Abstract
Mechanical wounding of plants triggers the release of a blend of reactive biogenic volatile organic compounds (BVOCs). During and after mowing and harvesting of managed grasslands, significant BVOC emissions have the potential to alter the physical and chemical properties of the atmosphere and lead to ozone and aerosol formation with consequences for regional air quality. We show that the amount and composition of BVOCs emitted per unit dry weight of plant material is comparable between laboratory enclosure measurements of artificially severed grassland plant species and in situ ecosystem-scale flux measurements above a temperate mountain grassland during and after periodic mowing and harvesting. The investigated grassland ecosystem emitted annually up to 130 mg carbon m−2 in response to cutting and drying, the largest part being consistently represented by methanol and a blend of green leaf volatiles (GLV). In addition, we report the plant species-specific emission of furfural, terpenoid-like compounds (e.g. camphor), and sesquiterpenes from cut plant material, which may be used as tracers for the presence of given plant species in the ecosystem.
Introduction
Mechanical wounding is a natural stress that plants endure repeatedly during their lifespan as a consequence of unfavorable environmental conditions such as hailstorms, strong winds or heavy snow loads, and grazing/feeding by animals. Mechanical wounding of plants is however also the result of agricultural practices such as pruning of horticultural plants, or harvesting of the above- and/or below-ground biomass of crops and grasslands.1 The biogenic volatile organic compounds (BVOCs) ubiquitously emitted from mechanically damaged plants include C6-alcohols, C6-aldehydes, acetate esters, methanol and acetaldehyde, as well as products of isoprene oxidation, especially methacrolein (MAC) and methyl vinyl ketone (MVK).2-4 Besides having fascinating ecological roles in plant protection, plant-plant and plant-insect communication,5-7 the complex blend of BVOCs released by wounded plants comprises short-lived reactive compounds that can actively take part in atmospheric chemistry and physics.8-10
In the Alps, grasslands cover over 85% of the agricultural area in more than 75% of all municipalities.11 As grasslands located at similar altitudes have similar growth rates, mowing and harvesting of grasslands usually takes place in a regionally coordinated fashion during warm and sunny high-pressure periods that allow for a rapid drying of the plant material in the field. Such a regionally coordinated harvest produces large-scale emission bursts of BVOCs, and in particular oxygenated volatiles (OVOCs), into the atmosphere. VOCs affect the oxidative capacity of the atmosphere through photolysis and reactions with ozone, hydroxyl and nitrate radicals. The products of these reactions lead to the formation of alkyl peroxy radicals which convert NO to NO2; photolysis of the latter results in the photochemical production of atmospheric ozone.11 In the presence of anthropogenic NOx from motorways and industries, BVOC emissions from agricultural management activities may thus directly impact regional air quality through the formation of ozone, as well as aerosols.9-14
However, so far, regional and global air chemistry models largely ignore the emission of BVOCs from agricultural practices, despite the fact that BVOC emissions caused by mechanical wounding and subsequent drying of plant matter have been repeatedly demonstrated.15-18 A possible reason for that are difficulties in up-scaling available leaf-level laboratory studies under controlled environmental conditions to the ecosystem-scale and real-world field conditions.
The main objective of the present paper is to close this knowledge gap by investigating leaf and ecosystem scale BVOC emissions after wounding of grassland species in a comparative fashion. To this end, we quantified BVOC emissions from three mountain grassland plant species after wounding and subsequent drying in enclosures under laboratory conditions and compared these with in situ BVOC emissions measured by means of the virtual disjunct eddy covariance method during and after harvesting of a temperate mountain grassland.
Experimental
PTR-MS field measurements
The study site is located near Neustift (47°07’N, 11° 19’E) in the Stubai Valley (Austria) at an elevation of 970 m a.s.l. in the middle of the flat valley. More details characterizing the study site are given by Hammerle et al. (2008), Wohlfahrt et al. (2008) and Bamberger et al. (2010, 2011).19-22 Briefly, in 2008 and 2009 volume mixing ratios (VMR) of different BVOCs were quantified by a high-sensitivity proton-transfer-reaction mass spectrometer (PTR-MS) before and after five cutting events.21,22 Simultaneously, the three wind components and the speed of sound were recorded at a time resolution of 20 Hz by a three-dimensional sonic anemometer (R3IA, Gill Instruments, Lymington, UK) and stored to a hard drive of a personal computer using the Eddymeas software (O. Kolle, Max Planck Institute for Biogeochemistry, Jena, Germany). Fluxes of BVOCs were then calculated using the virtual disjunct eddy covariance method (vDEC) as described by Karl et al. (2002).23
On average 25% of the data were missing due to quality control and regular zero calibration of the PTR-MS. In order to calculate cumulative VOC fluxes over 24 h periods these data gaps, which did not exceed 2 h, were filled by linear interpolation.
Further details about the flux calculation procedures and quality control of the flux data are given in the Supporting Information.
Mass of harvested plant material
The difference between the total above-ground phytomass immediately before and after cutting, corresponding to the mass of harvested plant material, was quantified by clipping of plants at the soil surface within square plots of 0.09 m2 (3-5 replicates) and dry weight (DW) determination after drying at 80°C for at least 3 days.
PTR-TOF enclosure measurements
Seeds of Trifolium repens L. (white clover), Ranunculus acris L. (buttercup), Dactylis glomerata L. (cocksfoot), representing the plant functional groups of legumes, forbs and C3 grasses, respectively, were collected at the same study site described above. All collected seeds were transferred to small pots (0.55 dm3) filled with commercial soil (resembling the structure and composition of the experimental site soil) and grown in the greenhouse facility of the Botanical Garden at the University of Innsbruck (Innsbruck, Austria). During plant growth light was artificially supplied by Osram-Power Start 1000 HQT lamps (Osram®, Munich, Germany) set to provide a light intensity of ~700 μmol photons m−2 s−1 during a 12-h photoperiod.
A custom made dynamic flow-through enclosure of 1 l volume was built of Teflon® low density material (Teflon® FEP 1000 A, Vector Foiltec GmbH, Bremen, Germany) to guarantee an inert surface for BVOC interactions and a clean background level. The enclosure was continuously flushed with 2 l min−1 VOC free zero air generated by passing ambient air through a home built catalytic converter kept at a constant temperature of 350 °C. The emission of VOCs from the wounded plant material was tracked for 24 h using a time-of-flight proton-transfer-reaction mass spectrometer (PTR-TOF) connected to the outlet of the enclosure (see SI). Prior to each experiment the background was quantified by sampling the empty enclosure and subtracted for the calculation of BVOC emission rates. PTR-TOF sensitivity was calibrated at ambient humidity using a gas standard mixture of BVOCs (Apel Riemer Inc., USA) diluted with an adjustable amount of zero air. During measurements, which were conducted at a temperature of 25 ± 3 °C, a light source of ~700 μmol photons m−2 s−1 was used to illuminate the enclosure with a 12-h photoperiod to simulate sunlight conditions and a circadian rhythm.
To simulate the effect of mowing in the laboratory, plants were first cut by excising the petiole at the soil level and then wounded outside the enclosure by repeatedly cutting with scissors placed over a small foil of Teflon to be easily and quickly inserted into the enclosure. At the end of each experiment the wounded plant material was removed from the enclosure and dried in an oven (60°C) for several days before the dry weight was determined.
Each cutting experiment was replicated four times for each of the measured plant species. The means of the four samples were statistically separated using Tukey’s post-hoc test. Different lowercase superscripts in Tables 1 and 2 indicate statistically different means between species (P < 0.05). All statistical analyses were conducted using SigmaPlot 11.0 (Systat Software Inc., Chicago IL, USA).
Table 1.
Chemical composition of the overall blend of BVOCs emitted during 24 h following cutting of three different plant species in the laboratory (columns 2-4), and as determined in the field with the disjunct eddy covariance method (column 5). For plant laboratory investigations the average ± standard error (three replicates) is given, while for field measurements the minimum to maximum range during the five cutting events observed 2008-2009 is given (§ indicates that these masses were observed only during two cuts). Values in brackets indicate the respective percentage contribution of single compounds to the total amount of BVOCs emitted ($ indicates tentative compound assignment according to Ruuskanen et al (2011)29). For eddy covariance measurements the total number of compounds quantified varied between nine (*) and six (**) in 2008 and 2009, respectively (see Supporting Information). Different lowercase superscripts indicate significant differences between species at P < 0.05.
| μmol BVOC g−1 DW |
Trifolium
repens |
Dactlylis glomerata | Ranunculus acris |
Field site fluxes
EC, PTR-MS |
|---|---|---|---|---|
|
Formaldehyde (m/z 31.018) CH2O-H+ |
0.060 ± 0.016a (1.37) |
0.007 ± 0.0003a (0.93) |
0.017 ± 0.001a (0.62) |
|
|
Methanol (m/z 33.033) CH3OH-H+ |
2.839 ± 0.522a (65.25) |
0.131 ± 0.016b (16.36) |
0.569 ± 0.006b (20.77) |
0.81 – 4.74 (67.99*-82.11**) |
|
Acetaldehyde (m/z 45.034) C2H4O-H+ |
0.171 ± 0.033a (3.93) |
0.017 ± 0.003b (2.17) |
0.069 ± 0.016b (2.51) |
0.07 – 0.52 (6.14**-13.94*) |
|
Acetone (m/z 59.049) C3H6O-H+ |
0.067 ± 0.009a (1.54) |
0.032 ± 0.005b (3.96) |
0.024 ± 0.005b (0.86) |
0.02 – 0.09 (1.46**-2.95*) |
|
Acetic Acid (m/z 61.029) C2H2O2-H+ |
0.043 ± 0.007a (0.98) |
0.020 ± 0.004b (2.51) |
0.061 ± 0.004a (2.24) |
|
|
e.g. Isoprene, pentenol fragment (m/z 69.070) C5H4O-H+ |
0.076 ± 0.021a (1.75) |
0.027 ± 0.005a (3.39) |
0.034 ± 0.003a (1.24) |
0.05 – 0.14 (1.71**-6.75*) |
|
MAC+MVK (m/z 71.049) C4H6O-H+ |
0.005 ± 0.001a (0.11) |
0.002 ± 0.0001a (0.21) |
0.003 ± 0.001a (0.12) |
|
|
Pentanal fragment (m/z 71.086) C5H11-H+ |
0.022 ± 0.004a (0.52) |
0.005 ± 0.001b (0.62) |
0.008 ± 0.001b (0.29) |
|
|
Butanone (m/z 73.065) C4H8O-H+ |
0.005 ± 0.001ab (0.11) |
0.002 ± 0.0004b (0.25) |
0.006 ± 0.001a (0.20) |
|
|
Pentenone (m/z 85.064) C5H8O-H+ |
0.021 ± 0.007a (0.48) |
0.003 ± 0.0001b (0.32) |
0.005 ± 0.001ab (0.20) |
|
|
Hexanol fragment (m/z 85.100) C6H12-H+ |
0.059 ± 0.009a (1.36) |
0.007 ± 0.001c (0.90) |
0.030 ± 0.003b (1.08) |
0.02 – 0.05§ (0.82*-1.29*) |
|
Pentenol (m/z 87.081) C5H10O-H+ |
0.019 ± 0.004a (0.43) |
0.003 ± 0.001b (0.41) |
0.006 ± 0.0004b (0.21) |
|
|
e.g. Furfural$ (m/z 97.028) C5H4O2-H+ |
0.001 ± 0.0001b (0.02) |
--- | 0.687 ± 0.117a (25.06) |
|
|
Hexenals (m/z 99.080) C6H10O-H+ |
0.417 ± 0.108a (9.59) |
0.114 ± 0.022b (14.21) |
0.224 ± 0.051ab (8.17) |
0.07 – 0.31 (3.38*-9.91**) |
|
Hexenols+Hexanal (m/z 101.096) C6H12O-H+ |
0.211 ± 0.037a (4.86) |
0.036 ± 0.008b (4.52) |
0.132 ± 0.017a (4.83) |
0.07 – 0.12§ (3.08*-3.43*) |
|
Monoterpenes (m/z 137.133) C10H16-H+ |
0.002 ± 0.0002a (0.03) |
0.0005 ± 0.0001b (0.06) |
0.001 ± 0.0001b (0.05) |
0.01 – 0.05 (0.46**-4.06**) |
|
Hexenyl acetates (m/z 143.107) C8H14O2-H+ |
0.324 ± 0.037b (7.45) |
0.375 ± 0.105b (46.89) |
0.844 ± 0.099a (30.80) |
0.10 – 0.11§ (2.72*-5.01*) |
|
Hexyl acetates (m/z 145.121) C8H16O2-H+ |
0.008 ± 0.001a (0.19) |
0.003 ± 0.001c (0.42) |
0.015 ± 0.001b (0.55) |
|
|
Terpenoids e.g. camphor$ (m/z 153.127) C10H16O-H+ |
--- | 0.014 ± 0.003 (1.78) |
--- | |
|
Sesquiterpenes (m/z 205.195) C15H24-H+ |
0.0012 ± 0.0005 (0.03) |
--- | --- | |
| Total | 3.487 ± 0.537a | 0.712 ± 0.092b | 2.736 ± 0.283a | 1.11 – 5.77 |
Table 2.
Total amount of carbon emitted by different grassland plant species during the complete 24 h drying process after cutting and relative percentage contribution by one oxygen containing VOCs (CxHYO), two oxygen containing VOCs (CxHYO2) and hydrocarbons (CxHY). Values represent means of four independent experiments replicated for each one of the three investigated plant species ± standard error of the mean. Different lowercase superscripts indicate significant differences between species at P < 0.05.
| Plant species | Total carbon emitted (μg C gDW−1) |
OVOCs (CxHYO) (%) |
OVOCs (CxHYO2) (%) |
Hydrocarbons (CxHY) (%) |
|---|---|---|---|---|
| Trifolium repens | 133.97 ± 27.89a | 66.80 | 24.76 | 8.44 |
| Dactlylis glomerata | 55.37 ± 10.75b | 28.89 | 66.68 | 4.43 |
| Ranunculus acris | 166.52 ± 25.28a | 21.82 | 75.30 | 2.87 |
Results
Laboratory plant enclosure measurements
BVOC emissions after wounding and drying of grassland species belonging to three different plant functional groups differed qualitatively and quantitatively between species (Table 1). In total, the release of 18-19 different BVOCs was observed when analyzing the full spectrum of volatile molecules by PTR-TOF. Quantitatively, methanol was the most representative compound of the blend, followed by the mixture of hexenols, hexanals and hexenyl acetates (collectively called green leaf volatiles, GLV), and by acetaldehyde and acetic acid (Table 1). Methanol was particularly abundant in T. repens where it contributed more than 65% of the total emission.
Differences between the three plant species included species-specific emission of high molecular weight compounds such as a sesquiterpenes (mass to charge ratio (m/z) 205.104) in T. repens and a terpenoid-like compound (e.g. camphor) (m/z = 153.125) in D. glomerata (Table 1). In contrast to the other species, wounded R. acris leaves emitted a large amount (~25%) of m/z 97.028 (e.g. furfural). Hexenyl acetates (m/z 143.107) represented a considerable fraction of the BVOCs collectivity emitted by D. glomerata (~47%) and R. acris (~31%), but accounted only for around 7% of the total BVOC emissions in T. repens (Table 1). Hexenals (m/z 99.080) and the summed amount of hexenols and hexanal (m/z 101.096) represented a similar percentage (5-10%) of the overall amount of emitted compounds for the three different plant species.
In all wounded plant species, traces of a compound with a m/z 31.018, which was assigned to formaldehyde, were detected.23,24 Formaldehyde is not constitutively emitted from vegetation and its biosynthesis remains unclear. However, our results confirm a recent study reporting the measurement of formaldehyde fluxes above a Ponderosa Pine forest.25
Our experiments also confirmed that all of the plant species released MVK and MAC (m/z 71.049) after wounding,4,23 which was separated from the contribution of a pentanal fragment having a different exact mass weight (m/z 71.086). Similarly, pentenone (m/z 85.064) emission was separated from the emission of a hexanol fragment (m/z 85.100). It was, however, not possible to unambiguously identify the compound having an exact mass weight of m/z 69.070, which was tentatively assigned to isoprene. The cumulative emission obtained through continuous measurements of BVOCs released during 24 h after wounding and drying, the first hour of which is shown in Fig. S1, amounted to 0.7 ± 0.1 μmol BVOCs g−1 DW in D. glomerata, followed by R. acris and T. repens (2.7 ± 0.3 and 3.5 ± 0.5 μmol BVOCs g−1 DW, respectively) (Table 1). The larger emission of longer-chain molecules (in particular Hexyl acetates) by R. acris as compared to T. repens caused the former species to emit a higher total amount of carbon (C) (167 ± 25 vs. 134 ± 28 μg C g−1 DW), despite a lower emission of BVOC molecules per unit of dry biomass (Table 1 and 2).
Partitioning C emissions between hydrocarbons (void of oxygen atoms, CxHy) and oxygenated compounds containing one or two oxygen atoms (CxHyO or CxHyO2) clearly showed that emissions of hydrocarbons were minor (<9%) for all plant species (Table 2). T. repens emitted the highest percentage of oxygenated compounds containing one oxygen atom, while R. acris and D. glomerata prevalently emitted compounds containing two oxygen atoms (Table 2).
Ecosystem eddy covariance flux measurements
The long-term average (2001-2009) of plant dry matter removed annually from the field site through harvesting was 720 ± 30 g m−2, while in 2008 and 2009 697 and 713 g m−2 were harvested, respectively. Each year, the grassland was cut three times. The amount of harvested plant dry material decreased from the first (2001-2009: 277 ± 14 g m−2; 2008: 284 g m−2, 2009: 271 g m−2), over the second (2001-2009: 239 ± 12 g m−2; 2008: 247 g m−2, 2009: 249 g m−2) to the third (2001-2009: 204 ± 26 g m−2; 2008: 166 g m−2, 2009: 193 g m−2) mowing.
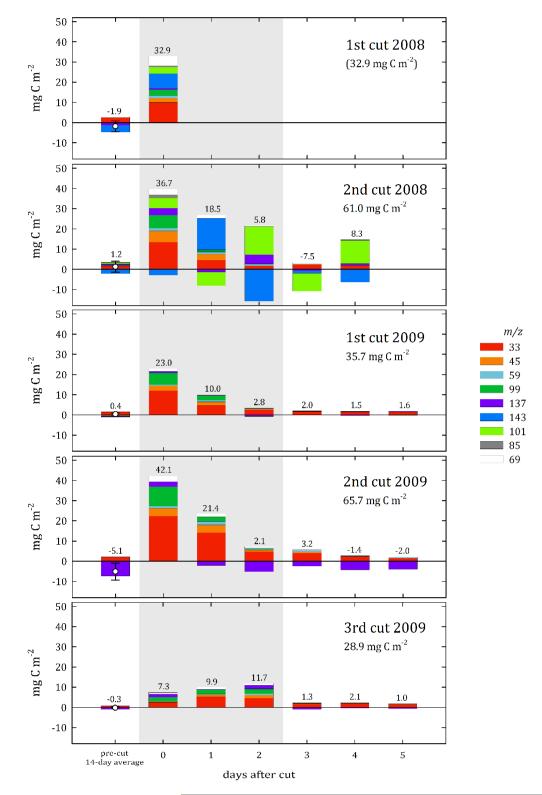
At a half-hourly time scale, harvesting caused large emission peaks of up to 6.3 mg C m−2 h−1 for methanol (m/z 33), acetaldehyde (m/z 45), acetone (m/z 59), isoprene/pentenol fragment (m/z 69) and the hexenals (m/z 99) (Fig. S2). On a daily basis the cumulative BVOC emissions amounted to 23-42 mg C m−2 on the day of cutting, except for the third mowing in 2009, when low temperatures and inclement weather did not lead to an observable increase in emissions (Fig. 1). After cutting, the exchange of the most responsive compounds followed a pronounced diurnal cycle (emissions during daytime, close to zero fluxes during nighttime), whose amplitude steadily decreased over time (Fig. S2). The total BVOC emission attained pre-cut values typically on day three or four after cutting (Fig. 1). During the day of cutting and the two subsequent days a total of 29-66 mg C m−2 was lost to the atmosphere as BVOCs, which corresponds to up to 56 days of BVOC emission prior to cutting (Fig. 1). Normalizing ecosystem BVOC emissions integrated over 24 h after cutting to the amount of harvested dry matter allows for a direct comparison of plant laboratory and ecosystem field measurements. As shown in Table 1, 1.1-5.8 μmol BVOCs g−1 DW were emitted in the field. Methanol (m/z 33) made the largest contribution to the emissions (>68 %), followed by acetaldehyde (m/z 45) and hexenals (m/z 99), while all other compounds individually accounted for a maximum of 7 % of the emissions by the ecosystem.
Figure 1.
Daily integrated volatile organic carbon flux before (pre-cut 14-day average), during (0), and up to five days after cutting of the investigated grassland quantified under field conditions by disjunct eddy covariance measurements. Differently colored bars refer to different m/z ratios as determined by the PTR-MS. The bars referring to carbon fluxes before cutting represent an average of the two weeks preceding the cut – error bars indicate the respective standard deviations. The numbers displayed above the bars represent the cumulative carbon flux in mg C m−2 of the respective bar, while the numbers in the upper right corner of each panel show the cumulative carbon flux during the day of cutting and the two subsequent days, graphically highlighted by grey shading. During 2008 instrumental problems prohibited reliable measurements after the day of the first cut and during the entire third cut.
Discussion
Our results demonstrate convergence between (i) the amount of BVOCs emitted per unit dry weight of plant material from artificially wounded leaves collected in laboratory enclosures (0.7-3.5 μmol BVOCs g−1 DW) and in situ ecosystem-scale flux measurements during regular mowing and drying events (1.1-5.8 μmol BVOCs g−1 DW), and (ii) the respective BVOC composition, which was dominated by methanol and the group of GLV. This suggests that leaf (or plant) enclosure measurements under laboratory conditions may be transferred to the ecosystem by scaling with the amount of harvested plant material, e.g. derived from agricultural census data.
The possibility of such a direct up-scaling is remarkable, as the conditions of laboratory leaf enclosure and in situ ecosystem-scale flux measurements are disparate and many examples in plant ecology have shown non-linear effects to preclude direct up-scaling of C fluxes based on the amount of biomass or leaf area.26 For example, in enclosures emissions are collected from well-defined plant parts only. In contrast, micrometeorological flux measurements combine (generally poorly known) contributions from different sources (and possibly sinks) within the footprint: wounded plant material immediately after cutting and during the following drying process in the field (when also fermentation may occur), the remaining standing plant parts (stubble) and the soil, which is known to represent both a sink and source for BVOCs.27 Furthermore, although the investigated plant species are representative of the three major functional plant groups found at the study site, more than 20 different plant species contribute to the total biomass in the field.28 In addition, under field conditions the degree to which plants are severed depends both on how and at which height above ground plants are cut and on whether additional practices such as turning of the harvested plant material to accelerate its drying are carried out.29 Environmental conditions in laboratory measurements with enclosures are usually held constant, whereas in the field variation occurs diurnally with solar forcing and from day to day in response to synoptic variability. As a consequence, the kinetics of BVOC emission observed in the field (Fig. S2) have larger diurnal variability with respect to laboratory enclosure measurements (Fig. S2). This reflects the temporal dynamics of the plant internal BVOC pools and circadian rhythms of the driving forces of drying (e.g. solar radiation and air temperature) and turbulent exchange (e.g. wind speed).
The amount of C injected into the atmosphere during and immediately after grassland cutting reached up to 65 mg C m−2 for a single mowing event, or 130 mg C m−2 during the entire vegetation period. This represents an appreciable amount when compared to the annual budget of methanol (329 mg C m−2),22 the quantitatively most significant compound emitted during undisturbed conditions from this site.30
Several other interesting insights about species-specific contributions to the total BVOC emission were collected in the laboratory, as the PTR-TOF provided an accurate description of the complex blend of volatiles characterizing plant species belonging to diverse functional plant groups: T. repens, a leguminous species, emitted the largest fraction of BVOCs as methanol, but did not form substantial amounts of GLV after wounding. On the other hand, a substantially larger amount of GLV, especially hexenyl acetates, was released by the monocotyledonous D. glomerata, which indicates larger membrane damage and large presence of lipoxygenases that readily oxidize compounds emitted from membrane lipid breakdown.3,4,31 The species-specific emission of semi-volatile terpenoids in the plant species that were examined suggests that these volatiles could be used as tracers for the presence of certain plant species. The same consideration applies to the wound-induced release of other high molecular weight volatiles, such as m/z 97.028. A recent study at the same experimental site reported ecosystem-scale fluxes of m/z 97.028 after cutting,29 which we suggest to be attributable to the presence of R. acris. Ruuskanen et al. (2011) reasoned m/z 97.028 to be associated with Furfural,29 which is emitted from degradation of high amounts of xylose and other five carbon sugars precursors that are known to be abundant in R. acris.32 In order to corroborate this finding, however, more of the >20 plant species present at this site need to be screened.
Although an explicit assignment of isoprene to the compound with m/z 69.070 was not possible, we assume this compound to be isoprene because MVK and/or MAC, the two compounds that are primarily formed from oxidation of isoprene in air,8 were also detected after leaf cutting. Isoprene might be produced in low amounts by the investigated species, possibly through non-enzymatic acid hydrolysis of its precursor DMADP.33 Since MAC and MVK were detected both from flux measurements at ecosystem level in the presence of atmospheric oxidants,29 and also from plant-scale enclosure measurements in absence of any oxidizing agents, it is suggested that isoprene oxidation occurs predominantly inside the leaves. One possible pathway is via the reaction with reactive oxygen species that are formed inside the leaf as a consequence of abiotic and biotic stress, including wounding.33-35
In conclusion, our results confirm a significant release of BVOCs as a consequence of agricultural practices for grassland management.18 Due to their quantitative relevance and recurrent emission, fluxes of GLVs and methanol should be included into regional air chemistry models to account for potential effects on the air quality. We were able to show that roughly 1-5 μmol BVOCs per gram harvested dry weight were emitted during the first 24h following cutting, when the largest part of emissions occurs, and that plant-scale emission factors can be scaled up to the ecosystem level based on the total amount of harvested biomass. Our study also revealed that while there are similarities between plant species both in terms of composition and rates of BVOC emissions, there are also significant differences in particular with regard to the fractional contribution of terpenoids and other higher molecular weight compounds to the total blend of emitted BVOCs that may be exploited as tracers for the presence of certain plant species.
Regional climate scenarios for the Alps predict further increases in air temperature that may be expected to cause a lengthening of the vegetation (i.e. growing) period and possibly an intensification of management, if farmers adapt to the expected additional plant growth by harvesting grasslands more frequently.36,37 On the basis of our analysis, we expect two major consequences from such a combined climate/land management scenario: first, we foresee an increase of the total amount of BVOC emitted into the atmosphere during and after more frequent cutting events, and second, we expect shifts in the BVOC blend composition if climate/land management scenarios affect plant species composition38 - both changes are expected to feed back on regional air quality.
Supplementary Material
Acknowledgements
This study was financially supported by Commission Marie Curie IAPP project 218065 “PTR-TOF”, the Austrian National Science Fund (FWF) under contracts P19849-B16 and P23267-B16 and the Tyrolean Science Fund under contract UNI-0404/486. Dr. Markus Müller is thanked for support with the post-processing of the PTR-TOF data, Family Hofer (Neustift, Austria) for granting us access to the study site, and the staff of the Botanical Garden of the Institute of Botany of the University of Innsbruck for raising the experimental plants.
Footnotes
Supporting Information Available Half-hourly ecosystem-scale BVOC fluxes before and after cutting, time courses of enclosure BVOC emissions after artificial cutting during the 24 h laboratory experiment, as well as further details pertaining to the description of the experimental setup. This information is available free of charge via the internet at http://pubs.acs.org/.
References
- (1).Hopkins A, Holz B. Grassland for agriculture and nature conservation: production, quality and multi-functionality. Agron. Res. 2006;4(1):3–20. [Google Scholar]
- (2).Hatanaka A. The biogeneration of green odor by green leaves. Phytochem. 1993;34:1201–1218. [Google Scholar]
- (3).Loreto F, Barta C, Brilli F, Nogués I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 2006;29:1820–1828. doi: 10.1111/j.1365-3040.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- (4).Brilli F, Ruuskanen TM, Schnitzhofer R, Müller M, Breitenlechner M, Bittner V, Wohlfahrt G, Loreto F, Hansel A. Detection of Plant Volatiles after Leaf Wounding and Darkening by Proton Transfer Reaction “Time-of-Flight” Mass Spectrometry (PTR-TOF) PLoS ONE. 2011;6(5):e20419. doi: 10.1371/journal.pone.0020419. DOI 10.1371/journal.pone.0020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ton J, D’Alessandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007;49:16–26. doi: 10.1111/j.1365-313X.2006.02935.x. [DOI] [PubMed] [Google Scholar]
- (6).Farag MA, Paré PW. C6-Green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochem. 2002;61:545–554. doi: 10.1016/s0031-9422(02)00240-6. [DOI] [PubMed] [Google Scholar]
- (7).Allmann S, Baldwin IT. Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science. 2010;329:1075–1078. doi: 10.1126/science.1191634. [DOI] [PubMed] [Google Scholar]
- (8).Atkinson R. Gas-phase tropospheric chemistry of volatile organic compounds: 1. Alkanes and alkenes. J. Phys. Chem. Ref. Data. 1997;26:215–290. [Google Scholar]
- (9).Atkinson R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000;34:2063–2101. [Google Scholar]
- (10).Chameides WL, Lindsay RW, Richardson J, Kiang CS. The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study. Science. 1998;241:1473–1475. doi: 10.1126/science.3420404. [DOI] [PubMed] [Google Scholar]
- (11).Tappeiner U, Bosdorf A, Tasser E, editors. Alpenatlas. Spektrum Akademischer Verlag; Heidelberg: 2008. [Google Scholar]
- (12).Prévôt ASH, Dommen J, Bäumle M, Furger M. Diurnal variations of volatile organic compounds and local circulation systems in an Alpine valley. Atmos. Environ. 2000;34:1413–1423. [Google Scholar]
- (13).Laaksonen A, Kulmala M, O’Dowd CD, Joutsensaari J, Vaattovaara P, Mikkonen S, Lehtinen KEJ, Sogacheva L, Dal Maso M, Aalto P, Petäjä T, Sogachev A, Yoon YJ, Lihavainen H, Nilsson D, Facchini MC, Cavalli F, Fuzzi S, Hoffmann T, Arnold F, Hanke M, Sellegri K, Umann B, Junkermann W, Coe H, Allan JD, Alfarra MR, Worsnop DR, Riekkola ML, Hyötyläinen T, Viisanen Y. The role of VOC oxidation products in continental new particle formation. Atmos. Chem. Phys. 2008;8:2657–2665. [Google Scholar]
- (14).Hallquist M, Wenger JC, Baltensperger U, Rudich Y, Simpson D, Claeys M, Dommen J, Donahue NM, George C, Goldstein AH, Hamilton JF, Herrmann H, Hoffmann T, Iinuma Y, Jang M, Jenkin ME, Jimenez JL, Kiendler-Scharr A, Maenhaut W, McFiggans G, Mentel Th. F., Monod A, Prévôt ASH, Seinfeld JH, Surratt JD, Szmigielski R, Wildt J. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys. 2009;9:5155–5236. [Google Scholar]
- (15).Kirstine W, Galbally I, Yerong Y, Hooper M. Emissions of volatile organic compounds (primarily oxygenated species) from pasture. J. Geophys. Res. 1998;103(9):10605–10619. [Google Scholar]
- (16).Karl T, Guenther A, Jordan A, Fall R, Lindinger W. Eddy covariance measurement of biogenic oxygenated VOC emissions from hay harvesting. Atmos. Environ. 2000;35:491–495. [Google Scholar]
- (17).Karl T, Guenther A, Lindinger C, Jordan A, Fall R, Lindinger W. Eddy covariance measurements of oxygenated volatile organic compound fluxes from crop harvesting using a redesigned proton-transfer-reaction mass spectrometer. J. Geophys. Res. 2001;106(20):24157–24167. A. [Google Scholar]
- (18).Davison B, Brunner A, Ammann C, Spirig C, Jocher M, Neftel A. Cut-induced VOC emissions from agricultural grasslands. Plant Biol. 2008;10:76–85. doi: 10.1055/s-2007-965043. [DOI] [PubMed] [Google Scholar]
- (19).Hammerle A, Haslwanter A, Tappeiner U, Cernusca A, Wohlfahrt G. Leaf area controls on energy partitioning of a temperate mountain grassland. Biogeosciences. 2008;5:421–431. doi: 10.5194/bg-5-421-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wohlfahrt G, Hammerle A, Haslwanter A, Bahn M, Tappeiner U, Cernusca A. Seasonal and inter-annual variability of the net ecosystem CO2 exchange of a temperate mountain grassland: Effects of weather and management. J. Geophys. Res. 2008;113 doi: 10.1029/2007jd009286. DOI 10.1029/2007JD009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Bamberger I, Hörtnagl L, Schnitzhofer R, Graus M, Ruuskanen TM, Müller M, Dunkl J, Wohlfahrt G, Hansel A. BVOC fluxes above mountain grassland. Biogeosciences. 2010;7:1413–1424. doi: 10.5194/bg-7-1413-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Bamberger I, Hörtnagl L, Ruuskanen TM, Schnitzhofer R, Müller M, Graus M, Karl T, Wohlfahrt G, Hansel A. Deposition fluxes of terpenes over grassland. J. Geophys. Res. 2011;116 doi: 10.1029/2010JD015457. DOI 10.1029/2010JD015457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Karl T, Spirig C, Rinne J, Stroud C, Prevost P, Greenberg J, Fall R, Guenther A. Virtual disjunct eddy covariance measurements of organic compound fluxes from a subalpine forest using proton transfer reaction mass spectrometry. Atmos. Chem. Phys. 2002;2:279–291. [Google Scholar]
- (24).DeGouw J, Carleton JH, Custer TG, Baker BM, Fall R. Proton-Transfer Chemical-Ionization Mass Spectrometry allows real-time analysis of volatile organic compounds released from cutting and drying of crops. Environ. Sci. Technol. 2000;34:2640–2648. [Google Scholar]
- (25).DiGangi JP, Boyle ES, Karl T, Harley P, Turnipseed A, Kim S, Cantrell C, Maudlin RL, Zheng W, Flocke F, Hall SR, Ullmann K, Nakashima Y, Paul JB, Wolfe GM, Desai AR, Kajii Y, Guenther A, Keutsch FN. First direct measurements of formaldehyde flux via eddy covariance: implications for missing in-canopy formaldehyde sources. Atmos. Chem. Phys. 2011;11:10565–10578. [Google Scholar]
- (26).Jarvis PG. Scaling processes and problems. Plant Cell Environ. 1995;18:1079–1089. [Google Scholar]
- (27).Asensio D, Penuelas J, Lüth C, Prieto P, Estiarte M, Filella I, Llusia J. Interannual and seasonal changes in the soil exchange rates of monoterpenes and other VOCs in a Mediterranean shrubland. Eur. J. Soil Sci. 2008;59(5):878–891. [Google Scholar]
- (28).Wohlfahrt G, Pilloni S, Hoertnagl L, Hammerle A. Estimating carbon dioxide fluxes from temperate mountain grasslands using broad-band vegetation indices. Biogeosciences. 2010;7:683–694. doi: 10.5194/bg-7-683-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Ruuskanen TM, Mueller M, Schnitzhofer R, Karl T, Graus M, Bamberger I, Hörtnagl L, Brilli F, Wohlfahrt G, Hansel A. Eddy covariance VOC emission and deposition fluxes above grassland using PTR-TOF. Atmos. Chem. Phys. 2011;11:611–625. doi: 10.5194/acp-11-611-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Hörtnagl L, Bamberger I, Graus M, Ruuskanen TM, Schnitzhofer R, Müller M, Hansel A, Wohlfahrt G. Biotic, abiotic, and management controls on methanol exchange above a temperate mountain grassland. J. Geophys. Res. 2011;116 doi: 10.1029/2011jg001641. DOI 10.1029/2011JG001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Fall R, Karl T, Hansel A, Jordan A, Lindinger W. Volatile organic compounds emitted after leaf wounding: on-line analysis by proton-transfer-reaction mass spectrometry. J. Geophys. Res. 1999;104:15963–15974. [Google Scholar]
- (32).Zeitsch KJ. The chemistry and technology of furfural and its many by-products. Elsevier Science B.V.; Amsterdam, The Netherlands: 2000. [Google Scholar]
- (33).Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009;5:283–291. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- (34).Jardine KJ, Monson RK, Abrell L, Saleska SR, Arneth A, Jardine A, Ishida FY, Serrano AM,Y, Artaxo P, Karl T, Fares S, Goldstein A, Loreto F, Huxman T. Within-plant isoprene oxidation confirmed by direct emission of oxidation products methyl vinyl ketone and methacrolein. Global Change Biol. 2012;18:973–984. [Google Scholar]
- (35).Loreto F, Schnitzler JP. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010;15(3):154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- (36).Calanca P, Fuhrer J, Karsten J, Torriani D, Keller F, Dueri S. Klimawandel und landwirtschaftliche Produktion. Agrarforschung. 2005;12:392–397. [Google Scholar]
- (37).Smiatek G, Kunstmann H, Knoche R, Marx A. Precipitation and temperature statistics in high-resolution regional climate models: Evaluation for the European Alps. J. Geophys. Res. 2009;114 DOI 10.1029/2008JD011353. [Google Scholar]
- (38).Niedrist G, Tasser E, Lüth C, Dalla Via J, Tappeiner U. Plant diversity declines with recent land use changes in European Alps. Plant Ecology. 2009;202(2):195–210. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.