Abstract
Purpose
Annual surveillance mammograms in older long-term breast cancer survivors are recommended, but this recommendation is based on little evidence and with no guidelines on when to stop. Surveillance mammograms should decrease breast cancer mortality by detecting second breast cancer events at an earlier stage. We examined the association between surveillance mammography beyond 5 years after diagnosis on breast cancer specific-mortality in a cohort of women aged ≥65 years diagnosed 1990-1994 with early stage breast cancer.
Methods
Our cohort included women who survived disease-free for ≥5 years (N=1,235) and were followed from year six through death, disenrollment, or 15 years after diagnosis. Asymptomatic surveillance mammograms were ascertained through medical record review. We used Cox proportional hazards regression stratified by follow-up year to calculate the association between time-varying surveillance mammography and breast cancer-specific and other-than-breast mortality adjusting for site, stage, primary surgery type, age and time-varying Charlson Comorbidity Index.
Results
The majority (85%) of the 1235 five-year breast cancer survivors received ≥1 surveillance mammogram in years 5–9 (yearly proportions ranged from 48–58%); 82% of women received ≥1 surveillance mammogram in years 10-14. A total of 120 women died of breast cancer and 393 women died from other causes (average follow-up 7.3 years). Multivariable models and lasagna plots suggested a modest reduction in breast cancer-specific mortality with surveillance mammogram receipt in the preceding year (IRR 0.82, 95%CI 0.56-1.19, p=0.29); the association with other-cause mortality was 0.95 (95%CI 0.78-1.17, p=0.64).
Conclusions
Among older breast cancer survivors, surveillance mammography may reduce breast-cancer specific mortality even after five years of disease free survival. Continuing surveillance mammography in older breast cancer survivors likely requires physician-patient discussions similar to those recommended for screening, taking into account comorbid conditions and life-expectancy.
Keywords: Surveillance mammography, breast carcinoma, survivorship
Background
The relative 5-year survival for female breast cancer patients 75 years of age or older is 88% [1, 2]. Older breast cancer survivors have a long period of time when they are at risk for recurrences, second primaries and late treatment effects because a healthy 75-year-old woman has a life expectancy of 17 years [3, 4]. Surveillance mammography following initial breast cancer treatment in women with early stage breast cancer is intended to prolong survival through the early detection of recurrences and second breast cancer primaries, with the goal of reducing their morbidity and management with the ultimate goal of reducing death from breast cancer. As with all studies on screening, there are major concerns about intermediate endpoints such as recurrence or second primary cancers, because of issues around lead and length-time bias [5]. For surveillance mammography to be successful for mortality reduction, women would need to be detected during the preclinical detectable phase.
Current surveillance guidelines [6-8] recommend annual mammography after completing primary surgery and adjuvant chemotherapy, which are analogous to guidelines for screening mammography in high risk women. Recommendations for when to stop surveillance for women with a personal history of breast cancer do not exist for women of any age, but older women are less likely to receive surveillance, independent of comorbidity and their providers’ clinical specialties [9-14]. Studies focused on the first 5 years following diagnosis have identified older women as being at risk for less than guideline surveillance receipt, independent of comorbidity and the type of providers seen [9-14]. There has been only limited examination of the potential consequences of this under-surveillance on mortality rates [15], leaving optimal surveillance management of long-term older survivors an open question [8].
We previously reported guideline surveillance mammography in the first 5 years following an early stage breast cancer diagnosis in older women was associated with reduced breast-cancer specific mortality risk, with protective effects primarily restricted to women with local recurrences or second primaries [15]. We are unaware of any studies that have examined surveillance mammography patterns beyond 5 years, nor whether such guideline care is effective in decreasing breast cancer mortality. This current analysis extends prior work [15] by lengthening follow-up to 15 years among 5-year survivors. Using a well-described cohort of women aged ≥65 years [13, 15-19] who had a minimum of 5 years of disease-free survival following an early stage breast cancer diagnosis, we investigated the association between surveillance mammography in years 6-14 post-diagnosis and mortality (breast and other-than-breast cancer).
METHODS
Design and study population
The Breast Cancer Treatment Effectiveness in Older Women (BOW) study was conducted within the Cancer Research Network (CRN) [20] and has been previously described in detail [13, 15-19]. The inception cohort included 1859 women aged ≥65 diagnosed with incident early stage breast cancer (stage I-II)] [21] between 1990 and 1994 within 6 health care organizations; all women received surgical therapy for their initial breast cancer diagnosis.
This study included women who survived disease-free for ≥5 years from initial diagnosis (N=1,235).
Data collection
Our study received a waiver of consent and was approved by institutional review boards at all participating sites, all of whom have assurance filed with and approved by the Department of Health and Human Services. Medical records were used to collect surveillance mammography, demographics, treatment, longitudinal comorbid conditions (to calculate Charlson Comorbidity Indices) [22], recurrence and second primary breast cancers [17]. Once a woman was diagnosed with a comorbid condition, the condition remained present through the end of follow-up. Date and cause of death were ascertained from the National Death Index through December 31, 2008 [15].
Variable definitions
Cause of death was determined by International Classification Diagnosis-9 and 10 codes from part 1 of the death certificate. Women were classified as having died of breast cancer (ICD-9 174 or ICD-10 code C50) or from another cause (all other codes).
Asymptomatic mammography receipt (”surveillance”) was evaluated from medical record review and was coded for each year of follow-up as “received” or “not received” in the preceding year; women could therefore change surveillance mammography status in each annual follow-up cycle. Reasons for mammograms coded as an: evaluation of a clinical finding, evaluation of a reported symptom without a clinical finding, work-up of abnormal finding, evaluation of breast after biopsy/surgery, or unknown were not counted as a surveillance mammogram [15, 23-25]. Surveillance mammograms had to be ≥9 months from the previously classified surveillance mammogram and mammograms were not counted after a diagnosis of a breast cancer recurrence or second primary [15, 23-25]. Women were classified as “exposed” in years 6-15 if they had a surveillance mammogram in years 5-14, respectively. We did not include surveillance mammograms in the 6 months before death [15].
We examined potential confounding variables known to be associated with mortality including age at the sixth year after breast cancer diagnosis (70-74, 75-79, or ≥80 years), Charlson Comorbidity Index [22] in the preceding year (0, 1, >1), tumor stage (I or II) [21], primary surgery type (mastectomy or breast cancer surgery), enrollment site diagnosis year, race/ethnicity (White non-Hispanic, Asian, African-American, and Hispanic), Charlson Comorbidity Index 5 years after breast cancer diagnosis, estrogen (ER) and progesterone receptor (PR) expression at diagnosis (ER- or PR-positive/ER- and PR-negative), completed adjuvant radiation therapy, systemic adjuvant therapy (none/tamoxifen/chemotherapy/both), and receipt of a surveillance mammogram 4-5 years after breast cancer diagnosis.
Statistical Analysis
We calculated the frequency and proportion of women who did, and did not, receive surveillance mammograms in the previous 12 months for the overall analytic sample within strata of covariate categories. Surveillance mammograms received in years 10–14 were collapsed due to small numbers. Frequency of death (due to breast cancer or causes other than breast cancer) and cumulative person-time were used to calculate the incidence rate of death.
To summarize patterns of surveillance mammography across the 1,235 women who survived 5-years after diagnosis, we used lasagna plots [26, 27] to graphically illustrate surveillance mammography receive over 10-years of follow-up (years 6 through 15 after diagnosis). The plots are sorted by breast-cancer specific mortality, other than breast cancer specific mortality and no mortality. The columns (x-axis) represent the prior 12-month exposure period (years 5 through 14 after diagnosis) for each follow-up year. Each plot includes a row (y-axis) for each woman included in the analysis. The number of surveillance mammograms ranged from a minimum of 0 (no surveillance mammograms) to a maximum of 10 (one surveillance mammogram in each year of follow-up). As the number of surveillance mammograms increase, the shades of blue become more intense to represent increasing density (i.e., few mammograms are represented by lighter shades and the more cumulative mammograms are represented by darker shades). The areas of white color indicate that the woman's follow-up time was censored due to death or disenrollment.
Women were followed from beginning of their sixth year after their breast cancer diagnosis date until death, disenrollment, or end of follow-up at 15 years after diagnosis, whichever came first. Hazard ratios (HR) and 95% confidence intervals (CI) for the association between surveillance mammography and breast cancer-specific mortality were estimated using Cox proportional hazard regression models, with duration of follow-up as the time scale. Cox proportional hazards regression analyses incorporated the Andersen-Gill data structure [28] to account for the time-dependent nature of surveillance mammography. We also calculated the association between surveillance mammography and mortality due to causes other than breast cancer. Models were stratified by follow-up cycle (1-year intervals) and also adjusted for site, age at year 6 after diagnosis, tumor stage, primary surgery type, and Charlson Comorbidity Index (treated as a time-dependent variable). The breast cancer mortality analysis censored women who died of other causes and the other-than-breast cancer mortality analysis censored women who died of breast cancer.
To address surveillance effectiveness, we examined surveillance mammography receipt in the prior year by stage distribution for recurrences (local/regional/distant/missing) and second primaries (stage I/II, III/IV, unstaged) and for women who did not experience a second breast event. For women without a second breast event, we used the median time to event for women with a second breast event (date of initial diagnosis+5 years+667.75 days) in years 6–10 as the time point from which to look back one year for receipt of surveillance exam; the same approach was used for years 11–15 (date of diagnosis+10 years+640 days).
RESULTS
Our prospective cohort comprised 1235 women who experienced no second breast cancer events in the 5 years after an initial breast cancer diagnosis, who contributed a total of 9013 person-years during 15 follow-up years. There were 120 women who died of breast cancer and 393 women died from another cause.
One-quarter of the cohort was aged ≥80 years six years after diagnosis, 34% aged 75–79, and 42% aged 70–74 (Table 1). Women aged 70–74 at the outset of follow-up comprised a greater proportion of the remaining cohort, because these women had the lowest overall mortality rate. Similarly, the proportion of women remaining with a Charlson score of 0 at the outset of follow-up increased over the follow-up period. There were no notable differences in stage, hormone receptor status, or primary and adjuvant therapy in the cohort over time.
Table 1.
Distribution of covariates by surveillance mammography receipt over the study period
| Receipt of surveillance mammogram by year following diagnosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year 6 | Year 7 | Year 8 | Year 9 | Year 10-14 | ||||||
| N=721 | N=514 | N=626 | N=522 | N=573 | N=508 | N=479 | N=512 | N=575 | N=129* | |
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| N (col %) | ||||||||||
| Diagnosis Year | ||||||||||
| 1990 | 131 (18) | 87 (17) | 116 (19) | 88 (17) | 111 (19) | 79 (16) | 94 (20) | 78 (15) | 100 (17) | 22 (17) |
| 1991 | 129 (18) | 84 (16) | 110 (18) | 88 (17) | 93 (16) | 97 (19) | 78 (16) | 101 (20) | 99 (17) | 20 (16) |
| 1992 | 154 (21) | 109 (21) | 116 (19) | 129 (25) | 111 (19) | 117 (23) | 98 (20) | 114 (22) | 124 (22) | 31 (24) |
| 1993 | 165 (23) | 120 (23) | 143 (23) | 117 (22) | 133 (23) | 108 (21) | 106 (22) | 114 (22) | 137 (24) | 30 (23) |
| 1994 | 142 (20) | 114 (22) | 141 (23) | 100 (19) | 125 (22) | 107 (21) | 103 (22) | 104 (21) | 115 (20) | 26 (20) |
| Age at year 6 | ||||||||||
| 70-74 | 302 (42) | 154 (30) | 264 (42) | 170 (33) | 259 (45) | 158 (31) | 232 (48) | 161 (31) | 274 (48) | 45 (35) |
| 75-79 | 242 (34) | 141 (27) | 215 (34) | 150 (29) | 210 (37) | 130 (26) | 167 (35) | 152 (30) | 206 (36) | 45 (35) |
| 80+ | 177 (25) | 219 (43) | 147 (23) | 202 (39) | 104 (18) | 220 (43) | 80 (17) | 199 (39) | 95 (17) | 39 (30) |
| Race/ethnicity | ||||||||||
| White non-Hispanic | 600 (83) | 407 (79) | 513 (82) | 421 (81) | 466 (81) | 410 (81) | 395 (82) | 400 (78) | 462 (80) | 96 (74) |
| Asian | 23 (3.2) | 9 (1.8) | 22 (3.5) | 10 (1.9) | 23 (4.0) | 9 (1.8) | 19 (4.0) | 11 (2.2) | 23 (4.0) | 4 (3.1) |
| African-American | 61 (8.5) | 64 (12) | 60 (9.6) | 56 (11) | 55 (9.6) | 54 (11) | 40 (8.4) | 66 (13) | 57 (9.9) | 15 (12) |
| Hispanic | 37 (5.1) | 33 (6.4) | 31 (5.0) | 35 (6.7) | 29 (5.1) | 35 (6.9) | 25 (5.2) | 35 (6.8) | 33 (5.7) | 14 (11) |
| Charlson Comorbidity Index at 5 yrs post-diagnosis | ||||||||||
| 0 | 423 (59) | 237 (46) | 365 (58) | 264 (51) | 341 (60) | 265 (52) | 311 (65) | 259 (51) | 363 (63) | 76 (59) |
| 1 | 244 (34) | 201 (39) | 214 (34) | 198 (38) | 191 (33) | 191 (38) | 141 (29) | 206 (40) | 183 (32) | 43 (33) |
| >1 | 54 (7.5) | 76 (15) | 47 (7.5) | 60 (11) | 41 (7.1) | 52 (10) | 27 (5.6) | 47 (9.2) | 29 (5.1) | 10 (7.6) |
| Tumor stage at diagnosis | ||||||||||
| I | 438 (61) | 309 (60) | 378 (60) | 321 (61) | 360 (63) | 299 (59) | 301 (63) | 314 (61) | 367 (64) | 78 (60) |
| IIA | 216 (30) | 153 (30) | 201 (32) | 139 (27) | 163 (28) | 160 (32) | 131 (27) | 154 (30) | 160 (28) | 36 (28) |
| IIB | 67 (9.3) | 52 (10) | 47 (7.5) | 62 (12) | 50 (8.7) | 49 (9.7) | 47 (9.8) | 44 (8.6) | 48 (8.4) | 15 (12) |
| ER/PR status | ||||||||||
| ER or PR + | 556 (77) | 380 (74) | 477 (76) | 394 (75) | 446 (78) | 373 (73) | 382 (80) | 377 (74) | 439 (76) | 97 (75) |
| ER/PR- | 87 (12) | 66 (13) | 80 (13) | 63 (12) | 70 (12) | 64 (13) | 53 (11) | 66 (13) | 77 (13) | 15 (12) |
| unknown | 78 (11) | 68 (13) | 89 (11) | 65 (12) | 57 (10) | 71 (14) | 44 (9.2) | 69 (13) | 59 (10) | 17 (13) |
| Primary surgery | ||||||||||
| BCS | 364 (50) | 239 (47) | 324 (52) | 238 (46) | 287 (50) | 243 (48) | 240 (50) | 252 (49) | 290 (50) | 58 (45) |
| Mastectomy | 357 (50) | 275 (54) | 302 (48) | 284 (54) | 286 (50) | 265 (52) | 239 (50) | 260 (51) | 285 (50) | 71 (55) |
| Radiation therapy complete | ||||||||||
| Yes | 333 (46) | 182 (35) | 298 (48) | 191 (37) | 279 (49) | 185 (36) | 232 (48) | 203 (40) | 280 (49) | 46 (36) |
| No | 388 (54) | 332 (65) | 328 (52) | 331 (63) | 294 (51) | 323 (64) | 237 (52) | 309 (60) | 295 (51) | 83 (64) |
| Adjuvant therapy | ||||||||||
| Tamoxifen, chemotherapy or both | 508 (70) | 351 (68) | 443 (71) | 363 (70) | 405 (71) | 362 (71) | 337 (70) | 369 (72) | 406 (71) | 87 (67) |
| Neither | 213 (30) | 163 (32) | 183 (29) | 159 (30) | 168 (29) | 146 (29) | 142 (30) | 143 (28) | 169 (29) | 42 (33) |
| Surveillance mammogram in year 4-5 following diagnosis | ||||||||||
| Yes | 555 (77) | 273 (53) | 478 (76) | 314 (60) | 452 (79) | 299 (59) | 381 (80) | 317 (62) | 446 (78) | 70 (54) |
| No | 166 (23) | 241 (47) | 148 (24) | 208 (40) | 121 (21) | 209 (41) | 98 (20) | 195 (38) | 129 (22) | 59 (46) |
| Number of surveillance mammograms in year 10-14 following diagnosis | ||||||||||
| 1 | - | - | - | - | - | - | - | - | 156 (27) | - |
| 2 | - | - | - | - | - | - | - | - | 103 (18) | - |
| 3 | - | - | - | - | - | - | - | - | 106 (18) | - |
| 4 | - | - | - | - | - | - | - | - | 121 (21) | - |
| 5 | - | - | - | - | - | - | - | - | 89 (15) | - |
The proportion receiving a surveillance mammogram decreased with each year, from 58% in Year 6 to 48% in year 9. In years 10-14, 82% of the remaining cohort (n=704) had ≥1 surveillance mammogram in any of the 5 years with roughly an equal proportion having 1-5 surveillance mammograms; median number of surveillance exams received in those years was 3 (range 0-10).
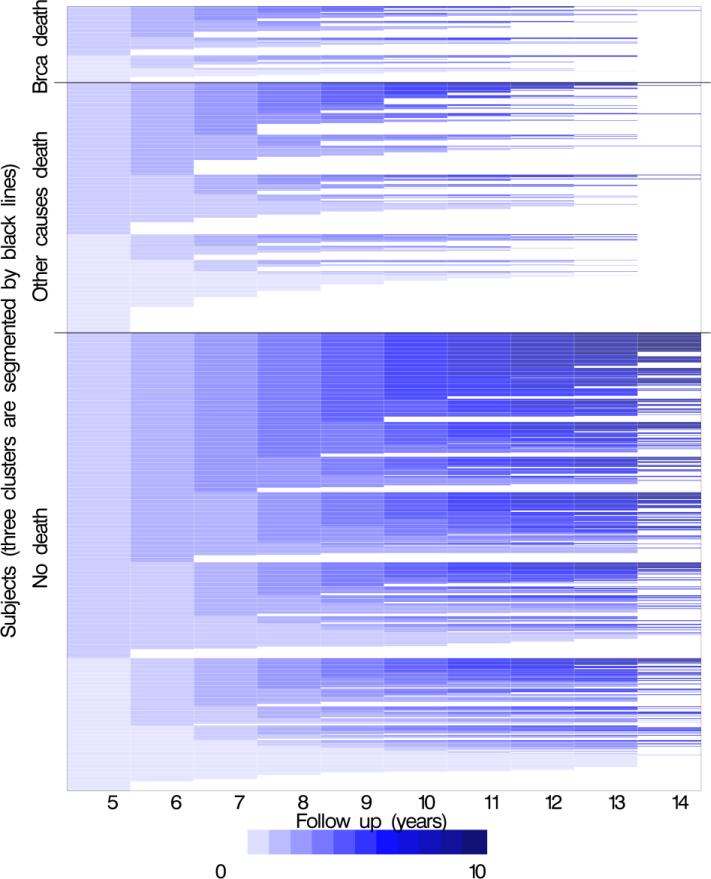
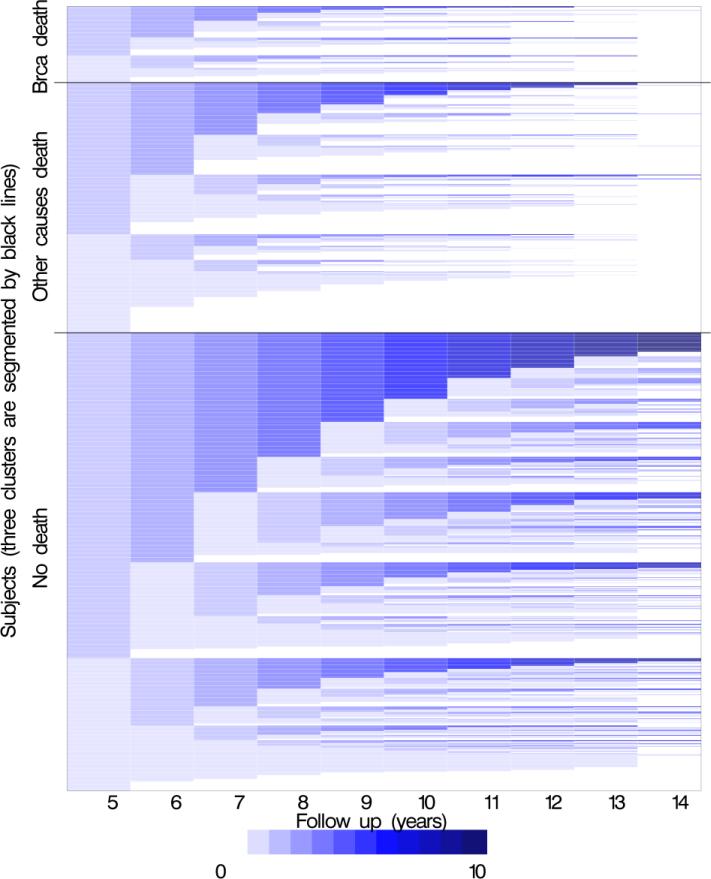
Most women survived the 10-year follow-up period (years 6-15 after diagnosis), and more women died from other-than breast cancer mortality than from breast cancer (Figures 1a/1b).
Figure 1.
Each woman was classified as having a surveillance mammogram (collected from medical record review and defined as asymptomatic, ≥9 months from previous mammogram) in follow-up year with adherence to surveillance able to change within each cycle. Surveillance mammograms were not counted in any follow-up period after a diagnosis of a recurrence or a second primary. Women could die between years 6–15, therefore to assess whether women were “exposed” to having a surveillance mammogram in the 12 months prior we look at receipt of surveillance mammograms in years 5–14. For the year 6 follow-up year, surveillance mammography would have had to been received in the 12 months prior (i.e. during year 5) so the plots show receipt of surveillance mammography in year 5-14 to correspond with the follow-up year of 6-15. The columns (x-axis) represent the prior 12-month exposure period (years 5 through 14 after diagnosis) for each follow-up year. Each plot includes a row (y-axis) for each woman included in the analysis. The number of surveillance mammograms ranged from a minimum of 0 (no surveillance mammograms) to a maximum of 10 (one surveillance mammogram in each year of follow-up). White segments of the graph represent censoring women at the end of observation (death or disenrollment). A total of 1,235 women are included with 9,015 person-years, including 120 women who died of breast cancer and 393 women who died of other causes of death.
*Figure 1a This plot depicts guideline adherence to surveillance mammography over follow-up by outcome (breast cancer specific mortality, other than breast cancer mortality and no mortality). Women with greater adherence are shown with accumulating darkness with each surveillance mammogram in a period. If a women skipped a surveillance mammogram in one period (e.g., year 7) but received a surveillance mammograms in the next period (e.g., year 8), the color would pick up with increasing darkness where it left off at year 5. The greater the proportion of women with lighter colors, the greater the proportion of non-adherence. The white portions represent the time women were censored.
*Figure 1b This plot depicts a proxy for surveillance clinical effectiveness over follow-up by outcome. In this figure, the surveillance mammography receipt is reset to the lightest shade in any year a woman did not receive a surveillance mammogram; whereas the color accumulates with receipt of each sequential surveillance mammogram. This strategy attempts to define a period during which a surveillance mammogram might be clinically effective for reducing breast cancer mortality and sets that period at annual intervals. The white portions represent the time women were censored.
Figure 1a depicts accumulating darkness for each additional surveillance mammogram, which provides a graphical overview of guideline adherence to annual mammography. Although annual surveillance mammography was obtained throughout the follow-up period in all groups, overall adherence was suboptimal. Receipt of one surveillance mammogram predicted receipt of subsequent surveillance mammograms throughout the follow-up period. Among women who had a surveillance mammogram in year 5, 77% also had a mammogram in year 6, whereas only 23% of those who did not have a surveillance mammogram in year 5 had a surveillance mammogram in year 6.
Figure 1b is designed to graphically depict surveillance mammography clinical effectiveness. In this figure, each surveillance examination is assumed to have a limited duration of clinical effectiveness (one year) and therefore surveillance mammography receipt is reset to the lightest shade in any year a woman did not receive a surveillance mammogram; whereas the color accumulates with receipt of each sequential surveillance mammogram. The color density pattern shows regular surveillance mammography was most common among the women who survived the entire follow-up period and least common among the women who died of breast cancer, with women who died of other-than-breast cancer displaying an intermediate color density.
Table 2 provides IRRs for breast cancer mortality and other-than-breast-cancer mortality associated with categories of all covariates and for time-varying surveillance mammography, and also provides multivariable adjusted mortality hazard ratios. Both mortality endpoints increased with age, with the oldest age more strongly associated with other-than breast cancer mortality (IRR 3.44, 95%CI 2.66-4.44) than breast cancer-specific mortality (IRR 2.09, 95%CI 1.34-3.26). Similarly, mortality was strongly associated with the time-varying Charlson Index (IRR 4.27, 95%CI 3.19-5.70 for other cause comparing >1 with 0, and IRR 1.94, 95%CI 1.19-3.15 for breast cancer-specific mortality). Higher stage was associated with breast cancer mortality even among these 5-year survivors (IRR 2.13 stage II versus I, 95%CI 1.45-3.14), but not with other-than breast cancer mortality (IRR 1.11, 95%CI 0.89-1.38).
Table 2.
Overall distribution of covariates and striated by breast cancer specific mortality and other cause mortality. Multivariable adjusted relationship between time-varying surveillance mammography and death
| Total | Breast cancer specific mortality | Other cause mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Follow-up Person-years (per 1,000) | Cases | IR | HRa (95% CI) | Cases | IR | HRa (95% CI) | |
| Overall | 1,235 | 9,015 | 120 | 13.3 | 393 | 43.6 | ||
| Time-varying surveillance mammography | 5,140 | 54 | 10.5 | 0.82 (0.56, 1.19) | 185 | 36.0 | 0.95 (0.78, 1.17) | |
| Age at year 6 | ||||||||
| 70-74 | 456 (37) | 3,804 | 41 | 10.8 | Reference | 92 | 24.2 | Reference |
| 75-79 | 383 (31) | 3,017 | 34 | 11.3 | 1.12 (0.71, 1.77) | 97 | 32.2 | 1.34 (1.01, 1.79) |
| 80+ | 396 (32) | 2,194 | 45 | 20.5 | 2.09 (1.34, 3.26) | 204 | 93.1 | 3.44 (2.66, 4.44) |
| Time-varying Charlson Comorbidity Index | ||||||||
| 0 | 3,882 | 37 | 9.5 | Reference | 72 | 18.6 | Reference | |
| 1 | 3,486 | 50 | 14.3 | 1.44 (0.94, 2.22) | 172 | 49.3 | 2.35 (1.78, 3.10) | |
| >1 | 1,647 | 33 | 20.0 | 1.94 (1.19, 3.15) | 149 | 90.5 | 4.27 (3.19, 5.70) | |
| Tumor stage at diagnosis | ||||||||
| I | 747 (60) | 5,574 | 50 | 9.0 | Reference | 250 | 44.9 | Reference |
| IIA | 369 (30) | 2,613 | 43 | 16.5 | 2.13 (1.45, 3.14) | 115 | 44.0 | 1.11 (0.89, 1.38) |
| IIB | 119 (9.6) | 827 | 27 | 32.6 | 28 | 33.8 | ||
| Primary surgery | ||||||||
| BCS | 603 (49) | 4,430 | 49 | 11.1 | 0.96 (0.65, 1.41) | 194 | 43.8 | 0.99 (0.80, 1.22) |
| Mastectomy | 632 (51) | 4,585 | 71 | 15.5 | Reference | 199 | 43.4 | Reference |
| Year of diagnosis | ||||||||
| 1990 | 218 (18) | 1,559 | 17 | 10.9 | 81 | 52.0 | ||
| 1991 | 213 (17) | 1,547 | 24 | 15.5 | 67 | 43.3 | ||
| 1992 | 263 (21) | 1,967 | 22 | 11.2 | 89 | 45.2 | ||
| 1993 | 285 (23) | 2,067 | 27 | 13.1 | 87 | 42.1 | ||
| 1994 | 256 (21) | 1,874 | 30 | 16.0 | 69 | 36.8 | ||
| Race/ethnicity | ||||||||
| White non-Hispanic | 1007 (82) | 7,214 | 101 | 14.0 | 317 | 44.0 | ||
| Asian | 32 (2.6) | 306 | 1 | 3.3 | 9 | 29.4 | ||
| African-American | 125 (10) | 943 | 12 | 12.7 | 44 | 46.7 | ||
| Hispanic | 70 (5.7) | 552 | 6 | 10.9 | 23 | 41.7 | ||
| Other/unknown | 1 (0.1) | 0 | 0 | - | 0 | - | ||
| Charlson Comorbidity 5 year post-index | ||||||||
| 0 | 660 (53) | 5,336 | 60 | 11.2 | 149 | 27.9 | ||
| 1 | 445 (36) | 3,015 | 45 | 14.9 | 178 | 59.1 | ||
| >1 | 130 (11) | 664 | 15 | 22.6 | 66 | 99.4 | ||
| ER/PR status | ||||||||
| ER or PR + | 936 (76) | 6,877 | 96 | 14.3 | 299 | 43.5 | ||
| ER/PR- | 153 (12) | 1,123 | 16 | 14.0 | 44 | 39.2 | ||
| unknown | 146 (12) | 1,015 | 8 | 7.9 | 50 | 49.3 | ||
| Radiation therapy complete | ||||||||
| Yes | 515 (42) | 4,004 | 37 | 9.2 | 139 | 34.7 | ||
| No | 720 (58) | 5,011 | 83 | 16.6 | 254 | 50.7 | ||
| Adjuvant therapy | ||||||||
| Tamoxifen, chemotherapy or both | 859 (70) | 6,353 | 102 | 16.1 | 263 | 41.4 | ||
| Neither | 376 (30) | 2,662 | 18 | 6.8 | 130 | 48.9 | ||
| Months since last surveillance mammogram at 5 years post-diagnosis | ||||||||
| Median (Min, Max) | 9.7 (0.01-60.9) | 10.0 (0.01- 60.9) | 10.5 (0.04-60.9) | |||||
| Surveillance mammogram in year 4-5 following diagnosis | ||||||||
| Yes | 828 (67) | 6,402 | 77 | 12.0 | 239 | 37.3 | ||
| No | 407 (33) | 2,613 | 43 | 16.5 | 154 | 59.0 | ||
| Number of surveillance mammograms in years 6+ following diagnosis | ||||||||
| 0 | 272 (22) | 1,095 | 38 | 34.7 | 138 | 126.0 | ||
| 1 | 171 (14) | 822 | 29 | 35.3 | 83 | 101.0 | ||
| 2 | 161 (13) | 992 | 22 | 22.2 | 70 | 70.6 | ||
| 3 | 109 (8.8) | 892 | 12 | 13.5 | 29 | 32.5 | ||
| 4 | 105 (8.5) | 893 | 8 | 9.0 | 27 | 30.2 | ||
| 5 | 79 (6.4) | 736 | 3 | 4.1 | 20 | 27.2 | ||
| 6 | 78 (6.3) | 795 | 3 | 3.8 | 10 | 12.6 | ||
| 7 | 88 (7.1) | 917 | 4 | 4.4 | 9 | 9.8 | ||
| 8 | 74 (6.0) | 799 | 1 | 1.3 | 6 | 7.5 | ||
| 9 | 73 (5.9) | 800 | 0 | - | 1 | 1.3 | ||
| 10 | 25 (2.0) | 299 | 0 | - | 0 | - | ||
| Median (Min, Max) | 3 (0-10) | 1 (0-8) | 1 (0-9) | |||||
Stratified by follow-up cycle and adjusted for: site, age at year 6, stage (I/IIA or IIB), primary surgery type, time-dependent Charlson comorbidity
There was a modest reduction in breast cancer-specific mortality with surveillance mammogram receipt in the preceding year (IRR 0.82, 95%CI 0.56-1.19, p=0.29), whereas the association with other-cause mortality was 0.95 (95%CI 0.78-1.17, p=0.64).
Sample sizes were small when examining the surveillance mammography distribution in the year before the second breast cancer event or the reference date for women with no second event by years 10 and 15 (Table 3). There were no systematic patterns in surveillance mammography receipt relative to extent of disease at second diagnosis by years 10 or 15. There was no association between recurrence stage and surveillance mammography receipt among women who remained alive at years 10 and 15 or among women who died of other causes.
Table 3.
Number and Percentage of Women Who Received a Surveillance Mammogram in the Year Preceding a Second Breast Cancer Event (by stage) and a Comparable Reference Datea for women without a recurrence or second primary stratified by women who died of breast cancer, had no death and who died of other-than breast cancer at 10 Years and 15 years
| 10 Year Outcomes | 15 Year Outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Death | Death | |||||||||||
| Event type | Alive | Breast Cancer | Other than breast-cancer | Alive | Breast Cancer | Other than breast-cancer | ||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Recurrence | ||||||||||||
| Local | 7 / 15 | 47 | 1 / 1 | 100 | 1 / 3 | 33 | 7 / 11 | 64 | 1 / 2 | 50 | 0 / 1 | 0 |
| Regional | 7 / 8 | 88 | 1 / 2 | 50 | - | - | 2 / 3 | 67 | 3 / 4 | 75 | - | - |
| Distant | 10 / 19 | 53 | 14 / 20 | 70 | 7 / 10 | 70 | 4 / 6 | 67 | 2 / 7 | 29 | 1 / 2 | 50 |
| Missing | 1 / 1 | 100 | ||||||||||
| Second Primary | ||||||||||||
| Stage I or II | 13 / 19 | 68 | - | - | 2 / 2 | 100 | 11 / 15 | 73 | - | - | - | - |
| Stage III or IV | - | - | - | - | - | - | - | - | - | - | - | - |
| Unstaged | 1 / 2 | 0 | 1 / 1 | 100 | 1 / 1 | 100 | 2 / 2 | 100 | 1 / 1 | 100 | 1 / 1 | 100 |
| Disease-freea | 519 / 878 | 59 | 13 / 42 | 31 | 62 / 212 | 29 | 269 / 642 | 42 | 3/ 40 | 8 | 31 / 161 | 19 |
For women without a second breast event, we used the median time to event for women with a second breast event (date of initial diagnosis+5 years+667.75 days) in years 6–10 as the time point from which to look back one year for receipt of surveillance exam; the same approach was used for years 11–15 (date of diagnosis+10 years+640 days).
DISCUSSION
In this cohort of older women who had survived disease free for 5 years after an early stage invasive breast cancer diagnosis, we observed a modest breast cancer-specific mortality reduction associated with surveillance mammography receipt among older women as far out as 6-15 years after initial early stage breast cancer diagnosis; mortality reductions were not suggested for other-than breast cancer. Breast cancer survivors are at higher risk for contralateral breast cancer than age-matched women with no breast cancer history [24], so surveillance mammography could be considered equivalent to screening mammography in a high risk population. Our result (IRR 0.82, 95%CI 0.56–1.19) is consistent with the most recent meta-analysis associating screening mammography with a reduced risk of breast cancer mortality (summary RR=0.84, 95% credible interval 0.77–0.91) [29].
Long-term surveillance is particularly challenging for older breast cancer survivors and their health care providers, because competing comorbid causes of death accumulate and the potential survival benefit of an early diagnosis of a recurrence or new primary decreases, making the potential harms [30] from screening weigh more heavily. Current screening mammography guidelines conclude there is insufficient evidence to recommend screening mammography to women aged ≥75 with no prior breast cancer [30]; there are no guidelines on when to stop surveillance for survivors, regardless of age [6, 7]. It is unlikely that a randomized controlled trial will be launched to address tailored surveillance stopping ages, so effectiveness studies must rest on observational studies such as these [15, 31-33] combined with extrapolation of screening mammography efficacy and effectiveness [6, 7].
Surveillance mammograms should decrease breast cancer mortality by detecting second breast cancer events at an earlier stage; surveillance mammograms should not be effective for diagnosing metastatic cancers, or preventing or delaying death from metastatic disease [5]. Our prior analysis during the first 5 years following diagnosis suggested that each additional surveillance mammogram post-diagnosis was associated with a one-third mortality reduction and also suggested reduced mortality could have resulted from detecting recurrences or second primary breast cancer at an earlier stage [15]. Our findings were subsequently criticized as potentially arising from the necessary association between accumulation of both surveillance mammograms and survival time itself [5]. We were aware of this potential, and addressed the concern in the original manuscript, concluding that reduced mortality rates likely resulted from detecting local recurrences or second primary breast cancer at an earlier stage with better prognosis, combined with the effect of better medical care in general, as evidenced by the lower mortality rate from other causes of death that were also associated with receipt of surveillance mammograms [15]. In the present analysis, our use of time-varying methods allowed an estimate of the association between surveillance mammogram receipt in the preceding year and death due to breast cancer or another cause in the current year. This analytic method more closely corresponds to the hypothesized effect implied by the guideline recommendations [6, 7] for annual surveillance mammograms. Our observed association between surveillance mammogram and breast cancer-specific mortality overlaps with our earlier estimate (OR per surveillance mammogram 0.69 for breast cancer mortality, 95%CI:0.52-0.92 [15]), but in years 6-15 we observed a near null association with other-than breast mortality. In this analysis, surveillance mammography did not appear to be associated with detecting local recurrences or second primary breast cancer at earlier stages in years 6-15 (table 3), although these analyses were based on very small numbers of events by stage, so may not reveal the expected pattern. Our earlier estimate was more precisely measured because it was based on the substantially larger initial cohort where surveillance mammograms and deaths due to breast cancer were more common.
The main advantages of our study are completeness and accuracy of surveillance mammograms ascertainment and follow-up over 15 years, homogeneity of access to care in these managed health care organizations, and the evaluation of breast cancer-specific and other-than breast cancer death. We were able to distinguish surveillance mammograms from mammograms ordered in response to symptoms. We were also able to examine guideline adherence to surveillance mammography by outcome as well as a proxy measure of clinical effectiveness of surveillance mammography by outcome through visual plots. The consistency of our findings regarding associations with covariates and with breast cancer-specific mortality (older age, increasing comorbidity and higher stage at initial diagnosis) and non-breast cancer mortality (older age and increasing comorbidity) are supported by other studies and demonstrate the face validity of our data. The large sample size and reliance on existing registries and medical records allowed all women to be included with negligible loss to follow-up, reducing the potential impact of selection biases and selective loss to follow-up. Finally, because all enrolled patients were ≥65 years at breast cancer diagnosis, they all had access to Medicare-financed health care with few financial barriers.
However, women received surveillance mammograms in the context of conventional patient-physician decision making, not by random assignment. An earlier study showed screening mammography prevalence declined among older women with no breast cancer history in direct relation to physician-estimated life-expectancy [33]. Although we had no measure of life-expectancy, we observed here and elsewhere [9, 13] that older patients and patients with comorbid conditions are less likely to receive surveillance mammograms and less-than-guideline care [34]. These patterns suggest that patient or physician preferences may impact surveillance mammogram receipt. We were unable to examine other potential downstream harms of surveillance mammography such as false positives, unnecessary biopsies and other adverse events, which are crucial measures in evaluating the harms and benefits of screening interventions and are likely to be even more important in older women with limited life-expectancies. While age and comorbidity and other factors were controlled analytically, unmeasured variables—such as healthy behaviors and physical function—may confound the relation. Many of the covariates examined were associated with mortality, but these were not associated with surveillance mammography, which is why they were not included in our multivariable model. Were the association between surveillance mammogram receipt and breast cancer mortality due entirely to confounding by healthy indications, we would have expected to see a similarly sized protective association between receipt of surveillance mammograms and death from causes other-than breast cancer.
We observed a modest breast cancer-specific mortality reduction associated with surveillance mammography receipt among older women as far out as 6-15 years after initial early stage breast cancer diagnosis. The decision to continue surveillance mammography in older breast cancer survivors likely requires physician-patient discussions similar to those recommended for screening, taking into account the perceived risks and benefits, comorbid conditions, and future life-expectancy.
Acknowledgements
Supported by a grant from the National Cancer Institute (R01 CA093772 R. Silliman, PI).
The Cancer Research Network is a consortium of 14 integrated health care delivery systems with more than ten million enrollees. The overall goal of the CRN is to assess and increase the effectiveness of preventive, curative and supportive interventions for major cancers through a program of collaborative research among diverse populations and health systems.
We would like to thank site principal investigators Hans Petersen and Meaghan St. Charles from Lovelace Clinic Foundation and Pamala Pawloski from Heath Partners and Hongyuan Gao for her work developing the lasagna plots. We would also like to thank our site project managers, programmers, and medical record abstractors: Group Health-Linda Shultz, Kristin Delaney, Margaret Farrell-Ross, Mary Sunderland, Millie Magner, and Beth Kirlin; Meyers Primary Care Institute and Fallon Clinic-Jackie Fuller, Doris Hoyer, and Janet Guilbert; Henry Ford Health System-Sharon Hensley Alford, Karen Wells, Patricia Baker, and Rita Montague; HealthPartners-Maribet McCarty and Alex Kravchik; Kaiser Permanente Southern California-Julie Stern, Janis Yao, Michelle McGuire, and Erica Hnatek-Mitchell; and Lovelace Health Plan-Judith Hurley, Hans Petersen, and Melissa Roberts.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Surveillance Epidemiology and End Results Relative Survival By Survival Time By Age At Diagnosis/Death Female Breast, All Races. Female. National Cancer Institute; 1988-2008. [August 20]. http://seer.cancer.gov/faststats/selections.php?run=runit&output=2&data=4&statistic=5&cancer=553&year=201204&race=1&sex=3&series=age&age=166#Output. [Google Scholar]
- 2.Reis LAG, Eisner MP. Cancer of the female breast. In: Reis LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner MJ, editors. SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215. Bethesda, MD: 2007. pp. 101–111. [Google Scholar]
- 3.Arias E. United States Life Tables, 2007. National Vital Statistics Reports. 2011;59:1–61. [PubMed] [Google Scholar]
- 4.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285:2750–2756. doi: 10.1001/jama.285.21.2750. doi: jsc00476 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Weiss NS. The analysis of case-control studies of the efficacy of screening for recurrence of cancer. J Clin Epidemiol. 2011;64:41–43. doi: 10.1016/j.jclinepi.2010.07.013. doi: S0895-4356(10)00302-1 [pii] 10.1016/j.jclinepi.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network . National Comprehensive Cancer Network: NCCN Guidelines. National Comprehensive Cancer Network; 2012. [August 26]. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 7.Khatcheressian JL, Wolff AC, Smith TJ, Grunfeld E, Muss HB, Vogel VG, Halberg F, Somerfield MR, Davidson NE, American Society of Clinical Oncology American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–5097. doi: 10.1200/JCO.2006.08.8575. doi: JCO.2006.08.8575 [pii] 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 8.Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, Hantel A, Henry NL, Muss HB, Smith TJ, Vogel VG, Wolff AC, Somerfield MR, Davidson NE. Breast cancer follow-up and management after primary treatment: american society of clinical oncology clinical practice guideline update. J Clin Oncol. 2013;31:961–965. doi: 10.1200/JCO.2012.45.9859. doi: 10.1200/JCO.2012.45.9859 JCO.2012.45.9859 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Lash TL, Silliman RA. Medical surveillance after breast cancer diagnosis. Med Care. 2001;39:945–955. doi: 10.1097/00005650-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Keating NL, Landrum MB, Guadagnoli E, Winer EP, Ayanian JZ. Surveillance testing among survivors of early-stage breast cancer. J Clin Oncol. 2007;25:1074–1081. doi: 10.1200/JCO.2006.08.6876. doi: 25/9/1074 [pii] 10.1200/JCO.2006.08.6876. [DOI] [PubMed] [Google Scholar]
- 11.Doubeni CA, Field TS, Ulcickas Yood M, Rolnick SJ, Quessenberry CP, Fouayzi H, Gurwitz JH, Wei F. Patterns and predictors of mammography utilization among breast cancer survivors. Cancer. 2006;106:2482–2488. doi: 10.1002/cncr.21893. [DOI] [PubMed] [Google Scholar]
- 12.Etim AE, Schellhase KG, Sparapani R, Nattinger AB. Effect of model of care delivery on mammography use among elderly breast cancer survivors. Breast Cancer Res Treat. 2006;96:293–299. doi: 10.1007/s10549-005-9141-4. doi: 10.1007/s10549-005-9141-4. [DOI] [PubMed] [Google Scholar]
- 13.Field TS, Doubeni C, Fox MP, Buist DS, Wei F, Geiger AM, Quinn VP, Lash TL, Prout MN, Yood MU, Frost FJ, Silliman RA. Under utilization of surveillance mammography among older breast cancer survivors. J Gen Intern Med. 2008;23:158–163. doi: 10.1007/s11606-007-0471-2. doi: 10.1007/s11606-007-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schapira MM, McAuliffe TL, Nattinger AB. Underutilization of mammography in older breast cancer survivors. Med Care. 2000;38:281–289. doi: 10.1097/00005650-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Lash TL, Fox MP, Buist DS, Wei F, Field TS, Frost FJ, Geiger AM, Quinn VP, Yood MU, Silliman RA. Mammography Surveillance and Mortality in Older Breast Cancer Survivors. J Clin Oncol. 2007;25:3001–3006. doi: 10.1200/JCO.2006.09.9572. [DOI] [PubMed] [Google Scholar]
- 16.Enger SM, Thwin SS, Buist DS, Field T, Frost F, Geiger AM, Lash TL, Prout M, Yood MU, Wei F, Silliman RA. Breast cancer treatment of older women in integrated health care settings. J Clin Oncol. 2006;24:4377–4383. doi: 10.1200/JCO.2006.06.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geiger AM, Thwin SS, Lash TL, Buist DS, Prout MN, Wei F, Field TS, Ulcickas Yood M, Frost FJ, Enger SM, Silliman RA. Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer. 2007;109:966–974. doi: 10.1002/cncr.22472. [DOI] [PubMed] [Google Scholar]
- 18.Yood MU, Owusu C, Buist DS, Geiger AM, Field TS, Thwin SS, Lash TL, Prout MN, Wei F, Quinn VP, Frost FJ, Silliman RA. Mortality impact of less-than-standard therapy in older breast cancer patients. J Am Coll Surg. 2008;206:66–75. doi: 10.1016/j.jamcollsurg.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Buist DS, Chubak J, Prout M, Yood MU, Bosco JL, Thwin SS, Gold HT, Owusu C, Field TS, Quinn VP, Wei F, Silliman RA. Referral, receipt, and completion of chemotherapy in patients with early-stage breast cancer older than 65 years and at high risk of breast cancer recurrence. J Clin Oncol. 2009;27:4508–4514. doi: 10.1200/JCO.2008.18.3459. doi: JCO.2008.18.3459 [pii] 10.1200/JCO.2008.18.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner EH, Greene SM, Hart G, Field TS, Fletcher S, Geiger AM, Herrinton LJ, Hornbrook MC, Johnson CC, Mouchawar J, Rolnick SJ, Stevens VJ, Taplin SH, Tolsma D, Vogt TM. Building a research consortium of large health systems: the Cancer Research Network. J Natl Cancer Inst Monogr. 2005:3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 21.Fleming ID, Cooper JS, Henson DE, Hutter RVP, Kennedy BJ, Murphy GP, et al. AJCC Cancer Staging Manual. Lippincott Williams & Wilkins; Philadelphia, PA: 1997. [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Houssami N, Abraham LA, Kerlikowske K, Buist DS, Irwig L, Lee J, Miglioretti DL. Risk factors for second screen-detected or interval breast cancers in women with a personal history of breast cancer participating in mammography screening. Cancer Epidemiol Biomarkers Prev. 2013;22:946–961. doi: 10.1158/1055-9965.EPI-12-1208-T. doi: 10.1158/1055-9965.EPI-12-1208-T1055-9965.EPI-12-1208-T [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houssami N, Abraham LA, Miglioretti DL, Sickles EA, Kerlikowske K, Buist DS, Geller BM, Muss HB, Irwig L. Accuracy and outcomes of screening mammography in women with a personal history of early-stage breast cancer. JAMA. 2011;305:790–799. doi: 10.1001/jama.2011.188. doi: 305/8/790 [pii] 10.1001/jama.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buist DS, Abraham LA, Barlow WE, Krishnaraj A, Holdridge RC, Sickles EA, Carney PA, Kerlikowske K, Geller BM, For the Breast Cancer Surveillance C Diagnosis of second breast cancer events after initial diagnosis of early stage breast cancer. Breast Cancer Res Treat. 2010;124:863–873. doi: 10.1007/s10549-010-1106-6. doi: 10.1007/s10549-010-1106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swihart BJ, Caffo B, James BD, Strand M, Schwartz BS, Punjabi NM. Lasagna plots: a saucy alternative to spaghetti plots. Epidemiology. 2010;21:621–625. doi: 10.1097/EDE.0b013e3181e5b06a. doi: 10.1097/EDE.0b013e3181e5b06a 00001648-201009000-00015 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao H, Buist DS, Lash TL, Bosco JL, Swihart B. Lasagna plots made in different (statistical) ovens. Epidemiology. 2012;23:934. doi: 10.1097/EDE.0b013e31826d08c7. doi: 10.1097/EDE.0b013e31826d08c7 00001648-201211000-00033 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. Springer-Verlag; New York: 2000. [Google Scholar]
- 29.Humphrey L, Chan BKS, Detlefsen S, Helfand M. Systematic Evidence Reviews. Database Agency for Healthcare Research and Quality; 2002. [March 3, 2013]. Screening for breast cancer [internet]. http://www.ncbi.nlm.nih.gov/books/NBK42742/. [PubMed] [Google Scholar]
- 30.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan B, Nygren P, L. H . Screening for Breast Cancer: Systematic Evidence Review Update for the U.S. Preventive Services Task Force. Evidence Review Update No. 74. Rockville, MD: 2009. [PubMed] [Google Scholar]
- 31.Lash TL, Clough-Gorr K, Silliman RA. Reduced rates of cancer-related worries and mortality associated with guideline surveillance after breast cancer therapy. Breast Cancer Res Treat. 2005;89:61–67. doi: 10.1007/s10549-004-1472-z. doi: 10.1007/s10549-004-1472-z. [DOI] [PubMed] [Google Scholar]
- 32.Grunfeld E, Noorani H, McGahan L, Paszat L, Coyle D, van Walraven C, Joyce J, Sawka C. Surveillance mammography after treatment of primary breast cancer: a systematic review. Breast. 2002;11:228–235. doi: 10.1054/brst.2001.0404. [DOI] [PubMed] [Google Scholar]
- 33.Koya DL, Chen JG, Smith TG, Moran WP. Screening mammography use in Medicare beneficiaries reflects 4-year mortality risk. Am J Med. 2011;124:369, e361–368. doi: 10.1016/j.amjmed.2010.11.019. doi: S0002-9343(10)01054-5 [pii] 10.1016/j.amjmed.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Field TS, Bosco JL, Prout MN, Gold HT, Cutrona S, Pawloski PA, Ulcickas Yood M, Quinn VP, Thwin SS, Silliman RA. Age, comorbidity, and breast cancer severity: impact on receipt of definitive local therapy and rate of recurrence among older women with early-stage breast cancer. J Am Coll Surg. 2011;213:757–765. doi: 10.1016/j.jamcollsurg.2011.09.010. doi: S1072-7515(11)01106-9 [pii] 10.1016/j.jamcollsurg.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]