in microsome and peroxisomes.
Investigators have identified a wide spectrum of effects on the underlying liver parenchyma, ranging from mild form of steatosis to severe steatohepatitis, and veno-occlusive disease termed sinusoidal obstruction syndrome (SOS) (5-7). As the histopathologic definitions of these changes evolve, studies have identified specific patterns of hepatic injury related to the various chemotherapeutic agents. 5-Fluorouracil has been linked to development of steatosis, which is possibly mediated by excessive production of reactive oxygen species resulting in the accumulation of lipid vesicles in hepatocytes (2, 8). Oxaliplatin has been associated with sinusoidal obstruction syndrome, possibly caused by sinusoidal endothelial cell damage (4, 7).
We postulated liver fibrosis is due to oxaliplatin other than 5-flurouracil as this case showed diffuse perisinusoidal fibrosis with necrosis without steatosis. SOS cannot be ruled out as a cause of fibrosis in this case as the initial lesion of SOS is perivenular necrosis followed by progressive fibrotic decrease in venule caliber and sharing mechanism of fibrosis. Centrilobular perivenular fibrosis is often described in SOS. However, oxaliplatin induced bridging fibrosis or cirrhosis is rarely reported.
The predominant mechanism of liver fibrosis is related to proliferation of hepatic stellate cells and their activation into myofibroblasts. We performed immunohistologic studies to investigate the pathogenesis of liver fibrosis. The most widely used and accessible marker of myofibroblast is the de novo expression of α-SMA (9). In addition, sinusoidal endothelial cells show phenotypic difference with vascular endothelium, but they assume vascular endothelium phenotypic properties in chronic liver disease and in hepatocelluar carcinoma (10). CD 31 is expressed by vascular endothelial cells and anti-CD31 has been used as a tool to identify the vascular origin of neoplasm. In current case, the immunohistochemical stains for α-SMA and CD31 were positive along the sinusoidal spaces, corresponding to the mechanism of liver fibrosis.
It has been suggested that hepatotoxicity caused by the chemotherapeutic drug may be time- and dose-dependent. Nakano et al. (11) reported an increased risk of sinusoidal dilatation with six or more cycles of oxaliplatin and Slade et al. (12) reported oxaliplatin induced hepatic sinusoidal injury and noncirrhotic portal hypertension with ten or more cycle of oxaliplatin on the dosing of 85 mg/m2 every 2 weeks. In our case liver fibrosis with splenomegaly developed after 12 cycles of FOLFOX regimen. In the case of preoperative chemotherapy, the optimal duration is about 4 month at most to maximize therapeutic benefit, while avoiding hepatotoxicity (13).
Considering effect of chemotherapy-associated liver injury on surgical outcome, sinusoidal injury with fibrosis and regenerative nodular hyperplasia might increase the risk of the bleeding from liver resection. A patient with chemotherapy-associated liver injury who needs major hepatic resection, the volume of the future liver remnant should be measured to minimize postoperative complication. To prevent adverse outcomes from chemotherapy-associated liver injury, long term chemotherapy should be avoided and response to therapy assessed as a prognostic indicator of outcome. For medical therapy, glutamine or glutathione has been studied for possible treatment for oxaliplatin associated liver injury.
Footnotes
The authors have no potential conflict of interest relevant to this article.
References
- 1.Kandutsch S, Klinger M, Hacker S, Wrba F, Gruenberger B, Gruenberger T. Patterns of hepatotoxicity after chemotherapy for colorectal cancer liver metastases. Eur J Surg Oncol. 2008;34:1231–1236. doi: 10.1016/j.ejso.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Khan AZ, Morris-Stiff G, Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg. 2009;16:137–144. doi: 10.1007/s00534-008-0016-z. [DOI] [PubMed] [Google Scholar]
- 3.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 4.Kneuertz PJ, Maithel SK, Staley CA, Kooby DA. Chemotherapy-associated liver injury: impact on surgical management of colorectal cancer liver metastases. Ann Surg Oncol. 2011;18:181–190. doi: 10.1245/s10434-010-1201-2. [DOI] [PubMed] [Google Scholar]
- 5.Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, DeMatteo RP, D'Angelica M, Blumgart LH, Jarnagin WR. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 7.Rubbia-Brandt L, Mentha G, Terris B. Sinusoidal obstruction syndrome is a major feature of hepatic lesions associated with oxaliplatin neoadjuvant chemotherapy for liver colorectal metastases. J Am Coll Surg. 2006;202:199–200. doi: 10.1016/j.jamcollsurg.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Mehta NN, Ravikumar R, Coldham CA, Buckels JA, Hubscher SG, Bramhall SR, Wigmore SJ, Mayer AD, Mirza DF. Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:782–786. doi: 10.1016/j.ejso.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terayama N, Terada T, Nakanuma Y. An immunohistochemical study of tumour vessels in metastatic liver cancers and the surrounding liver tissue. Histopathology. 1996;29:37–43. doi: 10.1046/j.1365-2559.1996.d01-484.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, Bachellier P, Jaeck D. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118–124. doi: 10.1097/SLA.0b013e31815774de. [DOI] [PubMed] [Google Scholar]
- 12.Slade JH, Alattar ML, Fogelman DR, Overman MJ, Agarwal A, Maru DM, Coulson RL, Charnsangavej C, Vauthey JN, Wolff RA, et al. Portal hypertension associated with oxaliplatin administration: clinical manifestations of hepatic sinusoidal injury. Clin Colorectal Cancer. 2009;8:225–230. doi: 10.3816/CCC.2009.n.038. [DOI] [PubMed] [Google Scholar]
- 13.White RR, Schwartz LH, Munoz JA, Raggio G, Jarnagin WR, Fong Y, D'Angelica MI, Kemeny NE. Assessing the optimal duration of chemotherapy in patients with colorectal liver metastases. J Surg Oncol. 2008;97:601–604. doi: 10.1002/jso.21042. [DOI] [PubMed] [Google Scholar]