Abstract
A 12-year-old spayed female mixed-bred dog presented with nasal bleeding of 2 days duration and a skin nodule in the left flank. No abnormalities were found in coagulation profiles and blood pressure. Cytological evaluation of the nodule revealed numerous characteristic round organisms having a nucleus and a bar within macrophages and in the background, consistent with leishmaniasis. In vitro culture was unsuccessful but PCR of the nodular aspirate identified the organisms as Leishmania infantum, and the final diagnosis was canine leishmaniasis. No history of travel to endemic countries was noted. Because the dog had received a blood transfusion 2 years before the illness, serological screening tests were performed in all donor dogs of the commercial blood bank using the commercial Leishmania ELISA test kit, and there were no positive results. Additional 113 dogs with hyperglobulinemia from Seoul were also screened with the same kits but no positive results were obtained. To the best of the author's knowledge this is the first autochthonous case of canine leishmaniasis in Korea.
Keywords: Leishmania infantum, autochthonous leishmaniasis, dog, Korea
INTRODUCTION
Leishmaniasis is a group of infectious diseases that affect people and domestic and wild animals worldwide and are caused by species of the genus Leishmania, transmitted by sandflies. About 30 species of Leishmania infect humans as well as domestic and wild animals in 88 countries. Dogs act as a reservoir host of the disease for human infections; they are considered to be the reservoir for Leishmania infantum infection in areas including Portugal, the Mediterranean basin, Central and South America, the Middle East, and China [1]. Human leishmaniasis is classified into cutaneous (CL), mucocutaneous (MCL), and visceral leishmaniasis (VL) according to their clinical manifestations, but dogs usually have both visceral and cutaneous involvement [1].
As the first detailed human case report in the Republic of Korea (=Korea), Heu [2] described 3 cases of Kala-azar (=visceral leishmaniasis) among returnees from northern China. Thereafter, more than 25 human cases of cutaneous and visceral forms of leishmaniasis have been reported in Korea [3-5]. However, most of them were imported from regions where the disease is endemic [2,4,5]. Only 1 case was autochthonous cutaneous leishmaniasis (CL) in a 70-year-old man in Kangwon Province who has never been abroad [3].
Neither autochthonous nor introduced canine leishmaniasis (CanL) has been reported previously in Korea to the best of the authors' knowledge. Here we report the first occurrence of an autochthonous case of CanL in Korea.
CASE DESCRIPTION
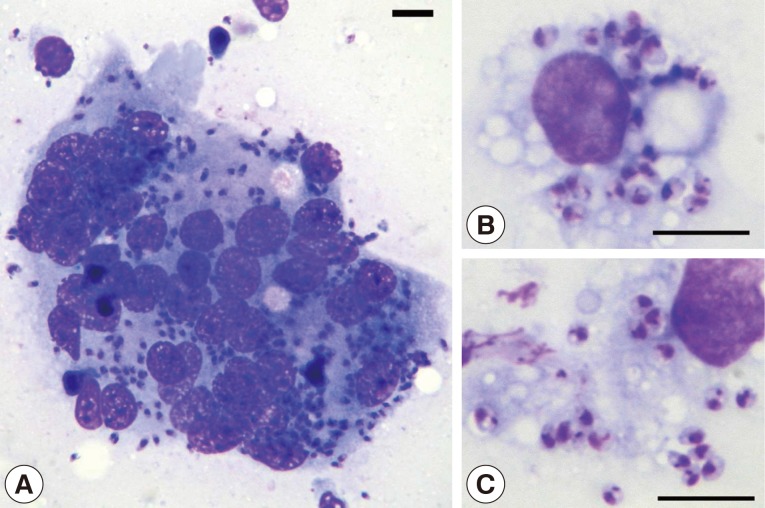
A 12-year-old neutered female mixed-bred dog weighing 5.9 kg presented to the Veterinary Medical Teaching Hospital, College of Veterinary Medicine, Seoul National University on July 24, 2006 after an episode of epistaxis and decreased appetite of 2 days' duration. The dog was born and raised in a residential area at Daeband-dong, Dongjak-gu, Seoul near where was Yongma Mountain and was living with its littermate and dam, none of which had been taken to a CanL endemic country. Physical examinations revealed pale mucous membranes (gingival and buccal) and a single diffuse firm round dermal nodule, 10×10 cm in diameter, extending from the lateral flank to the lateral abdomen. The skin nodule persisted for a year, and was tentatively diagnosed as panniculitis based on biopsy and histological examinations. Coagulation profiles and blood pressure were all within reference limits. The nodule was aspirated using a 23G needle and smears were cytologically stained. Numerous intracellular and extracellular organisms with basophilic nuclei and bar-shaped kinetoplasts were observed (Fig. 1), consistent with characteristic features of Leishmania sp.
Fig. 1.
Cytological findings. (A) A multinucleated giant cell containing Leishmania amastigotes (Bar=10 µm; Diff-Quik, ×1,000). (B, C) Macrophages containing numerous amastigotes having the characteristic bar-shaped kinetoplast and a nucleus (Bar=10 µm; Diff-Quik, ×1,200).
Based on cytological findings, tissue aspirates from the nodule were submitted for in vitro culture. Samples were first incubated in Dulbecco's Modified Eagle's Medium (DMEM) (Invitrogen, Carlsbad, California, USA) supplemented with 20% (v/v) heat-inactivated fetal calf serum (FCS) in an atmosphere of 5% CO2 and 95% air at 37℃ for 1 week, after which the medium was replaced with Schneider's Drosophila medium (GIBCO™ Schneider's Drosophila Medium 1X, liquid) (Invitrogen) with 20% heat-inactivated FCS, and incubated at 24℃ [6]. However, the culture was uninformative.
For PCR, DNA was extracted from the specimen using a commercial DNA Extraction Kit (AccuPrep® DNA Extraction Kit, Bioneer, Daejeon, Korea) with minor modifications [7,8]. In order to diagnose and obtain genomic data, PCR assays were performed with following primer pairs [7,8]; R174 (5'-GGTTCCTTTCCTGATTTACG-3') and R798 (5'-GGCCGGTAAAGGCCGAATAG-3') which were specific to the partial small subunit ribosomal RNA (SSU rRNA) gene (600 bp) of Leishmania, and N13A (5'-AACTTTTCTGGTCCTCCGGG-3') and N13B (5'-CCCCCAGTTTCCCGCCC-3') which were specific to a 120 bp fragment of the L. infantum kinetoplast DNA minicircle. The PCR products from each gene fragment were purified using a commercial gel extraction kit (QIAEX II Gel Extraction Kit, QIAGEN Inc., Valencia, California, USA). DNA sequencing was carried out using an automated DNA sequencer (ABI system 3700, Applied Biosystems, Inc., Foster City, California, USA). Using the DNA Basic module (DNAsis MAX, MiraiBio, Alameda, California, USA), gene sequences of the Korean canine Leishmania sp. (Leishmania infantum isolated from a dog in this study) were compared with those of the other known Leishmania species. The nucleotide sequences of the 600 bp fragment of the SSU rRNA gene and 120 bp fragment of the kinetoplast DNA minicircle gene from the Leishmania-infected dog described in this study were deposited in GenBank under accession numbers EU825208 and EU825207, respectively.
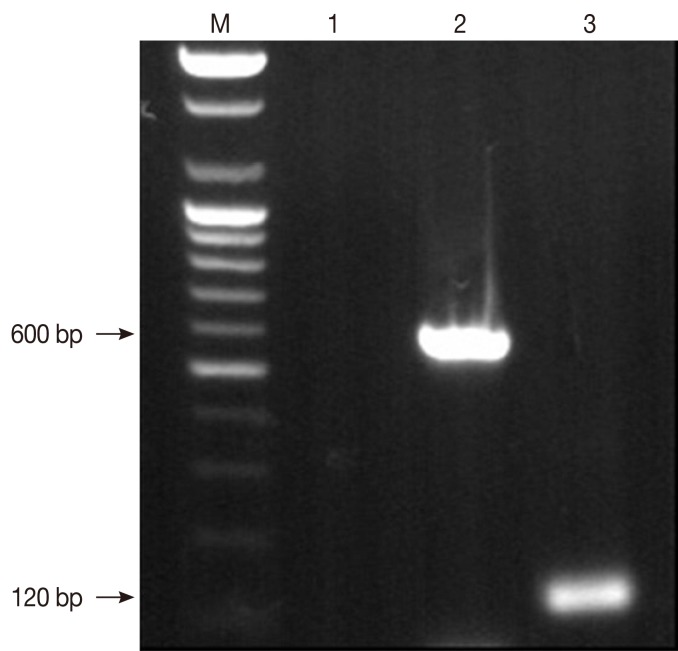
PCR analysis of the aspirated samples showed both the 600 bp band specific for Leishmania and 120 bp L. infantum-specific kinetoplast DNA minicircle (Fig. 2) [7-10]. Nucleotide sequence comparisons of the SSU rRNA gene of the organism with that of other parasites available in GenBank revealed that the organism shared greater than 99% identity with 10 of 13 strains of Leishmania spp. and above 98% identity with the other 3 Leishmania spp. (Table 1). The sequence comparison of the kinetoplast minicircle DNA of the organism with that of other parasites available in GenBank revealed that the organism shared 96.5-98.3 nucleotide identities with LLM-719, LEM-2298, and IPT1 strains of L. infantum. The organism also showed relatively high nucleotide identities of the kinetoplast minicircle DNA with Leishmania chagasi and Leishmania donovani, sharing 95.6-96.5% similarity (Table 2). However, the organism revealed to have very low nucleotide (69.7%) identities with Leishmania amazonensis (Table 2). Based on these data, the Korean Leishmania species from the dog described in this study was confirmed to be L. infantum and the dog was therefore diagnosed with CanL. Following the diagnosis, the dog was euthanized upon its owner's request.
Fig. 2.
Detection of Leishmania spp. in the canine skin specimen by PCR with primer pairs specific to Leishmania spp. Lane M; 100 bp DNA molecular marker. Lane 1; negative control without template DNA used for PCR assay with primer pair specific to the partial SSU rRNA gene. Lane 2; the partial SSU rRNA gene. Lane 3; kinetoplast DNA minicircle of Leishmania infantum isolated from a dog from Korea in this study.
Table 1.
Sequence comparison of a fragment of the SSU rRNA gene of L. infantum identified from a dog in Korea with that of some Leishmania species and different organisms
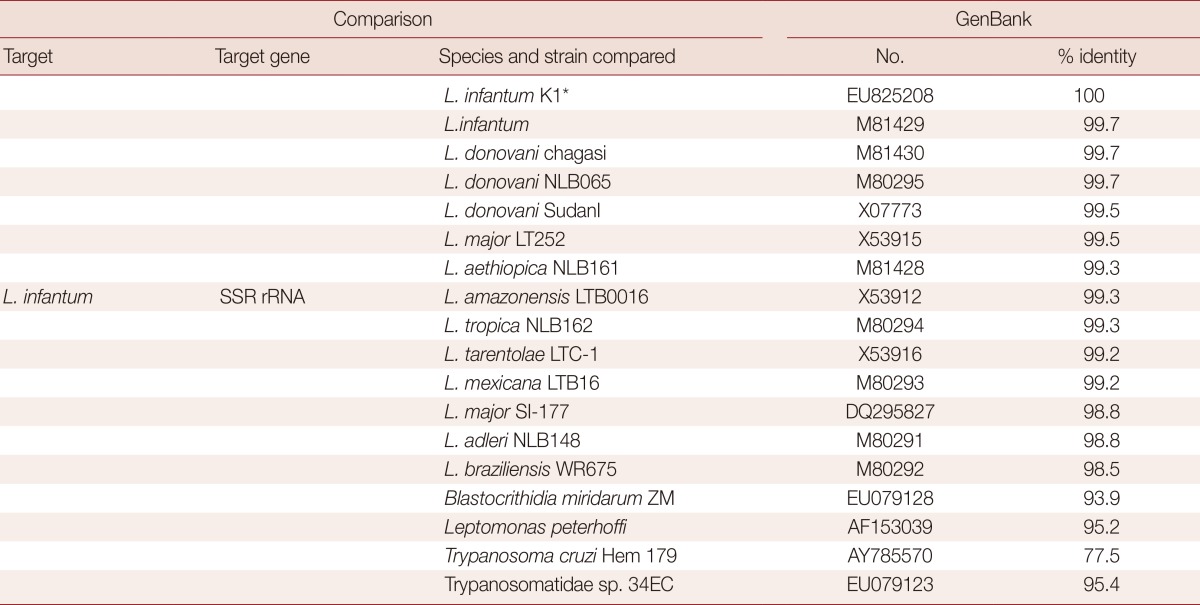
*Leishmania infantum isolated from a dog in Korea (this study).
Table 2.
Sequence comparison of a fragment of Leishmania kinetoplast DNA minicircle of L. infantum identified from a dog in Korea with that of the other Leishmania species
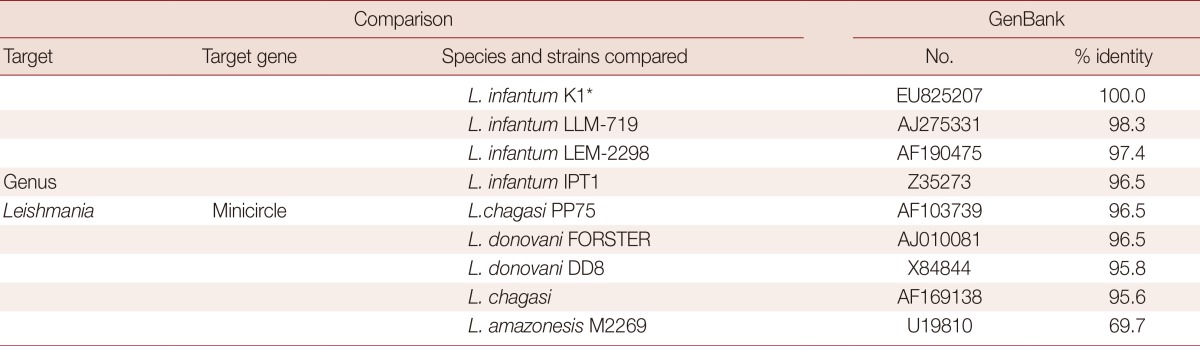
*Leishmania infantum isolated from a dog in Korea (this study).
DISCUSSION
In this report the diagnosis was made on direct visualization of specific Leishmania amastigotes in fine needle aspirate smears of the cutaneous lesion, positive results using PCR assay, and DNA sequencing. Cytological or histological identification of amastigotes is virtually 100% specific for the definitive diagnosis of leishmaniasis, and PCR demonstration of leishmanial DNA is sensitive both in human and animal patients [1,5,9-12].
Most cases of human and animal leishmaniasis in non-endemic areas have been associated with travel to endemic regions [2,4,5,13]. However, in this case of CanL, the dog had never been taken to an endemic region. In addition, known vector species for Leishmania spp. have not been recorded in Korea, and autochthonous animal infections have never been previously documented [1]. In a recent case of human autochthonous cutaneous leishmaniasis in Korea, the route of infection and the species of Leishmania responsible were not identified, and the patient had no history of travel to an endemic country [3], which were shared features with this canine case.
We can only speculate how this dog acquired its infection. One possibility is through congenital transmission [14,15]. Such transmission is rare, and is thought to occur through mothers who contracted severe CanL before or during pregnancy. The mother of the infected dog was not taken outside of Korea while pregnant and was subsequently proven to be free of Leishmania by PCR. The littermate also had a negative PCR result.
Another possible source of infection is from a blood transfusion that the dog received 2 years prior to the illness. Visceral leishmaniasis (VL) from blood transfusion has been documented previously [16,17]. The donor in this case was born in Korea, had never visited an endemic area, and was clinically healthy. None of other dogs that had received blood transfusion from the same donor showed clinical manifestations of leishmaniasis. Screening tests using a commercial ELISA test kit (SNAP® Leishmania test, IDEXX Laboratories, Wetherby, UK) for detection of Leishmania were performed on all donor dogs in the blood bank and no positive results were returned. Therefore, the route of infection through blood transfusion can be ruled out.
Because sandflies, the vectors of Leishmania, are intolerant of temperature changes, leishmaniasis acquired through accidental importation of sandflies is unlikely. The family who owned the dog had also not traveled to endemic countries. Further, the family was also unlikely to be the source of infection because all family members showed negative results on a serological test for detection of Leishmania. It has been suggested that sandflies may exist in Korea because of an increase in temperature and climate changes over the past few decades [3], but this has not been confirmed.
Although we were unable to identify where or how the dog described in this case study acquired Leishmania organism, it is possible that other cases of autochthonous leishmaniasis exist in Korea. Our findings suggest that more epidemiological investigations are needed, and clinicians in Korea should be alert to the possibility of leishmaniasis in animal patients presenting with cutaneous lesions and undiagnosed epistaxis, even if there is no history of travel to known endemic areas.
ACKNOWLEDGMENTS
This work was supported by the Brain Korea 21 Program for Veterinary Science. Further support was provided by the Research Institute of Veterinary Science, College of Veterinary Medicine, Seoul National University and Korean Research Foundation Grant (KRF-2006-005-J02902). Thanks to Dr. Barr for his help in this field at Cornell University.
Footnotes
We have no conflict of interest related with this study.
References
- 1.Baneth G. Leishmaniasis. In: Greene CE, editor. Infectious Disease of the dog and cat. St. Louis, USA: Saunders; 2006. pp. 685–695. [Google Scholar]
- 2.Heu IM. Three cases of Kala-azar, especially on the various serologic reaction. Korean J Intern Med. 1949;1:118–121. [Google Scholar]
- 3.Kim YJ, Hwang ES, You DS, Son SW, Uhm CS, Kim IH. A case of localized cutaneous leishmaniasis in a native Korean. Korean J Dermatol. 2004;42:884–888. [Google Scholar]
- 4.Ahn MH. Imported parasitic diseases in Korea. Infect Chemother. 2010;42:271–279. [Google Scholar]
- 5.Shin JY, Lee YB, Cho BK, Park HJ. New world cutaneous leishmaniasis treated with intralesional injection of pentavalent antimony. Ann Dermatol. 2013;25:80–83. doi: 10.5021/ad.2013.25.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merlen T, Sereno D, Brajon N, Rostand F, Lemesre JL. Leishmania spp.: completely defined medium without serum and macromolecules (CDM/LP) for the continuous in vitro cultivation of infective promastigote forms. Am J Trop Med Hyg. 1999;60:41–50. doi: 10.4269/ajtmh.1999.60.41. [DOI] [PubMed] [Google Scholar]
- 7.Francino O, Altet L, Sánchez-Roberta E, Rodriguezc A, Solano-Gallegoc L, Alberolac J, Ferrerd L, Sáncheza A, Rourab X. Advantages of real-time PCR assay for diagnosis and monitoring of canine leishmaniasis. Vet Parasitol. 2006;137:214–221. doi: 10.1016/j.vetpar.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Van Eys GJ, Schoone GJ, Kroon NC, Ebeling SB. Sequence analysis of small subunit ribosomal RNA genes and its use for detection and identification of Leishmania parasites. Mol Biochem Parasitol. 1992;51:133–142. doi: 10.1016/0166-6851(92)90208-2. [DOI] [PubMed] [Google Scholar]
- 9.da Silva ES, van der Meide WF, Schoone GY, Gontijo CM, Schallig HD, Brazil RP. Diagnosis of canine leishmaniasis in the endemic area of Belo Horizonte, Minas Gerais, Brazil by parasite, antibody and DNA detection assays. Vet Res Commun. 2006;30:637–643. doi: 10.1007/s11259-006-3324-2. [DOI] [PubMed] [Google Scholar]
- 10.Roura X, Sanchez A, Ferrer A. Diagnosis of canine leishmaniasis using a PCR technique. Vet Rec. 1999;144:262–264. doi: 10.1136/vr.144.10.262. [DOI] [PubMed] [Google Scholar]
- 11.Ashford DA, Bozza M, Freire M, Miranda JC, Sherlock I, Eulalio C, Lopes U, Fernandes O, Degrave W, Barker RH., Jr Comparison of the polymerase chain reaction and serology for the detection of canine visceral leishmaniasis. Am J Trop Med Hyg. 1995;53:251–255. doi: 10.4269/ajtmh.1995.53.251. [DOI] [PubMed] [Google Scholar]
- 12.Mathis A, DePlazes P. PCR and in vitro cultivation for detection of Leishmania spp. in diagnostic samples from humans and dogs. J Clin Microbiol. 1995;33:1145–1149. doi: 10.1128/jcm.33.5.1145-1149.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tánczos B, Balogh N, Király L, Biksi I, Szeredi L, Gyurkovsky M, Scalone A, Fiorentino E, Gramiccia M, Farkas R. First record of autochthonous canine leishmaniasis in Hungary. Vector Borne Zoonotic Dis. 2012;12:588–594. doi: 10.1089/vbz.2011.0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagliano P, Carannante N, Rossi M, Gramiccia M, Faella FS, Gaeta GB. Visceral leishmaniasis in pregnancy: a case series and a systematic review of the literature. J Antimicrob Chemother. 2005;55:229–233. doi: 10.1093/jac/dkh538. [DOI] [PubMed] [Google Scholar]
- 15.Rosypal AC, Troy GC, Zajac AM, Frank G, Lindsay DS. Transplacental transmission of a North American isolate of Leishmania infantum in an experimentally infected beagle. J Parasitol. 2005;91:970–972. doi: 10.1645/GE-483R.1. [DOI] [PubMed] [Google Scholar]
- 16.de Freitas E, Melo MN, da Costa-Val AP, Michalick MS. Transmission of Leishmania infantum via blood transfusion in dogs: potential for infection and importance of clinical factors. Vet Parasitol. 2006;137:159–167. doi: 10.1016/j.vetpar.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Kubar J, Quaranta JF, Marty P, Lelièvre A, Fichoux YL, Aufeuvre JP. Transmission of Leishmania infantum by blood donors. Nat Med. 1997;3:368. doi: 10.1038/nm0497-368. [DOI] [PubMed] [Google Scholar]