Abstract
Kernel chemical composition and fatty acids profile of three walnut cultivars (Toyserkan, Chaboksar and Karaj) was analyzed. Some physicochemical properties, total phenolics content (TPC), ortho-diphenols content (ODC) and total tocopherol concentration (TTC) of extracted oils from the walnuts were also determined. The antioxidant activity of oil was measured by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging capacity and β-carotene bleaching assays. The analysis of chemical composition revealed that protein and dietary fiber was highest in Toyserkan cultivar. Phosphorus was the most abundant element in the walnut kernels, followed by potassium, magnesium and calcium. The linoleic acid and linolenic contents ranged from 50.15% to 51.36% and 10.48% to 12.04%, respectively. Also, the results demonstrated that acid value, saponification value and viscosity of extracted oil had significantly varied between all cultivars. The extracted oil from Chaboksar cultivar illustrated more hydro peroxides and secondary products than those obtained from other cultivars. A positive correlation was found between Rancimat values and oleic acid content (r = 0.60), but considerably negative correlation with TTC (r = −0.81) and TPC (r = −0.92). The relationship between percentage of remaining DPPH radical and β-carotene of walnut oils showed high correlation among three selected cultivars (r = −0.94 to −0.97).
Keywords: Antioxidant activity, Fatty acid profile, Juglans regia L., Mineral content, Oil analysis, Phenolic compounds, Physicochemical properties, Proximate composition
Introduction
Walnut fruit belongs to the Juglandaceae family, Juglans variety Juglans regia L (Zwarts et al. 1999). Walnuts are widely distributed all over the world. World production of walnuts exceeds almost 1, 500, 000 metric tons. China, the United States and Iran are the major producers, with about 25%, 20% and 11% of total global production, respectively. In recent years, the production in these countries has increased rapidly (FAO 2006). Walnut has a high calorie level and rich nutrient composition. When it is consumed in sufficient quantity, it can significantly contribute to daily diet and has functional importance in medical biochemistry and physiology because of its specific compounds like polyunsaturated fatty acids. Walnut contains high levels of oil and it is consumed, fresh or toasted, alone or in other edible products. Their major constituents are triglycerides, in which monounsaturated (primarily oleic acid) and polyunsaturated fatty acids (linoleic and α-linolenic acids) are present in high amounts. The presence of other bioactive minor components, such as phenols, tocopherols and phytosterols, has been also documented (Martínez et al. 2006). Consumption of walnut and its oil due to existence of high concentration of natural antioxidants has been reported as being protective against certain types of cancer and may also decrease the risk of cardiovascular diseases (Miraliakbari and Shahidi 2008; Yang et al. 2009). The objective of this research was to analyze the chemical composition, certain physicochemical properties and antioxidant activity of oil extracted from Persian walnut cultivars.
Materials and methods
Collection site and plant material
Three cultivars of walnuts fruits (J. regia L.) were collected from commercial plantations at the same time during the 2008/2009 growing season in Toyserkan, Chaboksar and Karaj located in Hamedan (western Iran), Guilan (northern Iran) and Alborz (centeral Iran) provinces, respectively. Hamadan is situated at the foot of the central Zagros mountain range. The mean annual rainfall ranges between 320 and 350 mm. This region has elevation about 1700 m above sea level and semi-arid climate. Chaboksar is located in the southern borders of Caspian Sea with elevation of about 110 m above sea level and has hyrcanian climate. The mean value of annual precipitation is about 1600 mm, with rains mostly falling in autumn, wet, and short winters. Karaj also is surrounded by Alborz Mountains. Mean annual precipitation and air temperature are 243.8 ± 12.2 mm and 14.95 ± 3.41 °C, respectively.
The walnuts after harvest were immediately transported to the laboratory and held in an oven for 3 days at 30 °C. They were stored in the shell, closed in plastic bags, and frozen to −4 °C, until the time of tests. Before each of chemical analysis, the walnuts were manually cracked and shelled, and then chopped in a 643 MX mill (Moulinex, Spain).
Chemical composition
Dry matter of kernels was measured by drying the samples to a constant weight, in an oven at a temperature of 70 °C (AOAC 1990). In order to determine total ash, samples were kept in an oven at 550 °C for 12 h and then weighed. Protein content was determined by the micro-Kjeldahl method. Protein was calculated based on nitrogen conversion factor of 5.4 (AOAC 1990).
Crude fat was measured by the Soxhlet method. Crude fat was obtained by exhaustively extracting 5.0 g of each sample in a Soxhlet apparatus using n-hexane (boiling point range 55–60 °C) as the extractant (AOAC 1990).
Carbohydrate content was estimated by difference of the other components using the following formula: 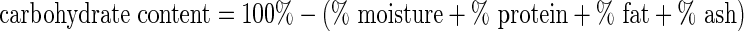 . Energy values were calculated from the data on protein, carbohydrates and fats by means of factors of 4, 4 and 9 kcal/g, respectively (Pereira et al. 2008).
. Energy values were calculated from the data on protein, carbohydrates and fats by means of factors of 4, 4 and 9 kcal/g, respectively (Pereira et al. 2008).
The total dietary fiber, acid detergent fiber (ADF) and neutral detergent fiber (NDF) contents were determined by the method applied by Savage (2001).
Samples were wet digested in a mixture of nitric and perchloride acids. Potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), manganese (Mn), zinc (Zn), copper (Cu) and sodium (Na) concentrations in the digest were determined by using atomic absorption spectrophotometer (Perkin-Elmer® Model 2380) (Kabas et al. 2007). The concentration of minerals was calculated with a standard curve for each element. Phosphorus (P) was measured by a UV visible spectrophotometer (Spectronic Genesys™ 10, GENEQ Inc., Montreal, Canada) at 430 nm wavelength and comparing the results to the standard curve (Kabas et al. 2007).
Fatty acid composition
Fatty acid was measured according to the method described by Savage et al. (1999). In order to evaluate fatty acid composition of walnut oil, a 30 m × 0.22 mm, 0.25 μm film thickness fused-silica capillary column DB-23 was connected to a Varian 3400 gas chromatograph (Palo Alto, CA) equipped with a flame ionization detector (FID) and split/splitless injector. Helium was used as the carrier gas at a velocity of 23 cm/s, and as the make-up gas at a rate of 30 mL/min. A temperature program of 158 °C for 5 min rising to 220 °C at a rate of 5 °C/min was used. The fatty acid methyl ester (FAME) dissolved in hexane was injected (1 μL) in a split mode of injection at a split ratio of 40:1. The injector and detector temperatures were 230 °C and 250 °C, respectively. A Varian 4270 integrator was used for recording the peak areas. No response factors were applied in calculating fatty acid composition, since the expand GC temperature program showed almost equal responses for different FAME standard mixtures.
Physicochemical properties of walnut oil
Acid, saponification, K270 and K232 values and unsaponifiable matter of the walnut oils were measured according to standard AOCS methods (AOCS 1998). Peroxide values (PV) were determined using the International Dairy Foundation method described in detail by Shantha and Decker (1994). The anisidine value (AnV) was determined at 350 nm in a 1.0 cm cell of a solution containing 1.0 g of oil in 100 mL of isooctane according to the method CD 18–90 of the AOCS (1998). The totox value was calculated as: 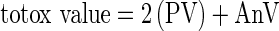 . Iodine values (IV) were calculated from fatty acid percentages by using the formula recommended by Martínez and Maestri (2008):
. Iodine values (IV) were calculated from fatty acid percentages by using the formula recommended by Martínez and Maestri (2008):
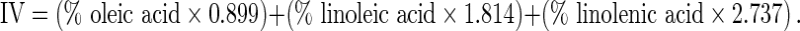 |
The oxidative stability was determined by Rancimat analysis. Air flow rate was set at 20 L/h and temperature of the heating block was maintained at 110 °C (Martínez et al. 2008).
Color value was determined using a Lovibond tintometer (Model F, Greenwich, England). The oils viscosity was determined using a Brookfield digital viscometer (model DV-II+Pro, USA). The oil density was determined by a digital densitometer (AP-PAAR DMA 46, Germany) with an accuracy of 10−4 g/cm3. The refractive index of oil samples was analyzed according to the AOAC (1990) by Abbe refractometer (model G manufactured by Carl-Zeiss, Germany).
Functional compounds of walnut oil
Tocopherol extraction
The tocopherol composition of oils obtained from different walnuts was analyzed as previously described by Maguire et al. (2004), with minor modifications. Briefly, in screwtop tubes fitted with Teflon lined screwtop, 40 mg oil with 300 mL of 50% KOH (w/v) and 2 mL of 1% ethanolic pyrogallol (w/v) was carefully mixed. The test tubes were kept in a thermostated water bath for 42.16 h at 70 °C. After cooling tubes, 1 mL of aerated distilled water and 4 mL hexane were slowly added. The tubes were agitated under high shaking rate and then centrifuged at 2000 rpm for 12 min. The hexane layer was removed and the extraction process iterated with a further 2 mL hexane. The combined hexane extracts were dried under nitrogen gas. The extract was redissolved in 200 mL ethanol, transferred to a plastic insert in a high-performance liquid chromatography (HPLC) vial and stored at −20 °C for later analysis through HPLC.
Tocopherol analysis
A Knauer (Berlin, Germany) HPLC system including a k-1001 HPLC pump, a k-1001 solvent organizer, an on-line degasser, a dynamic mixing chamber and a scanning HPLC fluorescence detector (Model RF-10XL) with the excitation and emission wavelength set at 290 nm and 330 nm, respectively were used for the analysis of tocopherols in walnut oil samples as previously explained by Pocklington and Dieffenbacher (1988). The separation was performed on a Lichrospher 100 RP-18 silica column (5 mm, 250 × 4 mm). The isocratic mobile phase used was hexane/2-propanol (99.5:0.05, v/v) at a flow rate of 2 mL/min. The chromatographic data were collected and recorded using EuroChrom 2000 software from Knauer, which was controlled by Windows XP. Tocopherol peaks were identified and quantified with the help of an in-house reference solution of α-tocopherol, β-tocopherol, γ-tocopherol and δ-tocopherol.
Phenolic compounds
The total phenolics content (TPC) of oil samples was determined according to the method applied by Singleton and Rossi (1965) using Folin-Ciocalteu’s reagent. Distilled water (3.16 mL) was mixed with a DMSO solution of the test compound (40 μL), followed by 200 μL of Folin- Ciocalteu’s reagent was added. Then, 600 μL of 20% sodium carbonate solution was added and the solutions were mixed again. After, the solutions were placed at room temperature for 2 h. Finally, the absorption of the developed blue color was measured at 765 nm, using a UV-visible spectrophotometer (DR/4000UHACH, USA). Results were expressed as milligrams of gallic acid equivalents (GAE) per gram of oil.
Ortho-diphenols content (ODC) in the walnut oils was evaluated in methanolic extract following the method described by Arranz et al. (2008) and expressed as millimol (mM) ortho-diphenols per kg of oil. Briefly, 2 mL of methanolic extract was taken and poured into a glass tube; followed by 0.5 mL of a 5% solution of sodium molybdate dihydrate in ethanol/water (1:1, v/v) was added. Then the mixture was shacked and incubated at room temperature for 15 min. The absorbance at was determined using a UV-visible spectrophotometer (DR/4000UHACH, USA) at 370 nm. Cathechol, hydroxytyrosol and hydroxytyrosyl acetate solutions were applied for building the calibration curve.
Antioxidant activity
DPPH radical-scavenging activity
Scavenging activities of walnut oil extracts towards the 1, 1-diphenyl-2-picrylhydrazyl (DPPH) free radical was evaluated as described by Martínez and Maestri (2008). Briefly, 100 mg of the oil in 1 mL toluene was vortexed for 20 s with 3.9 mL toluene solution (10−4 M) of the free stable DPPH (2,2 diphenyl 1-picrylhydrazyl) radical (DPPH●). The absorption at 515 nm was measured after 1, 30 and 60 min of mixing using a UV-visible spectrophotometer (DR/4000UHACH, USA) against a blank of pure toluene. The radical scavenging activity toward DPPH● was determined by the following equation:
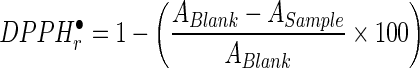 |
where A is the absorbance and DPPH●r is the amount of the remained radical in the medium after depletion of antioxidants in the walnut oil.
Also, the IC50 value defined as the concentration of an antioxidant in the reactive system necessary to decrease 50% of the initial DPPH concentration and was determined from the results. Therefore, a higher IC50 value indicates a lower antiradical activity.
Inhibition of β-carotene bleaching test
Analysis of the β-carotene bleaching is a practical method to measure the ability of a compound/mixture to inhibit the β-carotene oxidation. This experiment was carried out as described by Miraliakbari and Shahidi (2008). A solution of 5 mg/10 mL of β-carotene in chloroform was prepared and then 3 mL of this solution were pipetted into a 150 mL round bottom flask. Chloroform was eliminated by means of a rotary evaporator under vacuum at 40 °C, and after 100 mL of aerated distilled water, 40 mg linoleic acid, and 400 mg Tween 40 emulsifier were added to the flask under high shaking rate. Then, aliquots of 4.8 mL of this emulsion were transferred into a series of tubes containing 200 μL of the walnut oil or methanol (control). 10 μM of α-tocopherol was applied as the reference antioxidant. The zero time absorbance was determined at 470 nm using a UV-visible spectrophotometer (DR/4000UHACH, USA), immediately after the addition of the emulsion to each test tube. Subsequent absorbance readings were measured over a 2 h period at 5 min intervals by keeping the stoppered samples in a thermostated water bath at 50 °C. Blank samples devoid of β-carotene were prepared for background subtraction. The walnut oils capacity to protect against the β-carotene oxidation was measured as follows:
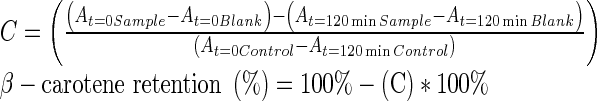 |
where A is the absorbance at a special time; and C is carotene depletion factor.
Statistical analysis
In this study, the sampling carried out three times for each cultivar. All chemical experiments were performed in triplicate and the results represented as a mean of the three values with the standard deviation. The results obtained were subjected to analysis of variance (ANOVA) and Duncan’s test using SPSS 13 (SPSS Inc., USA) software. Correlation analysis was carried out employing Pearson’s test.
Results and discussion
Proximate composition of walnut cultivars
Results related to chemical composition including dry matter, ash, protein, carbohydrate, fat, dietary fiber, acid detergent fiber (ADF), neutral detergent fiber (NDF) and energy of three Persian walnut cultivars are illustrated in Table 1. As expected, the walnut kernels had low moisture content (ranging from 3.23% for Chaboksar cultivar to 5.25% for Toyserkan cultivar). Low moisture content is key factor for keeping quality and shelf life of kernels as this parameter reduces the probability of microbial growth, and many undesirable biochemical changes normally associated with these processes (Venkatachalam and Sathe 2006). In the Table 1, the highest protein content was observed in Toyserkan cultivar (14.92%) and maximum fat and carbohydrate were detected in Chaboksar (67.35%) and Karaj (16.67%) cultivars, respectively. The difference in chemical composition was significant among the cultivars. Data of these components is crucial in process and product design in food and pharma industries. Ash content ranged from 2.09% (Toyserkan cultivar) to 2.24% (Chaboksar cultivar) (p > 0.05). The average content of dietary fiber, ADF and NDF was 4.2–4.6%, 2.4–2.8%, and 3.5–4.1%, respectively. It has been stated in many investigations that chemical composition is affected by variety, and environmental factors such as climate, geographical origin, harvest year and the methods of cultivation. The energy value ranged from 684.3 kcal/100 g (Karaj cultivar) to 714.8 kcal/100 g (Chaboksar cultivar). The findings reveal that walnut consumption led to a high input level of energetic value. The higher amount of energy value in Chaboksar cultivar can be contributed to high fat content in this cultivar.
Table 1.
Proximate chemical composition (g/100 g of dry matter) of walnut cultivars
| Compositions (mg/100 g) | Walnut cultivar | Significant level (P value) | Mean value | ||
|---|---|---|---|---|---|
| Chaboksar | Toyserkan | Karaj | |||
| Dry matter | 96.77 ± 0.22a | 94.75 ± 0.66b | 95.36 ± 0.24b | 0.039 | 95.62 |
| Crude protein | 13.77 ± 0.35b | 14.92 ± 0.29a | 14.25 ± 0.77a | 0.025 | 14.31 |
| Crude fat | 67.35 ± 0.11a | 64.9 ± 0.49b | 62.3 ± 0.54b | 0.019 | 64.85 |
| Total carbohydrates | 13.41 ± 0.81b | 12.84 ± 0.81c | 16.67 ± 0.33b | 0.008 | 14.30 |
| Ash | 2.24 ± 0.05 | 2.09 ± 0.07 | 2.14 ± 0.05 | ns | 2.15 |
| Dietary fiber | 4.3 ± 0.8b | 4.6 ± 0.15a | 4.2 ± 0.06b | 0.041 | 4.36 |
| ADF | 2.8 ± 0.8a | 2.7 ± 0.15a | 2.4 ± 0.06b | 0.044 | 2.63 |
| NDF | 3.5 ± 0.8c | 4.1 ± 0.15a | 3.8 ± 0.06b | 0.021 | 3.8 |
| Energy (kcal) | 714.87 ± 2.38 | 695.14 ± 2.52 | 684.38 ± 1.45 | ns | 698.13 |
All data represent the mean of three replications
ns not significant
a, b, c letters indicate the statistical difference in rows
Table 2 illustrates the mineral contents of the three walnut cultivars, expressed as mg 100 g−1 on a dry matter. Data indicated that the studied walnut cultivars contain many dietary essential minerals, such as sodium (0.30–0.41 mg/100 g), potassium (277–296 mg/100 g), calcium (68.15–75 mg/100 g), magnesium (71–94 mg/100 g), phosphorus (289–365 mg/100 g), iron (2.41–3.36 mg/100 g), zinc (1.92–3.02 mg/100 g), copper (0.65–1.11 mg/100 g) and manganese (2.21–2.43 mg/100 g). There were significant differences in mineral contents except for manganese (p < 0.05 and p < 0.01). The phosphorus is the most abundant element in the walnut kernels, followed by potassium, magnesium and calcium. In general, the values obtained in this work were similar to those observed in walnuts from Turkey (Cüaglarirmak 2003). The results also agree with those found by Lavedrine et al. (2000) except for magnesium. They studied the mineral composition of two walnut cultivars originating in France and California. The presence of the various mineral nutrients such as K, Ca, Mg, P, Fe, Zn, Mn and Cu are of biochemical importance to the physiology of the grains and nuts. In addition, they are an essential part of many important enzymes and they play roles as catalysts and antioxidants. For example, iron and copper are essential in blood formation and copper is involved in normal carbohydrate and lipid metabolism (Kabas et al. 2007).
Table 2.
Mineral concentrations of the three walnut cultivars
| Minerals (mg/100 g) | Walnut cultivar | Significant level (P value) | Mean value | ||
|---|---|---|---|---|---|
| Chaboksar | Toyserkan | Karaj | |||
| Calcium (Ca) | 68.15 ± 0.25c | 75 ± 0.65a | 71 ± 0.92b | 0.015 | 71.38 |
| Sodium (Na) | 0.41 ± 0.07a | 0.30 ± 0.08b | 0.32 ± 0.1b | 0.002 | 0.34 |
| Potassium (K) | 280 ± 0.76b | 296 ± 0.51a | 277 ± 0.14b | 0.011 | 284.3 |
| Magnesium (Mg) | 71 ± 0.2b | 94 ± 0.68a | 74 ± 0.21b | 0.005 | 79.6 |
| Iron (Fe) | 3.07 ± 0.34b | 3.36 ± 0.91a | 2.41 ± 0.14c | 0.004 | 2.94 |
| Zinc (Zn) | 1.92 ± 0.44c | 2.65 ± 0.75b | 3.02 ± 0.23a | 0.003 | 2.53 |
| Manganese (Mn) | 2.43 ± 0.35 | 2.21 ± 0.07 | 2.35 ± 0.13 | ns | 2.33 |
| Copper (Cu) | 0.65 ± 0.04b | 1.11 ± 0.06a | 0.98 ± 0.02a | 0.002 | 0.91 |
| Phosphorus (P) | 289 ± 0.65b | 365 ± 0.36a | 303 ± 0.77b | 0.014 | 319 |
All data represent the mean of three replications
ns not significant
a, b, c letters indicate the statistical difference in rows
Fatty acid compositions of walnut cultivars
Walnut kernels generally contain about 60% oil, but this can vary from 52% to 70% depending on the cultivar, location grown and irrigation rate (Beyhan et al. 1995). In this work, the average yield of total oil of the walnut kernels was found at 64.85%. Mean values and standard deviations of the fatty acid composition are presented in Table 3. Furthermore, fatty acids profile of Toyserkan walnut oil is depicted in Fig. 1. Myristic (14:0), palmitic (16:0), palmitoleic (C16:1), stearic (18:0), oleic (18:1), linoleic (18:2), linolenic (C18:3), and eicosenoic (20:1) acids were detected. Only myristic acid did not exhibit significant differences among walnut cultivars. As illustrated in Table 3, the major fatty acids found in the walnut oils are linoleic (50.15–51.36%), oleic (23.47–25.13%), and linolenic (10.48–12.04%). The ratios of these fatty acids are considered important for their economic and nutritional value. This might be important in selecting cultivars for particular applications; e.g. for the production of functional snack foods high in polyunsaturated fatty acids (PUFAs). Other fatty acids contents, myristic, palmitic, palmitoleic, and stearic acids, were observed at levels 0.39–0.43, 8.74–11.21, 0.146–0.245 and 2.52–4.45%, respectively (Table 3). Fatty acid analysis allowed the estimation of different nutritional fractions: saturated fatty acids (SFAs) and PUFAs. PUFAs due to the high content of linoleic acid were the main component of total fat extracted from the walnut cultivars. The SFAs content of oil extracted from analyzed walnut samples were the minor group (11.16–13.60%) (Table 3). The observations in the quantity of fatty acids in walnuts found in this study were similar to those reported in the literature (Martínez et al. 2008; Zwarts et al. 1999). Savage et al. (1999) studied 13 walnut varietal oils from New Zealand, Europe and the USA and obtained, on average, lower values of the PUFAs than those determined in the present research. Several factors such as variety type, climatic conditions, growing season, and locality may contribute to these differences.
Table 3.
Fatty acid composition of oil extracted from analyzed walnut samples
| Fatty acids (g/100 g) | Walnut cultivar | Significant level (P value) | Mean value | ||
|---|---|---|---|---|---|
| Chaboksar | Toyserkan | Karaj | |||
| Myristic (C14:0) | 0.43 ± 0.03 | 0.39 ± 0.02 | 0.41 ± 0.01 | ns | 0.41 |
| Palmitic (C16:0) | 11.21 ± 0.68a | 8.81 ± 1.40b | 8.74 ± 0.64b | 0.005 | 9.58 |
| Palmitoleic (C16:1) | 0.245 ± 0.06a | 0.146 ± 0.05c | 0.198 ± 0.02b | 0.030 | 0.196 |
| Stearic (C18:0) | 2.52 ± 0.35c | 3.09 ± 0.99b | 4.45 ± 0.33a | 0.001 | 3.35 |
| Oleic (C18:1) | 23.47 ± 0.22c | 25.13 ± 0.83a | 24.21 ± 0.49b | 0.041 | 24.27 |
| Linoleic (C18:2) | 51.36 ± 1.01a | 50.15 ± 0.61b | 50.55 ± 0.28b | 0.048 | 50.68 |
| Linolenic (C18:3) | 10.48 ± 0.44c | 12.04 ± 0.78a | 11.17 ± 0.32b | 0.034 | 11.23 |
| Eicosenoic acid (C20:1) | 0.22 ± 0.05a | 0.14 ± 0.04b | 0.17 ± 0.01b | 0.022 | 0.17 |
| P | 61.84 ± 0.14b | 62.19 ± 0.24a | 61.72 ± 0.57b | 0.047 | 61.91 |
| U | 85.7 ± 0.71 | 87.6 ± 0.94 | 86.3 ± 0.42 | ns | 86.55 |
| S | 11.16 ± 0.57c | 12.29 ± 1.22b | 13.60 ± 0.39a | 0.039 | 12.35 |
| P/S | 5.54 ± 0.12a | 5.06 ± 0.50a | 4.53 ± 0.29b | 0.040 | 5.04 |
| U/S | 7.68 ± 0.61a | 7.12 ± 0.67a | 6.34 ± 0.15b | 0.019 | 7.04 |
All data represent the mean of three replications
S Saturated fatty acid (C14:0 + C16:0 + C18:0), P Polyunsaturated fatty acid (C18:2 + C18:3), U Unsaturated fatty acid (C16:1 + C18:1 + C18:2 + C18:3+ C20:1)
ns not significant
a, b, c letters indicate the statistical difference in rows
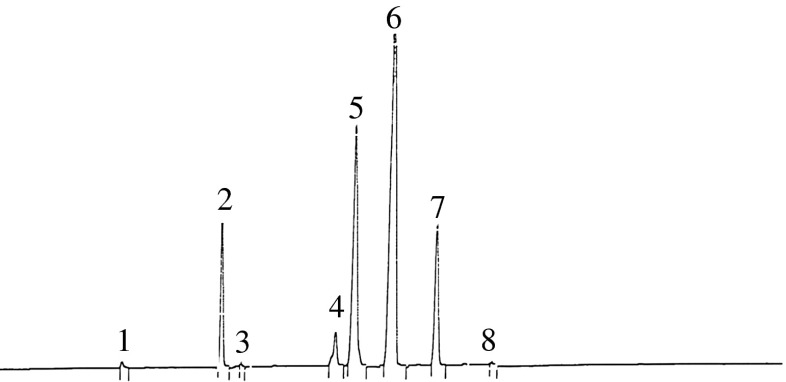
Fig. 1.
Partial GC chromatogram obtained from the Toyserkan walnut oil. For GC column and conditions see materials and methods. Peak identities: 1– Myristic acid; 2– Palmitic acid; 3– Palmitoleic acid; 4– Stearic acid; 5– Oleic acid; 6– Linoleic acid; 7– Linolenic acid; 8– Eicosenoic acid
Physicochemical properties of walnut oils
Physical and chemical characteristics of oil extracted from analyzed walnut samples are given in Table 4. The refractive index value at 25 °C ranged from 1.446 to 1.451 (p > 0.05). This index is a quick evaluation to estimate the level of suspected adulteration in edible oils (Adeeko and Ajibola 1990). The oils color is mainly attributed to the existence of which pigments are extracted along with the oil during extraction. The extracted walnut oils were yellowish in color. Lovibond values for red color of walnut oils extracted different cultivars were in the range of 1.15–1.2 (p > 0.05), whereas yellow color varied from 11 to 12 (p < 0.05). Martínez and Maestri (2008) have reported similar results in the case of walnut varieties grown in Argentina. The oils density insignificantly varied from 0.9212 to 0.9218 g/cm3 among different cultivars (p > 0.05). The findings indicated that Karaj cultivar had the highest level of oil viscosity (75 mPa.s), while the lowest viscosity of the walnut oil was related to Chaboksar cultivar (72.8 mPa.s).
Table 4.
Physical and chemical quality parameters of oil extracted from analyzed walnut samples
| Physical properties | Walnut cultivar | Significant level (P value) | Mean value | ||
|---|---|---|---|---|---|
| Chaboksar | Toyserkan | Karaj | |||
| Refractive index | 1.447 ± 0.002 | 1.451 ± 0.003 | 1.446 ± 0.001 | ns | 1.448 |
| Color (yellow) | 12 ± 0.001a | 11.6 ± 0.004ab | 11 ± 0.001b | 0.042 | 11.53 |
| Color (red) | 1.2 ± 0.01 | 1.2 ± 0.001 | 1.15 ± 0.001 | ns | 1.18 |
| Density | 0.9216 ± 0.0001 | 0.9218 ± 0.0003 | 0.9212 ± 0.0003 | ns | 0.9215 |
| Viscosity | 72.8 ± 0.7b | 73.4 ± 0.8b | 75 ± 0.4a | 0.012 | 73.73 |
| Chemical properties | |||||
| Acid value | 0.11 ± 0.02a | 0.07 ± 0.01b | 0.05 ± 0.01b | 0.045 | 0.07 |
| Peroxide value | 3.1 ± 0.32a | 1.9 ± 0.24b | 2.2 ± 0.1b | 0.009 | 2.4 |
| Anisidine value | 0.506 ± 0.009a | 0.475 ± 0.006b | 0.481 ± 0.002b | 0.049 | 0.487 |
| Totox value | 6.706 ± 0.92a | 4.275 ± 0.68c | 4.881 ± 0.51b | 0.003 | 5.287 |
| Iodine value | 142.95 ± 0.75b | 146.51 ± 0.73a | 144.03 ± 0.89b | 0.038 | 144.5 |
| Saponification value | 169.1 ± 7.04ab | 171.6 ± 5.63a | 167.6 ± 3.12b | 0.045 | 169.4 |
| Unsaponifiable matter | 4.67 ± 0.25 | 4.65 ± 0.41 | 4.69 ± 0.32 | ns | 4.67 |
| Oil stability | 3.01 ± 0.09c | 3.11 ± 0.14b | 3.14 ± 0.11a | 0.034 | 3.11 |
| K232 | 1.12 ± 0.01a | 1.08 ± 0.02b | 1.05 ± 0.02c | 0.042 | 1.08 |
| K270 | 0.06 ± 0.002 | 0.05 ± 0.003 | 0.05 ± 0.001 | ns | 0.05 |
All data represent the mean of three replications
Refractive index (at 25 °C); density (g/cm3); viscosity (10−3 × Pa.s); melting point (°C); acid value (% oleic acid); peroxide value (meq O2/kg oil); Unsaponifiable matter (g/kg oil); oil Stability (h); K232, conjugated dienes; K270, conjugated trienes
ns not significant
a, b, c letters indicate the statistical difference in rows
The oils obtained for all selected cultivars had low acid values (0.05–0.11% oleic acid) (p < 0.05). Peroxide value of oil is a valuable index to determine oil quality. Peroxide values of walnut oils extracted from Toyserkan, Chaboksar and Karaj cultivars were 1.9, 3.1 and 2.2 meq O2/kg of oil, respectively (p < 0.01). Therefore, the highest peroxide value observed was in the extracted oil from Chaboksar cultivar. Peroxide value depends on a number of factors including the oxidation state (level of oxygen consumed), the used method for oil extraction, the type of fatty acids and natural antioxidants present in the oil. The higher peroxide value in the oil extracted from walnut oil is probably due to low amount of bioactive compounds like phenolic substances (Fig. 2). However, these values were lower than generally recommended for commercial edible crude vegetable oils (≤ 10) (Özcan 2009). Anisidine values for the extracted oils were in the range of 0.475–0.506 (p < 0.05) and therefore, their Totox values varied between 4.881 and 6.706 (p < 0.01). The oil of Toysekan cultivar exhibited relatively higher iodine value (146.51), whereas that of Chaboksar cultivar was lower due to its lower unsaturated fatty acids content (p < 0.05).
Fig. 2.
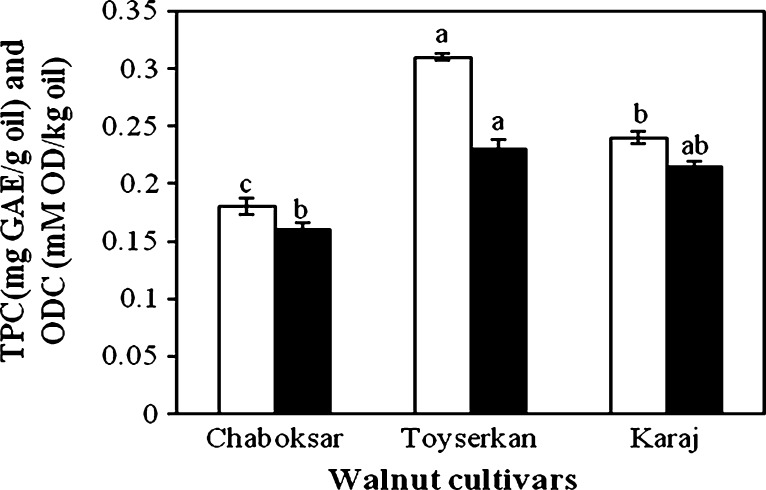
Total polyphenols (filled square) and ortho-diphenols (empty square) content of oil extracted from analyzed walnut cultivars
The mean saponification value of walnut oils for Toyserkan, Chaboksar and Karaj cultivars were 171.6, 169.1 and 167.6, respectively. The high saponification value showed that the oils have smaller molecular weight than other edible oils. This property of the oil depends on the presence of higher fatty acids and its unsaturation level (Bamgboye and Adejumo 2010). No significant difference (p > 0.05) was observed for the values of unsaponifiable matter (4.65–4.69 g/kg oil) among the walnut oils. These results were in agreement with those of Özcan (2009) for extracted oil from the walnut cultivated in Turkey.
The findings of the Rancimat test are illustrated in Table 4. The oil stability, expressed as the oxidation induction time, was about 3.01 h for Chaboksar’s cultivar oil, 3.11 h for Toyserkan’s cultivar oil, and about 3.14 for Karaj’s cultivar oil. This difference (p < 0.05) could be attributed to a higher content of natural antioxidants in the walnut oil obtained from Toyserkan cultivar (Fig. 2). The correlation analysis showed that the oxidative stability by Rancimat were negatively associated with total phenolics content (TPC) of the oil (r = −0.92). Moreover, a high positively correlation was found between Rancimat values and percentages of oleic acid (r = 0.60), but negatively correlated with linolenic acid content (r = −0.67). Aparicio et al. (1999) have described a significant correlation between phenols, oleic/linoleic ratio and tocopherols, and the oil stability for virgin olive oil. K232 and K270 extinction coefficients exhibited the oxidative deterioration and purity of the oils. The extracted oil from Chaboksar cultivar showed a higher absorptivity at 232 and 270 nm, thus containing more oxidation primary (hydro peroxides) and secondary products than the obtained oil from other cultivars.
Functional compounds and antioxidant activity of walnut oils
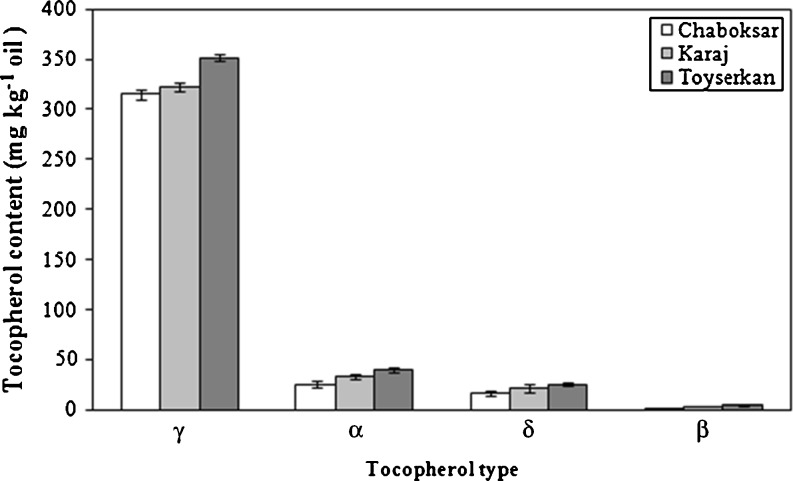
The total phenolics (TPC) and ortho-diphenols (ODC) contents of the oils from all selected cultivars are depicted in Fig. 2. It can be indicated in Fig. 2 that a highly significant variation among cultivars was obtained for TPC (p < 0.01) and ODC (p < 0.05). In general, the highest TPC and ODC were evaluated in extracted oil from Toyserkan cultivar (0.31 GAE/g, and 0.23 mM ortho-diphenols/kg, respectively), while the lowest TPC (0.18 GAE/g) and ODC (0.16 mM ortho-diphenols/kg) of the walnut oil was attributed to Chaboksar cultivar. Moreover, the total tocopherol concentration (TTC) of walnut oils extracted ranged from 359.2 to 420.6 mg/kg oil (p < 0.01). The walnut oil obtained from Toyserkan cultivar contained the highest TTC, followed by Karaj cultivar and then walnut oil extracted from Chaboksar cultivar. The TTC reported here are in agreement with those reported by Savage et al. (1999). In addition, a positive correlation was found between the oxidative stability values by Rancimat and TTC of walnut oils (r = 0.81, p < 0.01). Arranz et al. (2008) also obtained a significant correlation between Rancimat method and tocopherol amount (r = 0.91, p < 0.01) for hazelnut and pistachio oils. The major tocol in walnut oils was γ-tocopherol (315.3–351.2 mg/kg), followed by α-tocopherol (25.5–40.3 mg/kg) and then δ- (16.3–25.1 mg/kg) and β- (2.1–4.05 mg/kg) tocopherols (Fig. 3). However, β-tocopherol is a minor component of most nut oils, but it was present at low levels in walnut oils. Savage et al. (1999) found the β-tocotpherol amounts of cold press-extracted walnut oils were between 1 and 8.2 μg/g oil.
Fig. 3.
Individual tocopherol contents of oil extracted from analyzed walnut cultivars
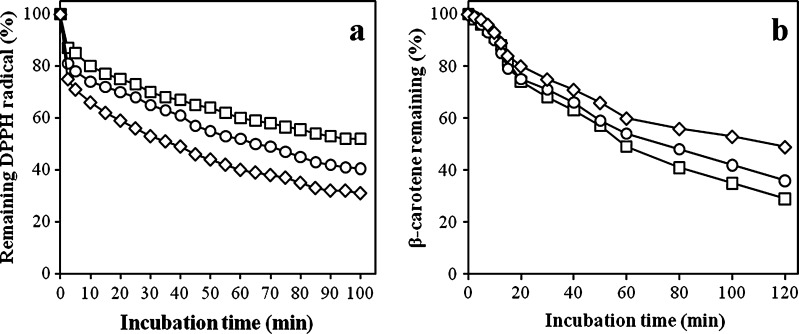
Also, a kinetic assay recorded by means of spectrophotometric absorbance to evaluate percentage of DPPH radical inhibition of the oils (Fig. 4a). The results showed that Toyserkan’s oil had the most efficiency and activity to scavenge DPPH radical. Walnut oil of Toyserkan cultivar had the highest IC50 values (585.5) followed by the oil obtained from Karaj’s cultivar (648.3) and finally Chaboksar cultivar’s oil (715.9). All IC50 values were observed to be lower than reported values by Li et al. (2007) for the extracted walnut oils from different origins. The IC50 values correlated significantly (p < 0.01) and inversely with the TPC (r = −0.91). Therefore, the oil stability of Toyserkan cultivar can be attributed to high antioxidant activity due to the phenolic compounds content in this oil.
Fig. 4.
Radical scavenging capacity during DPPH kinetic analyze (a), and kinetic assay for β-carotene remaining percentage (b) for walnut oils of different cultivars (empty square: Chaboksar; empty circle: Karaj; empty diamond: Toyserkan)
The analysis of β-carotene bleaching method can be useful particularly to evalaute lipophilic antioxidants and it is appropriate for the investigation of the antioxidant activity of walnut oils (Miraliakbari and Shahidi 2008). During incubation at 50 °C, linoleic acid produces hydroperoxide as free radicals that attack the 11 pairs of β-carotene double bonds, resulting in a bleaching of the reaction emulsion. In other words, the antioxidants present in the walnut oils quench hydroperoxide formed in this system. Therefore, the degradation rate of β-carotene depends on the antioxidant activity of the walnut oils by hindering the extent of β-carotene bleaching with acting on the free radicals formed in the system (Jayaprakasha et al. 2001).
The kinetic assay for the remaining percentage of β-carotene in the oil of walnut cultivars are given in Fig. 4b. Results of this experiment also indicated that the oil extracted from Toyserkan cultivar revealed the highest antioxidant activity, with 49% of β-carotene remaining after 120 min of test (p < 0.05). The oil of Karaj walnut obtained second rank by the activity (36% of β-carotene remaining after 120 min test), followed by the oil extracted from Chaboksar cultivar (29% of β-carotene remaining after 120 min assay). Moreover, the relationship between percentage of remaining β-carotene and DPPH radical of walnut oils illustrated high correlation among all cultivars (r = −0.94 to −0.97).
Conclusion
In the current study, chemical composition and the characteristics of certain physicochemical parameters of the kernel and oil of three Persian walnut cultivars were studied. The findings support the possible use of all investigated cultivars as natural antioxidants in the production of functional foods. However, Toyserkan walnut was the best of walnut among all investigated cultivars due to having the highest crude protein, dietary fiber, ADF, NDF, mineral contents such as Ca , P, K, Mg, Fe, and Cu, n-3 fatty acid, PUFAs, TPC, ODC, TTC, oil oxidative stability and antioxidant activity.
References
- Adeeko KA, Ajibola OO. Processing factors affecting yield and quality of mechanically expressed groundnut oil. J Agric Eng Res. 1990;45(1):31–43. doi: 10.1016/S0021-8634(05)80136-2. [DOI] [Google Scholar]
- Official methods of analysis. Washington: Association Official Analytical Chemists; 1990. [Google Scholar]
- Official methods and recommended practices of the American Oil Chemists’ Society. USA: AOCS; 1998. [Google Scholar]
- Aparicio R, Roda L, Albi MA, Gutierrez F. Effect of various compounds on virgin olive oil stability measured by Rancimat. J Agr Food Chem. 1999;47:4150–4155. doi: 10.1021/jf9812230. [DOI] [PubMed] [Google Scholar]
- Arranz S, Cert R, Pérez-Jiménez J, Cert A, Saura-Calixto F. Comparison between free radical scavenging capacity and oxidative stability of nut oils. Food Chem. 2008;110:985–990. doi: 10.1016/j.foodchem.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Bamgboye AI, Adejumo OI. Physicochemical properties of Roselle seed oil. Nutr Food Sci. 2010;40(2):186–192. doi: 10.1108/00346651011029219. [DOI] [Google Scholar]
- Beyhan OE, Kaya I, Sen SM, Dogan M. Fatty acid composition of walnut (Juglans regia L.) types selected in Darende. Turk J Agric For. 1995;19:299–302. [Google Scholar]
- Cüaglarirmak N. Biochemical and physical properties of some walnut genotypes (Juglans regia L.) Nahrung. 2003;47(1):28–32. doi: 10.1002/food.200390004. [DOI] [PubMed] [Google Scholar]
- Food Agriculture Organization of the United Nations. Italy: Rome; 2006. [Google Scholar]
- Jayaprakasha GK, Singh RP, Sakariah KK. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001;73:285–290. doi: 10.1016/S0308-8146(00)00298-3. [DOI] [Google Scholar]
- Kabas O, Yilmaz E, Ozmerzi A, Akinci I. Some physical and nutritional properties of cowpea seed (Vigna sinensis L.) J Food Eng. 2007;79:1405–1409. doi: 10.1016/j.jfoodeng.2006.04.022. [DOI] [Google Scholar]
- Lavedrine F, Ravela A, Villet A, Ducros V, Alary J. Mineral composition of two walnut cultivars originating in France and California. Food Chem. 2000;68:347–351. doi: 10.1016/S0308-8146(99)00204-6. [DOI] [Google Scholar]
- Li L, Tsao R, Yang R, Kramer JKG, Hernández M. Fatty acid profiles, tocopherol contents, and antioxidant activities of hearnut (Juglans ailanthifolia var. cordiformis) and Persian walnut (Juglans regia L.) J Agr Food Chem. 2007;55:1164–1169. doi: 10.1021/jf062322d. [DOI] [PubMed] [Google Scholar]
- Maguire LS, O’Sullivan M, Galvin K, O’Connor TP, O’Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr. 2004;55:171–178. doi: 10.1080/09637480410001725175. [DOI] [PubMed] [Google Scholar]
- Martínez ML, Maestri DM. Oil chemical variation in walnut (Juglans regia L.) genotypes grown in Argentina. Eur J Lipid Sci Technol. 2008;110:1183–1189. doi: 10.1002/ejlt.200800121. [DOI] [Google Scholar]
- Martínez ML, Mattea MA, Maestri DM. Varietal and crop year effects on lipid composition of walnut (Juglans regia L.) genotypes. J Am Oil Chem Soc. 2006;83:791–796. doi: 10.1007/s11746-006-5016-z. [DOI] [Google Scholar]
- Martínez ML, Mattea M, Maestri DM. Pressing and supercritical carbon dioxide extraction of walnut oil. J Food Eng. 2008;88:399–404. doi: 10.1016/j.jfoodeng.2008.02.026. [DOI] [Google Scholar]
- Miraliakbari H, Shahidi F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008;111:421–427. doi: 10.1016/j.foodchem.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Özcan MM. Some nutritional characteristics of fruit and oil of walnut (Juglans regia L.) growing in Turkey. Iran J Chem Chem Eng. 2009;28(1):57–62. [Google Scholar]
- Pereira JA, Oliveira I, Sousa A, Ferreira ICFR, Bento A, Estevinho L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem Toxicol. 2008;46:2103–2111. doi: 10.1016/j.fct.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Pocklington WD, Dieffenbacher A. Determination of tocopherols and tocotrienols in vegetable oils and fats by high performance liquid chromatography. Pure Appl Chem. 1988;60:877–892. doi: 10.1351/pac198860060877. [DOI] [Google Scholar]
- Savage GP. Chemical composition of walnuts (Juglans regia L.) grown in New Zealand. Plant Food Hum Nutr. 2001;56:75–82. doi: 10.1023/A:1008175606698. [DOI] [PubMed] [Google Scholar]
- Savage GP, Dutta PC, McNeil DL. Fatty acid and tocopherol contents and oxidative stability of walnut oils. J Am Oil Chem Soc. 1999;76(9):1059–1063. [Google Scholar]
- Shantha NC, Decker EA. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J AOAC Int. 1994;77(2):421–424. [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- Venkatachalam M, Sathe SK. Chemical composition of selected edible nut seeds. J Agr Food Chem. 2006;54:4705–4714. doi: 10.1021/jf0606959. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu RH, Halim L (2009) Antioxidant and antiproliferative activities of common edible nut seeds. LWT-Food Sci Technol 42:1–8
- Zwarts L, Savage GP, McNeil DL. Fatty acid content of New Zealand-grown walnuts (Juglans regia L.) Int J Food Sci Nutr. 1999;50:189–194. doi: 10.1080/096374899101229. [DOI] [PubMed] [Google Scholar]