Abstract
AIM: To assess the safety and utility of capsule endoscopy (CE) for children who are unable to swallow the capsule endoscope.
METHODS: The medical records of all of the children who underwent CE between 2010 and 2012 were retrospectively reviewed. The patients were divided into 2 groups: group A included patients who were unable to swallow the capsule endoscope, and group B included patients who were able to swallow it. For the patients who were unable to swallow the capsule endoscope, it was placed in the duodenum endoscopically. The small bowel transit time, endoscopic diagnosis and complications of the 2 groups were compared.
RESULTS: During the study period, 28 CE procedures were performed in 26 patients. Group A included 11 patients with a median age of 2 years (range 10 mo-9 years), and group B included 15 patients with a median age of 12 years (range 8 years-16 years). The lightest child in the study weighed 7.9 kg. The detection rates did not differ between the 2 groups. The median small bowel transit time was 401 min (range 264-734 min) in group A and 227 min (range 56-512 min) in group B (P = 0.0078). No serious complications, including capsule retention, occurred. No significant mucosal trauma occurred in the pharynx, esophagus, stomach or duodenum when the capsule was introduced using an endoscope.
CONCLUSION: CE is a safe and useful procedure for infants and young children who are unable to swallow the capsule endoscope.
Keywords: Capsule endoscopy, Retention, Infants, Children, Small bowel transit time, Complications
Core tip: We retrospectively reviewed the medical records of all children who underwent capsule endoscopy (CE) and compared the results of the patients who were unable to swallow the capsule (group A) with those of the patients who were able to swallow the capsule (group B). Although the mean small bowel transit time was significantly longer in group A, there were no significant differences between the 2 groups in the frequency of lesion detection or in the occurrence of adverse events. Capsule retention was not observed in either group. Thus, CE is a safe and useful procedure for infants and young children.
INTRODUCTION
The United States Food and Drug Administration (FDA) approved the use of capsule endoscopy (CE) for the evaluation of small bowel diseases in adults in 2001 and in patients 10 to 18 years of age in January 2004. In September 2009, CE was approved by the FDA for use in children 2 years of age and older[1].
CE provides a unique opportunity to visualize the entire small bowel, not only in adults but also in children. When the patient cannot swallow the endoscopic capsule, it can be safely introduced into the duodenum using various techniques or an introducing device[2-4]. Based on recent reports, the use of CE in the pediatric population is increasing[5-7]. However, its use in infants and young children who are unable to swallow the endoscopic capsule remains limited[8-12], and the safety and utility of CE in this age group are relatively unknown. The aim of this study was to clarify the safety and utility of CE in infants and young children who were unable to swallow the capsule endoscope, including patients younger than 1 year of age.
MATERIALS AND METHODS
All of the CE procedures performed at the Children’s Center for Health and Development at Saiseikai Yokohama City Tobu Hospital from August 2010 through March 2012 were retrospectively reviewed. Informed consent was obtained from the guardians of the children who underwent CE. The patients were divided into 2 groups: group A included patients who were unable to swallow the endoscopic capsule, and group B included patients who were able to swallow the capsule. We compared the 2 groups in terms of small bowel transit time, complications and endoscopic findings and diagnosis. The small bowel transit time was calculated from the first duodenal image to the first image of the cecum. Capsule retention was defined as a capsule endoscope remaining in the digestive tract for a minimum of 2 wk or requiring directed intervention or therapy to aid its passage[13].
Prior to the CE procedures, patients who were suspected of having a small bowel stricture were screened by small bowel X-ray in an attempt to decrease the risk of capsule retention. A standard PillCam® 2 (Given Imaging, Ltd., Yokneam, Israel) was used to perform all of the CE procedures. The PillCam® SB2 capsule is 11 mm × 26 mm in size and weighs 2.9 g. Its battery allows more than 8 h of work (usually approximately 13 h), during which the capsule photographs 2 images per second. All of the patients fasted overnight for at least 8 h prior to the delivery of the capsule. The patients were allowed to drink clear liquids 2 h after the procedure and to consume a light meal 4 h after the procedure.
If a patient was unable to swallow the capsule, it was placed into the duodenum endoscopically. These patients were anesthetized intravenously with midazolam and ketamine. A plastic hood was attached to the tip of a gastrointestinal endoscope (XQ260, Olympus, Tokyo, Japan). A net device for foreign body extraction was then introduced into the operative channel, and the capsule was placed into the net, pulled into the plastic hood and fixed. To avoid posterior pharyngeal damage during the introduction of the endoscope, a slight angle was created between the long axis of the capsule endoscope and that of the plastic hood (Figure 1). The pediatric endoscopist introduced the capsule into the stomach while observing both the real-time viewer of the endoscopic capsule and the video monitor of the endoscope. The endoscopic capsule was released into the stomach, caught with a polypectomy snare and delivered into the proximal portion of the duodenum (Figure 2).
Figure 1.
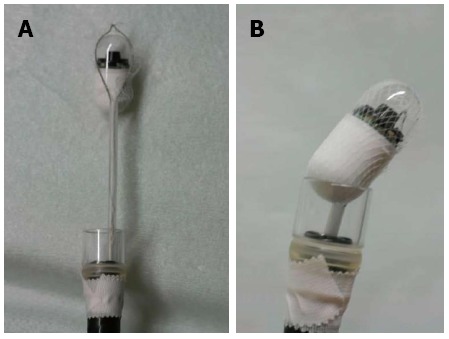
A capsule endoscope in a net device. A: A capsule endoscope in a net device for foreign body extraction; B: A capsule endoscope in a net device for foreign body extraction; the device has been retracted and is seated firmly against a clear plastic hood attached to the tip of the upper gastrointestinal endoscope.
Figure 2.
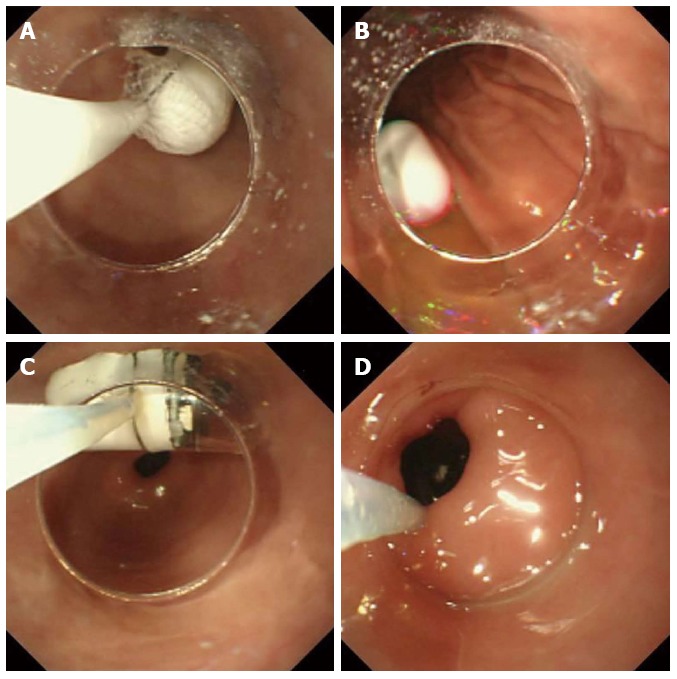
Capsule endoscope. A: The capsule endoscope was delivered into the stomach with endoscopic assistance; B: The capsule endoscope was released into the stomach; C: The capsule endoscope was caught by the polypectomy snare; D: The capsule endoscope was introduced into the duodenal bulb with the snare and was then pushed with the plastic hood into the proximal duodenum.
Statistical analysis
The results are reported as percentages or, in the case of continuous variables, as medians and ranges. The Mann-Whitney U test was used to evaluate differences in continuous variables between the 2 groups, and the chi-square test was used for dichotomous variables. A P value of less than 0.05 was considered statistically significant.
RESULTS
Twenty-eight CE procedures were performed in pediatric patients during the study period, August 2010 to March 2012. Thirteen males and 13 females underwent CE; the median age of the patients was 9.5 years (range 10 mo-16 years).
Group A included 11 patients (median age 2 years, range 10 mo-9 years), and group B included 15 patients (median age 12 years, range 8 years-16 years) (Table 1). The age distribution of the patients in each group is shown in Table 2. The lightest child, a group A patient, weighed 7.9 kg. Ten of the 11 procedures performed in group A used endoscopy to place the capsule endoscope into the duodenum. For a 9-year-old boy in group A, a net device for foreign body extraction was used to pass the capsule through the pharynx and into the stomach without endoscopy.
Table 1.
Patient demographics, transit times and complications
| Group A (n = 11) | Group B (n = 15) | P value | |
| Median age (yr) | 2 (0.8-9) | 12 (8-16) | 0.0006 |
| Male:female | 5:6 | 8:7 | NS |
| Median weight (kg) | 14 (7.9-24.1) | 41.8 (21.8-52.9) | 0.0003 |
| Median gastric transit time (min) | 10 (1-313) | 32 (4-446) | 0.0169 |
| Median small bowel transit time (min) | 401 (264-734) | 227 (56-512) | 0.0078 |
| Number of retentions | 0 | 0 | NS |
| Number of complications | 0 | 0 | NS |
The numbers in parentheses represent ranges. NS: Not significant.
Table 2.
Age distribution of the patients
| Age at examination, yr |
Cases (n) |
|
| Group A | Group B | |
| < 1 | 2 | 0 |
| 1 | 2 | 0 |
| 2 | 2 | 0 |
| 3 | 1 | 0 |
| 4 | 0 | 0 |
| 5 | 1 | 0 |
| 6 | 1 | 0 |
| 7 | 1 | 0 |
| 8 | 0 | 1 |
| 9 | 1 | 2 |
| 10 | 0 | 1 |
| 11 | 0 | 3 |
| 12 | 0 | 1 |
| 13 | 0 | 0 |
| 14 | 0 | 5 |
| 15 | 0 | 1 |
| 16 | 0 | 1 |
Of the 28 CE procedures, 26 were completed (92.9%) and 2 were not completed (7.1%). In one case, it took 9 h for a capsule endoscope placed in the stomach of a 2-year-old girl to pass through the stomach; the battery’s power became depleted before the entire small bowel could be observed. Therefore, the next day, we delivered the capsule into the duodenum using an endoscopic guide and successfully observed the entire small bowel without any problems. In another case, we misidentified the location of the capsule and terminated the examination before the capsule reached the cecum. The procedure was not repeated in the latter patient.
The median gastric transit time in group B was 32 min (range 4-446 min). The median small bowel transit time in group A (401 min, range 264-734 min) was significantly longer than that in group B (227 min, range 56-512 min) (Table 1).
The indications and findings of CE are summarized in Table 3. The CE examinations were performed for the following reasons: anemia or obscure gastrointestinal bleeding (OGIB) (n = 2), small bowel polyps with juvenile polyposis (n = 1), chronic or recurrent abdominal pain of unknown etiology (n = 8), evaluation of small bowel lesions associated with intractable Henoch-Schonlein purpura (HSP) (n = 3), recurrent vomiting (n = 1), diarrhea or steatorrhea (n = 3), ulcerative colitis (n = 4) and suspicion of Crohn’s disease (n = 4). Three patients with ulcerative colitis underwent CE to rule out Crohn’s disease, and 1 underwent CE to evaluate small bowel lesions caused by ulcerative colitis and portal hypertension due to primary sclerosing cholangitis. The most common indication in group B was abdominal pain (7 patients, 53.8%); among the patients in group A, the indications were more diverse.
Table 3.
Capsule endoscopy findings and outcomes n (%)
| n | Normal | New diagnosis | Suggestive of specific small bowel findings | Nonspecific small bowel findings | Change in therapy | |
| Group A | 11 | 4 (36.4) | 3 (27.3) | 5 (45.5) | 2 (18.2) | 0 (0.0) |
| Group B | 15 | 3 (20.0) | 3 (20.0) | 7 (46.7) | 5 (33.3) | 1 (6.6) |
Seven of the 26 capsule procedures (26.9%) were interpreted as normal. CE resulted in a new diagnosis for 6 patients (23.1%): 3 in group A (27.3%) and 3 in group B (20.0%). The CE small bowel findings were not sufficiently specific to permit a diagnosis in 12 patients (46.2%), 5 of in group A (45.5%) and 7 in group B (46.7%). The CE findings resulted in a change in therapy for 1 patient (6.6%) in group B. There was no significant difference in the incidence of abnormal findings between the 2 groups.
Of the 2 cases in which OGIB or anemia was investigated, CE identified angiodysplasia in both. The patient with juvenile polyposis was found to have no abnormal lesions in the small intestine. Of the 8 procedures performed to investigate abdominal pain, CE identified erosion in 1, duodenal ulcers in 1 and petechiae suggestive of HSP in 2. In a 3-year-old girl with intractable steroid-dependent HSP, CE revealed multiple erosions, extended ulcers and bleeding (Figure 3). In the child examined for recurrent vomiting, CE identified ulcers and aphthae (Figure 4). Of the 3 procedures in which diarrhea or steatorrhea were investigated, CE identified ulcers and erosion in 1. Of the 4 children with ulcerative colitis, 1 exhibited erosion and mucosal redness; 1 had a longitudinal ulcer and round ulcers; and 2 had mucosal edema. Of the 4 procedures undertaken for suspicion of Crohn’s disease, 1 revealed a linear ulcer; 1 showed a round region of redness; 1 revealed erosions with hematin; and 1 demonstrated white villi and erosion.
Figure 3.
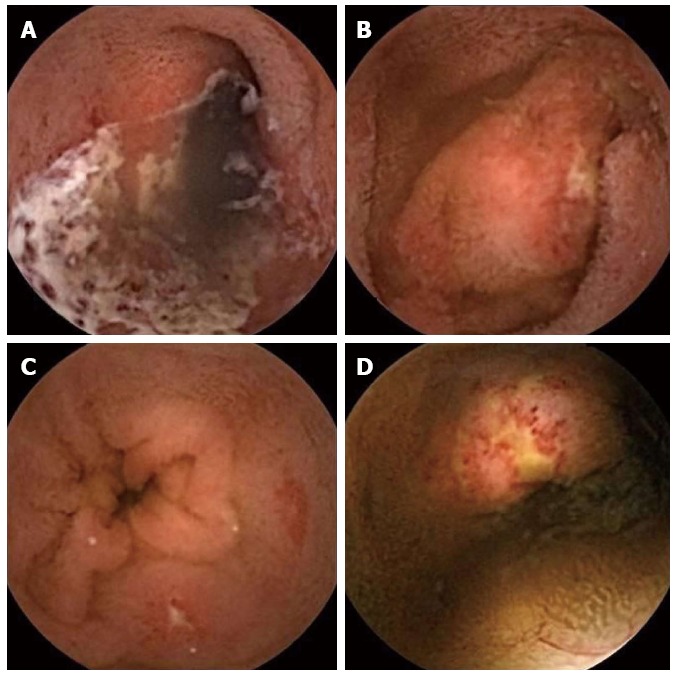
Three-year-old girl with Henoch-Schonlein purpura. A: Extended ulcer; B, C and D: Multiple erosions and ulcers.
Figure 4.
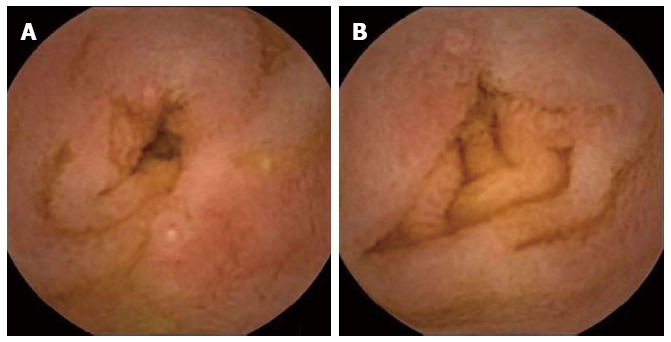
Ten-month-old girl with allergic gastroenteropathy (A and B) caused by the ingestion of cow’s milk.
No significant mucosal trauma to the pharynx, esophagus, stomach or duodenum occurred when the capsule was introduced using an endoscope. No further complications were noted; specifically, no capsule retention occurred.
DISCUSSION
CE is an endoscopic technique that offers an extremely safe approach for investigating small bowel pathology in adults. Although the US FDA has approved the use of CE in children 2 years of age and older, Japan’s Ministry of Health, Labor and Welfare has not officially approved CE for infants or young children who cannot swallow the capsule endoscope. Several techniques and devices for introducing the capsule endoscope into the duodenum have been described[2-4,14,15], and reports of the use of CE in children who are unable to swallow the capsule have been increasing[2,3,11,12]. Fritscher-Ravens et al[8] conducted a multicenter study to evaluate the feasibility and proper technique for using CE to identify small intestinal pathology in children < 8 years of age. No instances of severe complications or capsule retention occurred in the 85 examinations in that study, in which the smallest child weighed merely 10 kg. To our knowledge, the smallest child (an 8-mo-old) in whom the use of this technique has been reported weighed 9 kg[12].
In this study, we used a net device for foreign body extraction to introduce the capsule and a plastic hood adaptor to hold and stabilize the alignment of the capsule during its passage through the pharynx into the esophagus. It proved difficult to pass the capsule into the duodenum using a net device according to the method described by Barth et al[2]; therefore, we used a polypectomy snare to pass the capsule beyond the pylorus. Using this technique, we successfully performed CE in children who were unable to swallow the capsule. These children included 4 infants weighing < 10 kg, the lightest of whom weighed 7.9 kg and the youngest of whom was 10 mo old. The smallest child in group A weighed 7.9 kg; to our knowledge, this child is the smallest patient to be included in a study of CE.
Aparicio et al[16] reported that the patient’s age, sex, diabetes mellitus and body position after swallowing the capsule endoscope did not influence the gastric transit time or small intestinal transit time. In contrast, Fireman et al[17] reported age- and pathology-related effects on the small bowel transit time of the capsule endoscope. It has been unclear what factors influence small bowel transit time in children. We compared the small bowel transit time between group A, children who could not swallow the endoscopic capsule, and group B, children who could swallow the capsule, and found a significant difference between the 2 groups. This is the first report to compare small bowel transit times between 2 such groups. Small bowel transit time is thought to be influenced by the sphincter of the ileocecal valve, the length and luminal diameter of the small bowel and digestive peristalsis. Post-mortem studies have revealed that the capsule endoscope can traverse the pylorus and ileocecal valve in infants 1 year of age[8], suggesting that the size of the ileocecal valve may not be the primary factor influencing small bowel transit time during CE in children 1 year of age and older. Growth in intestinal length continues during early postnatal life; from approximately 1 year of age (75 cm body length) onward, this growth slows but continues to progress linearly with age until adulthood[18]. A study employing the lactulose hydrogen breath test showed that there was no correlation between age and transit time[19], although a test using solids might produce different results. We examined the relationships between small bowel transit time and age and body weight and found no significant relationships between these parameters. We demonstrated that small bowel transit time was significantly longer in group A than in group B. We hypothesize that the depression of digestive peristalsis caused by the anesthetic agents used during the placement of the capsule may have influenced the small bowel transit time.
CE is generally considered more sensitive than radiological and standard endoscopic modalities for the detection of small bowel lesions in children, especially for the distribution of Crohn’s disease lesions[20,21], the source of OGIB and the presence of polyps[8,22,23]. In our study, the source of bleeding was identified in all of the patients who underwent CE for OGIB or anemia. However, there was a low detection rate among the patients with juvenile polyposis or abdominal pain.
A major concern of both investigators and ethics review boards is the possibility of respiratory complications and/or CE retention in infants and young children. General endotracheal anesthesia was not required for the endoscopic placement of the capsule endoscope in any of the patients in group A, and no respiratory complications occurred in this group. There may be a risk of airway obstruction by the capsule or the endoscope during capsule introduction without general endotracheal anesthesia in infants and young children. The patient’s blood oxygen saturation, blood pressure, heart rate, electrocardiogram and respiratory rate must be monitored, and oxygen should be supplied via nasal cannula during capsule introduction in all cases. Our technique considerably reduces the risk of airway obstruction because it allows the accurate determination of the location of the CE: the use of the clear plastic hood permits the observation of both the real-time viewer of the capsule endoscope and the monitor of the endoscope.
A systematic review of CE studies, primarily in adults, reported retention rates of 1.4%, 1.2%, 2.6% and 2.1% for overall, OGIB, Crohn’s disease and neoplastic lesions, respectively[13]. A meta-analysis indicated that the CE retention rate in the pediatric population was 2.6% (stomach, 0.5%; small bowel, 1.9%), and pediatric patients showed similar risks of retention in OGIB, Crohn’s disease and polyp cases, with rates of 1.6% (n = 2/125), 2.5% (n = 9/359) and 1.3% (n = 1/75), respectively[24]. In a European multicenter study, Fritscher-Ravens et al[8] reported that no capsule retention was observed in 83 children younger than 8 years of age. These reports suggest that the retention rate is related to the indication for the procedure rather than to the age of the patient and that capsule retention is relatively uncommon in both adults and children. Atay et al[25] reported that the “red flags” for potential capsule retention included inflammatory bowel disease (IBD) (5.2% retention risk), previous small bowel follow-through demonstrating small bowel Crohn’s disease (37.5% retention risk) and body mass index < 5th percentile with known IBD (43% retention risk). To reduce the risk of capsule retention, small bowel follow-through should be performed prior to CE in all cases of suspected Crohn’s disease, especially in cases of growth failure. In our study, all of the patients with suspicion of Crohn’s disease underwent small bowel follow-through before CE, and no capsule retention occurred. If capsule retention were to occur, we would attempt to remove the capsule by double balloon endoscopy or by surgery if double balloon endoscopy could not be performed. Although the use of patency capsules in children has been reported[26], we did not use patency capsules prior to CE because such capsules are unavailable in Japan. Furthermore, the number of patients in this study was small, and any comparison of 2 groups with significant differences in age and weight would likely be of questionable validity. Because the number of pediatric patients who undergo endoscopy is far smaller than the number of adult patients who undergo this procedure, there are few data regarding the safety and utility of CE in infants and young children. We believe that our findings will encourage pediatric endoscopists to perform CE more frequently in infants and young children who cannot swallow capsules.
In conclusion, CE examinations of infants and young children who were unable to swallow the endoscopic capsule were performed without adverse events. In infants and young children, as in adults, CE is a useful and safe diagnostic method, especially in cases of gastrointestinal bleeding or anemia.
COMMENTS
Background
The United States Food and Drug Administration (FDA) approved capsule endoscopy (CE) for the evaluation of small-bowel diseases in adults in 2001 and for patients 10 to 18 years of age in January 2004. In September 2009, CE was approved by the FDA for use in children 2 years of age and older. However, the safety and utility of the use of CE in infants and young children who cannot swallow the capsule are unclear.
Research frontiers
The smallest child (an 8-mo-old) in whom the use of CE was previously reported weighed 9 kg. In this report, authors used a net device for foreign body extraction and a plastic hood adaptor to hold the capsule and stabilize its alignment during its passage through the pharynx into the esophagus. Using this technique, authors successfully performed CE in children who were unable to swallow the capsule, including an infant weighing 7.9 kg.
Innovations and breakthroughs
Authors compared the small bowel transit time between group A, consisting of children who could not swallow the endoscopic capsule, and group B, consisting of children who could swallow the capsule, revealing a significant difference between the 2 groups. This is the first report to compare the small bowel transit times in 2 such groups.
Applications
The capsule was endoscopically placed into the duodena of the patients who were unable to swallow the capsule. Thus, CE is a safe and useful procedure for infants and younger children who are unable to swallow the capsule endoscope.
Peer review
This is a valuable retrospective observational study. The study is very well thought out and should be useful to the readers in the Pediatric Group. If the retention of the capsule were to occur, what intervention or action would be undertaken by the clinician?
Footnotes
P- Reviewers: Akyuz F, Akiho H, Koh PS S- Editor: Gou SX L- Editor: A E- Editor: Ma S
References
- 1.Swaminath A, Legnani P, Kornbluth A. Video capsule endoscopy in inflammatory bowel disease: past, present, and future redux. Inflamm Bowel Dis. 2010;16:1254–1262. doi: 10.1002/ibd.21220. [DOI] [PubMed] [Google Scholar]
- 2.Barth BA, Donovan K, Fox VL. Endoscopic placement of the capsule endoscope in children. Gastrointest Endosc. 2004;60:818–821. doi: 10.1016/s0016-5107(04)02052-8. [DOI] [PubMed] [Google Scholar]
- 3.Bizzarri B, Fornaroli F, Cannizzaro R, de’ Angelis N, Vincenzi F, Maffini V, de’ Angelis GL. Endoscopic placement of video capsule in a pediatric population. Gastrointest Endosc. 2005;62:991. doi: 10.1016/j.gie.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 4.Shamir R, Eliakim R. Capsule endoscopy in pediatric patients. World J Gastroenterol. 2008;14:4152–4155. doi: 10.3748/wjg.14.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tokuhara D, Watanabe K, Okano Y, Tada A, Yamato K, Mochizuki T, Takaya J, Yamano T, Arakawa T. Wireless capsule endoscopy in pediatric patients: the first series from Japan. J Gastroenterol. 2010;45:683–691. doi: 10.1007/s00535-010-0209-5. [DOI] [PubMed] [Google Scholar]
- 6.de' Angelis GL, Fornaroli F, de’ Angelis N, Magiteri B, Bizzarri B. Wireless capsule endoscopy for pediatric small-bowel diseases. Am J Gastroenterol. 2007;102:1749–1757; quiz 1748, 1758. doi: 10.1111/j.1572-0241.2007.01209.x. [DOI] [PubMed] [Google Scholar]
- 7.Jensen MK, Tipnis NA, Bajorunaite R, Sheth MK, Sato TT, Noel RJ. Capsule endoscopy performed across the pediatric age range: indications, incomplete studies, and utility in management of inflammatory bowel disease. Gastrointest Endosc. 2010;72:95–102. doi: 10.1016/j.gie.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Fritscher-Ravens A, Scherbakov P, Bufler P, Torroni F, Ruuska T, Nuutinen H, Thomson M, Tabbers M, Milla P. The feasibility of wireless capsule endoscopy in detecting small intestinal pathology in children under the age of 8 years: a multicentre European study. Gut. 2009;58:1467–1472. doi: 10.1136/gut.2009.177774. [DOI] [PubMed] [Google Scholar]
- 9.Moy L, Levine J. Wireless capsule endoscopy in the pediatric age group: experience and complications. J Pediatr Gastroenterol Nutr. 2007;44:516–520. doi: 10.1097/MPG.0b013e3180335548. [DOI] [PubMed] [Google Scholar]
- 10.Orendain L, Rhee C, Fiore N, Kogut K, Baron H. Diagnostic use of video capsule endoscopy in a toddler with occult gastrointestinal bleeding. J Pediatr Gastroenterol Nutr. 2010;50:227–229. doi: 10.1097/MPG.0b013e3181a2e2d9. [DOI] [PubMed] [Google Scholar]
- 11.Kavin H, Berman J, Martin TL, Feldman A, Forsey-Koukol K. Successful wireless capsule endoscopy for a 2.5-year-old child: obscure gastrointestinal bleeding from mixed, juvenile, capillary hemangioma-angiomatosis of the jejunum. Pediatrics. 2006;117:539–543. doi: 10.1542/peds.2005-0710. [DOI] [PubMed] [Google Scholar]
- 12.Nuutinen H, Kolho KL, Salminen P, Rintala R, Koskenpato J, Koivusalo A, Sipponen T, Färkkilä M. Capsule endoscopy in pediatric patients: technique and results in our first 100 consecutive children. Scand J Gastroenterol. 2011;46:1138–1143. doi: 10.3109/00365521.2011.584900. [DOI] [PubMed] [Google Scholar]
- 13.Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280–286. doi: 10.1016/j.gie.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Seidman EG, Dirks MH. Capsule endoscopy in the pediatric patient. Curr Treat Options Gastroenterol. 2006;9:416–422. doi: 10.1007/BF02738531. [DOI] [PubMed] [Google Scholar]
- 15.Antao B, Bishop J, Shawis R, Thomson M. Clinical application and diagnostic yield of wireless capsule endoscopy in children. J Laparoendosc Adv Surg Tech A. 2007;17:364–370. doi: 10.1089/lap.2006.0114. [DOI] [PubMed] [Google Scholar]
- 16.Aparicio JR, Martínez J, Casellas JA. Right lateral position does not affect gastric transit times of video capsule endoscopy: a prospective study. Gastrointest Endosc. 2009;69:34–37. doi: 10.1016/j.gie.2008.03.1111. [DOI] [PubMed] [Google Scholar]
- 17.Fireman Z, Kopelman Y, Friedman S, Ephrath H, Choman E, Debby H, Eliakim R. Age and indication for referral to capsule endoscopy significantly affect small bowel transit times: the given database. Dig Dis Sci. 2007;52:2884–2887. doi: 10.1007/s10620-007-9789-1. [DOI] [PubMed] [Google Scholar]
- 18.Weaver LT, Austin S, Cole TJ. Small intestinal length: a factor essential for gut adaptation. Gut. 1991;32:1321–1323. doi: 10.1136/gut.32.11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy MS, Nelson R, Eastham EJ. Measurement of small intestinal transit time in children. Acta Paediatr Scand. 1988;77:802–806. doi: 10.1111/j.1651-2227.1988.tb10759.x. [DOI] [PubMed] [Google Scholar]
- 20.Ouahed J, Shagrani M, Sant’Anna A. Role of wireless capsule endoscopy in reclassifying inflammatory bowel disease in children. J Pediatr (Rio J) 2013;89:204–209. doi: 10.1016/j.jped.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Argüelles-Arias F, Caunedo A, Romero J, Sánchez A, Rodríguez-Téllez M, Pellicer FJ, Argüelles-Martín F, Herrerías JM. The value of capsule endoscopy in pediatric patients with a suspicion of Crohn’s disease. Endoscopy. 2004;36:869–873. doi: 10.1055/s-2004-825854. [DOI] [PubMed] [Google Scholar]
- 22.Thomson M, Fritscher-Ravens A, Mylonaki M, Swain P, Eltumi M, Heuschkel R, Murch S, McAlindon M, Furman M. Wireless capsule endoscopy in children: a study to assess diagnostic yield in small bowel disease in paediatric patients. J Pediatr Gastroenterol Nutr. 2007;44:192–197. doi: 10.1097/01.mpg.0000252196.91707.ff. [DOI] [PubMed] [Google Scholar]
- 23.Guilhon de Araujo Sant'Anna AM, Dubois J, Miron MC, Seidman EG. Wireless capsule endoscopy for obscure small-bowel disorders: final results of the first pediatric controlled trial. Clin Gastroenterol Hepatol. 2005;3:264–270. doi: 10.1016/s1542-3565(04)00715-3. [DOI] [PubMed] [Google Scholar]
- 24.Cohen SA, Klevens AI. Use of capsule endoscopy in diagnosis and management of pediatric patients, based on meta-analysis. Clin Gastroenterol Hepatol. 2011;9:490–496. doi: 10.1016/j.cgh.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Atay O, Mahajan L, Kay M, Mohr F, Kaplan B, Wyllie R. Risk of capsule endoscope retention in pediatric patients: a large single-center experience and review of the literature. J Pediatr Gastroenterol Nutr. 2009;49:196–201. doi: 10.1097/MPG.0b013e3181926b01. [DOI] [PubMed] [Google Scholar]
- 26.Cohen SA, Ephrath H, Lewis JD, Klevens A, Bergwerk A, Liu S, Patel D, Reed-Knight B, Stallworth A, Wakhisi T, et al. Pediatric capsule endoscopy: review of the small bowel and patency capsules. J Pediatr Gastroenterol Nutr. 2012;54:409–413. doi: 10.1097/MPG.0b013e31822c81fd. [DOI] [PubMed] [Google Scholar]


