Abstract
AIM: To establish the role of magnetic resonance cholangiography (MRC) in diagnosis of biliary anatomy in living-donor liver transplantation (LDLT) donors.
METHODS: A systematic review was performed by searching electronic bibliographic databases prior to March 2013. Studies with diagnostic results and fulfilled inclusion criteria were included. The methodological quality of the studies was assessed. Sensitivity, specificity and other measures of the accuracy of MRC for diagnosis of biliary anatomy in LDLT donors were summarized using a random-effects model or a fixed-effects model. Summary receiver operating characteristic (SROC) curves were used to summarize overall test performance. Publication bias was assessed using Deek’s funnel plot asymmetry test. Sensitivity analysis was adopted to explore the potential sources of heterogeneity.
RESULTS: Twelve studies involving 869 subjects were eligible to the analysis. The scores of Quality Assessment of Diagnostic Accuracy Studies for the included studies ranged from 11 to 14. The summary estimates of sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic OR of MRC in diagnosis of biliary anatomy in LDLT donor were 0.88 (95%CI: 0.84-0.92), 0.95 (95%CI: 0.93-0.97), 15.33 (95%CI: 10.70-21.95), 0.15 (95%CI: 0.11-0.20) and 130.77 (95%CI: 75.91-225.27), respectively. No significant heterogeneity was detected in all the above four measures. Area under SROC curve was 0.971. Little publication bias was noted across the studies (P = 0.557). Sensitivity analysis excluding a study with possible heterogeneity got a similar overall result, which suggested the little influence of this study on the overall results.
CONCLUSION: Our results suggest that MRC is a high specificity but moderate sensitivity technique in diagnosis of biliary anatomy in LDLT donors.
Keywords: Magnetic resonance imaging, Cholangiography, Biliary, Anatomy, Living donors
Core tip: The current findings on the value of magnetic resonance cholangiography (MRC) in diagnosis of biliary anatomy in living-donor liver transplantation (LDLT) donors are conflicting. This meta-analysis including 12 studies with 869 patients suggested that MRC has a high specificity in diagnosis of biliary anatomy in LDLT donors, but the sensitivity is moderate. This is the first meta-analysis to investigate the diagnostic accuracy of MRC in the detection of biliary anatomy in LDLT donors; and these results will provide valuable information to the doctors when they make a decision for the living liver donors.
INTRODUCTION
Adult living-donor liver transplantation (LDLT) is an alternative therapeutic option for patients with end-stage liver disease. Some transplantation centers have reported high rates of biliary complications following LDLT[1-3]. Biliary complications after LDLT are closely related to the complex anatomy of the donor’s biliary tree. Therefore, preoperative knowledge of the donor’s aberrant biliary anatomy can minimize postoperative morbidity in the recipient and maximize safety for the donor. Several techniques are currently being used in this setting; however, they all have some limitations. For example, endoscopic retrograde cholangiography (ERC) and intraoperative cholangiography (IOC) are both invasive techniques that can result in serious complications[4,5], while the non-invasive multidetector computed tomography (MDCT) technique exposes the potential donor to ionizing radiation and the risks associated with nephrotoxic contrast agents[6].
Magnetic resonance cholangiography (MRC) is a non-invasive imaging technique and has shown promise in the preoperative evaluation of the biliary anatomy of LDLT donors. Previously, two studies[7,8] reported meta-analyses on the value of MRC in the diagnosis of biliary complications after liver transplantation. However, neither evaluated the role of MRC in evaluating the biliary anatomy of LDLT donors. In addition, although some studies[9-12] reported a high diagnostic accuracy for MRC in the diagnosis of biliary anatomy, the sample sizes were small; thus, the results remained inconclusive. Given the importance of a preoperative evaluation of biliary anatomy and the uncertainty regarding the diagnostic accuracy of MRC, we performed a meta-analysis to determine the overall diagnostic accuracy of MRC in the evaluation of the biliary anatomy of LDLT donors.
MATERIALS AND METHODS
Search strategy and study selection
We systematically searched the Cochrane clinical trials database, MEDLINE/PubMed, and Embase to identify suitable studies prior to March 1, 2013. No starting date limit was applied. Articles were also identified using the related articles function in PubMed. References within the identified articles were also searched manually. The search terms included “magnetic resonance cholangiography” or “MRC,” “biliary anatomy,” and “donors.” The searches were limited to human studies. Potentially relevant articles were then screened by at least two independent reviewers. Disagreements were resolved by discussion or upon consensus from a third reviewer.
Inclusion and exclusion criteria
A study was included in the meta-analysis when it provided data on both the sensitivity and specificity of MRC for the diagnosis of the biliary anatomy of living donors, or when it provided values in a scatterplot form, allowing test results for individual study subjects to be extracted. Studies were excluded if they were review articles, case reports, or animal studies. In order to obtain a more reliable estimation of the accuracy of MRC, we only included studies that fulfilled at least nine items of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) criteria. Two reviewers independently judged the eligibility of the studies. Disagreements between reviewers were resolved by consensus. The authors of some publications were contacted for clarifications and additional information.
Data extraction and quality assessment
The final set of articles was assessed independently by two reviewers. The data retrieved included the authors, publication year, the country where the study was conducted, the number of patients and their mean age, the reference standard (gold standard), true-positive, false-negative, false-positive, and true-negative values, and the quality of the methodology. The methodological quality of the studies was assessed using QUADAS, an evidence-based quality assessment tool developed for use in systematic reviews of studies of diagnostic accuracy and fully described by Whiting et al[13], with a maximum score of 14.
Statistical analysis
Standard methods recommended for the meta-analysis of diagnostic test evaluations were used[14]. The following measurements of test accuracy were computed for each study: sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR). The analysis was based on a summary receiver operating characteristic curve (SROC); the results are described as the area under the curve (AUC) of the SROC, with its Q-point representing the maximal joint sensitivity and specificity[15,16]. The summary sensitivity, specificity, and other measures across MRC studies were calculated using a random-effects model and a fixed-effects model, respectively. A χ2 test and an inconsistency index (I2) were used to detect statistically significant heterogeneity across studies. Publication bias was assessed using Deeks’ Funnel Plot Asymmetry Test[17]. Analyses were performed using Meta-DiSc version 1.4 (Unit of Clinical Biostatistics, the Ramón y Cajal Hospital, Madrid, Spain) and Stata 11.2 (Stata Corporation, College Station, TX, United States) software.
RESULTS
Study selection
The primary literature search retrieved 25 studies that were considered eligible for the analysis. After a detailed evaluation, 13 studies were excluded, as six were laboratory studies, three were reviews, three provided insufficient data for calculations[1,10,18], and one was irrelevant to the current analysis. Consequently, 12 studies[9,11,12,19-27] involving 869 subjects were finally included in the present meta-analysis.
Characteristics of the studies and quality assessment
All included studies adequately described the MRC techniques used and the types of conventional and aberrant biliary anatomy; however, the screening techniques were different across the studies. All but one study were using IOC as the reference standard. Additional patient demographics from each of the included studies are listed in Table 1. The QUADAS scores for the included studies ranged from 11 to 14 (Table 2).
Table 1.
Characteristics of included studies
| Study | Country/years | No. of patients | Mean age | Study design | MRCP technique | Reference standard | QUADAS | TP | FP | FN | TN |
| Ayuso et al[9] | Spain/2004 | 25 | NS | Prospective | MnDPDP-enhanced, MIP | IOC | 12 | 15 | 0 | 1 | 9 |
| Limanond et al[11] | United States/2004 | 26 | 37 | Retrospective | T2 single-shot fast spin-echo, T2 HASTE | IOC | 13 | 5 | 2 | 2 | 17 |
| Kim et al[23] | Canada/2005 | 30 | 36 | Prospective | T2 weighted SSFSE, Mn-DPDP | IOC | 12 | 12 | 0 | 1 | 17 |
| An et al[12] | South Korea/2006 | 24 | 29 | Prospective | Gadobenate dimeglumine-enhanced T1-and T2-weighted, MIP | IOC | 14 | 13 | 1 | 1 | 9 |
| Sirvanci et al[25] | Turkey/2007 | 62 | 42 | Retrospective | RARE, HASTE, 3D TSE | IOC | 13 | 16 | 0 | 3 | 43 |
| Song et al[26] | South Korea/2007 | 111 | 29 | Prospective | Single-slab RARE or multislice HASTE | IOC | 12 | 42 | 3 | 2 | 64 |
| Basaran et al[19] | Turkey/2008 | 40 | 35 | Prospective | T2-weighted PACE turbo spin-echo | IOC | 12 | 13 | 3 | 0 | 24 |
| Kashyap et al[22] | United States/2008 | 36 | 38 | Retrospective | Thick and thin slab heavily T2 weighted | IOC | 14 | 16 | 0 | 3 | 17 |
| Artioli et al[27] | Italy/2010 | 32 | 38 | Prospective | T2-weighted | Surgery | 11 | 15 | 1 | 2 | 14 |
| Kim et al[24] | South Korea/2010 | 52 | 33 | Prospective | RARE, 3D SE T2-weighted sequences. | IOC | 14 | 15 | 3 | 3 | 31 |
| Hsu et al[21] | Taiwan/2011 | 203 | 32 | Retrospective | RARE thin-slab | IOC | 13 | 45 | 6 | 8 | 144 |
| Chiang et al[20] | Taiwan/2012 | 228 | 30 | Retrospective | T2-weighted GD-DTPA | IOC | 13 | 55 | 7 | 9 | 157 |
SSFSE: Single shot fast spin echo; RARE: Rapid acquisition with relaxation enhancement; SE: Spin-echo; Mn-DPDP: Mangafodipir trisodium; GD-DTPA: Diethylenetri aminepentaacetic acid; MIP: Maximum intensity projection; PACE: Prospective acquisition correction; SSD: Shaded surface display; HASTE: Half-Fourier Acquisition Single-Shot Turbo Spin-Echo; QUADAS: Quality Assessment of Diagnostic Accuracy Studies.
Table 2.
Quality assessment tool for diagnostic accuracy systematic review of quality criteria of included studies
| Ayuso et al[9] | Limanond et al[11] | Kim et al[23] | An et al[12] | Sirvanci et al[25] | Song et al[26] | Basaran et al[19] | Kashyap et al[22] | Artioli et al[27] | Kim et al[24] | Hsu et al[21] | Chiang et al[20] | |
| Patient spectrum representative | Yes | NA | Yes | Yes | NA | Yes | Yes | Yes | NA | Yes | NA | NA |
| Selection criteria described | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Reference standard appropriate | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Time between tests appropriate | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Uniform verification by reference standard | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Same reference test used | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Reference standard independent | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Reference standard described adequately | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Blinding to reference standard results | NA | Yes | NA | Yes | Yes | NA | NA | Yes | NA | Yes | Yes | Yes |
| Blinding to index test results | NA | Yes | NA | Yes | Yes | NA | NA | Yes | NA | Yes | Yes | Yes |
| Appropriate clinical data available | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Uninterpretable data reported | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Withdrawals explained | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| No. of criteria met out of 14 | 12 | 13 | 13 | 14 | 13 | 12 | 12 | 14 | 11 | 14 | 13 | 13 |
NA: Not available.
Meta-analysis
The overall analysis of the 12 studies showed that the summary sensitivity, specificity, PLR, NLR, and DOR were 0.88 (95%CI: 0.84-0.92), 0.95 (95%CI: 0.93-0.97), 15.33 (95%CI: 10.70-21.95), 0.15 (95%CI: 0.11-0.20), and 130.77 (95%CI: 75.91-225.27), respectively; no significant heterogeneity was detected in any of the above four measures (all P > 0.05). The AUC of the SROC was 0.971, suggesting a high diagnostic accuracy (Figures 1 and 2). The moderate sensitivity indicates that 12% of the cases with aberrant biliary anatomy could be missed, and the high specificity indicates a small probability of the presence of aberrant biliary anatomy when the MRC diagnosis is normal. A PLR of 15.33 suggests that patients with aberrant biliary anatomy have about a 15-fold higher chance of a positive test than those without. An NLR of 0.15 suggests that if the MRC result is negative, the probability of the patient having aberrant biliary anatomy is 15%.
Figure 1.
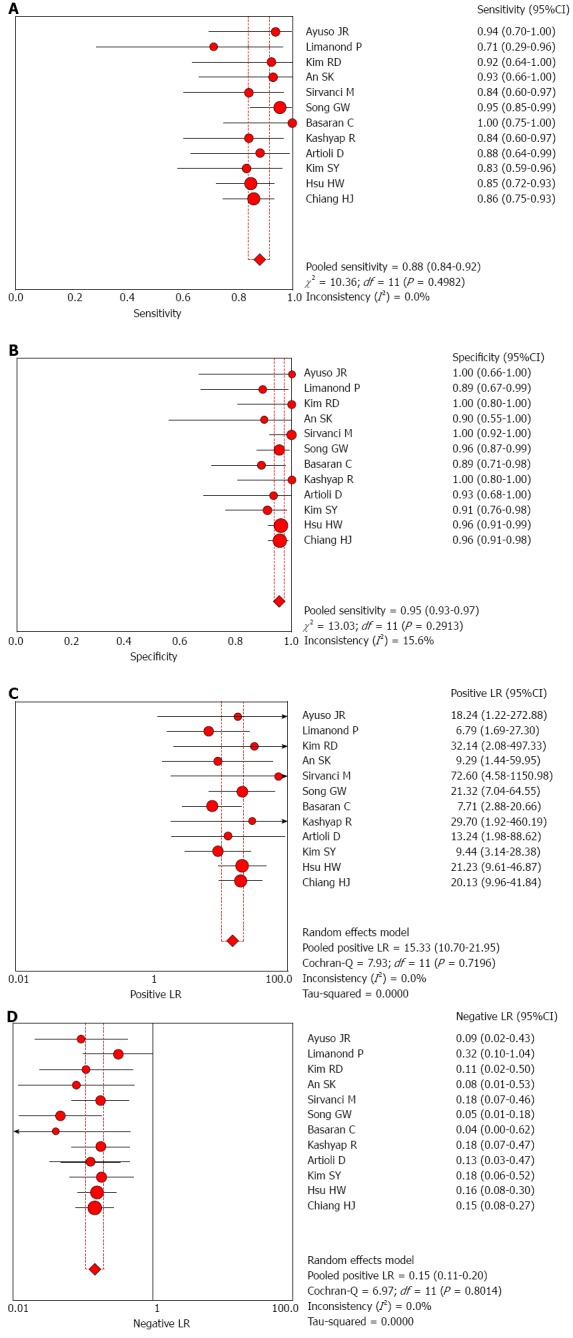
Forest plot of summary results of magnetic resonance cholangiography in the diagnosis of biliary anatomy in living-donor liver transplantation. A: Sensitivity; B: Specificity; C: Positive likelihood ratio; D: Negative likelihood ratio.
Figure 2.
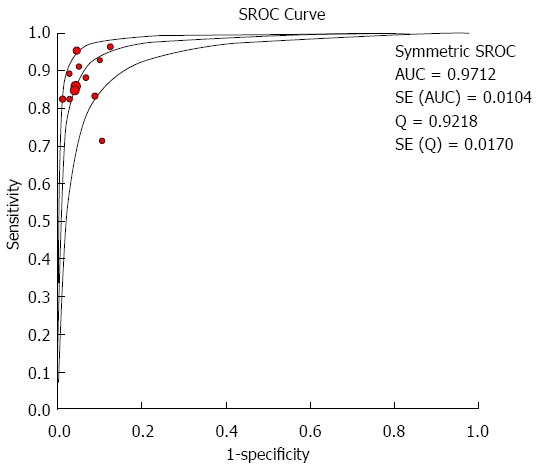
Summary receiver-operating characteristic curve of biliary anatomy in living-donor liver transplantation. SROC: Summary receiver-operating characteristic curve; AUC: Area under the curve.
Sensitivity analysis
Because one of the studies[27] used surgery as the reference, we excluded it from the sensitivity analysis. The results were similar to the overall results; the summary sensitivity, specificity, PLR, NLR, DOR, and AUC of the SROC were 0.88 (95%CI: 0.84-0.92), 0.96 (95%CI: 0.93-0.97), 15.41 (95%CI: 10.69-22.22), 0.148 (95%CI: 0.11-0.20), 132.19 (95%CI: 75.72-230.76), and 0.972, respectively, which suggested that the excluded study had little influence on the overall results.
Publication bias
Deeks’ Funnel Plot Asymmetry Test for the overall analysis showed that no significant publication bias was found (P = 0.557; Figure 3).
Figure 3.
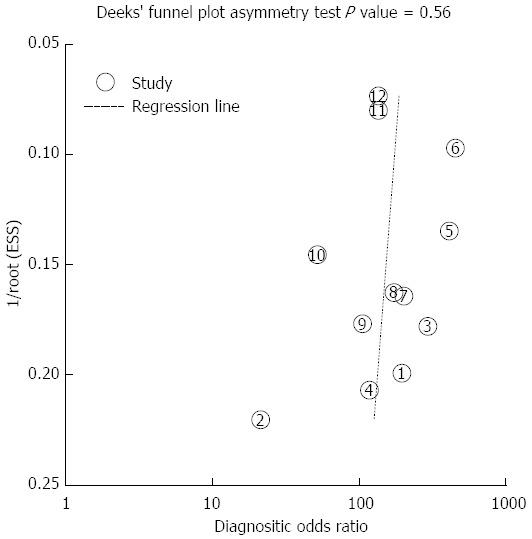
Deek’s funnel plot asymmetry test for identifying publication bias in the diagnosis of biliary anatomy in living-donor liver transplantation.
DISCUSSION
Generally, ERC, IOC, MRC, and MDCT cholangiography have been used to evaluate the biliary anatomy of liver donors. However, these techniques all have their inherent strengths and weaknesses. Although ERC is an accurate method to identify biliary anatomy, the high incidence of serious complications caused by the invasiveness of the procedure makes it excessively risky to perform on healthy donors. IOC is considered the gold standard for the imaging of the biliary anatomy; however, because it does not allow a three-dimensional (3D) view, this technique may not be helpful in obtaining a single bile duct orifice in some cases with anatomical variations. MDCT or MDCT cholangiography are both non-invasive techniques, and they have shown promise in delineating biliary anatomy in potential right lobe living donors[28,29]. However, compared with MRC, the obvious disadvantages of CT are the exposure to ionizing radiation and nephrotoxic contrast agents; especially, the intravenous contrast agent iodipamide meglumine is associated with poor patient tolerance and adverse reactions[6]. Several studies showed that MRC has a strong correlation with IOC in delineating the intrahepatic biliary anatomy. In addition to biliary anatomy imaging, MRC is also able to assess other aspects of a potential donor’s liver anatomy. It can perform an assessment of the vascular and biliary anatomy and provide an assessment of iron and fat depositions in the liver in a single examination[23].
One of the obvious advantages of the MRC technique is the elimination of the need for an invasive procedure, thus considerably reducing the cost and associated complications. However, this technique also has limitations. For example, MRC is more limited in temporal and spatial resolution than direct digital subtraction angiography and IOC. In addition, the conventional MRC technique (half-Fourier acquisition) provides a depiction of the biliary anatomy with limited detail, especially of non-dilated ducts. However, recent progress in MRC techniques seems to have overcome some of these limitations. Mangafodipir trisodium-enhanced 3D T1-weighted MRC is reported to provide an increased signal-to-noise ratio and a greater visualization rate of the ductal anatomy compared with T2-weighted MRC or two-dimensional (2D) gradient-echo images[30,31]. Gadobenate dimeglumine-enhanced T1-weighted MRC can provide information about the differentiation of cystic structures near bile ducts and the bile duct lumen[12]. In addition, gadobenate dimeglumine is easier to use and less expensive than mangafodipir trisodium.
The present meta-analysis suggests that MRC has a high diagnostic specificity and moderate sensitivity for biliary anatomy in LDLT donors. Likelihood ratios > 10 or < 0.1 generate large and often conclusive shifts from the pre-test to post-test probability[32,33]. DOR is a single indicator of test accuracy that combines the sensitivity and specificity data into a single number[34]. The SROC curve presents a global summary of test performance and shows the tradeoff between sensitivity and specificity[35]. The summary DOR and the AUC of the SROC were 130.77 and 0.971, respectively, indicating that the overall accuracy was as high as expected, and that MRC is helpful in the diagnosis of aberrant biliary anatomy.
To our knowledge, this is the first meta-analysis exploring the value of MRC in the detection of biliary anatomy of LDLT donors. Our study included more published articles with more subjects, which can provide a more reliable statistical power. In addition, the quality of the selected studies was higher, as all had QUADAS scores over 10. Furthermore, the negligible heterogeneity and publication bias suggest the robustness of the results. In the present study, although several MRC techniques were used and the diagnostic accuracy varied across the studies (the sensitivity ranged from 0.71 to 1.00), the screening techniques were not a significant factor contributing to differences in diagnosis. Even though both were using a conventional MRC technique, Limanond et al[11] reported the lowest sensitivity of 0.71, while Song et al[26] reported a higher sensitivity of 0.95. Likewise, Kim et al[23] and Chiang et al[20] both used GD-DTPA MRC techniques, and their diagnostic sensitivities were not similar, reporting 0.92 and 0.86, respectively. These differences suggest that there were other factors, such as sample size, imaging treatment technique (2D, 3D, or maximum intensity projection), or the variability among different imaging readers. These factors may have led to the differing levels of diagnostic accuracy.
There were several limitations to the present meta-analysis. First, the MRC screening techniques were heterogeneous across the studies, although the techniques are all considered good enough to make a correct diagnosis by our contributing radiologists (Min ZG and Zeng ZY). However, the heterogeneity of the MRC techniques across the studies may have led to a differential verification bias and could have falsely elevated the reported sensitivities. Second, five of the included studies[9,19,23,26,27] did not specifically state that the readers were blinded to the results of the MRC, thus, it might raise the possibility of a review bias. Third, five of the included studies[11,20-22,25] were retrospective in design; thus, the biases of retrospective design studies, such as selection bias and recall bias[36], should not be neglected. Fourth, although there were 12 studies included, the number of subjects was relatively small; a larger sample size of subjects is warranted to obtain more reliable results. Fifth, the eligible studies for the present study were all English publications, which may have led to the observed publication bias. Sixth, although all the included studies stated that the results of the MRC were evaluated by experienced radiologists, a reviewer bias caused by inter-observer variability among different readers should not be neglected.
In summary, this meta-analysis demonstrates that MRC is a diagnostic technique with high specificity, but moderate sensitivity, in the diagnosis of biliary anatomy in LDLT donors. Therefore, other techniques such as MDCT may be complementary methods to enhance the sensitivity of the evaluation.
COMMENTS
Background
Magnetic resonance cholangiography (MRC) is a non-invasive procedure in diagnosis of the biliary anatomy. The current findings on the value of MRC in diagnosis of biliary anatomy in living-donor liver transplantation (LDLT) donors are conflicting.
Research frontiers
This study suggests that MRC has a high specificity in diagnosis of biliary anatomy in LDLT donors, but the sensitivity is moderate.
Innovations and breakthroughs
This is the first meta-analysis to investigate the diagnostic accuracy of MRC in the detection of biliary anatomy in LDLT donors.
Applications
The results will provide valuable information to the doctors when they make a decision for the living liver donors.
Peer review
In LDLT, preoperative assessment of biliary duct is so important, especially in a case of posterior graft. Authors reported MRC is a high specificity but moderate sensitivity technique in diagnosis of biliary anatomy in LDLT donors. Other inspection of biliary duct is maybe invasive, and therefore MRC is a good tool for the first survey of the biliary tree. This report is informative for transplant surgeons, even though the sensitivity is moderate.
Footnotes
P- Reviewers: Alicioglu B, Hori T, Kayaalp C, Yan LN S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Lee VS, Morgan GR, Teperman LW, John D, Diflo T, Pandharipande PV, Berman PM, Lavelle MT, Krinsky GA, Rofsky NM, et al. MR imaging as the sole preoperative imaging modality for right hepatectomy: a prospective study of living adult-to-adult liver donor candidates. AJR Am J Roentgenol. 2001;176:1475–1482. doi: 10.2214/ajr.176.6.1761475. [DOI] [PubMed] [Google Scholar]
- 2.Marcos A, Ham JM, Fisher RA, Olzinski AT, Posner MP. Surgical management of anatomical variations of the right lobe in living donor liver transplantation. Ann Surg. 2000;231:824–831. doi: 10.1097/00000658-200006000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura T, Tanaka K, Kiuchi T, Kasahara M, Oike F, Ueda M, Kaihara S, Egawa H, Ozden I, Kobayashi N, et al. Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation. 2002;73:1896–1903. doi: 10.1097/00007890-200206270-00008. [DOI] [PubMed] [Google Scholar]
- 4.Ford JA, Soop M, Du J, Loveday BP, Rodgers M. Systematic review of intraoperative cholangiography in cholecystectomy. Br J Surg. 2012;99:160–167. doi: 10.1002/bjs.7809. [DOI] [PubMed] [Google Scholar]
- 5.Masci E, Toti G, Mariani A, Curioni S, Lomazzi A, Dinelli M, Minoli G, Crosta C, Comin U, Fertitta A, et al. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001;96:417–423. doi: 10.1111/j.1572-0241.2001.03594.x. [DOI] [PubMed] [Google Scholar]
- 6.Catalano C, Fraioli F, Danti M, Napoli A, Votta V, Lanciotti K, Bertoletti L, Passariello R. MDCT of the abdominal aorta: basics, technical improvements, and clinical applications. Eur Radiol. 2003;13 Suppl 3:N53–N58. doi: 10.1007/s00330-003-0008-y. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen JE, Waljee AK, Volk ML, Sonnenday CJ, Elta GH, Al-Hawary MM, Singal AG, Taylor JR, Elmunzer BJ. Is MRCP equivalent to ERCP for diagnosing biliary obstruction in orthotopic liver transplant recipients? A meta-analysis. Gastrointest Endosc. 2011;73:955–962. doi: 10.1016/j.gie.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaltenthaler EC, Walters SJ, Chilcott J, Blakeborough A, Vergel YB, Thomas S. MRCP compared to diagnostic ERCP for diagnosis when biliary obstruction is suspected: a systematic review. BMC Med Imaging. 2006;6:9. doi: 10.1186/1471-2342-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayuso JR, Ayuso C, Bombuy E, De Juan C, Llovet JM, De Caralt TM, Sánchez M, Pagés M, Bruix J, García-Valdecasas JC. Preoperative evaluation of biliary anatomy in adult live liver donors with volumetric mangafodipir trisodium enhanced magnetic resonance cholangiography. Liver Transpl. 2004;10:1391–1397. doi: 10.1002/lt.20281. [DOI] [PubMed] [Google Scholar]
- 10.Cheng YF, Chen CL, Huang TL, Chen TY, Lee TY, Chen YS, Wang CC, de Villa V, Goto S, Chiang YC, et al. Single imaging modality evaluation of living donors in liver transplantation: magnetic resonance imaging. Transplantation. 2001;72:1527–1533. doi: 10.1097/00007890-200111150-00010. [DOI] [PubMed] [Google Scholar]
- 11.Limanond P, Raman SS, Ghobrial RM, Busuttil RW, Lu DS. The utility of MRCP in preoperative mapping of biliary anatomy in adult-to-adult living related liver transplant donors. J Magn Reson Imaging. 2004;19:209–215. doi: 10.1002/jmri.10446. [DOI] [PubMed] [Google Scholar]
- 12.An SK, Lee JM, Suh KS, Lee NJ, Kim SH, Kim YJ, Han JK, Choi BI. Gadobenate dimeglumine-enhanced liver MRI as the sole preoperative imaging technique: a prospective study of living liver donors. AJR Am J Roentgenol. 2006;187:1223–1233. doi: 10.2214/AJR.05.0584. [DOI] [PubMed] [Google Scholar]
- 13.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devillé WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, Bezemer PD. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwig L, Macaskill P, Glasziou P, Fahey M. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol. 1995;48:119–30; discussion 131-2. doi: 10.1016/0895-4356(94)00099-c. [DOI] [PubMed] [Google Scholar]
- 16.Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med. 1993;12:1293–1316. doi: 10.1002/sim.4780121403. [DOI] [PubMed] [Google Scholar]
- 17.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Fulcher AS, Szucs RA, Bassignani MJ, Marcos A. Right lobe living donor liver transplantation: preoperative evaluation of the donor with MR imaging. AJR Am J Roentgenol. 2001;176:1483–1491. doi: 10.2214/ajr.176.6.1761483. [DOI] [PubMed] [Google Scholar]
- 19.Basaran C, Agildere AM, Donmez FY, Sevmis S, Budakoglu I, Karakayali H, Haberal M. MR cholangiopancreatography with T2-weighted prospective acquisition correction turbo spin-echo sequence of the biliary anatomy of potential living liver transplant donors. AJR Am J Roentgenol. 2008;190:1527–1533. doi: 10.2214/AJR.07.3006. [DOI] [PubMed] [Google Scholar]
- 20.Chiang HJ, Hsu HW, Chen PC, Chiang HW, Huang TL, Chen TY, Chen CL, Cheng YF. Magnetic resonance cholangiography in living donor liver transplantation: comparison of preenhanced and post-gadolinium-enhanced methods. Transplant Proc. 2012;44:324–327. doi: 10.1016/j.transproceed.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 21.Hsu HW, Tsang LL, Yap A, Huang TL, Chen TY, Lin TS, Concejero AM, Ou SY, Yu CY, Chen CL, et al. Magnetic resonance cholangiography in living donor liver transplantation. Transplantation. 2011;92:94–99. doi: 10.1097/TP.0b013e31821c1e33. [DOI] [PubMed] [Google Scholar]
- 22.Kashyap R, Bozorgzadeh A, Abt P, Tsoulfas G, Maloo M, Sharma R, Patel S, Dombroski D, Mantry P, Safadjou S, et al. Stratifying risk of biliary complications in adult living donor liver transplantation by magnetic resonance cholangiography. Transplantation. 2008;85:1569–1572. doi: 10.1097/TP.0b013e31816ff21f. [DOI] [PubMed] [Google Scholar]
- 23.Kim RD, Sakamoto S, Haider MA, Molinari M, Gallinger S, McGilvray ID, Greig PD, Grant DR, Cattral MS. Role of magnetic resonance cholangiography in assessing biliary anatomy in right lobe living donors. Transplantation. 2005;79:1417–1421. doi: 10.1097/01.tp.0000159793.02863.d2. [DOI] [PubMed] [Google Scholar]
- 24.Kim SY, Byun JH, Lee SS, Park SH, Jang YJ, Lee MG. Biliary tract depiction in living potential liver donors: intraindividual comparison of MR cholangiography at 3.0 and 1.5 T. Radiology. 2010;254:469–478. doi: 10.1148/radiol.09090003. [DOI] [PubMed] [Google Scholar]
- 25.Sirvanci M, Duran C, Ozturk E, Balci D, Dayangaç M, Onat L, Yüzer Y, Tokat Y, Killi R. The value of magnetic resonance cholangiography in the preoperative assessment of living liver donors. Clin Imaging. 2007;31:401–405. doi: 10.1016/j.clinimag.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Song GW, Lee SG, Hwang S, Sung GB, Park KM, Kim KH, Ahn CS, Moon DB, Ha TY, Kim BS, et al. Preoperative evaluation of biliary anatomy of donor in living donor liver transplantation by conventional nonenhanced magnetic resonance cholangiography. Transpl Int. 2007;20:167–173. doi: 10.1111/j.1432-2277.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 27.Artioli D, Tagliabue M, Aseni P, Sironi S, Vanzulli A. Detection of biliary and vascular anatomy in living liver donors: value of gadobenate dimeglumine enhanced MR and MDCT angiography. Eur J Radiol. 2010;76:e1–e5. doi: 10.1016/j.ejrad.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Fetzer DT, Furlan A, Wang JH, Kantartzis S, Sholosh B, Bae KT. Computed tomographic cholangiography in living liver transplant donors: factors determining the degree of contrast enhancement. J Comput Assist Tomogr. 2011;35:716–722. doi: 10.1097/RCT.0b013e318237284c. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder T, Malagó M, Debatin JF, Testa G, Nadalin S, Broelsch CE, Ruehm SG. Multidetector computed tomographic cholangiography in the evaluation of potential living liver donors. Transplantation. 2002;73:1972–1973. doi: 10.1097/00007890-200206270-00026. [DOI] [PubMed] [Google Scholar]
- 30.Papanikolaou N, Prassopoulos P, Eracleous E, Maris T, Gogas C, Gourtsoyiannis N. Contrast-enhanced magnetic resonance cholangiography versus heavily T2-weighted magnetic resonance cholangiography. Invest Radiol. 2001;36:682–686. doi: 10.1097/00004424-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Lee VS, Rofsky NM, Morgan GR, Teperman LW, Krinsky GA, Berman P, Weinreb JC. Volumetric mangafodipir trisodium-enhanced cholangiography to define intrahepatic biliary anatomy. AJR Am J Roentgenol. 2001;176:906–908. doi: 10.2214/ajr.176.4.1760906. [DOI] [PubMed] [Google Scholar]
- 32.Deeks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323:157–162. doi: 10.1136/bmj.323.7305.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaeschke RGG, Lijmer J. Diagnostic tests. In: Guyatt G, Rennie D, editors. Users’ Guides to the Medical Literature. A Manual for Evidence-Based Clinical Practice. Chicago (IL): AMA Press; 2002. pp. 121–140. [Google Scholar]
- 34.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–1135. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 35.Gatsonis C, Paliwal P. Meta-analysis of diagnostic and screening test accuracy evaluations: methodologic primer. AJR Am J Roentgenol. 2006;187:271–281. doi: 10.2214/AJR.06.0226. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann RG, Lim HJ. Observational study design. Methods Mol Biol. 2007;404:19–31. doi: 10.1007/978-1-59745-530-5_2. [DOI] [PubMed] [Google Scholar]


