Abstract
In this study, whole wheat bread (WWB) prepared by whole wheat flour (WWF) which its branny fraction (35 ± 1% w/w whole flour) previously was stabilized with different processes. Branny fractions obtained by milling of two different Bezostaja-1 wheat samples (medium and high strong) at 65 ± 1% wheat flour extraction ratio. These fractions were stabilized using autoclave (AU), microwave (MW), infrared (IR) and ultraviolet-C (UV-C) methods. Then, WWF obtained by remixing of stabilized branny fraction (35 ± 1% w/w) and wheat flour (65 ± 1% w/w) of same wheat samples. Following this process, WWB was made from WWF. WWB were analyzed to determine their nutritional properties as crude protein, in vitro protein digestibility (IVPD), phytic acid content, total and HCl-extractable mineral concentrations, total phenolic content (TPC), antioxidant activity and total dietary fiber (TDF). While IVPD, TPC and antioxidant activity of WWB increased together with all stabilization methods, a significant (P < 0.05) loss was observed on phytic acid content of the WWB. Especially, UV-C and IR treatments had positive effects on TPC and antioxidant activity. AU and MW stabilization methods increased total mineral and HCl-extractable minerals of WWB. As a result of this study, all stabilization processes had an improving effect on nutritional characteristic of WWB.
Keywords: Whole wheat bread, Microwave, Infrared, Ultraviolet-C, Stabilization
Introduction
Bread and bakery products have an important role in human nutrition all over the World. Generally, wheat bread is considered to be a good source of energy and irreplaceable nutrients for human body. This is especially true for the products made from whole wheat flour or high-yield flour types. Bread prepared from refined wheat flour is nutritionally much poorer and does not adequately meet the requirements for many macro or micro nutrients (Skrbic and Filipcev 2008). Since milling of wheat efficiently separates the bran, germ and endosperm to produce refined flour (Venn and Mann 2004). Dietary fiber, antioxidant and phenolic components like most nutritive ingredients are chiefly present in the bran layers and germ of the wheat kernel (Beta et al. 2005). Especially, wheat germ contains highly concentrated nutrients: 3 times as much protein, 7 times as much fat, 15 times as much sugar and 6 times as much mineral when compared to refined flour (Sudha et al. 2007).
Whole wheat flour is a very cheap micronutrients and dietary fiber source besides to be the main food stuff for human. It receives increasing demand year to year due to the decreasing effect on the risk of heart disease, hypertension, colon cancer, diabetes and obesity (Miller et al. 2000; Slavin 2000; Adam et al. 2003; Liu 2007). On the other hand, it has some antioxidants like phytic acid, glutathione, and tocopherols, and some essential fatty acids (Slavin 2000; Liu 2007). Also, it is used to make some foods with lower glycemic index and calorie (Adam et al. 2003). It has the proteins with good nitrogen balance, and the high amount of starch with the best suitable and cheap energy source (Slavin 2000). Also, some epidemiological studies indicate that the consumption of whole-grain and whole-grain products is related to reduction in total mortality (Jacobs et al. 2001).
However wheat bran and germ have poor shelf life due to the presence of unsaturated fatty acids, and oxidative and hydrolytic enzymes, rendering the product highly susceptible to rancidity since biologic and enzymatic activities of outer layer of the wheat kernel influence the whole wheat flour stability and bread properties during storage (Sudha et al. 2007; Srivastava et al. 2007). In literature, there are some studies about the effect of different stabilization methods, MW, AU steaming, roasting, toasting, IR heating, on bran or germ fraction of wheat kernel (Sivri et al. 1992; Vetrimani et al. 1992; Kermasha et al. 1993; Zwingelberg and Fretzdorff 1996; Pınarlı et al. 2004; Srivastava et al. 2007). For this reason, to apply heat treatment of the outer tissue layers for improving and stabilizing shelf life of cereal grains have been achieved (Srivastava et al. 2007; Sudha et al. 2007).
Thermal processing is the most extensively used method of food preservation to destroy microorganisms thereby extending its shelf-life. However, thermal processing has long been perceived to generally increase the digestibility of foods but with loss of certain heat-labile nutrients thus lowering the nutritional value (Randhir et al. 2008).
In this study branny fractions of two different types of Bezostaja-1 wheat were fine ground and stabilized by using some physical treatment methods such as AU, MW, IR and UV-C versus the control. Then, the treated “branny fractions” are remixed with the separated “wheat flour”, and “whole wheat flour” is regenerated. The obtained WWB were analyzed to determine their some nutritional properties as crude protein, IVPD, phytic acid content, total mineral and HCl extractable mineral concentrations, TPC, antioxidant activity and TDF content.
Materials and methods
Materials
Two different strong (medium and high strong) Bezostaja-1 wheat materials obtained from a local commercial flour mill (Alaybeyi Flour Mill, Konya, Turkey).
Experimental design
Two diffent stong Bezostaja-1 wheats (Bezostaja-1 (A) and Bezostaja-1 (B)) and five stabilization methods (AU, UV-C, MW, IR and control) were used as factors with two replications in the experiments, according to the completely randomized design of (2 × 5) ×2 factorial plan.
Stabilized WWF production
Wheat samples were milled on a lab-roller flour mill (Chopin CD 1, Chopin Technologies, Villeneuve La Garenne, France) at 65 ± 1% “wheat flour” extraction rate. After this processing, the remaining milling fractions called as “branny fractions” containing germ, coarse and fine bran, and a small amount flour. These fractions were milled into fine material on a hammer mill (FN-3100 Laboratory Mill, Perten Instruments AB, Huddinge, Sweden) with 500-micron-sieve opening. On these materials, different stabilization treatments (AU, MW, IR and UV-C) were applied. To prepare the remix flour, the fine ground and heat treated “branny fractions” and “wheat flour” were mixed thoroughly. Thus, stabilized WWF was obtained.
Stabilization methods
Autoclave (AU) stabilization Each batch, consisted of 100 g of branny fractions, were put in autoclavable bags, and steamed in AU at 121 °C for 15 min.
Microwave (MW) stabilization Each batch consisted of 100 g of branny fractions were put in a glass plate to achieve 3 mm height and subjected to thermal process using MW-oven (Arçelik ARMD580 oven, İstanbul, Türkiye) at 750 watt and 70 °C. Stabilization process was carried out by allowing the branny fractions in a glass plate to keep in the MW-oven for 1 min after their temperature level reached to 70 °C.
Infrared (IR) Stabilization Each batch consisted of 100 g of branny fractions were spread on a glass table with dimensions of 0.2 × 20 × 20 cm to achieve 3 mm of height and subjected to thermal process using IR-lamp (Philips, IR-250R 250 W lamp, Eindhoven, Holland) to reach 70 °C of internal temperature of the materials. Then, they were kept further at this temperature level for 1 min to achieve IR stabilization. In order to apply the thermal process to underside of the materials, approximately 1 cm2 of gaps were left. A mirror apparatus having the same dimensions was placed under the glass table.
Ultraviolet (UV-C) stabilization Each batch was spread on a flat surface as described in the IR stabilization method and subjected to UV-C using UV-lamp (30 W, 254 nm) for 3 min.
Proximate analysis
The AACC methods were used for the determination of moisture (method 44-19), crude ash (method 08-01), crude fat (method 30-25), crude fiber (method 32-10), and crude protein (method 46-12) contents of the WWF samples (AACC 1990). For determination of gluten quality of the WWF, wet gluten (ACCC method 38-12), gluten index (AACC method 38-12) and Zeleny sedimentation (ICC method 116/1) tests were used. The color parameters “L*”, “a*” and “b*” were obtained by using Hunter Lab Color Quest II Minolta CR 400 (Konica Minolta Sensing, Inc., Osaka, Japan). The color measurements were determined according to the CIELab color space system (Francis 1998). In the measurements of the rheological properties of the WWF, farinograph (ICC method 115/1) and extensograph (ICC method 114/1) are used according to ICC standard methods (ICC 2002).
Bread making
WWB samples were obtained by the modification of AACC method 10-10B (AACC 1990). Fermentation time was “30 + 30” min for bulk fermentation and was 50 min for proofing. Then, the samples were dried at 50 °C for 18 h in an air-convection oven (Nüve FN-500, Ankara, Türkiye) and these samples were crushed using a laboratory type grinder (Moulinex Super Junior S, Paris, France). Afterwards the samples were packaged in sealed bags and stored at 5 °C, until used.
Nutritional analysis
The Kjeldahl method was used to determine the crude protein content of the WWB samples with a conversion factor of 6.25 (AACC 1990). In vitro protein digestibility (IVPD) tests followed the methods of Bookwalter et al. (1987). Phytic acid content was measured by a colorimetric method according to Haug and Lantzsch (1983). Phytic acid in the sample was extracted with a solution of HCl (0.2 N) and precipitated with solution of Fe (III) (ammonium iron (III) sulphate.12 H2O). TPC was determined using the Folin-Ciocalteau method (Singleton and Rossi 1965) as modified by Beta et al. (2005). The total phenolic content was used a Hitachi-U1800 spectrophotometer (Hitachi High-Technologies, Tokyo, Japan) at 725 nm. The results were expressed as mg gallic acid equivalents per g sample. Antioxidant activity was measured using 2.2-diphenyl, 1-picrylhydrazyl free radical (DPPH) as modified by Brand-Williams et al. (1995). Total dietary fiber (TDF) contents of WWB samples were analyzed in duplicate using the AACC enzymatic-gravimetric method 32-07 (AACC 1990). Total mineral concentrations of the samples were determined by spectroscopic method using a ICP-AES (Vista series, Varian International AG, Switzerland) as described by Bubert and Hagenah (1987). HCl extractabilities of the minerals were carried out according to Saharan et al. (2001).
Data analysis
A commercial software program (TARIST, version 4.0; Izmir, Turkey) was used to perform statistical analyses. Data were assessed by analysis of variance. Means that were statistically different from each other were compared using Duncan’s multiple range tests at 5% confidence interval.
Results and discussion
Analytical results
The results of the analysis for the WWF of Bezostaja-1 are shown in Table 1. Crude protein, crude fiber, wet gluten, gluten index values of Bezostaja-1 (B) flours were found higher than those of the Bezostaja-1 (A) flours. In addition, Bezostaja-1 (B) wheat samples were better than Bezostaja-1 (A) wheat samples in quality in term of Farinograph and Extensograph parameters. As seen in Table 1, Bezostaja-1 (B) is red and strong wheat in good bread making quality.
Table 1.
Quality characteristics of Bezostaja-1 whole wheat floura
| Parameters | Bezostaja-1 (A)b | Bezostaja-1 (B)b | |
|---|---|---|---|
| Color | L* | 84.3 ± 0.17a | 83.7 ± 0.19b |
| a* | 1.7 ± 0.02b | 1.8 ± 0.01a | |
| b* | 14.3 ± 0.04a | 14.2 ± 0.13a | |
| Moisture (%) | 11.1 ± 0.21a | 10.7 ± 0.11b | |
| Ash (%)c | 1.5 ± 0.04a | 1.5 ± 0.06a | |
| Crude protein (%)c,d | 13.1 ± 0.52b | 15.0 ± 0.28a | |
| Crude fat (%)c | 2.8 ± 0.08a | 2.7 ± 0.07a | |
| Crude fiber (%)c | 2.8 ± 0.04b | 2.9 ± 0.01a | |
| Wet gluten (%)c | 29.7 ± 0.42b | 34.0 ± 1.27a | |
| Gluten index (%)c | 70.2 ± 3.11b | 79.8 ± 3.39a | |
| Zeleny sedimentation (cc)e | 25.0 ± 1.41b | 33.0 ± 0.71a | |
| Farinograph | Water absorption (%) | 69.1 ± 0.14a | 69.2 ± 0.14a |
| Stability (min) | 3.9 ± 0.14b | 6.4 ± 0.28a | |
| Degree of softening (BU)f | 73.0 ± 4.24a | 65.0 ± 2.83b | |
| Extensograph | Energy (cm2) | 36.0 ± 1.41b | 43.0 ± 1.41a |
| Extensibility (mm) | 105.5 ± 6.36b | 121.0 ± 5.66a | |
| Maximum resistance (BU)f | 228.5 ± 13.44b | 245.0 ± 4.24a | |
aThe means with the same letter in column are not significantly different (P < 0.05) (According to one way ANOVA)
b(A): Medium-strong wheat samples; (B): High-strong wheat samples (n = 2)
cBased on dry matter
d N × 5.70
eBased on 14% moisture
fBU: Brabender Unit
Nutritional properties
Protein and IVPD of WWB
Protein and IVPD values of the WWB samples are given in Table 2. Protein values were not significantly (P > 0.05) affected by stabilization methods. Similar results have been reported by some researchers (Sun et al. 2006; Pınarlı et al. 2004; Vadivambal et al. 2007). As can be seen from Table 2, the protein level was high in the breads produced from whole flour of Bezostaja-1 (B) wheat with high protein quality and content (Elgün and Ertugay 1995).
Table 2.
Nutritional properties of WWBa
| n | Crude protein (g/100 g) | IVPD (%) | Phytic acid (mg/100 g) | TPC (mg GAE/g) | Antioxidant activity (%) | TDF (g/100 g) | |
|---|---|---|---|---|---|---|---|
| Wheat cultivar | |||||||
| Bezostaja-1(A)b | 10 | 13.9b ± 0.10 | 76.4a ± 1.93 | 462.8b ± 36.14 | 1.3a ± 0.07 | 15.8b ± 1.58 | 11.9b ± 0.05 |
| Bezostaja-1(B)b | 10 | 15.9a ± 0.06 | 70.8b ± 3.55 | 544.7a ± 55.53 | 1.3a ± 0.12 | 16.7a ± 1.76 | 12.1a ± 0.04 |
| Stabilization method | |||||||
| Control | 4 | 14.9a ± 1.21 | 69.3d ± 4.77 | 566.5a ± 60.56 | 1.2b ± 0.03 | 13.3c ± 0.43 | 12.0a ± 0.14 |
| Autoclave | 4 | 14.9a ± 1.20 | 75.5a ± 3.12 | 454.8d ± 33.02 | 1.2b ± 0.01 | 16.2b ± 047 | 12.0a ± 0.12 |
| Ultraviolet-C | 4 | 14.9a ± 1.16 | 72.9c ± 4.88 | 536.8b ± 48.31 | 1.4a ± 0.03 | 17.6a ± 0.63 | 12.0a ± 0.11 |
| Microwave | 4 | 14.9a ± 1.15 | 74.7b ± 0.72 | 461.0d ± 31.80 | 1.2b ± 0.04 | 16.8ab ± 0.71 | 12.0a ± 0.15 |
| Infrared | 4 | 14.9a ± 1.20 | 75.6a ± 2.88 | 504.8c ± 48.28 | 1.4a ± 0.08 | 17.2a ± 0.96 | 12.0a ± 0.12 |
aThe means with the same letter in column are not significantly different (P < 0.05) (According to two way ANOVA)
b(A): Medium-strong wheat samples; (B): High-strong wheat samples
WWB whole wheat bread; IVPD In-vitro protein digestibility; TPC total phenolic content; TDF total dietary fiber
IVPD is an important factor when the nutritional status of a food product is assessed. In this study, IVPD values of WWB samples increased with stabilization methods. The highest protein digestibility values are obtained through the IR (75.6%) and AU (75.5%) stabilization methods and these are followed by MW (74.7%) and UV-C (72.9%) stabilization methods (Table 2). Previously published studies report that, heating processes applied for stabilization clearly increased the protein digestibility (Abu-Tarboush 1998; Krishnamurthy et al. 2008). Also, IVPD values decreased of WWB made from high strong wheat samples. This result may be attributed to the high content of phytic acid of WWB made from high-strong Bezostaja-1 (B) wheat flour (Table 2). Since, phytic acid is an important antinutrient due to reducing effect on some minerals and also protein bioavailability (Rickard and Thompson 1997).
Phytic acid content of WWB
Phytic acid is found in cereals, legumes and seeds as a natural component. Phytic acid makes complex with necessary minerals important for human nutrition by hindering absorption. In addition, phytates formed with association of phytic acid and minerals affect the protein absorption negatively (Özkaya 2004). Phytic acid content in cereal and legume grain can be decreased using, germination, soaking, fermentation and enzymatic methods (Bilgiçli and Elgün 2005). The effects of wheat cultivar and stabilization methods on the phytic acid content of WWB are given in Table 2. Phytic acid content of WWB changed between 454.8 and 566.5 mg/100 g. As seen in Table 2, the average phytic acid content of WWB made from Bezostaja-1 (A) and Bezostaja-1 (B) were 462.8 mg/100 g and 544.70 mg/100 g, respectively. Özkaya (2004) has described that WWB made from WWF with 14.3% protein content have 708.3 mg/100 g phytic acid and ones with 11.1% protein have 642.3 mg/100 g of phytic acid content. There was a big difference between the phytic acid contents of WWB samples. Phytic acid content of WWB, when ordered from lowest to highest, were obtained as AU (454.8 mg/100 g), MW (461.0 mg/100 g), IR (504.8 mg/100 g), UV-C (536.8 mg/100 g) stabilizations and control (566.5 mg/100 g) group. The best results were obtained by AU and MW stabilization methods. Many researches in literature shows that thermal and radiation processes decrease the phytic acid content of food (Ahn et al. 2003; Al-Kaisey et al. 2003; Masud et al. 2007; Krishnamurthy et al. 2008).
TPC of WWB
Phenolic compounds (phenolic acids, simple flavonoids, and proanthocyanidins) form a major group of phytochemicals found in plants. Numerous studies have indicated the positive association between consumption of foods rich in phenolic phytochemicals and health. The interest in phenolics has been spurred by recent reports of their antioxidant activity (Beta et al. 2005; Randhir et al. 2008).
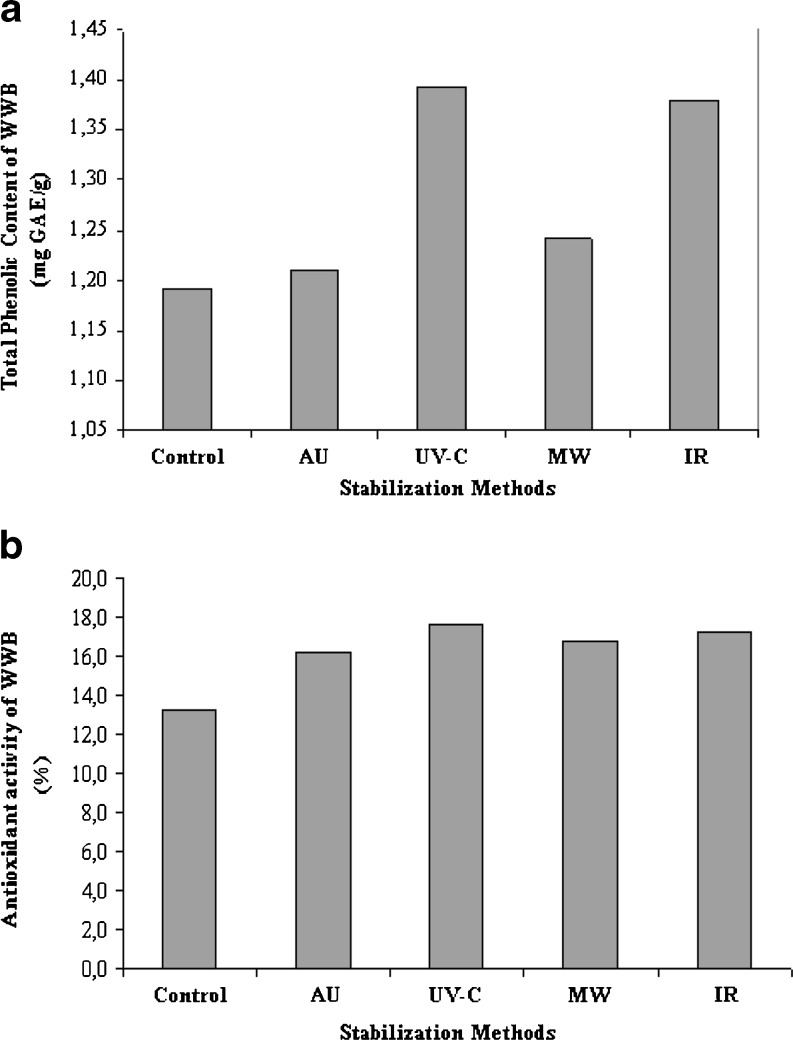
Table 2 shows the TPC of WWB. TPC of WWB ranged between 1.2 and 1.4 mg GAE/g. TPC in the WWB samples was not affected when wheat cultivar from different strong wheat used in this study. These changes are due to environmental and genetic factors. TPC and antioxidant activity of wheat grain have been reported to be dependent on environmental and genetic factors. Further, the TPC of wheat varieties grown at different locations produced significant differences when compared with each other (Liyana-Pathirana and Shahidi 2006). Effect of stabilization methods on TPC of WWB was given in Fig. 1a. As seen in Fig. 1a, TPC markedly increased in UV-C and IR stabilization methods. Nevertheless, TPC was not affected by AU and MW stabilization methods. As descriptive, all the stabilization applications increased TPC of the WWB samples. Many antioxidant phenolic compounds in plants, however, are most frequently present as covalently bound forms (Peleg et al. 1991). These phenolics are also known to accumulate in the cellular vacuoles and thermal processing may release these contained unavailable phenolics (Randhir et al. 2008). The increase in TPC as observed in WWB may be due to these bound phenolic compounds from the breakdown of cellular constituents and cell walls by thermal processing.
Fig. 1.
Effect of stabilization methods on total phenolic content (a) and antioxidant activity (b) of WWB. (n = 20). AU: Autoclave, UV-C: Ultraviolet-C, MW: Microwave, IR: Infrared
Antioxidant activity of WWB
Antioxidants in grains and its products are known, but their potential contribution to health through the diet has essentially been ignored. Free radicals are believed to trigger the initiation phase of several diseases. The ability of natural antioxidants to react with free radicals makes them of special interest (Miller et al. 2000).
In this study, DPPH is used as a free radical to evaluate antioxidant activity of WWB. Antioxidant activity was expressed as % (Table 2). As seen in Table 2, antioxidant activity of WWB varied between 13.3 and 17.6%. Antioxidant activity of WWB made from Bezostaja-1 (A) was found to be lower than WWB of Bezostaja-1 (B). It may be due to, higher phytic acid contents present in bread made from stronger wheat (Bezostaja-1 (B)). It’s known that, phytic acid have the good antioxidant properties (Graf et al. 1987). Effect of stabilization methods on antioxidant activity of WWB was given in Fig. 1b. As seen in Fig. 1b, it was found that WWB branny fractions of which were stabilized had higher antioxidant activity than control. The highest antioxidant activity was found in UV-C (17.6%) stabilization method and followed by IR (17.2%), MW (16.8%), AU (16.2%) and control (13.3%) samples, respectively. Many studies in literature show that both thermal and radiation processes increased the antioxidant acitivity (Lee et al. 2006; Lemoine et al. 2007; Randhir et al. 2008; Hayat et al. 2010). Its reason could be higher content of phenolic compounds. Beta et al. (2005) reported high correlation between TPC and antioxidant activity of wheat and wheat fractions. Other reasons could be due to Maillard reaction (non-enzymatic browning) and caramelization products (Randhir et al. 2008).
TDF of WWB
Cereals are important source of dietary fiber. Cereals, containing high concentrations of TDF, are known to have a cholesterol-lowering effect, regulating blood glucose level and insulin response in diabetics and even reducing cancer risk (Slavin 2000). TDF of the WWB samples are presented in Table 2. Wheat cultivar was found significant on TDF of the WWB samples, while on the contrary stabilization methods were not found significant on these values of WWB samples. This result has shown that TDF of WWB was preserved. Similar result was reported by Siljestrom et al. (1986). Also, TDF of WWB made from high-strong Bezostaja-1 (B) wheat was higher than those of Bezostaja-1 (A) wheat. Similarly phytic acid content of WWB made from Bezostaja-1 (B) was found high. Idouraine et al. (1996) reported that the phytic acid in cereals take part in TDF. It may be due to higher phytic acid content.
Total and HCl-extractability of minerals of WWB
Total and HCl-extractability of minerals of WWB samples are presented in Table 3 and 4. Stabilization methods and wheat cultivars showed a significant effect on total “Zn”, “Ca”, “K”, “Mg” and “P” contents. HCl-extractability of WWB made from high-strong wheat samples were lower than in WWB made from medium-strong wheat samples. The most effective factor may be high phytic acid content of high-strong wheat samples. Therefore, mineral bioavailability in this WWB was lower than in WWB made from medium-strong wheat samples.
Table 3.
Total mineral content 1 (mg/100 g) of WWBa
| n | Zn | Fe | Ca | K | Mg | P | |
|---|---|---|---|---|---|---|---|
| Wheat cultivar | |||||||
| Bezostaja-1(A)b | 10 | 1.60a ± 0.06 | 1.98b ± 0.15 | 40.5a ± 1.23 | 436.0b ± 14.67 | 57.5b ± 2.09 | 373.5b ± 14.75 |
| Bezostaja-1(B)b | 10 | 1.54b ± 0.04 | 3.03a ± 015 | 40.8a ± 0.32 | 488.3a ± 5.30 | 60.7a ± 1.41 | 399.5a ± 8.60 |
| Stabilization method | |||||||
| Control | 4 | 1.52b ± 0.04 | 2.38b ± 0.64 | 39.8b ± 0.66 | 453.3c ± 34.43 | 57.5d ± 1.45 | 373.0d ± 15.70 |
| Autoclave | 4 | 1.60a ± 0.02 | 2.52ab ± 0.53 | 41.1a ± 0.50 | 461.7bc ± 34.16 | 59.5b ± 2.84 | 385.1bc ± 20.56 |
| Ultraviolet-C | 4 | 1.55b ± 0.04 | 2.44ab ± 0.62 | 40.2b ± 0.69 | 455.4bc ± 33.77 | 58.1cd ± 2.11 | 379.7c ± 18.21 |
| Microwave | 4 | 1.64a ± 0.08 | 2.69a ± 0.64 | 41.7a ± 0.67 | 478.4a ± 20.83 | 61.9a ± 0.68 | 404.0a ± 6.88 |
| Infrared | 4 | 1.54b ± 0.03 | 2.49ab ±0.64 | 40.3b ± 0.66 | 461.9b ± 29.24 | 58.7bc ± 2.41 | 390.6b ± 14.68 |
aThe means with the same letter in column are not significantly different (P < 0.05) (According to two way ANOVA)
b(A): Medium-strong wheat samples; (B): High-strong wheat samples
WWB whole wheat bread
Table 4.
HCl-extractability (%) of minerals of WWBa
| n | Zn | Fe | Ca | K | Mg | P | |
|---|---|---|---|---|---|---|---|
| Wheat cultivar | |||||||
| Bezostaja-1(A)b | 10 | 78.1a ± 4.99 | 60.8a ± 3.85 | 67.7a ± 2.11 | 82.3a ± 2.63 | 83.8a ± 2.95 | 72.3a ± 2.35 |
| Bezostaja-1(B)b | 10 | 70.8b ± 5.24 | 57.2b ± 3.05 | 62.5b ± 0.71 | 81.7b ± 2.28 | 82.9b ± 3.15 | 71.7b ± 1.84 |
| Stabilization method | |||||||
| Control | 4 | 68.1e ± 4.11 | 54.2d ± 2.35 | 62.8d ± 1.70 | 78.6d ± 0.43 | 80.0e ± 0.79 | 69.4d ± 0.23 |
| Autoclave | 4 | 80.7a ± 3.11 | 62.5a ± 2.99 | 66.5a ± 3.79 | 84.7a ± 0.75 | 87.9a ± 0.55 | 74.9a ± 0.53 |
| Ultraviolet-C | 4 | 70.0d ± 4.10 | 56.7c ± 1.36 | 64.7c ± 2.88 | 80.2c ± 0.15 | 80.9d ± 0.67 | 70.6c ± 0.16 |
| Microwave | 4 | 78.6b ± 4.67 | 62.3a ± 3.13 | 66.4a ± 3.80 | 84.2a ± 0.66 | 85.0b ± 0.46 | 73.4b ± 1.19 |
| Infrared | 4 | 74.9c ± 5.28 | 58.8b ± 1.02 | 65.2b ± 2.87 | 82.2b ± 0.33 | 82.9c ± 0.81 | 71.5c ± 0.34 |
aThe means with the same letter in column are not significantly different (P < 0.05) (Two way ANOVA)
b(A): Medium-strong wheat samples; (B): High-strong wheat samples
WWB whole wheat bread
Generally, total mineral content and HCl-extractable minerals of WWB increased with stabilization methods of branny fractions. The most distinctive effect was observed with AU and MW applications which have higher heat penetration properties. It was previously reported that UV-C, AU, IR and MW stabilizations methods increased or prevented the mineral content of food (McCurdy 1992; Yue et al. 1998; Porres et al. 2003).
The Recommended Dietary Allowances (RDAs) for adult males are 800 mg of calcium, 10 mg of iron, 1.6–2.0 g of potassium, 350 mg of magnesium, 800 mg of phosphorus and 15 mg of zinc. When 100-g (dry matter) WWB made from stabilized branny fractions in AU were consumed, 5.1% of RDA for Ca, 25.2% of RDA for Fe, 25.6% of RDA for K, 17.0% RDA for Mg, 48.1% of RDA for P and 10.8% of RDA for Zn were taken by the human body. These RDA ratios were 5.2% of Ca, 26.9% of Fe, 26.6% of K, 17.7% Mg, 50.5% of P and 10.9% of Zn in WWB made from stabilized branny fractions in MW treatment. These ratios are very important to overcome mineral deficiency, especially in terms of Fe and P. On the other hand, the bioavailability of these minerals also increased by stabilization application.
Conclusion
In this study, some changes in the nutritional properties of WWB made from stabilized branny fractions of Bezostaja-1 wheat were studied. As conclusion, nutritional properties of WWB improved with all stabilization methods. Especially, UV-C and IR treatments were found to have positive effects on TPC and antioxidant activity. AU and MW treatments not only decreased phytic acid content, but they also increased total mineral content and HCl-extractability of minerals in WWB. Generally, stabilization methods improved nutritional properties of WWB.
Acknowledgements
This study is a part of the PhD. thesis by Mustafa Kürşat DEMİR and was supported by the Selçuk University Scientific Research Projects Coordinatorship (Project No: 07101028).
References
- Abu-Tarboush HM. Irradiation inactivation of some antinutritional factors in plant seeds. J Agr Food Chem. 1998;46:2698–2702. doi: 10.1021/jf980102x. [DOI] [Google Scholar]
- Approved methods of the American Association of Cereal Chemists. 8. St. Paul: AACC International; 1990. [Google Scholar]
- Adam A, Lopez HW, Leuillet M, Demigne C, Remesy C. Whole wheat flour exerts cholesterol-lowering in rats in its native form and after use in bread-making. Food Chem. 2003;80:337–344. doi: 10.1016/S0308-8146(02)00269-8. [DOI] [Google Scholar]
- Ahn HJ, Kim JH, Yook HS, Byun MW. Irradiation effects on free radical scavenging and antioxidant activity of phytic acid. J Food Sci. 2003;68(7):2221–2224. doi: 10.1111/j.1365-2621.2003.tb05750.x. [DOI] [Google Scholar]
- Al-Kaisey MT, Alwan AKH, Mohammad MH, Saeed AH. Effect of gamma irradiation on antinutritional factors in broad bean. Radiat Phys Chem. 2003;67:493–496. doi: 10.1016/S0969-806X(03)00091-4. [DOI] [Google Scholar]
- Beta T, Nam S, Dexter JE, Sapirstein HD. Phenolic content and antioxidant activity of pearled wheat and roller-milled fractions. Cereal Chem. 2005;82:390–393. doi: 10.1094/CC-82-0390. [DOI] [Google Scholar]
- Bilgiçli N, Elgün A. Changes in some physical and nutritional properties of tarhana, a turkish fermented cereal food, added various phytase sources. Food Sci Technol Int. 2005;11(5):383–389. doi: 10.1177/1082013205058602. [DOI] [Google Scholar]
- Bookwalter GN, Kırleis AW, Mertz ET. In vitro digestibility of protein in milled sorghum and other processed cereals with and without soy fortification. J Food Sci. 1987;52(6):1577–1579. doi: 10.1111/j.1365-2621.1987.tb05882.x. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Bubert H, Hagenah WD. Detection and measurement. In: Boumans PWJM, editor. Inductively coupled plasma emission spectroscopy. New York: Wiley-Interscience Publishers; 1987. pp. 536–567. [Google Scholar]
- Elgün A, Ertugay Z (1995) Tahıl İşleme Teknolojisi. Atatürk University Publications No:718, Erzurum, Turkey, pp 73–83
- Francis FJ. Colour analysis. In: Nielsen SS, editor. Food analysis. Maryland: An Aspen Publishers; 1998. pp. 599–612. [Google Scholar]
- Graf E, Empson KL, Eaton JW. Phytic acid: a natural antioxidant. J Biol Chem. 1987;262:11647–11650. [PubMed] [Google Scholar]
- Haug W, Lantzsch HJ. Sensitive method for the rapid determination of phytate in cereals and cereal product. J Sci Food Agr. 1983;34:1423–1426. doi: 10.1002/jsfa.2740341217. [DOI] [Google Scholar]
- Hayat K, Zhang X, Farooq U, Abbas S, Xia S, Jia C, Zhong F, Zhang J. Effect of microwave treatment on phenolic content and antioxidant activity of citrus mandarin pomace. Food Chem. 2010;123:423–429. doi: 10.1016/j.foodchem.2010.04.060. [DOI] [Google Scholar]
- ICC (2002) International Association for Cereal Science and Technology, ICC-Vienna
- Idouraine A, Khan MJ, Weber CW. In vitro binding capacity of wheat bran, rice bran, and oat fiber for Ca, Mg, Cu and Zn alone and in different combinations. J Agric Food Chem. 1996;44:2067–2072. doi: 10.1021/jf960151e. [DOI] [Google Scholar]
- Jacobs DR, Meyer HE, Solvoll K. Reduced mortality among whole grain bread eaters in men and women in the Norwegian county study. Eur J Clin Nutr. 2001;55:137–143. doi: 10.1038/sj.ejcn.1601133. [DOI] [PubMed] [Google Scholar]
- Kermasha S, Bisakowski B, Ramaswamy H, Van de Voort F. Comparison of microwave, conventional and combination heat treatment on wheat germ lipase activity. Int J Food Sci Tech. 1993;28:617–623. doi: 10.1111/j.1365-2621.1993.tb01313.x. [DOI] [Google Scholar]
- Krishnamurthy K, Khurana HK, Jun S, Irudayaraj J, Demirci A. Infrared heating in food processing: an overview. Compr Rev Food Sci F. 2008;7:2–13. doi: 10.1111/j.1541-4337.2007.00024.x. [DOI] [Google Scholar]
- Lee SC, Jeong SM, Kim SY, Park HR, Nam KC, Ahn DU. Effect of far-infrared radiation and heat treatment on the antioxidant activity of water extracts from peanut hulls. Food Chem. 2006;94:489–493. doi: 10.1016/j.foodchem.2004.12.001. [DOI] [Google Scholar]
- Lemoine ML, Civello PM, Martnez GA, Chaves AR. Influence of postharvest UV-C treatment on refrigerated storage of minimally processed broccoli (Brassica oleracea var. Italica) J Sci Food Agr. 2007;87:1132–1139. doi: 10.1002/jsfa.2826. [DOI] [Google Scholar]
- Liu RH. Whole grain phytochemicals and health. J Cereal Sci. 2007;46:207–219. doi: 10.1016/j.jcs.2007.06.010. [DOI] [Google Scholar]
- Liyana-Pathirana CM, Shahidi F. Antioxidant properties of commercial soft and hard winter wheats (Triticum aestivum L.) and their milling fractions. J Sci Food Agr. 2006;86(3):477–485. doi: 10.1002/jsfa.2374. [DOI] [Google Scholar]
- Masud T, Mahmood T, Latif A, Sammi S, Hameed T. Influence of processing and cooking methodologies for reduction of phytic acid content in wheat (Triticum aestivum) varieties. J Food Process Pres. 2007;31:583–594. doi: 10.1111/j.1745-4549.2007.00147.x. [DOI] [Google Scholar]
- McCurdy SM. Infrared processing of dry peas, canola, and canola screenings. J Food Sci. 1992;57(4):941–944. doi: 10.1111/j.1365-2621.1992.tb14329.x. [DOI] [Google Scholar]
- Miller HE, Rigelhof F, Marquart L, Prakash A, Kanter M. Antioxidant content of whole grain breakfast cereals, fruits and vegetables. J Am Coll Nutr. 2000;19:312–319. doi: 10.1080/07315724.2000.10718966. [DOI] [PubMed] [Google Scholar]
- Özkaya B (2004) The effect of variety and extraction on phytic acid content of bread. Ankara University, Scientific Research Projects, Project Number:2002-07-11-064, Ankara
- Peleg H, Naim M, Rouseff RL, Zehavi U. Distribution of bound and free phenolic acids in oranges (Citrus sinensis) and grapefruit (Citrus paradise) J Sci Food Agric. 1991;57:417–426. doi: 10.1002/jsfa.2740570312. [DOI] [Google Scholar]
- Pınarlı İ, İbanoglu Ş, Öner MD. Effect of storage on the selected properties of macaroni enriched with wheat germ. J Food Eng. 2004;64:249–256. doi: 10.1016/j.jfoodeng.2003.10.005. [DOI] [Google Scholar]
- Porres JM, López-Jurado M, Aranda P, Urbano G. Effect of heat treatment and mineral and vitamin supplementation on the nutritive use of protein and calcium from lentils (Lens culinaris M.) in growing rats. Nutrition. 2003;19(5):451–456. doi: 10.1016/S0899-9007(02)01011-0. [DOI] [PubMed] [Google Scholar]
- Randhir R, Kwon YI, Shetty K. Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. Innovat Food Sci Emerg Tech. 2008;9:355–364. doi: 10.1016/j.ifset.2007.10.004. [DOI] [Google Scholar]
- Rickard ES, Thompson LU. Interactions and effects of phytic acid. In: Shahidi F, editor. Antinutrients and phytochemicals in food. Washington DC: American Chemical Society; 1997. pp. 294–312. [Google Scholar]
- Saharan K, Khetarpaul N, Bishnoi S. HCl-extractability of minerals from ricebean and fababean: influence of domestic processing methods. Innovat Food Sci Emerg Tech. 2001;2:323–325. doi: 10.1016/S1466-8564(01)00044-3. [DOI] [Google Scholar]
- Siljestrom M, Westerlunds E, Bjorck I, Holm J, Asp NG, Theander O. The effects of various thermal processes on dietary fiber and starch content of whole grain wheat and white flour. J Cereal Sci. 1986;4(4):315–323. doi: 10.1016/S0733-5210(86)80035-2. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Viticult. 1965;16(1):144–158. [Google Scholar]
- Sivri D, Köksel H, Özkaya H. The possibilities of improving the baking qualities of wheat germ fortified wheat flour. Gıda. 1992;17(4):219–226. [Google Scholar]
- Skrbic B, Filipcev B. Nutritional and sensory evaluation of wheat breads supplemented with oleic-rich sunflower seed. Food Chem. 2008;108(1):119–129. doi: 10.1016/j.foodchem.2007.10.052. [DOI] [Google Scholar]
- Slavin JL. Mechanisms for the impact of whole grain foods on cancer risk. J Am Coll Nutr. 2000;19(3):300–307. doi: 10.1080/07315724.2000.10718964. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Sudha ML, Baskaran V, Leelavathi K. Studies on heat stabilized wheat germ and its influence on rheological characteristics of dough. Eur Food Res Technol. 2007;224:365–372. doi: 10.1007/s00217-006-0317-x. [DOI] [Google Scholar]
- Sudha ML, Srivastava AK, Leelavathi K. Studies on pasting and structural characteristics of thermally treated wheat germ. Eur Food Res Technol. 2007;225:351–357. doi: 10.1007/s00217-006-0422-x. [DOI] [Google Scholar]
- Sun S, Watts BM, Lukow OM, Arntfield SD. Effects of micronization on protein and rheological properties of spring wheat. Cereal Chem. 2006;83(4):340–347. doi: 10.1094/CC-83-0340. [DOI] [Google Scholar]
- Vadivambal R, Jayas DS, White NDG. Wheat disinfestation using microwave energy. J Stored Prod Res. 2007;43:508–514. doi: 10.1016/j.jspr.2007.01.007. [DOI] [Google Scholar]
- Venn BJ, Mann JI. Cereal grains, legumes and diabetes. Eur J Clin Nutr. 2004;58:1443–1461. doi: 10.1038/sj.ejcn.1601995. [DOI] [PubMed] [Google Scholar]
- Vetrimani R, Jyothirmayi N, Rao PH, Ramadoss CS. Inactivation of lipase and lipoxygenase in cereal bran, germ and soybean by microwave treatment. Lebensm Wiss Technol. 1992;25:532–535. [Google Scholar]
- Yue M, Li Y, Wang X. Effects of enhanced ultraviolet-B radiation on plant nutrients and decomposition of spring wheat under field conditions. Environ Exp Bot. 1998;40(3):187–196. doi: 10.1016/S0098-8472(98)00036-7. [DOI] [Google Scholar]
- Zwingelberg H, Fretzdorff B. Effect of microwave treatment on the keeping characteristics of food grade wheat germ. Getredie Mehl und Brot. 1996;50:214–218. [Google Scholar]