Abstract
AIM: To determine the role of the fecal immunochemical test (FIT), used to evaluate fecal hemoglobin concentration, in the prediction of histological grade and risk of colorectal tumors.
METHODS: We enrolled 17881 individuals who attended the two-step colorectal cancer screening program in a single hospital between January 2010 and October 2011. Colonoscopy was recommended to the participants with an FIT of ≥ 12 ngHb/mL buffer. We classified colorectal lesions as cancer (C), advanced adenoma (AA), adenoma (A), and others (O) by their colonoscopic and histological findings. Multiple linear regression analysis adjusted for age and gender was used to determine the association between the FIT results and colorectal tumor grade. The risk of adenomatous neoplasia was estimated by calculating the positive predictive values for different FIT concentrations.
RESULTS: The positive rate of the FIT was 10.9% (1948/17881). The attendance rate for colonoscopy was 63.1% (1229/1948). The number of false positive results was 23. Of these 1229 cases, the numbers of O, A, AA, and C were 759, 221, 201, and 48, respectively. Regression analysis revealed a positive association between histological grade and FIT concentration (β = 0.088, P < 0.01). A significant log-linear relationship was found between the concentration and positive predictive value of the FIT for predicting colorectal tumors (R2 > 0.95, P < 0.001).
CONCLUSION: Higher FIT concentrations are associated with more advanced histological grades. Risk prediction for colorectal neoplasia based on individual FIT concentrations is significant and may help to improve the performance of screening programs.
Keywords: Colorectal cancer, Fecal immunochemical test, Screening, Risk prediction, Performance
Core tip: The fecal immunochemical test (FIT) for hemoglobin is specific for detecting colorectal lesions. With adjustment for age and gender of 17 881 attendees at a colorectal cancer screening program in a single hospital, we demonstrated that higher FIT concentrations were associated with more advanced histological grades (β = 0.088, P < 0.01). A significant log-linear relationship was found between the FIT concentration and positive predictive value of the FIT for predicting colorectal tumors (R2 > 0.95, P < 0.001). Risk stratification for colorectal neoplasia based on individual FIT concentration may help to improve the performance of screening programs.
INTRODUCTION
Colorectal cancer (CRC) accounts for the highest number of newly diagnosed cancer cases in Taiwan[1]. Most CRC develops via the well-known adenoma-carcinoma sequence that averages 10-15 years for progression and enables clinicians to detect neoplasms at the precancerous or early stages through adequate screening of the average-risk population[2].
Several population-based studies suggest that screening for colorectal neoplasms using the fecal occult blood test can reduce mortality[3,4]. Occult blood may be measured using either the guaiac fecal occult blood test or the fecal immunochemical test (FIT). The FIT has several advantages. For example, the FIT can provide a measurable value of the fecal hemoglobin concentration and can predict colorectal bleeding more specifically[5-7]. Notably, the quantitative FIT value may be converted using a defined cut-off value into a “negative” or “positive” qualitative result, which facilitates subsequent management. Despite its widespread use, little is known about the clinical significance and application of the quantitative value of the FIT. Recently, Omata et al[8] reported that the performance of the FIT for detecting CRC could be promising by choosing an optimal cut-off value of the quantitative FIT concentration[8]. Chen et al[9] investigated the association between baseline fecal hemoglobin concentration and the risk of incident colorectal neoplasia using a cut-off fecal hemoglobin concentration of 100 ng/mL to classify attendees as negative or positive. In subjects with a negative FIT, the adjusted hazard ratios (HRs) increased from 1.43 for a baseline fecal hemoglobin concentration of 20-39 ng/mL, to 3.41 for a baseline concentration of 80-99 ng/mL (t test P < 0.0001), relative to 1-19 ng/mL. They concluded that quantitative fecal hemoglobin concentration at baseline could predict subsequent risk of incident colorectal neoplasia[9]. These important findings suggest that quantitative FIT could be applied more widely in clinical practice.
The Taiwan Bureau of National Health Insurance began population-based CRC detection programs in 2006 for average-risk individuals aged between 50 and 69 years. A two-step approach was applied: a biennial 1-d FIT was used as a screening tool; subjects with a “positive” FIT underwent follow-up endoscopy to detect asymptomatic CRC. The effectiveness of the program appeared encouraging as a significant number of CRCs were detected in the early stages. However, a substantial portion of the participants did not adhere to the screening protocol because they were asymptomatic and/or worried about the discomfort and risk associated with colonoscopy. In addition, some physicians and health providers were less inclined to recommend feces-based CRC screening to patients. These factors may limit the outcomes of the project[10-12]. To improve the performance of CRC screening, a more personalized approach on the basis of risk stratification may be helpful[13]. Here, we evaluated the quality of colonoscopy in the screening program and determined the association between the FIT and colorectal neoplasia with the aim of determining whether the risk of CRC could be deduced from the quantitative value of the FIT.
MATERIALS AND METHODS
Subjects and feces analysis
We enrolled individuals who attended the national colorectal cancer screening program in a single medical center from January 2010 through October 2011. The participants were interviewed by a cancer screening specialist nurse during a face-to-face meeting to explain the purpose of CRC screening, the procedure for feces collection, and to sign the consent form. They were asked to provide one sample of feces in a stool container (Kyowa, Tokyo, Japan) to enable an accurate and quantitative evaluation by decreasing the variations in feces sampling levels. Storage of the samples was suggested at 4 °C and they were to be returned to the hospital within 3 d. Feces specimens were analyzed on a fully automated analyzer. The reagents, calibrators and controls for the samples were prepared according to the manufacturer’s guidance (Kyowa, Tokyo, Japan). The FIT measurements were converted to the proposed standardized reporting units: 2.5 (ngHb/mL buffer) = 1 (μgHb/g feces)[14,15].
Colonoscopy within 2 months was recommended to the subjects if their FIT was ≥ 12 ngHb/mL buffer. If a polyp with a diameter > 0.5 cm was observed, polypectomy or biopsy was performed to obtain histological results. The Hospital Ethics Committee approved this study.
Quality indicators
Several quality indicators were chosen according to the National Health Service bowel cancer screening program. guideline to investigate the performance of the screening program[16]. The colonoscopic attendance rate was determined as: No. of colonoscopies/No. of positive FITs. Cecal intubation rate was defined as: No. of photographic documentations of the cecal landmark/No. of colonoscopies. The adenoma detection rate was calculated as: no. of cases with adenomatous tumors/No. of colonoscopies. The cancer detection rate was calculated as: no. of cases with cancer/No. of colonoscopies. The incidence of complications was calculated as: No. of cases with major bleeding or perforation/No. of colonoscopies).
Cancer stage
The cancer stage was recorded according to the American Joint Committee on Cancer 7th edition. To determine whether cancer detected by screening (CS) was at an earlier stage compared with the hospital cancer registry (CR), the stage migration was calculated as the difference in the proportion of tumors at each stage between CS and CR.
Classifications used in the study
The colorectal findings were classified as cancer (C), advanced adenoma (AA), adenoma (A), and other (O) on the basis of their endoscopic and histological findings. Advanced adenoma was defined by the presence of any of the following characteristics: adenoma with a villous component, high-grade dysplasia, and polyp size ≥ 1.0 cm. If more than two lesions were found in one examination, we grouped the cases according to the most advanced findings.
The FIT data of all participants with colonoscopies were recorded. We transformed the continuous F value (ngHb/mL buffer) into ordinal scales: 12.1-25.0, 25.1-50.0, 50.1-100.0, 100.1-200.0, 200.1-400.0, 400.1-800.0, and > 800.0. To determine the associations between age, gender, FIT concentration and tumor grade, these non-normally distributed data were compared among the groups using the Kruskal-Wallis test. Subsequently, a multiple linear regression analysis adjusted for age and gender was used to determine whether the FIT concentration was independently associated with the histologic grade of colorectal tumors.
Statistical analysis
To determine the risk of colorectal neoplasia with regard to the FIT concentration, we evaluated the positive predictive value (PPV) of the FIT concentration (ngHb/mL buffer) at different cut-off concentrations (12, 25, 50, 100, 200, 400, 800 and 1000) for detecting A + AA + C, AA + C, and C. After log(2) transformation of the FIT results, a simple linear regression analysis was performed to determine the relationship. All statistical analyses were conducted using the software SPSS 19.0 to compare the differences among groups.
RESULTS
Baseline characteristics and performance of the CRC screening program
A flowchart of the study participants is shown in Figure 1. A total of 17881 participants underwent the FIT for colorectal cancer screening. The positive rate of the FIT was 10.9% (1948/17881). The number of false positive results was 23. The indicators for the quality of colonoscopy are listed in Table 1. The attendance rate for colonoscopy was 63.1% (1229/1948). The cecum-reaching rate was 98.1% (1206/1229). The polyp detection rate was 57.2% (703/1229). The adenoma detection rate was 35.7% (422/1181). The cancer detection rate was 3.9% (48/1229). The incidence of complications was 0.24% (3 cases of bleeding requiring hemostasis intervention and no perforations). The other endoscopic findings included normal, hemorrhoids, colitis, ulcers, diverticulum, and submucosal lesions.
Figure 1.
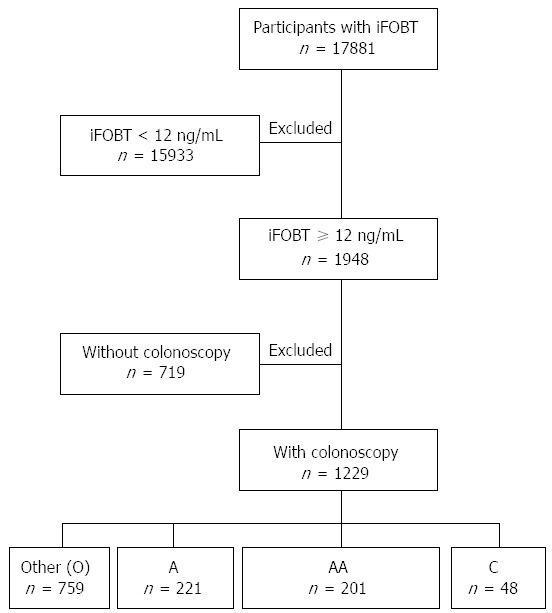
Flowchart of study participants recruited from the two-step colorectal cancer screening program. O: Other; A: Adenoma; AA: Advanced adenoma; C: Cancer.
Table 1.
Quality measures of the colorectal cancer screening program in this study
| Quality indicator | Outcomes | 1Standard | Quality |
| Colonoscopy attendance rate | 63.10% | ≥ 85% | Inadequate |
| Cecum intubation rate | 98.10% | ≥ 90% | Adequate |
| Polyp detection rate | 57.20% | - | - |
| Adenoma detection rate | 35.70% | ≥ 35% | Adequate |
| Cancer detection rate | 2.7‰ screened by FIT | ≥ 2‰ screened by fecal occult blood test | Auditable outcome |
| 3.9% screening colonoscopies | ≥ 11% screening colonoscopies | ||
| Major bleeding | 0.24% | < 1% | Adequate |
| Perforation | 0 | < 1‰ | Adequate |
1NHS BCSP quality assurance guidelines for colonoscopy 2011. NHS: National Health Service; BCSP: bowel cancer screening program.
Cancer stage migration
The stage migration of colorectal cancer is shown in Figure 2. Of the 48 malignancies, 1 was a lymphoma and 47 were adenocarcinomas. Of the 47 cases, the proportions of stages 0, I, II, III, and IV were 25.5%, 31.9%, 6.4%, 25.5%, and 10.6%, respectively. In the hospital cancer registration database, the proportions of stages 0, I, II, III, and IV were 14.1%, 18.1%, 22.8%, 30.9%, and 14.1%, respectively. Therefore, the corresponding differences for stages 0, I, II, III, and IV were 11.4%, 13.9%, -16.4%, -5.4%, and -3.5%, respectively, indicating that cancers tended to be detected at an earlier stage by screening.
Figure 2.
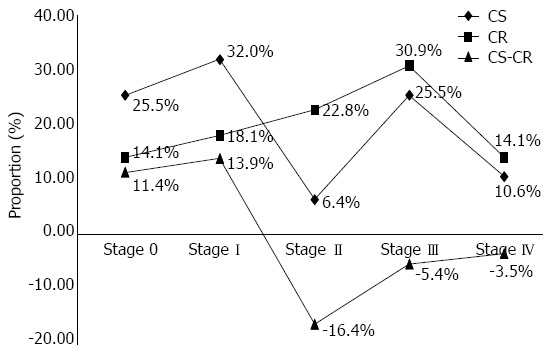
Cancer stage difference between the colorectal cancer screening program and cancer registry database. The cancer stage (X-axis) and proportion (Y-axis) of screening-detected cancers (CS) and the hospital cancer registry (CR) database. The stage migration was calculated as the difference in cancer proportions between the two databases (CS-CR).
Age, gender, and FIT in association with histologic grade of colorectal tumors
The patient numbers in the 4 groups O, A, AA, and C were 759, 221, 201, and 48, respectively. Figure 3 shows the differences in age, gender, and FIT concentrations in these groups. The median age of the 1229 patients was 59 years. The median ages of patients with O, A, AA, and C were 58, 59, 60 and 61 years, respectively. Male patients accounted for 55.7% of the 1229 patients. The percentages of men with O, A, AA, and C were 47.3%, 66.1%, 69.7% and 83.3%, respectively. The median FIT concentration (ngHb/mL buffer) in the 1229 cases was 37.5. The median FIT values in patients with O, A, AA, and C were 34.0, 35.9, 51.0 and 298.9, respectively. Comparing the distributions of age, gender, and FIT concentration among the groups, the histological grade of the colorectal tumors increased with increased age, FIT level and male gender (Kruskal-Wallis test, all P < 0.001). Subsequent multiple linear regression analysis revealed a significant association between ordinal scales of the FIT and histological grade of colorectal tumors after adjusting for age and gender (β = 0.088, P < 0.001) (Table 2).
Figure 3.
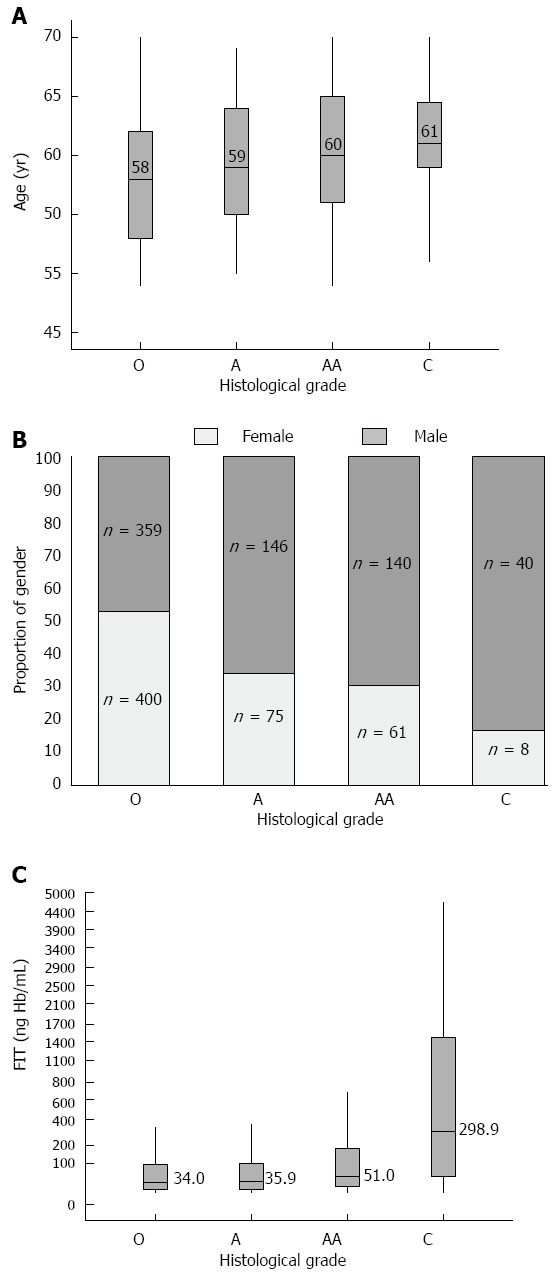
Age, gender, and fecal immunochemical test concentration in association with histological grade of colorectal tumor. A: Age; B: gender; C: FIT. The differences in age, gender, and FIT concentrations (Y-axis) in the different histological groups (X-axis). (Kruskal-Wallis test, all P < 0.001). FIT: fecal immunochemical test; O: Other; A: Adenoma; AA: Advanced adenoma; C: Cancer.
Table 2.
Age, gender, and fecal immunochemical test concentration in association with histological grade of colorectal tumor: multiple linear regression analysis
| 1Multiple linear regression model | Beta (95%CI) | P value | |
| Age (yr) | 50-69 | 0.021 (0.012-0.030) | < 0.001 |
| Gender | Female: 0, Male: 1 | 0.342 (0.245-0.44) | < 0.001 |
| 2FIT (ngHb/mL) | Scale 1: 12.1-25 | 0.088 (0.062-0.114) | < 0.001 |
| Scale 2: 25.1-50 | |||
| Scale 3: 50.1-100 | |||
| Scale 4: 100.1-200 | |||
| Scale 5: 200.1-400 | |||
| Scale 6: 400.1-800 | |||
| Scale 7: > 800 | |||
1The model revealed a significant association between histological grade of colorectal tumors and three independent variables: age, gender and FIT concentrations;
We converted the continuous FIT value into an ordinal variable after a log(2) transformation of the FIT concentrations. FIT: Fecal immunochemical test.
Risk prediction for colorectal tumors based on the FIT
The PPVs for detecting A + AA + C, AA + C, and C were elevated from 38.2%-58.7%, 20.3%-42.7% and 4.0%-21.3%, respectively, as the FIT concentration (ngHb/mL buffer) increased from 12 to 1000. After log(2) transformation of the FIT values, a linear relationship between quantitative level and PPV of the FIT for predicting colorectal adenomatous polyps and malignancies was observed (all R2 > 0.95, P < 0.001). The linear relationship reached a plateau when the FIT concentration was > 800 (Figure 4).
Figure 4.
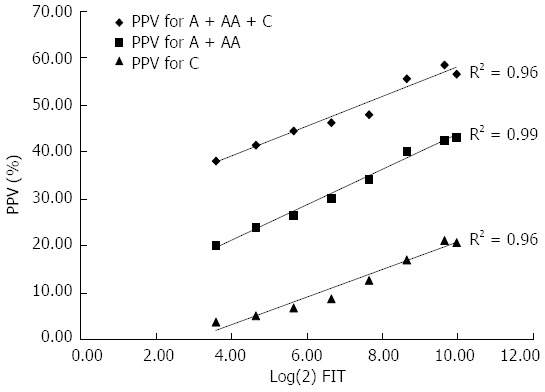
Fecal immunochemical test concentration in the prediction of colorectal neoplasia: linear regression analysis. The association between FIT concentration with log(2) transformation (X-axis) and PPV (Y-axis). (all R2 > 0.95, P < 0.001). FIT: Fecal immunochemical test; O: Other; A: Adenoma; AA: Advanced adenoma; C: Cancer; PPV: Positive predictive value.
DISCUSSION
According to these results, the performance of the two-step CRC screening strategy in our study appeared satisfactory as most of the quality indicators were adequate[16], and a stage migration phenomenon of the detected cancer was observed in the screening population. However, the following issues remain to be clarified: (1) whether colonoscopy should be recommended in asymptomatic subjects only on the basis of a “positive” feces test; (2) whether subjects with “positive” feces tests, but different FIT concentrations have the same risk of significant tumors; and (3) how to improve the performance of the CRC screening program.
Compared with the hospital cancer registry, we observed a stage migration phenomenon in detected cancer in the screening program. The transition zone was between stages I and II reflecting early cancer detection (Figure 2). This result was similar to several cohort studies which showed that feces-based screening programs may offer better prognosis and a reduced CRC mortality rate due to early diagnosis[17,18]. Whether early detection translates to improved overall survival may be disputed. CRC occurs predominantly in elderly patients, who may have comorbid conditions. CRC screening mainly identifies slower-growing lesions, and as most symptomatic populations have more advanced stages, they will not attend the screening program. Therefore, the lead-time bias and length bias cannot be neglected in the migration phenomenon[19-21]. Even so, the CRC screening program was still effective in detecting precancerous lesions and preventing tumor progression to more advanced stages. Taking these advantages together, colonoscopy should be recommended in individuals in the screening population with a positive feces test.
Consistent with previous reports, our research showed that the FIT concentration increases as disease becomes more serious, from non-significant findings to non-advanced adenomas to advanced adenomas to cancer[6,22,23]. This trend is independent after adjustment for age and gender which are important influencing factors[24]. The biological explanation for this association is complex. In summary, a more advanced tumor may be larger and more friable, may have increased vascularity in the stroma, and may have significant inflammation in the surrounding tissues[25,26]. These changes in the characteristics and environment of the tumor may result in significant occult bleeding and an increased FIT concentration. These interesting findings suggest that interpretation of the FIT should be extended beyond “negative” or “positive” and should be tailored according to concentration.
Colonoscopy compliance in participants who were positive for the FIT was 63.1% in this study. This inadequate performance has been frequently reported in other studies. The reasons for non-adherence to the protocol may be due to lack of awareness in the patient or inadequate recommendation by the physicians[27]. The present study demonstrated a dose-dependent relationship between the FIT level and the risk of colorectal tumors. This relationship between the FIT level and colorectal neoplasia could be used to offer more personalized care in risk stratification and management[7,8,28]. As we increased the FIT cut-off values, the trend in predicting advanced colorectal neoplasia increased from 20.3% to 42.7% (Figure 4). These data allow both participants and physicians to be aware of the possibility of occult tumors at each FIT concentration. Accordingly, patients may be more willing to undergo colonoscopy if they are aware of the risk of advanced neoplasia according to the FIT concentration.
There are some limitations in this study. First, the study design was retrospective. Second, the lifestyles, BMI, and family history of colorectal cancer patients, which could be weighted risk factors for colorectal tumors, were incomplete and not included in the present study due to recall bias and missing records. Subsequent prospective studies will be needed to determine the validity of risk prediction for advanced colorectal neoplasia based on the FIT.
In summary, the adenoma detection rate and cancer stage migration support the effectiveness of the FIT as the first step in the current screening strategy. In addition, higher FIT concentrations were associated with more advanced colorectal neoplasia in the average-risk population. Risk prediction for colorectal neoplasia based on the FIT was significant and may help to improve the performance of the colorectal cancer screening program.
COMMENTS
Background
The fecal immunochemical test (FIT) for hemoglobin is specific for detecting colorectal lesions. Screening for colorectal neoplasms using the fecal occult blood test can reduce mortality.
Research frontiers
Despite its widespread use, little is known about the clinical significance and application of the quantitative value of the FIT.
Innovations and breakthroughs
This study used a linear regression model to determine the associations between key variables (age, gender and FIT concentration) and the pathology of colorectal neoplasia. The result demonstrated a dose-dependent relationship between the FIT level and the risk of colorectal tumors.
Applications
FIT concentrations are associated with histological grades and can improve risk prediction for colorectal neoplasia in screening programs.
Peer review
The paper contains interesting results. Authors reported a linear relationship between quantitative level and positive predictive value of FIT for predicting colorectal adenomatous polyps and cancers. Furthermore, this study includes a very large cohort of individuals and it is therefore possible to assume that the results actually reflect the trend of the general population.
Footnotes
P- Reviewers: Flsch UR, Pirazzini C S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Chiang CJ, Chen YC, Chen CJ, You SL, Lai MS. Cancer trends in Taiwan. Jpn J Clin Oncol. 2010;40:897–904. doi: 10.1093/jjco/hyq057. [DOI] [PubMed] [Google Scholar]
- 2.Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut. 2012;61:1036–1040. doi: 10.1136/gutjnl-2011-300774. [DOI] [PubMed] [Google Scholar]
- 3.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 4.Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 5.Duffy MJ, van Rossum LG, van Turenhout ST, Malminiemi O, Sturgeon C, Lamerz R, Nicolini A, Haglund C, Holubec L, Fraser CG, et al. Use of faecal markers in screening for colorectal neoplasia: a European group on tumor markers position paper. Int J Cancer. 2011;128:3–11. doi: 10.1002/ijc.25654. [DOI] [PubMed] [Google Scholar]
- 6.Fraser CG, Mathew CM, McKay K, Carey FA, Steele RJ. Automated immunochemical quantitation of haemoglobin in faeces collected on cards for screening for colorectal cancer. Gut. 2008;57:1256–1260. doi: 10.1136/gut.2008.153494. [DOI] [PubMed] [Google Scholar]
- 7.Fraser CG. Screening for colorectal neoplasia with faecal tests. Lancet Oncol. 2011;12:516–517. doi: 10.1016/S1470-2045(11)70106-1. [DOI] [PubMed] [Google Scholar]
- 8.Omata F, Shintani A, Isozaki M, Masuda K, Fujita Y, Fukui T. Diagnostic performance of quantitative fecal immunochemical test and multivariate prediction model for colorectal neoplasms in asymptomatic individuals. Eur J Gastroenterol Hepatol. 2011;23:1036–1041. doi: 10.1097/MEG.0b013e32834a2882. [DOI] [PubMed] [Google Scholar]
- 9.Chen LS, Yen AM, Chiu SY, Liao CS, Chen HH. Baseline faecal occult blood concentration as a predictor of incident colorectal neoplasia: longitudinal follow-up of a Taiwanese population-based colorectal cancer screening cohort. Lancet Oncol. 2011;12:551–558. doi: 10.1016/S1470-2045(11)70101-2. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell AE, Crespi CM, Antonio CM, Lu P. Explaining disparities in colorectal cancer screening among five Asian ethnic groups: a population-based study in California. BMC Cancer. 2010;10:214. doi: 10.1186/1471-2407-10-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damery S, Clifford S, Wilson S. Colorectal cancer screening using the faecal occult blood test (FOBt): a survey of GP attitudes and practices in the UK. BMC Fam Pract. 2010;11:20. doi: 10.1186/1471-2296-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong MC, Lam AT, Li DK, Lau JT, Griffiths SM, Sung JJ. Factors associated with practice of colorectal cancer screening among primary care physicians in a Chinese population: a cross-sectional study. Cancer Epidemiol. 2009;33:201–206. doi: 10.1016/j.canep.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Yeoh KG, Ho KY, Chiu HM, Zhu F, Ching JY, Wu DC, Matsuda T, Byeon JS, Lee SK, Goh KL, et al. The Asia-Pacific Colorectal Screening score: a validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut. 2011;60:1236–1241. doi: 10.1136/gut.2010.221168. [DOI] [PubMed] [Google Scholar]
- 14.Fraser CG, Allison JE, Halloran SP, Young GP. A proposal to standardize reporting units for fecal immunochemical tests for hemoglobin. J Natl Cancer Inst. 2012;104:810–814. doi: 10.1093/jnci/djs190. [DOI] [PubMed] [Google Scholar]
- 15.Allison JE, Fraser CG, Halloran SP, Young GP. Comparing fecal immunochemical tests: improved standardization is needed. Gastroenterology. 2012;142:422–424. doi: 10.1053/j.gastro.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Lee TJ, Rutter MD, Blanks RG, Moss SM, Goddard AF, Chilton A, Nickerson C, McNally RJ, Patnick J, Rees CJ. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut. 2012;61:1050–1057. doi: 10.1136/gutjnl-2011-300651. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Ge Z, Dai J, Li X, Gao Y. Effectiveness of the immunofecal occult blood test for colorectal cancer screening in a large population. Dig Dis Sci. 2011;56:203–207. doi: 10.1007/s10620-010-1264-8. [DOI] [PubMed] [Google Scholar]
- 18.Pox C. Colon cancer screening: which non-invasive filter tests? Dig Dis. 2011;29 Suppl 1:56–59. doi: 10.1159/000331127. [DOI] [PubMed] [Google Scholar]
- 19.Ekelund G, Manjer J, Zackrisson S. Population-based screening for colorectal cancer with faecal occult blood test--do we really have enough evidence? Int J Colorectal Dis. 2010;25:1269–1275. doi: 10.1007/s00384-010-1027-1. [DOI] [PubMed] [Google Scholar]
- 20.Lindqvist M. [The Christian viewpoint of therapy in human development processes] Sairaanh Vuosik. 1978;15:253–269. [PubMed] [Google Scholar]
- 21.MacDonald AJ, McEwan H, McCabe M, Macdonald A. Age at death of patients with colorectal cancer and the effect of lead-time bias on survival in elective vs emergency surgery. Colorectal Dis. 2011;13:519–525. doi: 10.1111/j.1463-1318.2009.02183.x. [DOI] [PubMed] [Google Scholar]
- 22.Levi Z, Rozen P, Hazazi R, Vilkin A, Waked A, Maoz E, Birkenfeld S, Leshno M, Niv Y. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med. 2007;146:244–255. doi: 10.7326/0003-4819-146-4-200702200-00003. [DOI] [PubMed] [Google Scholar]
- 23.Ciatto S, Martinelli F, Castiglione G, Mantellini P, Rubeca T, Grazzini G, Bonanomi AG, Confortini M, Zappa M. Association of FOBT-assessed faecal Hb content with colonic lesions detected in the Florence screening programme. Br J Cancer. 2007;96:218–221. doi: 10.1038/sj.bjc.6603534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald PJ, Strachan JA, Digby J, Steele RJ, Fraser CG. Faecal haemoglobin concentrations by gender and age: implications for population-based screening for colorectal cancer. Clin Chem Lab Med. 2012;50:935–940. doi: 10.1515/CCLM.2011.815. [DOI] [PubMed] [Google Scholar]
- 25.McLean MH, Murray GI, Stewart KN, Norrie G, Mayer C, Hold GL, Thomson J, Fyfe N, Hope M, Mowat NA, et al. The inflammatory microenvironment in colorectal neoplasia. PLoS One. 2011;6:e15366. doi: 10.1371/journal.pone.0015366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 27.Yabroff KR, Klabunde CN, Yuan G, McNeel TS, Brown ML, Casciotti D, Buckman DW, Taplin S. Are physicians’ recommendations for colorectal cancer screening guideline-consistent? J Gen Intern Med. 2011;26:177–184. doi: 10.1007/s11606-010-1516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Rossum LG, van Rijn AF, Laheij RJ, van Oijen MG, Fockens P, Jansen JB, Verbeek AL, Dekker E. Cutoff value determines the performance of a semi-quantitative immunochemical faecal occult blood test in a colorectal cancer screening programme. Br J Cancer. 2009;101:1274–1281. doi: 10.1038/sj.bjc.6605326. [DOI] [PMC free article] [PubMed] [Google Scholar]


