Abstract
Small heat shock proteins (sHSPs) are the most diverse but also the most poorly known family of molecular chaperones, and they play essential roles in various biological processes. The striped stem borer, Chilo suppressalis (Insecta: Lepidoptera: Pyralidae), is one of the most serious pests of rice, causing extensive damage and yield loss. In this study, we isolated and characterized five members of the sHSPs family—Cshsp19.8, Cshsp21.4, Cshsp21.5, Cshsp21.7a, and Cshsp21.7b—from C. suppressalis. The cDNAs of these genes encoded proteins of 177, 187, 191, 191, and 191 amino acids with isoelectric points of 7.0, 5.6, 6.1, 6.3, and 6.3, respectively. While Cshsp19.8, Cshsp21.5, and Cshsp21.7b had no introns, Cshsp21.4 and Cshsp21.7a contained one and two introns, respectively. Structural analysis indicated that all five Cshsps possessed conserved arginine and a V/IXI/V motif, which is related to hydrophobic characteristics of sHSPs. The five heat shock proteins can be classified into two main groups: an orthologous type (Cshsp21.4 and Cshsp21.7a) and a species-specific type (Cshsp19.8, Cshsp21.5, and Cshsp21.7b). Real-time quantitative PCR analyses revealed that Cshsp19.8, Cshsp21.5, Cshsp21.7a, and Cshsp21.7b all exhibited their highest expression levels within Malpighian tubules or the hindgut, while such levels were found in the head for Cshsp21.4. The expression of Csshsps at different developmental stages revealed that the mRNA levels of Cshsp19.8, Cshsp21.4, Cshsp21.5, and Cshsp21.7b peaked in adults, whereas the highest level of Cshsp21.7a was observed in first instar larvae. Cshsp19.8 and Cshsp21.7b were both upregulated dramatically by heat and cold, and Cshsp21.5 could be induced by cold stress. Neither Cshsp21.4 nor Cshsp21.7a responded to heat or cold. These results demonstrated that different Csshsps play distinctive roles in the regulation of the physiological activities in C. suppressalis.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-013-0437-8) contains supplementary material, which is available to authorized users.
Keywords: sHSPs, Chilo suppressalis, Genome, Structure, Expression, Temperature
Introduction
Previous studies suggest that small heat shock proteins (sHSPs) are abundant and ubiquitous in almost all organisms (Kim et al. 1998; Aevermann and Waters 2008; Waters et al. 2008). Compared to other types of HSPs, sHSPs exhibit a greater variation in sequence, structure, size, and function (de Jong et al. 1998; Franck et al. 2004). They are a superfamily of proteins that contain an α-crystallin domain with molecular weights of 12–43 kDa, depending on the variable N- and C-terminal extensions (Stromer et al. 2004), and exist under physiological conditions as large oligomers of up to 50 subunits and 1.2 MDa in mass (Gusev et al. 2002; Horwitz 1992). Structurally, sHSPs remain poorly understood, mainly because members of this protein family are extremely dynamic and heterogeneous (Horwitz 2003; Haslbeck et al. 2005). Some studies have found sHSP chaperone activity to be involved with rearrangement of the sHSP oligomer (Giese and Vierling 2002; Morris et al. 2008), and surface-exposed hydrophobic sites of sHSP oligomer on the sHSPs bound to partially unfolded proteins (van Montfort et al. 2001). They are able to bind to denatured proteins and prevent irreversible protein aggregation during stresses, such as extreme temperatures, oxidation, and heavy metals (van Montfort et al. 2002; Haslbeck et al. 2005; Reineke 2005). In addition to stress response, sHSPs have been suggested to be involved in the apoptosis and autophagy, actin and intermediate filament dynamics, organization of the cytoskeleton, and membrane fluidity (Haslbeck 2002; Quinlan 2002; Sun and MacRae 2005; Tsvetkova et al. 2002). They are also therapeutic targets and biomarkers for many diseases (Arrigo et al. 2007). In insects, they are assumed to play an important role in the heat stress, metamorphosis, normal development, diapause, and immune responses (Gu et al. 2012; Hayward et al. 2005; Huang and Kang 2007; Jakob and Buchner 1994; Rinehart et al. 2007; Song et al. 2006), but their functional roles remain unclear. Different organisms have different numbers of sHSPs, ranging from only one up to 19 (Haslbeck et al. 2005). Insects are one of the most successful organisms, having evolved a strong ability to adapt to various habitats. Sixteen shsps in Bombyx mori, 11 in Drosophila melanogaster, 10 in Apis mellifera, and 7 in Anopheles gambiae have been identified (Li et al. 2009). However, studies of sHSPs in other insects are not as extensive and penetrating as in model insects.
Temperature is one of the most important factors determining the distribution and abundance of insects (Bale et al. 2002). When exposed to extreme temperatures, insects may respond in different ways, adopting behaviors to avoid or escape extreme temperatures or altering their physiology to better withstand them (Hoffmann and Parsons 1991). One important behavior is to synthesize a small set of proteins called heat shock proteins (HSPs) that participate in the refolding and relocalization of proteins damaged by such stresses (Sørensen et al. 2003).
The striped stem borer, Chilo suppressalis (Walker) (Insecta: Lepidoptera: Pyralidae), is one of the most economically damaging insect pests of rice in Asia, northern Africa, and southern Europe. In the district of Yangzhou (32.39°N, 119.42°E), Jiangsu Province, China, this insect now completes two full and a partial third generation each year, undergoing diapause in the larval stage (Lu et al. 2012). Moths of the overwintering generation lay egg masses on rice leaves and larvae, which undergo six instars and bore into rice leaf sheaths and stems to feed. Recently, increasing damage and yield loss has been caused by C. suppressalis. Three large and one small hsps genes in C. suppressalis have been identified, and it has been suggested that they could be related to temperature stress (Cui et al. 2010a, b; Sonoda et al. 2006a, b). However, whether other sHSPs exist in C. suppressalis and what differences they display in genome aspect, structure, and function are as yet unknown.
Here, we identified five sHSP genes in C. suppressalis and described their genomic and structural characteristics. In addition, we compared the differential abundance of five shsps among tissues or organs and developmental stages. In order to evaluate the potential relationships between regulation of the five shsps and temperature, we also studied shsps expression under various temperatures.
Materials and methods
Insects
The population of C. suppressalis used in this study was collected from a suburb of Yangzhou (32.39°N, 119.42°E). The rice stem borers were reared in an environmental chamber at 28 ± 1 °C, a 16:8 (light/dark) photoperiod, and a relative humidity of 70 ± 5 % as per Shang et al. (1979).
Cloning and RACEs
Total RNA was extracted using the SV Total RNA isolation system (Promega Z3100) combined with DNase digestion to eliminate DNA contamination. Total cDNA was synthesized by oligo(dT)18 primer (TaKaRa). The full-length cDNAs of the small heat shock protein genes were determined using 5′- and 3′-RACE (SMART RACE, Clontech). The primers used are shown in Supplemental Data 1. The full length sequences of genes were confirmed by RACE 5′ cDNA.
Characterization of the genomes
The genomic DNA of C. suppressalis was extracted by AxyprepTM multisource Genomic DNA Kit (Axygen, USA). According to the full-length cDNA of the five small heat shock protein genes, the pairs of specific primers (Supplemental Data 1) were designed to amplify Csshsps genomic fragments. The products were purified using a gel extraction kit (Axygen, USA), cloned into PGEM-T Easy vector (Promega, USA), and transformed into competent Escherichia coli DH5α cells for sequencing.
Sample preparation
For the first experiment (focused on eight tissues within one life stage), randomly selected fifth instars were used. The larvae used in the experiments were all fifth instars of similar body size and were assigned randomly to each experimental group. Each experimental group contained ten larvae, and each experiment was repeated three times. Larvae were anesthetized on ice before dissection. Heads (HE), epidermis (EP), fat body (FB), foregut (FG), midgut (MG), hindgut (HG), Malpighian tubules (MT), and hemocytes (HC) were collected from larvae and rinsed with a 0.9 % sodium chloride solution. For the second experiment (focused on different life stages and sexes), relevant stages (egg masses; the first, second, third, fourth, fifth, and sixth instar larvae; pupae [male and female]; and 1-day-old adults [male and female]) were randomly selected for the experiment. The samples were frozen immediately in liquid nitrogen and stored at −70 °C until the experiment.
Temperature stress
Larvae were confined individually in glass tubes, and experimental groups of ten were exposed to a given temperature ( −11, −8, −6, −3, 0, 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36, 39, and 42 °C) for 2 h in a constant temperature subzero incubator (DC-3010, Jiangnan equipment). The larvae were recovered at 27 ± 1 °C for 2 h, after which surviving larvae were frozen in liquid nitrogen and stored at −70 °C. The larvae set at 27 ± 1 °C were regarded as control. Each treatment included more than three surviving larvae.
Quantitative real-time PCR (qPCR) analysis
Total RNA was extracted using the SV Total RNA isolation system (Promega Z3100), followed by DNase treatment to eliminate DNA contamination. The integrity of the RNA in all samples was verified by comparing the ribosomal RNA bands in ethidium bromide-stained gels. RNA sample purity was estimated using spectrophotometric measurements at 260 and 280 nm (Eppendorf BioPhotometer plus). Real-time PCR reactions were performed in a 20-μl total reaction volume comprised of 10 μl of 2× SYBR® Premix EXTaqTM (TaKaRa, Dalian, China) master mix, 0.8 μl of each gene-specific primer (Supplemental Data 1), 0.4 μl of Rox reference dye, and 2 μl of cDNA templates. Reactions were carried out on a CFX-96 real-time PCR system (Bio-Rad). The efficiencies of the target and reference genes were similar (Bustin et al. 2009; Nolan et al. 2006). The quantity of Csshsps mRNA was calculated using the 2−ΔΔCt method and normalized to the abundance of the 18SrRNA gene. Hemocytes, eggs, and larvae at 27 ± 1 °C were described respectively as a control (Schmittgen and Livak 2008; http://www.ambion.com/techlib/basics/rtpcr/index.html). Following qPCR, the homogeneity of the PCR products was confirmed by melting curve analysis, which was read each 5 s per 0.5 °C increment from 65 to 95 °C. Every treatment included three replicates, and each reaction was run in triplicate.
Bioinformatic analysis
The open reading frames (ORFs) were identified with the aid of the ORF Finder software (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The deduced amino acid sequences were aligned using ClustalX software. Sequence analysis tools of the ExPASy Molecular Biology Server of the Swiss Institute of Bioinformatics, including Translate, Compute pI/MW, and Blast, were used to analyze the deduced small heat shock protein sequences. Amino acid sequences were used to estimate phylogeny using neighbor-joining, minimum evolution, maximum likelihood, and maximum parsimony methods. Phylogenetic trees were constructed with 1,000 bootstrap replicates using MEGA version 5.0 (Tamura et al. 2011).
Computational molecular modeling
Homology models were generated using Protein Homology/analogy recognition engine V 2.0 (http://www.sbg.bio.ic.ac.uk/~phyre2/html) (Kelley and Sternberg 2009). The small heat shock protein sequences of C. suppressalis were aligned using Phyre2, and the best model of human αB-crystallin V* structure (PDB ID: 2YGD) was used for modeling analyses (Braun et al. 2011). The Chimera tool was used to visualize the 3D coordinates for the atoms of the model (Pettersen et al. 2004).
Statistical analysis
Homogeneity of variances among different groups was evaluated by Levene’s test. The significance of differences between treatments was identified with either an LSD test (homogeneity of variances) or Dunnett’s C test (nonhomogeneous) for multiple comparisons. The data were analyzed using SPSS16.0 software (Pallant 2005) and denoted as means ± SE (standard error).
Results
Isolation, cloning, sequencing, and structure of five shsps from C. suppressalis larvae
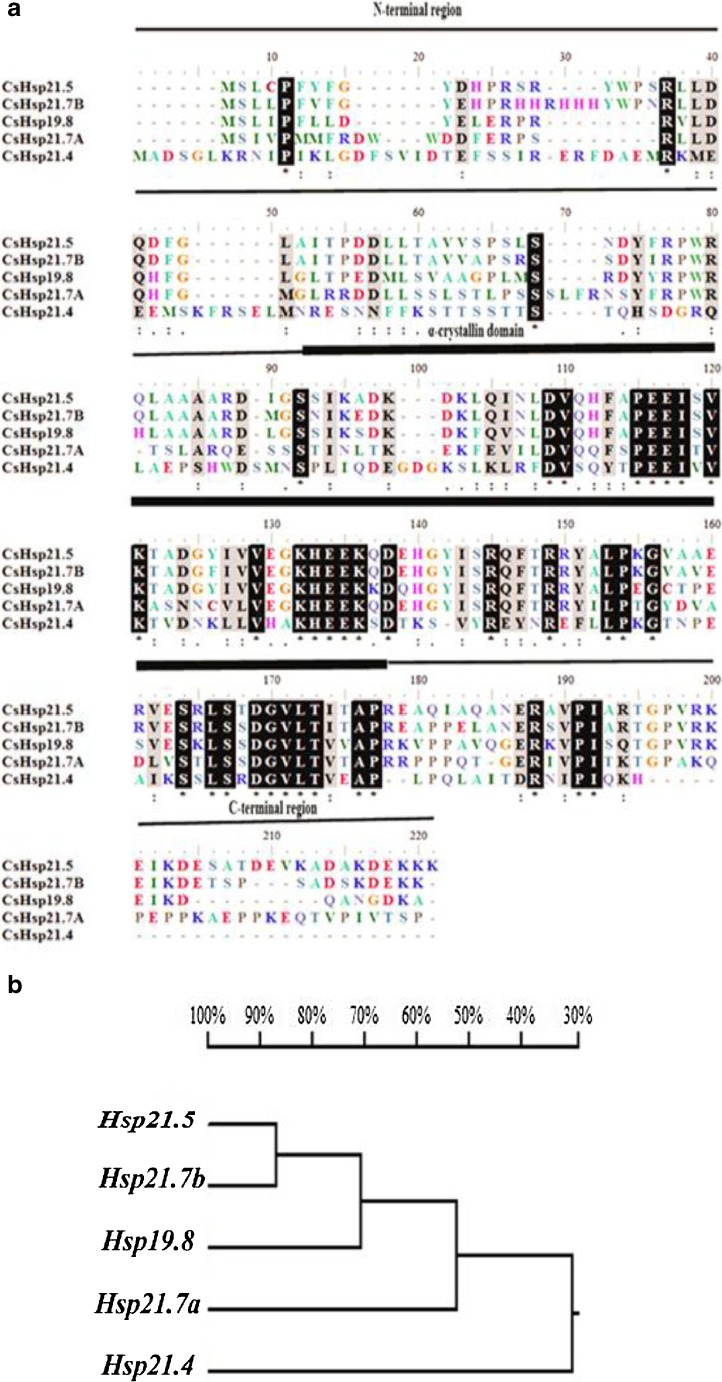
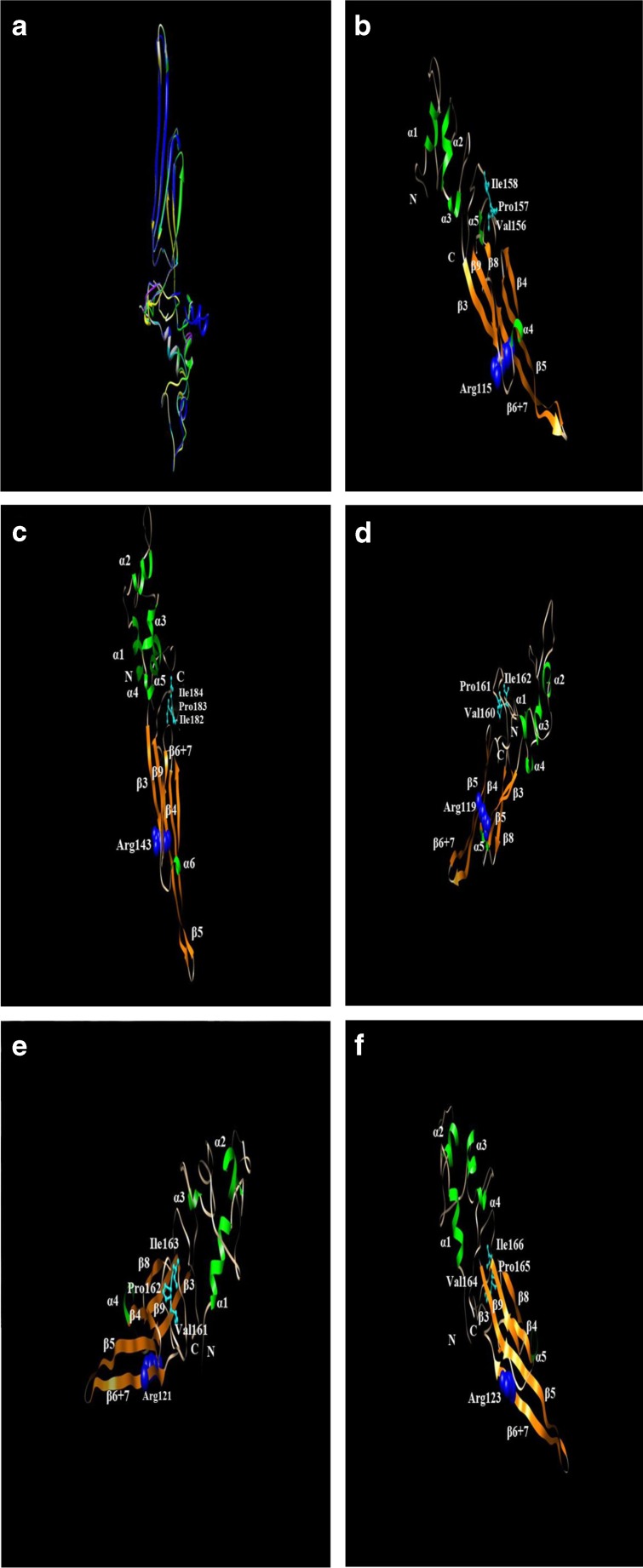
In this study, five small heat shock protein genes—Cshsp19.8, Cshsp21.4, Cshsp21.5, Cshsp21.7a, and Cshsp21.7b—were obtained from C. suppressalis (GenBank accession nos. JX491641, JX491642, KC710018, JX491640, and KC710019, respectively). They possessed the ORFs of 177, 187, 191, 191, and 191 amino acids with an isoelectric point of 7.0, 5.6, 6.1, 6.3, and 6.3, respectively. All five shsps contained a conserved α-crystallin domain, a typical characteristic of shsps (Fig. 1a). Cshsp21.5 and Cshsp21.7b had the highest sequence similarity, while the other three shsps shared only low levels of similarity (Fig. 1b). To investigate the structural characteristics of these five small heat shock proteins from C. suppressalis, we generated their homology models with Phyre using human αB-crystallin V* structure (PDB ID: 2YGD) as a template (Braun et al. 2011). The structures of all five Csshsps models exhibited low homology, compared to that of αB-crystallin (Fig. 2). Alignment analyses demonstrated that CsHSP19.8, CsHSP21.4, CsHSP21.5, CsHSP21.7a, and CsHSP21.7b possessed 37, 25, 36, 41, and 35 % identity, respectively. All five CssHSPs also consisted of α-helixes and β-strands. For example, CsHSP21.5 only included seven β-strands, which differed from the other CssHSPs, which possessed nine β-strands (Fig. 2). CsHSP19.8, CsHSP21.5, and CsHSP21.7b contained five α-helixes, while CsHSP21.4 contained six and CsHSP21.7a contained four. The conserved Arg was found in the β6 + β7 of all five CssHSPs (Arg115, Arg143, Arg119, Arg121, and Arg123, respectively). The carboxy-terminal extensions of all five CssHSPs contained a V/IXI/V motif, and CsHSP19.8, CsHSP21.5, CsHSP21.7a, and CsHSP21.7b contained a V/P/I motif (Val156/Pro157/Ile158, Val160/Pro161/Ile162, Val161/Pro162/Ile163, and Val164/Pro165/Ile166). Meanwhile, CsHSP21.4 contained an I/P/I motif (Ile182/Pro183/ Ile184) (Fig. 2).
Fig. 1.
Alignment of the deduced amino acid sequences of five shsps from Chilo suppressalis. a The horizontal line indicates the conserved α-crystallin domain bordered by variable amino- and carboxy-terminal regions. (−) no amino acid residue; (*) identical residues; (:) strong positive residues; (.) weaker positive residues. b Sequence similarities of C. suppressalis
Fig. 2.
Structure analyses of five small heat shock proteins from C. suppressalis. a Homology modeling analyses of the CsHSP19.8 (yellow), CsHSP21.4 (blue), CsHSP21.5 (cyan), CsHSP21.7a (purple), and CsHSP21.7b (green) with human αB-crystallin V* structure (PDB ID: 2YGD) (white) as template. b–e The α-helix shown in yellow and β-strand shown in orange. The conserved Arg is shown in blue sphere and the V/IXI/V motif is shown in cyan stick and ball. b CsHSP19.8, c CsHSP21.4, d CsHSP21.5, e CsHSP21.7a, and f CsHSP21.7b
Phylogenetic analysis
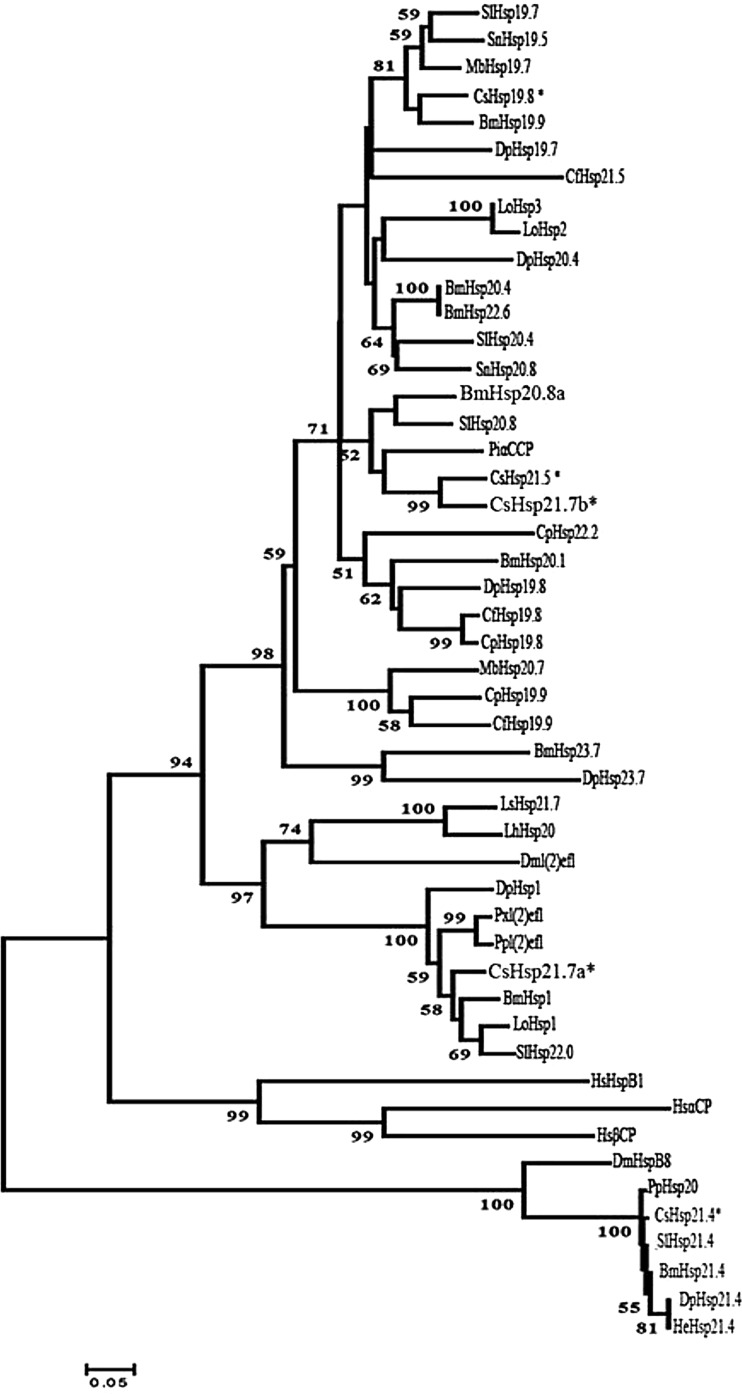
The deduced amino acid sequences of the five shsps displayed a high degree of homology with those of other insects reported so far (Table 1). We used CLUSTALX and MEGA 5.0 phylogenetic analysis to compare Csshsps with other shsps and crystallins through the neighbor-joining, minimum evolution, maximum likelihood, and maximum parsimony methods, and four phylogenetic trees exhibited a consistent trend. As shown in Fig. 3, the tree was separated into three clusters. Cshsp19.8, Cshsp21.5, and Cshsp21.7b, all with high sequence similarity to each other, fell into a well-supported cluster. Meanwhile, Cshsp21.4 belonged to the group of the lepidopteran hsp21.4, and Cshsp21.7a and l(2)efl proteins were placed together (Fig. 3). For Cshsp21.5, the highest amino acid identity of 75 % was observed with the α-crystallin cognate protein gene of Plodia interpunctella; Cshsp21.4 had 98 % identity with the hsp21.4 of Spodoptera litura.
Table 1.
sHsps sequences collected from the database from which the phylogenetic tree was constructed
| No. | Species | Name | Accession number |
|---|---|---|---|
| 1 | Chilo suppressalis | CsHsp19.8 | JX491641 |
| 2 | CsHsp21.4 | JX491642 | |
| 3 | CsHsp21.5 | KC710018 | |
| 4 | CsHsp21.7a | JX491640 | |
| 5 | CsHsp21.7b | KC710019 | |
| 6 | Bombyx mori | BmHsp19.9 | Q5R1P7 |
| 7 | Bmhsp20.1 | Q5R1P6 | |
| 8 | BmHsp20.4 | Q9GSB6 | |
| 9 | BmHsp20.8a | Q9GN07 | |
| 10 | BmHsp21.4 | Q5R1P5 | |
| 11 | BmHsp22.6 | B9VTS8 | |
| 12 | BmHsp23.7 | Q5R1P4 | |
| 13 | BmHsp1 | F1C929 | |
| 14 | Danaus plexippus | DpHsp19.7 | G6CMJ5 |
| 15 | DpHsp19.8 | G6DDW4 | |
| 16 | DpHsp20.4 | G6DR93 | |
| 17 | DpHsp21.4 | G6D9T7 | |
| 18 | DpHsp23.7 | G6D7N2 | |
| 19 | DpHsp1 | G6DC87 | |
| 20 | Spodoptera litura | SlHsp19.7 | E2F3A1 |
| 21 | SlHsp20.4 | E2F399 | |
| 22 | SlHsp20.8 | E2F397 | |
| 23 | SlHsp21.4 | E2F396 | |
| 24 | SlHsp22.0 | I3QQD5 | |
| 25 | Lonomia obliqua | LoHsp1 | Q5MGP0 |
| 26 | LoHsp2 | Q5MGL1 | |
| 27 | LoHsp3 | Q5MGN8 | |
| 28 | Choristoneura fumiferana | CfHsp19.8 | Q1AMF5 |
| 29 | CfHsp19.9 | Q1AMF6 | |
| 30 | CfHsp21.5 | Q1AMF7 | |
| 31 | Cydia pomonella | CpHsp19.8 | F1C928 |
| 32 | CpHsp19.9 | F1C929 | |
| 33 | CpHsp22.2 | F1C930 | |
| 34 | Papilio polytes | PpHsp20 | I4DM98 |
| 35 | Ppl(2)efl | I4DLZ6 | |
| 36 | Sesamia nonagrioides | SnHsp19.5 | B2ZHW3 |
| 37 | SnHsp20.8 | Q2LCS7 | |
| 38 | Mamestra brassicae | MbHsp19.7 | Q0KKB1 |
| 39 | MbHsp20.7 | Q0KKB2 | |
| 40 | Heliconius erato | HeHsp21.4 | A7KCX9 |
| 41 | Plodia interpunctella | α-Crystallin cognate protein | O18634 |
| 42 | Papilio xuthus | Pxl(2)efl | I4DIM9 |
| 43 | Drosophila melanogaster | DpHspB8 | Q9VWG1 |
| 44 | Dpl(2)efl | P82147 | |
| 45 | Liriomyza sativae | LsHsp21.7 | Q1PCB6 |
| 46 | Liriomyza huidobrenisis | LhHsp20 | Q1PCB7 |
| 47 | Homo sapiens | HSPB1 | NM_001540 |
| 48 | α-Crystallin | NM_000394 | |
| 49 | β-Crystallin | NM_001885 |
Fig. 3.
Neighbor-joining phylogenetic tree of lepidopteran shsps. The Chilo suppressalis shsps are labeled with asterisks. The Homo sapiens HSPB1, α-crystalline and β-crystallin were used as the outgroup. Numbers on the branches are the bootstrap values obtained from 1,000 replicates (only bootstrap values >50 are shown). The accession numbers and abbreviation for the species names are listed in Table 1
Genomic structure of C. suppressalis
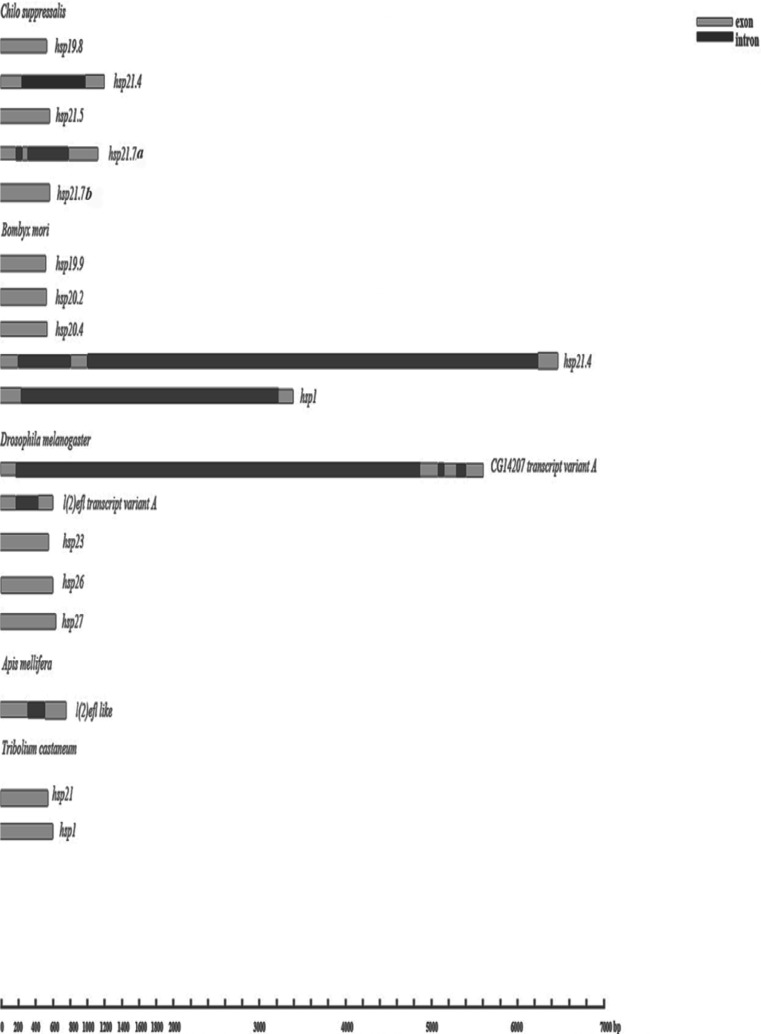
The genomic DNA sequences of the five Csshsps were identified (GenBank accession nos. KC210020, KC210021, KC210022, KC210023, and KC210024, respectively). The positions and sizes of the introns were noted by aligning the cDNA sequences of the five Csshsps with the genomic DNA sequences. The nucleotide sequences at the splice junctions are consistent with the canonical GT-AG rule. We found that Cshsp19.8, Cshsp21.5, and Cshsp21.7b had no introns, similar to the homology sequences of the other insects (Figs. 3 and 4), while Cshsp21.4 and Cshsp21.7a had one and two introns, respectively (Fig. 4). Although Cshsp21.4 and Bmhsp21.4 (B. mori) had the high identity mentioned above, they had different numbers and sizes of introns. Cshsp21.4 had one intron with 746 bp, while Bmhsp21.4 had two introns with 720 and 5531 bp. And while we found only one intron position in the alignment of the orthologous Cshsp21.7a sequences, the position of Bmhsp1 is at 242–3219 and that for Dpl(2)efl of D. melanogaster was at 176–239. However, position of the two CsHsp21.7a introns was at 177–243 and 314–786, respectively (Figs. 3 and 4).
Fig. 4.
Schematic representation of five insect shsps genomes. The species names and accession numbers of genomic DNA sequences were listed in Supplemental Data 3. Light gray and black rectangles are used to highlight the exons and introns, respectively
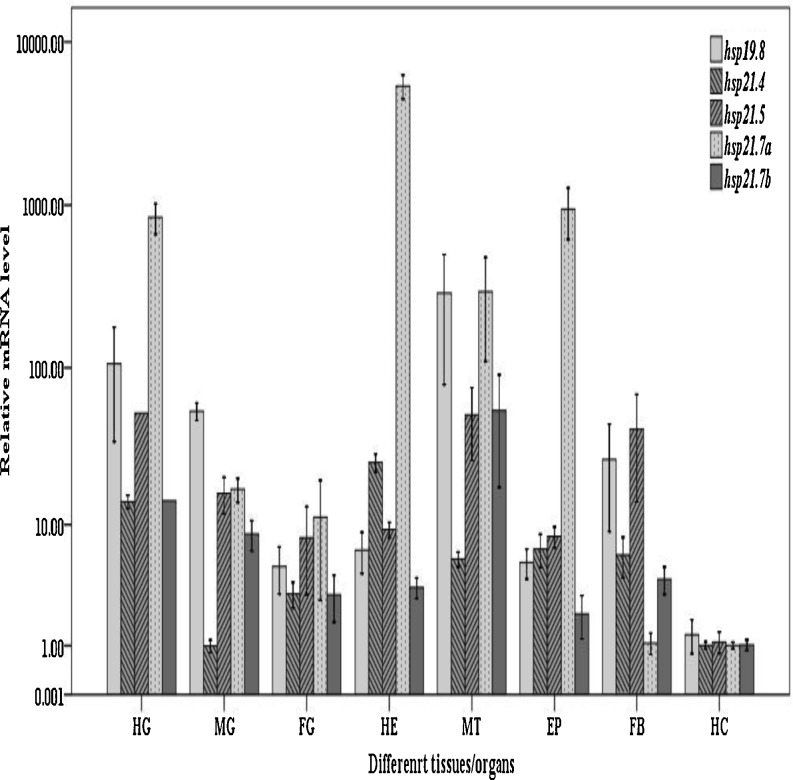
Tissue (organ) distribution of mRNA expression in C. suppressalis larvae
Real-time PCR was used to study the tissue (organ)-specific expressions of Cshsp19.8, Cshsp21.4, Cshsp21.5, Cshsp21.7a, and Cshsp21.7b in C. suppressalis larvae. All five small heat shock protein genes were commonly expressed in all tissues or organs though they exhibited different expression patterns. For example, the lowest mRNA levels of Csshsps were observed consistently in the hemocytes. The highest expression level of Cshsp21.7a was in the heads, which was as high as 5,382.51-fold that of the level in the hemocytes. Also, the epidermis, Malpighian tubules, and hindgut also exhibited abundant levels. Three Cshsps (Cshsp19.8, Cshsp21.5, and Cshsp21.7b) all exhibited the highest expression levels in the Malpighian tubules or hindguts. The mRNA level of Cshsp19.8 in the midguts was significantly higher than that of the foreguts (F7, 16 = 9.956, P < 0.001). Meanwhile, Cshsp21.4 was expressed more highly in the heads than in the other tissues (organs) (F7, 16 = 28.227, P < 0.001) (Fig. 5).
Fig. 5.
Relative mRNA expression levels of the Cshsp19.8, Cshsp21.4, Cshsp21.5, Cshsp21.7a, and Cshsp21.7b genes in different tissues (organs) of Chilo suppressalis. Abbreviations: HG hindgut, MG midgut, FG foregut, HC hemocytes, MT Malpighian tubules, EP epidermis, FB fat body, HE head
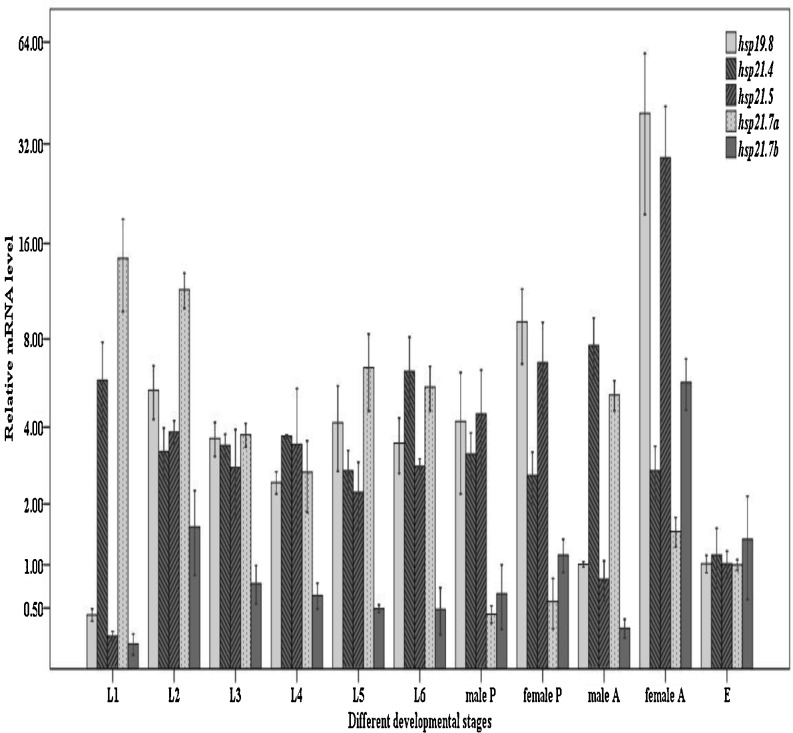
Expression of different shsps in the developmental stages of C. suppressalis
We investigated the mRNA level of five shsps through the developmental stage of C. suppressalis, including eggs, larvae (first, second, third, fourth, fifth, and sixth instar larvae), pupae (male and female), and adults (male and female). We found that Cshsp19.8, Cshsp21.4, Cshsp21.5, Cshsp21.7a, and Cshsp21.7b all showed irregular expression variations throughout the developmental stages (F10, 18 = 29.595, P < 0.001; F10, 21 = 3.487, P = 0.008; F10, 17 = 10.730, P < 0.001; F10, 19 = 17.790, P < 0.001; F10, 20 = 5.239, P = 0.001). We observed the highest expression levels for Cshsp19.8, Cshsp21.4, Cshsp21.5, and Cshsp21.7b in adults and in the first instar larvae for Cshsp21.7a. Moreover, the first instar larvae contained the lowest levels of Cshsp19.8, Cshsp21.5, and Cshsp21.7b. Interestingly, the expression levels of five Csshsps all showed significant differences between male and female adults, but between male and female pupae, only Cshsp19.8 had a significant difference (P < 0.050) (Fig. 6).
Fig. 6.
Relative mRNA expression levels of Cshsp19.8, Cshsp21.4, Cshsp21.5, Cshsp21.7a, and Cshsp21.7b in different developmental stages of Chilo suppressalis. Abbreviations: E eggs, L1 first instar larvae, L2 second instar larvae, L3 third instar larvae, L4 fourth instar larvae, L5 fifth instar larvae, L6 sixth instar larvae, P pupae, A adults
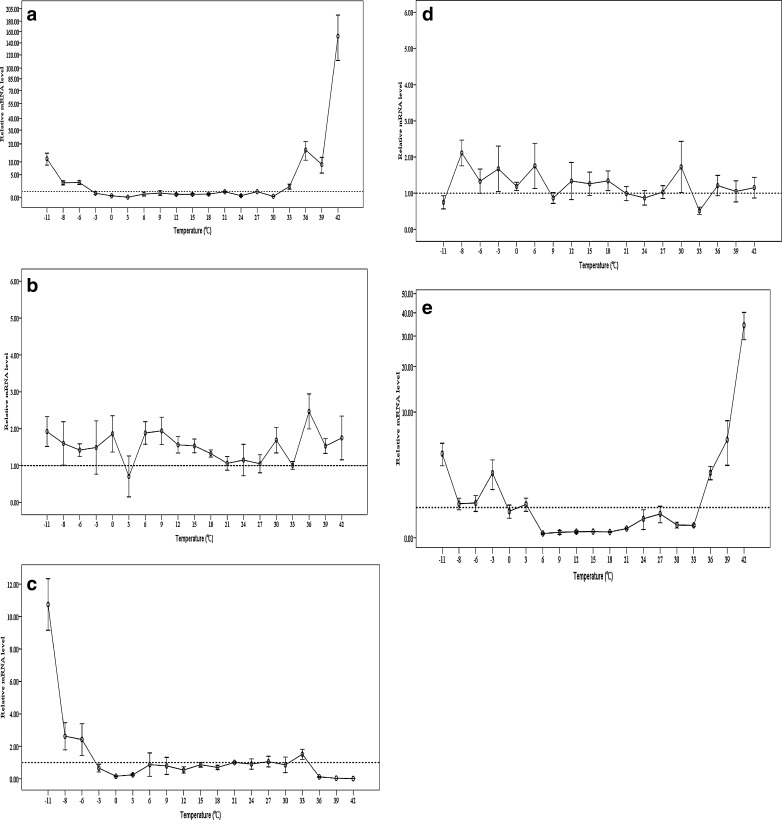
Expression of five shsps from C. suppressalis in response to temperatures
The relative mRNA levels of the five shsps were observed at temperature gradients from −11 to 42 °C. Different Csshsps possessed various expression patterns under temperatures (Fig. 7). For example, Cshsp19.8 and Cshsp21.7b were both upregulated dramatically by heat and cold, with an intensive response to heat, and their mRNA level increased by 151.72- and 34.39-fold, respectively, after 2 h at 42 °C. However, they could not be induced by mild cold and heat (Fig. 7a, e). Although the survival rate of larvae of C. suppressalis only reached to 55.56 % after 2 h at −11 °C and was 0.00 % after 2 h at 45 °C (Supplemental data 2), Cshsp21.4 and Cshsp21.7a did not respond to the heat and cold (Fig. 7b, d). While Cshsp21.5 could be induced by cold stress and the mRNA level increased by 10.75-fold after 2 h at −11 °C, it did not respond to heat (Fig. 7c).
Fig. 7.
Relative expression levels of small heat shock protein genes in Chilo suppressalis under temperature treatments. a Relative expression levels of Cshsp19.8 gene. b Relative expression levels of Cshsp21.4 gene. c Relative expression levels of Cshsp21.5 gene. d Relative expression levels of Cshsp21.7a gene. e Relative expression levels of Cshsp21.7b gene
Discussion
Previous research has suggested that small heat shock proteins participate in diverse physiological processes, one of the best known of which is to increase the heat and cold tolerance of an organism (Liu et al. 2012; Sun and MacRae 2005). In addition to stress response, sHSPs are produced during normal developmental and diapause stages in insects (Feder and Hofmann 1999; Gkouvitsas et al. 2008; Hayward et al. 2004; Kokolakis et al. 2009; Shirk et al. 1998).
In this study, five new members of the shsps family have been identified from the rice stem borer, C. suppressalis: Cshsp19.8, Cshsp21.4, Cshsp21.5, Cshsp21.7a, and Cshsp21.7b. The cDNA sequences contained open reading frames that encoded 177, 187, 191, 191, and 191 amino acids for the genes, respectively. The predicted amino acid sequences for Cshsp19.8, Cshsp21.4, Cshsp21.5, Cshsp21.7a, and Cshsp21.7b shared considerable sequence similarity with shsps from other insects and α-crystallin proteins from vertebrate lenses. The Csshsps share low levels of similarity among themselves, and the neighbor-Joining dendrogram placed the predicted proteins of the five Csshsps in different clusters. In general, shsps from different insect species were phylogenetically closer than those from the same insect. In addition, Cshsp21.4 belonged to the group of the lepidopteran hsp21.4, an orthologous cluster that includes one sHSP from each insect, whereas most insect sHSPs are species specific (Li et al. 2009). This may imply that Cshsp21.4 might have experienced a specific evolutionary process. The five Csshsps all have a conserved structural organization of α-helixes and β-strands (Kim et al. 1998; van Montfort et al. 2001). The highly conserved arginine was found to correlate to surface-exposed hydrophobic sites of sHSPs (Clark et al. 2011; Jehle et al. 2011). The five CssHSPs contained Arg115, Arg143, Arg119, Arg121, and Arg123, respectively, in the β6 + β7. In the carboxy-terminal extensions, every CssHSP had a V/IXI/V motif, which was one of several features that determined the assembly of sHSP dimers into a variety of oligomeric structures (Basha et al. 2012). Two of the five Csshsps contained introns, and their number and size differ from their homology sequences of insects. The negative correlation between intron size and gene expression level was suggested in some studies, and the shorter or no intron genes had highly expressed level (Comeron 2004). The shorter or no intron genes also could be sensitive to various environmental stresses. According to the information of chromosome location and intron number, the sHSP genes were roughly divided into two types: orthologous and species specific (Li et al. 2009). Thus, we considered five small heat shock protein genes from C. suppressalis and classified them into two groups: orthologous type that contained introns (Cshsp21.4 and Cshsp21.7a) and species-specific type with no intron (Cshsp19.8, Cshsp21.5, and Cshsp21.7b).
The role of small heat shock proteins in the tissues or organs is not well understood. One possibility is that sHSP genes expressed in special tissues or organs may play an important role in maintaining the normal organism functioning (Gu et al. 2012). In this study, five small Cshsps (Cshsp19.8, Cshsp21.5, Cshsp21.4, Cshsp21.7a, and Cshsp21.7b) all exhibited abundant levels within the Malpighian tubules or hindgut. Similar results were found in S. litura and Apis cerana cerana (Liu et al. 2012; Shen et al. 2011). All five Csshsps in C. suppressalis demonstrated tissue (organ)-specific expressions. Hindgut and Malpighian tubules reabsorb water, salts, and other substances before excretion by the insect, and it remains unclear why shsps should be highly expressed in either. One explanation is that shsps protect these tissues or organs from toxic injury. However, Cshsp21.4 exhibited the highest mRNA level in the head, similar to the high expression levels of Bm19.1 and Bm22.6 in the head (Li et al. 2009). Thus, it is evident that different shsps play different roles in insect biological processes.
sHSPs also play important roles in insect development. Previous studies have indicated that they were involved in the regulation of development in insects (Concha et al. 2012; Shen et al. 2011; Takahashi et al. 2010). For instance, l(2)efl, a type of small hsp, reached its maximum value in the third larval instar of D. melanogaster (Kurzik-Dumke and Lohmann 1995). In Lucilia cuprina, the hsp24 gene was expressed at its lowest level in the third instar larvae (Concha et al. 2012). In this study, the highest expression level of Cshsp21.7a was observed in the first instar larvae, while those for Cshsp19.8, Cshsp21.4, Cshsp21.5, and Cshsp21.7b were observed in adults, which corresponded with the expression of hsp19.7, hsp20, and hsp20.7 in S. litura and hsp19.7 and hsp19.8 in Cydia pomonella (Garczynski et al. 2011; Shen et al. 2011). However, we found the adult phase of C. suppressalis possessed the lowest thermotolerance in our previous study. Therefore, we speculated that Cshsp19.8, Cshsp21.4, Cshsp21.5, and Cshsp21.7b also correlated with the reproduction of C. suppressalis. Conversely, the hsp20.4 of S. litura and three shsps of Liriomyza sativa had their lowest expression levels in adults (Huang et al. 2009; Shen et al. 2011). However, the hsp21.4 of S. litura exhibited a constitutive expression pattern during all developmental stages (Shen et al. 2011). In general, small heat shock proteins of different insects could have evolved respectively specific roles in the development. The expression levels of five small heat shock protein genes from C. suppressalis exhibited differences based on sex, and a similar phenomenon was observed in B. mori and A. mellifera (Aamodt 2008; Li et al. 2009).
Recent reports have stressed the fact that regulation and expression levels of HSPs contribute to the thermotolerance of organisms (Sørensen et al. 2003; Queitsch et al. 2002). All five Csshsps (Cshsp19.8, Cshsp21.4, Cshsp21.5, Cshsp21.7a, and Cshsp21.7b) differed in their expression profiles. Cshsp19.8 and Cshsp21.7b were both upregulated dramatically by heat and cold, with an intensive response to heat, which is similar to hsp20.4 and hsp20.8 from S. litura, which were also significantly upregulated by both heat and cold treatments. Five shsps from B. mori and three shsps from C. pomonella demonstrated increased expression to heat shock stress (Garczynski et al. 2011; Li et al. 2009; Sakano et al. 2006). However, none of the five Csshsps were induced by only mild cold or heat, a phenomenon shared by the three shsps of L. sativa and two shsps of L. cuprina (Concha et al. 2012; Huang et al. 2009). However, hsp19.7 and hsp20.7 of S. litura responded only to heat treatment, not to cold stress (Shen et al. 2011). In contrast to hsp19.7 and hsp20.7, our study found that Cshsp21.5 only responded to cold stress. Meanwhile, neither Cshsp21.4 nor Cshsp21.7a responded to heat or cold, similar to hsp20 and hsp21.4 of S. litura and hsp21.4 of B. mori, all of which were insensitive to thermal stress (Li et al. 2009; Shen et al. 2011). Because overexpression of HSP may cause some deleterious effects in organisms, it is very important to understand the balance between benefits and costs (Krebs and Feder 1998; Sørensen et al. 2003). In order to balance, C. suppressalis could regulate different shsps under different temperature stresses.
The more studies there are on the levels of such proteins, the better we will understand the roles of these genes in insect behavior and development. Future investigations of shsps will help reveal the underlying physiological mechanisms of C. suppressalis, allowing for improved management of this important pest.
Electronic supplementary material
Primer sequences used in this study (DOC). (DOCX 18 kb)
The survival rates of Chilo suppressalis under different temperatures (DOC). (DOC 40 kb)
The species name and accession number of genomic DNA sequences (DOC). (DOCX 15 kb)
Acknowledgments
This work was supported by the National Basic Research and Development Program of China (2012CB114100), the Ph.D. Programs Foundation of the Ministry of Education (20113250110008), and the Key Study Project of Education Department of Anhui Province (KJ2011A211).
References
- Aamodt RM. The caste- and age-specific expression signature of honeybee heat shock genes shows an alternative splicing-dependent regulation of Hsp90. Mech Ageing Dev. 2008;129:632–637. doi: 10.1016/j.mad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Aevermann BD, Waters ER. A comparative genomic analysis of the small heat shock proteins in Caenorhabditis elegans and briggsae. Genetica. 2008;133:307–319. doi: 10.1007/s10709-007-9215-9. [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A, Guillet D, Moulin M, Diaz-Latoud C, Vicart P. Hsp27 (HspB1) and alpha B-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Bale JS, Masters GJ, Hodkinson ID, Awmak C, Bezemer TM, Brown V, Butterfield J, Buse A, Coulson JC, Farrar J, Good JEG, Hartley R, Jones TH, Lindroth RL, Press MC, Symrnioudis I, Watt A, Whittaker JB. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biol. 2002;8:1–16. doi: 10.1046/j.1365-2486.2002.00451.x. [DOI] [Google Scholar]
- Basha E, O’Neill H, Vierling E. Small heat shock proteins and α-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci. 2012;37:106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, Zachariasb M, Peschek J, Kastenmüller A, Zou J, Hanzlik M, Haslbeck M, Rappsilber J, Buchner J, Weinkauf S. Multiple molecular architectures of the eye lens chaperone αB-crystallin elucidated by a triple hybrid approach. Proc Natl Acad Sci USA. 2011;108:20491–20496. doi: 10.1073/pnas.1111014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubisa M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Clark AR, Naylor CE, Bagnéris C, Keep NH, Slingsby C. Crystal structure of R120G disease mutant of human αB-crystallin domain dimer shows closure of a groove. J Mol Biol. 2011;408:118–134. doi: 10.1016/j.jmb.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeron JM. Selective and mutational patterns associated with gene expression in humans: influences on synonymous composition and intron presence. Genet. 2004;167:1293–1304. doi: 10.1534/genetics.104.026351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha C, Edman RM, Belikoff EJ, Schiemann AH, Carey B, Scott MJ. Organization and expression of the Australian sheep blowfly (Lucilia cuprina) hsp23, hsp24, hsp70 and hsp83 genes. Insect Mol Bio. 2012;21:169–180. doi: 10.1111/j.1365-2583.2011.01123.x. [DOI] [PubMed] [Google Scholar]
- Cui YD, Du YZ, Lu MX, Qiang CK. Cloning of the heat shock protein 60 gene from the stem borer, Chilo suppressalis, and analysis of expression characteristics under heat stress. J Insect Sci. 2010;10:1–13. doi: 10.1673/031.010.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YD, Du YZ, Lu MX, Qiang CK. Cloning of the heat shock protein 70 gene from Chilo suppressalis and the analysis of its expression characteristics under heat stress. Acta Entomol Sin. 2010;53:841–848. doi: 10.1673/031.010.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong WW, Caspers GJ, Leunissen JA. Genealogy of the alpha-crystallin-small heat-shock protein superfamily. Int J Biol Macromol. 1998;22:151–162. doi: 10.1016/S0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Franck E, Madsen O, van Rheede T, Ricard G, Huynen MA, de Jong W. Evolutionary diversity of vertebrate small heat shock proteins. J Mol Evol. 2004;59:792–805. doi: 10.1007/s00239-004-0013-z. [DOI] [PubMed] [Google Scholar]
- Garczynski SF, Unruh TR, Guédot C, Neven LG. Characterization of three transcripts encoding small heat shock proteins expressed in the codling moth, Cydia pomonella (Lepidoptera: Tortricidae) Insect Sci. 2011;18:473–483. doi: 10.1111/j.1744-7917.2010.01401.x. [DOI] [Google Scholar]
- Giese KC, Vierling E. Changes in oligomerization are essential for the chaperone activity of a small heat shock protein in vivo and in vitro. J Bio Chem. 2002;277:46310–46318. doi: 10.1074/jbc.M208926200. [DOI] [PubMed] [Google Scholar]
- Gkouvitsas T, Kontogiannatos D, Kourti A. Differential expression of two small Hsps during diapause in the corn stalk borer Sesamia nonagrioides (Lef.) J Insect Physiol. 2008;54:1503–1510. doi: 10.1016/j.jinsphys.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Gu J, Huang LX, Shen Y, Huang LH, Feng QL. Hsp70 and small Hsps are the major heat shock protein members involved in midgut metamorphosis in the common cutworm, Spodoptera litura. Insect Mol Bio. 2012;5:535–543. doi: 10.1111/j.1365-2583.2012.01158.x. [DOI] [PubMed] [Google Scholar]
- Gusev NB, Bogatcheva NV, Marston SB. Structure and properties of the small heat shock proteins (sHsp) and their interaction with cytoskeleton proteins. Biochem Mosc. 2002;67:511–519. doi: 10.1023/A:1015549725819. [DOI] [PubMed] [Google Scholar]
- Haslbeck M. sHsps and their role in the chaperone network. Cell Mol Life Sci. 2002;59:51–60. doi: 10.1007/PL00012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Hayward SAL, Rinehart JP, Denlinger DL. Desiccation and rehydration elicit distinct heat shock protein transcript responses in flesh fly pupae. J Exp Biol. 2004;207:963–971. doi: 10.1242/jeb.00842. [DOI] [PubMed] [Google Scholar]
- Hayward SAL, Pavlidesb SC, Tammariellob SP, Rineharta JP, Denlinger DL. Temporal expression patterns of diapause-associated genes in flesh fly pupae from the onset of diapause through post-diapause quiescence. J Insect Physiol. 2005;51:631–640. doi: 10.1016/j.jinsphys.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Parsons PA. Evolutionary genetics and environmental stress. New York: Oxford University Press; 1991. [Google Scholar]
- Horwitz J. α-Crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/S0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- Huang LH, Kang L. Cloning and inter-specific altered expression of heat shock protein genes in two leaf miner species in response to thermal stress. Insect Mol Biol. 2007;16:491–500. doi: 10.1111/j.1365-2583.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- Huang LH, Wang CZ, Kang L. Cloning and expression of five heat shock protein genes in relation to cold hardening and development in the leaf miner, Liriomyza sativa. J Insect Physiol. 2009;55:279–285. doi: 10.1016/j.jinsphys.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Jakob U, Buchner J. Assisting spontaneity: the role of Hsp90 and small Hsps as molecular chaperones. Trends Biochem Sci. 1994;19:205–211. doi: 10.1016/0968-0004(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Jehle S, Vollmar BS, Bardiaux B, Dove KK, Rajagopal P, Gonen T, Oschkinat H, Klevita RE. N-terminal domain of αB-crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc Natl Acad Sci USA. 2011;108:6409–6414. doi: 10.1073/pnas.1014656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJE. Protein structure prediction on the web: a case study using the phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Krebs RA, Feder ME. Hsp70 and larval thermotolerance in Drosophila melanogaster: how much is enough and when is more too much? J Insect Physiol. 1998;44:1091–1101. doi: 10.1016/S0022-1910(98)00059-6. [DOI] [PubMed] [Google Scholar]
- Kokolakis G, Kritsidima M, Tkachenko T, Mintzas AC. Two hsp23 genes in the Mediterranean fruit fly, Ceratitis capitata: structural characterization, heat shock regulation and developmental expression. Insect Mol Biol. 2009;18:171–181. doi: 10.1111/j.1365-2583.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- Kurzik-Dumke U, Lohmann E. Sequence of the new Drosophila melanogaster small heat-shock-related gene, lethal (2) essential for life [l(2)efl], at locus 59F4, 5. Gene. 1995;154:171–175. doi: 10.1016/0378-1119(94)00827-F. [DOI] [PubMed] [Google Scholar]
- Li ZW, Li X, Yu QY, Xiang ZH, Kishino H, Zhang Z (2009) The small heat shock protein (sHSP) genes in the silkworm, Bombyx mori, and comparative analysis with other insect sHSP genes. BMC Evol Biol 215 [DOI] [PMC free article] [PubMed]
- Liu ZH, Xi DM, Kang MJ, Guo XQ, Xu BH. Molecular cloning and characterization of Hsp27.6: the first reported small heat shock protein from Apis cerana cerana. Cell Stress Chaperones. 2012;17:539–551. doi: 10.1007/s12192-012-0330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MX, Liu ZX, Wang X, Du YZ. Seasonal cold tolerance of Chilo suppressalis (Walker) in Yangzhou, China. Ann Entomol Soc America. 2012;105:479–483. doi: 10.1603/AN11171. [DOI] [Google Scholar]
- Morris AM, Treweek TM, Aquilina JA, Carver JA, Walker MJ. Glutamic acid residues in the C-terminal extension of small heat shock protein 25 are critical for structural and functional integrity. FEBS J. 2008;275:5885–5898. doi: 10.1111/j.1742-4658.2008.06719.x. [DOI] [PubMed] [Google Scholar]
- Nolan T, Hand RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1:1559–1552. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- Pallant J. SPSS Survival Manual: A Step by Step Guide to Data Analysis using SPSS for Windows (Version 12) Maidenhead: Open University Press; 2005. [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- Quinlan R. Cytoskeletal competence requires protein chaperones. Prog Mol Subcell Biol. 2002;28:219–234. doi: 10.1007/978-3-642-56348-5_12. [DOI] [PubMed] [Google Scholar]
- Reineke A. Identification and expression of a small heat shock protein in two lines of the endoparasitic wasp Venturia canescens. Comp Biochem Physiol A. 2005;141:60–69. doi: 10.1016/j.cbpb.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Rinehart JP, Li AQ, Yocum GD, Robich RM, Hayward SAL, Denlinger DL. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc Natl Acad Sci USA. 2007;104:11130–11137. doi: 10.1073/pnas.0703538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano D, Li B, Xia QY, Yamamoto K, Fujii H, Aso Y. Genes encoding small heat shock proteins of the silkworm, Bombyx mori. Bios Biotech Bioch. 2006;70:2443–2450. doi: 10.1271/bbb.60176. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shang ZZ, Wang YS, Zou YH. Study on rearing method of rice stem borer Chilo suppressalis Walker. Acta Entomol Sin. 1979;2:164–167. [Google Scholar]
- Shen Y, Gu J, Huang LH, Zheng SC, Liu L, Xu WH, Feng QL, Kang L. Cloning and expression analysis of six small heat shock protein genes in the common cutworm, Spodoptera litura. J Insect Physiol. 2011;57:908–914. doi: 10.1016/j.jinsphys.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Shirk PD, Broza R, Hemphill M, Perera OP. α-Crystallin protein cognates in eggs of the moth, Plodia interpunctella: possible chaperones for the follicular epithelium yolk protein. Insect Biochem Mol Biol. 1998;28:151–161. doi: 10.1016/S0965-1748(97)00111-2. [DOI] [PubMed] [Google Scholar]
- Song KH, Jung SJ, Seo YR, Kang SW, Han SS. Identification of upregulated proteins in the hemolymph of immunized Bombyx mori larvae. Comp Biochem Phys D. 2006;1:260–266. doi: 10.1016/j.cbd.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Fukumoto K, Izumi Y, Ashfaq M, Yoshida H, Tsumuki H. A small HSP gene is not responsible for diapause and cold tolerance acquisition in Chilo suppressalis. J Appl Entomol. 2006;130:309–313. doi: 10.1111/j.1439-0418.2006.01067.x. [DOI] [Google Scholar]
- Sonoda S, Fukumoto K, Izumi Y, Yoshida H, Tsumuki H. Cloning of heat shock protein genes (hsp90 and hsc70) and their expression during larval diapause and cold tolerance acquisition in the rice stem borer, Chilo suppressalis Walker. Arch Insect Biochem. 2006;63:36–47. doi: 10.1002/arch.20138. [DOI] [PubMed] [Google Scholar]
- Sørensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol Lett. 2003;6:1025–1037. doi: 10.1046/j.1461-0248.2003.00528.x. [DOI] [Google Scholar]
- Stromer T, Fischer E, Richter K, Haslbeck M, Buchner J. Analysis of the regulation of the molecular chaperone Hsp26 by temperature-induced dissociation: the N-terminal domail is important for oligomer assembly and the binding of unfolding proteins. J Biol Chem. 2004;279:11222–11228. doi: 10.1074/jbc.M310149200. [DOI] [PubMed] [Google Scholar]
- Sun Y, MacRae TH. Small heat shock proteins: molecular structure and chaperone function. Cell Mol Life Sci. 2005;62:2460–2476. doi: 10.1007/s00018-005-5190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi KH, Rako L, Takano-Shimizu T, Hoffmann AA, Lee SF. Effects of small Hsp genes on developmental stability and micro-environmental canalization. BMC Evol Biol. 2010;10:284. doi: 10.1186/1471-2148-10-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkova NM, Horvath I, Torok Z, Wolkers WF, Balogi Z, Shigapova N, Crowe LM, Tablin F, Vierling E, Crowe JH, Vigh L. Small heat shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci USA. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Montfort RLM, Basha E, Friedrich KL, Slingsby C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Mol Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- van Montfort RLM, Slingsby C, Vierling E. Structure and function of the small heat shock protein/alpha-crystallin family of molecular chaperones. Adv Protein Chem. 2002;59:105–156. doi: 10.1016/S0065-3233(01)59004-X. [DOI] [PubMed] [Google Scholar]
- Waters ER, Aevermann BD, Sanders-Reed Z. Comparative analysis of the small heat shock proteins in three angiosperm genomes. Cell Stress Chaperones. 2008;13:127–142. doi: 10.1007/s12192-008-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences used in this study (DOC). (DOCX 18 kb)
The survival rates of Chilo suppressalis under different temperatures (DOC). (DOC 40 kb)
The species name and accession number of genomic DNA sequences (DOC). (DOCX 15 kb)