Abstract
An experiment was conducted to evaluate the role of different lipotropes in modulating immunity and biochemical plasticity under conditions of sublethal low-dose pesticide-induced stress in fish. Labeo rohita fish fingerlings were divided in two sets with one set of fish continuously exposed to low-dose endosulfan (1/10th of 96-h LC50) for 21 days, the other was unexposed, and both sets of fish were fed with practical diets supplemented with either 2 % lecithin, 0.5 % betaine, or 0.1 % choline and compared against unsupplemented diet. Low-dose endosulfan exposure had adverse effects (P < 0.05/P < 0.01) on hematological profile (erythrocyte count, hemoglobin, and hematocrit), serum protein (total protein, albumin, and globulin) and lipid profile (cholesterol and triglyceride), anti-oxidative status (ascorbic acid content of muscle, liver, brain, and kidney and activity of anti-oxidative enzymes: catalase and superoxide dismutase), neurotransmission (acetylcholinesterase activity in muscle and brain), immunological attributes (WBC count, albumin to globulin ratio, phagocytic activity, and serum cortisol), and metabolic plasticity as revealed from enzyme activities (muscle lactate dehydrogenase, liver and kidney glucose-6-phosphatase dehydrogenase-G6PDH activity). Dietary lipotropes prevented these effects completely or partially and the effects were lipotrope dependent. Kinetics (maximum velocity value Vmax, catalytic efficiency and Michaelis constant Km) of G6PDH enzyme from crude extracts of liver and kidney indicated inhibition due to endosulfan but lipotropes could protect enzyme and showed a stabilizing effect. The supplements also helped maintain integrity of histoarchitecture of the hepatocytes in endosulfan-exposed fish to a great extent. Feeding lipotropes to fish reared in endosulfan-free water also improved hematological and serum protein and lipid profiles and were immunostimulatory. In conclusion, dietary lipotropes, especially betaine and lecithin at the levels used, improve erythropoiesis, serum protein and lipid profile, anti-oxidant status, immunocompetence, neurotransmission, and protect the livers of L. rohita fingerlings even when continuously exposed to low-dose endosulfan.
Keywords: Lipotropes, Pesticide stress, Hematological profile, Serum protein and lipid profile, Anti-oxidant status, Immunocompetence, Acetylcholinesterase activity, Biochemical plasticity, G6PDH kinetics, Labeo rohita, Immunostimulants
Introduction
Aquatic pollution and water quality issues are important global concerns in the twenty-first century. A Gallup poll taken in 2009 revealed that pollution of drinking water is the primary environmental concern in the U.S. (Saad 2009). Two major sources of freshwater pollution are industries and agriculture. Industrial waste-water contains numerous chemicals. In many parts of the world, especially emerging economies, these wastewaters are still untreated or undergo only treatment that does not effectively remove the majority of the pollutants present (Shao et al. 2006). Several million tons of pesticides are used each year in agriculture (Bockstaller et al. 2009), and subsequent run-off leads to contamination of water resources in catchment areas of agricultural land. This causes continuous exposure of humans and biota to biologically active chemicals, which is a matter of a great concern. Because of widespread aquatic pollution (Gilliom 2007), fish living in polluted niches experience chronic stress.
While a little amount of stress within physiological limits is vital for maintenance of normal life processes, it turns from being physiological to being pathological if a high dose of stress beyond the coping capability of an individual is encountered. A small stress-induced alteration may have significant effects on functional outcomes including disease resistance. Animals living in polluted niches are natural models of chronic oxidative stress conditions. Unfortunately, there are enormous consequences and the cost associated with pesticide-induced stress is decreased immunocompetence leading to biodiversity loss. In India, out of the 46 % of species evaluated from 700 total freshwater fish species, 70 % are threatened (Kumar et al. 2000). Uncovering new knowledge in the prevention, detection, diagnosis, and treatment of oxidative stress can lead to enormous benefits. Chronic stress alters nutrient requirements and physiological response to stress is modified by nutritional status. Studies in fish in this regard can be used for resurrecting declining species under controlled conditions while gaining some useful insights for extrapolating the findings to other species.
Deficiencies of some vitamins, minerals, and other nutritional components have been shown to reduce the immunocompetence of fish (Bell et al. 1984; Hardie et al. 1990, 1991). Current aquaculture practice is invariably associated with various stresses which cause immunosuppression in fish (Cruz et al. 1991). Lipotropes like lecithin, betaine, and choline are nutritional supplements used in fish/animal feed having methyl-donating properties, and there are reports in other species (fowl) which indicate that methyl donors increase resistance to several types of stress (Cook 1990). However, information on role of methyl donors in the immunity of fish and shellfish is scanty (Muona and Virtanen 1993).
Methyl groups are needed for many metabolic reactions which transfer CH3 groups to DNA, RNA, protein, carnitine, creatine, lipid, and many other important CH3-containing metabolic compounds (Smolin 1989; Frontiera 1994). Research on methyl group metabolism and regulation has shown a significant relationship between methyl donors, metabolism and disease. For example, interest in the methylation of macromolecules has recently been heightened by a growing body of evidence which suggests deficiency of methyl groups in animals may lead to undermethylation of DNA and concomitant activation of oncogenes (Newberne et al. 1993). Methyl donors like choline, betaine, and lecithin (phosphatidyl choline) are metabolically interrelated.
Lecithin, a source of phospholipids, is a feeding attractant, increases resistance to stress and can reduce the oxidation of fat soluble vitamins. Betaine, being a compatible osmolyte, increases the water retention of cells, replaces inorganic salts, and protects intracellular enzymes against osmotically or temperature-induced inactivation (osmoprotectant) (Yancey et al. 1982). Choline is a precursor of betaine, acetylcholine (neurotransmitter), and phosphatidylcholine. Choline is also an important component of some plasmalogens, sphingomyelins, and lecithin and acts as a source of methyl groups, via betaine, for the synthesis of various methylated metabolites. Choline deficiency has been shown to produce poor growth and fatty liver (Ketola 1976; Ogino et al. 1970; Griffin et al. 1994), renal hemorrhage (Griffin et al. 1994), anorexia, and hemorrhagic areas in the kidneys, liver, and intestine (Wilson and Poe 1988).
Labeo rohita is one of the most preferred freshwater species among the Indian Major Carps in the Indian subcontinent, contributing 80–90 % of the carp polyculture. With changes in practice of agriculture and aquaculture, fish are exposed to various stressors that can cause immunosuppression, decreased productivity, and also lead to species decline. Hence, nutritional supplementation studies are a promising area of research in the prevention of infectious diseases of fish (Kumar and Dey 1988). This experiment was designed to study the health beneficial properties of metabolically related lipotropes in fish. We compared the health of L. rohita fingerlings that were either unexposed (control) or exposed to and continuously challenged with a low dose of endosulphan (1/10th of 96-h LC50) but supplemented with lecithin, betaine, and choline.
Materials and methods
Preparation of the diet
Four isoproteinous (34 % crude protein) and isocaloric (3.7 kcal DE/g) practical diets were prepared by inclusion of lipotropes (as supplements) such as 20 g/kg lecithin, 5 g/kg betaine, and 1 g/kg choline to the basal diet (Table 1), which served as control. While choline (as choline hydrochloride) (SD Fine Chemicals Ltd, Mumbai, India) and betaine (as betaine hydrochloride) (HIMEDIA, Mumbai, India) substituted wheat flour in the basal diet, 2 % soy lecithin (lipid) (HIMEDIA, Mumbai, India) substituted oil component in the diet to add all dietary components to 100 %. The supplements were of 99 % purity. Betaine and choline were first dissolved in water and incorporated along with a vitamin–mineral premix, whereas lecithin was dissolved in oil. For formulation of the pelleted diet, good quality fish meal, soybean meal, sunflower meal, wheat flour, wheat bran, and sunflower oil were procured from a local market. The dough was mixed properly and was pelleted, air dried for some time and kept in hot air oven at 60 °C until dry and was subsequently stored at 4 °C until required for feeding. The diets were the same for both the experiments.
Table 1.
Composition of basal diet
| Ingredients | Percent (%) |
|---|---|
| Soybean meal | 45.5 |
| Fish meal | 10.00 |
| Sunflower meal | 10.00 |
| Wheat flour | 14.8 |
| Wheat bran | 10.00 |
| Sunflower oil | 4.00 |
| Cod liver oil | 2.00 |
| CMC | 1.00 |
| Vitamin + mineral mixa | 2.00 |
| Vitamin B complexb | 0.10 |
| Vitamin Cc | 0.10 |
| Chromic oxide | 0.50 |
CMC carboxyl methyl cellulose
aComposition of vitamin mineral mix (EMIX PLUS) (quantity/2.5 kg): vitamin A 55,00,000 IU; vitamin D3 11,00,000 IU; vitamin B2 2,000 mg; vitamin E 750 mg; vitamin K 1,000 mg; vitamin B6 1,000 mg; vitamin B12 6 mcg; calcium pantothenate 2,500 mg; nicotinamide 10 g; choline chloride 150 g; Mn 27,000 mg; I 1,000 mg; Fe 7,500 mg; Zn 5,000 mg; Cu 2,000 mg; Co 450 mg; Ca 500 g; P 300 g; l-lysine 10 g; dl-methionine 10 g; selenium 50 ppm; selenium 50 ppm; Satwari 250 ppm; (Lactobacillus 120 million units and yeast culture 3,000 crore units)
bComposition of vitamin B complex (quantity per gram): thiamine mononitrate 20 mg; riboflavin 20 mg; pyridoxine hydrochloride 6 mg; vitamin B12 30 mcg; niaciamide 200 mg; Ca pantothenate 100 mg; folic acid 3 mg; biotin 200 mcg
cAscorbyl phosphate (SRL Ltd., Mumbai) as a source of vitamin C
Ethics statement
The research undertaken complies with the current animal welfare laws in India. The care and treatment of animals used in this study were in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals [Ministry of Environment and Forests (Animal Welfare Division), Government of India] on care and use of animals in scientific research. As the experimental fish L. rohita is a commercially important and not endangered fish, the provisions of the Government of India’s Wildlife Protection Act of 1972 are not applicable for experiment on this fish.
Fish and experimental design
Fingerlings of L. rohita were procured from Palgar Fish Seed Farm (Maharastra, India), stocked in a circular tank (1,000 L) after being given a prophylactic dip in KMnO4 solution (50 mg L−1) for 2 min. They were acclimatized for a period of 15 days and fed with a basal diet containing 30 % crude protein. A set of uniform fingerlings were distributed in four distinct groups with three replicates of 12 fish in each treatment, in plastic containers (60 × 40 × 47 cm) of 100-L capacity, following a completely randomized design. This set of fish was exposed to a sublethal concentration (1/10th of 96-h LC50) of endosulfan for 21 days. The LC50 dose was determined in the same set of fishes used for this study as reported earlier (Muthappa et al. 2011). Another identical set of fingerlings was distributed in four treatments with three replicates in each (12 fish per replicate) and reared in normal water (unexposed). The fish in four treatments in each set were offered diets supplemented with three different lipotropes or an unsupplemented control diet. Water exchange was carried out manually every alternate day with the separate addition of an estimated quantity of endosulfan to water to be replenished so as to maintain fish exposure at 1/10th level of LC50. Bore-well chlorine-free water was used for rearing fishes. Experimental diets were fed twice daily (0900 and 1700 hours) to approximate satiation. Round-the-clock aeration was provided to all the containers from a compressed air pump. The physiochemical parameters of water analyzed every week following the standard methods (APHA-AWWA-WEF 1998) were within the optimum range as required for the L. rohita fingerlings. Water quality parameters recorded during the study were as follows: dissolved oxygen 6.5–7.8 mg L−1, temperature 26.4 to 28.7 °C (dissolved oxygen and temperature meter, Merck, Germany), pH 7.5–8.6 (digital pH meter, Labindia, Mumbai), negligible free carbon dioxide (titrimetric method); total hardness 236–245 mg L−1 (carbonate hardness test kit, Merck, Germany), ammonia 0.14–0.27 mgL−1 (at 635 nm by phenate method), nitrite 0.001–0.005 mg L−1, and nitrate 0.02–0.07 mg L−1 (543-nm wavelength).
Blood collection
At the end of the experimental trial, sampling was carried out for the analysis of the different blood parameters. The sampling was performed 12 h after the last feeding. Three fish from each replicate were anesthetised with clove oil (50 μl L−1) and blood was collected from the caudal vein using a syringe with EDTA as anticoagulant. The blood was then transferred immediately to an Eppendorf tube containing a pinch of dry EDTA powder and shaken gently and kept at 4 °C. The blood was used for determination of hemoglobin content, total erythrocyte, and leukocyte count and for nitroblue tetrazolium (NBT) assay. For serum, another three fish from each replicate were anesthetised and blood was collected without anticoagulant and allowed to clot for 2 h followed by collection of straw-colored serum with a micropipette. All determinations were performed within 24 h.
Hematology
Hematological parameters were studied in both the sets of fish: unexposed and those exposed to endosulfan. The hemoglobin percentage was determined by estimating cyanomethemoglobin using Drabkin’s fluid (Qualigens, India). Five milliliters of Drabkin’s working solution were taken in a clean and dry test tube and 20 μl of blood were added to it. The absorbance was measured using a Nicolet Evolution 100 UV/vis spectrophotometer (Thermo Electron Corp, Cambridge, UK) at a wavelength of 540 nm. The final concentration was calculated by comparing with standard cyanmethemoglobin (Qualigens, India). The hemoglobin concentration was calculated as: Hemoglobin (gm dL−1) = [OD (T)/OD (S)] [251/1,000] × 60; where OD (T) = absorbance of test and OD (S) = absorbance of standard.
Total erythrocytes and leucocytes were counted in a hemocytometer using erythrocyte and leucocyte diluting fluids (Qualigens, India), respectively. Twenty microliters of blood were mixed with 3,980 μl of diluting fluid in a clean glass test tube. The mixture was shaken well to suspend the cells uniformly in the solution. Then the cells were counted using a hemocytometer. The following formula was used to calculate the number of RBC and leukocytes per ml of the blood sample: Number of cells per milliliter = [Number of cells counted × dilution]/[Area counted × depth of fluid].
Respiratory burst activity
Respiratory burst activity of phagocytes was quantified by using the reduction of NBT to formazan as a measure of superoxide anion (O2−) production as described by Secombes (Secombes et al. 1990) and modified by Stasiack and Bauman (1996). In short, 50 μL of blood were placed in the wells of microtiter plates and incubated at 37 °C for 1 h to facilitate adhesion of cells. Then the supernatant was removed and the loaded wells were washed three times in PBS. After washing, 50 μl of 0.2 % NBT was added and was incubated for a further 1 h. The cells were then fixed with 100 % methanol for 2–3 min and again washed thrice with 30 % methanol. The plates were then air dried. Sixty microliters of 2N potassium hydroxide and 70 μl of dimethyl-sulphoxide were added into each well to dissolve the formazan blue precipitate formed. The OD of the turquoise blue-colored solution was then read in an ELISA reader at 540 nm.
Serum proteins
Serum protein was estimated by Biuret method and BCG dye binding method (Reinhold 1953) using the kit (total protein and albumin kit, Qualigens Diagnostics). Albumin was estimated by the bromocresol green binding method (Doumas et al. 1971). The absorbance of the standard and test was measured against a blank in a spectrophotometer at 630 nm. Globulin was calculated by subtracting the albumin values from the total serum protein.
Serum lipids
Serum total cholesterol was measured using a commercially available kit (Sigma-Aldrich, St. Louis, MO). In the presence of cholesterol esterase in the reaction mixture, the assay detects total cholesterol. In brief, serum and reaction mixture (containing assay buffer, cholesterol probe, cholesterol enzyme mix, and cholesterol esterase) was mixed well and incubated at 37 °C at for an hour as per manufacturer’s instructions. The absorbance of samples was measured at 570 nm against the reagent blank value. Serum triglycerides were measured using commercially available kit (Sigma-Aldrich), which is used for quantitative enzymatic measurement of glycerol, true triglycerides, and total triglycerides in serum at 540 nm. In brief, 10 μl of water, glycerol standard and serum were taken in three cuvettes containing 1 ml of triglyceride working reagent. Readings were taken after incubations as per protocol. The increase in absorbance due to the formation the quinoneimine dye was measured at 540 nm, which was directly proportional to the triglyceride concentration in the sample. The procedure for free glycerol was similar except the reaction mixture contained 1 ml of the triglyceride reagent blank instead of the working reagent. The increase in absorbance at 540 nm due to the same-colored compound is directly proportional to the glycerol concentration of the sample. True serum triglycerides were calculated by subtracting the free glycerol concentration in the sample from total triglycerides.
Estimation of ascorbate
Ascorbic acid was estimated from liver, brain, kidney, and muscle by the method of Roe and Keuther (1943). Following weighing, wet tissue was transferred to the test tube containing 10 ml of chilled 6 % trichloro acetic acid. The sample was then homogenized and activated charcoal (200–300 mg) was added to the test tubes and mixed thoroughly. The solution was filtered using Whatman filter paper (No. 42). Five ml of aliquot were taken in test tubes and one drop of 10 % thio-urea solution and 1 ml of 2, 4 dinitrophenylhydrazine were added, and test tubes were then transferred to an ice bath and 5 ml of 85 % H2SO4 was added drop by drop. The test tubes were allowed to stand for 30 min after removing them from ice; the OD was taken at 540 nm against standard ascorbic acid.
Estimation of cortisol
Cortisol in fish plasma was estimated by using radioimmunoassay (RIA or Coat-A-count) kit purchased from Immunotech (Beckman Coulter Company) as per manufacturer’s instructions. The kit is intended for the quantitative direct determination of cortisol concentration. Samples and standards were incubated in monoclonal antibody-coated tubes with 125I-labelled cortisol tracer. Each sample and standard was pipetted into two tubes. After 1-h incubation at room temperature (under horizontal shaking—400 rpm) the liquid contents of the tubes were aspirated to the waste and the radioactivity bound to the antibody was measured using a gamma counter. Concentration of samples was obtained from the calibration curve. Plasma cortisol was expressed as nanogram per milliliter.
Preparation of tissue homogenate and protein estimation
The tissues of the fish were removed carefully and were weighed. Tissue homogenate was prepared with chilled sucrose solution (0.25 M) in a glass tube using a Teflon-coated mechanical tissue homogenizer. The tube was continuously kept in ice to avoid heating. The homogenate was centrifuged at 5,000 rpm for 10 min at 4 °C in a cooling centrifuge machine. The supernatant was stored at 4 °C until use. A 5 % homogenate was prepared for muscle and liver. Quantification of protein in the different tissues was carried out using Lowry’s method (Lowry et al. 1951). Tissue homogenate (0.1 ml) was taken and precipitated using 1 ml of 10 % TCA. The protein residue was obtained by discarding the supernatant produced after centrifugation at 5,000 rpm for 20 min. The residue was dissolved in 0.5 ml of 0.1 N NaOH. An amount of 0.1 ml of the dissolved protein residue was used for further analysis. Alkaline copper sulphate (5 ml) was added and left for 10 min. To this 1 N Folin’s reagent was added and incubated for 30 min in the dark. Reading was taken at 660 nm against the blank. Bovine serum albumin was used as a standard.
Neurotransmitter enzyme activities
The acetylcholinesterase (AChE) enzyme (E.C.3.1.1.7) was assayed according to the method of Hestrin (1949) modified by Augstinasso (1957). Reaction mixture of AChE system comprising 1.0 ml of M/15 phosphate buffer (pH 7.2), 1 ml acetylcholine (4 mM, pH 4.0) substrate buffer mixture (1:9 dilution), and 0.2 ml of homogenate was incubated for 30 min at 37 °C. Alkaline hydroxylamine (2.0 ml) was added to terminate the reaction. The solution was mixed thoroughly and 1 ml of HCl (2:1) was added followed by thorough mixing. Enzyme solution was then added to the control tubes. The color was developed by addition of 1 ml of FeCl3 (10 %) and OD was recorded at 540 nm after thorough mixing. In this assay, mixing the solution in every step was very essential to avoid trapping of air bubbles.
Antioxidant enzyme activities
Catalase activity was estimated according to the method of Takahara et al. (1960). To 2.45 ml of phosphate buffer (50 mM, pH 7.0), 50 μl of the tissue homogenate was added and the reaction was started by the addition of 1.0 ml of H2O2 solution. The decrease in absorbance was measured at 240 nm at 30-s intervals for 2 mins. The enzyme blank was run simultaneously with 1.0 ml of distilled water instead of hydrogen peroxide. The enzyme activity was expressed as n moles of H2O2 decomposed per minute per milligram protein
Superoxide dismutase (SOD) activity was estimated by the method of Misra and Fridovich (1972). The assay is based on the oxidation of epinephrine-adrenochrome transition by the enzyme. The reaction mixture consisted of 50 μl of sample, 1.5 ml phosphate buffer, and 0.5 ml epinephrine. The solutions were mixed well and immediately read for the change in optical density at 480 nm for 2 mins in a Shimadzu—UV spectrophotometer. One unit of SOD activity was the amount of protein required to give 50 % inhibition of epinephrine auto oxidation.
Enzyme of carbohydrate metabolism
The lactate dehydrogenase (LDH; E.C. 1.1.1.27) activity was assayed by the method of Wroblewski and Ladue (1955). The total 3 ml of the reaction mixture was composed of 2.7 ml of 0.1 M phosphate buffer (pH 7.5), 0.1 ml of NADH solution (2 mg NADH dissolved in 1 ml of phosphate buffer solution), 0.1 ml of tissue homogenate, and 0.1 ml of sodium pyruvate. The reaction was started after addition of substrate sodium pyruvate. The OD was recorded at 340 nm at 30-s intervals. The enzymatic activity was expressed as units per milligram of protein per minute at 25 °C where 1 unit was equal to Δ0.01 OD/min.
Enzyme of pentose phosphate pathways
The G6PDH (E.C.1.1.1.49) activity in different tissues was assayed by the method of DeMoss (1955). The total 3 ml of the reaction mixture was composed of 1.5 ml of 0.1 M Tris buffer (pH 7.8), 0.2 ml of 2.7 mM NADP, 0.1 ml of tissue homogenate, 1.05 ml of distilled water, and 0.1 ml of 0.02 M glucose-6-phosphate (G6P). The reaction was started by adding G6P as substrate. The OD was recorded at 340 nm at 15-s interval against distilled water. The G6PDH activity was expressed as units per milligram of protein per minute. One unit was equal to Δ0.01OD/min/ml at 25 °C.
Transminases providing gluconeogenic substrates
The aspartate aminotransferase (AST; E.C.2.6.1.1) activity was assayed in different tissue homogenates as described by Wooten (1964). The substrate was composed of 0.2 M d,l-aspartic acid and 2 mM α-ketoglutarate in 0.05 M phosphate buffer (pH 7.4). In the experimental and control tubes, 0.5 ml of substrate was added. The reaction was started by adding 0.1 ml of tissue homogenate. The assay mixture was incubated at 37 °C for 60 min. The reaction was terminated by adding 0.5 ml of 1 mM 2, 4 dinitrophenyl hydrazine (DNPH). In the control tubes, the enzyme source was added after DNPH solution. The tubes were held at room temperature for 20 min with occasional shaking. Then 5 ml of 0.4 ml NaOH solution was added, and the contents were thoroughly mixed. After 10 min, the OD was recorded at 540 nm against blank. The procedure adopted for alanine aminotransferase (ALT; E.C.2.6.1.2) activity was the same as used for AST activity except the substrate was composed of 0.2 M d,l-alanine instead of aspartic acid.
Enzyme of energy metabolism
The ATPase activity (E.C. 3.6.1.3) was assayed according to the method of Post and Sen (1967) with minor modification. The reaction mixture was composed of 1.0 ml of 0.1 M Tris–HCl buffer (pH 7.8), 0.1 ml of 100 mM NaCl, 0.1 ml of 20 mM KCl, 0.1 ml of 3 mM of MgCl2, 0.5 ml of 5 mM ATP, and 0.1 ml homogenate. The mixture was incubated for 15 min and the reaction was terminated by the addition of 1 ml 10 % TCA. After centrifugation, 2.0 ml of supernatant was processed for estimation of inorganic P by the method of Fiske and Subbarow (1925).
Kinetics of G6PDH
Kinetics study for the liver and kidney G6PDH enzyme was done to know the effect of exposure of fish to sub-lethal concentration of pesticide, and the protective effects of lipotropes. Crude extract prepared as mentioned above was used for the kinetic study. The assay was done by the method of De Moss (1053) by measuring the reduction of NADP at 340 nm. The range of substrate concentration used was 0.05, 0.1, 0.5, 1.0 and 3.0 mM of glucose 6 phosphate. All assays were performed at temperature 25 ± 0.05°C. The data were analyzed by nonlinear regression methods described by the Michaelis–Menten equation: V = Vmax[S]/(Km + [S]). The nonlinear plot was constructed with the aid of computer a program (Prism 5). The catalytic efficiency was determined at saturating substrate concentration and is given as: Catalytic efficiency = Vmax/Km.
Histology
Liver tissue samples were fixed in neutral buffered formalin, embedded in paraffin wax, cut at 5 mm, and sections were stained by hematoxylin and eosin (H&E) as described by Roberts (1989).
Statistical analysis
Effects of diets in unexposed or endosulfan exposure conditions were analyzed by one-way ANOVA independently. Post hoc tests were carried out using Duncan’s multiple comparison procedures, if there were significant differences. Student’s t test was performed to assess the significance between means of two corresponding treatments or while comparing mean in exposed group with unexposed control. All the statistical analyses were performed using statistical software, SPSS version 11.0 for Windows.
Results
Hematology
Hematological profiles of the unexposed and endosulfan-exposed sets of fish are given in Table 2. Fish from the unexposed set revealed significant improvement in RBC (P = 0.06), hemoglobin (Hb) content (P < 0.05), hematocrit (Hct) (P < 0.01) in soy lecithin and either betaine or choline-fed group. Comparison of the unexposed and endosulfan-exposed control fish revealed that RBC (P < 0.01), hemoglobin content (Hb) (P < 0.01), and haematocrit (Hct) (P < 0.05) values were significantly reduced by endosulfan exposure. Endosulfan-exposed but lipotrope-fed fish had RBC count improved (P < 0.05) over endosulfan-exposed diet-controlled group, but still the values were lower (P < 0.01) than unexposed control fish. While the Hb and Hct content in lecithin-fed endosulfan-exposed fish was on par with unexposed control (P > 0.05), the values were lower for groups fed with betaine and choline (P < 0.05). Comparison of the corresponding lipotrope-fed fish revealed that endosulfan-exposed fish had lower RBC, Hb and Hct (P < 0.01) than unexposed counterparts.
Table 2.
Hematological profile of fish on 21-days continuous exposure to nonlethal dose of endosulfan (1/10 dose of LC50) against unexposed control and fed with diets containing lecithin, betaine, and choline during experimental period
| Parameter | Exposure | Soylecithin | Betaine | Choline | Control | P |
|---|---|---|---|---|---|---|
| RBC (106 cells/mm3) | Control | 2.69c ± 0.09 | 2.05b ± 0.14 | 2.15b ± 0.05 | 1.74a ± 0.01 | 0.06 |
| Endosulfan | 1.61c##,** ± 0.01 | 1.46b##,** ± 0.04 | 1.58bc##,** ± 0.02 | 1.39a** ± 0.05 | <0.05 | |
| Hemoglobin (g/dl) | Control | 11.55b ± 0.45 | 10.05ab ± 0.65 | 9.85ab ± 0.75 | 8.15a ± 0.15 | <0.05 |
| Endosulfan | 7.72c** ± 0.12 | 6.80b#,** ± 0.10 | 7.61c#,** ± 0.11 | 5.85a** ± 0.15 | <0.01 | |
| Hematocrit (%) | Control | 29.10c ± 0.80 | 19.30a ± 0.10 | 23.35b ± 0.15 | 18.50a ± 0.60 | <0.01 |
| Endosulfan | 17.35c** ± 0.15 | 14.75b#,** ± 0.25 | 15.45b#,** ± 0.45 | 13.35a** ± 0.35 | <0.01 |
Mean values in the same row (one-way ANOVA) with different lowercase letters (a, b, c, d) differ significantly.
Data expressed as mean ± SE, N = 3
*P < 0.05, **P < 0.01—comparison of unexposed vs. corresponding endosulfan-exposed group
# P < 0.05, ## P < 0.01—indicates significant differences between endosulfan-exposed and methyl donor-fed and unexposed control fish
Serum protein and lipid profile
Serum protein and lipid profiles of the fish are given in Table 3. Fish from the unexposed set fed with lecithin and betaine had increased serum total proteins, and increased globulin for lecithin-fed fish. Albumin was not affected by lecithin and betaine but choline decreased it compared to unexposed control. Comparison of the unexposed and endosulfan-exposed control fish revealed that serum total protein and globulin were decreased (P < 0.01) but albumin was increased (P < 0.01) with endosulfan exposure. Endosulfan-exposed but lipotrope-fed fish had unaffected serum albumin (P > 0.05) but significantly increased total protein and globulin than the endosulfan-exposed control group. The levels of total protein (in choline and lecithin-fed fish), albumin (in all lipotropes) and globulin (in lecithin) were even higher in supplemented groups than unexposed control fish. Comparison of unexposed and exposed lipotrope-fed fish revealed that endosulfan-exposed fish had lower serum globulin (in all lipotropes fed) and lower total protein (P < 0.01) (in lecithin and betaine), with increased albumin in betaine and choline-fed fish than in corresponding unexposed fish. With regard to serum lipids in unexposed fish, all lipotropes decreased cholesterol (P < 0.01) and increased triglyceride (P < 0.01). Serum cholesterol and triglyceride were decreased (P < 0.01) with endosulfan exposure compared to unexposed fish fed with control diet. However, endosulfan-exposed lipotrope-fed fish had significantly increased TG (P < 0.01) and decreased cholesterol (only in lecithin) than the endosulfan-exposed control group. However, in none of the supplemented endosulfan-exposed groups could this increase in TG restore values to be on par with the unexposed control. Comparison of unexposed and endosulfan exposed but lipotrope-fed fish revealed that serum TG was lower in exposed than unexposed fish. Serum cholesterol was lower in the betaine-fed group but was same in soy lecithin and choline-fed exposed fish.
Table 3.
Serum protein and lipid profile of fish on 21-days continuous exposure to nonlethal dose of endosulfan (1/10 dose of LC50) against unexposed control and fed with diets containing lecithin, betaine, and choline during experimental period
| Parameter | Exposure | Soylecithin | Betaine | Choline | Control | P |
|---|---|---|---|---|---|---|
| Total protein (g/dl) | Control | 4.97b ± 0.11 | 4.70b ± 0.22 | 2.97a ± 0.16 | 2.89a ± 0.03 | <0.01 |
| Endosulfan | 3.62c##,** ± 0.11 | 3.03b** ± 0.16 | 3.05b# ± 0.03 | 2.24a** ± 0.08 | <0.01 | |
| Albumin (g/dl) | Control | 0.87b ± 0.03 | 0.88b ± 0.05 | 0.63a ± 0.01 | 0.94b ± 0.01 | <0.01 |
| Endosulfan | 1.18# ± 0.18 | 1.46# ± 0.08** | 1.39## ± 0.09** | 1.41 ± 0.04** | NS | |
| Globulin (g/dl) | Control | 4.11b ± 0.13 | 3.82b ± 0.27 | 2.34a ± 0.17 | 1.95a ± 0.04 | <0.01 |
| Endosulfan | 2.45c##,** ± .07 | 1.57b#,** ± 0.08 | 1.66b** ± 0.12 | 0.84a** ± 0.12 | <0.01 | |
| Cholesterol (mg/dl) | Control | 67.41a ± 6.91 | 103.43b ± 6.58 | 93.49ab ± 1.62 | 173.12c ± 10.53 | <0.01 |
| Endosulfan | 53.49a## ± 7.93 | 82.6ab##,** ± 2.92 | 88.94b## ± 1.48 | 105.68b** ± 0.13 | <0.05 | |
| Triglyceride (mg/dl) | Control | 161.39d ± 3.8 | 107.69b ± 2.57 | 129.8c ± 4.55 | 93.14a ± 2.31 | <0.01 |
| Endosulfan | 77.92c#,** ± 2.45 | 57.22b#,** ± 3.04 | 53.98b#,** ± 3.73 | 39.27a** ± 3.45 | <0.01 |
Mean values in the same row (one-way ANOVA) with different lowercase letters (a, b, c, d) differ significantly
Data expressed as mean ± SE, N = 3
*P < 0.05, **P < 0.01—comparison of unexposed vs. corresponding endosulfan-exposed group
# P < 0.05, ## P < 0.01—indicates significant differences between endosulfan-exposed and methyl donor-fed and unexposed control fish
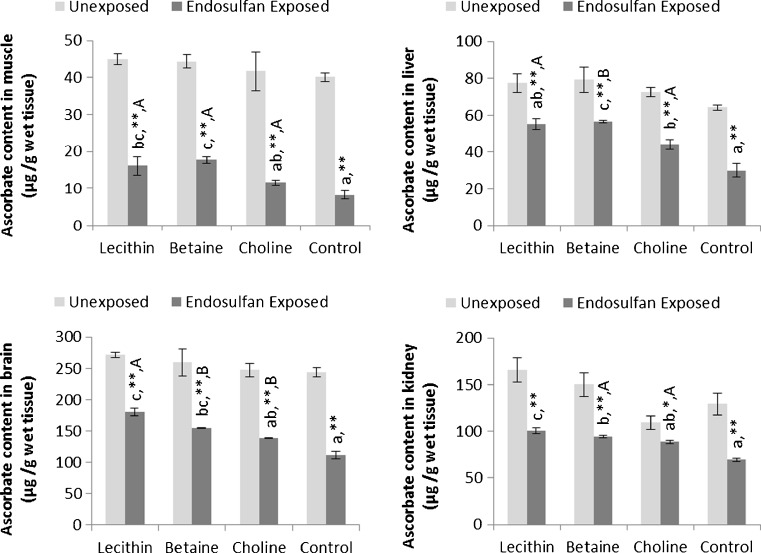
Ascorbic acid content of tissues
The ascorbic acid content of different tissues of fish unexposed and exposed to stressful conditions is given in Fig. 1. Ascorbic acid content of muscle, liver, brain, and kidney from the unexposed set of fish were unaffected by dietary supplements (P > 0.05). Endosulfan exposure significantly decreased (P < 0.01) the ascorbic acid content of muscle, liver, brain, and kidney tissues in the unsupplemented (control) group. However, compared to endosulfan-exposed control, significantly higher ascorbic acid content in muscle and brain (P < 0.05) and kidney (P < 0.01) in lecithin and betaine-fed groups and in liver (P < 0.05) of all three lipotrope-fed fish was observed. However, lipotropes could not restore ascorbic acid content in these tissues on par with unexposed control fishes. A comparison of corresponding unexposed and endosulfan-exposed fish fed with respective supplements revealed that ascorbic acid content of all tissues in endosulfan-exposed fish was significantly depleted (P < 0.01). Unlike in the unexposed set of fish, where catalase activity in liver and muscle were unaffected (P > 0.05), catalase in the muscle of fish fed with lecithin (P < 0.05) and in the liver of fish fed with lecithin and betaine was lowered compared to endosulfan-exposed control. In the endosulfan-exposed and lecithin and betaine-fed fish activity of muscle SOD (P < 0.05) and liver SOD (P < 0.06) was significantly reduced. Comparison of the unexposed and endosulfan-exposed fish from corresponding groups revealed that muscle catalase activity was enhanced but liver catalase activity was reduced (P < 0.05 or P < 0.01).
Fig. 1.
Ascorbic acid content of different tissues of fish following 21-days continuous exposure to nonlethal dose of endosulfan (1/10 dose of LC50) against unexposed control and fed with diets containing lecithin, betaine, and choline during experimental period. Mean values in the same set (one-way ANOVA) with different superscript letters (a, b, c, d) differ significantly. * P < 0.05, **P < 0.01—comparison of unexposed vs. corresponding endosulfan-exposed group.A P < 0.05 and B P < 0.01—indicates significant differences between endosulfan-exposed and methyl donor-fed and unexposed control fish. Data expressed as mean ± SE, N = 3
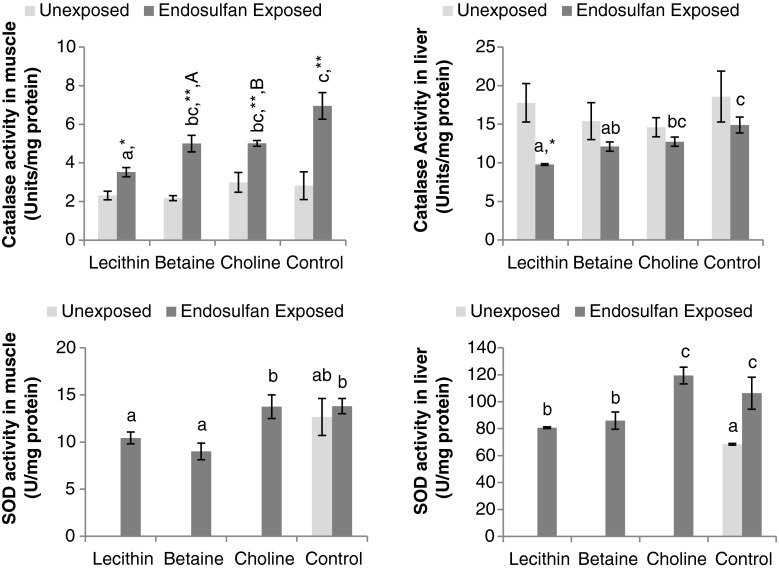
Anti-oxidant enzyme activities
Tissue anti-oxidative status in terms of catalase and superoxide dismutase activity is given in Fig. 2. Feeding of lipotropic compounds had no effect (P > 0.05) on catalase activity in liver and muscle in the unexposed set of fish. Endosulfan exposure increased catalase activity in the muscle (P < 0.01), and lecithin helped restore activity which was on par with unexposed controls. Endosulfan exposure had no effect on catalase in liver (P > 0.05) and activities were comparable in exposed and unexposed controls. However, betaine and lecithin reduced (P < 0.05) catalase activity in the liver compared to endosulfan exposed control. Nonetheless, catalase activity in the liver in each methyl donor-fed group was not significantly different from unexposed control fish. The activity of muscle catalase in endosulfan-exposed fish fed with lipotropes was less (P < 0.05 or P < 0.01) than corresponding methyl donor-fed unexposed fish. Choline and betaine had no influence on liver catalase, while soy lecithin reduced activity in endosulfan-exposed group than corresponding unexposed group. The activity of SOD in liver was increased due to endosulfan exposure (P < 0.05), and although lecithin and betaine feeding lowered it significantly, it could not be restored at par with unexposed control. While SOD activity in muscle was unaffected by endosulfan exposure, lecithin and betaine lowered it significantly (P < 0.05).
Fig. 2.
Activities of anti-oxidant enzymes catalase and superoxide dismutase (SOD) of different tissues of fish following 21-days continuous exposure to nonlethal dose of endosulfan (1/10 dose of LC50) against unexposed control and fed with diets containing lecithin, betaine and choline during experimental period mean values in the same set (one-way ANOVA) with different superscript letters (a, b, c, d) differ significantly (P < 0.05). *P < 0.05, **P < 0.01—comparison of unexposed vs. corresponding endosulfan-exposed group. A P < 0.05, B P < 0.01—indicates significant differences between endosulfan-exposed and methyl donor-fed and unexposed control fish. Data expressed as mean ± SE, N = 3
Neurotransmitter acetylcholine esterase activity
Activity of AChE in two tissues of unexposed and endosufan-exposed fish and fed with lipotropes containing diet is given in Fig. 3. While AChE activity in brain and liver from unexposed set of fish were unaffected by the dietary supplements (P > 0.05), AChE activity was lowered (P < 0.01) by endosulfan exposure (controls compared), but all lipotropes significantly elevated (P < 0.05) AChE activities in both the tissues. This increase in AChE activity in brain in all lipotrope-fed groups and in muscle of exposed fish fed with betaine and lecithin brought activities at par with unexposed unsupplemented control fish (P > 0.05). Comparison of corresponding unexposed and endosulfan-exposed fish fed with lipotropes showed no effects on activity of brain AChE activity but AChE activity in muscle of lipotrope-fed endosulfan-exposed fish was lower than corresponding unexposed fish (P < 0.05 or P < 0.01).
Fig. 3.
Activities of acetylcholine esterase (AChE) activity in brain and muscle tissues of fish following 21-days continuous exposure to nonlethal dose of endosulfan (1/10 dose of LC50) against unexposed control and fed with diets containing lecithin, betaine and choline during experimental period. Mean values in the same set (one-way ANOVA) with different superscript letters (a, b, c, d) differ significantly. *P < 0.05, **P < 0.01—comparison of unexposed vs. corresponding endosulfan-exposed group. A P < 0.05, B P < 0.01—indicates significant differences between endosulfan-exposed and methyl donor-fed and unexposed control fish. Data expressed as mean ± SE, N = 3
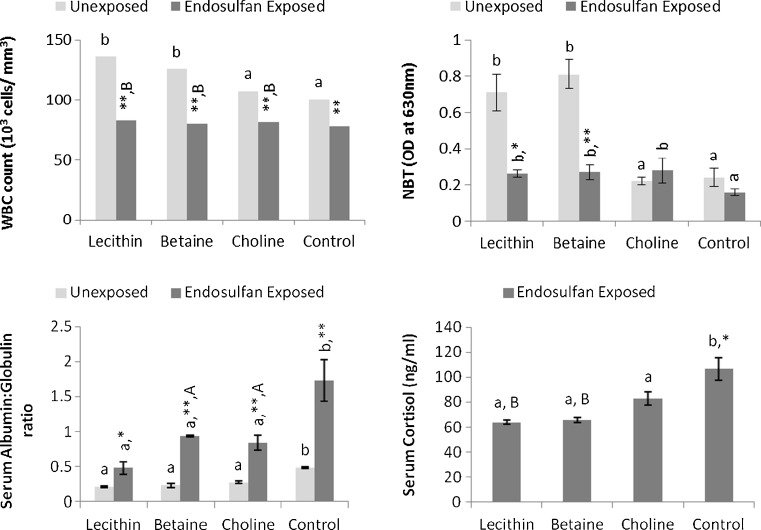
Immunological response and serum cortisol
The immunological response of fish fed with lipotropes under unexposed and endosulfan-exposed conditions is given in Fig. 4. Increased (P < 0.05) WBC count and respiratory burst (in lecithin and betaine) and decreased A/G ratio (P < 0.05) (in all three lipotropes) in unexposed fish were noticed. Endosulfan exposure significantly (P < 0.01) decreased WBC count and increased A/G ratio in fish (controls compared). This decrease in WBC due to endosulfan exposure was not rectified by any lipotropic compound supplement (P > 0.05), but phagocytic activity (as indicated by NBT reduction scores) and A/G ratio were significantly (P < 0.05) improved by lipotropes in endosulfan-exposed fish. While all lipotropes could restore phagocytic activity, only lecithin did this in endosulfan-exposed fish on par with unexposed controls (P > 0.05). Comparison of corresponding treatments in unexposed and endosulfan-exposed groups indicated decreased WBC count (P < 0.01) and increased A/G ratio in lecithin-fed (P < 0.05), betaine-fed, and choline-fed (P < 0.01) groups and decreased NBT in lecithin-fed (P < 0.05) and betaine-fed (P < 0.01) fish. The stress hormone cortisol was decreased (P < 0.05) in methyl donor supplemented groups compared to the unsupplemented endosulfan-exposed group.
Fig. 4.
Immunological attributes of fish following 21-days continuous exposure to nonlethal dose of endosulfan (1/10 dose of LC50) against unexposed control and fed with diets containing lecithin, betaine, and choline during experimental period. Mean values in the same set (one-way ANOVA) with different superscript letters (a, b, c, d) differ significantly. *P < 0.05, **P < 0.01—comparison of unexposed vs. corresponding endosulfan-exposed group. A P < 0.05, B P < 0.01—indicates significant differences between endosulfan-exposed and methyl donor-fed and unexposed control fish. Serum cortisol values compared with value (85.88 ± 2.11) estimated by RIA method in our lab from same species and same size fish reared in the water procured from the same source and fed with balanced diet (Akhtar et al. 2010). Data expressed as mean ± SE, N = 3
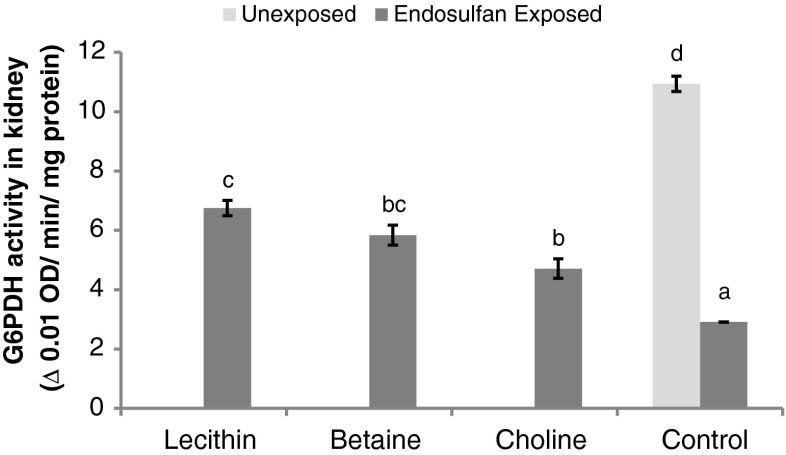
Metabolic enzyme activities
Biochemical plasticity in terms of changes in the activity of enzymes involved in carbohydrate (anaerobic) metabolism (LDH), gluconeogenic enzymes (ALT, AST), enzymes of the pentose phosphate pathways (glucose 6-phosphate dehydrogenase), and enzymatic markers of general energetics (ATPase) are given in Table 4. There were no significant differences in the activity of LDH in liver and muscle tissues in either the unexposed group or the group exposed to endosulfan and fed with lipotropes. While LDH was reduced in the liver of choline-fed fish (P < 0.05), it was increased in the muscle of endosulfan-exposed control and in betaine- and lecithin-fed fish. While liver LDH was unaffected, muscle LDH activity was enhanced (P < 0.01) due to endosulfan exposure and this remained unaffected by feeding lipotropes consequently, but the activity in lipotrope-fed groups was higher than in unexposed controls (P < 0.05). Among the lipotropic compound-fed groups, liver LDH was lowered (P < 0.05) in choline-fed fish and muscle LDH was increased (P < 0.01) in the choline and betaine-fed groups with endosulfan exposure compared with corresponding unexposed groups. Liver ALT and AST activities were unaffected by methyl donor feeding in both the unexposed and exposed sets, as was also the case for muscle AST. While muscle ALT was higher (P < 0.05) in the choline-fed unexposed group, endosulfan exposure had no effect on its activity (unexposed vs. exposed control). While muscle ALT activity in the betaine-fed groups was on par with each other, the activity in lecithin- and choline-fed exposed groups was higher than unexposed control. Endosulfan-exposed choline-fed (P < 0.05) and lecithin-fed (P < 0.01) fish had higher muscle ALT than corresponding unexposed groups. The activity of G6PDH in muscle was not influenced by the feeding of methyl donors in unexposed or stress-exposed groups (P > 0.05). However, the activity in fish exposed and fed with choline was reduced compared to unexposed controls. G6PDH in muscle in endosulfan-exposed groups was less in betaine- and choline-fed groups than in corresponding unexposed groups. The activity of G6PDH in liver was increased in the lipotrope-fed unexposed fish (P < 0.05). Moreover, endosulfan exposure decreased (P < 0.01) G6PDH in liver and there was no effect of lipotropes on its activity in endosulfan-exposed fish. Consequently, the activity of G6PDH in the liver of fish fed with lipotropes was less than in unexposed control. G6PDH in the liver in endosulfan-exposed, lipotrope-fed groups was lower (P < 0.01) than in corresponding unexposed groups. Compared to unexposed controls, endosulfan exposure decreased G6PDH in the kidneys. Lipotrope supplementation improved this activity to a significant extent (Fig. 5), but could not restore it. The overall cellular energetic picture in terms of ATPase activity revealed that ATPase activity was not influenced by feeding lipotropes to unexposed fish nor was it affected by endosulfan exposure. The ATPase in the liver of unexposed fish was not affected by lipotropes feeding (P > 0.05). Endosulfan exposure had no significant effect on liver ATPase (unexposed vs endosulfan-exposed control), though it decreased liver ATPase slightly. However, all endosulfan-exposed but lipotrope-fed groups had liver ATPase activity similar to unexposed controls. The ATPase levels in the liver of endosulfan-exposed groups fed with lecithin was higher (P < 0.05) and in those fed with choline was lower (P < 0.05) than in corresponding unexposed groups.
Table 4.
Metabolic enzyme activities in different tissues of fish on 21-days continuous exposure to nonlethal dose of endosulfan (1/10 dose of LC50) against unexposed control and fed with diets containing lecithin, betaine and choline during experimental period
| Enzyme | Exposure | Soy lecithin | betaine | Choline | Control | P |
|---|---|---|---|---|---|---|
| Liver LDH | Control | 605 ± 111.9 | 565 ± 104.6 | 616 ± 35.64 | 456 ± 52.75 | NS |
| Endosulfan | 485 ± 66.16 | 406 ± 4.14 | 466* ± 19.56 | 587 ± 32.38 | NS | |
| Muscle LDH | Control | 647 ± 65.9 | 597 ± 43.06 | 612 ± 134.9 | 711 ± 18.6 | NS |
| Endosulfan | 870# ± 93.67 | 1,040#,** ± 59.21 | 1,112#,** ± 10.91 | 1,011** ± 52.8 | NS | |
| Liver ALT | Control | 10.68 ± 0.83 | 14.07 ± 3.31 | 10.22 ± 0.03 | 10.13 ± 1.99 | NS |
| Endosulfan | 6.18* ± 1.08 | 6.56 ± 0.69 | 8.17 ± 0.88 | 7.22 ± 0.55 | NS | |
| Liver AST | Control | 41.72 ± 0.48 | 49.44 ± 6.07 | 42.35 ± 14.16 | 42.97 ± 4.13 | NS |
| Endosulfan | 55.09* ± 3.23 | 40.37 ± 7.21 | 45.11 ± 3.79 | 42.75 ± 2.70 | NS | |
| Muscle ALT | Control | 4.95a ± 0.32 | 7.21b ± 0.20 | 7.3c ± 0.80 | 5.37ab ±0.65 | <0.05 |
| Endosulfan | 15.30##,** ± 0.61 | 12.21 ± 2.51 | 16.32##,* ± 1.96 | 11.55 ± 2.62 | NS | |
| Muscle AST | Control | 56.60 ± 5.20 | 56.47 ± 3.04 | 67.09 ± 10.12 | 61.26 ± 3.67 | NS |
| Endosulfan | 62.43 ± 8.03 | 52.84 ± 8.53 | 62.69 ± 10.59 | 64.38 ± 2.41 | NS | |
| Liver G6PDH | Control | 97.88b ± 12.79 | 87.89b ± 8.00 | 88.86 b ± 10.56 | 41.46a ± 6.15 | <0.05 |
| Endosulfan | 7.99c#,** ± 0.75 | 7.95c#,** ± 0.89 | 6.08b#, ** ± 1.66 | 2.0a** ± 0.26 | <0.05 | |
| Muscle G6PDH | Control | 1.63 ± 0.13 | 2.16 ± 0.25 | 1.86 ± 0.11 | 1.74 ± 0.15 | NS |
| Endosulfan | 1.89 ± 0.46 | 1.05* ± 0.28 | 0.63##,** ± 0.14 | 1.50 ± 0.44 | NS | |
| Liver ATPase | Control | 1.52 ± 0.08 | 1.86 ± 0.08 | 1.52 ± 0.09 | 1.54 ± 0.35 | NS |
| Endosulfan | 2.56c* ± 0.26 | 1.85bc ± 0.19 | 1.25ab* ± 0.02 | 0.79a ± 0.01 | <0.01 | |
| Muscle ATPase | Control | 1.22 ± 0.07 | 1.29 ± 0.09 | 1.45 ± 0.11 | 1.39 ± 0.19 | NS |
| Endosulfan | 1.22 ± 0.07 | 1.26 ± 0.09 | 1.45 ± 0.11 | 1.40 ± 0.19 | NS |
ALT specific activities expressed as n moles of sodium pyruvate released/min/mg protein, AST specific activities expressed as n moles of oxaloacetate released/min/mg protein, LDH specific activity expressed as unit per minute per milligram protein, ATPase microgram phosphorus released/min/mg protein at 37 °C, G6PDH specific activities expressed as Δ0.01 OD/min/mg protein
Mean values in the same row (one-way ANOVA) with different lowercase letters (a, b, c, d) differ significantly
Data expressed as mean ± SE, N = 3
*P < 0.05, **P < 0.01—comparison of unexposed vs. corresponding endosulfan-exposed group
# P < 0.05; ## P < 0.01—significant differences between endosulfan-exposed and methyl donor fed and unexposed control fish
Fig. 5.
Glucose-6-phosphate dehydrogenase activity in kidney of L. rohita fingerling following 21-days continuous exposure to nonlethal dose of endosulfan (1/10 dose of LC50) against unexposed control and fed with diets containing lecithin, betaine and choline during experimental period. Mean values with different superscript letters (a, b, c, d) differ significantly (one-way ANOVA)
Enzyme kinetics assay
The G6PDH enzyme kinetic parameters in liver and kidney are given in Tables 5 and 6 and the respective nonlinear regression curves (Michaelis–Menten) are given in Figs. 6 and 7. In liver tissue, there was significant (P < 0.05) decrease in the Vmax and increase in Km values in the endosulfan-treated fish compared to the untreated controls; however, lipotropes helped protect enzyme activity in endosulfan-exposed fish with significant effects on improvement of Km and Vmax values. The catalytic efficiency of the enzyme was reduced in the fish exposed to pesticide. However, among lipotropes, only lecithin helped improve catalytic efficiency of G6PDH in the liver. In kidney tissue, lipotropes also helped bring the Vmax value in endosulfan-exposed groups on par with unexposed controls, which was decreased (P < 0.05) with sublethal exposure. The decreased (P < 0.05) catalytic efficiency due to endosulfan exposure was improved with lecithin only.
Table 5.
Kinetics parameters of G6PDH in liver of L. rohita fingerling on 21-days continuous exposure to nonlethal dose of endosulfan (1/10 dose of LC50) against unexposed control and fed with diets containing lecithin, betaine and choline during experimental period
| Michaelis–Menten | Endosulfan exposed | Control | |||
|---|---|---|---|---|---|
| Lecithin | Betaine | Choline | Control | ||
| Vmax | 40.43c ± 3.89 | 45.81c ± 3.76 | 29.81b ± 3.01 | 13.69a ± 1.08 | 74.23d ± 5.29 |
| K m | 1.75b ± 0.15 | 4.23d ± 0.40 | 3.56d ± 0.23 | 2.63c ± 0.24 | 0.77a ± 0.06 |
| Catalytic efficiency | 23.15b ± 1.98 | 10.82a ± 0.97 | 8.37a ± 0.66 | 5.21a ± 0.33 | 95.92c ± 7.91 |
Values in the same row with different lowercase letter differ significantly (P < 0.05), data expressed as mean ± SE, N = 3
Table 6.
Kinetics parameters of G6PDH in kidney of L. rohita fingerling on 21-days continuous exposure to nonlethal dose of endosulfan (1/10 dose of LC50) against unexposed control and fed with diets containing lecithin, betaine and choline during experimental period
| Michaelis–Menten | Endosulfan exposed | Control | |||
|---|---|---|---|---|---|
| Soylecithin | Betaine | Choline | Control | ||
| V max | 5.75b ± 0.55 | 5.89b ± 0.39 | 4.15ab ± 0.03 | 2.97a ± 0.03 | 5.23b ± 0.04 |
| K m | 0.14a ± 0.01 | 0.44b ± 0.03 | 0.46b ± 0.02 | 0.20a ± 0.01 | 0.38b ± 0.01 |
| Catalytic efficiency | 40.63b ± 2.86 | 13.34a ± 1.21 | 9.04a ± 0.53 | 15.14a ± 0.76 | 13.45a ± 1.28 |
Values in the same row with different lowercase letter differ significantly (P < 0.05), data expressed as mean ± SE, N = 3
Fig. 6.
Effect of lipotropes on the kinetics of glucose-6-phosphate dehydrogenase in the liver of L. rohita fingerlings exposed to sub-lethal concentration of endosulfan and a control not exposed to pesticide
Fig. 7.
Effect of lipotropes on the kinetics of glucose-6-phosphate dehydrogenase in the kidneys of L. rohita fingerlings exposed to sub-lethal concentration of endosulfan and a control not exposed to pesticide
Liver histology
Liver sections of L. rohita fingerlings from the group that was exposed to endosulfan but fed with the control diet (without any supplementation) showed extensive vacuolation and marked disruption of cordal arrangements of hepatocytes (Fig. 8a). But a protective effect from betaine- and lecithin-based diets was visible, although mild disarray of hepatic cords and moderate vacuolation was noticed (Fig. 8b, c). Liver sections of unexposed fish fed with lipotropes were similar to those of unexposed controls (figure not given).
Fig. 8.
Liver section (H&E, ×40) of L. rohita fingerlings from a group that was: a control (unexposed) and fed with control diet with normal hepatic tissue structure; b exposed to endosulfan and fed with control diet showing extensive vacuolation and marked disruption of cordal arrangements of hepatocytes; c exposed to endosulfan and fed with betaine diet showing mild disarray of hepatic cords and moderate vacuolation; d exposed to endosulfan and fed with lecithin diet showing mild disarray of hepatic cords and moderate vacuolation
Discussion
Endosulfan is an organochlorine pesticide, and organochlorines are known to cause oxidative stress (Videla et al. 1990). We used methyl donors to counter the oxidative stress of low-dose endosulfan. Methyl donors have important functions in the biological synthesis of important compounds and in methylation of genes, proteins and other moieties. The importance of methylation of genes in stress-modulating hypothalamo-pitutary circuitry such as the neuropeptide corticotrophin-releasing factor (Elliot et al. 2010) and immune system (Suarez-Alvarez et al. 2012) in stress modulation is being increasingly realized. However, literature on applied nutrition studies employing lipotropes (methyl donors) in stress context is scarce. Suggested requirements in freshwater fish for soy lecithin (phospholipid), betaine and choline are 20 g/kg (Geurden et al. 1995; 1997), 1 to 2 g/kg (Przyby et al. 1999; Wu and Davis 2005), and 1 to 3 g/kg (NRC 1993; Ogino et al. 1970; Kasper et al. 2000) respectively. Thus, selection of dietary supplement levels was based on our assessment of culture conditions, literature on the subject and the context of the study. Moreover, the compounds have metabolic interrelationships. Choline is a precursor of betaine, acetylcholine and phosphatidylcholine. Low-dose endosulfan has an inhibitory role in phospholipid biosynthesis and different component of phospholipids are highly impacted in endosulfan toxicity in fish (Singh and Singh 2006) and animals (Goldbergen et al. 1964). Thus, supplementation of lecithin, which contains 20 % each of phosphatidylcholine, phosphatdylinositol, and phosphatidylethanolamine is very appropriate in endosulfan-induced oxidative stress. The benefits of lecithin are especially pronounced in the diets of young aquatic species as in early life stages the digestive tracts have a very limited ability to synthesize adequate quantities of phospholipid to enhance growth and survival.
Both the sets of fishes in the present study, unexposed and exposed to low dose of endosulphan, exhibited stimulated erythropoiesis in methyl donor-supplemented groups that was manifested in the form of higher RBC count, Hb concentration, and Ht level. Erythropoietic properties of lecithin have been reported previously (Rehuluka and Minarik 2003). Fernandes et al. (2010) reported no effect from feeding 0.12 % choline on the hematic response of Nile tilapia fed with diets supplemented with choline and subjected to a stimulus of 17 °C temperature. Decreased Hb level due to 5.5 ppm Arsenic (through water) was improved with 50 g excess methionine alone or 25 g methionine + 25 g betaine per 100 kg of feed in poultry without effect on total erythrocytes (Halder et al. 2009). The remarkable capacity of methyl donors, especially lecithin, to normalize the decreased Hb resulting from low doses of pesticide is not reported in literature. RBC and Hb are related to the oxygen-carrying capacity of the blood.
The concentration of total protein in blood serum is used as a basic index for the health status of fish (Mulcahy 1971). Increases in the serum protein, albumin, and globulin levels are thought to be associated with a stronger innate response in fishes (Wiegertjes et al. 1996). In fish reared in normal water (unexposed fish), lecithin- and betaine-fed groups had higher total protein and globulin than the control. But in fishes exposed to endosulfan, all three methyl donors resulted in increase in total protein and globulin.
Phosphatidylcholine (PC) in lecithin is linked to cholesterol because of their role in lipid metabolism. PC is a structural component of the enzyme lecithin-cholesterol acyltransferase which is synthesized in the liver and converts free cholesterol into cholesterol esters for transport by lipoproteins (Sealey et al. 2001). PC appears to be crucial in the formation of very low-density lipoproteins (VLDL) during the intestinal absorption of neutral lipids and thus increases the amount of energy available for growth (Field and Mathur 1995). Lipotrope-supplemented unexposed fish were found to have (p < 0.01) lower serum cholesterol and higher triglyceride (TG) levels. But following endosulfan exposure, only the lecithin supplement lowered cholesterol with the TG trend being similar to that in unexposed fish. Significantly lower cholesterol in endosulfan-exposed groups indicates a possibility for cholesterol processing and breakdown after transportation to the liver. Field and Mathur (1995) reported that lecithin did not consistently affect plasma, liver cholesterol, and TGs though there was a change in phospholipid levels. TG-rich lipoproteins (VLDL and chylomicrons) are components of the innate, nonadaptive immune defense mechanism and the link between the innate immune defense mechanism and lipid metabolism is underappreciated. Production of TG-rich lipoproteins (which is normally cytokine mediated) is an indication of an improved host immune response under given circumstances (Barcia and Harris 2005). Thus, the better serum lipid profile of the fish given dietary lipotropes complements the better immune status of the fish. While a study on nonspecific immune stimulation with vitamin C (l-ascorbic acid) was accompanied by a decrease in total cholesterol and triglyceride in red sea bream, Pagrus major (Ren 2008), in another similar study with vitamin C in cobia fish triglyceride was found to increase along with enhanced immune status (Zhou et al. 2012).
Ascorbic acid, popularly known as vitamin C, plays an important role in protection and has a number of biological functions. In addition to the normal function of collagen synthesis, it helps in preventing peroxidation in cells as an antioxidant, helps in metabolism of steroids, takes part in detoxification processes and may alter cellular function. Ascorbate is the most abundant hydrophilic antioxidant, with a low reduction potential in the body fluids combating free radicals and protecting biomembranes from peroxidative damage induced by pollution (Skŕivan et al. 2012). There is considerable evidence demonstrating the depletion of antioxidants like ascorbic acid and glutathione in living organisms subjected to oxidative stress. However, the former matters most as needs to be replaced through dietary supplementation. Evidence of the loss of ascorbic acid under oxidative stress from endosulfan comes from direct estimation of ascorbic acid content of the brain and kidneys in fish exposed to low-dose endosulfan and subsequent replenishment through dietary vitamin C supplementation in spotted murrel fish, Channa punctatus. It was also shown to be rapidly depleted in fish subjected to sub-lethal levels of organic and inorganic substances (Heath 1995), which was also found in this study after low-dose endosulfan exposure. Thomas et al. (1982) reported a 50 % drop in ascorbate levels in hepatic tissue after 6 h of cadmium exposure, and continued exposure resulted in more or less steady depletion. This indicates that ascorbate might have been used up for detoxification processes or for preventing peroxidation of cells (Winston and Diguilo 1991) in endosulphan-exposed fishes. So this might have caused a reduction in ascorbate content in all the organs. The increased use of ascorbate for detoxification of polychlorinated biphenyl Aroclor in brook trout fry caused a functional deficiency of ascorbate (Mauck et al. 1978). Competition for vitamin C between collagen metabolism in bone and microsomal mixed-function oxidase for detoxification would also decrease vitamin C (Mehrle et al. 1982). Ascorbic acid levels in all the tissues of endosulfan-exposed fish were higher in the lecithin- and betaine-supplemented groups than in endosulfan-exposed controls; however, this could not bring ascorbate content on par with unexposed controls. This indicates that lipotropes help conserve to some extent ascorbate content of tissues during stress. There are no reports to substantiate these observations.
Oxidative stress results when antioxidant defenses are overcome by pro-oxidant forces and reactive oxygen species are not adequately removed (Sies et al. 1986). Living organisms are protected from reactive oxygen species by several defense mechanisms, such as the antioxidant enzymes SOD, catalase, glutathione peroxidase (GSH-Px), glutathione-s-transferase (GST), and glutathione reductase (Paulino et al. 2012), and hormones like melatonin (Sabrina et al. 2011), etc. Catalase and SOD are considered the first line of defense against oxygen toxicity. SOD constitutes the primary defense against the toxic effects of superoxide radicals generated in mitochondria and peroxisomes in aerobic organisms. SOD catalyses the transformation of superoxide radicals to H2O2 and O2 and is the first enzyme to cope with oxiradicals (Kappus 1985; Kohen and Nyska 2002). Catalase is a peroxisomal enzyme that facilitates the removal of hydrogen peroxide, which is metabolized to molecular oxygen (O2) and water (van der Oost et al. 2003). The significant increase in the liver SOD and muscle catalse activities in this study may be meant to scavenge the overproduction of superoxide anions under the oxidative stress induced by low-dose endosulfan. Akhtar et al. (2010) reported significantly increased SOD in the gills and liver of fish given diets deficient in pyridoxine and which were exposed to the same level of the same endosulfan as used in this study. When pyridoxine in the diet of the endosufan-exposed group was on par with control, SOD levels in the gills were higher, but not in the liver. Regardless of the regular dietary level of pyridoxine, catalase was unaffected in the gills, but was reduced in the liver of fish exposed to endosulfan. There are numerous other reports supporting our findings. Hu et al. (2008) also reported increased SOD in the liver in response to exposure to endosulfan. Significant increase in the SOD enzyme activity occurred in fish gonads following chlorpyriphos exposure (Oruc 2010; Yonar et al. 2012). In studies by Matos et al. (2007) on carbaryl (an organochlorine pesticide) at sublethal toxicity, compared to control, liver SOD activity was decreased up to the 14th day but increased on 21st day while catalase remained significantly decreased from days 7 through 21. Studies involving propiconazole (Li et al. 2010) found significantly increased activity of catalase in treated fish at the 7th day but decreased at the 20th day, and SOD was likewise decreased at the 20th day. During 40 days of exposure to 1/5th level of LC50 of atrazine, chlorpryrifos, or a combination of the two, Xing et al. (2012) found a decrease in catalase, SOD, and GSH-Px in the brain and kidneys, but exposure to 1/50th level of LC50 of atrazine had no effect on catalase activities in brain and kidney. Furthermore, 1/50th level of LC50 dose of chlorpyriphos alone or in a mixture of atrazine and chloripyriphos led to a decrease not only of catalase but also SOD in the brain and kidneys. However, GSH activity was increased in fish exposed to 1/50th level of LC50 dose of either of pesticides or combinations. Paulino et al. (2012) evaluated the impact of subchronic (14 days) exposure to environmentally realistic atrazine concentrations (2, 10, and 25 μg L−1) on the gills of Prochilodus lineatus. Subchronic exposure to atrazine at 2 or 25 μg L−1 did not change the activities of GST, SOD, CAT, or GSH-Px or the concentrations of GSH and LPO; however, subchronic exposure to 10 μg L−1 increased the activity of GST, SOD, and CAT and the LPO level. In male mice, the oxidative stress of a sublethal dose (1/10 LD50) of imidacloprid revealed increased lipid peroxidation level, increased liver catalase, increased liver SOD, increased liver GSH-Px, and increased liver GST (El-Gendy et al. 2010). Sometimes animals may be in stress but may have normal lipid peroxidation and antioxidant enzyme profiles. In one such study, Miller et al. (2007) noticed higher cortisol levels but normal lipid peroxidation levels and hepatic antioxidant (glutathione peroxidase) enzyme levels following 30 days of exposure to waterborne selenite.
The above facts indicate that the anti-oxidative stress response is a complex phenomenon and the cumulative animal response per se is an outcome of the actions of several enzymes, hormones and the body’s defense mechanisms aimed at reducing stress. However, the activity of a specific antioxidant enzyme may be enhanced, inhibited or remain unchanged under pesticide stress depending on the intensity and the duration of the stress applied, type of tissue, sex of species, physiological profile, and season as well as the susceptibility of the exposed species to the stressor. While one anti-oxidant enzyme among the circuitry of anti-oxidant enzymes may be downregulated, another enzyme may be upregulated and vice versa. Accordingly, Vega-Lopez et al. (2008) observed four characteristic of patterns of the oxidative stress response, a complex phenomenon, in Girardinichthys viviparus fish: (1) increased lipid peroxidation (LPOX), depressed SOD, and increased catalase; (2) an increase in all three biomarkers; (3) reduced LPOX, unchanged SOD, and increased catalase; (4) increased LPOX and depressed SOD and catalase. Thus, increased SOD in liver and catalase in muscle tissue, or unchanged SOD in muscle and catalase in liver along with increased cortisol concurs with the findings of others.
Feeding lipotropes under low-dose pesticide toxicity partially or completely reversed the activities of SOD, catalase and other enzymes in this study. This decrease in SOD and catalase activity can be attributed to the inhibition of superoxide radical formation or enhancement of the free radical scavenging activity by other moieties in body. Lipotropes used in the studies also had methyl donor properties and methylation of genes is known to reduce stress. Widespread dynamic DNA methylation including methylation of genes involved in defense mechanims in response to biotic stress has been observed in Arabidopsis thaliana (Dowen et al. 2012). Methylation of genes in stress-modulating limbic–hypothalamic–pituitary–adrenal circuitry such as the neuropeptide corticotrophin-releasing factor (Elliot et al. 2010), oxytocin and brain-derived neurotrophic factors (Unternaehrer et al. 2012), and the immune system (Suarez-Alvarez et al. 2012) has been observed. In animals, methylation of FOXO3 reduces oxidative stress-induced and neuronal apoptosis (Xie et al. 2012). The effect of lipotrope-feeding on the modulation of some of the anti-oxidant enzyme profiles was observed. Such a protective effect in terms of restoring anti-oxidant enzymes activities has been noted by feeding pyridoxine for endosulfan-induced toxicity in fish (Akhtar et al. 2010), propolis feeding for chlorpyrifos toxicity in fish (Yonar et al. 2012), vitamin C (200 mg/100 g feed) feeding for sublethal chlorpyriphos toxicity in fish (Ozkan et al. 2012) and vitamin C (200 mg/kg bw) in sublethal (1/10th of LD50) toxicity of imidacloprid in mice (El-Gendy et al. 2010).
AChE is one of the most widely-used enzymes as a biomarker for environmental pollution. Coppage and Mathews (1975) opined that the correlation between the extent of inhibition of AChE activity and mortality could be used as a diagnostic tool, and they felt that the greater extent of inhibition of brain AChE caused by sub-lethal exposure of organophosphate compounds facilitate early detection of pollution by anti-AChE compounds. In general, fish can tolerate about 70–80 % inhibition of AChE activity before death. Devaraj et al. (1991) observed 80 % inhibition of AChE in Oreochromis mossambicus with behavioural changes like sluggish movement, loss of balance, etc., but without any mortality. According to them, this may be due to maximum inhibition of AChE activity in the cerebellum, since the cerebellum controls muscular coordination and any change in its activity causes changes in the behavior of the organism. Das (1997) reported significant reduction (75.43 %) of AChE activity in brain tissue upon endosulfan (1/10 LC50) exposure over a period of 45 days. Although AChE levels in the brain and muscle in endosulfan-exposed fish in this study were decreased, methyl donor supplementation significantly improved AChE activity in the stressed and exposed fish to the extent that AChE activity in the brain of fish fed with all three lipotropes and activity in betaine- and lecithin-fed fish was on par with unexposed control fish.
Several parameters like leukocyte (WBC) count, phagocytic activity, albumin:globulin ratio, and serum cortisol are indicators of improved immunocompetence. Leukocytes have a role in nonspecific or innate immunity (Roberts 1978). The number of circulating lymphocytes in peripheral blood is an index of the functional ability of lymphoid organs and lymphocytes are the main agents of immunogenic response in animals. It is generally accepted that fish phagocytes are able to generate superoxide anion (O2−) and its reactive derivatives (i.e., hydrogen peroxide and hydroxyl radicals) during a period of intense oxygen consumption called the respiratory burst (Rehulka and Minarik 2003). Increased respiratory burst activity can be correlated with increased pathogen-killing activity of phagocytes (Sharp 1993) and hence a better immunity. The nonspecific cellular immune response is often reported as a function of macrophage activity such as phagocytosis and chemotaxis. In the present study, the respiratory burst activity of phagocytes was measured by reduction of NBT by intracellular superoxide radicals produced by leucocytes. Decreased serum A/G ratio implies an increased quantity of immunoglobulins in blood. Cortisol produced in response to activation of the hypothalamus–pituitary–interrenal axis, the functional analogue of the mammalian hypothalamus–pituitary–adrenal axis, is the major steroid in stress physiology and is produced in response to various stressors (Mommsen et al. 1999; Wendelaar Bonga 1997). Stress often inhibits immune responses. Increased cortisol reduces the number of circulating B-lymphocytes and decreases the antibody response after immunization in vivo (Verburg-van Kemenade et al. 1999); cortisol inhibits inflammatory cytokine expression in vitro (Saeij et al. 2003). Cortisol and lipopolysacharide synergistically stimulate the expression of interleukin 1β (IL-1β) mRNA in head kidney phagocytes (Engelsma et al. 2003).
Significantly (P < 0.01) decreased WBC count and increased A/G ratio and lowered respiratory burst (substantial but nonsignificant) and serum cortisol in exposed fish clearly indicated that endosulfan even at sublethal doses was immunosuppressive in fish. This agrees with Girón-Pérez et al. (2008) who reported decreased phagocytic activity. Akhtar et al. (2010) also reported decreased phagocytic activity, increased A/G ratio, increased cortisol and reduced survival in response to bacterial challenge (17 % less than control). However, complete prevention of this endosulfan-induced decrease in phagocytic activity (NBT/respiratory burst) and increased cortisol (by all lipotropes) and increase in A/G ratio (in lecithin) and partial prevention as exhibited with improved A/G ratio in choline- and betaine-supplemented fish indicated that lipotropes have immunostimulatory properties. Immunostimulants can increase the nonspecific immunity by either increasing the number of phagocytes or activating phagocytosis and respiratory burst (Shoemaker and Klesius 1997). Compared to endosulfan-exposed control, there was significant increase in respiratory burst in exposed but lipotrope-fed fish which attests to this fact. Lipotropes like methionine and betaine have been found to possess immunomodulating properties in chicks (Hess et al. 1998; Klasing et al. 2002). Their importance in immune responses may be due to their role in DNA methylation occurring during immune recognition and antibody production (Sano et al. 1988; Bestor 1993). In addition, the metabolic product of betaine, dimethylglycine has been shown to enhance both the humoral and cell-mediated immune responses in humans (Graber et al. 1981) and in mice (Reap and Lawson 1990), although the mechanisms of the effect are unknown. Klasing et al. (2002) has also demonstrated a modulatory effect of dietary betaine on the pathogenesis of Eimeria acervulina infection in chicks and attributed the protective effect of betaine to enhancement of monocyte chemotaxis and nitrous oxide production by heterophils and macrophages. Whether betaine exerts similar effect in fish is not established. However, WBC count in endosulfan-exposed fish was similar (P > 0.05).
Stress is an energy-demanding process, and the animal has to mobilize energy substrates to metabolically cope with stress (see review Iwama 1998). The production of glucose during stress assists the animal by providing energy substrates to tissues such as brain, gills, and muscles in order to cope with the increased energy demand. The liver is the main site of glucose production, which is achieved by glycogenolysis and/or gluconeogenesis. Cortisol has been shown to increase glucose production in fish and plays an important role in long-term maintenance of glucose post-stress in fish (see review Iwama 1998). The extent, magnitude, and direction of biochemical/metabolic changes to compensate for increased energetic demand (glucose production) in response to low-dose environmental stress decide how an animal will perform or perish in the hostile environment. The unique biochemical plasticity of fish, in terms of changes in activity of enzymes involved in anaerobic metabolism of carbohydrates (LDH), transaminases in amino acid metabolism providing gluconeogenic substrates (ALT and AST), enzymes of pentose phosphate pathways (G6PDH), and enzyme markers of general energetics (ATPase) and controlling hormone cortisol, was revealed in fish exposed to low-dose pesticide. During stress, protein catabolism increases to supply more glucose (energy) via upregulation of AST and ALT, which play role in gluconeogenesis via a transamination reaction. So, we used these as markers along with several others.
The activity of the LDH (terminal glycolytic enzyme) and ALT (tranaminase providing gluconeogenic substrate) in liver and muscle was differentially regulated in endosulfan-exposed groups. While LDH and ALT in the liver was reduced in one of the group in the exposed set, it was upregulated in the muscle in one of the group in the endosufan-exposed set, with other groups being unaffected. This is because liver and muscle have different functions and the latter has limited energy sources. Increased LDH activity on the 21st day in fish exposed to a sublethal dose (1/10th of 96-h LC50) of arsenic trioxide (Lavanya et al. (2011) and distillery effluent (Ramakritinan et al. 2005) has been shown. Such an increase is result of significantly depleted (P < 0.01) energy sources like total carbohydrates and glycogen in muscle, liver, and brain after 21 days, which was also evident from increased lactate in fish exposed to 1/10th of a sublethal (1/10th of 96-h LC50) toxic dose (Ramakritinan et al. 2005). Chronic exposure of fish to stressors affects the energy requirements by changing anaerobic oxidation. This was indicated by elevated LDH activity in muscle, indicating a shift towards anaerobic metabolism (utilizing stored metabolites like glycogen) during sublethal intoxication (Ellis 1937). Skeletal muscle has high energy demands, and to fulfill this demand, the activity of the gluconeogenic substrate-providing enzyme, ALT, must have been increased. Our observation also agrees with Sarma et al. (2009), who reported no variation in the liver but decreased LDH in the muscle of fish exposed for 90 days to a similar dose of endosulfan as used in this study. They also found variations in anaerobic metabolism governed by nutritional status.
In this study, exposure to endosulfan for 21 days had no significant effect on ALT and AST activity in muscle and liver (controls compared). Muscle ALT was higher in numerical but not statistical terms in endosulfan-exposed groups than in the unexposed groups, but muscle ALT in choline- and lecithin-fed endosulfan groups was higher than in unexposed groups. Akhtar et al. (2010) reported significantly elevated ALT and AST in muscle (but no effect on ALT and AST in liver) in the same species of fish exposed to the same dose of endosulfan during a 60-day study and fed with a standard control diet, but higher pyridoxine in the diet prevented this effect. In another study on sublethal chlorine exposure, while liver and muscle ALT activity on the 14th day at 26 °C was unaffected, AST activity was decreased and on 21st day both ALT and AST were decreased (Verma et al. 2007). Sarma et al. (2009) reported unaffected liver AST but decreased muscle AST and liver AST and ALT in fish exposed to a similar dose of endosulfan, the effect which could be prevented by high protein or vitamin C feeding. Thus influence on transaminases providing gluconeogenic substrates is the sum total of overall metabolic energetic demand and environment, and fish attempts to supply that by modulating enzymatic activities and micronutrient content.
ATPase enzyme is associated with the active transport of Na+ and K+ in cells (Skou 1975) and is also essential for the integrity of the cellular membrane and for the stabilization of branchial permeability (Isai and Masoni 1976). In the present study, the endosulfan exposure reduced ATPase activity in the liver almost by half, but methyl donor feeding in endosulfan-exposed fish improved this, with betaine and lecithin groups having ATPase activities on par with unexposed control. Thus, methyl donors protect ATPase activity. Similar protective effects of vitamin C, high protein (Sarma et al. 2009), and pyridoxine (Akhtar et al. 2010) were noticed in sublethal endosulfan toxicity.
Glucose-6-phosphate dehydrogenase (d-glucose-6-phosphate: NADP+oxidoreductase EC 1.1.1.49; G6PD) catalyzes the first and rate-limiting step of the pentose phosphate pathway, an important source of cellular reduced nicotinamide adenine dinucleotide phosphate (NADPH) which is essential for many types of biosyntheses as well as DNA/RNA synthesis and three to seven carbon sugars or sugar phosphates for many other uses (Carvalho et al. 1993; Kletzien et al. 1994). NADPH is used to generate the reduced glutathione and thioredoxin that are primary sources of reducing power for antioxidant reactions. Thus, NADPH participates in cell membrane protection and detoxification of xenobiotics in hepatic and other cells through the gluthatione reductase–peroxidase system and mixed function oxidases (Barroso et al. 1999; Diez-Fernandez et al. 1996). Decreased G6PDH activity in the liver of fish from endosulfan-exposed groups compared to the unexposed group is an indication that endosulfan had an inhibitory effect on the active site of G6PD enzyme in the liver, a dynamic metabolic organ. This inhibition might reduce production of NADPH (Beutler 1994), compromising antioxidant defense mechanisms (Reznick et al. 1998) as NADPH plays an important role in regeneration of reduced glutathione. Decreased G6PDH activity in fish due to sublethal endosulfan exposure (Tripathi and Verma 2004) and sublethal endosulfan and disulfoton exposure (Arnold et al. 1995) and in rats in 21 days of exposure to atrazine (Singh et al. 2011 and references therein) in addition to other chemicals has been observed. However, Akhtar et al. (2012) found increased G6PDH activity in fish exposed to sublethal doses of endosulfan for 60 days. Findings of Verma et al. (2007) based on estimation of G6PDH activity on 14th and 28th day following exposure of fish to an oxidative agent (chlorine) at four temperatures indicates that G6PDH activity probably increases initially for a few days but subsequently decreases. Thus, fish attempt to supply NADPH for anabolic processes, but subsequently the cellular machinery are exhausted upon longer-duration exposure. NADPH produced in the pentose phosphate pathway is also required by NADPH oxidase for producing superoxide anions for destroying phagocytosised material. Therefore, the enzyme activity is expected to increase with increased phagocytosis (Das et al. 2002). This was also reflected in the activities of free radical scavengers like catalase and SOD. The increased concentration of cortisol is also reported to inhibit the activity of G6PDH in fish (Tripathi and Verma 2004; Verma et al. 2007). The G6PDH activity also corresponds with the cortisol level, which was high in the control.
The inhibition of G6PDH in the liver was reflected in reduced maximum velocity values (Vmax) and catalytic efficiency and increased Michelis constants (Km). A reduced Vmax and increase in Km value reflects the mixed competitive-noncompetitive nature of endosulfan. Mishra and Shukla (1997) have reported a mixed competitive-noncompetitive nature of endosulfan inhibition on LDH activity in Clarius batrachus. Similarly, the enzyme was inhibited in kidney tissue in endosulfan-exposed groups which was reflected in reduced Vmax, Km, and catalytic efficiency of enzyme from crude extract during the kinetic study. While there was a significant increase in G6PDH activity in the livers of groups of fish fed with a methyl donor-supplemented diet compared to the endosulfan-exposed control, the activity could not be restored on par with unexposed control, and similar effects were observed on Vmax, Km, and catalytic efficiency values in endosulfan-exposed but lipotrope-fed groups in liver tissue. A similar protective effect of lipotropes on G6PDH enzyme activity in the kidneys was noticed. In endosulfan-exposed fish, all lipotropes restored Vmax values, betaine and choline resored Km and catalytic efficiency was higher in lecithin-fed fish.
Liver tissue from fish fed with control and experimental diets did not show any discernible changes. Histology represents a useful tool to assess the degree of pollution, particularly for sub-lethal and chronic effects. Necrosis, cordal disarrangement, cytoplasmic disintegration, and individualization of hepatocytes were major damages seen in the livers of fish exposed to different pesticides (Urdaneta 1989; John et al. 1993). In the present experiment, after exposure to sub-lethal levels of pesticide for 21 days, there were marked changes in liver histology of the fish fed with the control diets with extensive vacuolation and marked disruption of cordal arrangement of the hepatocytes. However in the lecithin- and betaine-fed groups, the changes were mild compared to the control. There was mild disarray of hepatic cords with moderate vacuolation. This indicates the protective role of lecithin and betaine in the liver tissue and was also supported by the various biochemical, enzymatic, and immunological parameters discussed earlier.
Conclusions
Results of improved erythropoiesis, serum protein and lipid profile, anti-oxidant status, immunocompetence, nerve transmission, and protection of hepatic tissue of L. rohita fingerlings fed with dietary lipotropes even when continuously challenged with low-dose pesticide stress indicates that dietary lipotropes promote immunobiochemical plasticity and protect fish against low-toxicity dose stress in fish. Some of these properties can be attributed to stabilization of functionality of cellular proteins as noticed from kinetics of metabolic enzymes.
Acknowledgments
Authors are thankful to the Director, Central Institute of Fisheries Education, Mumbai, India, for providing all the facilities required for the present work.
References
- Akhtar MS, Pal AK, Sahu NP, Alexander C, Gupta SK, Choudhary AK, Jha AK, Rajan MG. Stress mitigating and immunomodulatory effect of dietary pyridoxine in Labeo rohita (Hamilton) fingerlings. Aquacult Res. 2010;41:991–1002. [Google Scholar]
- Akhtar MS, Pal AK, Sahu NP, Alexander C, Gupta SK. Effects of dietary pyridoxine on growth and biochemical responses of Labeo rohita fingerlings exposed to endosulfan. Pestic Biochem Physiol. 2012;103:23–30. [Google Scholar]
- APHA-AWWA-WEF (1998) Standard methods for the estimation of water and waste water, 20th edn. In: Clesceri, L. S., Greenberg, A. E., Eaton, A. D. (eds.). American Public Health Association, American Water Works Association, Water Envirionment Federation, Washington DC
- Arnold H, Pluta HJ, Braunbeck T. Simultaneous exposure of fish to endosulfan and disulfoton in vivo: ultrastructural, stereological and biochemical reactions in hepatocytes of male rainbow trout (Oncorhynchus mykiss) Aquat Toxicol. 1995;33:17–43. [Google Scholar]
- Augustinsson KB. The reaction of acetyl choline esters and other carboxylic acid derivatives with hydroxylamine and its analytical application. J Biol Chem. 1957;180:249–261. [PubMed] [Google Scholar]
- Barcia AM, Harris HW. Triglyceride-rich lipoproteins as agents of innate immunity. Clin Infect Dis. 2005;41:S498–503. doi: 10.1086/432005. [DOI] [PubMed] [Google Scholar]
- Barroso JB, Peragon J, Garcıa-Salguero L, De la Higuera M, Lupianez JA. Variations in the kinetic behaviour of the NADPH-production systems in different tissues of the trout when fed on an amino-acid-based diet at different frequencies. Int J Biochem Cell Biol. 1999;31B:277–290. [Google Scholar]
- Bell GR, Higgs DA, Traxler GS. The effect of dietary ascorbate, zinc, and manganese on the development of experimentally induced bacterial kidney disease in sockeye salmon (Oncorhynchus nerka) Aquaculture. 1984;36:293–311. [Google Scholar]
- Bestor TH. Methylation pattern in the vertebrate genome. J NIH Res. 1993;5:57–60. [Google Scholar]
- Beutler E. Glucose-6-phosphate dehydrogenase deficiency. Blood. 1994;84:3613–3636. [Google Scholar]
- Bockstaller C, Guichard L, Keichinger O, Girardin P, Galan MB, Gaillard G. Comparison of methods to assess the sustainability of agricultural systems. A review. Agron Sustain Dev. 2009;29:223–35. [Google Scholar]
- Carvalho SD, Negrao N, Bianco AC. Hormonal regulation of malic enzyme and glucose-6-phosphate dehydrogenase in brown adipose tissue. Am J Physiol. 1993;264(6):E874–E881. doi: 10.1152/ajpendo.1993.264.6.E874. [DOI] [PubMed] [Google Scholar]
- Cook ME (1990) Methionine and immune response of the domestic fowl. Proceedings of Degussa Technological Symposium, Indianpolis, IN, pp 8
- Coppage DO, Mathews E. Brain acetyl cholinesterase inhibition in marine teleost during lethal and sublethal exposure to 1,2-dibromo-2,2-dichloroethyl dimethyl phosphate (naled) in sea water. Toxicol Appl Pharmacol. 1975;31:128. doi: 10.1016/0041-008x(75)90060-5. [DOI] [PubMed] [Google Scholar]
- Cruz EM, Ridha M. Production of the tilapia, Oreochromis spilurus (Gunther), stocked at different densities in sea cages. Aquaculture. 1991;99:95–105. [Google Scholar]
- Das BK (1997) Studies on the effect of some pesticides and common used chemicals on Indian major carp and their ecosystem. PhD Thesis. Orissa University of Agriculture and Technology, Bhubaneswar, pp 211
- Das D. Metabolism of lipids. In: Das D, editor. Biochemistry. Calcutta: Academic Publishers; 2002. pp. 505–566. [Google Scholar]
- DeMoss RD. Glucose-6-phosphate and 6-phosphogluconic dehydrogenase from Leuconostoc mesenteroides. In: Colowick SP, Kalpan NO, editors. Methods in enzymology. I. New York: Academic Press; 1955. pp. 328–332. [Google Scholar]
- Devraj P, Selvarajan VR, Durairaj S. Relationship between acetyl cholinesterase and monoamine oxidase in bran regions of O. mossambicus exposed to phosalone. Ind J Exp Bio. 1991;29:790–792. [PubMed] [Google Scholar]
- Diez-Fernandez C, Sanz N, Cascales M. Changes in glucose-6-phosphate dehydrogenase and malic enzyme gene expression in acute hepatic injury induced by thiochetamide. Biochem Pharmacol. 1996;51:1159–1163. doi: 10.1016/0006-2952(96)00030-5. [DOI] [PubMed] [Google Scholar]
- Doumas BT, Watson W, Biggs HG. Albumin standards and measurement of serum albumin with bromocresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Dowen RH, Pelizzola M, Schmitz RJ, Lister R, Dowen JM, Nery JR, Dixon JE, Ecker JR. Widespread dynamic DNA methylation in response to biotic stress. Proc Natl Acad Sci USA. 2012;109(32):E2183–91. doi: 10.1073/pnas.1209329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gendy KS, Aly NM, Mahmoud FH, Kenawy A, El-Sebae AK. The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem Toxicol. 2010;48(1):215–21. doi: 10.1016/j.fct.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Elliott E, Ezra NG, Regev L, Neufeld CA, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13(11):1351–3. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- Ellis MM. Detection and measurement of stream pollution. Bull Bur Fish. 1937;48:365–437. [Google Scholar]
- Engelsma MY, Stet RJ, Saeij JP, Verburg-van Kemenade BM. Differential expression and haplotypic variation of two interleukin-1beta genes in the common carp (Cyprinus carpio L.) Cytokine. 2003;22:21–32. doi: 10.1016/s1043-4666(03)00102-9. [DOI] [PubMed] [Google Scholar]
- Fernandes AC, Pezzato LE, Guimaraes IG, Teixeira CP, Koch JFA, Barros MM. Hematic response of Nile tilapia fed diets supplemented with choline and submitted to stimulus by low temperature. Rev Bras Zootec. 2010;39:1619–1625. [Google Scholar]
- Field FG, Mathur SN. Intestinal lipoprotein synthesis and secretion. Prog Lipid Res. 1995;34:185–98. doi: 10.1016/0163-7827(95)00005-k. [DOI] [PubMed] [Google Scholar]
- Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- Frontiera MS, Stabler SP, Kolhouse JF, Allen RH. Regulation of methionine metabolism: effect of nitrous oxide and excess methionine. J Nutr Biochem. 1994;5:28–38. [Google Scholar]
- Geurden I, Radunz-Neto J, Bergot P. Essentiality of dietary phospholipids for carp (Cyprinus carpio L.) larvae. Aquaculture. 1995;131:303–314. [Google Scholar]
- Geurden I, Charlon N, Marion D, Bergot P. Influence of purified soybean phospholipids on early development of carp Cyprinus carpio L. Aquacult Int. 1997;5:137–149. [Google Scholar]
- Gilliom, RJ (2007) The quality of our nation’s waters: pesticides in the nation’s streams and ground water, 1992–2001, Chapter 1, US Geological Survey. http://pubs.usgs.gov/circ/2005/1291/pdf/circ1291_chapter1.pdf pp 4. Accessed 3 October 2012
- Girón-Pérez MI, Montes-López M, García-Ramírez LA, Romero-Bañuelos CA, Robledo-Marenco ML. Effect of sub-lethal concentrations of endosulfan on phagocytic and hematological parameters in Nile tilapia (Oreochromis niloticus) Bull Environ Contam Toxicol. 2008;80:266–269. doi: 10.1007/s00128-008-9358-0. [DOI] [PubMed] [Google Scholar]
- Goldbergen M, Kublitskene O, Malak-Hovskis A (1964) The problem of the antitoxic action of choline in the poisoning of animals with carbon tetrachloride—a biochemical and histochemical study. Information on the 3rd Scientific Coordinating Meeting on Parasitological Problems of the Lithuanian, Latvian, and Estonian SSR From: REF ZH Otd Vypusk Farmakol Toksikol, No. 22.54.439
- Graber CD, Goust JM, Glassman AD, Kendall R, Loadhot CB. Immunomodulating properties of dimethylglycine in human. J Infect Dis. 1981;143:101–5. doi: 10.1093/infdis/143.1.101. [DOI] [PubMed] [Google Scholar]
- Griffin ME, Wilson KA, White MR, Brown PB. Dietary choline requirement of juvenile hybrid striped bass. J Nutr. 1994;124:1685–89. doi: 10.1093/jn/124.9.1685. [DOI] [PubMed] [Google Scholar]
- Halder G, Roy B, Samanta G. Haematologic aspects of arsenic intoxication with and without supplemental methionine and betaine in layer chicken. Indian J Poult Sci. 2009;44(2):269–272. [Google Scholar]
- Hardie LJ, Fletcher TC, Secombes CJ. The effect of vitamin E in the immune response of the Atlantic salmon (Salmo salar) Aquaculture. 1990;87:1–13. [Google Scholar]
- Hardie LJ, Fletcher TC, Secombes CJ. The effect of dietary vitamin C on the immune response of the Atlantic salmon (Salmo salar L.) Aquaculture. 1991;95:201–14. [Google Scholar]
- Health AG. Water pollution and fish physiology. 2. London: Lewis Publishers; 1995. [Google Scholar]
- Hess JB, Eckman MK, Bilgili SF. Influence of betaine of broilers challenged with two levels of Eimeria acervulina. Poult Sci. 1998;77:43. [Google Scholar]
- Hestrin S. Modified by Augustinsson (1957) The reaction of acetyl choline esters and other carboxylic acid derivatives with hydroxyline and its analytical application. J Biol Chem. 1949;180:249–261. [PubMed] [Google Scholar]
- Hu GC, Gan L, Wu TS, Qiu JL, Dai JY, Cao H, Xu MQ. Toxicological effects of endosulfan on Danio rerio. Chin J Zool. 2008;43:1–6. [Google Scholar]
- Isai J, Masoni A. The effects of calcium and magnesium on water and ionic permeabilities in the sea water adapted eel, Anguilla anguilla. J Comp Physiol. 1976;109:221–223. [Google Scholar]
- Iwama GK. Stress in fish. Ann N Y Acad Sci. 1998;851:304–310. [Google Scholar]
- John KR, Jayabalan N, George MR (1993) Impact of sublethal concentrations of endosulfan on the histology of Cyprinus carpio liver and kidney. In: Proceedings of the national seminar on aquaculture development in India-problems and prospect, pp. 27–29
- Kappus H. Lipid peroxidation: mechanisms, analysis, enzymology and biological relevance. In: Sies H, editor. Oxidative stress: oxidants and antioxidants. London: Academic Press; 1985. pp. 273–310. [Google Scholar]
- Kasper CS, White MR, Brown PB. Choline is required by tilapia when methionine is not in excess. J Nutr. 2000;130:238–42. doi: 10.1093/jn/130.2.238. [DOI] [PubMed] [Google Scholar]
- Ketola HG. Choline metabolism and nutritional requirement of lake trout (Salvelinus namaycush) J Anim Sci. 1976;43:474–77. doi: 10.2527/jas1976.432474x. [DOI] [PubMed] [Google Scholar]
- Klasing KC, Adler KL, Remus JC, Calvert CC. Dietary betaine increases intraepithelial lymphocytes in the duodenum of coccidia-infected chicks and increases functional properties of phagocytes. J Nutr. 2002;132:2274–82. doi: 10.1093/jn/132.8.2274. [DOI] [PubMed] [Google Scholar]
- Kletzien RF, Harris PKW, Foellmi LA. Glucose-6-phosphate dehydrogenase: a “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J. 1994;8(2):174–181. doi: 10.1096/fasebj.8.2.8119488. [DOI] [PubMed] [Google Scholar]
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- Kumar D, Dey RK (1988) Fish diseases in India. In: Sinha VR, Shrivastava HC, editors. Proceedings of the symposium on aquaculture productivity. Calcutta, India, pp. 315–343
- Kumar A, Walker S, Molur S. Prioritisation of endangered species. In: Singh S, Sastry ARK, Mehta R, Uppal V, editors. Setting biodiversity priorities for India. New Delhi: World Wide Fund for Nature, India; 2000. pp. 341–386. [Google Scholar]
- Lavanya S, Ramesh M, Kavitha C, Malarvizhi A. Hematological, biochemical and ionoregulatory responses of Indian major carp Catla catla during chronic sublethal exposure to inorganic arsenic. Chemosphere. 2011;82(7):977–85. doi: 10.1016/j.chemosphere.2010.10.071. [DOI] [PubMed] [Google Scholar]
- Li ZH, Zlabek V, Grabic R, Li P, Machova J, Velisek J, Randak T. Effects of exposure to sublethal propiconazole on the antioxidant defense system and Na+–K+–ATPase activity in brain of rainbow trout, Oncorhynchus mykiss. Aquat Toxicol. 2010;98:297–303. doi: 10.1016/j.aquatox.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Ronebrough NJ, Farr AL, Randell RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–276. [PubMed] [Google Scholar]
- Matos P, Fontaınhas-Fernandes A, Peixoto F, Carrola J, Rocha E. Biochemical and histological hepatic changes of Nile tilapia Oreochromis niloticus exposed to carbaryl. Pestic Biochem Physiol. 2007;89:73–80. [Google Scholar]
- Mauck WL, Mehrle PM, Mayer FL. Effect of the polychlorinated biphenyl Arochor® 1254 on growth, survival and bone development in brook trout, Salvelinus fontinalis. J Fish Res Board Can. 1978;35:1084. [Google Scholar]
- Mehrle PM, Haines TA, Hamilton S, Ludke L, Mayar FL, Ribick MA. Realtionship between body contaminants and bone development in east coast stripped bass. Trans Am Fish Soc. 1982;111:231. [Google Scholar]
- Miller LL, Wang F, Palace VP, Hontela A. Effects of acute and subchronic exposures to waterborne selenite on the physiological stress response and oxidative stress indicators in juvenile rainbow trout. Aquat Toxicol. 2007;83:263–271. doi: 10.1016/j.aquatox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Mishra R, Shukla SP (1997) Impact of endosulfan on lactate dehydrogenase from the freshwater catfish, Clarius batrachus. Pestic Biochem Physiol 57:220–234 [DOI] [PubMed]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- Mommsen TP, Vijayan MM, Moon TW. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish. 1999;9:211–268. [Google Scholar]
- Mulcahy MF. Serum protein changes associated with ulcerative dermal necrosis (UDN) in the trout Salmo trutta L. J Fish Biol. 1971;3:199–201. [Google Scholar]
- Muona M. Virtanen E. Effect of dimethylgylcine and trimethylglycine (betaine) on the response of Atlantic salamon (Salmo salar L.) smolts to experimental Vibrio anguillarum infection. Fish Shellfish Immunol. 1993;3:439–49. [Google Scholar]
- Muthappa NA, Jadhao SB, Gupta S. Acute toxicity of endosulfan and its effect on activity of some enzymes in Labeo rohita fingerlings. Natl Acad Sci Let India. 2011;34(5–6):211–218. [Google Scholar]
- National Research Council (NCR) (1993) Nutrient requirements of fish. National Academy Press, Washington, DC
- Newberne PM. The methyl deficiency model: history characteristic and research direction. J Nutr Biochem. 1993;4:618–24. [Google Scholar]
- Ogino C, Uki N, Watanabe T, Iida Z, Ando KB. Vitamin requirements of carp. IV. Requirement for choline. Bull Jpn Soc Sci Fish. 1970;36:1140–46. [Google Scholar]
- Oruç EÖ. Oxidative stress, steroid hormone concentrations and acetylcholine esterase activity in Oreochromis niloticus exposed to chlorpyrifos. Pestic Biochem Physiol. 2010;96:160–166. [Google Scholar]
- Ozkan F, Gündüz SG, Berköz M, Hunt AO, Yalın S. The protective role of ascorbic acid (vitamin C) against chlorpyrifos-induced oxidative stress in Oreochromis niloticus. Fish Physiol Biochem. 2012;38(3):635–43. doi: 10.1007/s10695-011-9544-6. [DOI] [PubMed] [Google Scholar]
- Paulino MG, Souza NES, Fernandes MN. Subchronic exposure to atrazine induces biochemical and histopathological changes in the gills of a Neotropical fresh water fish, Prochilodus lineatus. Ecotoxicol Environ Saf. 2012;80:6–13. doi: 10.1016/j.ecoenv.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Post RL, Sen AK (1967) Methods in enzymology. vol. In: Colowick SP, Kaplan NO (eds). Academic Press Inc., New York, p 762
- Przyby A, Mazurkiewicz J, Madziar M, Hallas M. Effect of betafin addition on selected indices of carp fry rearing in ponds. Arch Ryb Polish. 1999;7:321–328. [Google Scholar]
- Ramakritinan CM, Kumaraguru AK, Balasubramanian MP. Impact of distillery effluent on carbohydrate metabolism of freshwater fish, Cyprinus carpio. Ecotoxicol. 2005;14:693–707. doi: 10.1007/s10646-005-0019-3. [DOI] [PubMed] [Google Scholar]
- Reap EA, Lawson JW. Stimulation of the immune response by dimethylglycine, a nontoxic metabolite. J Lab Clin Med. 1990;115:481–86. [PubMed] [Google Scholar]
- Rehulka J, Minarik B. Effect of lecithin on the haematological and condition indices of the rainbow trout Oncorhynchus mykiss (Walbaum) Aquacult Res. 2003;34:617–27. [Google Scholar]
- Reinhold JG. Manual determination of serum total protein, albumin and globulin fractions by Biuret method. In: Reiner M, editor. Standard method of clinical chemistry. New York: Academic Press; 1953. p. 88. [Google Scholar]
- Ren T. Effects of dietary vitamin c on blood chemistry and nonspecific immune response of juvenile red sea bream, Pagrus major. J World Aqua Soc. 2008;39:797–803. [Google Scholar]
- Reznick AZ, Packer L, Sen CK. In: Oxidative stress in skeletal muscle. Reznick AZ, Packer L, Sen CK, Holloszy JO, Jackson MJ, editors. Basel: Birkhäuser; 1998. pp. 43–58. [Google Scholar]
- Roberts RJ. The pathophysiology and systemic pathology of teleosts. In: Roberts RJ, editor. Fish pathology. London: Bailliere Tindal; 1978. pp. 55–91. [Google Scholar]
- Roberts RJ. Nutritional pathology of teleosts. In: Roberts RJ, editor. Fish pathology. London: Bailliere Tindall; 1989. pp. 337–62. [Google Scholar]
- Roe JH, Keuther CA. The determinations of ascorbic acid in whole blood and urine through the 2,4-dinitrophenylhydrazine (DNPH) derivative of dehydroascorbic acid. J Biol Chem. 1943;147:399–407. [Google Scholar]
- Saad L (2009) Water pollution Americans’ top green concern. http://www.gallup.com/poll/117079/waterpollution-americans-top-green-concern.aspx. Accessed 30 Oct 2012
- Saeij JP, Verburg-vanKemenade LB, vanMuiswinkel WB, Wiegertjes GF. Daily handling stress reduces resistance of carp to Trypanoplasma borreli: in vitro modulatory effects of cortisol on leukocyte function and apoptosis. Dev Comp Immunol. 2003;27:233–245. doi: 10.1016/s0145-305x(02)00093-9. [DOI] [PubMed] [Google Scholar]
- Sano H, Imokawa M, Sager R (1988) Detection of heavy methylation in human repetitive DNA subsets by monoclonal antibody against 5-methylcytosine. Biochim Biophys Acta 951:157–65 [DOI] [PubMed]
- Sarabia L, Espinoza-Navarro O, Maurer I, Ponce C, Bustos-Obregón E. Protective effect of melatonin on damage in the sperm parameters of mice exposed to diazinon. Int J Morphol. 2011;29(4):1241–1247. [Google Scholar]
- Sarma K, Pal AK, Sahu NP, Ayyappan S, Baruah K. Dietary high protein and vitamin C mitigates endosulfan toxicity in the spotted murrel, Channa punctatus (Bloch, 1793) Sci Total Environ. 2009;407:3668–73. doi: 10.1016/j.scitotenv.2009.02.031. [DOI] [PubMed] [Google Scholar]
- Sealey WM, Craig SR, Gatlin DM. Dietary cholesterol and lecithin have limited effects on growth and body composition of hybrid striped bass (Morone chrysops × M. saxatilis) Aquacult Nutr. 2001;7:25–31. [Google Scholar]
- Secombes CJ. Isolation of salmonid macrophages and analysis of their killing ability. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, Van WB, Winkel M, editors. Techniques in fish immunology. New Jersey: SOS Publication; 1990. pp. 137–52. [Google Scholar]
- Shao M, Tang XY, Zhang YH, Li WJ. City clusters in China: air and surface water pollution. Front Ecol Environ. 2006;4:353–61. [Google Scholar]
- Sharp GJE, Secombes CJ. The role of reactive oxygen species in the killing of the bacterial fish pathogen Aeromonas salmonicida by rainbow trout macrophages. Fish Shellfish Immunol. 1993;3:119–29. [Google Scholar]
- Shoemaker CA, Klesius PH, Plumb JA. Killing of Edwardsiella ictaluri by macrophages from channel catfish immune system and susceptible to enteric- septicemia of catfish. Vet Immunol Immunopathol. 1997;58:181–90. doi: 10.1016/s0165-2427(97)00026-3. [DOI] [PubMed] [Google Scholar]
- Sies H. Biochemistry of oxidative stress, Angew. Chem Int Ed. 1986;25:1058–1071. [Google Scholar]
- Singh PB, Singh V. Impact of endosulfan on the profiles of phospholipids at sublethal concentration in the male Heteropneustes fossilis (Bloch) J Environ Biol. 2006;27(3):509–514. [PubMed] [Google Scholar]
- Singh M, Sandhir R, Kiran R. Effects on antioxidant status of liver following atrazine exposure and its attenuation by vitamin E. Exp Toxicol Pathol. 2011;63:269–276. doi: 10.1016/j.etp.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Skou JC. The Na+−K+ activated enzyme system its relationship to transport of sodium and potassium. Q Rev Biophys. 1975;7:401–434. doi: 10.1017/s0033583500001475. [DOI] [PubMed] [Google Scholar]
- Skŕivan M, Marounek M, Englmaierová M, Skr¡ivanová E. Influence of dietary vitamin C and selenium, alone and in combination, on the composition and oxidative stability of meat of broilers. Food Chem. 2012;130:660–664. [Google Scholar]
- Smolin LA, Benevega NJ. Methionine, homocysteine, cysteine-metabolic interrelationships. In: Mendel-Friedman Z, editor. Absorption and utilization of amino acids, vol. 1. Boca Raton: CRC Press; 1989. pp. 155–87. [Google Scholar]
- Stasiack AS, Bauman CP (1996) Neutrophil activity as a potent indicator of concomitant analysis. Fish and Shell-Fish Immunol 37:539
- Suarez-Alvarez B, Rodriguez RM, Fraga MF, López-Larrea C. DNA methylation: a promising landscape for immune system-related diseases. Trends Genet. 2012;28(10):506–14. doi: 10.1016/j.tig.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Takahara S, BH H, JV N, Ogura Y, Nishimura ET. Hypocatalasemia, a new genetic carrier state. J Clin Invest. 1960;39:610–619. doi: 10.1172/JCI104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Bally M, Nefe JM. Ascorbic acid status of mullet, Mugil cephalus. (Linn) exposed to cadmium. J Fish Biol. 1982;20:183–193. [Google Scholar]
- Tripathi G, Verma P. Endosulfan-mediated biochemical changes in the freshwater fish, Clarias batrachus. Biomed Environ Sci. 2004;17:47–56. [PubMed] [Google Scholar]
- Unternaehrer E, et al. Dynamic changes in DNA methylation of stress-associated genes (OXTR, BDNF) after acute psychosocial stress. Transl Psychiatry. 2012;2(8):e150. doi: 10.1038/tp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdaneta H. Organochlorine compound in ponds of a fish culture center Paez Municipality, Zulia State Venezuela. Bol Cent Invest Biol (Maracaibo) 1989;23:1–10. [Google Scholar]
- van der Oost R, Beyer J, Vermeulen NP. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol. 2003;13:57–149. doi: 10.1016/s1382-6689(02)00126-6. [DOI] [PubMed] [Google Scholar]
- Vega-Lopez A, Jimenez-Orozco FA, Garcia-Latorre E, Domınguez-Lopez MA. Oxidative stress response in an endangered goodeid fish (Girardinichthys viviparus) by exposure to water from its extant localities. Ecotoxicol Environ Saf. 2008;71:94–103. doi: 10.1016/j.ecoenv.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Verburg-van Kemenade BML, Nowak B, Engelsma MY, Weyts FAA. Differential effects of cortisol on apoptosis and proliferation of carp B-lymphocytes from head kidney, spleen and blood. Fish Shellfish Immunol. 1999;9:405–415. [Google Scholar]
- Verma AK, Pal AK, Manush SM, Das T, Dalvi RS, Chandrachoodan PP, Ravi PM, Apte SK. Persistent sub-lethal chlorine exposure elicits the temperature induced stress responses in Cyprinus carpio early fingerlings. Pestic Biochem Physiol. 2007;87:229–237. doi: 10.1016/j.fsi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Videla LA, Barros SB, Junqueira VB. Lindane-induced liver oxidative stress. Free Rad Biol Med. 1990;9:169–179. doi: 10.1016/0891-5849(90)90120-8. [DOI] [PubMed] [Google Scholar]
- Wendelaar Bonga SE. The stress response in fish. Physiol Rev. 1997;77:591–625. doi: 10.1152/physrev.1997.77.3.591. [DOI] [PubMed] [Google Scholar]
- Wiegertjes GF, Stet RJM, Parmentier HK, Van Muiswinkel WB. Immunogenetics of disease resistance in fish: a comparable approach. Dev Comp Immunol. 1996;20:365–81. doi: 10.1016/s0145-305x(96)00032-8. [DOI] [PubMed] [Google Scholar]
- Wilson RP, Poe WE. Choline nutrition of fingerling channel catfish. Aquaculture. 1988;68:65–71. [Google Scholar]
- Winston G, Diguila R. Pro-oxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol. 1991;19:137. [Google Scholar]
- Wooten IDP (1964) Microanalysis in medical biochemistry. J & A Churchill Ltd, London, pp 101–103
- Wroblewski F, Ladue JS. LDH activity in blood. Proc Soc Exp Biol Med. 1955;90:210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Wu GB, Davis DA (2005) Interrelationship among methionine, choline, and betaine in channel catfish Ictalurus punctatus. J World Aqua Soc 36:337–345
- Xie Q, Hao Y, Tao L, Peng S, Rao C, Chen H, You H, Dong MQ, Yuan Z. Lysine methylation of FOXO3 regulates oxidative stress-induced neuronal cell death. EMBO Rep. 2012;13(4):371. doi: 10.1038/embor.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing H, Li S, Wang Z, Gao X, Xu S, Wang X (2012) Histopathological changes and antioxidant response in brain and kidney of common carp exposed to atrazine and chlorpyrifos. Chemosphere 88:377–383 [DOI] [PubMed]
- Yancey PH, Clar ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evaluation of osmolyte system. Science. 1982;217:1214–23. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Yonar ME, Yonar SM, Ural MS, Silici S, Dusukcan M. Protective role of propolis in chlorpyrifos-induced changes in the haematological parameters and the oxidative/antioxidative status of Cyprinus carpio carpio. Food Chem Toxicol. 2012;50:2703–2708. doi: 10.1016/j.fct.2012.05.032. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang L, Wang H, Xie F, Wang T. Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum) Fish Shellfish Immunol. 2012;32:969–75. doi: 10.1016/j.fsi.2012.01.024. [DOI] [PubMed] [Google Scholar]