Abstract
Protein hydrolysates were produced from shrimp waste mainly comprising head and shell of Penaeus monodon by enzymatic hydrolysis for 90 min using four microbial proteases (Alcalase, Neutrase, Protamex, Flavourzyme) where PR(%) and DH (%) of respective enzymes were compared to select best of the lot. Alcalase, which showed the best result, was used to optimize hydrolysis conditions for shrimp waste hydrolysis by response surface methodology using a central composite design. A model equation was proposed to determine effects of temperature, pH, enzyme/substrate ratio and time on DH where optimum values found to be 59.37 °C, 8.25, 1.84% and 84.42 min. for maximum degree of hydrolysis 33.13% respectively. The model showed a good fit in experimental data because 92.13% of the variability within the range of values studied could be explained by it. The protein hydrolysate obtained contained high protein content (72.3%) and amino acid (529.93 mg/gm) of which essential amino acid and flavour amino acid were was 54.67–55.93% and 39.27–38.32% respectively. Protein efficiency ratio (PER) (2.99) and chemical score (1.05) of hydrolysate was suitable enough to recommend as a functional food additive.
Keywords: Protein hydrolysate, Enzymatic hydrolysis, Protein recovery, Degree of hydrolysis, Shrimp waste, Alcalase
Introduction
Over the past two decades the shellfish processing industry has experienced a significant expansion and in the year 08-09’ 280872 tonnes tonnes of shrimp used for production of frozen products of which it has been estimated that nearly 186368 tonnes is waste generated from processing plants in India (MPEDA 2009). About 35–45% by weight of shrimp raw material is discarded as waste depending on the species and processing method applied (INFOFISH 1991). With increasing competition on world markets there is a need to develop value-added products from the waste material to help maintain the economic viability of the industry as well as reduce environmental pollution (Gildberg and Stenberg 2001) Shrimp head and shell generally contain good percentage of protein with balanced amino acid profile and minerals like Ca, P, Na and Zn (Ibrahim et al. 1999). Recovery of protein fraction from the shrimp waste by enzymatic hydrolysis has been widely studied (Synowiecki and Alkhateeb 2000; Mizani et al. 2005) which has advantages since accelerated hydrolysis allows for control of hydrolysis and thus minimizes undesirable reactions. Protein digesting enzymes breakdown protein in smaller peptide, making hydrolysates most available amino acid source for protein biosynthesis (Gildberg and Stenberg 2001). Enzymatic hydrolysis modify physicochemical ,functional and /or sensory properties of native protein without loosing nutritional value (Kristinsson and Rasco 2000). Enzymes from microbial sources operating at alkaline pH, such as Alcalase, Neutrase, Protamex, Flavourzyme, are efficient in the hydrolysis of shellfish proteins (Aunstrup 1980). The critical parameters for optimizing degree of enzymatic hydrolysis are temperature (T), time (t) of hydrolysis, enzyme/substrate (E/S) ratio and pH (Diniz and Martin 1997a; Deng et al. 2002). When many factors and interactions affect a desired response, response surface methodology (RSM) is an effective tool for optimizing the process (Box and Wilson 1951) and the main advantage of it is the reduced number of experimental trials needed to optimize the parameters (Giovanni 1983). Shrimp waste hydrolysates produced under controlled conditions yield desirable functional properties, high nutritive value and reduced bitterness (Kristinsson and Rasco 2000). The objective of the present investigation was to optimize extraction procedure of protein hydrolysates from shrimp waste mainly comprising P. monodon using microbial proteases and study the influence of physical parameters viz. pH, temperature, substrate concentration and time on the protein hydrolysis reaction. Also proximate composition and amino acid profile was studied of the protein hydrolysate obtained.
Material and methods
Raw material (10 kg) comprising head, shell and tail of P. monodon were washed thoroughly under running water, milled (Electrolux 70 mesh mill) to eliminate foreign particles if any and then dried in solar drier maintaining (32 °C) temperature for 30 min. The dried sample was ground in a National meat grinder (MK-G5NS, Japan) through a 5 mm grind plate and the resulting ground shell was sieved (20–60 mesh) and final material of (9.4 kg) was obtained with a size range of 0.25–0.85 mm. It was then divided in four parts and each part was packed in LDPE bag which were kept in frozen storage at −20 °C till further use.
Materials
The enzymes used for the hydrolysis of raw shrimp waste were provided by Novozymes A/S (Bagsvaerd, Denmark). Protamex is a Bacillus protease complex; Alcalase 2.4 L is a bacterial serine endopeptidase prepared from a strain of Bacillus lichenformis. Flavourzyme 500 MG is a fungal protease/peptidase complex produced by submerged fermentation of a strain of Aspergillus oryzae. It exhibits both endoprotease and exoprotease activities. Neutrase1 0.5 L (EC 3.4.24.28) was provided by Biosis, India. The chemicals and reagents used in the experiment were of analytical or food grade quality.
Enzymatic hydrolysis
Shrimp waste protein hydrolysate prepared according to method of Holanda and Netto (2002) with slight modification. Freeze-dried raw waste thawed and suspended (1:1, w/v) in distilled water in Sorvall bottles and the mixture was heated at a temperature of 90 °C for 30 min to inactivate the endogenous hydrolyzing enzyme. The mixture was then homogenized and pH was adjusted with 1 N NaOH at 60 °C and hydrolysed upto optimum temperature, pH and E/S ratio of the respective enzyme given in Table 1. The Sorvall bottles were then preheated in a water bath to optimum temperature for the respective enzyme used. Reactions were carried out in duplicates in 1 L polyethylene—jacketed glass vessel in a thermostatically controlled water bath with an automatic temperature compensator (ATC) probe, a pH electrode, a mixer shaft for addition of alkali. Enzymes were added and during hydrolysis reaction temperature was controlled and pH was monitored by pH stat method (Adler-Nissen 1986) using automatic Mettler DL 25 titration unit. Hydrolysis was continued with Alcalase, Neutrase, Protamex, Flavourzyme for 30, 45, 60, 75 and 90 min after which reaction was stopped by heating upto 90 °C for 5 min. Samples were cooled and then centrifuged at 16000 g for 15 min at 4 °C. The supernatants were collected, concentrated and freeze dried to obtain in dry powdered form referred as protein hydrolysis. It was stored in dark glass bottle at refrigerated storage at 4 °C until used.
Table 1.
Conditions for hydrolysis using four microbial proteases
| Alcalase | Neutrase | Protamex | Flavourzyme | |
|---|---|---|---|---|
| pH | 7.0–8.0 | 6.3–6.5 | 7.2–8.0 | 5.5–7.5 |
| Temperature | 56–60 | 47–50 | 50–52 | 50–55 |
| [E]/[S] ratio | 30a | 30a | 100a | 125b |
aExpressed as AU kg −1 protein
bExpressed as LAPU kg −1 protein
Determination of protein recovery and degree of hydrolysis
After hydrolysis reaction, the supernatant was obtained by centrifuging at 3000 g for 10 min. The dense lipid layer was skimmed using two-layers of cheese cloth. The volume of soluble fraction was recorded and total protein in supernatant was determined using Kjeldahl method (AOAC, 1995). PR (%) was calculated using the following equation:
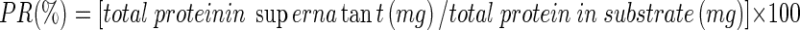 |
1 |
The content of α-amino acids in the supernatant obtained after each hydrolysis methods were determined using 2,4,6- trinitrobenzenesulfonic acid (TNBS) method (Benjakul and Morrisey 1997). The absorbance was measured at 420 nm and ά -amino acid was expressed in terms of L-leucine. The DH was determined using the modified method of Beak and Cadwallader (1995) and defined as follows:
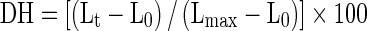 |
2 |
where Lt correspond to the amount of ά-amino acid released at time t. L0 was the amount of ά -amino acid in original raw shrimp waste. L max was the maximum amount of ά -amino acid in shrimp waste protein hydrolysate obtained after each hydrolysis method.
Experimental design
To standardize hydrolysis procedure, reaction parameters were optimized using response surface methodology (RSM). The central composite design (CCD) was employed in this regard. The range and center point values of three independent variables presented in Table 2 were based on the results of preliminary experiments. CCD in the experimental design consists of twelve factorial points, nine axial points and nine replicates of the central point (Table 3). Reaction temperature (A), pH (B), reaction time (C), Enzyme substrate ratio (D) were chosen for independent variables. Degree of hydrolysis was selected as the response for the combination of the independent variables given in Table 3. Experimental runs were randomized to minimize the effects of unexpected variability in the observed responses. The behavior of the system was explained by the following quadratic equation:
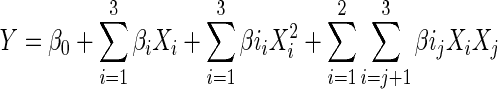 |
3 |
Table 2.
Hydrolysis variables and experimental design levels for response surface analysis
| Design levels | Independent factors | |||
|---|---|---|---|---|
| T (C) | pH | E/S (%) | T (min.) | |
| −1 | 50 | 7 | 0.10 | 30 |
| 0 | 55 | 8 | 1.05 | 60 |
| 1 | 60 | 9 | 2.00 | 90 |
T temperature; E/S enzyme/substrate ratio (%v/w of shrimp waste); t time (minutes)
Table 3.
Box–behnken design matrix and the responses of the dependent variable degree of hydrolysis (%DH) for shrimp waste hydrolysis by Alcalase
| Run | T | pH | E/S | t | DH % |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 0 | 0 | 29.9 |
| 2 | 0 | 0 | −1 | −1 | 4.2 |
| 3 | 0 | 0 | −1 | 1 | 17.3 |
| 4 | 0 | 0 | 1 | −1 | 15.4 |
| 5 | 0 | 0 | 1 | 1 | 22.5 |
| 6 | −1 | 0 | 0 | −1 | 2.3 |
| 7 | −1 | 0 | 0 | 1 | 11.4 |
| 8 | −1 | −1 | 0 | 0 | 25.0 |
| 9 | −1 | 1 | 0 | 0 | 23.1 |
| 10 | 1 | −1 | 0 | 0 | 10.1 |
| 11 | 1 | 0 | 0 | −1 | 32.9 |
| 12 | 1 | 0 | 0 | 1 | 7.6 |
| 13 | 0 | −1 | −1 | 0 | 19.1 |
| 14 | 0 | −1 | 1 | 0 | 14.5 |
| 15 | 0 | 1 | −1 | 0 | 24.8 |
| 16 | 0 | 1 | 1 | 0 | 19.5 |
| 17 | 1 | 0 | −1 | 0 | 16.2 |
| 18 | 1 | 0 | 1 | 0 | 28.0 |
| 19 | 0 | −1 | 0 | −1 | 7.8 |
| 20 | 0 | −1 | 0 | 1 | 15.4 |
| 21 | 0 | 1 | 0 | −1 | 13.0 |
| 22 | 0 | 1 | 0 | 1 | 27.6 |
| 23 | 0 | 0 | 0 | 0 | 20.6 |
| 24 | 0 | 0 | 0 | 0 | 20.2 |
| 25 | 0 | 0 | 0 | 0 | 20.4 |
| 26 | −1 | 0 | −1 | 0 | 13.5 |
| 27 | −1 | 0 | 1 | 0 | 24.0 |
| 28 | −1 | 1 | 1 | 0 | 23.1 |
| 29 | −1 | −1 | 0 | −1 | 19.0 |
| 30 | 1 | 0 | 0 | 0 | 28.0 |
*Average of duplicate determinations from different experiments.T, temperature; E/S, enzyme/substrate ratio (%v/w of shrimp waste); t, time
Where, Y is the dependent variable (degree of hydrolysis in real value), β0 is constant, βi , βii and βij are coefficients estimated by the model. Xi , Xj are levels of the independent variables. They represent the linear, quadratic and cross product effects of the A, B, C and D factors on the response, respectively. The model evaluated the effect of each independent variable to a response. Analysis of the experimental design and calculation of predicted data were carried out using Design Expert Software (version 8.0, trial Statease Inc., Silicon Valley, CA, USA) to estimate the response of the independent variables. Statistical testing of the regression model has been done by the Fisher’s statistical test for ANOVA (analysis of variance) for quadratic model. F value, R2 value, P value, Residual error, Pure error and Lack of fit were calculated for the model. Thus a model equation was proposed from the outcome of the study, for optimizing hydrolysis condition which will produce maximum degree of hydrolysis for obtaining shrimp waste protein hydrolysate from raw waste.
Proximate composition
Moisture and ash content were analyzed in triplicate using AOAC (1995) standard methods 930.15 and 942.05, respectively. The total crude protein (N × 6.25) content of the samples was determined using the Kjeldahl method (AOAC 1995). Total lipid extraction from the samples was done using methanol (Bligh and Dyer 1959).
Amino acid composition
The amino acid composition of hydrolysate samples produced by alcalase hydrolysis after 30, 60 and 90 min were quantified using amino acid analyzer (Waters, USA) by employing PICO.TAG column and work station following method of Ghosh et al. 1995. Detection for eighteen amino acids in the sample were done, which were aspartic acid (Asp), glutamic acid (Glu), serine (Ser), glycine (Gly), histidine (His), arginine (Arg), threonine (Thr), alanine (Ala), proline (Pro), tyrosine (Tyr), valine (Val), methionine (Met), isoleucine (Ile), leucine (Leu) and phenylalanine (Phe). 20 mg sample was extensively dialyzed and then hydrolysed by 6N HCl containing 1% phenol for 22 h at 105 °C. Hydrolysed samples were then derivatized by phenyl isothyocyanate (PITC) solution for 20 min at 25 °C which were then analysed by HPLC at 38 °C as per PICO.TAG manual. Amino acids present in unknown sample was determined quantitatively by comparing the peak areas of amino acids present in standard amino acid mixture in the unit of pico mol by multiplying with 6.25 and finally expressed in percentage. Tryptophan content was determined separately by colorimetric analysis (UV-1700, Shimadzu Co., Kyoto, Japan) at 400 nm after mixing with 4.2 M NaOH (100 ml) and 0.3 ml triglycerine under the condition of pH 5.0–5.5, column oven temperature 55 °C, reactor temperature 100 °C, and reaction time 10–15 min (Deng et al. 2002). Essential amino acid index (EAA), Flavour amino acid index (FAA) and protein efficiency ratio (PER) was calculated by consideration of the content of 10 designated amino acids from the equation developed by Lee et al. (1978).
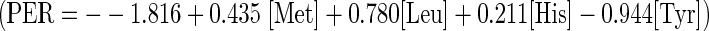 |
4 |
Chemical score of the protein hydrolysate extracted was computed according to the formulae of Vidotti et al. 2003, considering essential amino acid in standard protein as per FAO/WHO (1990).
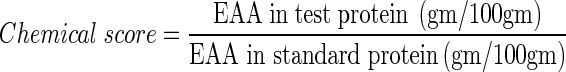 |
5 |
Results and discussion
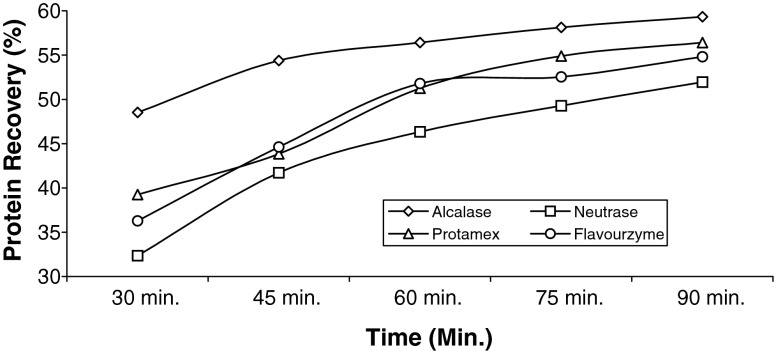
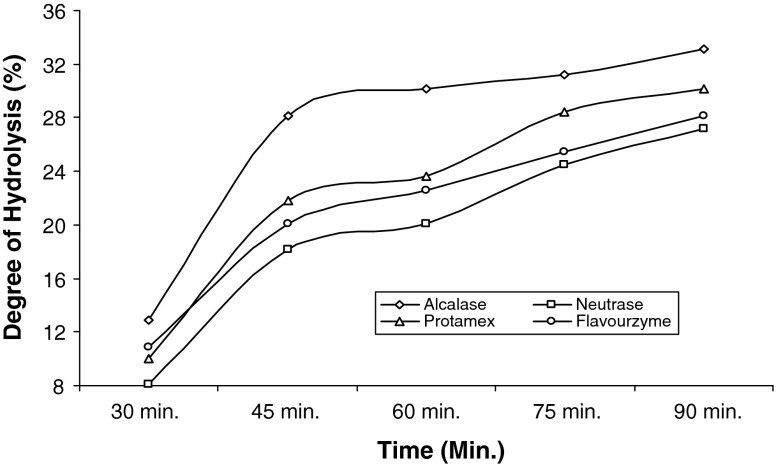
The degree of hydrolysis and corresponding protein recovery after 30, 45, 60, 75 and 90 min during enzymatic hydrolysis are presented in Figs. 1 and 2. From the result it was evident that, after 90 min of hydrolysis, shrimp waste hydrolysed with Alcalase showed mean protein recovery in the range of 59–60%, which was significantly (p ≤ 0.05) higher than Neutrase and also higher than Protamex and Flavourzyme by 5.31% and 8.20%. Figure 1 showed that protein recoveries for all the enzymes increased with time of hydrolysis but the rate of increase get slower at the later stage. Various authors reported that, when compared to other proteolytic enzymes, Alcalase resulted in higher protein recovery, which was again proved for the Paeneus monodon processing waste (Beak and Cadwallader 1995; Shahidi et al. 1995, Mizani et al. 2005). The decrease in protein recovery in the later stage of hydrolysis can be explained by the slower rate of cleavage of peptide bonds with the elapse of time. Figure 2 showed the hydrolysis curve against time for shrimp waste using four microbial proteases. The curve was characterized with high initial reaction rates followed by decrease in reaction rate upto a stationary phase, where apparently hydrolysis no longer occurred. The profile could be explained as, at the initial stage loosely bound polypeptide chains were cleaved from insoluble protein peptides whereas in later stage the soluble peptides or compounds inhibition act as an effective substrate competitor for the non-hydrolysed proteins (Rebeca et al. 1991). Alcalase showed highest DH % i.e. 32.88% after 90 min of hydrolysis, which was higher than other proteases. Although there is a relationship between PR (%) and DH (%) (Beak and Cadwallader 1995), previous work has shown that PR (%) did not improve significantly at DH values higher than 12% (Holanda and Netto 2002). In the present study also significant increase of PR (%) with increase in DH (%) was not observed at the later stage, however this was observed in the initial stage of hydrolysis.
Fig. 1.
Protein recovery during different stages of enzymatic hydrolysis using four microbial proteases. (n = 3)
Fig. 2.
Degree of Hydrolysis during different stages of enzymatic hydrolysis using four microbial proteases. (n = 3)
Response surface methodology
From Figs. 1 and 2 it was evident that Alcalase was the most effective enzyme in terms of PR (%) and DH (%) for shrimp waste hydrolysis so response surface methodology (RSM) on its hydrolysis process. RSM has been used successfully as a statistical technique to optimize a desired response affected by several factors during protein hydrolysis (Cheison et al. 2007). Preliminary experiments were performed to determine values of three independent variables for corresponding levels for the experiment. The following quadratic model explains the dependence of DH on the independent variables and the parameters of the equation were obtained by multiple regression analysis of the experimental data.
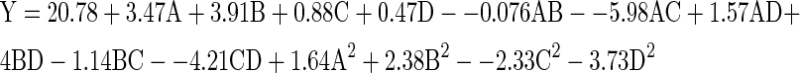 |
6 |
The empirical model showed a good fit with the experimental data because the adjusted coefficient of determination (R2adj) is 0.9213 indicating 92.1% of the variability in behavior within the range of values studied could be explained by the model. ANOVA of the quadratic model presented in Table 5. F value is the ratio of mean square due to regression to the mean square due to residual which is 20.51. In general, the calculated F value should be several times greater than the tabulated value for a good model. If the F value is greater than tabulated F 0.05 (3.02), then the null hypothesis is rejected at the a level of significance and implies that the variation accounted for by the model is significantly greater than the unexplained variation. The probability (P) value of the regression model was less than 0.0001, with no significant lack-of fit (P = 0.14999). The determination coefficient (R2 = 0.9554) was satisfactory, having a low experimental error according to ANOVA (Table 4). The pH (B) and substrate concentration (D) had a highly significant effect (P < 0.001) at the maximum DH. The pH-temperature (AB) and time temperature (AC) interaction term had a significant effect (P < 0.05), and the quadratic terms (A2 and C2) also had a highly significant effect (P < 0.001). The 3D response surfaces and the 2D contour plots of the response using Eq. 3 when one of the variables is fixed at the central point and the other two are allowed to vary are shown in Fig. 3. The maximum predicted value is indicated by the surface confined in the smallest ellipse in the contour diagram. It indicates that there is relatively significant interaction between pH and temperature corresponding to the response surface, which is consistent with the results of the ANOVA for quadratic model. The optimal conditions were extracted by Design Expert Software with its optimization menus: A = 4.37, B = 0.25, C = 24.42, D = 0.79. The real values were temperature at 59.37 °C, pH at 8.25, reaction time at 84.42 min. and substrate concentration at 1.84%. The maximum DH obtained by using the above optimized concentrations of the variables is 33.13%. The maximum DH obtained experimentally was found to be 35.06%. This is obviously in close agreement with the model prediction. The non-linear relation between t and %DH implies that the hydrolytic reaction depends on the availability of susceptible peptide bonds on which the primary enzymatic attack is concentrated and on the physical structure of the protein molecule (Raghunath 1993). The observed responses in Table 4 were all obtained at 1.5 h for hydrolysis reaction. To check whether 1.5 h was really optimal, time course experiments were performed at optimal pH, temperature and substrate concentration. The results clearly showed that hydrolysis increases nonlinearly with time and reached a plateau after 1.5 h. It indicated that the hydrolysis reaction was nearly finished in the initial 1.5 h.
Table 5.
Proximate composition of raw shrimp waste and protein hydrolysate prepared by different enzymes
| Sample | Moisture (%) | Crude protein (%) | Fat (%) | Ash (%) | Chitin (%) |
|---|---|---|---|---|---|
| Raw shrimp waste | 67.4 ± 0.45 | 13.7 ± 0.91 | 1.7 ± 0.04 | 8.4 ± 0.12 | 3.5 ± 0.2 |
| PH by Protamex | 13.7 ± 0.91 | 65.8 ± 0.06 | 3.0 ± 0.05 | 14.6 ± 0.08 | – |
| PH by Alcalase | 1.7 ± 0.04 | 72.3 ± 0.04 | 2.4 ± 0.06 | 16.6 ± 0.06 | – |
| PH by Flavourzyme | 8.4 ± 0.12 | 59.8 ± 0.08 | 2.6 ± 0.4 | 13.2 ± 0.04 | – |
| PH by Neutrase | 9.8 ± 0.14 | 60.2 ± 0.03 | 2.6 ± 0.02 | 15.1 ± 0.01 | – |
Mean of 3 determinations ± standard deviation
Table 4.
Analysis of variance for the response of degree of hydrolysis
| Source | Degree of freedom | Sum of squares | Mean square | F-value | P value |
|---|---|---|---|---|---|
| Model | 14 | 1205.20 | 86.09 | 2.51 | <0.0001 |
| A (Reaction temperature) | 1 | 152.20 | 152.20 | 4.43* | 0.17456 |
| B (pH) | 1 | 105.16 | 105.16 | 3.06* | <0.0001 |
| C (Reaction time) | 1 | 8.45 | 8.45 | 0.25* | 0.08546 |
| D (Enzyme/substrate ratio) | 1 | 2.06 | 2.06 | 0.60* | <0.0001 |
| AB | 1 | 0.022 | 0.022 | 6.537** | 0.00856 |
| AC | 1 | 111.89 | 111.89 | 3.26** | 0.00254 |
| AD | 1 | 10.95 | 10.95 | 0.32** | 0.17652 |
| BC | 1 | 52.99 | 52.99 | 1.54** | 0.84634 |
| BD | 1 | 3.77 | 3.77 | 0.11** | 0.45233 |
| DC | 1 | 112.86 | 112.86 | 3.29** | 0.94572 |
| A2 | 1 | 15.63 | 15.63 | 0.46* | 0.23659 |
| B2 | 1 | 30.94 | 30.94 | 0.90* | <0.0001 |
| C2 | 1 | 24.04 | 24.04 | 0.70* | 0.23451 |
| D2 | 1 | 59.44 | 59.44 | 1.73* | <0.0001 |
| Residual | 15 | 514.93 | 34.33 | 34.33 | – |
| Pure error | 4 | 55.63 | 13.91 | 13.91 | – |
| Lack of fit | 11 | 459.30 | 41.75 | 41.75 | 0.14999 |
| Total | 29 | 1720.14 |
% variability explained R2 = 0.9554, R2 adj = 0.9213
* Significant at α = 0.01
** Significant at α = 0.05
Fig. 3.
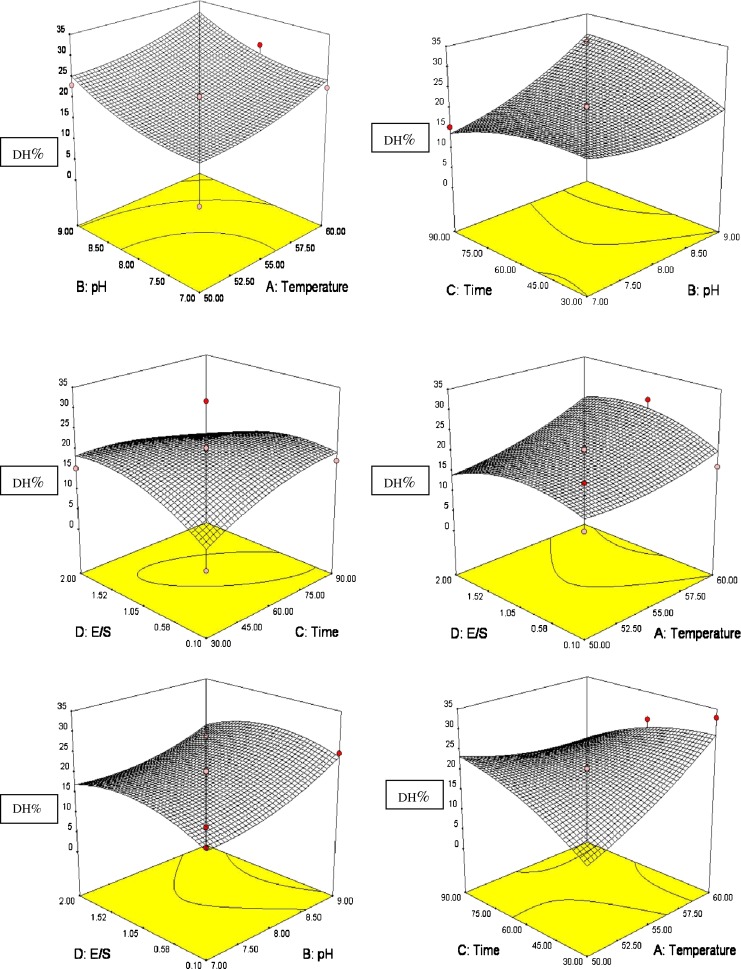
Response surfaces graphs depicting effects of independent variables [Temperature (A), pH (B), time (C) and Enzyme substrate ratio (D)] on DH% during Alcalase hydrolysis of shrimp waste
Proximate composition
The proximate composition of raw shrimp waste and protein hydrolysates prepared are given in Table 5. The freeze-dried protein hydrolysate was produced following the critical values for maximizing DH for the hydrolysis factors: T = 56.75 °C, pH = 7.84, E/S = 1.61% (%v/w of shrimp waste) and t = 79.64 min. The DH obtained was 33.13%. The fat content of the freeze-dried hydrolysate greatly reduced by 56.11% when compared with that in the starting material because lipids were most likely excluded with insoluble protein fraction by centrifugal separation. Therefore there will be no lipids to contribute to lipid oxidation. This may in turn enhance the storage stability of the products (Shahidi et al. 1995; Diniz and Martin 1997b). A high protein recovery by Alcalase in the range of 59–60% and its low cost may provide an incentive for using it in commercial operations. Ash content is normally high in fish protein hydrolysates (Kristinsson and Rasco 2000) and the ash content reflect rich mineral content in the shrimp shell and head. The protein content of raw shrimp waste was 13.7% (in dry weight) which increased in the alcalase hydrolysate upto 72.3% showing a steep increase in the protein percentage. Previous reports also showed protein content of shrimp hydrolysates ranging from 62 to 90% (Shahidi et al. 1995; Benjakul and Morrisey 1997; Kristinsson and Rasco 2000).
Amino acid profile
Amino acid composition of alcalase hydrolysate at varied time of hydrolysis has been presented in Table 6 which depicts time of hydrolysis affected release of 18 amino acids in the protein hydrolysate extracted. Most amino acids exhibited greater variation in the initial 30 min. apart from cystine, all of which appeared to exhibit a tendency toward an increase in concentration with the passage of time as hydrolysis continued. Among amino acids analyzed herein, the content of Glycine (14.09%), Valine (11.07%), Isoleucine + Leucine (16.21%) and Lysine (18.13%) tended to predominate in the hydrolysates at different DH, which are considered to be among the eight essential amino acids for human being diet. The essential amino acids make up 54.67–55.93% of all amino acids which also increased with the increase of DH (p ≤ 0.05). The values exceed the reference values of 40% recommended by FAO/WHO (1990) for infants. The hydrolysates had an extremely high content of the flavor enhancers, glutamic acid, aspartic acid, glycine and alanine (39.27–38.32% of the total amino acids), which may account for the good taste. The hydrolysates contain very high levels of lysine (16.78–18.13% of the total amino acids) which is extremely rare in cereals and required for proper development and acts as precursor for production of carnitine, a nutrient with roles in converting fatty acids into energy and regulating cholesterol levels. Arginine is classified as a semi-essential or conditionally essential amino acid and it participates in protein synthesis and other physiological functions such as detoxification and energy conversion (Morris 2005). The Nutritional quality of hydrolysate was also confirmed by high protein efficiency ratio (PER) values, calculated according to the equation developed by Lee et al. (1978). The PER value of protein hydrolysate was 2.99 as compared to that for protein hydrolysate from Capelin (2.64) and Beef muscle protein (2.81) (Synowiecki and Alkhateeb 2000). Chemical score measures protein quality based on the amino acid requirements of humans. Criteria needed for chemical score are approximate nitrogen composition, essential amino acid profile and true digestibility. According to this method, chemical score of an ideal protein meeting all the essential amino acid requirements of the human body has a value of 1.00. Shrimp waste protein hydrolysate having chemical score of 1.05; values greater than 1.00 are considered to indicate that the protein contains essential amino acids in excess of the human requirements. This excess can serve to complement the essential amino acid profile of food that may have deficiencies and result in a more nutritious prepared/processed food or meal. Thus, the nutritional value the hydrolysates of the shrimp waste was high and could be expected to be excellent functional food stuff or a good taste enhancer.
Table 6.
Amino acid composition of shrinp waste protein hydrolysate prepared at sifferent stages of hydrolysis
| Amino acids | Amount dry basis (% pico mol × 6.25) expressed as percentage of total amino acid composition in samples prepared at different degree of hydrolysis* | |||
|---|---|---|---|---|
| 0 h | 0.5 h | 1 h | 1.5 h | |
| Aspartic acid | 6.4 ± 0.35 | 8.0 ± 0.35 | 8.6 ± 0.05 | 9.00 ± 0.32 |
| Threonine | 1.0 ± 0.04 | 1.2 ± 0.04 | 1.1 ± 0.22 | 1.0 ± 0.22 |
| Serine | 1.8 ± 0.45 | 1.8 ± 0.45 | 2.00 ± 0.45 | 2.0 ± 0.05 |
| Glutamic acid | 9.6 ± 0.02 | 10.5 ± 0.02 | 11.1 ± 0.06 | 11.4 ± 0.04 |
| Proline | 1.7 ± 0.11 | 0.56 ± 0.11 | 0.45 ± 0.01 | 0.40 ± 0.06 |
| Glycine | 11.8 ± 0.03 | 16.4 ± 0.03 | 14.8 ± 0.45 | 14.0 ± 0.08 |
| Alanine | 8.7 ± 0.05 | 4.3 ± 0.05 | 4.2 ± 0.22 | 3.8 ± 0.88 |
| Cystine | 4.6 ± 0.01 | 2.2 ± 0.01 | 2.4 ± 0.05 | 2.5 ± 0.56 |
| Valine | 7.9 ± 0.38 | 9.9 ± 0.38 | 10.0 ± 0.01 | 11.1 ± 0.02 |
| Methionine | 2.5 ± 0.23 | 3.9 ± 0.23 | 3.00 ± 0.08 | 2.1 ± 0.01 |
| Isoleucine + Leucine | 12.3 ± 0.01 | 14.4 ± 0.01 | 15.3 ± 0.05 | 16.2 ± 0.09 |
| Tyrosine | 2.3 ± 0.62 | 1.4 ± 0.62 | 1.4 ± 0.06 | 1.2 ± 0.15 |
| Phenylalanin | 3.0 ± 0.04 | 3.8 ± 0.04 | 3.0 ± 0.55 | 2.4 ± 0.02 |
| Lysine | 15.2 ± 0.01 | 16.8 ± 0.01 | 17.5 ± 0.34 | 18.1 ± 0.06 |
| Histidin | 1.2 ± 0.09 | 2.0 ± 0.09 | 2.4 ± 0.01 | 2.4 ± 0.54 |
| Arginine | 1.2 ± 0.07 | 1.9 ± 0.07 | 1.9 ± 0.48 | 1.1 ± 0.12 |
| Tryptophan | 2.3 ± 0.05 | 1.2 ± 0.05 | 1.3 ± 0.05 | 1.3 ± 0.03 |
| Essential amino acid (EAA) | – | 54.7 ± 0.01 | 55.2 ± 0.01 | 55.9 ± 0.01 |
| Flavour amino acids (FAA) | – | 39.3 ± 0.01 | 38.7 ± 0.01 | 38.3 ± 0.01 |
| Protein efficiency ratio(PER) | – | 2.8 ± 0.06 | 2.8 ± 0.01 | 3.0 ± 0.01 |
| Chemical score | – | 0.87 | 0.97 | 1.05 |
Mean of 3 determinations ± standard deviation.
Conclusions
Microbial proteases were efficient to extract protein hydrolysate of which Alcalase showed highest protein recovery. The DH of shrimp waste was significantly influenced by the hydrolysis conditions that included time, temperature, pH of the substrate and substrate concentration. The conditions were optimized by RSM and optimum values for T, pH, E/S ratio and t were found to be 59.37 °C, 8.25, 1.84% and 84.42 min, respectively for maximum DH. The application of RSM may therefore provide useful information in the development of economic and efficient processes in food protein hydrolysis systems. Protein hydrolysates in powdered form was produced from the shrimp processing waste with good protein recovery, high crude protein content and rich amino acid profile having a good potential as food additives. Thus it can show a roadmap for effective alternative usage of shrimp processing waste as well as take more profits to shrimp processors.
References
- Adler-Nissen J. Enzymatic hydrolysis of food proteins. London: Elsevier Applied Science Publishers Ltd; 1986. p. 427. [Google Scholar]
- Official methods of analysis. 16. Arlington: Association of Official Analytical Chemists; 1995. [Google Scholar]
- Aunstrup K (1980). Proteinases. In Microbial enzymes and bioconvertions (Edited by Rose A. Academic Press, New York, pp. 50–112
- Beak HH, Cadwallader KR. Enzymatic hydrolysis of crayfish processing byproducts. J Food Sci. 1995;60:929–935. doi: 10.1111/j.1365-2621.1995.tb06264.x. [DOI] [Google Scholar]
- Benjakul S, Morrisey T. Protein hydrolysates from Pacific whiting solid wastes. J Agric Food Chem. 1997;45:3423–3430. doi: 10.1021/jf970294g. [DOI] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Box GEP, Wilson KG. On the experimental attainment of optimum conditions. J R Stat Soc. 1951;13:1–45. [Google Scholar]
- Cheison SC, Wang Z, Xu SY. Use of response surface methodology to optimise the hydrolysis of whey protein isolate in a tangential flow filter membrane reactor. J Food Eng. 2007;80:1134–1145. doi: 10.1016/j.jfoodeng.2006.09.014. [DOI] [Google Scholar]
- Deng SG, Peng ZY, Yang P, Xia XZ. Application of multienzymatic method in fermented fish sauce production from Harengula zunasi’s offal. Food Ferment Ind. 2002;28:32–36. [Google Scholar]
- Diniz FM, Martin AM. Effects of the extent of enzymatic hydrolysis on the functional properties of shark protein hydrolysate. Lebensm Wiss Technol. 1997;30:266–272. doi: 10.1006/fstl.1996.0184. [DOI] [Google Scholar]
- Diniz FM, Martin AM. Fish protein hydrolysates by enzymatic processing. Agro Food Ind Hi Tec. 1997;8:9–13. [Google Scholar]
- FAO/WHO Protein quality evaluation. Reports of a joint FAO/WHO expert consultation. Rome Food and Agriculture Organization of the United Nations. Food Nutr Paper. 1990;51:1–66. [Google Scholar]
- Ghosh AK, Naskar AK, Jana ML, Khowala S, Sengupta S. Biotechnol Prog USA. 1995;11:452–456. doi: 10.1021/bp00034a013. [DOI] [Google Scholar]
- Gildberg A, Stenberg E. A new process for advanced utilization of shrimp waste. Process Biochem. 2001;36:809–812. doi: 10.1016/S0032-9592(00)00278-8. [DOI] [Google Scholar]
- Giovanni M. Response surface methodology and product optimization. Food Technol. 1983;37:41–45. [Google Scholar]
- Holanda HD, Netto FM (2002) Optimization of the conditions for the enzyme hydrolysis of shrimp residue, using response surface methodology (RSM). In book of abstracts, 2002 IFT Annual Meeting, Anaheim, Calif., USA, P 194
- Ibrahim HM, Salama MF, El-Banna HA. Shrimp waste: chemical composition, nutritional value and utilization. Nahrung. 1999;43:418–23. doi: 10.1002/(SICI)1521-3803(19991201)43:6<418::AID-FOOD418>3.0.CO;2-6. [DOI] [Google Scholar]
- INFOFISH (1991) Shrimp Waste Utilization , INFOFISH, technical handbook series 4, kualalampur, Malayasia
- Kristinsson HG, Rasco BA. Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle hydrolyzed with various alkaline proteases. J Agric Food Chem. 2000;48:657–666. doi: 10.1021/jf990447v. [DOI] [PubMed] [Google Scholar]
- Lee YB, Eliot JG, Richansrud DA, Hugberg EC. Predicting protein efficiency ratioby chemical determination of connective tissue content in meat. J Food Sci. 1978;43:1359–1362. doi: 10.1111/j.1365-2621.1978.tb02490.x. [DOI] [Google Scholar]
- Mizani M, Aminlari M, Khodabandeh M. An effective method for producing a nutritive protein extract powder from shrimp-head waste. Sci Technol Int. 2005;11(1):2005. [Google Scholar]
- Morris SM. Arginine metabolism in vascular biology and disease. Vasc Med. 2005;10:S83–S87. doi: 10.1177/1358836X0501000112. [DOI] [PubMed] [Google Scholar]
- MPEDA (2009) Marine export review. Available from (www.mpeda.com ). 20th Oct 2009
- Raghunath MR. Enzymatic protein hydrolysate from tuna canning wastes standarisation of hydrolysis parameters. Fish Technol Soc. 1993;30(1):40–45. [Google Scholar]
- Rebeca B, Pena-Vera MI, Diaz-Castaneda M. Production of fish protein hydrolysates with bacterial proteases: yield and nutritional value. J Food Sci. 1991;56:309–14. doi: 10.1111/j.1365-2621.1991.tb05268.x. [DOI] [Google Scholar]
- Shahidi F, Han XQ, Synowiecki J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus) Food Chem. 1995;53:285–293. doi: 10.1016/0308-8146(95)93934-J. [DOI] [Google Scholar]
- Synowiecki J, Alkhateeb N. The recovery of protein hysrolysate during enzymatic isolation of chitin from shrimp Crangon crangon processing discards. Food Chem. 2000;68:147–152. doi: 10.1016/S0308-8146(99)00165-X. [DOI] [Google Scholar]
- Vidotti RM, Viegas EMM, Cariero DJ. Amino acid composition of processed fish silage using different raw materials. Anim Feed Sci Technol. 2003;105:199–204. doi: 10.1016/S0377-8401(03)00056-7. [DOI] [Google Scholar]