Abstract
Encapsulation is a technique used in foods that may protect some compounds with sensory impact, in particular flavoring as liquid smoke. We used the dripping method, obtaining two different layers for encapsulation of liquid smoke: calcium alginate and calcium alginate–chitosan. The results show that the load capacity of liquid smoke encapsulation reached values above 96 %. The beads exhibit syneresis at room temperature, but in opposite side, refrigeration temperature stabilizes the hydrogel of beads, allowing the samples loss weight less than 3 % after 72 h. Heated capsules with liquid smoke released several volatile compounds in the headspace and may identify 66 compounds. Among these volatile compounds, phenols derivatives can be considered sensory descriptors to contribute to the specific flavor of smoke. We conclude that the dripping method is highly efficient to encapsulate liquid smoke and released several volatile compounds, although it is necessary to minimize syneresis at room temperature.
Keywords: Liquid smoke, Encapsulation, Ca-alginate, Chitosan
Introduction
Smoke flavorings have several advantages compared to traditional smoking techniques: ease of application, speed, uniformity of the product, reproducibility of the characteristics obtained in the final smoked food, cleanliness of application and a decrease of the content of certain polycyclic aromatic hydrocarbons (Simon et al. 2005).
Flavor, as smoke flavorings, plays an important role in consumer satisfaction and influences further consumption of foods. However, manufacturing and storage processes, packaging materials and ingredients in foods often cause modifications in overall flavor by reducing aroma compound intensity or producing off-flavor components. Therefore, to limit aroma degradation or loss during processing and storage, it is beneficial to encapsulate volatile ingredients prior to use in foods or beverages (Madene et al. 2006).
Encapsulation may be defined as a process to entrap one substance within another substance, thereby producing particles with diameters of a few nm to a few mm. Some benefits of encapsulated ingredients in the food industry could be: superior handling of the active agent, immobility of active agent in food processing systems, improved stability in final product and during processing (i.e., less evaporation of volatile active agent and/or no degradation or reaction with other components in the food product such as oxygen or water), off-taste masking, controlled release (differentiation, release by the right stimulus) (Zuidam and Shimoni 2010). The smoke flavor is a sensory characteristic sought in some foods such as seafood and some meat products, currently achieved with conventional smoking or with the addition of liquid smoke (Montazeri et al. 2013). Liquid smoke encapsulating would give the desired smoke flavor with the advantages already mention that gives a flavor encapsulation.
Flavor encapsulation could be accomplished by a variety of methods. The two majors industrial processes are spray-drying and dripping (Madene et al. 2006). Encapsulation of flavors via dripping in glassy carbohydrate matrices has been used for volatile and unstable flavors. The principal advantage of the dripping method is the stability of the flavor against oxidation. Carbohydrate matrices have very good barrier properties and dripping is a convenient process enabling the encapsulation of flavors (Gouin 2004).
In this study, calcium alginate gel was employed as the matrix for flavor encapsulation. Alginate has been approved as a coating material by the US Food and Drug Administration and European Food Safety Authority. One of the major advantages of flavor encapsulation in alginate beads is that the encapsulation does not adversely affect the release of the flavor during consumption of the product. The beads provide a sustained release of the flavor to the product during storage and prior to consumption (De Roos 2006).
An additional encapsulating agent used in this study was chitosan. Chitosan, obtained from crab exoesquelets, has several polar groups such as –OH and –NH2 which can act as electron donors. Whereas alginates have been largely employed in drug delivery systems, there is increased interest in studying chitosan for its biological applications due to its mucous adhesiveness, no toxicity, biocompatibility and biodegradability (Sinha and Kumria 2001). Chitosan and alginate can react together by coacervation due to their opposite charges. The easy solubility of chitosan at low pH is prevented by the alginate network since alginate is insoluble at low pH conditions. The possible dissolution of alginate at higher pH is prevented by the chitosan which is stable at higher pH ranges (George and Abraham 2006). Alginate-chitosan capsules have been proposed to encapsulate natural antioxidants from yerba mate (Deladino et al. 2008) and some micronutrients such as ferrous fumarate, ascorbic acid and β-carotene (Han et al. 2008).
Thus, the objectives of this work were to encapsulate liquid smoke in calcium alginate beads, with and without a chitosan layer; to analyze the loading capacity, the syneresis process at ambient conditions, the stability of samples at refrigeration (4 °C), and the aromatic profiles of beads.
Materials and methods
Encapsulating agents
The encapsulating agents used were 1 % (w/v) sodium alginate (Sigma–Aldrich, USA, mannuronic acid to guluronic acid ratio 1.56, medium viscosity), and chitosan 1 % (w/v) in acetic acid (Sigma–Aldrich, USA, deacetylation degree higher than 75 %, density: 0.15–0.3 g/cm3).
Capsules formation
Capsules formation was carried out according to the dripping method of Deladino et al. (Deladino et al. 2008), with slight modifications. Beads were obtained by mixing mechanically the active component (commercial water-soluble liquid smoke, 1 % w/v) with the sodium alginate, forming a stable solution. Once homogenized, the alginate-liquid smoke solution was pumped with a peristaltic pump (Masteflex, model 7521–10, Barrington, Illinois, USA) at 0 60 cm3/min to a calcium chloride solution (0.05 M, Merck, Darmstadt, Germany). The beads formed in this process were maintained in the gelling bath to harden for 15 min. Then, they were filtered through a Whatman #1 paper and washed with buffer solution (acetic/acetate, pH 5.5). The beads, once filtered and washed, were allowed to stabilize (remove excess surface moisture) at ambient conditions (approx. 22 °C and 65 % relative humidity) for 15 min. Finally, a portion of the beads was immersed in a chitosan solution for 30 min to analyze the effect of an additional layer formed by complex coacervation alginate-chitosan. As a summary, four types of capsules were prepared: capsules of ca-alginate, ca-alginate beads with liquid smoke, ca-alginate-chitosan capsules and ca-alginate-chitosan beads with liquid smoke.
Beads characterization
Loading capacity of beads with the active component was determined gravimetrically according to the indirect method of Chang and Dobashi (Chang and Dobashi 2003) and Jiamrungraksa and Charuchinda (Jiamrungraksa and Charuchinda 2010), drying the beads in an oven at 120 °C for 2 h. The percentage of loading efficiency was calculated with the following equation:
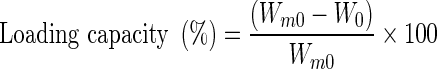 |
Where Wm0 and W0 denote the weight of beads measured before, and after complete evaporation of active component, respectively.
The weight loss (by syneresis or drying) of beads was determined at ambient conditions (approx. 22 °C) and at refrigeration (4 °C in a refrigerator) by weight difference (gravimetrically) for 72 h, respectively. All measurements were repeated at least in triplicate on bead samples from the same prepared batch.
Instrumental aromatic profiles of the samples
About 3 g of alginate and alginate-chitosan beads in triplicate, both with liquid smoke, were introduced separately into vials with screw cap and PTFE silicone septum (Supelco, Bellefonte, PA, USA) with 10 ml of physiological saline solution (1 % w/v NaCl). The samples were heated in a thermo block (Equilab 2050-ICE, Paris, France) during 60 min at 30 °C to equilibrate the headspace for to reach equilibrium. Instrumental aromatic profiles of the samples were obtained from the aromas to be released in the headspaces of the tubes, by the technique of solid phase microextraction (SPME) where the volatiles were adsorbed by the fiber Carboxen/Polydimethylsiloxane (Car/PDMS) and were subsequently released into the injection port of gas chromatograph Shimadzu GC-17 series equipped with a mass selective detector GCMS QP5050A Shimadzu (Tokyo, Japan) for the corresponding separation, identification and analysis (Gianelli et al. 2002, 2003). The fiber was exposed for 5 min in a normal position open purge valve (splitless mode) for complete desorption at 230 °C, after the time required SPME device was removed. Separated compounds using helium gas linear velocity of 28.3 cm s-1 in a capillary column DB-624 of 60 m in length, 0.25 mm inside and 1.4 μm of film (J & W Scientific; 60 m, 0.32 mm ID 1.8 μm, Folsom, USA). For the separation of the compounds was used with onset temperature program of 13 min at 38 °C. It was noted during this time the rise in temperature to 180 °C at 8 °C/min was performed also a second rise of 21.25 min until a maximum temperature of 230 °C at 4 °C/min. The time required for this experimental phase required 52 min (Gianelli et al. 2009).
Statistical analysis
Statistical analysis of data were performed through analysis of variance (ANOVA) using Statgraphics Centurion XVI Software. Differences among mean values were established by the least significant difference (LSD) at 5 %.
Results and discussion
Beads characterization
The diameter of capsules formed with ca-alginate and ca-alginate-chitosan (with and without liquid smoke) was approximately 4 mm (visual observation). Comparing with other studies that encapsulated flavors as vainilline ethyl (Manojlovic et al. 2008) or volatile tea-tree oil (Yeh et al. 2011), the size of ca-alginate capsules obtained was greater, due to the absence of factors as a small diameter syringe and/or high voltage which allows to decrease significantly the diameter of the capsules.
Capsules whit liquid smoke showed a high load capacity, 96.57 ± 0.02 and 98.11 ± 0.0 for ca-alginate and ca-alginate-chitosan, respectively; similarly, Deladino et al. (Deladino et al. 2008) obtained values higher than 85 % in ca-alginate loaded capsules with antioxidant extracts of yerba mate. Similar results were obtained by Bajpai and Tankhiwale (Bajpai and Tankhiwale 2006). However, these authors reported an important loss of active compound during immersion of capsules in chitosan, effect no observed in this study. The high efficiency of liquid smoke encapsulation in ca-alginate and ca-alginate-chitosan could be attributed to the water-soluble characteristic and low concentration of commercial liquid smoke used.
Syneresis is commonly seen in many biopolymer gel systems as ca-alginate gels and macroscopically is detected as a release of water from the gel (Donati and Paoletti 2009). The gel retains water through hydrogen bonds, but if the gel network contracts, some water will be squeezed out by diffusion (Helgerud et al. 2010). As observed, Fig. 1a shows the gradual syneresis of samples over time, showing a statistically significant increase of values over time for each treatment, reaching syneresis values at 72 h near of 8 % and 13 % for samples whit and without chitosan, respectively. The high values of syneresis can be attributed to a relative excess of calcium ions (0.05 M) in the gel formation step, tending to give gels with external gelation (Helgerud et al. 2010). On the other hand, Fig. 1a shows a significant reduction in syneresis of the samples by incorporating an additional layer of chitosan, could be associated to a reinforcement of the bead structure; the chitosan could bind to free alginate sites by cooperative ionic bounds.
Fig. 1.
Syneresis (%) of beads over time at room temperature (a), and Weight loss (%) of beads over time at refrigeration temperature (b). Each observation is a mean ± standard deviation of at least triplicate experiments. Letters represent a least significant difference (LSD) test. Ca-alginate: calcium-alginate; ca-alginate, liquid smoke: calcium-alginate with liquid smoke, ca-alginate-chitosan: calcium-alginate with a chitosan layer; ca-alginate-chitosan, liquid smoke: calcium-alginate with a chitosan layer and liquid smoke
Figure 1b shows the weight loss over time at refrigeration temperature, observing a reduced weight loss (minor of 3 % at 72 h in all samples), indicates that low temperature stabilizes the hydrogel in samples with, and without chitosan, disappearing syneresis. This behavior could be attributed to the temperature directly influences the rheological properties of materials, in particular experiencing a gradual molecular rearrangement and increasing the strength of calcium alginate gels when subjected to prolong cooling treatments (Papageorgiou et al. 1994).
Aromatic profiles of the samples
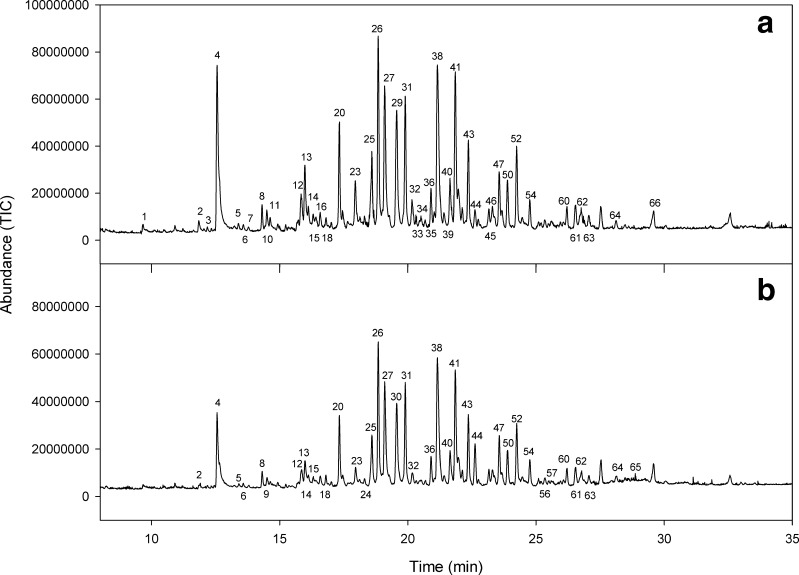
Figure 2 shows the chromatograms of volatile compound identified form headspace of capsules with liquid smoke. Table 1 lists the compounds identified, 66 in total, of which 59 were identified from the samples with chitosan (see Fig. 2a) and 60 from the samples without chitosan (see Fig. 2b). Among the substances identified, compounds arising from liquid smoke and specifically from the thermal degradation of the three main classes of wood components, i.e. carbonyls, furans and pyrans derivatives, as well as some compounds that could mainly arise from cellulose and hemicellulose pyrolysis (Guillén et al. 1995). Among these volatile compounds, phenols derivatives such as eugenol, guaiacol (in this case p-ethylguaiacol), cresol and dimethyl-phenol (see Table 1) can be considered sensory descriptors to contribute to the specific flavor of smoke (according to the review by (Simon et al. 2005)).
Fig. 2.
Chromatogram obtained by SPME-GC-MS after fibre CAR/PDMS exposition for 1 h at 30 °C in the headspace of ca-alginate beads with liquid smoke beads without (a) and with (b) a chitosan layer. The numbers represent compounds identified and listed in Table 1. SPME-GC-MS: Solid Phase Microextraction-Gas Chromatograph- Mass Selective CAR/PDMS: Carboxen/Polydimethylsiloxane
Table 1.
Volatile compounds identified in the headspace of ca-alginate with liquid smoke beads with and without a chitosan layer using SPME-GC-MS and CAR/PDMS
| N°a | Compoundb | Trc | Without chitosan | With chitosan | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aread | DSe | %Areaf | Aread | DSe | %Areaf | ||||
| 1 | 2,3-Dihydro-3-methyl-furan | 9.662 | 11578401 | 95771 | 0.22 | 5831328 | 466262 | 0.16 | |
| 2 | BCH | 11.843 | 23895215 | 2728274 | 0.45 | 7141351 | 2961590 | 0.20 | (Guillen et al. 1995) |
| 3 | 5-Methyl-2(5H)-furanone | 12.181 | 6072394 | 254826 | 0.11 | 3497023 | 438930 | 0.10 | |
| 4 | Furfural | 12.560 | 507395584 | 74669666 | 9.58 | 186358024 | 69785027 | 5.12 | (Guillen et al. 1995; Meier 2009) |
| 5 | Pentan-2-one | 13.386 | 9032961 | 1526339 | 0.17 | – | – | – | |
| 6 | Ethylene | 13.410 | – | – | – | 5038574 | 1835822 | 0.14 | |
| 7 | Heptanal | 13.582 | – | – | – | 6403195 | 1801316 | 0.18 | |
| 8 | 2-Methyl-2-cyclopenten-1-one | 14.319 | 32261566 | 501489 | 0.61 | 25129879 | 3477631 | 0.69 | (Guillen et al. 1995; Meier 2009) |
| 9 | 1-(2-furanyl)-1-Heptanone | 14.426 | 4617095 | 2375484 | 0.09 | – | – | 0.00 | |
| 10 | 1-(2-furanyl)-Ethanone, | 14.495 | 33017859 | 6243563 | 0.62 | 23648880 | 11935306 | 0.65 | (Guillen et al. 1995; Sung et al. 2007) |
| 11 | 5-Methyl-2-furfural | 14.629 | 17656262 | 7157390 | 0.33 | 9919541 | 7551758 | 0.27 | (Guillen et al. 1995; Meier 2009; Sung et al. 2007) |
| 12 | Benzaldehyde | 15.843 | 83698562 | 10669095 | 1.58 | 40852832 | 10758537 | 1.12 | |
| 13 | 5-Methyl-2-furfural | 15.979 | 123476336 | 10184382 | 2.33 | 58673022 | 1661305 | 1.61 | (Guillen et al. 1995; Meier 2009; Sung et al. 2007) |
| 14 | Octanal | 16.120 | 48208638 | 248513 | 0.91 | 22714179 | 10820335 | 0.62 | |
| 15 | 2,3-Benzofuran | 16.313 | 31020124 | 16634694 | 0.59 | 17274521 | 12157214 | 0.47 | |
| 16 | 3-Methyl-2-cyclopenten-1-one | 16.416 | 33895301 | 1158154 | 0.64 | – | – | – | (Guillen et al. 1995; Kostyra and Barylko-Pikielna 2006; Meier 2009) |
| 17 | 2,3-Dimethyl-2-cyclopenten-1-one | 16.574 | 35854182 | 5686148 | 0.68 | 24072308 | 9631759 | 0.66 | (Meier 2009; Sung et al. 2007) |
| 18 | 2-Ethyl-1-hexanol | 16.805 | 29943294 | 5468244 | 0.57 | 18670085 | 8305772 | 0.51 | |
| 19 | BCH | 17.021 | 18026609 | 10764101 | 0.34 | 11280184 | 10904442 | 0.31 | |
| 20 | Phenol | 17.330 | 195022011 | 167897 | 3.68 | 130974173 | 10813942 | 3.60 | (Guillen et al. 1995; Kostyra and Barylko-Pikielna 2006; Meier 2009; Sung et al. 2007) |
| 21 | BCH | 17.465 | 36536245 | 11751074 | 0.69 | 27390328 | 1454229 | 0.75 | |
| 22 | 3-Methyl-1,2-cyclopentanedione | 17.649 | 26392343 | 23355974 | 0.50 | 22580993 | 8278752 | 0.62 | (Guillen et al. 1995; Kostyra and Barylko-Pikielna 2006) |
| 23 | 2-Hydroxy-benzaldehyde | 17.955 | 93256347 | 26397546 | 1.76 | 46533158 | 111686 | 1.28 | |
| 24 | 5-Ethyl-2-furaldehyde | 18.311 | 21372376 | 7123980 | 0.40 | 25682110 | 2390320 | 0.71 | |
| 25 | Nonanal | 18.597 | 163765051 | 21789489 | 3.09 | 125077875 | 12155338 | 3.43 | |
| 26 | 2-Methylphenol(o-Cresol) | 18.847 | 378525870 | 4848340 | 7.15 | 291121987 | 23755079 | 7.99 | (Guillen et al. 1995; Kostyra and Barylko-Pikielna 2006; Meier 2009; Sung et al. 2007) |
| 27 | 2-Methoxy-phenol | 19.102 | 344884536 | 29101825 | 6.51 | 256166463 | 30069657 | 7.03 | (Guillen et al. 1995; Kostyra and Barylko-Pikielna 2006; Meier 2009) |
| 28 | BCH | 19.267 | – | – | – | 21329852 | 2867259 | 0.59 | |
| 29 | 3-Methylphenol | 19.563 | 309446840 | 5524007 | 5.85 | – | – | – | (Guillen et al. 1995) |
| 30 | 4-Methylphenol | 19.569 | – | – | – | 225001676 | 13529714 | 6.18 | (Guillen et al. 1995; Sung et al. 2007) |
| 31 | 2,6-Dimethylphenol | 19.901 | 277377673 | 10105398 | 5.24 | 218001136 | 19938202 | 5.99 | (Meier 2009) |
| 32 | 2-Hydroxy-4-methylbenzaldehyde | 20.169 | 61258353 | 14053698 | 1.16 | 36284039 | 2260354 | 1.00 | |
| 33 | BCH | 20.340 | 14840447 | 870018 | 0.28 | 12242113 | 1156621 | 0.34 | |
| 34 | BCH | 20.522 | 25329096 | 5903907 | 0.48 | 23534052 | 27722 | 0.65 | |
| 35 | 1,3-Dimethoxybenzene | 20.698 | 18729946 | 6553074 | 0.35 | 13022534 | 470820 | 0.36 | (Sung et al. 2007) |
| 36 | 2-Ethylphenol | 20.908 | 77990925 | 11250054 | 1.47 | 64163413 | 11231097 | 1.76 | (Guillen et al. 1995; Meier 2009) |
| 37 | Unknown | 21.033 | 26487257 | 4105222 | 0.50 | 14299323 | 250164 | 0.39 | |
| 38 | 2,4-Dimethylphenol | 21.156 | 488187860 | 33929962 | 9.22 | 374696935 | 39143494 | 10.29 | (Guillen et al. 1995; Kostyra and Barylko-Pikielna 2006; Meier 2009) |
| 39 | 1-(2-Methylphenyl)-ethanone | 21.411 | 29300453 | 16698957 | 0.55 | 30917915 | 2763154 | 0.85 | |
| 40 | Naphthalene | 21.652 | 106911541 | 19237935 | 2.02 | 91953746 | 7164535 | 2.53 | (Kostyra and Barylko-Pikielna 2006) |
| 41 | 2-Methoxy-4-methylphenol (p-Methylguaiacol) | 21.852 | 332958768 | 20972600 | 6.29 | 248265618 | 25557305 | 6.82 | (Guillen et al. 1995; Kostyra and Barylko-Pikielna 2006; Meier 2009) |
| 42 | 2-Ethyl-phenol | 21.960 | 101380482 | 6338682 | 1.92 | 75256746 | 5123074 | 2.07 | (Guillen et al. 1995) |
| 43 | 2,4,5-trimethylphenol | 22.356 | 183127976 | 5517947 | 3.46 | 147420825 | 26582533 | 4.05 | (Meier 2009) |
| 44 | 3,4-Dimethylphenol | 22.755 | 20387918 | 5085791 | 0.39 | – | – | – | (Guillen et al. 1995) |
| 45 | 3-Ethyl-5-methylphenol | 23.160 | 40388048 | 9374124 | 0.76 | 33751630 | 10040535 | 0.93 | |
| 46 | 4-Ethyl-2-methoxyphenol | 23.372 | 16468359 | 6241374 | 0.31 | (Guillen et al. 1995; Meier 2009; Sung et al. 2007) | |||
| 47 | 4-(1-Methylethylphenol)phenol | 23.564 | 123829964 | 2.34 | – | – | – | ||
| 48 | 1-Ethyl-4-methoxybenzene | 23.568 | 102179530 | – | 1.93 | 96892187 | 13435809 | 2.66 | |
| 49 | 3,5-Diethylphenol | 23.664 | 38510588 | 3261678 | 0.73 | 27626510 | 6877429 | 0.76 | (Guillen et al. 1995; Meier 2009) |
| 50 | 1,4-Bimethoxy-2-methybenzene | 23.891 | 87451406 | 10307139 | 1.65 | 67681597 | 9712379 | 1.86 | (Guillen et al. 1995) |
| 51 | 1-Methoxy-4-propylbenzene | 24.084 | 7180357 | – | 0.14 | 6856333 | 702480 | 0.19 | |
| 52 | 4-Ethyl-2-methoxyphenol (p-Ethylguaiacol) | 24.251 | 168730013 | 24704559 | 3.19 | 122989676 | 19412247 | 3.38 | (Guillen et al. 1995; Kostyra and Barylko-Pikielna 2006; Meier 2009; Sung et al. 2007) |
| 53 | 2,4,6-trimethyl phenol | 24.459 | 23494068 | 12087515 | 0.44 | 11660976 | 1331829 | 0.32 | (Meier 2009) |
| 54 | 2-methyl-5-(1-methylethyl)phenol | 24.753 | 55017812 | 14085262 | 1.04 | 46876225 | 6262767 | 1.29 | |
| 55 | (2-Propynyloxy)benzene | 25.188 | 10214222 | 711819 | 0.19 | – | – | – | |
| 56 | 2-Methylnaphthalene | 25.354 | – | – | – | 13604769 | 916057 | 0.37 | |
| 57 | Diethylphenol | 25.499 | – | – | – | 4759703 | 179286 | 0.13 | |
| 58 | 3-Methoxy-4,5,6-trimethylphenol | 25.950 | 10267882 | 1192968 | 0.19 | 5526530 | 834030 | 0.15 | |
| 59 | 1-Methoxy-4-propylbenzene | 26.060 | 13979375 | 3753282 | 0.26 | 11383581 | 3584877 | 0.31 | |
| 60 | 4-(1,1-dimethylethyl)-1,2-Benzenediol | 26.211 | 39580375 | 3822889 | 0.75 | 31898957 | 3892558 | 0.88 | |
| 61 | 2-Methoxy-4-(2-propenyl)phenol(Eugenol) | 26.546 | 52247408 | 5267905 | 0.99 | 40852731 | 6683998 | 1.12 | (Kostyra and Barylko-Pikielna 2006; Meier 2009) |
| 62 | 2-Methoxy-4-(1-propyl)phenol(Guaiacylpropane) | 26.768 | 52885924 | – | 1.00 | 41168817 | 2334223 | 1.13 | (Guillen et al. 1995; Kostyra and Barylko-Pikielna 2006) |
| 63 | 2-Methyl-propanoic acid | 27.066 | 23595182 | 619244 | 0.45 | 23614267 | 3267230 | 0.65 | |
| 64 | 3-Allyl-6-methoxyphenol | 28.131 | 12904473 | 2450298 | 0.24 | 14205115 | 231142 | 0.39 | |
| 65 | 1,2-Dimethoxy-4-(2-propenyl)benzene | 28.617 | 5353596 | 1016535 | 0.10 | – | – | – | |
| 66 | Methylesterdodecanoicacid | 29.588 | 26348661 | 24449268 | 0.50 | 51593732 | 8264385 | 1.42 | |
| Total | 5293747929 | 100 | 3641365257 | 100 | |||||
a Number of peak (Fig. 2); bBCH: Branched Chain Hydrocarbon; cTime retention (min) for capillary column (J & W Scientific; 60 m, 0.32 mm ID. 1.8 μm) installed on a gas chromatograph equipped with detector, chromatographic conditions detailed in Materials and Methods; dResults expressed as mean of three replicates of total ion current (TIC); eStandard Deviation; fPercentage of total TIC area
SPME-GC-MS Solid Phase Microextraction-Gas Chromatograph-Mass Selective; CAR/PDMS Carboxen/Polydimethylsiloxane
On the other hand, Fig. 2 show greater abundance (TIC) of volatile compounds in the samples without chitosan, showing a greater total area than samples with chitosan (see Table 1), which could be attributed to the chitosan can decrease the permeability of the capsules by crosslinking with the calcium alginate gel. Furthermore, it is confirmed that the temperature applied (30 °C for 1 h) was sufficient for the release of volatile compounds from samples, some of which have been recognized as responsible for smoke flavor. Relative to the effect of temperature, Serp et al. (2002), Manojlovic et al. (2008) and Yeh et al. (2011) clearly demonstrated that calcium alginate capsules change their physical properties and in particular significantly increase their permeability, which can explains the release of volatile compounds in the present study. In particular, one can consider that the model by Yeh et al. (2011) can explain the diffusion of volatile compounds from the beads with liquid smoke, where a volatile liquid compound first evaporates inside the capsule and as vapor diffuses outside.
Conclusions
Capsules of ca-alginate and ca-alginate-chitosan whit liquid smoke showed a high load capacity (over 96 %). However, the beads exhibit high values of syneresis at room temperature after 72 h, syneresis that is reduced in samples than have the additional layer of chitosan (8 % versus 13 % in samples with and without chitosan, respectively). A factor that allows to stabilize the hydrogel of beads is refrigerated storage (4 °C), allowing the samples loss weight less than 3 % after 72 h. On the other hand, it is confirmed that the temperature applied was sufficient for the release of volatile compounds from samples, and the chromatograms show greater abundance (TIC) of volatile compounds in the samples without chitosan, showing a greater total area than samples with chitosan, which could be attributed to the chitosan can decrease the permeability of the capsules by crosslinking with the calcium alginate gel. Among these volatile compounds, phenols derivatives such as eugenol, p-ethylguaiacol, cresol and dimethyl-phenol can be considered sensory descriptors to contribute to the specific flavor of smoke. In summary, the dripping method is highly efficient to encapsulate liquid smoke and the beads diffuse volatile compounds with sensory impact on the flavor of smoke, although it is necessary to minimize syneresis at room temperature through a better balance calcium/alginate or considered refrigerated storage to increase the stability of the beads.
Acknowledgements
We thank the Universidad del Bío-Bío, project DIUBB 114022 3/R for its financial support.
References
- Bajpai SK, Tankhiwale R. Investigation of dynamic release of vitamin B2 from calcium alginate/chitosan multilayered beads: part II. React Funct Polym. 2006;66:1565–1574. doi: 10.1016/j.reactfunctpolym.2006.05.007. [DOI] [Google Scholar]
- Chang CP, Dobashi T. Preparation of alginate complex capsules containing eucalyptus essential oil and its controlled release. Colloids Surf B: Biointerfaces. 2003;32:257–262. doi: 10.1016/j.colsurfb.2003.07.002. [DOI] [Google Scholar]
- De Roos KB. Understanding and controlling the behavior of aroma compounds in thermally processed foods. Trends Food Sci Technol. 2006;17(5):236–243. doi: 10.1016/j.tifs.2005.11.008. [DOI] [Google Scholar]
- Deladino L, Pablo SA, Anbidner A, Navarro N, Miriam NM. Encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr Polym. 2008;71:126–134. doi: 10.1016/j.carbpol.2007.05.030. [DOI] [Google Scholar]
- Donati I, Paoletti S. Material properties of alginates. In: Rehm BHA, editor. Alginates: biology and applications. Berlin: Springer; 2009. pp. 1–53. [Google Scholar]
- George M, Abraham E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan – a review. J Controlled Release. 2006;114:1–14. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Gianelli MP, Flores M, Toldra F. Optimization of solid phase microextraction (SPME) for the analysis of volatile compounds in dry-cured ham. J Sci Food Agric. 2002;82:1703–1709. doi: 10.1002/jsfa.1249. [DOI] [Google Scholar]
- Gianelli MP, Flores M, Toldra F. Interaction of solid phase microextraction (SPME) for the analysis of volatile compounds in dry cured ham. J Agric Food Chem. 2003;51:6828–6834. doi: 10.1021/jf0303666. [DOI] [PubMed] [Google Scholar]
- Gianelli MP, Valdebenito P, Fritz M, Flores M. Fat acts as reservoir for volatile compounds. Effect of the cooking process on the development of volatile compounds in “longaniza” sausage from Chillán, Chile. Fleischwirtschaft Int. 2009;24:39–44. [Google Scholar]
- Gouin S. Microencapulation: industrial appraisal of existing technologies and trends. Trends Food Sci Technol. 2004;15:330–347. doi: 10.1016/j.tifs.2003.10.005. [DOI] [Google Scholar]
- Guillén MD, Manzanos MJ, Zabala L. Study of a commercial liquid smoke flavoring by means of gas chromatography/mass spectrometry and Fourier transform infrared spectroscopy. J Agric Food Chem. 1995;43:463–468. doi: 10.1021/jf00050a039. [DOI] [Google Scholar]
- Han J, Guenier A-S, Salmieri S, Lacroix M. Alginate and chitosan functionalization for micronutrient encapsulation. J Agric Food Chem. 2008;56:2528–2535. doi: 10.1021/jf703739k. [DOI] [PubMed] [Google Scholar]
- Helgerud T, Gåserød O, Fjæreide T, Andersen PO, Larsen CK. Alginates. In: Imeson A, editor. Food stabilizers, thickeners and gelling agents. Iowa: Willey-Blackwell; 2010. pp. 50–56. [Google Scholar]
- Jiamrungraksa T, Charuchinda S. Preparation and characteristics of galangal essential oil/alginate microcapsules. J Met, Mater Miner. 2010;20(2):89–92. [Google Scholar]
- Kostyra E, Barylko-Pikielna N. Volatiles composition and flavour profile identity of smoke flavourings. Food Qual Prefer. 2006;17:85–95. doi: 10.1016/j.foodqual.2005.06.008. [DOI] [Google Scholar]
- Madene A, Jacquot M, Scher J, Desobry S. Flavour encapsulation and controlled release- a review. Int J Food Sci Technol. 2006;41:1–21. doi: 10.1111/j.1365-2621.2005.00980.x. [DOI] [Google Scholar]
- Manojlovic V, Rajic N, Djonlagic J, Obradovic B, Nedovic V, Bugarski B. Application of electrostatic extrusion flavour encapsulation and controlled release. Sensors. 2008;8:1488–1496. doi: 10.3390/s8031488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier D. Additives: smoke flavorings. In: Nollet L, Toldrá F, editors. Handbook of processed meats and poultry analysis. Boca Raton: CRC Press; 2009. pp. 109–128. [Google Scholar]
- Montazeri N, Oliveira ACM, Himelbloom BH, Leigh MB, Crapo CA. Chemical characterization of commercial liquid smoke products. Food Sci Nutr. 2013;1:102–115. doi: 10.1002/fsn3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou M, Stefan K, Michelle GG. Structural and textural properties of calcium, hot-made alginate gels. Carbohydr Polym. 1994;24:199–207. doi: 10.1016/0144-8617(94)90131-7. [DOI] [Google Scholar]
- Serp D, Mueller M, von Stockar U, Marison IW. Low-temperature electron microscope for the study of polysaccharide ultrastructure in hydrogels. II. Effect of temperature on the structure of Ca2+-alginate beads. Biotechnol Bioeng. 2002;79:253–259. doi: 10.1002/bit.10287. [DOI] [PubMed] [Google Scholar]
- Simon R, de la Calle B, Palme S, Meier D, Anklam E. Composition and analysis of liquid smoke flavouring primary products. J Sep Sci. 2005;28:871–882. doi: 10.1002/jssc.200500009. [DOI] [PubMed] [Google Scholar]
- Sinha VR, Kumria R. Polysaccharides in colon specific drug delivery. Int J Pharm. 2001;224:19–38. doi: 10.1016/S0378-5173(01)00720-7. [DOI] [PubMed] [Google Scholar]
- Sung W-C, Huang C-H, Sun F-M. Volatile components detected in liquid smoke flavoring preparations from two types of rice hull. Chia-nan Ann Bull. 2007;33:13–20. [Google Scholar]
- Yeh KW, Chang CP, Yamamoto T, Dobashi T. Release model of alginate microcapsules containing volatile tea-tree oil. Colloids Surf. 2011;380:152–155. doi: 10.1016/j.colsurfa.2011.02.043. [DOI] [Google Scholar]
- Zuidam JN, Shimoni E. Overview of microencapsulation for use in food products or processes and methods. In: Nedovics VA, Zuidam JN, editors. Encapsulation technologies for active food ingredients and food processing. New York: Springer; 2010. pp. 1–6. [Google Scholar]