Abstract
Clathrin-mediated endocytosis is a central and well-studied trafficking process in eukaryotic cells. How this process is initiated is likely to be a critical point in regulating endocytic activity spatially and temporally, but the underlying mechanisms are poorly understood. During the early stages of endocytosis three components—adaptor and accessory proteins, cargo, and lipids—come together at the plasma membrane to begin the formation of clathrin-coated vesicles. Although different models have been proposed, there is still no clear picture of how these three components cooperate to initiate endocytosis, which may indicate that there is some flexibility underlying this important event.
Introduction
Clathrin-mediated endocytosis is the process by which cargo-containing clathrin-coated vesicles bud off from the plasma membrane and are taken up into the cell. This process is critical for intercellular signaling, uptake of nutrients, and membrane recycling. Endocytosis requires the coordinated assembly of a large number of proteins at the plasma membrane, the timing and composition of which are very regular (Kaksonen et al., 2005; Taylor et al., 2011). Despite the large amount of research dedicated to the understanding of this assembly sequence, the way the assembly is triggered is not well understood.
Although the budding of clathrin-coated vesicles in vitro has recently been shown to only require the presence of clathrin, an adaptor protein, and dynamin (Dannhauser and Ungewickell, 2012), endocytosis in vivo is a lot more complex. To gain a full understanding of how vesicle budding is regulated in vivo we need to determine how the initiation of this process is controlled.
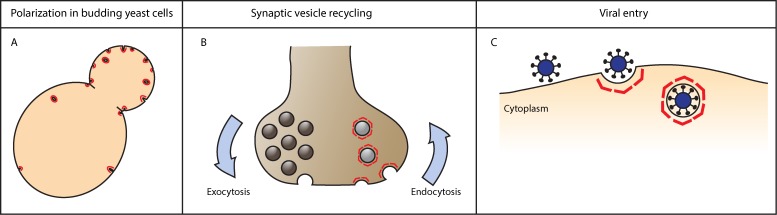
The location or timing of endocytosis can be tightly regulated, as highlighted in the following examples: In budding yeast Saccharomyces cerevisiae, a larger number of endocytic events occur in the growing bud compared with the mother cell (Fig. 1 A). This polarization of endocytosis in yeast is important for maintaining overall cellular polarity (Bi and Park, 2012). Another example is the neuronal synapse, where the level of endocytosis is tightly coupled to exocytic activity in a process called synaptic vesicle recycling, in order to maintain the pool of synaptic vesicles (Fig. 1 B; Murthy and De Camilli, 2003). Also, during entry into a host cell, viruses can exploit the mechanisms of endocytic initiation in order to induce their own uptake (Fig. 1 C; Sieczkarski and Whittaker, 2002).
Figure 1.
Examples of regulation of the initiation of endocytosis. (A) In yeast Saccharomyces cerevisiae, clathrin-mediated endocytosis is polarized to the growing bud. (B) After signal propagation at the synapse, exocytosis of the neurotransmitter is accompanied by a compensatory increase in clathrin-mediated endocytosis to maintain synaptic plasma membrane area and recycle synaptic vesicle components. (C) Many viruses enter the host cell by clathrin-mediated endocytosis. The virus particle may exploit the cell’s regulation of endocytosis in order to induce its own uptake.
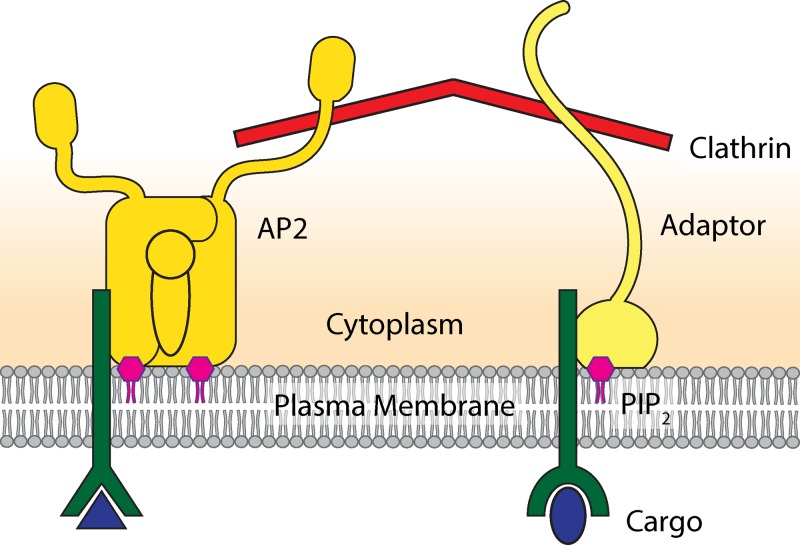
In this review we will discuss studies addressing the question of how endocytic sites are initiated. Although lots of data are available, there are a number of inconsistent results that have led to uncertainty about the mechanisms of initiation. When studying the initiation of endocytosis we need to focus on the earliest stages of the sequence of protein assembly. The key players involved in these stages are clathrin adaptors and accessory proteins, cargo, and lipids (Fig. 2). We will discuss how these components have been suggested to function in the initiation of endocytosis.
Figure 2.
The key players involved in the initiation of endocytosis. Adaptor proteins, cargo, lipids, and clathrin accumulate at the plasma membrane to make up the nascent endocytic site. Adaptor proteins, including the AP2 complex, bind to the inner leaflet of the plasma membrane. The adaptors interact with the plasma membrane via lipid-binding domains, many of which bind specifically to PIP2. The tails of adaptor proteins bind and recruit clathrin triskelions to the plasma membrane where they polymerize to form the clathrin coat. Cargo—consisting of transmembrane receptors and extracellular soluble ligands—is taken up by clathrin-coated vesicles. Adaptors bind to signaling motifs on the intracellular side of the receptors.
Adaptors: Bringing everything together
Clathrin, the main coat component of the endocytic machinery, is unable to directly interact with the lipids or proteins of the plasma membrane (Maldonado-Báez and Wendland, 2006). Therefore, adaptor proteins are required to link the clathrin coat to the membrane (see Table 1 for a summary of endocytic adaptors). These adaptors are also able to specifically bind to endocytic cargo, thereby ensuring cargo uptake into coated vesicles. Many adaptors are among the earliest proteins to arrive at the endocytic site (Taylor et al., 2011; Carroll et al., 2012), making them good candidates to play a role in initiation. Additionally, their ability to interact with other endocytic proteins could be important for recruitment of further components to the plasma membrane at the early stages of endocytosis. Although two-color live-cell microscopy has been used to arrange the endocytic process into a temporal sequence, it has proved challenging to determine one protein that consistently arrives first at the site (Kaksonen et al., 2005; Henne et al., 2010; Taylor et al., 2011; Carroll et al., 2012). Nevertheless, it is plausible that there could be one factor that is essential for the initiation of endocytosis.
Table 1.
Adaptor and accessory proteins involved in endocytosis
| Mammalian protein | Yeast homologue | Lipid-binding domain | Clathrin binding | Example of specific cargo uptake | Reference for cargo uptake |
| AP2 (α, β2, μ2, σ2) | AP2 (Apl3, Apl1, Apm4, Aps2) | α-Core domain and μ2 C-terminal domain | Yes | Transferrin receptor | Motley et al., 2003 |
| FCHo1/2 | Syp1 | F-BAR domain | – | Mid2 in yeast | Reider et al., 2009 |
| AP180/CALM | Yap1801/2 | ANTH | Yes | SNAREs | Miller et al., 2011 |
| Epsin1/2/3 | Ent1/2 | ENTH | Yes | Notch ligands | Meloty-Kapella et al., 2012 |
| Eps15 | Ede1/End3 | – | – | – | – |
| Intersectin1/2 | Sla1/Pan1 | PH/C2 | Yes (for Sla1 in yeast) | Ste2 in yeast | Howard et al., 2002 |
| HIP1/HIP1R | Sla2 | ANTH | Yes | – | – |
| Dab2 | – | PTB | Yes | LDLR | Maurer and Cooper, 2006 |
| ARH | – | PTB | – | LDLR | Maurer and Cooper, 2006 |
| Numb | – | PTB | – | Notch | Santolini et al., 2000 |
| Stonin 2 | – | – | – | Synaptotagmin | Diril et al., 2006 |
| β-Arrestin 1/2 | – | Arginine/lysine residues | Yes | GPCRs | Ferguson et al., 1996 |
ANTH, AP180 N-terminal homology; ENTH, epsin N-terminal homology; GPCR, G-protein–coupled receptor; LDLR, low-density lipoprotein receptor; PH, pleckstrin homology; PTB, phosphotyrosine binding. “–” indicates none known.
Search for the master initiator.
The heterotetrameric adaptor protein complex AP2 is one of the best-studied components of the endocytic machinery. The AP2 complex consists of four different subunits, α, β2, σ2, and μ2, which assemble into a core domain with two appendages (Fig. 2; Collins et al., 2002; Jackson et al., 2010). AP2 has multiple binding partners, including phosphatidylinositol 4,5-bisphosphate (PIP2), clathrin, several endocytic accessory proteins, and two signaling motifs present on some cargo receptors (see Traub, 2009 for a detailed review). The AP2 complex has classically been considered to be the master initiator of clathrin-mediated endocytosis through its role in recruiting clathrin molecules to the membrane. However, several lines of evidence question this idea.
If the AP2 complex has an essential role in initiation then its presence would be required for the formation of endocytic sites. However, in yeast the endocytosis of mating pheromone α-factor is unaffected in strains lacking functional AP2 (Huang et al., 1999; Yeung et al., 1999). More recently, live-cell imaging in yeast has shown that deletion of the AP2 complex has no effect on the general dynamics of endocytosis (Carroll et al., 2009). These results indicate that AP2 is not essential for the initiation of endocytosis in yeast.
In contrast, the AP2 complex is essential in animals. A lack of AP2 in both Mus musculus and Caenorhabditis elegans causes embryonic lethality (Shim and Lee, 2000; Mitsunari et al., 2005). However, this does not indicate whether AP2 is required at initiation or later during endocytosis. The role of AP2 has been studied in detail in cultured mammalian cells, but it is not clear whether AP2 depletion completely prevents the formation of endocytic sites. One study using RNAi depletion showed that, in the absence of AP2, clathrin sites still formed and contained other endocytic proteins, although these sites were smaller and less abundant (Motley et al., 2003). An alternative method to down-regulate AP2, by mutating the regulatory kinase AAK1, produced similar results (Conner and Schmid, 2003). However, another study using RNAi showed no plasma membrane clathrin sites and suggested that any cargo that is still taken up in the absence of AP2 may use a clathrin-independent pathway (Hinrichsen et al., 2003). It has also been suggested that an incomplete depletion by RNAi could mean that all remaining endocytic sites could still contain some AP2 (Boucrot et al., 2010). These discrepancies may be due to difficulties in completely depleting proteins in cell culture and challenges in imaging the very early stages of endocytosis.
To analyze the exact molecular composition of nascent endocytic sites, highly sensitive live-cell microscopy is needed to visualize the low numbers of molecules present. A pioneering single-molecule imaging study looked at the early stages of endocytosis from the point of view of AP2 and clathrin, and proposed that endocytic sites begin with the simultaneous plasma membrane binding of two AP2 molecules with one clathrin triskelion (Cocucci et al., 2012). Extending this powerful approach to other early-arriving proteins could provide important information on their relative arrival times. Other recent microscopy studies imply that AP2 may not be required at the earliest stages of endocytosis (Henne et al., 2010; Aguet et al., 2013). One study showed that AP2 arrives later than the F-BAR domain proteins FCHo1/2 to the plasma membrane (Henne et al., 2010). Another study showed a large decrease in the number of productive endocytic sites after AP2 depletion, but very low intensity, transient clathrin sites were still detected (Aguet et al., 2013). The initiation rate of these transient clathrin sites was comparable to the initiation rate of normal endocytic events in control cells. Although further work is required to determine the exact nature of these clathrin structures, these results suggest that AP2 is not required for the initiation step but rather for a later stage of endocytic progression. In summary, the importance of AP2 at the very earliest stages of endocytosis remains to be elucidated.
The requirement for AP2 in animals may also be due to its role in the uptake of specific cargo. A cargo-specific role for AP2 is seen in yeast, where it is not essential, but is required for the uptake of killer toxin (Carroll et al., 2009). In mammalian cells, depletion of AP2 subunits leads to a general block in transferrin uptake (Hinrichsen et al., 2003; Motley et al., 2003). However, a number of studies have presented conflicting results on whether AP2 depletion also blocks the uptake of other major cargos, including the low-density lipoprotein receptor (LDLR), the epidermal growth factor receptor (EGFR), and the influenza virus (Motley et al., 2003; Huang et al., 2004; Lakadamyali et al., 2006). These discrepancies may be due to the different experimental conditions in which uptake assays were performed, for example the effectiveness of the RNAi depletion and the ligand incubation conditions. If not all cargos require AP2 for their uptake it could mean that the differing requirement for AP2 between organisms is due to the types of cargo that depend on it for their uptake.
The studies described clearly support the conclusion that AP2 has an important role in the progression of clathrin-mediated endocytosis and in cargo selection. Some of the results, however, indicate that other factors in addition to AP2 are likely to contribute to the initiation of endocytosis.
Another protein that has recently been proposed to be the master initiator of endocytosis is FCHo. FCHo1 and FCHo2 are homologous proteins that contain a membrane-binding F-BAR domain that can induce membrane curvature. FCHo1/2 were shown to arrive at endocytic sites before the arrival of AP2 or clathrin (Henne et al., 2010). Crucially, upon depletion of FCHo1/2 by RNAi, there was a loss of endocytic sites marked by AP2 and clathrin patches, and uptake of transferrin was inhibited. Upon overexpression of FCHo1/2, there was a higher density of endocytic sites, a higher nucleation rate, and increased transferrin uptake. Conversely, after AP2 depletion, FCHo1/2 sites were still present at the membrane. FCHo1/2 interact and share similar assembly dynamics with two endocytic scaffold proteins, Eps15 and intersectin, which have both been shown to be important for the progression of endocytosis (Benmerah et al., 1999; Koh et al., 2007; Pechstein et al., 2010). In the absence of Eps15 and intersectin, FCHo1/2 have a diffuse localization at the plasma membrane (Henne et al., 2010), suggesting that Eps15 and intersectin cluster FCHo1/2 molecules that then initiate endocytosis, possibly by generating initial membrane bending.
However, two recent studies have questioned the role of FCHo1/2 in endocytic initiation. The first study showed that depletion of FCHo1 in zebrafish embryos leads to a developmental phenotype due to a failure in uptake of specific cargo and not due to a general endocytic defect (Umasankar et al., 2012). The second study followed fluorescent AP2 in cultured mammalian cells and in this study AP2 patches were still seen after FCHo1/2 depletion. The lifetime of these patches was drastically reduced but the initiation rate of AP2 sites was not decreased. This result indicates that FCHo1/2 may be critical for maturation of endocytic sites, but not for their initiation (Cocucci et al., 2012).
FCHo1/2 therefore may not be the sought-after essential initiator. However, these proteins may still contribute to initiation. The yeast homologues of FCHo1/2 and Eps15—Syp1 and Ede1—are also among the first proteins to arrive at the endocytic site and seem to have a role in site initiation (Carroll et al., 2012). Syp1 deletion prevents the formation of endocytic sites specifically in the yeast bud neck region, whereas Ede1 deletion decreases the number of endocytic events throughout the cell (Stimpson et al., 2009). Ede1 deletion also leads to a diffuse plasma membrane localization of many of the other early-arriving endocytic proteins (Reider et al., 2009; Stimpson et al., 2009; Carroll et al., 2012). These results suggest that both Syp1 and Ede1 can promote initiation of endocytosis: Syp1 locally at the bud neck and Ede1 globally. However, as endocytosis still proceeds in the absence of these proteins, neither of them are essential for initiation. Taken together, these results point to the idea that no single protein is essential for the initiation of endocytosis but rather a number of proteins share the initiation function.
Redundancy in initiation?
The function of an endocytic adaptor depends on its ability to bind to the plasma membrane and recruit both clathrin and cargo. Various in vitro studies have shown that clathrin can be recruited to a membrane by a number of different adaptors other than the AP2 complex. The adaptor proteins AP180 and epsin are able to recruit clathrin lattices to a synthetic lipid monolayer (Ford et al., 2001, 2002). In fact, linking clathrin to the membrane using a nickel-chelating lipid and a His-tagged epsin fragment, lacking the PIP2-binding domain but containing clathrin-binding motifs, is all that is needed for clathrin lattices to assemble and drive vesicle budding in vitro (Dannhauser and Ungewickell, 2012). These results suggest that any adaptor that can support clathrin lattice assembly might be able to initiate endocytosis. Increasing numbers of endocytic proteins, which are able to bind to both clathrin and the plasma membrane, are being defined as clathrin adaptors (see Table 1). Some of these adaptors also bind to, and therefore may work in cooperation with, AP2. In the case of two LDLR adaptors, ARH requires the presence of AP2 but Dab2 functions independently of AP2 (Keyel et al., 2006; Maurer and Cooper, 2006; Mettlen et al., 2010). In addition, clathrin-mediated endocytosis of Notch ligand is dependent on the alternative clathrin adaptors, epsins and CALM, but not AP2 (Meloty-Kapella et al., 2012). Interestingly, it has been shown that there is no competition for uptake in the case of some distinct cargos, even though the internalization of one specific cargo can be saturated (Warren et al., 1997). This suggests that different cargo can depend on different clathrin adaptors, which can function independently in mediating endocytosis. It is possible that a variety of adaptors are able to initiate endocytosis with different efficiencies.
Can cargo initiate its own endocytosis?
The main function of clathrin-mediated endocytosis is to traffic cargo—transmembrane receptors and their extracellular ligands—from the plasma membrane into the cell. It is therefore reasonable to hypothesize that cargo molecules might regulate their own uptake under certain conditions, by initiating the assembly of the endocytic machinery. To answer this question, several studies have visualized whether cargo is present at the plasma membrane site before or after the endocytic machinery.
The clearest experimental evidence for cargo-initiated endocytic assembly comes from studies in which the cargo is a viral particle entering the host cell by endocytosis. Viruses have multiple receptor binding sites, allowing them to bind to, and therefore cluster, a number of receptors at the cell surface. This ability could be a mechanism to induce the assembly of the endocytic machinery, allowing the virus particle to force its own entry into the cell. Both influenza and vesicular stomatitis virus were shown to stably bind to the cell surface before the first detection of a colocalized clathrin signal (Rust et al., 2004; Cureton et al., 2009). The formation rate of new clathrin-coated pits at sites of virus binding was higher than elsewhere on the membrane. These viruses therefore seem to be able to induce the nucleation of clathrin sites. However, not all viruses have this effect on the endocytic machinery. Reovirus particles were also shown to bind to the plasma membrane before the appearance of clathrin, but in this case the colocalized assembly of a clathrin site was probably due to random initiation rather than induction by the virus (Ehrlich et al., 2004). Other viruses including canine parvovirus and dengue virus have been shown to diffuse randomly on the cell surface until they join a preexisting clathrin site (van der Schaar et al., 2008; Cureton et al., 2012). These differences in methods of viral uptake may depend on the receptor-binding capability of different viruses.
Viral uptake by endocytosis is an atypical situation, so these results do not necessarily apply to normal physiological cargo. In mammalian cells fluorescently labeled ligands, transferrin and LDL, were shown to diffuse randomly on the cell surface until they reached stationary, preexisting clathrin sites (Ehrlich et al., 2004). Upon colocalization with clathrin the ligands remained stationary while the clathrin signal increased, then both signals disappeared simultaneously. Equally in yeast, tracking of fluorescently labeled α-factor revealed that it too was incorporated into preexisting sites (Toshima et al., 2006, 2009). These physiological cargos seem to rely on passive diffusion to a preformed site.
Transferrin and LDL receptors are constitutive cargos, which are also taken up in the absence of their ligands. Some receptors, including EGFR, nerve growth factor receptor (NGFR), and G protein–coupled receptors (GPCRs) require ligand binding for their recruitment into clathrin-coated vesicles. A number of studies on EGF and NGF signaling report that, upon addition of these ligands, there is an increase in recruitment of clathrin and other accessory proteins to the membrane, a larger number of clathrin-coated sites, and an increase in uptake by endocytosis (Connolly et al., 1981; Wilde et al., 1999; Beattie et al., 2000; Johannessen et al., 2006). However, it was not clear whether ligand-induced signaling resulted in more endocytic sites assembling specifically at the position of activated cargo or in a general increase in endocytosis. Live-cell imaging of fluorescently tagged EGFR and GPCRs showed that the addition of ligand resulted in the clustering of previously diffusely localized receptors. However, these receptors were then incorporated into clathrin sites that were present before signaling (Santini et al., 2002; Scott et al., 2002; Rappoport and Simon, 2009).
The suggestion that cargo is recruited to preformed sites would mean that the initiation of endocytosis is independent of cargo recruitment. This idea is strengthened by the fact that all endocytic sites have the same assembly dynamics in the first stages (Mettlen et al., 2010). This could mean that normal cargo molecules are passive players in their own uptake. However, cargo has been shown to affect endocytosis later in the process: for example, the size of endocytic sites, maturation rate, productivity, and downstream pathways (Lakadamyali et al., 2006; Puthenveedu and von Zastrow, 2006; Soohoo and Puthenveedu, 2013).
Other studies have aimed to determine whether an increased amount of cargo can alter the rate of endocytic initiation. Overexpression of the transferrin receptor using an inducible promoter did not increase the initiation of new endocytic sites, nor did it increase the membrane recruitment of clathrin or AP2 (Loerke et al., 2009). Overexpression of LDLR, on the other hand, increased the membrane recruitment of its adaptor proteins Dab2 and ARH and the initiation rate of new sites (Mettlen et al., 2010). These results suggest that the ability to influence endocytic initiation could be cargo dependent and differences in cargo content could affect endocytic dynamics.
Although overexpression of receptors can lead to an increase in their concentration at the plasma membrane, they may remain diffusely localized. A recent study used a more specific technique to induce the local clustering of transferrin receptors. Fluorescent streptavidin was used to multimerize biotinylated transferrin receptors and this method did lead to an increase in clathrin site density and initiation rate (Liu et al., 2010). Additionally clathrin was shown to assemble at a site already marked by the clustered cargo.
Taken together, although cargo-regulated signaling may cause an increase in the rate of initiation, it seems that many cargos enter the endocytic pathway by diffusing into preformed endocytic sites. The clustering of receptors at the plasma membrane, which could locally concentrate endocytic adaptors on the cytosolic side, is a potential mechanism for initiating endocytosis. Some viral cargos can initiate the assembly of endocytic sites by this mechanism. Whether or not physiological cargos can control their own uptake in a similar manner remains to be clarified.
A role for lipids?
The assembly of the endocytic machinery within the cell is restricted to the inner leaflet of the plasma membrane. Lipids are known to be important at different stages of clathrin-mediated endocytosis, but our understanding of the specific functions of different lipids is still quite limited, due to the difficulties in visualizing and manipulating the levels of specific lipids. Many of the early-arriving proteins including AP2, epsins, and AP180 have domains that bind to PIP2, a lipid enriched in the plasma membrane (Table 1). PIP2 is the best-studied lipid in endocytosis and it is known to be important during the early stages (Jost et al., 1998; Malecz et al., 2000).
Incubation of cells with butanol or ionomycin causes a general decrease in PIP2 levels (Boucrot et al., 2006; Zoncu et al., 2007). These treatments disrupt endocytosis, as can be seen by a decrease in uptake of transferrin, the disassembly of endocytic sites, marked by AP2 and clathrin, and the inhibition of the formation of new sites (Boucrot et al., 2006; Zoncu et al., 2007). To affect the levels of PIP2 in a more specific manner Antonescu et al. (2011) used siRNA knockdown and overexpression of inositol 5-phosphate kinases. They found that the synthesis of PIP2 by a PIP 5-kinase is important for initiation of sites, whereas its turnover by an inositol 5-phosphatase is important during a later stage of vesicle budding. The phosphatase was shown to accumulate at endocytic sites but there was no accumulation of PIP2 or the PIP 5-kinase, suggesting that general PIP2 levels are needed for initial clathrin site assembly and a more localized PIP2 turnover controls later stages in maturation.
An even more controlled method for reducing the levels of PIP2 involves the anchoring of an inositol 5-phoshatase to the plasma membrane by light- or drug-induced heterodimerization (Zoncu et al., 2007; Abe et al., 2008; Idevall-Hagren et al., 2012). Both methods caused an acute decrease in PIP2 levels at the plasma membrane. This was almost immediately followed by a relocalization of AP2 to the cytoplasm, which resulted in a block in transferrin uptake. These results clearly show that PIP2 is required for AP2 localization. However, these studies on PIP2 depletion reported different effects on clathrin assembly. In one study the number of clathrin structures was reduced, and those that were left were smaller and mainly stable over time (Zoncu et al., 2007). In another, the dynamic assembly of clathrin at the plasma membrane was only weakly affected (Abe et al., 2008). In this case initiation of new clathrin sites was not inhibited and the intensity and turnover of sites was relatively unaffected. These conflicting results could be due to differences in the degree of PIP2 depletion and varied sensitivities of proteins to PIP2 concentrations. This discrepancy also echoes the differences seen in the previously discussed AP2 depletion studies. PIP2 is clearly required for AP2 recruitment and possibly for the recruitment of other PIP2-binding adaptors or accessory proteins. What remains unresolved is whether AP2 or any other adaptors are essential for the initiation of endocytosis or whether redundancy in function means that endocytosis can proceed in the absence of any specific adaptor protein.
Although much of the work on the role of lipids in endocytosis has focused on PIP2, other lipids, including phosphatidic acid (Antonescu et al., 2010) and phosphatidylserine (PS; Sun and Drubin, 2012) have been suggested to play a role. A recent study focusing on the differing functions of PIP2 and PS in yeast showed that PIP2 was not needed for the formation of endocytic sites, but rather at a later stage in vesicle budding (Sun and Drubin, 2012). Endocytic sites could form at the plasma membrane in PIP2-depleted yeast cells. However, these sites had much longer lifetimes and final vesicle budding was inhibited. The fact that many proteins that have PIP2-binding domains, including the AP2 complex, were still able to bind to PIP2-deficient membranes implies that there may be another mechanism to recruit these proteins. Sun and Drubin (2012) went on to show that in a PS-deficient strain the number of endocytic sites was reduced and the polarization of endocytic sites was lost. This suggests a role for PS in the recruitment and localization of endocytic sites. This is consistent with the proposal that the negative charge of PS contributes to the recruitment of positively charged proteins to the endocytic pathway (Yeung et al., 2008). Interestingly, however, endocytic sites were still present when both PS and PIP2 were depleted, indicating that other lipids may have a role in the initiation of endocytosis.
The presence of specific lipids, in particular PIP2, is clearly critical for endocytosis. What is not yet understood is whether these lipids are able to regulate the initiation of endocytosis. Lipids can diffuse relatively freely in the plasma membrane and might therefore be unable to provide spatial or temporal information for the control of endocytosis. On the other hand, specific lipid compositions in microdomains or over concentration gradients may be able to regulate the location or rate of initiation. The possibility of a regulatory role for lipids in initiation is an interesting area for further research.
The elusive mechanisms of initiation
So far, no clear consensus has emerged from the studies addressing the mechanisms of endocytic site initiation. Adaptor and accessory proteins clearly have an important role in initiation, as they are able to recruit clathrin and the other endocytic components to the plasma membrane. Although FCHo1/2 and AP2 have both been proposed to be the essential master initiator of endocytosis, it may be that a variety of adaptor proteins are able to initiate endocytosis. But what are the actual molecular mechanisms that initiate endocytic events? We summarize here a number of different models that have been proposed in the studies discussed.
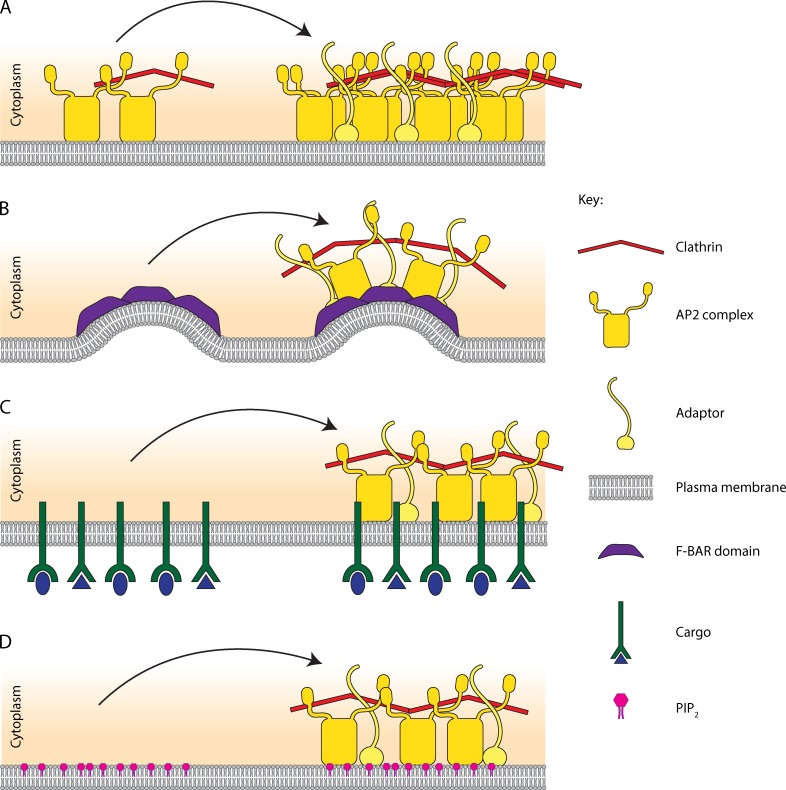
One possible model is that endocytosis begins with stochastic association of adaptors at the membrane. The transient clustering of a few membrane-bound adaptors could, if stable for long enough, act as a nucleus that would recruit further endocytic components. This model suggests that adaptor proteins are constantly sampling the plasma membrane and that endocytic sites initiate randomly—an idea supported by data showing the random spatial distribution of endocytic sites (Ehrlich et al., 2004). Cocucci et al. (2012) suggested that the assembly of two AP2 complexes with a clathrin triskelion is enough to nucleate an endocytic site (Fig. 3 A). This stochastic assembly model could also easily accommodate many different adaptors, which could form a nucleus either independently or together with AP2 and scaffold proteins.
Figure 3.
Possible mechanisms for the initiation of endocytosis. (A) Stochastic interactions of adaptors and lipids at the plasma membrane can form a nucleus that recruits further endocytic components. One such assembly that has been proposed is two AP2 complexes along with a clathrin triskelion (Cocucci et al., 2012). (B) Membrane bending may be required or aid in the initiation of endocytosis. Protein domains such as the F-BAR domain have been suggested to induce membrane bending as a mechanism for initiating endocytic site formation (Henne et al., 2010). (C) Clustering of cargo molecules has been shown to be able to induce the assembly of endocytic components de novo (Liu et al., 2010). (D) Specific lipids forming a lipid gradient or at a membrane domain could increase the recruitment of adaptors to the plasma membrane, leading to increased initiation of endocytosis.
Additionally, the establishment of membrane bending could be the rate-limiting step for the formation of the initial nucleus (Fig. 3 B; Henne et al., 2010). Many endocytic proteins have the potential ability to induce membrane curvature, and this ability has been demonstrated for some in vitro (Ford et al., 2002; Henne et al., 2010). In addition to the F-BAR domains of FCHo1/2 proteins, other membrane-bending mechanisms include insertion of an amphipathic helix, for example that of the ENTH domain of epsin (Ford et al., 2002), spontaneous membrane curvature induced by lipid composition (Zimmerberg and Kozlov, 2006), protein crowding (Stachowiak et al., 2012), and recruitment of the clathrin scaffold (Dannhauser and Ungewickell, 2012; see Kirchhausen, 2012 for detailed review). However, recent work on the ultrastructure of endocytosis has shown that endocytic assembly in yeast cells is initiated on a flat plasma membrane and that membrane bending occurs much later in the assembly pathway (Kukulski et al., 2012). This finding shows that membrane bending, although essential for endocytosis, can be uncoupled from the initiation of an endocytic site.
Besides from the stochastic interaction of adaptors or accessory proteins with the plasma membrane, it may be possible that the recruitment of adaptors to the membrane is regulated in some way. Receptor signaling of the EGFR was followed by an increase in the recruitment of endocytic components to the membrane (Johannessen et al., 2006). An increase in phosphorylation of clathrin was seen alongside this response, leading to the suggestion that it was mediated by a kinase signaling pathway (Wilde et al., 1999).
Furthermore, the recruitment of adaptors to the membrane may be increased by high local concentrations of cargo receptors (Fig. 3 C) or specific lipids (Fig. 3 D). Local clustering of receptors seems to increase adaptor recruitment (Liu et al., 2010)—a mechanism that could be used by viruses to ensure their own uptake into the host cell (Fig. 1 C). PIP2 and PS gradients have been demonstrated between mother and daughter cells in yeast (Garrenton et al., 2010; Fairn et al., 2011; Vernay et al., 2012) and it is possible that these gradients contribute to the polarization of endocytic sites in the growing daughter cell (Fig. 1 A).
Finally, it is possible that other membrane components are able to recruit endocytic adaptor proteins. This suggestion could explain the presence of so-called hotspots that have been seen in mammalian cells, where multiple endocytic events bud from one position (Nunez et al., 2011). These hotspots could be composed of endocytic proteins, which are retained at the plasma membrane after vesicle budding, and can subsequently recruit a new round of endocytosis. In a similar mechanism, during synaptic vesicle recycling, components of exocytic vesicles delivered to the plasma membrane after fusion may be able to recruit the endocytic machinery (Fig. 1 B).
Conclusions and future directions
Clearly, more work is needed in order to gain a full understanding of how endocytic events initiate. Some inconsistencies in the literature may have arisen from technical limitations, such as the variability in the effectiveness of depletion by RNAi and the level of overexpression of fluorescently labeled proteins. Another challenge in the study of endocytic initiation has been the visualization of the low levels of protein present at the earliest stages of endocytosis. Improvements in imaging and image analysis have allowed visualization at single-molecule sensitivity (Cocucci et al., 2012). This is an important advancement and needs to be applied to further adaptor, accessory, and cargo proteins to give an exact picture of the composition of the nascent endocytic site. Additionally, improvements in methods for visualizing and altering lipid composition may give new insight into the roles of lipids. Given the potential variability between different sites at the initiation step, it is important to observe individual endocytic events rather than rely on bulk measurements. Also, a distinction has to be made between roles in initiation and later stages of maturation, as end point measurements are hard to interpret in the context of initiation. This point has proved critical in the study of both the AP2 complex and FCHo1/2 proteins, which are both essential for productive endocytosis in mammalian cells and have both been proposed to have a key role in initiation. Recently, advanced microscopy techniques have indicated that after the depletion of either AP2 or FCHo1/2 short-lived endocytic sites are still present with the same rate of initiation, leading to the question of which stage of endocytosis these proteins are essential at (Cocucci et al., 2012; Aguet et al., 2013). Yeast genetics provide a powerful tool for genome manipulation and recent technical advances have allowed the introduction of similar approaches for mammalian cell lines (Doyon et al., 2011). This could reduce our reliance on the study of exogenously overexpressed fluorescently tagged proteins and provide unambiguous gene deletions.
There is a certain level of confusion surrounding our current understanding of endocytic initiation, but perhaps this reflects the true nature of the process. Although the assembly of the endocytic machinery and subsequent vesicle budding proceeds very regularly, it may be that there is more than one way to initiate the process. We would like to propose a working hypothesis that a number of different factors, including adaptor proteins, cargo, and lipids are able to promote the nucleation of an endocytic site. These factors may act synergistically and in different combinations and therefore provide many different levels of regulation. This could mean that, depending on the circumstances, individual endocytic sites could be initiated by different mechanisms, ensuring flexibility in different physiological conditions and over evolution.
Acknowledgments
We thank Michal Skruzny and Dominik Boeke for critical reading of the manuscript and all the members of our laboratory for interesting discussions.
Footnotes
Abbreviations used in this paper:
- EGFR
- epidermal growth factor receptor
- GPCR
- G protein–coupled receptor
- LDLR
- low-density lipoprotein receptor
- NGFR
- nerve growth factor receptor
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- PS
- phosphatidylserine
References
- Abe N., Inoue T., Galvez T., Klein L., Meyer T. 2008. Dissecting the role of PtdIns(4,5)P2 in endocytosis and recycling of the transferrin receptor. J. Cell Sci. 121:1488–1494 10.1242/jcs.020792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguet F., Antonescu C.N., Mettlen M., Schmid S.L., Danuser G. 2013. Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev. Cell. 26:279–291 10.1016/j.devcel.2013.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonescu C.N., Danuser G., Schmid S.L. 2010. Phosphatidic acid plays a regulatory role in clathrin-mediated endocytosis. Mol. Biol. Cell. 21:2944–2952 10.1091/mbc.E10-05-0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonescu C.N., Aguet F., Danuser G., Schmid S.L. 2011. Phosphatidylinositol-(4,5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size. Mol. Biol. Cell. 22:2588–2600 10.1091/mbc.E11-04-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie E.C., Howe C.L., Wilde A., Brodsky F.M., Mobley W.C. 2000. NGF signals through TrkA to increase clathrin at the plasma membrane and enhance clathrin-mediated membrane trafficking. J. Neurosci. 20:7325–7333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A., Bayrou M., Cerf-Bensussan N., Dautry-Varsat A. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303–1311 [DOI] [PubMed] [Google Scholar]
- Bi E., Park H.-O. 2012. Cell polarization and cytokinesis in budding yeast. Genetics. 191:347–387 10.1534/genetics.111.132886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucrot E., Saffarian S., Massol R., Kirchhausen T., Ehrlich M. 2006. Role of lipids and actin in the formation of clathrin-coated pits. Exp. Cell Res. 312:4036–4048 10.1016/j.yexcr.2006.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucrot E., Saffarian S., Zhang R., Kirchhausen T. 2010. Roles of AP-2 in clathrin-mediated endocytosis. PLoS ONE. 5:e10597 10.1371/journal.pone.0010597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S.Y., Stirling P.C., Stimpson H.E.M., Giesselmann E., Schmitt M.J., Drubin D.G. 2009. A yeast killer toxin screen provides insights into a/b toxin entry, trafficking, and killing mechanisms. Dev. Cell. 17:552–560 10.1016/j.devcel.2009.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S.Y., Stimpson H.E.M., Weinberg J., Toret C.P., Sun Y., Drubin D.G. 2012. Analysis of yeast endocytic site formation and maturation through a regulatory transition point. Mol. Biol. Cell. 23:657–668 10.1091/mbc.E11-02-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E., Aguet F., Boulant S., Kirchhausen T. 2012. The first five seconds in the life of a clathrin-coated pit. Cell. 150:495–507 10.1016/j.cell.2012.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B.M., McCoy A.J., Kent H.M., Evans P.R., Owen D.J. 2002. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 109:523–535 10.1016/S0092-8674(02)00735-3 [DOI] [PubMed] [Google Scholar]
- Conner S.D., Schmid S.L. 2003. Differential requirements for AP-2 in clathrin-mediated endocytosis. J. Cell Biol. 162:773–779 10.1083/jcb.200304069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J.L., Green S.A., Greene L.A. 1981. Pit formation and rapid changes in surface morphology of sympathetic neurons in response to nerve growth factor. J. Cell Biol. 90:176–180 10.1083/jcb.90.1.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cureton D.K., Massol R.H., Saffarian S., Kirchhausen T.L., Whelan S.P.J. 2009. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog. 5:e1000394 10.1371/journal.ppat.1000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cureton D.K., Harbison C.E., Cocucci E., Parrish C.R., Kirchhausen T. 2012. Limited transferrin receptor clustering allows rapid diffusion of canine parvovirus into clathrin endocytic structures. J. Virol. 86:5330–5340 10.1128/JVI.07194-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannhauser P.N., Ungewickell E.J. 2012. Reconstitution of clathrin-coated bud and vesicle formation with minimal components. Nat. Cell Biol. 14:634–639 10.1038/ncb2478 [DOI] [PubMed] [Google Scholar]
- Diril M.K., Wienisch M., Jung N., Klingauf J., Haucke V. 2006. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev. Cell. 10:233–244 10.1016/j.devcel.2005.12.011 [DOI] [PubMed] [Google Scholar]
- Doyon J.B., Zeitler B., Cheng J., Cheng A.T., Cherone J.M., Santiago Y., Lee A.H., Vo T.D., Doyon Y., Miller J.C., et al. 2011. Rapid and efficient clathrin-mediated endocytosis revealed in genome-edited mammalian cells. Nat. Cell Biol. 13:331–337 10.1038/ncb2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M., Boll W., Van Oijen A., Hariharan R., Chandran K., Nibert M.L., Kirchhausen T. 2004. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 118:591–605 10.1016/j.cell.2004.08.017 [DOI] [PubMed] [Google Scholar]
- Fairn G.D., Hermansson M., Somerharju P., Grinstein S. 2011. Phosphatidylserine is polarized and required for proper Cdc42 localization and for development of cell polarity. Nat. Cell Biol. 13:1424–1430 10.1038/ncb2351 [DOI] [PubMed] [Google Scholar]
- Ferguson S.S.G., Downey W.E., III, Colapietro A.-M., Barak L.S., Ménard L., Caron M.G. 1996. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 271:363–366 10.1126/science.271.5247.363 [DOI] [PubMed] [Google Scholar]
- Ford M.G.J., Pearse B.M., Higgins M.K., Vallis Y., Owen D.J., Gibson A., Hopkins C.R., Evans P.R., McMahon H.T. 2001. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 291:1051–1055 10.1126/science.291.5506.1051 [DOI] [PubMed] [Google Scholar]
- Ford M.G.J., Mills I.G., Peter B.J., Vallis Y., Praefcke G.J.K., Evans P.R., McMahon H.T. 2002. Curvature of clathrin-coated pits driven by epsin. Nature. 419:361–366 10.1038/nature01020 [DOI] [PubMed] [Google Scholar]
- Garrenton L.S., Stefan C.J., McMurray M.A., Emr S.D., Thorner J. 2010. Pheromone-induced anisotropy in yeast plasma membrane phosphatidylinositol-4,5-bisphosphate distribution is required for MAPK signaling. Proc. Natl. Acad. Sci. USA. 107:11805–11810 10.1073/pnas.1005817107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne W.M., Boucrot E., Meinecke M., Evergren E., Vallis Y., Mittal R., McMahon H.T. 2010. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 328:1281–1284 10.1126/science.1188462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen L., Harborth J., Andrees L., Weber K., Ungewickell E.J. 2003. Effect of clathrin heavy chain- and alpha-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J. Biol. Chem. 278:45160–45170 10.1074/jbc.M307290200 [DOI] [PubMed] [Google Scholar]
- Howard J.P., Hutton J.L., Olson J.M., Payne G.S. 2002. Sla1p serves as the targeting signal recognition factor for NPFX(1,2)D-mediated endocytosis. J. Cell Biol. 157:315–326 10.1083/jcb.200110027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.M., D’Hondt K., Riezman H., Lemmon S.K. 1999. Clathrin functions in the absence of heterotetrameric adaptors and AP180-related proteins in yeast. EMBO J. 18:3897–3908 10.1093/emboj/18.14.3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Khvorova A., Marshall W., Sorkin A. 2004. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J. Biol. Chem. 279:16657–16661 10.1074/jbc.C400046200 [DOI] [PubMed] [Google Scholar]
- Idevall-Hagren O., Dickson E.J., Hille B., Toomre D.K., De Camilli P. 2012. Optogenetic control of phosphoinositide metabolism. Proc. Natl. Acad. Sci. USA. 109:E2316–E2323 10.1073/pnas.1211305109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.P., Kelly B.T., McCoy A.J., Gaffry T., James L.C., Collins B.M., Höning S., Evans P.R., Owen D.J. 2010. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell. 141:1220–1229 10.1016/j.cell.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen L.E., Pedersen N.M., Pedersen K.W., Madshus I.H., Stang E. 2006. Activation of the epidermal growth factor (EGF) receptor induces formation of EGF receptor- and Grb2-containing clathrin-coated pits. Mol. Cell. Biol. 26:389–401 10.1128/MCB.26.2.389-401.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M., Simpson F., Kavran J.M., Lemmon M.A., Schmid S.L. 1998. Phosphatidylinositol-4,5-bisphosphate is required for endocytic coated vesicle formation. Curr. Biol. 8:1399–1402 10.1016/S0960-9822(98)00022-0 [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C.P., Drubin D.G. 2005. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 123:305–320 10.1016/j.cell.2005.09.024 [DOI] [PubMed] [Google Scholar]
- Keyel P.A., Mishra S.K., Roth R., Heuser J.E., Watkins S.C., Traub L.M. 2006. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol. Biol. Cell. 17:4300–4317 10.1091/mbc.E06-05-0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. 2012. Bending membranes. Nat. Cell Biol. 14:906–908 10.1038/ncb2570 [DOI] [PubMed] [Google Scholar]
- Koh T.-W., Korolchuk V.I., Wairkar Y.P., Jiao W., Evergren E., Pan H., Zhou Y., Venken K.J.T., Shupliakov O., Robinson I.M., et al. 2007. Eps15 and Dap160 control synaptic vesicle membrane retrieval and synapse development. J. Cell Biol. 178:309–322 10.1083/jcb.200701030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukulski W., Schorb M., Kaksonen M., Briggs J.A.G. 2012. Plasma membrane reshaping during endocytosis is revealed by time-resolved electron tomography. Cell. 150:508–520 10.1016/j.cell.2012.05.046 [DOI] [PubMed] [Google Scholar]
- Lakadamyali M., Rust M.J., Zhuang X. 2006. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 124:997–1009 10.1016/j.cell.2005.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A.P., Aguet F., Danuser G., Schmid S.L. 2010. Local clustering of transferrin receptors promotes clathrin-coated pit initiation. J. Cell Biol. 191:1381–1393 10.1083/jcb.201008117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loerke D., Mettlen M., Yarar D., Jaqaman K., Jaqaman H., Danuser G., Schmid S.L. 2009. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 7:e57 10.1371/journal.pbio.1000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Báez L., Wendland B. 2006. Endocytic adaptors: recruiters, coordinators and regulators. Trends Cell Biol. 16:505–513 10.1016/j.tcb.2006.08.001 [DOI] [PubMed] [Google Scholar]
- Malecz N., McCabe P.C., Spaargaren C., Qiu R., Chuang Y., Symons M. 2000. Synaptojanin 2, a novel Rac1 effector that regulates clathrin-mediated endocytosis. Curr. Biol. 10:1383–1386 10.1016/S0960-9822(00)00778-8 [DOI] [PubMed] [Google Scholar]
- Maurer M.E., Cooper J.A. 2006. The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J. Cell Sci. 119:4235–4246 10.1242/jcs.03217 [DOI] [PubMed] [Google Scholar]
- Meloty-Kapella L., Shergill B., Kuon J., Botvinick E., Weinmaster G. 2012. Notch ligand endocytosis generates mechanical pulling force dependent on dynamin, epsins, and actin. Dev. Cell. 22:1299–1312 10.1016/j.devcel.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettlen M., Loerke D., Yarar D., Danuser G., Schmid S.L. 2010. Cargo- and adaptor-specific mechanisms regulate clathrin-mediated endocytosis. J. Cell Biol. 188:919–933 10.1083/jcb.200908078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.E., Sahlender D.A., Graham S.C., Höning S., Robinson M.S., Peden A.A., Owen D.J. 2011. The molecular basis for the endocytosis of small R-SNAREs by the clathrin adaptor CALM. Cell. 147:1118–1131 10.1016/j.cell.2011.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunari T., Nakatsu F., Shioda N., Love P.E., Grinberg A., Bonifacino J.S., Ohno H. 2005. Clathrin adaptor AP-2 is essential for early embryonal development. Mol. Cell. Biol. 25:9318–9323 10.1128/MCB.25.21.9318-9323.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A., Bright N.A., Seaman M.N.J., Robinson M.S. 2003. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162:909–918 10.1083/jcb.200305145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy V.N., De Camilli P. 2003. Cell biology of the presynaptic terminal. Annu. Rev. Neurosci. 26:701–728 10.1146/annurev.neuro.26.041002.131445 [DOI] [PubMed] [Google Scholar]
- Nunez D., Antonescu C., Mettlen M., Liu A., Schmid S.L., Loerke D., Danuser G. 2011. Hotspots organize clathrin-mediated endocytosis by efficient recruitment and retention of nucleating resources. Traffic. 12:1868–1878 10.1111/j.1600-0854.2011.01273.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechstein A., Bacetic J., Vahedi-Faridi A., Gromova K., Sundborger A., Tomlin N., Krainer G., Vorontsova O., Schäfer J.G., Owe S.G., et al. 2010. Regulation of synaptic vesicle recycling by complex formation between intersectin 1 and the clathrin adaptor complex AP2. Proc. Natl. Acad. Sci. USA. 107:4206–4211 10.1073/pnas.0911073107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu M.A., von Zastrow M. 2006. Cargo regulates clathrin-coated pit dynamics. Cell. 127:113–124 10.1016/j.cell.2006.08.035 [DOI] [PubMed] [Google Scholar]
- Rappoport J.Z., Simon S.M. 2009. Endocytic trafficking of activated EGFR is AP-2 dependent and occurs through preformed clathrin spots. J. Cell Sci. 122:1301–1305 10.1242/jcs.040030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reider A., Barker S.L., Mishra S.K., Im Y.J., Maldonado-Báez L., Hurley J.H., Traub L.M., Wendland B. 2009. Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation. EMBO J. 28:3103–3116 10.1038/emboj.2009.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust M.J., Lakadamyali M., Zhang F., Zhuang X. 2004. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat. Struct. Mol. Biol. 11:567–573 10.1038/nsmb769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini F., Gaidarov I., Keen J.H. 2002. G protein-coupled receptor/arrestin3 modulation of the endocytic machinery. J. Cell Biol. 156:665–676 10.1083/jcb.200110132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolini E., Puri C., Salcini A.E., Gagliani M.C., Pelicci P.G., Tacchetti C., Di Fiore P.P. 2000. Numb is an endocytic protein. J. Cell Biol. 151:1345–1352 10.1083/jcb.151.6.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M.G.H., Benmerah A., Muntaner O., Marullo S. 2002. Recruitment of activated G protein-coupled receptors to pre-existing clathrin-coated pits in living cells. J. Biol. Chem. 277:3552–3559 10.1074/jbc.M106586200 [DOI] [PubMed] [Google Scholar]
- Shim J., Lee J. 2000. Molecular genetic analysis of apm-2 and aps-2, genes encoding the medium and small chains of the AP-2 clathrin-associated protein complex in the nematode Caenorhabditis elegans. Mol. Cells. 10:309–316 [PubMed] [Google Scholar]
- Sieczkarski S.B., Whittaker G.R. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535–1545 [DOI] [PubMed] [Google Scholar]
- Soohoo A.L., Puthenveedu M.A. 2013. Divergent modes for cargo-mediated control of clathrin-coated pit dynamics. Mol. Biol. Cell. 24:1725–1734: S1–S12 10.1091/mbc.E12-07-0550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak J.C., Schmid E.M., Ryan C.J., Ann H.S., Sasaki D.Y., Sherman M.B., Geissler P.L., Fletcher D.A., Hayden C.C. 2012. Membrane bending by protein-protein crowding. Nat. Cell Biol. 14:944–949 10.1038/ncb2561 [DOI] [PubMed] [Google Scholar]
- Stimpson H.E.M., Toret C.P., Cheng A.T., Pauly B.S., Drubin D.G. 2009. Early-arriving Syp1p and Ede1p function in endocytic site placement and formation in budding yeast. Mol. Biol. Cell. 20:4640–4651 10.1091/mbc.E09-05-0429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Drubin D.G. 2012. The functions of anionic phospholipids during clathrin-mediated endocytosis site initiation and vesicle formation. J. Cell Sci. 125:6157–6165 10.1242/jcs.115741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.J., Perrais D., Merrifield C.J. 2011. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 9:e1000604 10.1371/journal.pbio.1000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima J.Y., Toshima J., Kaksonen M., Martin A.C., King D.S., Drubin D.G. 2006. Spatial dynamics of receptor-mediated endocytic trafficking in budding yeast revealed by using fluorescent alpha-factor derivatives. Proc. Natl. Acad. Sci. USA. 103:5793–5798 10.1073/pnas.0601042103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima J.Y., Nakanishi J., Mizuno K., Toshima J., Drubin D.G. 2009. Requirements for recruitment of a G protein-coupled receptor to clathrin-coated pits in budding yeast. Mol. Biol. Cell. 20:5039–5050 10.1091/mbc.E09-07-0541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L.M. 2009. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 10:583–596 10.1038/nrm2751 [DOI] [PubMed] [Google Scholar]
- Umasankar P.K., Sanker S., Thieman J.R., Chakraborty S., Wendland B., Tsang M., Traub L.M. 2012. Distinct and separable activities of the endocytic clathrin-coat components Fcho1/2 and AP-2 in developmental patterning. Nat. Cell Biol. 14:488–501 10.1038/ncb2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaar H.M., Rust M.J., Chen C., van der Ende-Metselaar H., Wilschut J., Zhuang X., Smit J.M. 2008. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog. 4:e1000244 10.1371/journal.ppat.1000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernay A., Schaub S., Guillas I., Bassilana M., Arkowitz R.A. 2012. A steep phosphoinositide bis-phosphate gradient forms during fungal filamentous growth. J. Cell Biol. 198:711–730 10.1083/jcb.201203099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R.A., Green F.A., Enns C.A. 1997. Saturation of the endocytic pathway for the transferrin receptor does not affect the endocytosis of the epidermal growth factor receptor. J. Biol. Chem. 272:2116–2121 10.1074/jbc.272.4.2116 [DOI] [PubMed] [Google Scholar]
- Wilde A., Beattie E.C., Lem L., Riethof D.A., Liu S.H., Mobley W.C., Soriano P., Brodsky F.M. 1999. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 96:677–687 10.1016/S0092-8674(00)80578-4 [DOI] [PubMed] [Google Scholar]
- Yeung B.G., Phan H.L., Payne G.S. 1999. Adaptor complex-independent clathrin function in yeast. Mol. Biol. Cell. 10:3643–3659 10.1091/mbc.10.11.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T., Gilbert G.E., Shi J., Silvius J., Kapus A., Grinstein S. 2008. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 319:210–213 10.1126/science.1152066 [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Kozlov M.M. 2006. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 7:9–19 10.1038/nrm1784 [DOI] [PubMed] [Google Scholar]
- Zoncu R., Perera R.M., Sebastian R., Nakatsu F., Chen H., Balla T., Ayala G., Toomre D., De Camilli P.V. 2007. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc. Natl. Acad. Sci. USA. 104:3793–3798 10.1073/pnas.0611733104 [DOI] [PMC free article] [PubMed] [Google Scholar]