Abstract
Functional properties (anthocyanins, antioxidant, ascorbic acid and tannin) and sensory score were determined in pomegranate fruits at two storage temperatures (3 and 5 °C) after treatment with 2 mM putrescine and 1 : 10 carnauba wax (carnauba wax : water). The treatments (putrescine and carnauba wax) were given by immersion method followed by storage up to 60 days. Both treatments retained significantly higher anthocyanins, antioxidant, ascorbic acid, tannin and sensory qualities as compared with control fruits under both the storage conditions. Combined application of putrescine + carnauba wax showed better response in retaining functional properties than putrescine treated or nontreated fruits. The impacts of putrescine and carnauba wax treatments were found more pronounced after 30 days at 3–5 °C storage temperature in retaining functional and sensory qualities. After 60 days of storage, putrescine + carnauba wax retained about 25% higher antioxidant activity both at 3 and 5 °C storage temperatures.
Keywords: Polyamines, Carnauba wax, Anthocyanins, Antioxidant activity, Ascorbic acid, Tannins
Introduction
Pomegranate (Punica granatum L.) fruit is highly valued mainly due to its nutritional and sensory properties and grown almost in all tropical and subtropical countries (López-Rubira et al. 2005). The plant has bushy growing habit and belongs to the Punicaceae family. Fruits are usually consumed as fresh seeds (arils), which contain around 80% of juice and 20% of seed. The juice is rich in several minerals and pigments, especially anthocyanins, which are known for its strong chemopreventive activities such as antimutagenicity, antihypertention, antioxidative potential and reduction of liver injury (Hertog et al. 1997; Lansky et al. 1998). Different compounds have been addressed as contributors to the antioxidant capacity of pomegranate. In juice, galloylglucose, ellagic acid, anthocyanin glucosides (delphinidin and cyanidin) and tannin have been found associated with antioxidant activity (Gil et al. 2000). The shelf life extension is one of the most intensively studied topics in postharvest literature of pomegranate. Mukherjee (1958) reported the viability of stored pomegranate for 7 months at 0 °C with a relative humidity of 80%. Later, Elyatem and Kader (1984) reported that at 2.2 °C the pomegranate present their lowest level of respiration and endogenous ethylene production. But these studies have hardly tells on internal functional fruit quality which was undertaken at a range of storage temperatures. Now it is well known that during postharvest operations important quality losses occurs, the main problem being desiccation and browning in both peel and arils, which further increases with storage temperature below 5 °C. However, storage at higher temperature leads to reduction of shelf life by acceleration of physiological and pathological activities, which makes necessary the storage at low temperatures. Moreover, losses of anthocyanins and ascorbic acid were reported in fruits at higher temperature that would reduce the potential of antioxidant activity (Wang and Zheng 2001; Lee and Kader 2000).
The naturally occurring polyamines putrescine (PUT), spermidine (SPD) and spermine (SPM) are involved in many developmental processes, and in the case of fruits, the pre-storage application of polyamines had maintained functional qualities (Mirdehghan et al. 2007a; Valero et al. 2002; Serrano et al. 2003; Martínez-Romero et al. 2002). Exogenous application of putrescine (usually under pressure infiltration) induced low softening and delayed color changes in lemon, peach, apricot and plum (Valero et al. 1998; Martínez-Romero et al. 2000, 2002; Serrano et al. 2003). These effects clearly retarded the ripening process and maintained higher functional properties rendering fruits more attractive and palatable, and it seems related to their antisenescence properties.
The beneficial additive role of carnauba wax is well known for retaining functional properties of several tropical, subtropical and temperate fruits and vegetables during cold storage (Eum et al. 2009; Khuyen et al. 2008; Ergun et al. 2005; Koley et al. 2009).
Generally for fruit shelf life extension, polyamines have been administered through pressure infiltration to ensure polyamine intake, which seems costly and difficult in comparison to immersion method. As far as we know, no information is yet available about the combine effect of carnauba wax and putrescine (PUT) on textural and functional properties of the pomegranate arils. ‘Mridula’ is an Indian cultivar with extra soft delicious sweet aril suited for export (Asrey et al. 2008). Looking into economic importance of this cultivar, the aim of the present work was to study the effect of the application of PUT and carnauba wax by immersion treatment on the textural and functional properties contributing compounds (total anthocyanins, total antioxidant activity, ascorbic acid and aril tannin content) of pomegranate stored at 3 °C or 5 °C followed by 3 days at 20 °C.
Materials and methods
Plant material and experimental design
Pomegranates (Punica granatum L. cv. Mridula) were picked on January, 2009 from a commercial orchard at Rahuri (Maharashtra). Fruits were harvested at commercial maturity (average TSS 12 °Brix) and immediately transported to the laboratory. Pomegranates with defects (sunburn, cracks, bruises and cuts in the husk) were discarded. The remaining selected fruits were randomized and divided into 3 lots of 60 fruits for the following treatments in 3 replicates (each replicate contained 20 individual fruit). One lot was treated with 2 mM putrescine (PUT), another with 2 mM putrescine in combination with carnauba wax and the rests with distilled water which served as control. Treatments were performed by dipping in 10 L solution of 2 mM putrescine containing Tween-20 (2 g L−1) as surfactant at 25 °C for 8 min. and in carnauba wax emulsion (1 : 10) at 40 °C for 2 min. After treatments, fruits were air-dried and stored in a temperature-controlled chamber (3 and 5 °C) with a relative humidity of 90 ± 5%. After 15, 30, 45 and 60 days, 5 fruits for each treatment and replicate were sampled and further stored at 20 °C for 3 days. Then, husk (peel) was carefully cut at the equatorial zone with sharpened knives, arils were taken out and from these arils juice was extracted manually for analysis.
Total anthocyanins content
The total anthocyanins content was determined on a UV-visible spectrophotometer by the pH-differential method (Wrolstad et al. 2005) using two buffer systems- potassium chloride buffer, pH 1.0 (0.025 M) and sodium acetate buffer, pH 4.5 (0.4 M). Juice extracts were centrifuged at 10,000 rpm for 10 min. at 18 °C. The supernatant were diluted in pH 1.0 and pH 4.5 buffers, and absorbance measurements were made at 510 and 700 nm using 1.0 cm path length cuvettes. The pigment content was calculated and expressed as milligrams equivalent delphinidin-3,5-diglucoside 100 g−1 aril fresh weight, using an extinction coefficient of 795 L cm−1 mol−1 and a molecular weight of 465.2 g mol−1.
Ascorbic acid
Ascorbic acid was quantitatively determined by using a relatively simple and fast 2,6-dichlorophenolindophenol- dye method as described by Jones and Hughes (1983) with slight modifications. For each sample, 10 g arils were homogenized with 10 mL of 3% metaphosphoric acid (volume-to-volume ratio). The extract was made up to a volume of 100 mL and centrifuged at 3,000 rpm for 15 min. at room temperature. Ten milliliters of supernatant were titrated against standard 2,6-dichlorophenolindophenol dye, which had already been standardized against standard ascorbic acid. Results were expressed as mg 100 g−1 on aril weight basis.
Tannin content
Tannin content was determined by colorimetric method (AOAC 2000) using a standard curve of gallic acid. Juice was extracted from arils for each sample and 2 mL of juice was taken and made up to 250 mL with distilled water and boiled for 5 min. Then in a 100 mL volumetric flask 5 mL of diluted sample, 5 mL Folin-Denis reagent and 10 mL Na2CO3 solution were added and volume was made up to 100 mL with distilled water, mixed well, kept for 30 min and absorbance was measured at 760 nm in spectrophotometer using an experimental blank (5 mL Folin-Denis reagent + 10 mL Na2CO3 + 85 mL distilled water) for zero setting. In the similar way standard curve was performed with gallic acid (0–10 mL) and results were expressed as milligrams equivalent gallic acid 100 g−1.
Antioxidant activity
Antioxidant activity was measured by cupric reducing antioxidant capacity (CUPRAC) method of Apak et al. (2004). Juice was extracted from the arils and samples were prepared by taking 2 mL juice and adjusting volume up to 15 mL with 80% ethanol. Then, to a test tube 1 mL of the following reagents were added: copper chloride solution (10−2 M), neocuproine solution of 7.5 × 10−3 M and ammonium acetate buffer (pH 7.0) solutions. Antioxidant sample (or standard) solution (100 μL) and distilled water (1 mL) were added to the initial mixture. The tubes were capped and after 1 h, the absorbance at 450 nm was recorded against a reagent blank. The standard calibration curve of antioxidant compound was constructed in this manner as absorbance versus concentration. The molar absorptivity of the CUPRAC method for antioxidant was found from the slope of the calibration line concerned and the antioxidant activity was expressed as μmol equivalent Trolox g−1.
Sensory analysis
Twenty members of non-trained sensory panel rated the arils using a 9-point hedonic scale with 1, dislike extremely; 2, dislike very much; 3, dislike moderately; 4, dislike slightly; 5, neither like nor dislike; 6, like slightly; 7, like moderately; 8, like very much and 9, like extremely (Resende et al. 2008). The evaluated parameters were color, aroma, taste, firmness, juiciness and overall appearance.
Statistical analysis
The data obtained from 3 replicates under different treatments in respect to various functional properties during storage were subjected to analysis of variance (ANOVA). Sources of variation were treatment and storage temperature. All analyses were performed with SPSS software package.
Results and discussion
Total anthocyanins content
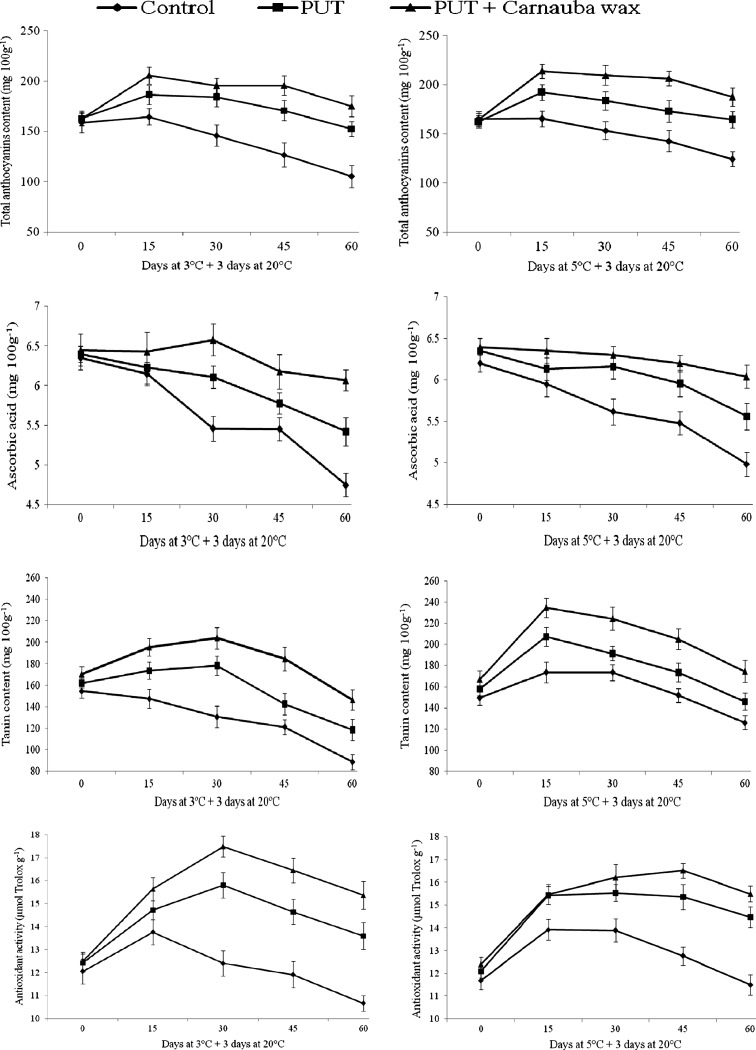
Anthocyanin (pigment) is the key color and antioxidant contributing constituent present in pomegranate juice. Internal breakdown and aril browning (pigment degradation) is main bottleneck during storage of pomegranate at low temperature (below 5 °C). Regardless to treatments and storage temperatures (3 and 5 °C), the total anthocyanins content was initially found increased up to 15 days but later on a definite declining trend was observed (Fig. 1). Irrespective to storage intervals and temperatures, total anthocyanins content remained higher in treated fruits than control. Comparing the treatment effects, the putrescine in combination with carnauba wax was more effective than PUT alone at both the storage temperatures. At the end of the storage period, total anthocyanins content differences between control and treated fruits were significantly higher especially at 3 °C and found to be 175.07 ± 4.55 mg equiv delphinidin-3,5-diglucoside 100 g−1 for PUT 2 mM + carnauba; 152.54 ± 6.47 mg equiv delphinidin-3,5-diglucoside 100 g−1 for PUT alone and 105.35 ± 4.01 mg equiv delphinidin-3,5-diglucoside 100 g−1 for control arils. Under both storage conditions, the retention of total anthocyanins were about 30–40% higher in PUT 2 mM + carnauba treated fruits compared to control after 60 days of storage period.
Fig. 1.
Effect of putrescine and carnauba wax treatments on chemical changes in pomegranate arils during cold storage (3 and 5 °C) + 3 days at 20 °C. Data are means ± S.E. of three replicate determination (n = 3)
The exogenous application of polyamines (PUT) mainly protects the membrane lipid from being converted in liquid-crystalline to a solid-gel state (induce chilling injury) by preventing lipid peroxidation (Mirdehghan et al. 2007b). This could explain the reason behind the high anthocyanins content in PUT treated pomegranate fruit by lowering anthocyanins degradation.
The difference between the sole and combine impact of PUT and carnauba wax may be attributed to the internal CO2 and O2 level of carnauba wax coated pomegranate fruit. The carnauba wax coating tends to lower internal O2 level. Thus it is clear that the lower O2 contributing role reported for carnauba wax coating (Bai et al. 2003) would have a role in lower oxidation and thus might contributed towards higher anthocyanins retention under combined application with PUT.
Ascorbic acid
The effect of treatments on ascorbic acid content revealed that there was a definite declining trend in the ascorbic acid content of fruit from the initial days at both the storage temperatures (Fig. 1). However, ascorbic acid declining trend was much pronounced in control as compared to treated fruit arils. Among the applied treatments, combined application of PUT and carnauba wax given best results in terms of ascorbic acid retention of the stored fruit arils both at 3 and 5 °C storage temperatures. At the 60th day of storage, PUT + carnauba wax treated fruit arils retained highest ascorbic acid (6.07 ± 0.13 and 6.04 ± 0.14 mg 100 g−1) content at 3 and 5 °C storage temperatures. PUT + carnauba wax shown continuously best results up to 60th day under both the storage conditions, while PUT alone could not retained higher amount of ascorbic acid up to end of the storage period. On 60th day of storage, combined application of PUT and carnauba wax retained more than 20% higher ascorbic acid as compared to control.
Polyamines (PUT, SPD and SPM) are known for delaying ripening process and extending shelf life of several fruits and vegetables (Valero et al. 1998; Serrano et al. 2003). Furthermore, the losses of ascorbic acid and increase in ascorbate oxidase in tomato development, ripening and senescence have been associated with decrease in polyamine content (Yahia et al. 2001). In this way, it is clear that the antisenescence nature reported for polyamine would have a role in cell integrity and thus would lower down the depletion of ascorbic acid in treated fruits. Furthermore, comparatively higher retention of aril ascorbic acid in PUT + carnauba wax treatment may be explained by the carnauba coating effect on lowering down respiration process (Purvis 1994).
Tannin content
It is evident from the results that all the treatments significantly influenced the tannin content of the stored fruit aril during storage at both 5 and 3 °C temperature (Fig. 1). Up to 15th day of storage, all treatments exerted similar response both at 3 and 5 °C storage temperatures that is, an increase in tannins concentration, and afterward a different response pattern was observed. The initial increase was much higher at 5 °C under all the treatments up to 15th day of storage in comparison to 3 °C. The highest aril tannin content was obtained in fruits treated with PUT along with carnauba wax (235.0 ± 7.5 mg equiv gallic acid 100 g−1) followed by PUT alone (207.5 ± 7.0 mg equiv gallic acid 100 g−1) 15 days after storage at 5 °C. Further, at 60th day of storage, PUT + carnauba wax treated fruits maintained significantly higher tannin content than putrescine treated or control arils.
Largely, the tannin content was found increased initially and diminished later on. This fluctuating phenomenon in the stored fruit may be explained by migration of tanin (rind to aril) and ripening processes. Tannins are basically polyphenolic compound and their level of concentration in different parts (leaf, flower, rind, aril and seed) of pomegranate affected by several pre and postharvest factors (Wang 2006). Punicalagin, punicalin and punicafolin are important component of tannins (Jurenka 2008). The reduction in total tannin content has been reported with the progression of ripening in pomegranate (Kulkarni and Aradhya 2005). This might be attributed to the conversion of tannins into other organic acids (citric, malic, oxalic and succinic acid) during advanced stage of fruit ripening.
Antioxidant activity
Irrespective of treatments and storage temperatures, all fruits showed an increasing trend in total antioxidant activity up to 15th day (Fig. 1). Thereafter, juice obtained from PUT and carnauba wax treated fruits further shown an increasing trend up to 30th day in antioxidant activity while control gave a declining pattern. Combined application of PUT (2 mM) and carnauba wax had registered most sharp increasing trend in antioxidant activity (17.49 ± 0.45 μmol equiv Trolox g−1) even up to 30th day at 3 °C storage temperature followed by PUT (15.81 ± 0.55 μmol equiv Trolox g−1) and control (12.40 ± 0.55 μmol equiv Trolox g−1). By and large, the treatments have shown a declining trend in respect to total antioxidant activity of the fruit juice after 30th day of storage. The treatment effect on antioxidant activity was much pronounced at 3 °C compared to 5 °C storage temperature.
In our study, the application of putrescine either alone or in combination with carnauba wax gave effective results in maintaining the higher concentration of antioxidant activity in treated fruits compared to control. Antioxidant capacity of plant produce is mainly because of the presence of pigments, vitamins (mainly ascorbic acid) and tannins. The reasons for higher retention of total antioxidant activity may be explained by lowering losses of anthocyanins, ascorbic acid and tannins. Kulkarni et al. (2004) reported that anthocyanins, ascorbic acid and phenolics are responsible for the antioxidant activity, either alone or in combination in several pomegranate cultivars. In pomegranate, major phenolic compound that contributes to antioxidant activity is punicalagin (Kulkarni et al. 2004), while delphinidin, cyanidin and pelargonidin were also suggested as participating constituents in antioxidant activity of arils (Noda et al. 2002). Another reason for higher antioxidant retention in PUT and carnauba wax treated arils could be attributed due to putrescine’s capacity to act as scavenger of free radicals (Drolet et al. 1986) and oxidation suppression activity of carnauba wax.
Sensory analysis
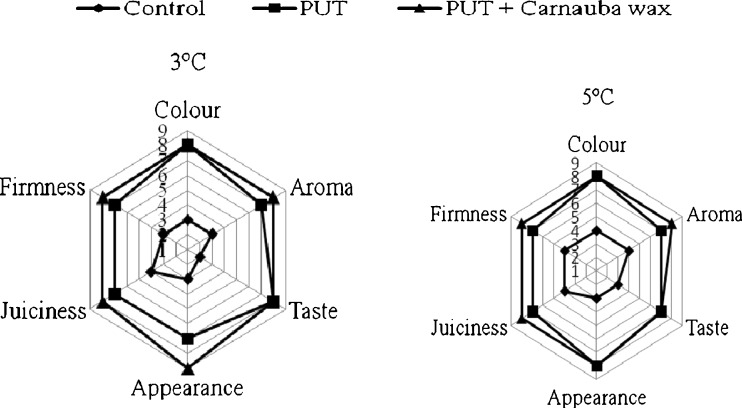
In sensory score study performed at 60th day of storage shown that score given in respect to color, aroma, taste, appearance, juiciness and firmness was much higher in PUT and carnauba wax treated fruits compared to control (Fig. 2). Untreated fruits lost their sensory qualities (color, aroma, taste and aril firmness) much faster at 3 °C compared to 5 °C storage temperature. Higher sensory score was awarded in respect to PUT + carnauba wax treated fruit arils followed by PUT and control under both the storage conditions.
Fig. 2.
Effect of putrescine and carnauba wax treatments on sensory attributes of pomegranate arils during cold storage (3 and 5 °C) + 3 days at 20 °C. Data are means ± S.E. of three replicate determination (n = 3)
Several parameters contribute to the overall quality and sensory attributes of fruits. Sensory attributes such as appearance, color, texture, aroma or some of the most important criteria used by a consumer to evaluate the immediate quality of fruits or vegetables (Nunes et al. 2007). Under both the storage conditions, fruit treated with PUT or PUT + carnauba wax got the highest sensory score. As shown earlier, PUT and carnauba wax application retained higher concentration of anthocyanins (color), ascorbic acid and tannins during storage, these attributes contributed towards better overall aril appearance and thus higher sensory score.
Conclusion
In conclusion, exogenous application of PUT and carnauba wax is an effective means for maintaining higher level of anthocyanins, antioxidant activity, ascorbic acid, tannins and sensory score of pomegranate during storage at 3 and 5 °C temperature, which otherwise leads to loss in functional properties of arils. As demonstrated in our results, combined application of PUT + carnauba wax could be a simple technique to maintain the functional properties of pomegranate during low temperature and long storage of pomegranate fruits. Further research is required to ascertain impact of PUT and carnauba wax treatment on extracted arils (minimally processed) in relation to health promoting/functional properties during prolonged storage.
Contributor Information
Kalyan Barman, Phone: +91-11-25848414, FAX: +91-11-25842177, Email: barman.kalyan@gmail.com.
Ram Asrey, Phone: +91-11-25848414, FAX: +91-11-25842177, Email: ramu_211@yahoo.com.
R. K. Pal, Phone: +91-11-25848414, FAX: +91-11-25842177, Email: rkrishnapal@gmail.com
Charanjit Kaur, Phone: +91-11-25848414, FAX: +91-11-25842177, Email: charanjitkaur6@gmail.com.
S. K. Jha, Phone: +91-11-25848414, FAX: +91-11-25842177, Email: skj_age@gmail.com
References
- Official methods of analysis. 17. Gaithersburg: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Apak R, Guclu K, Ozyurek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenol and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine, CUPRAC method. J Agric Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Asrey R, Barman K, Kumar A. Enjoying quality pomegranate for a longer period. Indian Hort. 2008;53:30–32. [Google Scholar]
- Bai J, Hagenmaier RD, Baldwin EA. Coating selection for ‘Delicious’ and other apples. Postharvest Biol Technol. 2003;28:381–390. doi: 10.1016/S0925-5214(02)00201-6. [DOI] [Google Scholar]
- Drolet G, Dumbroff EB, Legge RL, Thompson JE. Radical scavenging properties of polyamines. Phytochem. 1986;25:367–371. doi: 10.1016/S0031-9422(00)85482-5. [DOI] [Google Scholar]
- Elyatem SM, Kader AA. Post harvest physiology and storage behaviour of pomegranate fruits. Sci Hortic. 1984;24:287–298. doi: 10.1016/0304-4238(84)90113-4. [DOI] [Google Scholar]
- Ergun M, Sargent SA, Fox AJ, Crane JH, Huber DJ. Ripening and quality responses of mamey sapote fruit to postharvest wax and 1-methylcyclopropene treatments. Postharvest Biol Technol. 2005;36:127–134. doi: 10.1016/j.postharvbio.2004.12.002. [DOI] [Google Scholar]
- Eum HL, Hwang DK, Linke M, Lee SK, Zude M. Influence of edible coating on quality of plum (Prunus salicina Lindl. cv. ‘Sapphire’) Eur Food Res Technol. 2009;229:427–434. doi: 10.1007/s00217-009-1054-8. [DOI] [Google Scholar]
- Gil M, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Hertog MGL, Van-Poppel G, Verhoeven D. Potentially anticarcinogenic secondary metabolites from fruit and vegetables. In: Tomás-Barberán FA, Robins RJ, editors. Phytochemistry of fruit and vegetables. Oxford: Clarendon; 1997. pp. 313–329. [Google Scholar]
- Jones E, Hughes RE. Foliar ascorbic acid in some angiosperms. Phytochem. 1983;22:2493–2499. doi: 10.1016/0031-9422(83)80147-2. [DOI] [Google Scholar]
- Jurenka J. Therapeutic applications of pomegranate (Punica granatum L.): a review. Alternative Med Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- Khuyen THD, Singh Z, Swinny EE. Edible coatings influence fruit ripening, quality, and aroma biosynthesis in mango fruit. J Agric Food Chem. 2008;56:1361–1370. doi: 10.1021/jf072208a. [DOI] [PubMed] [Google Scholar]
- Koley TK, Asrey R, Pal RK, Samuel DVK. Shelf-life extension in pointed gourd (Trichosanthes dioica Roxb.) by post-harvest application of sodium hypochlorite, potassium metabisulphite and carnauba wax. J Food Sci Technol. 2009;46(6):581–584. [Google Scholar]
- Kulkarni AP, Aradhya SM. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem. 2005;93:319–324. doi: 10.1016/j.foodchem.2004.09.029. [DOI] [Google Scholar]
- Kulkarni AP, Aradhya SM, Divakar S. Isolation and identification of a radical scavenging antioxidant- punicalagin from pith and carpellary membrane of pomegranate fruit. Food Chem. 2004;87:551–557. doi: 10.1016/j.foodchem.2004.01.006. [DOI] [Google Scholar]
- Lansky E, Shubert S, Neeman I. Pharmacological and therapeutical properties of pomegranate. In: Melgarejo P, Martínez JJ, Martínez J, editors. Proceedings 1st International Symposium on Pomegranate. Orihuela: CIHEAM; 1998. pp. 231–235. [Google Scholar]
- Lee SK, Kader AA. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol. 2000;20:207–220. doi: 10.1016/S0925-5214(00)00133-2. [DOI] [Google Scholar]
- López-Rubira V, Conesa A, Allende A, Artés F. Shelf life and overall quality of minimaly processed pomegranate arils modified atmosphere packaged and treated with UV-C. Postharvest Biol Technol. 2005;37:174–185. doi: 10.1016/j.postharvbio.2005.04.003. [DOI] [Google Scholar]
- Martínez-Romero D, Valero D, Serrano M, Burló F, Carbonell A, Burgos L, Riquelme F. Exogenous polyamines and gibberellic acid effects on peach (Prunus persica L.) storability improvement. J Food Sci. 2000;65:288–294. doi: 10.1111/j.1365-2621.2000.tb15995.x. [DOI] [Google Scholar]
- Martínez-Romero D, Serrano M, Carbonell A, Burgos L, Riquelme F, Valero D. Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J Food Sci. 2002;67:1706–1712. doi: 10.1111/j.1365-2621.2002.tb08710.x. [DOI] [Google Scholar]
- Mirdehghan SH, Rahemi M, Castillo S, Martínez-Romero D, Serrano M, Valero D. Pre-storage application of polyamines by pressure or immersion improves shelf life of pomegranate stored at chilling temperature by increasing endogenous polyamine levels. Postharvest Biol Technol. 2007;44:26–33. doi: 10.1016/j.postharvbio.2006.11.010. [DOI] [Google Scholar]
- Mirdehghan SH, Rahemi M, Serrano M, Guillén F, Martínez-Romero D, Valero D. The application of polyamines by pressure or immersion as a tool to maintain functional properties in stored pomegranate arils. J Agric Food Chem. 2007;55:755–760. doi: 10.1021/jf062985v. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK. Storage of pomegranates (Punica granatum L.) Sci Cult. 1958;24:91. [Google Scholar]
- Noda Y, Kaneyuki T, Mori A, Packer L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyanidin and pelargonidin. J Agric Food Chem. 2002;50:166–171. doi: 10.1021/jf0108765. [DOI] [PubMed] [Google Scholar]
- Nunes MCN, Emond JP, Brecht JK, Dea S, Proulx E. Quality curves for mango fruit (cv. Tommy Atkins and Palmer) stored at chilling and nonchilling temperatures. J Food Qual. 2007;30:104–120. doi: 10.1111/j.1745-4557.2007.00109.x. [DOI] [Google Scholar]
- Purvis AC. Interaction of waxes and temperature in retarding moisture loss and chilling injury of cucumber fruits during storage. Pra Fla State Hort Soc. 1994;107:257–260. [Google Scholar]
- Resende JTV, Camargo LKP, Argandoña EJS, Marchese A, Camargo CK. Sensory analysis and chemical characterization of strawberry fruits. Hortic Brasileira. 2008;26:371–374. doi: 10.1590/S0102-05362008000300015. [DOI] [Google Scholar]
- Serrano M, Martínez-Romero D, Guillén F, Valero D. Effects of exogenous putrescine on improving shelf life of four plum cultivars. Postharvest Biol Technol. 2003;30:259–271. doi: 10.1016/S0925-5214(03)00113-3. [DOI] [Google Scholar]
- Valero D, Martínez-Romero D, Serrano M, Riquelme F. Influence of postharvest treatment with putrescine and calcium on endogenous polyamines, firmness, and abscisic acid in lemon (Citrus lemon L. Burm cv. Verna) J Agric Food Chem. 1998;46:2102–2109. doi: 10.1021/jf970866x. [DOI] [Google Scholar]
- Valero D, Martínez-Romero D, Serrano M. The role of polyamines in the improvement of the shelf life of fruit. Trends Food Sci Technol. 2002;13:228–234. doi: 10.1016/S0924-2244(02)00134-6. [DOI] [Google Scholar]
- Wang SY. Effect of pre-harvest conditions on antioxidant capacity in fruits. Acta Hort. 2006;712:299–305. [Google Scholar]
- Wang SY, Zheng W. Effect of plant growth temperature on antioxidant capacity in strawberry. J Agric Food Chem. 2001;49:4977–4982. doi: 10.1021/jf0106244. [DOI] [PubMed] [Google Scholar]
- Wrolstad RE, Durst RW, Lee J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci Technol. 2005;16:423–428. doi: 10.1016/j.tifs.2005.03.019. [DOI] [Google Scholar]
- Yahia EM, Contreras-Padilla M, Gonzalez-Aguilar G. Ascorbic acid content in relation to ascorbic acid oxidase and polyamine content in tomato and bell pepper fruits during development, maturation and senescence. Lebensm-Wiss Technol. 2001;34:452–457. doi: 10.1006/fstl.2001.0790. [DOI] [Google Scholar]