Abstract
Various cell transfection techniques exist and these can be broken down to three broad categories: viral, chemical and mechanical. This protocol describes a mechanical method to temporally permeabilize adherent cells using an inert gas jet that can facilitate the transfer of normally non-permeable macromolecules into cells. We believe this technique works by imparting shear forces on the plasma membrane of adherent cells, resulting in the temporary formation of micropores. Once these pores are created, the cells are then permeable to genetic material and other biomolecules. The mechanical forces involved do run the risk of permanently damaging or detaching cells from their substrate. There is, therefore, a narrow range of inert gas dynamics where the technique is effective. An inert gas jet has proven efficient at permeabilizing various adherent cell lines including HeLa, HEK293 and human abdominal aortic endothelial cells. This protocol is appropriate for the permeabilization of adherent cells both in vitro and, as we have demonstrated, in vivo, showing it may be used for research and potentially in future clinical applications. It also has the advantage of permeabilizing cells in a spatially restrictive manner, which could prove to be a valuable research tool.
Keywords: Bioengineering, Issue 79, Chemical Engineering, Chemistry, Biochemistry, Cellular Biology, Molecular Biology, Biomedical Engineering, Biophysics, Transfection, Permeabilization, transfection, shear stress, adherent cells, SEM, cell, microscopy, imaging
Introduction
With the evolution of biomedicine and the understanding of cell mechanics, the delivery of biomolecules into cells has become vital to many research fields and medical therapies. Different techniques have been developed to introduce foreign molecules through the plasma membrane and into the cell cytosol. These can be generally classified as either: viral, chemical or mechanical techniques. Viral techniques can transfect genetic material such as RNA and DNA using viral vectors1. Viral techniques tend to have high efficiencies in certain cell lines, however they do have the potential for immune and inflammatory reactions2. Common chemical transfection methods include precipitation with calcium phosphate3, bacterial exotoxins4 and lipofection5. These techniques have proven to be effective; however, certain problems arise such as cell toxicity and non-specificity. Furthermore, techniques such as calcium phosphate precipitation have been found to have transfection efficiencies up to 70% but only in certain cell lines, rarely in primary cells6. This is of note as increasing research efforts are being put into primary cells, especially in the case of designing various treatments for clinical use and the study of DNA functioning.
Temporal disruption of the cell membrane through mechanical stimuli is an alternative for introducing foreign molecules into cells. Techniques include: microinjection of molecules7, electroporation using an electric field to disrupt the membrane8,9, sonoporation using ultrasound waves to disrupt the cell membrane10-12, particle bombardment as demonstrated by the "gene gun", which shoots particles bound with genes into the cell13, and more recently, the application of fluid shear stress that has been shown to temporally permeabilize mammalian cells14,15. Although mechanical methods avoid some of the aforementioned issues, they are typically accompanied by lower efficiencies and very complex and specialized setups. An atmospheric pressure glow discharge torch (APGD-t) was developed at McGill with the initial goal of functionalizing surfaces and detaching adherent cells in vitro16. Serendipitously, it was discovered that a live/dead stain being used was somehow being taken in by cells without a permeabilizing agent being added. Furthermore, this seemed to have occurred only in restricted areas of the wells which happened to match up with the torch path. Investigation into the permeabilization capabilities of the APGD-t continued and in control studies, it was found that the carrier inert gas jet of the plasma without excitation was also able to permeabilize cells. This contradicted the initial hypothesis that the reactive species created by the plasma jet temporarily impaired the membrane function and suggested that simply the mechanical forces were sufficient to cause cell permeabilization. From here, studies continued in our lab to try and quantify and characterize the permeabilization efficiency of an inert gas jet as well as look at its transfection capabilities16.
Through these studies, it has been found that micropores do in fact form in the plasma membrane, and these pores tend to reseal within approximately 5 sec15. We have demonstrated the technique's utility in vitro and in vivo in the chorioallantoic membrane of a chick embryo.
Protocol
1. Development of LabView Program
A mass flow controller (MKS M100B Mass-Flo Controller) is attached to the helium gas line to ensure a precise gas flow rate. This unit is controlled by an input voltage, ranging between 0 and 5 V. A control loop in the LabView program determines the required voltage at any given time. Interfacing between the computer and the mass flow controller is performed by a data acquisition device (NI USB-6009), and a 12 V power supply provides power to the mass flow controller.
Two positioning linear slides are used to control the movement of the 6-well plate, one for each direction. Each assembly consists of a MA15-series Velmex Unislide Assembly coupled with a stepping motor, and both assemblies are controlled by a single Velmex VXM Stepping Motor Controller. The controller allows for manual jogging of each motor or accepts a string of external serial commands, the combination of which allow for specific movement patterns. Several patterns can be programmed into the LabView program, requiring the user to provide only the desired torch speed as well as the path dimensions.
2. Preparation of Equipment
Open both the helium cylinder and regulator.
Turn on the motor controller.
Open the LabView program and verify all conditions.
Make sure the platform is centered. To check, look at each motor stage and verify that each carriage is not near either stage end. Either have the program center the carriages or manually adjust by using the jogging controls on the front of the motor controller.
If this is the first run of the day, it is recommended to run the program once without cells, to ensure the setup is working correctly.
3. Preparation of the Gas Jet Capillary Nozzle Height
Remove the capillary from the holder and zero the height dial.
Place the 6-well plate on the platform.
Replace and lower the gas jet capillary to the bottom of the well until it hits the cover slip. Secure the capillary in the holder.
Using the height dial, raise the capillary 3 mm. Mark this height on the capillary base.
Raise the capillary to a safe height and then remove the 6-well plate.
4. Cell Permeabilization Protocol
Culture adherent cell line to confluence on glass cover slips in a 6-well plate using standard culturing techniques. For HeLa cells, seed 6-well plates with 2 x 105 cells per well and incubate for 48 hr before treatment.
Prepare appropriate volume of solution containing the desired vector. For hrGFPII-1, a concentration of 50 ng/μl in 1x PBS provides a strong fluorescent signal in HeLa cells 24 hr after transfection. For dextran studies, 80 μg/ml of 10 kDa green fluorescent dextran is recommended. Henceforth, this solution will be referred to as "vector solution".
Remove cell culture media from well and rinse three times with 1x PBS.
Pipette 1,370 μl of vector solution prepared in step 4.2 into well. This should result in a liquid depth of approximately 1.3 mm.
Place the well containing the vector solution in the center of the platform. Lower the gas jet nozzle to the previously marked level indicating a nozzle height of 3 mm above the slide.
Set helium flow rate in the LabView program and select the desired movement pattern to be used. Select "Run" when ready to begin the permeabilization protocol. There will be an initial lag period during which the mass flow controller is preparing to provide the necessary flow rate. Once the appropriate flow rate has been reached, the LabView program will activate the stepper motors to move the well in the desired permeabilization pattern. Once the platform has stopped, wait approximately 30 sec and remove the vector solution. Note: Optimal permeabilization occurs near a dynamic pressure of 100 to 200 Pa at the nozzle outlet, depending on the capillary diameter.
Wash well three times with 1x PBS.
Fill well with fresh culture media.
Repeat steps 4.3 - 4.6 for desired number of experiments. Note: Be sure to include control samples which consist of wells being filled with the vector solution without the gas jet being run over them. Leave the solution in the control wells for approximately 1 min then proceed to steps 4.5 and 4.6.
If running an hrGFP transfection experiment, return 6-well plate to incubator for 24 hr to allow transfected material to be transcribed by the cells. If running a dextran permeabilization experiment, return 6-well plate to incubator for 15 min.
5. Cell Imaging
If desired, immediately before imaging, counterstain with appropriate live/dead stain to evaluate cell death.
Image cells with appropriate microscope to investigate transfection/ permeabilization efficacy.
Representative Results
Permeabilization of HeLa cells with 10 kDa green fluorescent dextran using a helium gas jet with a 0.86 mm inner diameter capillary is shown in Figure 1. Cells were counterstained immediately after permeabilization with 2 μl/ml EthD-1 solution (LIVE/DEAD Viability/Cytotoxicity Kit) to visualize cell death. Immediately following counterstaining, cover slips were mounted and imaged. This figure shows results of running the helium gas jet at three different outlet pressures compared to a control sample. Note that the permeabilization track width and efficiency increased with a higher dynamic pressure and cell death only showed a slight increase (A and B). However, once a high dynamic pressure was reached, HeLa cells were stripped from the cover slips and very little peripheral permeabilization occurred. A more detailed analysis of cell stripping and transfection efficiency was previously conducted by Chouinard-Pelletier et al.15
Through scanning electron microscopy studies, micropores were observed in the cell membrane (Figure 2). Permeabilization of HeLa cells was performed using a helium gas jet with a 0.86 mm inner diameter capillary at an outlet pressure of 300 Pa. Following the permeabilization protocol, the cells were immediately fixed with a 1% paraformaldehyde solution in 1x PBS.
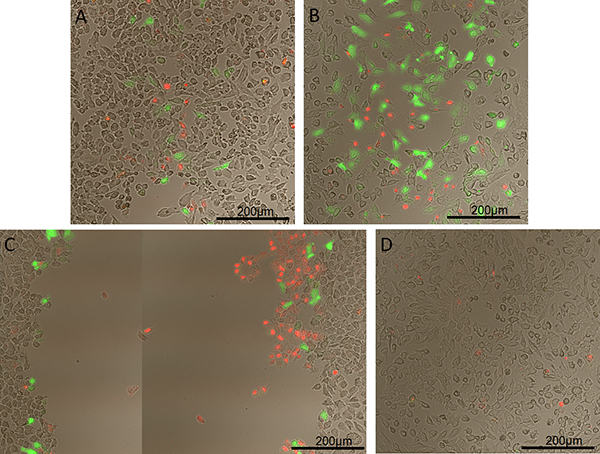 Figure 1. Permeabilization of HeLa cells with 10 kDa green fluorescent dextran using a helium gas jet with a capillary nozzle inner diameter of 0.86 mm. Dextran fluoresces green and dead cells fluoresce red. A) Dynamic pressure of 100 Pa, B) of 135 Pa, and C) of 175 Pa. D) Control with dextran added but no exposure to helium gas jet.
Figure 1. Permeabilization of HeLa cells with 10 kDa green fluorescent dextran using a helium gas jet with a capillary nozzle inner diameter of 0.86 mm. Dextran fluoresces green and dead cells fluoresce red. A) Dynamic pressure of 100 Pa, B) of 135 Pa, and C) of 175 Pa. D) Control with dextran added but no exposure to helium gas jet.
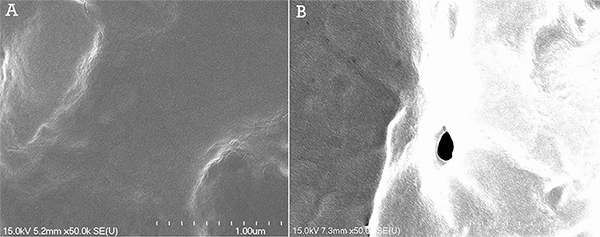 Figure 2. SEM images at a magnification of 50,000X of the surface of HeLa cells following gas jet permeabilization at 300 Pa.A) Control sample exhibiting a smooth cell surface. B) Surface of permeabilized cell exhibiting a pore with an 84 nm diameter. Click here to view larger figure.
Figure 2. SEM images at a magnification of 50,000X of the surface of HeLa cells following gas jet permeabilization at 300 Pa.A) Control sample exhibiting a smooth cell surface. B) Surface of permeabilized cell exhibiting a pore with an 84 nm diameter. Click here to view larger figure.
Discussion
Inert gas jet permeabilization is a useful technique for adherent cell transfection. It provides the ability to transfer biomolecules into cells with the use of mechanical forces, which eliminates the need for potentially harmful chemicals or viral vectors. The technique could potentially give researchers and clinicians an efficient and relatively simple way to precisely transfect cells. The selectivity of the permeabilization is also unique allowing researchers to only treat certain cells in a single colony which could be useful in multiple applications.
Several parameters can be adjusted for individual experimentation such as capillary nozzle diameter and angle, gas flow rate, liquid and capillary height, cell line, target biomolecule to be used, and type of inert gas. A set liquid level and capillary height have been described in the procedure and these values were chosen to minimize the number of variables involved in our experiments. Currently, we are modeling the system using experimental flow visualization and computational fluid dynamics. This will give us a better understanding of the forces imparted on the cells, allowing us to determine the permeabilization's dependency on variables such as the liquid properties.
It is important to take note of the maximum dynamic pressure under which the cells can survive. It was found that once dynamic pressures neared 200 Pa for a gas jet nozzle height of 3 mm, HeLa cell stripping became likely. This agrees with other studies such as Lu et al. who observed WT NR6 fibroblast cell detachment at 200-265 Pa17. If large amounts of stripping occur, a lower gas flow rate should alleviate this issue. Similarly, high flow rates result in cell death and false-positive permeabilization results. Once cells have died they will become permeable to molecules and therefore dead cells can be mistaken for permeabilized live cells. It is therefore recommended to conduct a live/dead assay to ensure that the permeabilized cells are in fact still alive. Death increases with increasing pressure and our lab found that at 390 Pa the majority of permeabilized HeLa cells were dead15. Cell viability over an extended period of time has also been investigated, with hrGFPII-1 expression being observed up to 72 hr post-permeabilization (data not shown). Further studies are currently underway to investigate viability at even longer time points.
Our hypothesis is that fluid shear stresses on the cells, created by the jet of gas and the displacement of the media, cause temporary disruption of the cell plasma membrane. This is the same mechanism proposed for the other more complex physical methods such as microbubble collapse and sonoporation18. The operating conditions for this method are dependent on the type of cells, attachment to the substrate, mechanical properties of the cell and cell-wall, molecule of interest, properties of the liquid and gas, and the physical dimensions of the setup. Through size exclusion studies, we have found that dextran molecules larger than 40 kDa show very low permeabilization efficiency and that an optimal dextran size is 10 kDa for experimentation15. This is important as it limits the size of molecules that can be permeabilized into cells using this protocol. Thus far, we have only investigated the transfection properties of plasmids. Further studies will ascertain whether larger pieces of genetic material are capable of being transfected using this technique.
Disclosures
The authors declare they have no competing financial interests.
Acknowledgments
The authors would like to thank the following funding sources: Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council (NSERC).
References
- Rosenberg SA, et al. Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. The New England Journal of Medicine. 1990;323:570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Molecular and cellular biology. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walev I, et al. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3185–3190. doi: 10.1073/pnas.051429498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner PL, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm A, Krott N, Breibach I, Blindt R, Bosserhoff AK. Efficient transfection method for primary cells. Tissue engineering. 2002;8:235–245. doi: 10.1089/107632702753725003. [DOI] [PubMed] [Google Scholar]
- Rauth S, Kucherlapati RS. Expression of DNA Transferred into Mammalian-Cells. J. Bioscience. 1984;6:543–567. [Google Scholar]
- Spandidos DA. Electric field-mediated gene transfer (electroporation) into mouse Friend and human K562 erythroleukemic cells. Gene analysis techniques. 1987;4:50–56. doi: 10.1016/0735-0651(87)90018-5. [DOI] [PubMed] [Google Scholar]
- Chu G, Hayakawa H, Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Research. 1987;15:1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechheimer M, et al. Transfection of mammalian cells with plasmid DNA by scrape loading and sonication loading. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:8463–8467. doi: 10.1073/pnas.84.23.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Pohl P, Cobet U, Rainov NG. Ultrasound enhancement of liposome-mediated cell transfection is caused by cavitation effects. Ultrasound in Medicine & Biology. 2000;26:897–903. doi: 10.1016/s0301-5629(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Greenleaf WJ, Bolander ME, Sarkar G, Goldring MB, Greenleaf JF. Artificial cavitation nuclei significantly enhance acoustically induced cell transfection. Ultrasound in Medicine & Biology. 1998;24:587–595. doi: 10.1016/s0301-5629(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Yang NS, Burkholder J, Roberts B, Martinell B, McCabe D. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9568–9572. doi: 10.1073/pnas.87.24.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallow DM, et al. Shear-induced intracellular loading of cells with molecules by controlled microfluidics. Biotechnology and Bioengineering. 2008;99:846–854. doi: 10.1002/bit.21651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard-Pelletier GL, Guay M, Coulombe D, Leask S, L R, Jones E. Use of inert gas jets to measure the forces required for mechanical gene transfection. Biomedical Engineering Online. 2012;11 doi: 10.1186/1475-925X-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc M, Coulombe S, Leask RL. Atmospheric Pressure Plasma Jet Deposition of Patterned Polymer Films for Cell Culture Applications. Ieee T. Plasma Sci. 2009;37:927–933. [Google Scholar]
- Lu H, et al. Microfluidic shear devices for quantitative analysis of cell adhesion. Analytical Chemistry. 2004;76:5257–5264. doi: 10.1021/ac049837t. [DOI] [PubMed] [Google Scholar]
- Sankin GN, Yuan F, Zhong P. Pulsating tandem microbubble for localized and directional single-cell membrane poration. Physical Review Letters. 2010;105:078101. doi: 10.1103/PhysRevLett.105.078101. [DOI] [PMC free article] [PubMed] [Google Scholar]


