Abstract
Synbiotics offer potential in the prophylactic management of gastrointestinal disorders. Therefore, present study evaluates the effect of prebiotics (inulin and gum acacia) on Lactocaillus plantarum for developing a freeze-dried synbiotic product from the selected culture. L. plantarum exhibited the highest specific growth rate (0.23/h) in presence of inulin followed by gum acacia (0.22/h) and glucose (0.22/h). Preparation of the lyophilized synbiotic powder incorporating inulin or gum acacia and using non fat dry milk as base material was standardized. Throughout refrigerated storage for 90 days, viable counts (i. e. 8 to 9 log cfu/g) of the probiotic bacteria in the product remained high, while a considerable reduction in the counts was observed in the product stored at room temperature (25 ± 1 °C).
Keywords: Lactobacillus plantarum, Synbiotic, Prebiotics, Gum acacia, Inulin
Introduction
Probiotics have established their efficacy as health promoting organisms. These are defined as live microbial cultures, which on ingestion in sufficient numbers provide beneficial effects to consumers beyond basic inherent nutrition. To be selected a probiotic, a culture must undergo various in vitro as well as in vivo tests (Mishra and Prasad 2005; Mishra et al. 2008) of which establishment of the organism in gastrointestinal tract is necessary (Sudha et al. 2006). There have been continuous efforts in improving the functional performance of probiotics in gastro-intestinal tract using dietary ingredients known as prebiotics. These are non digestible ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of bacteria in colon that can improve host health (Gibson and Roberfroid 1995; Liong 2008; Stoyanova et al. 2010). Non-digestible carbohydrates, oligo- and poly- saccharides, occur naturally and meet the criteria of prebiotics (Ziemer and Gibson 1998). Certain non-digestible carbohydrates such as inulin seem authentic prebiotics. A number of other non-digestible oligosaccharides have now been developed of which gum acacia is a potential candidate. Gum acacia has been demonstrated as highly fermentable in vivo both in humans (McLeanRoss et al. 1983) and in rats (McLeanRoss et al. 1984) and in vitro using human faecal flora (Bourquin et al. 1993).
To increase the microbial management in gastrointestinal tract, probiotics and prebiotics are combined to develop synbiotics. Evidences suggest that combination of suitable prebiotics with probiotics enhance the survival and activity of the organism (Gibson et al. 1995; Nagpal et al. 2007). The mixture could improve the survival of the probiotic organisms as its specific substrate, prebiotic, is readily available for fermentation. Synbiotic has been found to exert a synergistic effect in reducing colon carcinogenesis compared to when both were used individually (Liong 2008). As application of probiotic cultures requires processing and subsequent storage, maintaining the viability of cultures is a major challenge. Frozen or dried form, as either freeze-dried or spray-dried powders, is considered suitable mode of delivery for application in food (Holzapfel et al. 2001) due to longer shelf-life along with reductions in the costs of transport and storage and improved culture stability. Freeze drying is considered most convenient method for the production of dried probiotic/synbiotic preparations. The present research has been carried out to develop a freeze-dried synbiotic formula using a selected probiotic culture viz., Lactobacillus plantarum (Dhewa et al. 2010) and inulin and gum acacia as prebiotics .
Materials and methods
Bacterial strains
Lactic culture: Lactobacillus plantarum of human faecal origin was procured from Department of Microbiology, Dolphin (PG) Institute of Biomedical and Natural Sciences, Dehradun. The strain was characterized by morphological, physiological, biochemical and sugar utilization pattern tests and the data obtained for genus and species identification were also subjected to software called PIBWin (Bryant 2004). Lactic cultures were maintained in glycerol stocks at −20 °C and sub-cultured in MRS broth when required. The culture was studied for in vitro probiotic parameters such as acid and bile tolerance, cell surface hydrophobicity etc. (Dhewa et al. 2010).
Indicator strains: Organisms viz., Escherichia coli MTCC443, Bacillus cereus NCDC240, Enterococcus faecalis MTCC439, Salmonella typhimurium NCDC113 and Staphylococcus aureus MTCC87 procured from National Collection of Dairy Cultures (NCDC), National Dairy Research Institute, Karnal and Microbial Type Culture Collection (MTCC) Chandigarh, were used as indicator organisms for antimicrobial activity. Indicator cultures were stored on nutrient agar slant at refrigeration temperature (4 °C) and routine sub culturing was done. The purity of cultures was tested regularly by Gram staining.
Growth of L. plantarum in presence of prebiotics: Carbohydrate free modified MRS broth containing Bromocresol Purple (30 mg/l) was used as the basal medium to study the utilization pattern of different prebiotics (inulin and gum acacia @ 0.5, 1.0, 3.0 and 5.0%) by L. plantarum. Ten ml of each medium was transferred aseptically in sterile test tubes and tubes were inoculated with active lactobacilli cultures containing approximately 1010 cfu/ml. Medium containing glucose as sole energy source was taken as control. The inoculated tubes were incubated aerobically at 37 °C for 24 h. pH and viable counts of L. plantarum were determined at an interval of 6, 12, 18, 24 h. For viable counts, 1 ml of culture from each tube was taken immediately and serial dilutions were prepared in 0.85% sterile saline. Appropriate dilutions were pour plated in sterile MRS agar. The plates were incubated in inverted position aerobically at 37 °C and colonies developed were counted after incubation period. Specific growth rate (μ) and mean doubling time (Td) for lactobacilli grown in inulin and gum acacia were calculated (Kaplan and Hutkins 2003).
Antibacterial Activity of the lactic culture against enteric organisms in the presence of prebiotics: The antimicrobial activity of the L. plantarum grown in modified MRS containing inulin and gum acacia added @ 3% was determined and compared with that grown in modified MRS containing glucose by agar well method. Nutrient agar containing 0.1% Tween-80 was seeded with 100 μL of 24 h old culture of indicator organism (containing approximately 108 cells/ml), stirred gently and poured into the plates. These were then allowed to solidify. Wells (6 mm dia) were bored at equal distance on the solidified agar medium in each of those plates. A sterile cylindrical hollow stainless steel gel- cutter (6 mm dia) was used for this purpose. Then holes were filled (except central hole) with 50 μL of the 24 h old culture of L. plantarum grown in modified MRS containing either glucose or inulin and/or gum acacia as the sole carbon source. The central hole filled with sterile distilled water was used as a control. The plates were kept at room temperature until the liquid was absorbed, then incubated at 37 °C for 48 h and the diameter (mm) of inhibition zone measured.
Development of a lyophilized synbiotic preparation containing lactobacilli and prebiotics: The freeze dried synbiotic formulation was developed on the basis of the method followed earlier by Collins and Hall (1984) and Crittenden et al. (2006) with suitable modifications. The selected Lactobacillus culture was inoculated into modified MRS broth @ 2% and incubated at 37 °C for 48 h under aerobic conditions. The cells were harvested by centrifugation at 14,500 g for 10 min at 4 °C, washed once in sterilized distilled water and resuspended at the rate of 1010 cfu/ml in a sterilized mix containing 5% skim milk powder, 10 and 20% inulin or gum acacia, 8% sucrose and 1.5% gelatin (gelatin was separately sterilized). The mix thus obtained was poured into sterile petri plates and freeze-dried at room temperature; the condenser was cooled to −55 °C.
Storage studies of synbiotic product: In order to carry out storage studies, the synbiotic product was aseptically transferred into sterile moisture proof 30 ml glass roll tubes and stored at room (25 ± 1 °C) and refrigeration temperatures (4 ± 1 °C). Viability of probiotic organisms was determined on 0, 7, 14, 21, 30, 60 and 90 days of storage. Freeze-dried samples were taken at random. For viable counts, 1 g of synbiotic product was immediately suspended in 9 ml of normal saline (0.85%) and manually homogenized under aseptic conditions. One ml of culture from each tube was taken immediately and serial dilutions were prepared in 0.85% sterile saline. Appropriate dilutions were pour plated in sterile MRS agar. The plates were incubated in inverted position aerobically at 37 °C and colonies developed were counted after incubation period.
Statistical analysis
Data obtained were statistically analyzed using the software SPSS 12.0 (SPSS, Inc., Chicago, IL). Paired samples T test was used for normal distribution and differences were considered significant if the P value was <0.05.
Results and discussion
Growth of Lactobacilli cultures in presence of prebiotics
Lactobacillus has complex nutritional requirements which vary markedly from species to species. Incorporation of prebiotics has been proved to improve the viability of probiotics by selectively stimulating their growth and several of the experimental prebiotics, have been shown to be beneficial in favorably modulating the microbial ecology of the gut, protecting against various intestinal pathogens and in some instances, boosting gastrointestinal immunity (Grizard and Barthomeuf 1999). A primary screening for the growth of the L. plantarum on selected prebiotic substances i.e. inulin and gum acacia revealed successful growth of the organism indicated by development of a yellow color around bacterial spotting.
Effect of inulin and gum acacia on the growth of lactobacilli cultures
During present investigation, potential probiotic strain was allowed to grow in different prebiotics (inulin and gum acacia) at different concentrations (0.5, 1.0, 3.0 and 5.0%) for a time period of 0–24 h. Varied results were observed with the prebiotics and the μ and Td for lactobacillus grown in inulin and gum acacia were calculated (Kaplan and Hutkins 2003).
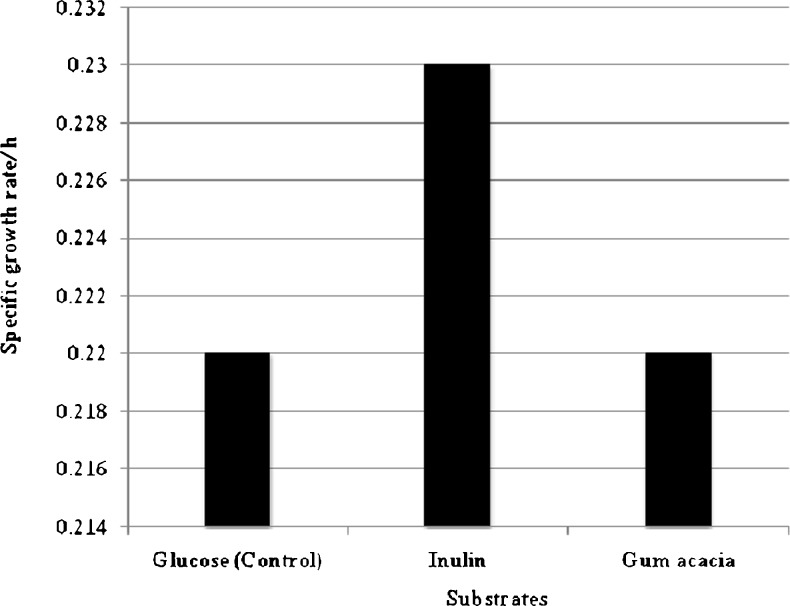
Data on the effect of different concentrations of prebiotics (inulin and gum acacia) on the specific growth rate L. plantarum are shown in Table 1. The strain gave promising results as it could utilize the inulin and gum acacia with maximum μ at 3% of concentration for the period of 0–6, 6–12, 12–18 and 18–24 h, respectively. Results indicated that 3% of prebiotic concentration showed maximum growth and activity of L. plantarum though difference with other concentrations was non significant. Figure 1 exhibited that the μ (0.23/h) of L. plantarum was highest in presence of inulin followed by gum acacia (0.22/h) and glucose (0.22/h). There were several reports (Gibson and Roberfroid 1995; MacBrain and MacFarlane 1997; Hopkins et al.1998; Kolida et al.2002) indicating in vitro effect of prebiotic on lactobacilli and bifidobacteria. Gibson and Wang (1994) confirmed the prebiotic effects of inulin and oligofructose in an in vitro study. Kaplan and Hutkins (2000) screened a selection of 28 LAB and bifidobacteria for their ability to ferment inulin and oligofructose on MRS agar. Twelve of 16 Lactobacillus strains and seven of eight Bifidobacterium strains tested were able to ferment the substrate (Kaplan and Hutkins 2000). Through molecular techniques it was demonstrated that inulin and oligofructose were selectively fermented not only by bifidobacteria but also by lactobacilli (Sghir et al. 1998).
Table 1.
Effect of the prebiotic concentrations (inulin and gum acacia) on the specific growth rate* (μ) of L. plantarum
| Incubation (h) | Control 1 glucose (1%) | Inulin | Gum acacia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (%) | Concentration (%) | ||||||||
| 0.5 | 1.0 | 3.0 | 5.0 | 0.5 | 1.0 | 3.0 | 5.0 | ||
| Specific growth rate (μ) | |||||||||
| 6 | 0.34 ± 0.15aA | 0.59 ± 0.23aB | 0.59 ± 0.23aB | 0.66 ± 0.22aB | 0.58 ± 0.11aB | 0.47 ± 0.01aC | 0.55 ± 0.22aC | 0.59 ± 0.03aC | 0.54 ± 0.02aC |
| 12 | 0.25 ± 0.21aA | 0.36 ± 0.23aB | 0.37 ± 0.23aB | 0.39 ± 0.14aB | 0.38 ± 0.17aB | 0.26 ± 0.01aC | 0.28 ± 0.12aC | 0.49 ± 0.02aC | 0.38 ± 0.01aC |
| 18 | 0.08 ± 0.27aA | 0.22 ± 0.18aB | 0.23 ± 0.16aB | 0.39 ± 0.15aB | 0.29 ± 0.12aB | 0.07 ± 0.01aC | 0.10 ± 0.15aC | 0.20 ± 0.12aC | 0.15 ± 0.02aC |
| 24 | 0.00 ± 00aA | 0.02 ± 0.01aB | 0.02 ± 0.01aB | 0.01 ± 0.00aB | 0.01 ± 0.00aB | 0.01 ± 0.00aC | 0.02 ± 0.01aC | 0.04 ± 0.03aC | 0.05 ± 0.02aC |
*Mean±SD; n = 4
Different small superscripts show significant (P > 0.05) difference within the column
Different capital superscripts show significant (P > 0.05) difference within rows
Fig. 1.
Effect of prebiotics (3%) on the specific growth rate of L. plantarum after 24 h of incubation
Gum acacia, is traditionally utilised by African and Indian populations (Cherbut et al. 2003) to prevent and treat intestinal disorders. A few reports (Cherbut et al. 2003; Calame et al. 2008) indicate that gum acacia is able to selectively raise the proportions of LAB and bifidobacteria in healthy subjects. Therefore, it was considered as a prebiotic dietary fibre (Phillipsa et al. 2006). There is evidence that gum feeding can not only improve intestinal transit and provide digestive comfort (Cherbut et al.2003) but also produce less abdominal side effects than FOS (Goetze et al. 2008). Such traditional health promoting characteristics of dietary fibre have been used and accepted. It is given to lactating mothers in India as part of a special diet and in medical practice used in the treatment of diarrhoea and diabetes (Meance 2004). The present investigation confirms that gum acacia can be growth stimulatory (Table 1) . L. plantarum could use inulin and gum acacia as the sole carbon source, suggesting better colonization chances of the organism on consuming together with inulin and gum acacia as a synbiotic.
Antibacterial activity of selected lactobacillus culture in the presence of prebiotics
In order to test whether the incorporation of inulin and gum acacia into the growth media has any enhancing effect on the antibacterial activity of the selected culture, a comparison was made between the antibacterial activity exhibited by the culture grown in modified MRS containing either glucose or inulin and gum acacia @ 3% as the sole carbon source. L. plantarum grown in medium containing either inulin and gum acacia or glucose exhibited inhibitory effect against all the tested enteric organisms. The antibacterial activity exhibited by the organism grown in inulin and gum acacia added media was found significant (P < 0.05) in gum acacia while non significant in glucose and inulin (P > 0.05) (Table 2). In vitro experiments conducted by Oyarzabal and Conner (1995) to evaluate the ability of Bifidobacterium, Lactobacilli and Salmonella species grown in media containing fructooligosaccharides (FOS-50 or FOS pure formulation), as the only carbohydrate source, showed clear inhibition of six Salmonella serotypes (S. california, S. enteritidis, S. heidelberg, S. mission, S. senftenberg and S. typhimurium) grown in media containing the pure formulation of FOS as the only carbohydrate source. Similarly Basavanna and Prapulla (2011) reported inhibition of common pathogens by L. fermentum CFR 2195 grown in media containing FOS. In the present study, inhibition of growth of E. coli, B. cereus, E. faecalis, S. typhimurium and S. aureus by culture supernatants obtained by growing L. plantarum culture in the medium containing inulin and gum acacia as the sole carbon source was observed. Hence, these prebiotics may contribute towards the inhibition of pathogens by the stimulation of growth and the metabolic activity of lactobacilli in the gut.
Table 2.
Antimicrobial activity (diameter of zone of inhibition*) of the L. plantarum against indicator organisms in the presence of inulin and gum acacia
| Indicator strain | Diameter of zone of inhibition* (mm) | ||
|---|---|---|---|
| Modified MRS+glucose | Modified MRS+inulin | Modified MRS+gum acacia | |
| E. coli MTCC443 | 14.2 ± 0.27a | 14.2 ± 0.54a | 14.1 ± 0.30b |
| B. cereus NCDC240 | 12.2 ± 0.71a | 12.1 ± 0.26a | 12.0 ± 0.21b |
| E. faecalis MTCC439 | 12.5 ± 0.39a | 12.4 ± 0.48a | 12.4 ± 0.85a |
| S. typhimurium NCDC113 | 14.9 ± 0.67a | 14.8 ± 0.64a | 14.5 ± 0.76b |
*Mean±SD; n = 5 (a, b Means in row with different superscript differ significantly (P < 0.05)
Development of a lyophilized synbiotic preparation containing lactobacilli and prebiotics and storage studies
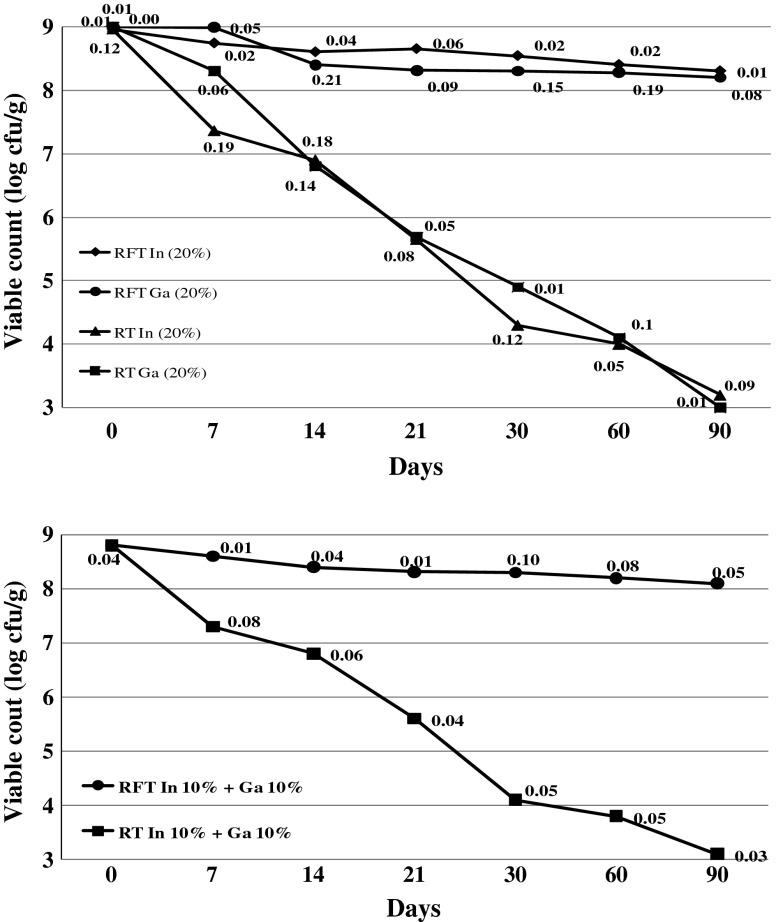
The freeze dried synbiotic formulation was developed by incorporating the tested probiotic culture and prebiotic compounds. Optimal performance of strains incorporated in a synbiotic product must at first guarantee their potential to survive during freeze drying and retain the viability after a long storage, so as to ensure eventual stabilization of their metabolic activity (Baati et al. 2000). Therefore, the accomplishment of probiotic food development depends on the maintenance of suitable level of viable cells during storage. In order to exert maximum beneficial effects, probiotic cultures must remain viable and active in the product during storage until consumption (Gilliland 1989; Hull et al. 1992). Storage of the synbiotic powder at room temperature (25 ± 1 °C) resulted in marked reduction in the count of L. plantarum, the count decreased by 5 to 6 logs after 90 days of storage (Fig. 2). This reduction was observed in all prebiotic combinations (20% inulin, 20% gum acacia or 10% inulin+10% gum acacia). However, no significant reduction was observed in the count of L. plantarum in the synbiotic powder of all prebiotic combinations, stored under refrigerated conditions (4 ± 1 °C). The count remained in the range of 8 to 9 log cfu/g even after 90 days of refrigerated storage. There were no significant differences among L. plantarum survival during dried storage when synbiotic preparation was in 20% inulin, 20% gum acacia or 10% inulin+10% gum acacia. These observations indicate that a combination (in different concentration) or single incorporation of prebiotics may not affect the survivability of lactobacilli. These findings are similar with that of Carvalho et al. (2004), who reported no effect of various complex sugars on survival of freeze–dried preparation of L. delbrueckii subsp. bulgaricus during storage at room temperature. It was also reported that non-digestible polyols (prebiotics) had no cryoprotective effect on the survival rate of freeze–dried form of L. plantarum ATCC 8014 in nonfat skim milk (Graciela et al. 1983).
Fig. 2.
Effect of storage temperature (RFT: Refrigeration (4 °C); RT: Room (25 °C) temp.) and concentration of prebiotics on viable count of L. plantarum n = 4
It has been recommended that probiotic functional food should contain at least 7 log live microorganisms/g or/ml (FAO/WHO 2001) at the time of consumption for obtaining maximum health benefits to the consumer. Based on the results obtained from the storage study, the developed synbiotic preparation product showed satisfactory count of organisms even after 90 days of storage at refrigeration temperature. These findings are also comparable with an earlier report, in which no significant decrease in viability (1010 cfu/g) of freeze-dried form of L. acidophilus during storage for 6 months at 4 °C was observed (Fazeli et al. 2006). Maintaining good viability, stability and functionality of probiotics during processing, formulation and storage is essential for delivering the health benefits of these organisms to consumers. Development of synbiotic formulations play a considerable role in this direction.
Optimal performance of strains incorporated in synbiotic product must at first guarantee their potential to survive during freeze drying and retain the viability after a long storage. For that reason, the development of products in which a suitable level of viable probiotic cells are retained for a longer period is one of the key research and development areas for probiotic foods. As prebiotics selectively enhance the multiplication of desirable bacteria, consumption of these substances along with probiotics will greatly help in achieving the desired effect of the health promoting organisms.
Conclusion
A freeze-dried synbiotic formulation was prepared incorporating L. plantarum and prebiotics using non fat dry milk as base material. The product contained high numbers of viable probiotics even after storage for 90 days at 4 ± 1 °C and thus can be of great help in the development of probiotic food.
References
- Baati L, Fabre-Ga C, Auriol D, Blanc PJ. Study of the cryotolerance of Lactobacillus acidophilus: effect of culture and freezing conditions on the viability and cellular protein levels. Int J Food Microbiol. 2000;59:241–247. doi: 10.1016/S0168-1605(00)00361-5. [DOI] [PubMed] [Google Scholar]
- Basavanna G, Prapulla SG (2011) Evaluation of functional aspects of Lactobacillus fermentum CFR 2195 isolated from breast fed healthy infants’ fecal matter. J Food Sci Technol; doi 10.1007/s13197-011-0345-9. [DOI] [PMC free article] [PubMed]
- Bourquin LD, Titgemeyer EC, Fahey GC, Garleb KA. Fermentation of dietary fibre by human colonic bacteria: disappearance of, short-chain fatty acid from and potential water-holding capacity, various substrates. Scand J Gastroenterol. 1993;28:249–255. doi: 10.3109/00365529309096081. [DOI] [PubMed] [Google Scholar]
- Bryant TN. PIBWin-software for probabilistic identification. J Appl Microbiol. 2004;97:1326–1327. doi: 10.1111/j.1365-2672.2004.02388.x. [DOI] [PubMed] [Google Scholar]
- Calame W, Weseler AR, Viebke C, Flynn C, Siemensma AD. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Brit J Nutr. 2008;100:1269–1275. doi: 10.1017/S0007114508981447. [DOI] [PubMed] [Google Scholar]
- Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P. Effects of various sugars added to growth and drying media upon thermo tolerance and survival throughout storage of freeze-dried Lactobacillus delbrueckii spp bulgaricus. Biotechnol Prog. 2004;20:248–254. doi: 10.1021/bp034165y. [DOI] [PubMed] [Google Scholar]
- Cherbut C, Michel C, Raison V, Kravtchenko T, Meance S. Gum acacia is a bifidogenic dietary fibre with high digestive tolerance in healthy humans. Microb Ecol Health Dis. 2003;15:43–50. doi: 10.1080/08910600310014377. [DOI] [Google Scholar]
- Collins EB, Hall BJ. Growth of bifidobacteria in milk and preparation of Bifidobacterium infantis for a dietary adjunct. J Dairy Sci. 1984;67:1376–1380. doi: 10.3168/jds.S0022-0302(84)81451-4. [DOI] [Google Scholar]
- Crittenden R, Weerakkody R, Sanguansri L, Augustin MA. Synbiotic microcapsules that enhance microbial viability during nonrefrigerated storage and gastrointestinal transit. Appl Environ Microbiol. 2006;3:2280–2282. doi: 10.1128/AEM.72.3.2280-2282.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhewa T, Bajpai V, Saxena RK, Pant S, Mishra V. Selection of lactobacillus strains as potential probiotics on basis of in vitro attributes. Int J Prbo Preb. 2010;5(1):45–52. [Google Scholar]
- FAO/WHO Experts’ Report (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria: 1–10
- Fazeli MR, Toliyat T, Samadi N, Hajjaran S, Jamalifer H. Viability of Lactobacillus acidophilus in various vaginal tablet formulations. DARU. 2006;14:172–177. [Google Scholar]
- Gibson GR, Wang X. Bifidogenic properties of different types of fructooligosaccharides. Food Microbiol. 1994;11:491–498. doi: 10.1006/fmic.1994.1055. [DOI] [Google Scholar]
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Beatty ER, Wand X, Cumming JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterol. 1995;108:975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Gilliland SE. Acidophilus milk products: a review of potential benefits to consumers. J Dairy Sci. 1989;72:2483–2494. doi: 10.3168/jds.S0022-0302(89)79389-9. [DOI] [PubMed] [Google Scholar]
- Goetze O, Fruehauf H, Pohl D, Giarre M, Rochat F, Ornstein K, Menne D, Fried M, Thumshirn M. Effect of a prebiotic mixture on intestinal comfort and general wellbeing in health. Brit J Nutr. 2008;100:1077–1085. doi: 10.1017/S0007114508960918. [DOI] [PubMed] [Google Scholar]
- Graciela FV, Graciela SG, Aida APRH, Guillermo O. Protective effect of adonitol on Lactic acid Bacteria subjected to freeze–drying. Appl Environ Microbiol. 1983;45(1):302–304. doi: 10.1128/aem.45.1.302-304.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grizard D, Barthomeuf C. Non-digestible oligosaccharides used as prebiotic agents: mode of production and beneficial effects on animal and human health. Reprod Nutr Dev. 1999;39:563–588. doi: 10.1051/rnd:19990505. [DOI] [PubMed] [Google Scholar]
- Holzapfel WH, Haberer P, Geisen R, Bjorkroth J, Schillinger U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am J Clin Nutr. 2001;73:365S–373S. doi: 10.1093/ajcn/73.2.365s. [DOI] [PubMed] [Google Scholar]
- Hopkins MJ, Cumming JH, Macfarlane GT. Interspecies differences in maximum specific growth rates and cell yields of bifidobacteria cultured on oligosaccharides and other simple carbohydrate sources. J Appl Microbiol. 1998;85:381–386. doi: 10.1046/j.1365-2672.1998.00524.x. [DOI] [Google Scholar]
- Hull RR, Conway PL, Evans AJ. Probiotic foods: a new opportunity. Food Aust. 1992;44:112–113. [Google Scholar]
- Kaplan H, Hutkins RW. Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl Environ Microbiol. 2000;66:2682–2684. doi: 10.1128/AEM.66.6.2682-2684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H, Hutkins RW. Metabolism of fructo- oligosaccharides by Lactobacillus paracasei 1195. Appl Environ Microbiol. 2003;69(4):2217–2222. doi: 10.1128/AEM.69.4.2217-2222.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolida S, Tuohy K, Gibson GR. Prebiotic effects of inulin and oligofructose. Br J Nutr. 2002;87(Suppl 2):S193–S197. doi: 10.1079/BJN/2002537. [DOI] [PubMed] [Google Scholar]
- Liong MT. Roles of probiotics and prebiotics in colon cancer prevention: postulated mechanisms and in vivo evidence. Int J Mol Sci. 2008;9:854–863. doi: 10.3390/ijms9050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacBrain AJ, MacFarlane GT. Investigations of bifidobacterial ecology and oligosaccharide metabolism in a three-stage compound continuous culture system. Scand J Gastoenterol. 1997;32:32–40. doi: 10.1080/00365521.1997.11720715. [DOI] [PubMed] [Google Scholar]
- McLeanRoss AH, Eastwood MA, Brydon WG, Anderson JR, Anderson DMW. A study of the effects of dietary gum arabic in humans. Ame J Clin Nutr. 1983;37:368–375. doi: 10.1093/ajcn/37.3.368. [DOI] [PubMed] [Google Scholar]
- McLeanRoss AH, Eastwood MA, Brydon WG, Busuttil A, McKay LF. A study of the effects of dietary gum arabic in the rat. Brit J Nutr. 1984;51:47–56. doi: 10.1079/BJN19840008. [DOI] [PubMed] [Google Scholar]
- Meance S (2004) Gum acacia (Fibregum TM), a very well tolerated specific natural prebiotic having a wide range of food applications. Agro Food 1:24–28; 2:32–35
- Mishra V, Prasad DN. Application of in vitro methods for selection of Lactobacillus casei strains as potential probiotics. Int J Food Microbiol. 2005;103(1):109–115. doi: 10.1016/j.ijfoodmicro.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Mishra V, Goswami P, Dabur R, Prasad DN. Antibiotic susceptibility and in vivo studies of selected probiotic strains of Lactobacillus casei. Int J Prob Preb. 2008;3(1):15–20. [Google Scholar]
- Nagpal R, Yadav H, Puniya AK, Singh K, Jain S, Marotta F. Potential of probiotics and prebiotics for synbiotic functional dairy foods: an overview. Int J Prob Preb. 2007;2:75–84. [Google Scholar]
- Oyarzabal OA, Conner DE. In vitro fructooligo-saccharides utilization and inhibition of Salmonella spp. by selected bacteria. Poult Sci. 1995;74:1418–1425. doi: 10.3382/ps.0741418. [DOI] [PubMed] [Google Scholar]
- Phillipsa GO, Ogasawarab T, Ushida K (2006) The regulatory and scientific approach to defining gum arabic (Acacia senegal and Acacia seyal) as a dietary fibre. Food Hydrocoll Special Rev:1–10
- Sghir A, Chow JM, Mackie RI. Continuous culture selection of bifidobacteria and lactobacilli from human faecal samples using fructooligosaccharides as a selective substrate. J Appl Microbiol. 1998;85:769–777. doi: 10.1111/j.1365-2672.1998.00590.x. [DOI] [PubMed] [Google Scholar]
- Stoyanova S, Geuns J, Hideg E, Van Den Ende W (2010) The food additives inulin and stevioside counteract oxidative stress. Int J Food Sci Nutr. doi:10.3109/09637486.2010.523416 [DOI] [PubMed]
- Sudha N, Shobha Rani P, Agrawal R. Studies on the stability and viability of a local probiotic isolate Pediococcus pentosaceus (MTCC 5151) under induced gastrointestinal tract conditions. J Food Sci Technol. 2006;43(6):677–678. [Google Scholar]
- Ziemer CJ, Gibson GR. An overview of probiotics, prebiotics and synbiotics in the functional food concept: perspective and future strategies. Int Dairy J. 1998;8:473–479. doi: 10.1016/S0958-6946(98)00071-5. [DOI] [Google Scholar]