Abstract
Complicated malaria is mainly caused by Plasmodium falciparum, but, increasingly, Plasmodium vivax is also being reported as a cause. Since the reemergence of indigenous vivax malaria in 1993, cases of severe malaria have been steadily reported in Korea. Herein, we report a case of vivax malaria complicated by adult respiratory distress syndrome (ARDS) that was successfully managed with extracorporeal membrane oxygenation (ECMO). A 59-year-old man presented at our hospital with fever and abdominal pain, which had persisted for 10 days. On admission, the patient had impaired consciousness, shock, hypoxia and haziness in both lungs, jaundice, thrombocytopenia and disseminated intravascular coagulation, metabolic acidosis, and acute kidney injury. A peripheral blood smear and a rapid diagnostic test verified P. vivax mono-infection. Ten hours after admission, hypoxia became more severe, despite providing maximal ventilatory support. The administration of antimalarial agents, ECMO, and continuous venovenous hemofiltration resulted in an improvement of his vital signs and laboratory findings. He was discharged from the hospital 7 weeks later, without any sequelae.
Keywords: Plasmodium vivax, vivax malaria, case report, shock, acute kidney injury, adult respiratory distress syndrome (ARDS), extracorporeal membrane oxygenation (ECMO)
INTRODUCTION
Malaria is one of the major health problems in the world. It is known to have caused an estimated 655,000 deaths in 2010, mainly due to Plasmodium falciparum infection. These fatalities usually occurred in patients with severe complications of malaria, such as severe acute lung injury or adult respiratory distress syndrome (ARDS), cerebral malaria, hemoglobinuria, abnormalities in blood coagulation and thrombocytopenia, cardiovascular collapse and shock, acute kidney injury, severe anemia, metabolic acidosis, hyperlactatemia, and hypoglycemia [1]. Vivax malaria has been recognized as a benign and self-limited illness, but it can be severe and fatal in some cases, especially in endemic areas, and in patients with existing comorbidities [2,3]. It was widely believed that Plasmodium vivax was incapable of cytoadherence to endothelial cells and microvascular sequestration. However, it was recently documented that P. vivax-infected erythrocytes can also cytoadhere to endothelial cells in vitro [4]. Further, an autopsy study revealed that scattered parasitized erythrocytes were observed inside pulmonary capillaries, suggesting some sequestration in the lungs [5].
In the Republic of Korea (=Korea), there have been increasing reports of vivax malaria and its related complications since the first case of reemerging malaria was reported in 1993 near the demilitarized zone (DMZ) [6]. Severe or life-threatening complications have also been reported, some of which include multiorgan failure and pulmonary hemorrhage [7], shock and pulmonary edema [8], shock and seizure [9], acute renal failure and jaundice [10], and splenic rupture [11]. Among these reported cases, 1 patient who presented with multi-organ failure and pulmonary hemorrhage died eventually [7].
Extracorporeal membrane oxygenation (ECMO) is a therapeutic tool for providing life support in patients whose lungs or hearts cannot maintain sufficient oxygenation for the body. ECMO involves placing the patients on a vascular circuit with a membrane oxygenator that temporarily takes over the gas exchange function of the lungs. There have been an increasing number of clinical reports documenting the use of ECMO in severe ARDS patients who failed to respond to conventional mechanical ventilation therapy [12]. Further case reports have indicated that extracorporeal life support was used in 2 patients with falciparum malaria [13,14]. Herein, we describe a case of severe P. vivax infection complicated by multi-organ failure that was successfully managed with ECMO and continuous venovenous hemofiltration (CVVH).
CASE RECORD
A 59-year-old man presented with fever and abdominal pain. One month prior, he had returned from a trip to Kanghwa-do (Island), a high-risk region of vivax malaria, located on the west coast and just below the DMZ of South Korea. Apart from hypertension, which had been managed for 5 years, he was otherwise healthy. Three days before presentation to our hospital, he visited a nearby hospital for evaluation of fever and abdominal pain that he had been experiencing for 7 days. An abdominal computed tomography (CT) scan revealed edematous wall thickening of the distended gallbladder, splenomegaly, and fluid collection in the pelvic cavity. Under the tentative diagnosis of acute cholecystitis, he was treated with intravenous (IV) antibiotics (meropenem and metronidazole); however, his clinical condition did not improve. After 2 days, his dyspnea became severe and a chest radiograph revealed haziness in both lungs. He was then intubated and transferred to our hospital.
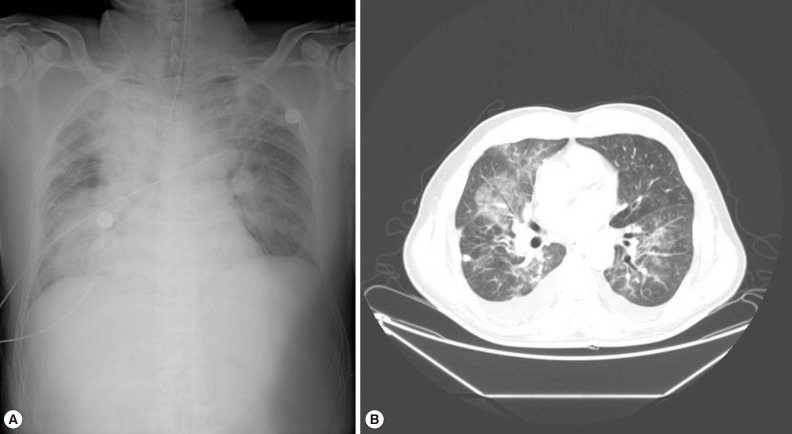
On admission, his body temperature was 37.3℃, blood pressure was 109/65 mmHg, and pulse rate was 180 beats per min. He exhibited a drowsy mental status and irritability. While the patient was maintained on a continuous mandatory ventilation (CMV) mode with tidal volume of 450 ml, respiration rate of 20/min, FiO2 of 0.8, and positive end-expiratory pressure (PEEP) of 15 cmH2O, arterial blood gas analysis (ABGA) revealed the following findings: pH, 7.22; PaCO2, 36.3 mmHg; PaO2, 101 mmHg; HCO3, 14.4 mmol/L; and O2 saturation, 95.7%. Central venous pressure determined from the right jugular central venous catheter was 10 cmH2O. His hemoglobin level was 11.1 g/dl, hematocrit was 33.6%, WBC count was 9,910/µl, and platelet count was 37,000/µl. Serum blood urea nitrogen (BUN) and creatinine levels were 38.1 mg/dl and 1.35 mg/dl, respectively. Total and direct bilirubin levels were 9.3 mg/L and 7.8 mg/L, respectively; aspartate aminotransferase, 183 IU/L; alanine aminotransferase, 115 IU/L; and alkaline phosphatase, 423 IU/L. Other laboratory tests revealed glucose, 161 mg/dl; C-reactive protein, 210.1 mg/L; lactic acid, 2.8 mmol/L; activated partial thromboplastin time, 48.9 sec; prothrombin time, 18.9 sec; and D-dimer, 14.20 µg/ml. A test for anti-HIV antibody was negative. The levels of muscle enzymes for detection of myocardial infarction were normal, and an electrocardiogram showed sinus tachycardia and there was no evidence of myocardial ischemia. A chest radiograph (Fig. 1A) and a chest CT scan (Fig. 1B) revealed haziness in both lungs. The malaria rapid antigen test (SD Bioline Ag P.f/pan Rapid) (SD BIOLINE, Seoul, Korea) was performed; the test was positive for P. vivax but negative for P. falciparum.
Fig. 1.
A chest radiograph (A) and a contrast-enhanced chest CT scan (B) taken on admission are showing haziness in both lungs; confluent haziness in the right lung and patchy alveolar infiltrates in the left lung. A perihilar distribution of pulmonary infiltrates suggests noncardiogenic pulmonary edema.
Microscopic examinations of a peripheral blood smear revealed P. vivax at a density of 16,380/µl. Chloroquine (10 mg of base/kg followed by 5 mg/kg at 12, 24, and 36 hr) was administered via a Levin tube. An IV quinine (20 mg/kg followed by 10 mg/kg every 8 hr) was administered because there was a possibility of poor intestinal absorption of chloroquine. Intravenous meropenem was added to empirically cover bacterial coinfection. Physiologic doses of steroid (hydrocortisone, 200 mg/day) were also administered as an adjuvant therapy for refractory shock. Ten hours after admission, his systolic blood pressure could be maintained between 100 and 110 mmHg when the following vasopressors were used simultaneously: dopamine, up to 30 µg/kg/min; dobutamine, up to 30 µg/kg/min; norepinephrine, up to 43 µg/min; and vasopressin, up to 2 units/hr.
A follow-up ABGA revealed an exacerbated pulmonary status: pH, 7.11; PaCO2, 54.0 mmHg; PaO2, 69.0 mmHg; HCO3, 17.2 mmol/L; and oxygen saturation, 86.0%. The patient was on a CMV mode with a tidal volume of 430 ml, respiratory rate of 26/min, FiO2 of 1.0, and PEEP of 15 cmH2O. His BUN and creatinine levels increased to 72.5 mg/dl and 3.9 mg/dl, respectively, and urine output decreased to less than 10 ml/hr. To deliver sufficient oxygen and to avoid pulmonary oxygen toxicity, venovenous ECMO was instituted. In addition, CVVH of blood drawn from the ECMO circuits was initiated to reduce fluid overload and to maintain renal function during acute kidney injury. Over 3 days after the initiation of ECMO and CVVH, radiographic abnormalities, vital signs, and arterial oxygenation progressively improved. On hospital day (HD) 4, ECMO and CVVH were discontinued because bleeding from the catheter sites was uncontrollable and the ECMO catheter lumen was blocked by blood clots despite the use of regional heparinization. Bloody secretion through an endotracheal tube was also observed, even though platelets and fresh frozen plasma were replaced daily from HD 2 to HD 6.
After removal of the ECMO catheters, conventional venovenous hemodialysis via a central femoral venous catheter was continued for an additional 4 days, and the mechanical ventilator was applied on a synchronized intermittent mandatory ventilation mode with tidal volume of 400 ml, respiration rate of 20/min, FiO2 of 0.5, and PEEP of 10 cmH2O. Thereafter, with the above ventilator settings, adequate oxygenation could be maintained. On HD 5, his urinary output began to increase to 50 ml/hr. On HD 14, he became alert and was weaned from the ventilator completely.
The parasitized erythrocytes levels decreased as follows: 16,380/µl on HD 1, 2,411/µl on HD 3, 1,552/µl on HD 4, and 166/µl on HD 5. On HD 6 and thereafter, P. vivax was not observed in blood films. Chloroquine was administered daily until HD 6, and the IV quinine was discontinued on HD 8. The cultures of blood, sputum, and urine taken on admission revealed no growth of pathogens, and pneumococcal and legionella antigens were also not detected in the urine. Intravenous meropenem was discontinued on HD 14, and primaquine (15 mg/day) was administered from HD 18 for 14 days. He was discharged to home without sequelae after a total of 7 weeks in the hospital.
DISCUSSION
We experienced a case of P. vivax infection complicated by multi-organ failure in an adult patient who, with the exception of hypertension, had no comorbidities. The patient had no travel history abroad and, therefore, there was no possibility of undiagnosed mixed P. falciparum infection, given that indigenous P. falciparum infection is not possible in Korea. Fatal cases of vivax malaria have usually been reported in countries like Indonesia, Papua New Guinea, Brazil, Thailand, and India. These fatalities occurred in patients with comorbid conditions and conditions of compromised immunity such as pregnancy, childhood, or HIV infection [2,3].
The clinical features of the present case was similar to those of a previously reported Korean patient, who had fever for 10 days and abdominal pain for 1 day and presented to a hospital with shock, acute kidney injury, jaundice, and coagulation failure. Forty-eight hr after admission, ARDS and pulmonary hemorrhage also developed [7]. In the current and previously reported Korean cases, the above mentioned risk factors were apparently absent; however, from an immunological point of view, Korean adults are affected similarly as are children in areas where vivax malaria is hyperendemic. The absence of vivax malaria for 17 years, i.e., between 1977 and 1993, in Korea has resulted in a large population of non-immune adults, which seems to be an important contributing factor for the severity of vivax malaria in the present case. Hypertension might predispose the patient to clinical decompensation. The patient alleged that he had managed his hypertension well, although complications of arterial hypertension such as ischemic heart disease or renal damage might have been present. Further, the patient presented with abdominal pain, and the abdominal CT scan suggested acalculous cholecystitis, a condition secondary to serious clinical conditions such as sepsis, which made the diagnosis difficult and delayed administration of appropriate treatment. Although vivax malaria is generally known to be a self-limited illness, delayed treatment may result in relatively high-density parasitemia as was observed before [15], and may have contributed to the severe course in the present case. The patient's age may also have contributed to the severity, given that fatality rates are higher in older patients with severe malaria [3,16]. Additionally, older patients with vivax malaria have higher levels of inflammatory cytokines than do younger adults [17]. Finally, pulmonary hemorrhage secondary to coagulation failure may be superimposed on ARDS in the present case.
To treat ARDS caused by P. vivax, oxygen supplementation, noninvasive positive pressure ventilation, or artificial ventilation with high PEEP [18] is usually sufficient to maintain arterial oxygenation. However, in certain patients, as in the case of our patient who presented with shock and oliguria in addition to ARDS, the outcome could be fatal, as previously reported [7, 19]. The presentation of shock necessitates an infusion of large volumes of fluid, which may aggravate pulmonary edema. Further, oliguria makes the management of volume status difficult. Even though volume overload can be managed with CVVH, concurrent hypoxia refractory to maximal ventilator care could be life-threatening, and oxygen toxicity is an irreversible complication associated with the administration of high concentrations of oxygen for an extended period of time. For these reasons, ECMO is a good treatment modality for malaria patients with ARDS refractory to conventional ventilator therapy, especially if ARDS is accompanied with shock or acute kidney injury.
In the present case, ECMO was useful as a bridge to conventional ventilator therapy. However, we experienced some difficulties during ECMO. In particular, bleeding was excessive in our patient, and it was thought that platelet alteration along with disseminated intravascular coagulation due to malaria, the bleeding tendency associated with renal failure, and hypoprothrombinemia due to the antibiotics were responsible. Because all these abnormalities could not be corrected immediately, bleeding aggravated in the patient despite replacement of platelets and fresh frozen plasma. Another factor to be considered in such a case is the pharmacokinetics of the medications administered. ECMO involves massive influx and efflux of fluids via catheters, consequently altering the volumes of distribution, elimination half-lives, and protein bindings of drugs. Furthermore, adsorption of the drugs into ECMO circuits may lower the plasma levels of the drugs. For these reasons, dose requirements may change if ECMO is being instituted. Since there are only a limited number of studies in this area of practice, current dosing recommendations are mainly for antibiotics and only for neonates [20]. No study has elucidated the dosage adjustment of chloroquine or quinine in adults during ECMO. Furthermore, concomitant use of CVVH with ECMO made the dosage adjustment more complicated. In fact, the clearance of parasitemia in the present case seemed to be delayed by 1 to 2 days, possibly due to the low plasma levels of chloroquine and quinine. Because the pharmacokinetic parameters of chloroquine and quinine during ECMO could not be predicted, we administered the antimalarial agents until the parasitemia was resolved. For patients with severe malaria who will be managed with ECMO or other life support procedures, therapeutic drug monitoring may be helpful in affirming the therapeutic blood levels of antimalarial agents.
ACKNOWLEDGMENTS
This work was financially supported by Inha University.
Footnotes
We have no conflict of interest related with this study.
References
- 1.World Health Organization. Management of severe malaria: a practical handbook. 3rd ed. Geneva, Switzerland: WHO Press; 2012. pp. 1–68. [Google Scholar]
- 2.Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. Plasmodium vivax: clinical spectrum, risk factors and pathogenesis. Adv Parasitol. 2012;80:151–201. doi: 10.1016/B978-0-12-397900-1.00003-7. [DOI] [PubMed] [Google Scholar]
- 3.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 2008;5:e128. doi: 10.1371/journal.pmed.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho BO, Lopes SC, Nogueira PA, Orlandi PP, Bargieri DY, Blanco YC, Mamoni R, Leite JA, Rodrigues MM, Soares IS, Oliveira TR, Wunderlich G, Lacerda MV, del Portillo HA, Araújo MO, Russell B, Suwanarusk R, Snounou G, Rénia L, Costa FT. On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect Dis. 2010;202:638–647. doi: 10.1086/654815. [DOI] [PubMed] [Google Scholar]
- 5.Lacerda MV, Fragoso SC, Alecrim MG, Alexandre MA, Magalhães BM, Siqueira AM, Ferreira LC, Araújo JR, Mourão MP, Ferrer M, Castillo P, Martin-Jaular L, Fernandez-Becerra C, del Portillo H, Ordi J, Alonso PL, Bassat Q. Postmortem characterization of patients with clinical diagnosis of Plasmodium vivax malaria: to what extent does this parasite kill? Clin Infect Dis. 2012;55:e67–e74. doi: 10.1093/cid/cis615. [DOI] [PubMed] [Google Scholar]
- 6.Chai JY. Re-emerging Plasmodium vivax malaria in the Republic of Korea. Korean J Parasitol. 1999;37:129–143. doi: 10.3347/kjp.1999.37.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SW, Kim DW, Park JW, Lee SI, Shin YH, Kim EC, Oh MD, Choe KW. A case of fatal Plasmodium vivax malaria with multi-organ failure. Infect Chemother. 2005;37:111–115. [Google Scholar]
- 8.Song JY, Park CW, Jo YM, Kim JY, Kim JH, Yoon HJ. Two cases of Plasmodium vivax malaria with the clinical picture resembling toxic shock. Am J Trop Med Hyg. 2007;77:609–611. [PubMed] [Google Scholar]
- 9.Yoon SG, Kim MH, Jung ES, Han KH, Kwak YG, Cho CR, Um TH, Kim ES. A case of vivax malaria with seizure and shock. Infect Chemother. 2007;39:226–229. [Google Scholar]
- 10.Kim HJ, Lee SH, Koo TY, Kim KM, Jang SS, Lee SK. A case of Plasmodium vivax malaria complicated with acute renal failure, jaundice and thrombocytopenia. Korean J Nephrol. 2010;29:792–795. [Google Scholar]
- 11.Shin DH, Kim KS, Kim YJ, Lee SH, Kim SJ, Cho CK, Shin JH, Ryang DW, Lee JH. A case of spontaneous splenic rupture in vivax malaria. Korean J Infect Dis. 1999;31:176–179. [Google Scholar]
- 12.Hemmila MR, Rowe SA, Boules TN, Miskulin J, McGillicuddy JW, Schuerer DJ, Haft JW, Swaniker F, Arbabi S, Hirschl RB, Bartlett RH. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004;240:595–605. doi: 10.1097/01.sla.0000141159.90676.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neurath M, Benzing A, Knolle P, Grundmann H, Dippold W, Meyer zum Büschenfelde KH. Acute respiratory failure in tropical malaria during pregnancy. Successful treatment using extracorporeal CO2 elimination. Dtsch Med Wochenschr. 1993;118:1060–1066. doi: 10.1055/s-2008-1059426. [DOI] [PubMed] [Google Scholar]
- 14.Vandroux D, Leaute B, Hoarau N, Ursulet L, Djouhri S, Braunberger E, Gaüzère BA. High frequency oscillation ventilation and extracorporeal membrane oxygenation during pernicious malaria. Med Mal Infect. 2011;41:209–212. doi: 10.1016/j.medmal.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Andrade BB, Reis-Filho A, Souza-Neto SM, Clarêncio J, Camargo LM, Barral A, Barral-Netto M. Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar J. 2010;9:13. doi: 10.1186/1475-2875-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dondorp AM, Lee SJ, Faiz MA, Mishra S, Price R, Tjitra E, Than M, Htut Y, Mohanty S, Yunus EB, Rahman R, Nosten F, Anstey NM, Day NP, White NJ. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis. 2008;47:151–157. doi: 10.1086/589287. [DOI] [PubMed] [Google Scholar]
- 17.MacMullin G, Mackenzie R, Lau R, Khang J, Zhang H, Rajwans N, Liles WC, Pillai DR. Host immune response in returning travellers infected with malaria. Malar J. 2012;11:148. doi: 10.1186/1475-2875-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkar S, Saha K, Das CS. Three cases of ARDS: an emerging complication of Plasmodium vivax malaria. Lung India. 2010;27:154–157. doi: 10.4103/0970-2113.68323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacerda MV, Mourão MP, Alexandre MA, Siqueira AM, Magalhães BM, Martinez-Espinosa FE, Filho FS, Brasil P, Ventura AM, Tada MS, Couto VS, Silva AR, Silva RS, Alecrim MG. Understanding the clinical spectrum of complicated Plasmodium vivax malaria: a systematic review on the contributions of the Brazilian literature. Malar J. 2012;11:12. doi: 10.1186/1475-2875-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buck ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin Pharmacokinet. 2003;42:403–417. doi: 10.2165/00003088-200342050-00001. [DOI] [PubMed] [Google Scholar]