Abstract
We review observational, experimental and model results on how plants respond to extreme climatic conditions induced by changing climatic variability. Distinguishing between impacts of changing mean climatic conditions and changing climatic variability on terrestrial ecosystems is generally underrated in current studies. The goals of our review are thus (1) to identify plant processes that are vulnerable to changes in the variability of climatic variables rather than to changes in their mean, and (2) to depict/evaluate available study designs to quantify responses of plants to changing climatic variability. We find that phenology is largely affected by changing mean climate but also that impacts of climatic variability are much less studied but potentially damaging. We note that plant water relations seem to be very vulnerable to extremes driven by changes in temperature and precipitation and that heatwaves and flooding have stronger impacts on physiological processes than changing mean climate. Moreover, interacting phenological and physiological processes are likely to further complicate plant responses to changing climatic variability. Phenological and physiological processes and their interactions culminate in even more sophisticated responses to changing mean climate and climatic variability at the species and community level. Generally, observational studies are well suited to study plant responses to changing mean climate, but less suitable to gain a mechanistic understanding of plant responses to climatic variability. Experiments seem best suited to simulate extreme events. In models, temporal resolution and model structure are crucial to capture plant responses to changing climatic variability. We highlight that a combination of experimental, observational and /or modeling studies have the potential to overcome important caveats of the respective individual approaches.
Keywords: climate change, plant phenology, plant physiology, observations, experiments, models, combined approaches
1. Introduction
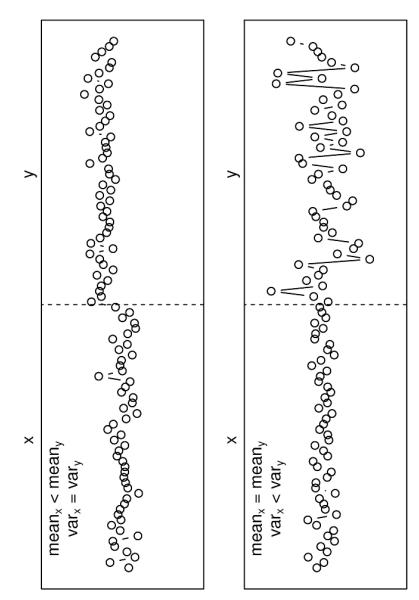
Although the spatial and temporal extent of future climatic changes is still partly uncertain (IPCC 2007a), it is likely that the adaptive capacity of terrestrial plants and ecosystems will be exceeded in many regions (IPCC 2007b). Already today, responses to climate change can be observed for individual species and ecosystems (e.g. Allen & Breshears 1998; Gitlin et al. 2006) but also across species and organizational scales (e.g. Walther et al. 2002; Allen et al. 2010; Lindner et al. 2010). Climate change may manifest itself in two fundamentally different ways: in a change in the mean of for example temperature or precipitation, and in a change in their variability (i.e. variance and/or distribution, Fig. 1; Rummukainen 2012; Seneviratne et al. 2012). These terms relate to steady-state systems. The climate system and ecosystems however are in permanent transition and therefore the term ‘mean’ and ‘variability’ only make sense relative to well-defined spatial and temporal scales. Moreover, it is important to note that mean and variability may not be fully independent (e.g. an increasing mean value often implies increasing standard errors). Here, we still treat changes in mean and variability as two separate aspects, defining changes in the mean as changes over longer time periods (e.g. inter-annual changes) and changes in variability as changes over medium/short term periods (e.g. inter-daily changes) of climatic variables. We define extreme events from this climatological perspective as increasing climatic variability (i.e. increasing variance and/or changing distribution) in contrast to changes in mean climate. Our aim is to emphasize the generally unrecognized distinction between impacts of changing mean climate and changing climatic variability on terrestrial ecosystems.
Figure 1. The two theoretical cases of changing climatic drivers: (1) changes in the mean but not the variance (upper panel), (2) changes in the variance but not the mean of a variable (lower panel).
A third case is conceivable where both the variance and the mean remain comparable, but rare, very extreme events occur, changing essentially the nature of the distribution. Importantly, any discussion of means vs. extremes requires a temporal reference, as a short-term increase in the mean may turn out a long-term increase in the variance.
We center but do not limit our synthesis on a plant’s perspective of temperature and precipitation extremes, since these are the most important climatic determinants of plant growth and survival globally (e.g. Boisvenue & Running 2006). Observations since 1950 show that the length of warm spells and heat waves increased (e.g. Barriopedro et al. 2011; Rahmstorf & Coumou 2011; Seneviratne et al. 2012). More intense and longer droughts are observed but at the same time the number of heavy precipitation events increased (Seneviratne et al. 2012 and references therein). Future projections on changes in climatic variability show strong spatial and temporal heterogeneity (Giorgi et al. 2004; Orlowsky & Seneviratne 2012) and are highly uncertain (Seneviratne et al. 2012). Using multi-model experiments, Barriopedro et al. (2011) for instance found that the probability of summer heatwaves may increase by a factor of 5-10 in the future while Schär et al. (2004) predict that temperature variability will increase by a factor of 2 in Europe. Projected changes in extreme precipitation events (droughts or flooding) are even more uncertain. Orlowsky & Seneviratne 2011 derived from their simulations with an ensemble of Global Circulation Models (GCMs) robust projections on increasing droughts over the Mediterranean and increasing heavy precipitation over the Northern high latitudes.
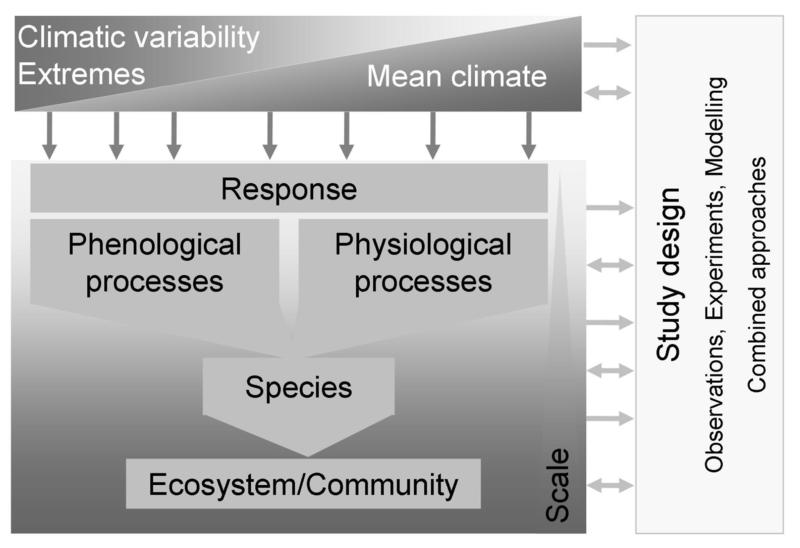
While changes in the mean values are important, there is evidence that plant distribution (Chapin et al. 1993; Bokhorst et al. 2007), survival (van Peer et al. 2004) or net primary productivity and species diversity (Knapp et al. 2002) respond to extreme rather than to average conditions Jentsch & Beierkuhnlein 2008). Additionally to that, different physiological processes such as photosynthesis, water relations or nutrient uptake at the species, community or ecosystem level affect the response of plants to climatic variability (Fig. 2). To account, e.g., for changing precipitation distributions, Knapp et al. (2002) decreased precipitation frequency but not its total amount in a mesic grassland leading to more intense precipitation events. They found reduced carbon turnover but increased species diversity. Drier conditions also tend to decrease evapotranspiration, which leads to lower evaporative cooling (Teuling et al. 2010). In combination, warming and drought can therefore lead to additional warming of an ecosystem (Seneviratne et al. 2006; Fischer et al. 2007; Kuster et al. 2012).
Figure 2. Conceptual overview of the different processes and scales affected by extremes and the methods to study them.
In addition to the impacts of changing climatic variability, the physiological response of terrestrial plants depends also on interactions between species (Thorpe et al. 2011) and their ability to adapt and acclimate. The water available for plants depends on the water holding capacity of the soil (Kramer & Boyer 1995; Porporato et al. 2004; Leuzinger & Körner 2010; Raz-Yaseef et al. 2010), competition with other plants (Casper & Jackson 1997) and precipitation patterns (Knapp et al. 2008). The latter has different effects on soils with high or low water holding capacity (i.e. a stronger or weaker buffer against drought; Knapp et al. 2008) or on flood occurrence, which is an important driver of plant distribution (Crawford 1992; Colmer & Flowers 2008; Parolin & Wittmann 2010). Furthermore, interactions between changing climatic variables as well as thereby induced community shifts may affect the response of plants to new conditions (Langley & Megonigal 2010; de Boeck et al. 2011). For example, a drier and warmer climate will exert stronger constraints on plant growth than a warmer but also wetter climate; or rising CO2 may alleviate the impact of drought (Morgan et al. 2004; Holtum & Winter 2010). Moreover, more prolonged dry periods will alternate with more intensive rainfall events, both within and between years, which will change soil moisture dynamics (Weltzin et al. 2003; Porporato et al. 2004; Fay et al. 2008; Knapp et al. 2008; Bartholomeus et al. 2011a). Eventually, it is also crucial how quickly plant communities adapt genetically to the imposed changes. The IPCC (2007b) concluded that the rate of natural adaptation will be slower than the rate of climate change. Natural adaptation differs between species: while species with short generation times may adapt within years, e.g. Rehfeldt et al. (2001) estimate that it will take 2-12 generations (an equivalent of 200-1200 years) for a coniferous trees species to show genetic adaptation in response to climatic change. All these factors determine whether plants at a specific site will experience changing climatic variability as extreme or not.
Thus, the vulnerability of terrestrial plants to climate change will, besides changes in the mean, largely depend on the changes in the climatic variability and the occurrence of extreme events. The understanding of this difference in experiments and model simulations requires very good knowledge of the baseline or control climate (especially the background variability to which plants are adapted to). This complies with the fact that extreme conditions per se have shaped ecosystems for a long time (Körner 1998, 2003) and may also foster adaptation and thus decrease sensitivity (Hegerl et al. 2011). The plant’s response to specific environmental conditions produces their specialized set of traits which allows them to prevail over competitors and occupy a specific habitat (Körner 1998, 2003). We use the term ‘stress’ throughout this review according to Lortie et al. (2004) to refer to situations in which plants experience critical environmental conditions beyond what they experience normally (Chapin 1991) such that damage to vital function occurs (see Gaspar et al. 2002).
In this paper we strive to answer the following questions:
Which plant processes are vulnerable to changes in the variability of climatic drivers rather than to changes in their mean?
How can we quantify responses of plants to changing climatic variability?
We present evidence from experiments, observations and modeling studies that help to understand the current and future responses of individuals and communities to changing variability, with a particular focus on temporal and spatial patterns. These examples also help to identify important research gaps. We do not aim to cover the literature on these topics systematically.
2. Which plant processes are vulnerable to changes in the variability of climatic drivers rather than to changes in their mean?
The vulnerability of plants refers to their susceptibility to adverse effects of environmental change (IPCC 2007b). Estimates of vulnerability depend on the definitions (e.g. the definition of death (Zeppel et al. 2011)) and the spatiotemporal scale considered. The ultimate limit to withstanding environmental stress from an individual plant’s perspective is mortality due to physiological failure (“You can only die once”) but at the community level, already reductions in growth and subsequently competitiveness may constitute a limit to species fitness. For commercial crops it may even be a critical reduction in productivity so that cultivation is discontinued.
In the following sections, we discuss the vulnerability of phenological and (individual and interacting) physiological processes to changes in the climatic variability rather than the mean of climatic drivers and we highlight how these play out at the species and the community level (see schematic overview in Fig. 2). Our list of examples is not exhaustive but meant to illustrate this important difference between changes in climatic variability rather than the mean.
2.1. Phenological processes
One of the well-studied responses of plant species or communities to environmental change is phenology, which tracks seasonal events in generative and vegetative plant growth. Given the predominant influence of climate (with the important exception of photoperiodism, see Körner & Basler 2010), phenology has emerged as a key tool in identifying fingerprints of anthropogenic climate change in nature (Menzel et al. 2006). Observed large-scale phenological changes such as an earlier onset of leaf unfolding/ flowering (Menzel & Fabian 1999; Walther et al. 2002; Parmesan & Yohe 2003; Root et al. 2003; Menzel et al. 2006) are mainly driven by changes in mean climatic conditions especially temperature (Vitasse et al. 2009; Polgar & Primack 2011; see also Table 1).
Table 1. Examples of observed plant vulnerabilities to changes in the mean climate and climate variability.
| Process | Changing mean | Effect / Response | Reference | Changing variability | Effect / Response | Reference |
|---|---|---|---|---|---|---|
| Phenology | Increase in mean temperature | Prolongation of growing season, earlier onset of leaf unfolding and first flowering, delay of leaf senescence | Menzel & Fabian 1999; Walther et al. 2002; Parmesan & Yohe 2003; Root et al. 2003; Menzel et al. 2006; Polgar & Primack 2011; Vitasse et al. 2009 | Early and late frosts, warm spells, drought, heavy rain | Frost damage, possibly fatal damage to opportunistic species, second or extended flowering, advanced mid- flowering, decreased flowering length | Leuzinger et al. 2011a; Luterbacher et al. 2007; Jentsch et al. 2009 |
| Soil organic matter decomposition | Increase in mean temperature | Potentially increase in soil organic matter decomposition | Saxe et al. 2001 | Droughts/heatwaves | Increase in soil water repellency lead to reduced decomposition of soil organic matter | Goebel et al. 2011 |
| Water relations | Increase in night- time warming (and mean temperature) | Slight increases in stomatal conductance | Albert et al. 2011 | Drought | Stomatal closure and carbon starvation (isohydric plants) hydraulic failure (anisohydric plants) | Breda et al. 2006; McDowell et al. 2008 |
Phenological changes in response to changing climatic variability are much less studied although they clearly interact with phenological changes induced by changing mean climate. For example, in the temperate and boreal zones which are often temperature limited, a central trade-off revolves around maximizing the vegetation period while avoiding frost damage (Kramer et al. 2010). An untimely response to early warm spells may be fatal but can bring enormous advantages for early successional or opportunistic species (r-strategists, Leuzinger et al. 2011a). In contrast, long-lived, late successional species often have chilling requirements and photoperiodic safety mechanisms (Heide 1993) and thus may be in a position to avoid increasing risks of late frost due to changing climatic variability but would also benefit less from early warm spells. This is supported by the fact that the risk of damage due to late frost events has not increased so far for several coniferous and broad-leaved species in Central Europe (Scheifinger et al. 2003; Menzel et al. 2003). Besides this example, there is further evidence, that extreme events may alter phenological responses depending on their timing and strength (e.g. Jentsch et al. 2009; Menzel et al. 2011). This can lead to unexpected effects such as second flowering in autumn or extended flowering until the beginning of winter for some species (Luterbacher et al. 2007). Moreover, extreme warm spells decreased the differences in spring phenology between urban and rural sites (Jochner et al. 2011). Furthermore, only half of the trees reached leaf maturity in an extreme drought experiment in the Mediterranean (Misson et al. 2011). Overall, the response of phenology to climatic variability seems to be less well understood than to changing mean climate although increasing climatic variability may have a strong damaging potential.
2.2. Physiological processes
We here focus on the response of plant water relations such as transpiration to climatic variability (drought/heat waves and excess water). Increasing temperatures and/or heat waves combined with less precipitation or more variable precipitation events lead to prolonged dry periods and high atmospheric demand for plant transpiration, which determine drought stress of plants beyond changes in mean climate (Schimper 1903; Porporato et al. 2004). Barriopedro et al. (2011) predict such an increase in drought events for the 21st century and the consequences for plant water relations are well documented (e.g. Leuzinger et al. 2005; Bréda et al. 2006; Granier et al. 2007) although not all mechanism are fully understood. There is an ongoing debate about two competing response strategies to drought: Isohydric plants may respond by closing their stomates thus reducing their water loss but eventually facing carbon starvation, whereas anisohydric plants keep their stomates open thus running the risk of hydraulic failure (Mc Dowell et al. 2008; Sala et al. 2010; Zeppel et al. 2011). Furthermore, Craine et al. (2012) highlighted the importance of the timing of an extreme event for grassland productivity. The response of plants to drought is of such an importance that Hartmann (2011) refers to it as a “change of evolutionary forces” from competition for light to competition for water and carbon. The responses of plants to climatic variability and particularly drought have important consequences for net primary productivity (NPP) and hence carbon cycling even at large spatial scales such as Europe (Ciais et al. 2005; Dury et al. 2011). Thus, plant responses to increasing drought events and heat waves influence plant functioning across spatial and temporal scales.
Also climatic variability resulting in excess water (i.e. flooding or waterlogging), can induce important physiological responses by terrestrial plants. Due to waterlogging O2 diffusion and supply to the roots is reduced, and the oxygen demand of plant roots, (i.e. root respiration – oxygen consumption in the roots), cannot be fulfilled (Lloyd & Taylor 1994; Blom & Voesenek 1996; Kozlowski 1997; Amthor 2000). This results in waterlogging/ oxygen stress, i.e. lack of oxygen due to high soil moisture contents (Bartholomeus et al. 2008). Both the oxygen supply and demand may be affected by a more extreme climate, due to more intense precipitation and higher temperatures (respiration increases with temperature), respectively. Therefore, to analyze the effects of low soil oxygen availability on species performance, it is necessary to integrate the soil physical and plant physiological processes, thus accounting for both the oxygen supply to and oxygen demand of plant roots (Bartholomeus et al. 2011b). Besides reduced root respiration rates, the decrease of water absorption due to waterlogging stress causes sensitive plants to wilt in a similar way to drought (Jackson & Drew 1984). Many species already growing in flood-prone habitats have developed different strategies to survive hypoxia, by producing aerenchyma and/or adventitious roots in response to an increase in the concentration of ethylene and auxin (Blom & Voesenek 1996). Flooding can also give rise to detrimental effects at leaf level, by inducing stomatal closure and, consequently, limiting gas exchange and plant growth (Kramer 1951; Chen et al. 2005; Rengifo et al. 2005; Fernandez 2006). Thus, similarly to drought, extremes of excess water, in combination with higher temperatures, strongly alter plant physiological processes such as carbon uptake and transpiration.
In conclusion, we note that plant water relations seem to be very vulnerable to increasing variability in temperature and precipitation and that changing heatwaves and flooding have stronger impacts on physiological processes than changing mean climate (see also Table 1).
2.3. Interacting physiological processes
The interaction of physiological processes such as photosynthesis, nutrient uptake and water relations may strongly affect the response of plants to changing climatic variability. Furthermore, interactions among several global change drivers or between global change drivers and other environmental variables, may result in other growth-limiting factors (e.g. soil type) becoming less important. Drought periods for example may have the potential to not only determine growth or mortality in an ecosystem but also to cause shifts in growth-limiting factors, e.g. nutrient limitations. For example, in an experiment of Kuster et al. (2012) oaks were grown on two different soil types with different nutrient availabilities. Under well-watered conditions, growth on one soil was lower due to nutrient-limiting conditions, whereas under repeated drought periods these differences disappeared. This shows that growth-limiting factors such as nutrient availability can become less important under changing climatic variability, while they may be overlooked if only changes in mean climate are considered. There are many other examples of interacting processes under changing climatic variability such as ozone stress during periods of high temperature (Matyssek et al. 2010; Pretzsch & Dieler 2011).
The interactions of physiological processes can however be even more intriguing. In coastal habitats (i.e. the interface of terrestrial and aquatic habitats) which are not only saline, but are also prone to flooding (e.g. mangroves and salt marshes) (Colmer & Flowers 2008) Tamarix africana Poir., for example, showed a reduction of CO2 assimilation rates only in young Tamarix africana Poir. leaves after 45 days under continuous flooding with saline water (200 mM), while old leaves and the aboveground relative growth rate were not affected by the treatment (Abou Jaoudé et al. 2012). Thus, while parts of the plants actually responded to flooding, this was not the case for the entire plant. This example is rather related to changes in mean climatic conditions (i.e. temperature-induced rising sea levels) but it highlights that changing climatic variability is likely to interact with an already complex interplay of physiological processes.
2.4. Species-level processes
At the species level, responses of different genotypes to climate provide information how a species may react to changing climatic variability. Since genotypic variation results in different sensitivity thresholds of distinct ecotypes to changing climatic variability it can partly substitute lacking data of changing climatic variability for a specific genotype. In an ecotype study (Klein et al. submitted) that included all three climate types (meso-Mediterranean (MM), thermo-Mediterranean (TM), and semi-arid (SA) within the natural distribution of the forest tree Pinus halepensis Mill. (and hence three very different combinations of mean climate and climate variability), two major physiological adjustments were identified: (1) shortening of the growing season length (from 165 to 100 days) to match a shorter rainy season and (2) increasing water use efficiency (from 80, to 95, to 110 μmol CO2 mol-1 H2O under MM, TM, and SA climates respectively). However the sensitivity threshold differed in between ecotypes: Northern ecotypes mainly responded to the change MM to TM, whereas Southern ecotypes responded to the change TM to SA. At the species level, the study showed that higher xylem sensitivity to embolism in specific ecotypes matched previous reports (Atzmon et al. 2004; Schiller et al. 2009) of significantly higher mortality rates in these ecotypes under yet harsher conditions. These observations suggest that while hydraulic constraints in response to climatic variability limited the distribution of a tree species, plasticity in water use efficiency and growth phenology enabled its success under a wide range of climatic conditions.
2.5. Community-level processes
At the community level, phenological, physiological and species-level processes as well as their interaction culminate in complex responses to changing mean climate and climatic variability (Fig. 2). Species range shifts have been associated with changes in mean climate (Lenoir et al. 2008; Harsch et al. 2009) but also with changing climatic variability (Kelly & Goulden 2008; Doak & Morris 2010). They lead to a disruption of ecological communities and species interactions due to different dispersal speed and success. These processes differ between the trailing and the leading edge of a population (Kramer et al. 2010; Doak & Morris 2010). From a community’s perspective such range shifts may entail positive (e.g. release from competition) and negative (e.g. loss of important pollinator) consequences. Despite these importance consequences of range shifts, it is yet unclear whether changing mean climate or changing climatic variability will be the more important driver of range shifts.
At community level, for annual plants, the variability of rainfall is important for the success of germination. Increasing climate variability can have both negative and positive effects on species persistence and thus plant population dynamics (Levine et al. 2008). Climatic fluctuations, for example, may enable species to avoid interspecific competition if species differ in the years in which they perform (e.g. reproduce or grow) best (Levine & Rees 2004). Dormancy and germination biology determine whether temporal variability favors or inhibits species persistence (Levine & Rees 2004) and can thus be limiting for a species (Godefroid et al. 2011). Temporal variation in resource availability as induced by climatic variability may reduce the effects of competitive exclusion, allowing more species to coexist (Knapp et al. 2002).
A combination of extremes/multiple stresses may not only hamper performance but may also drive extinctions (Smith & Huston 1989; Niinemets & Valladares 2006). As functional trade-offs exist in adjusting to multiple environmental limitations (Holmgren et al. 1997; Silvertown et al. 1999), adapting to one stressor may go at the cost of adapting to another (Holmgren et al. 1997; Niinemets & Valladares 2006). This trade-off among the tolerances to multiple environmental limitations hampers niche differentiation (Niinemets & Valladares 2006). Bartholomeus et al. (2011a) demonstrated that the interaction between both the wet and dry extremes of plant water stress (oxygen/waterlogging and drought stress) is particularly detrimental to the survival of specialists and of endangered plant species. Both wet and dry weather extremes may increase due to changing climatic variability, thus increasing the risk of a combination of these stressors to occur at a site (Knapp et al. 2008; Bartholomeus et al. 2011a). This may favor generalists over specialists and rare species and thus influence vegetation dynamics and associated ecosystem services in response to changing climatic variability at the community level.
3. How can we quantify responses of plants to changing climatic variability?
Just as responses to global change in general (Rustad 2008), the responses of plants to changing climatic variability can be assessed in observational, experimental and modeling studies and combinations of these approaches (Fig. 2). All these approaches have their limitations in assessing a plant’s perspectives of extremes: on the one hand, observational studies are by definition ‘opportunistic’ in the sense that extreme conditions such as a long-lasting drought can not be planned (Smith 2011). On the other hand, scaling and higher-order interactions are an important issue in experimental and modeling studies (Leuzinger et al. 2011b; Wolkovich et al. 2012). Furthermore, it is crucial for any type of study that claims to assess climate variability to report whether changing mean climate and/or changing climatic variability have truly been measured and what the background variability of the system is over a well-defined time period. We qualitatively show this in Table 2 for a number of studies cited above as a first attempt to foster consistent reporting of studies dealing with climatic variability.
Table 2. Are we measuring the impact of mean climate or climate variability? Non-exhaustive list of the studies cited in the text and their testing amplitude in comparison to the background variability in the respective study system.
The last column indicates in a qualitative way how well the testing amplitude accounts for climatic variability in terms of the background variability.
| Study system | Testing amplitude | Background variability | Study type | Reference | Testing climate variability? |
|---|---|---|---|---|---|
| European grassland & heath species | Drought: 32 days | Local 100-year extreme drought (number of days with precipitation < 1mm), 33 days of drought in 1976 | Experiment | Jentsch et al. 2009 | Yes, 100-year-event |
| European grassland & heath species | Precipitation: 170mm over 14 days | Local 100-year rainfall extreme, 152mm of precipitation over 14 days in 1977 | Experiment | Jentsch et al. 2009 | Yes, 100-year-event |
| European plant phenology | +1.5 (warm), +3 (very warm), −1.5 (cold) and −3 (very cold) standard deviations from the long-term mean at the respective grid point to classify warm and cold spells | Long-term mean | Observation | Menzel et al. 2011 | Yes, +/− 3 standard deviations from mean |
| Grasslands | 6-8 large precipitation events per growing season (mean per event = 42 mm) | The large size and low frequency of precipitation events in the altered precipitation treatment are well within the range of documented precipitation regimes of the past 100 years in this region | Experiment | Knapp et al. 2002 | Yes, but less than 100-year- event |
| Young oak stands (3 species (Quercus robur, Quercus petraea, Quercus pubescens), 4 provenances each) | Amount of irrigation water in drought- treated stands was 60% lower than the long-term mean precipitation (728 mm during the growing season from April to October) in 2007 and 43% lower in 2008 and 2009. Experimental droughts were imposed by stopping irrigation for several consecutive weeks during selected periods in the growing season | Compared to the long-term mean of the site, the amount of irrigation in the control was 16% lower in 2007, 26% higher in 2008 and 30% higher in 2009 | Experiment | Kuster et al. 2012 | Unclear but testing amplitude much larger than variability in control |
| Mixed broadleaved forest in Central Europe | Seasonal precipitation: 50% of the 10- year mean from 1989 to 1999, Spring precipitation: below the mean, Mean monthly temperatures: exceeded the long-term mean (1989–1999) (e.g., + 6.8 °C for June). | Long-term mean | Observation | Leuzinger et al. 2005 | Unclear (background variability not further specified) but likely |
| Pinus halepensis stands (3 contrasting sites, 5 provenances) | Precipitation in Rome, 766+-156mm; Tel Aviv, 557+-184mm, and Yatir (semi-arid), 279+-88mm | Long-term mean (differences in mean climate are very large hence testing amplitude equals high background variability but no explicit testing of climate variability) | Transplantation | Klein et al. submitted | Locally unclear but over the species distribution range probably yes |
| Tamarix africana Poiret | Continuous soil flooding with fresh and saline water during 45 days | Not explicitly mentioned, plant survived 45 days of flooding | Experiment | Abou Jaoudé et al. 2012 | Unclear |
3.1. Observational studies
Observational studies elucidate plant’s perspectives of extremes, if by chance they cover extremes. This makes them inherently opportunistic (Smith 2011) unless they involve some retrospective elements such as dendrochronology. Observations from ‘extreme’ (from a plant’s perspective) sites (e.g. from the leading and trailing edge of population (Doak & Morris 2010)) can help us learning about the limits and coping range of plants. To this end, GIS mapping of ‘extreme’ sites within a species’ distribution requires careful interpolation of weather/climate data collected at appropriately distributed climate stations. However, ‘extreme’ sites are sometimes only poorly studied since they represent marginal ecosystems, whose services are not fully valued by society and have thus been outside the main focus of researchers. The psamophilic plants and vegetation of the beaches and dunes of the Portuguese coast, for example, are highly adapted to very specific environmental conditions and directly exposed to sea level rise, storms and severe erosion processes. Unless their ecological requirements, functioning as communities and most influential physical drivers are understood, it will be difficult to study their responses to future climate change (Martins et al. 2011). It is however important to note, that in some disciplines there is a strong focus on extreme sites (such as on cold, high elevation or very dry sites in dendrochronological studies (e.g. Gruber et al. 2012)) which in turn may complicate studying mean climate impacts.
Generally, observational studies are well suited to study plant responses to changing mean climate, since long-term ecological data can be matched with increasingly available climatic observations. They are less suitable to gain a mechanistic understanding of plant responses to climatic variability since usually too many factors are involved and not all are measured.
3.2. Experimental studies
Experiments allow for controlled conditions and factorial experiments in the field and laboratory, have a long history in ecological research and are of crucial importance for global change studies (Luo et al. 2011)). When quantifying climate change impacts however, field experiments can usually only test a limited number of factors and their combinations due to financial and logistic constraints (Templer & Reinmann 2011). Therefore, interactions can often not be fully assessed (e.g. Wolkovich et al. 2012). Furthermore, to provide answers to the question of how extreme climatic events impact on ecosystems, experimenters should make sure the applied treatment is indeed ‘extreme’ beyond the current background variability of the system over a well-defined time period, running the risk of killing plants (Leuzinger & Thomas 2011; Beier et al. 2012).
Also, the temporal scale influences the outcome of an experiment. A comparable set of factors and a minimal experimental duration, for example, for all drought experiments would therefore be desirable. However, even then, most experiments would have to stop after few years. This raises the question whether the experiment actually simulates extreme situations or long-term change and whether the system recovers after the experiment ends. The high diversity in the response of growth parameters of oaks to drought as discussed in Kuster et al. (2012), shows that in experimental conditions, e.g. treatment duration and intensity, tree age or experimental set up, have to be considered in the evaluation of drought effects on trees. Thus it is crucial to assess what degree of change and what temporal scale experiments cover if we want to evaluate whether they actually simulate responses to changing climatic variability, or rather to changing mean climate.
In a transplantation study, for example, the effect of a drying and warming trend was obtained by comparing tree performance in Rome (Italy), Tel Aviv (Israel) and Yatir (Israel) along a precipitation gradient (Klein et al. submitted). The sites differed significantly in their mean annual precipitation, each representing a different climate type, but the responses were interpreted as drought acclimation. Results from this study captured many plant adjustments that were induced by both phenotypic plasticity and locally adapted ecotypes. Such transplantation experiments along altitudinal or latitudinal gradients do not require manipulation of the environment and may be an alternative to laboratory/greenhouse experiments. So far, transplantation experiments have not been considered in comparative studies of different artificial warming methods (e.g. Aronson & McNulty 2009). However, such experiments seem to be well adapted especially for long term experiments, as they project a realistic simulation of future climate conditions considering also the length of the growing period, one of the most important limiting factors in alpine plant growth (Jonas et al. 2008). Similar to laboratory/greenhouse experiments it is crucial that the results are interpreted in terms of changing mean climate and changing variability over well-defined temporal scales.
3.3. Modeling
Models can be used as diagnostic and predictive tools that integrate results from experiments and observation to gain mechanistic understanding and allow testing hypothesis generated from field data, experiments and theory (Leuzinger & Thomas 2011; Luo et al. 2011). Models have to be designed for a specific purpose and here we discuss which are suitable to simulate plant responses to changing climate variability. This is a highly relevant question, since models that account for extremes may require a different structure, e.g. an appropriate time resolution to capture an extreme precipitation event. Many forest models for example use monthly input data and are thus unable to account for short-term extreme events (e.g. Bugmann 2001). Forcing such a model with daily weather instead with monthly climate data improved its performance (Stratton et al. 2012). Zimmermann et al. (2009) argue that for capturing some ecosystem responses even daily climate data may be insufficient since they smooth meteorological extremes.
Generally, effects of climate change on ecosystems are analyzed by driving simulation models with output from general circulation models (GCMs) and regional climate models (RCMs). To account for the uncertainty of climate change projections, besides different scenarios, also several GCMs (e.g. Buisson et al. 2010) and different realizations of a scenario may be used. Many models do not use the original GCM/RCM data at hourly resolution (which may also not always be available) but only daily or monthly aggregations and thus strictly speaking miss some of the meteorological variability. The CARAIB dynamic vegetation model (Otto et al. 2002; Laurent et al. 2008; Dury et al. 2011), for example, derives daily values of meteorological variables, as usual in large-scale simulations, from monthly mean outputs from GCM/RCMs using a stochastic weather generator (Hubert et al. 1998). The sequences of daily temperature or precipitation produced by the stochastic generator are renormalized to the monthly values generated by the RCMs. Thus the precise day-to-day sequence of an extreme event in the model, such as a drought period or a succession of heat wave days (Beniston et al. 2007; Déqué 2007), depends on the distribution functions used in the stochastic generator, although the monthly values of the climate model are not altered. While evidently it is challenging for such large scale modeling efforts to integrate high-frequency climate variability, these studies are necessary to assess different feedbacks of vegetation types (e.g. feedbacks of ecosystem response to drying on near-surface temperature differ between forest and grassland ecosystems (Teuling et al. 2010)) at the global scale.
Also, species distribution models face the challenge of including changing climate variability. Usually, they use information on species distribution (both potential from expert knowledge or forest communities, and actual from inventories and landcover-data) together with climate data to construct bioclimatic ranges (also called climate envelopes). They show a two dimensional frequency distribution of e.g. temperature and precipitation, indicating the mean climatic range, in which the analyzed species (potentially) exist. Extrapolation of this information allows identifying regions with comparable climate to e.g. estimate a (extended) potentially occupied habitat (Guisan & Zimmermann 2000) or new growing areas outside the recent (actual or potential) distribution (Miller et al. 2004; Peters et al. 2004). Also the match of actual and future suitable ranges can be identified, classifying species into tolerant or intolerant to expected climatic conditions (Dunk et al. 2004; Gibson et al. 2004). This provides further understanding about expanding or shrinking habitats under changing climate (Erasmus et al. 2002; Midgley et al. 2006). Usually, climate envelopes are derived from mean values (e.g. mean temperature) and are thus designed to assess impacts of changes in mean climate. Consequently especially regions at the edge of the distribution range may appear suitable, but in reality maximum or minimum precipitation or temperature may determine the distribution range (or other, non-climatic factors such as soil type or herbivory). This can partly be circumvented by including standard deviations as variables (Zimmermann et al. 2009), and species distribution models could also be built with extremes (e.g. maximum temperature or minimum precipitation) to enhance the predictive power. Zimmermann et al. (2009) for example found that incorporating climatic extremes slightly improved models of species range limits, since it corrected local over- and underprediction, but they also argue that climate variability rather complements the response to mean climate. Thus including climate variability is one uncertainty of species distribution models that has to be considered to assess compliance of climate envelopes (Gloning et al. in prep.).
While generally process-based modeling is required to derive climate-robust relationships to predict vegetation characteristics (Franklin 1995; Guisan & Zimmermann 2000; Schwalm & Ek 2001; Botkin et al. 2007; Suding et al. 2008; Hajar et al. 2010), this is even more evident when considering changing climate variability in particular. Bartholomeus et al. (2011b) demonstrated that, in contrast to process-based relationships between site factors and vegetation characteristics, relations based on indirect site factors produce systematic prediction errors when applied outside their calibration rate, and so cannot be used for climate projections. Mean groundwater level, for example, is only an indirect site factor related to plant performance, as it is the interaction between soil-water-plant-atmosphere that essentially determines if plants suffer from drought stress or oxygen/waterlogging stress. When, for example, soil moisture availability is too low to meet the water demand for transpiration, a plant suffers from drought stress (Reddy et al. 2004; Schimper 1903). This so-called physiological drought (Schimper 1903), implies that not only water availability but also vegetation’s demand for water has to be considered. Instead, more process-based explanatory variables are needed to predict the effects of changing climate variability on the species composition of the vegetation. These explanatory variables should consider the interacting meteorological, soil physical, microbial, and plant physiological processes in the soil-plant-atmosphere system. Bartholomeus et al. (2011a) did so for water related stressors, by simulating respiration reduction (reflecting the combined effect of high temperature and low oxygen availability), and transpiration reduction (reflecting the combined effect of high atmospheric water demand and low water availability) for a reference vegetation. The simulated stress for reference vegetation acts as a habitat characteristic, i.e. a measure for the moisture regime of the soil to which the actual vegetation will adapt. The use of reference vegetation improves the applicability of models in which stress measures are implemented, especially in predicting climate change effects (Dyer 2009).
3.4. Combined approaches
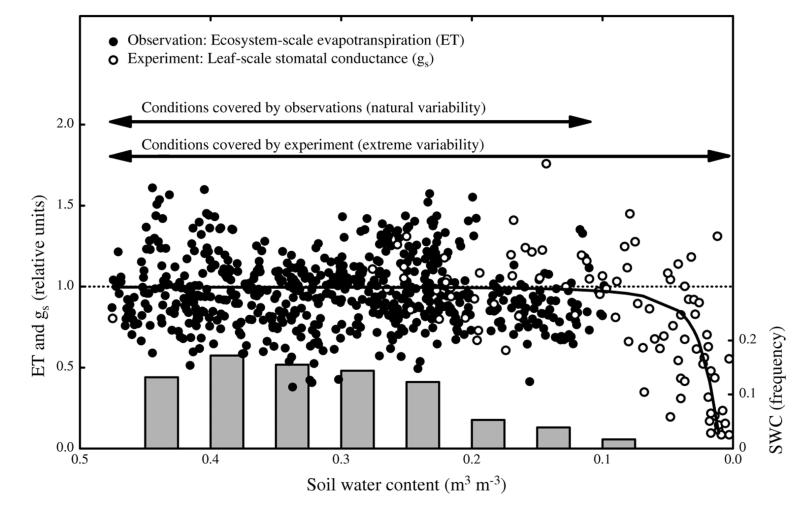
Combined approaches unite experimental, observational and/or modeling studies. A recent meta-analysis shows that the temperature sensitivity of phenology in warming experiments is underestimated in comparison to observations (Wolkovich et al. 2012). It highlights that observational studies are crucial to test whether experimental results match observations in natural systems. A combination of laboratory and field studies is necessary to determine whether thresholds detected in the laboratory, are also likely to occur in the field. This is especially relevant when calculating the effects of changing climatic variability. We take leaf gas exchange and ecosystem flux measurement data from Brilli et al. (2011) as an example of how to link experiments and observation at different scales and how an experiment can complement observations to study plant responses to climate variability. Fig. 3 shows that evapotranspiration measured in the field with the eddy covariance method, was insensitive to soil drying over the range of soil water contents occurring in the field. The leaf gas exchange measurements during the laboratory drought experiment when extended to much drier conditions showed that the plant species occurring at this site start to down-regulate stomatal conductance at soil water contents close to the wilting point – conditions that have never been reached in the field during the observational period of 2001-2009. Back-of-the-envelope calculations suggest that ca. 10 additional rain-free days would have been required even during the 2003 and 2006 droughts in order for plants at this site to experience gas exchange limitations. Such information is crucial to assess whether responses to changing mean climate or to changing climate variability are measured.
Figure 3. Evapotranspiration measured in the field with the eddy covariance method (black filled dots) over the range of soil water contents (grey bars) occurring in the field and stomatal conductance measured in a laboratory experiment (black open dots).
Data from and further descriptions available in Brilli et al. (2011). SWC = Soil Water Content.
Moreover results can be extended to a larger spatial scale, by combining simulation models with research tools like raster GIS (Minacapilli et al. 2009; Bonfante et al. 2011) and Digital Elevation Model (DEM) derived analysis (MacMillan et al. 2000). Furthermore, studies that combine observational or experimental results - at field scale - with simulation models of hydro-thermal regime - at landscape scale - allow to quantify the effects of changing climate variability (Bonfante et al. 2010). Riccardi et al. (2011) assessed the adaptive capacity of olive cultivars to future climate by means of a data base of cultivars’ climatic requirements, combined with a spatially distributed model of the soil–plant–atmosphere system. They set up a database on climatic requirements and defined critical environmental conditions using two quantitative indicators of soil water availability (the relative evapotranspiration deficit, i.e. the ratio of actual to maximum evapotranspiration of the crop, and the relative soil water deficit, i.e. the ratio between the actual and the maximum volume of soil water available to plants taking into account the water retention characteristics, to get a comparable indicator across soil types). The response in terms of yield of several olive cultivars to these indicators was determined through the re-analysis of experimental data derived from scientific literature (Moriana et al. 2003; Tognetti et al. 2006). This database on cultivars’ requirements was used in combination with a plant-soil-atmosphere model (SWAP, van Dam et al. 2008). The model was used to describe the soil water regime at landscape scale under future climate scenarios from statistically down-scaled GCMs, resulting in several realizations (Tomozeiu et al. 2007). The indicators of soil water availability were thus determined in different soil units, and were compared with the limits set for each cultivar. A cultivar was considered tolerant to expected climatic conditions when the indicator values resulted above critical values in at least 90% of realizations. While Riccardi et al. (2011) did not further specify the climate scenarios and realizations in terms of changing mean or climate variability, such analysis could be easily linked to the soil water availability indicators and the related limits for cultivars under climate change.
4. Conclusions
In this review, we have emphasized that changing climatic variability and the resulting extreme (climatic) conditions are highly relevant for different plant processes at different scales in comparison to changes in mean climate (although mean and variability may not be fully independent of each other). We have also shown how to quantify responses of plants to changing climate variability: While experiments seem to be well-suited to study the effects of changing climatic variability it is important to remember that they only control a limited number of factors. For modeling studies we stress that the model structure should allow integrating extreme events (e.g. by having the appropriate temporal resolution). These points highlight the importance of linking experiments, observations, and modeling studies as well as assessing study results in light of the background variability of the system and the temporal scale considered. We also identified the several research gaps. While knowledge of plant responses to changing climatic variability for individual processes has to be consolidated, we still lack knowledge on how interactions of these processes and other environmental variables play out at different hierarchical levels and in combination with changing mean climatic conditions. Similarly, while there is room to improve individual methods to study changing climatic variability, there is a particular need to integrate observations, experiments and models results across scales.
Ultimately, the information on extremes and corresponding vulnerability of plants are crucial to identify which species and regions (and thus which ecosystem services and functions) are most at risk from climate change. Moreover, designing ecosystem-based adaptation strategies to climate change relies on understanding the interactions between species’ natural adaptive capacity and climate change. Analyzing plant responses to climate variability is important to determine drivers of ecosystem dynamics over time (slow vs. fast processes) and highlights the importance of extremes to assess the impacts of environmental change on socio-ecological systems.
Acknowledgement
This review synthesizes and expands the results from a session which was held during the 2011 European Geoscience Union (EGU) general assembly (BG2.7). We are grateful to all participants of this session for the valuable discussions. CR acknowledges funding from the EC FP7 MOTIVE project (grant agreement no. 226544). SL was funded by EC FP7 ACQWA. AR acknowledges funding from the EU FP-7 project CARBO-Extreme (grant agreement no. 226701). FDL acknowledges funding from the MIPAAF-IT project AGROSCENARI. We are grateful to one anonymous reviewer for intelligent comments on an earlier version of this paper.
References
- Abou Jaoudé R, De Dato G, Palmegiani M, De Angelis P. Impact of fresh and saline water flooding on leaf gas exchange in two Italian provenances of Tamarix africana Poiret. Plant Biology. doi: 10.1111/j.1438-8677.2012.00597.x. (in press) [DOI] [PubMed] [Google Scholar]
- Albert KR, Ro-Poulsen H, Mikkelsen TN, Michelsen A, Van Der Linden L, Beier C. Effects of elevated CO2, warming and drought episodes on plant carbon uptake in a temperate heath ecosystem are controlled by soil water status. Plant, Cell & Environment. 2011;34:1207–1222. doi: 10.1111/j.1365-3040.2011.02320.x. [DOI] [PubMed] [Google Scholar]
- Allen CD, Breshears DD. Drought-induced shift of a forest woodland ecotone: Rapid landscape response to climate variation. Proceedings of the National Academy of Sciences. 1998;95:14839–14842. doi: 10.1073/pnas.95.25.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management. 2010;259:660–684. [Google Scholar]
- Amthor JS. The McCree–de Wit–Penning de Vries–Thornley respiration paradigms: 30 Years Later. Annals of Botany. 2000;86:1–20. [Google Scholar]
- Aronson EL, Mcnulty SG. Appropriate experimental ecosystem warming methods by ecosystem, objective, and practicality. Agricultural and Forest Meteorology. 2009;149:1791–1799. [Google Scholar]
- Atzmon N, Moshe Y, Schiller G. Ecophysiological response to severe drought in Pinus halepensis Mill. trees of two provenances. Plant Ecology. 2004;171:15–22. [Google Scholar]
- Barriopedro D, Fischer EM, Luterbacher J, Trigo RM, García-Herrera R. The Hot Summer of 2010: Redrawing the Temperature Record Map of Europe. Science. 2011;332:220–224. doi: 10.1126/science.1201224. [DOI] [PubMed] [Google Scholar]
- Bartholomeus R, Witte J-P, Van Bodegom P, Van Dam J, Aerts R. Climate change threatens endangered plant species by stronger and interacting water-related stresses. Journal of Geophysical Research. 2011a;116:G04023. [Google Scholar]
- Bartholomeus R, Witte J-P, Van Bodegom P, Van Dam J, De Becker P, Aerts R. Process-based proxy of oxygen stress surpasses indirect ones in predicting vegetation characteristics. Ecohydrology. 2011b [Google Scholar]
- Bartholomeus R, Witte J-P, Van Bodegom P, Van Dam J, Aerts R. Critical soil conditions for oxygen stress to plant roots: Substituting the Feddes-function by a process-based model. Journal of Hydrology. 2008;360:147–165. [Google Scholar]
- Beier C, Beierkuhnlein C, Wohlgemuth T, et al. Precipitation manipulation experiments – challenges and recommendations for the future. Ecology Letters. 2012 doi: 10.1111/j.1461-0248.2012.01793.x. [DOI] [PubMed] [Google Scholar]
- Beniston M, Stephenson D, Christensen O, et al. Future extreme events in European climate: an exploration of regional climate model projections. Climatic Change. 2007;81:71–95. [Google Scholar]
- Bertoldi G, Della Chiesa S, Niedrist G, Hell V, Tasser E, Tappeiner U. Modelling the water and energy budget in alpine environments at plot and catchment scale. Geophysical Research Abstracts. 2011;13:EGU2011–10152. [Google Scholar]
- Blom C, Voesenek L. Flooding: the survival strategies of plants. Trees. 1996;11:290–295. doi: 10.1016/0169-5347(96)10034-3. [DOI] [PubMed] [Google Scholar]
- Blumwald E, Aharon G, Apse M. Sodium transport in plant cells. Biochimica et Biophysica Acta. 2000;1465:140–151. doi: 10.1016/s0005-2736(00)00135-8. [DOI] [PubMed] [Google Scholar]
- Boisvenue C, Running S. Impacts of climate change on natural forest productivity – evidence since the middle of the 20th century. Global Change Biology. 2006;12:862–882. [Google Scholar]
- Bokhorst S, Huiskes A, Convey P, Aerts R. The effect of environmental change on vascular plant and cryptogam communities from the Falkland Islands and the Maritime Antarctic. BMC Ecology. 2007;7:15. doi: 10.1186/1472-6785-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante A, Basile A, De Lorenzi F, Langella G, Terribile F, Menenti M. The adaptative capacity of a viticultural area (Valle Telesina, Southern Italy) to climate changes. Proceedings VIII International Terroir Congress; June 14-18; Soave (VR) Italy: 2010. pp. 96–101. [Google Scholar]
- Bonfante A, Basile A, Langella G, Manna P, Terribile F. A physically oriented approach to analysis and mapping of terroirs. Geoderma. 2011;167-168:103–117. [Google Scholar]
- Botkin DB, Saxe H, Araujo MB, et al. Forecasting the Effects of Global Warming on Biodiversity. Bioscience. 2007;57:227–236. [Google Scholar]
- Bréda N, Huc R, Granier A, Dreyer E. Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Annals of Forest Science. 2006;63:625–644. [Google Scholar]
- Brilli F, Hörtnagl L, Hammerle A, Haslwanter A, Hansel A, Loreto F, Wohlfahrt G. Leaf and ecosystem response to soil water availability in mountain grasslands. Agricultural and Forest Meteorology. 2011;151:1731–1740. doi: 10.1016/j.agrformet.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks P, Vivoni E. Mountain ecohydrology: quantifying the role of vegetation in the water balance of montane catchments. Ecohydrology. 2008;1:187–192. [Google Scholar]
- Bugmann H. A Review of Forest Gap Models. Climatic Change. 2001;51:259–305. [Google Scholar]
- Buisson L, Thuiller W, Casajus N, Lek S, Grenouillet G. Uncertainty in ensemble forecasting of species distribution. Global Change Biology. 2010;16:1145–1157. [Google Scholar]
- Casper B, Jackson R. Plant Competition Underground. Annual Review of Ecology and Systematics. 1997;28:545–570. [Google Scholar]
- Chapin F., III Integrated Responses of Plants to Stress. Bioscience. 1991;41:29–36. [Google Scholar]
- Chapin F, III, Rincon E, Huante P. Environmental responses of plants and ecosystems as predictors of the impact of global change. Journal of Biosciences. 1993;18:515–524. [Google Scholar]
- Chen H, Qualls R, Blank R. Effect of soil flooding on photosynthesis, carbohydrate partitioning and nutrient uptake in the invasive exotic Lepidium latifolium. Acquatic Botany. 2005;82:250–268. [Google Scholar]
- Ciais P, Reichstein M, Viovy N, et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature. 2005;437:529–533. doi: 10.1038/nature03972. [DOI] [PubMed] [Google Scholar]
- Colmer T, Flowers T. Flooding tolerance in halophytes. New Phytologist. 2008;179:964–974. doi: 10.1111/j.1469-8137.2008.02483.x. [DOI] [PubMed] [Google Scholar]
- Craine JM, Nippert JB, Elmore AJ, Skibbe AM, Hutchinson SL, Brunsell NA. Timing of climate variability and grassland productivity. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1118438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. Oxygen availability as an ecological limit to plant distribution. Advances in Ecological Research. 1992;23:93–185. [Google Scholar]
- De Boeck HJ, Dreesen FE, Janssens IA, Nijs I. Whole-system responses of experimental plant communities to climate extremes imposed in different seasons. New Phytologist. 2011;189:806–817. doi: 10.1111/j.1469-8137.2010.03515.x. [DOI] [PubMed] [Google Scholar]
- Déqué M. Frequency of precipitation and temperature extremes over France in an anthropogenic scenario: Model results and statistical correction according to observed values. Global and Planetary Change. 2007;57:16–26. [Google Scholar]
- Doak DF, Morris WF. Demographic compensation and tipping points in climate-induced range shifts. Nature. 2010;467:959–962. doi: 10.1038/nature09439. [DOI] [PubMed] [Google Scholar]
- Dunk J, Zielinski W, Preisler H. Predicting the occurrence of rare mollusks in northern California forests. Ecological Applications. 2004;14:713–729. [Google Scholar]
- Dury M, Hambuckers A, Warnant P, Henrot A, Favre E, Ouberdous M, François L. Responses of European forest ecosystems to 21st century climate: assessing changes in interannual variability and fire intensity. iForest. 2011;4:82–99. [Google Scholar]
- Dyer J. Assessing topographic patterns in moisture use and stress using a water balance approach. Landscape Ecology. 2009;24:391–403. [Google Scholar]
- Erasmus BFN, Van Jaarsveld AS, Chown SL, Kshatriya M, Wessels KJ. Vulnerability of South African animal taxa to climate change. Global Change Biology. 2002;8:679–693. [Google Scholar]
- Fay P, Kaufman D, Nippert J, Carlisle J, Harper C. Changes in grassland ecosystem function due to extreme rainfall events: implications for responses to climate change. Global Change Biology. 2008;14:1600–1608. [Google Scholar]
- Fernandez M. Changes in photosynthesis and fluorescence in response to flooding in emerged and submerged leaves of Pouteria orinocoensis. Photosynthetica. 2006;44:32–38. [Google Scholar]
- Fischer E, Seneviratne S, Vidale P, Luthi D, Schar C. Soil moisture - Atmosphere interactions during the 2003 European summer heat wave. Journal of Climate. 2007;20:5081–5099. [Google Scholar]
- Franklin J. Predictive vegetation mapping: geographic modelling of biospatial patterns in relation to environmental gradients. Progress in Physical Geography. 1995;19:474–499. [Google Scholar]
- Gaspar T, Franck T, Bisbis B, Kevers C, Jouve L, Hausman J, Dommes J. Concepts in plant stress physiology. Application to plant tissue cultures. Plant Growth Regulation. 2002;37:263–285. [Google Scholar]
- Gibson LA, Wilson BA, Cahill DM, Hill J. Modelling habitat suitability of the swamp antechinus (Antechinus minimus maritimus) in the coastal heathlands of southern Victoria, Australia. Biological Conservation. 2004;117:143–150. [Google Scholar]
- Giorgi F, Bi X, Pal J. Mean, interannual variability and trends in a regional climate change experiment over Europe. II: climate change scenarios (2071–2100) Climate Dynamics. 2004;23:839–858. [Google Scholar]
- Gitlin A, Sthultz C, Bowker M, et al. Mortality gradients within and among dominant plant populations as barometers of ecosystem change during extreme drought. Conservation Biology. 2006;20:1477–1486. doi: 10.1111/j.1523-1739.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- Gloning P, Taeger S, Seifert H, Schäffler U, Kölling C, Schilcher M, Menzel A. Comparison of four different types of European tree species distribution maps and their correlation to weather and climate. (in preparation)
- Godefroid S, Rivière S, Waldren S, Boretos N, Eastwood R, Vanderborght T. To what extent are threatened European plant species conserved in seed banks? Biological Conservation. 2011;144:1494–1498. [Google Scholar]
- Granier A, Reichstein M, Bréda N, et al. Evidence for soil water control on carbon and water dynamics in European forests during the extremely dry year: 2003. Agricultural and Forest Meteorology. 2007;143:123–145. [Google Scholar]
- Gruber A, Pirkebner D, Florian C, Oberhuber W. No evidence for depletion of carbohydrate pools in Scots pine (Pinus sylvestris L.) under drought stress. Plant Biology. 2012;14:142–148. doi: 10.1111/j.1438-8677.2011.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisan A, Zimmermann NE. Predictive habitat distribution models in ecology. Ecological Modelling. 2000;135:147–186. [Google Scholar]
- Hajar L, François L, Khater C, Jomaa I, Déqué M, Cheddadi R. Cedrus libani (A. Rich) distribution in Lebanon: Past, present and future. Comptes Rendus Biologies. 2010;333:622–630. doi: 10.1016/j.crvi.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Harfouche A, Meilan R, Altman A. Tree genetic engineering and applications to sustainable forestry and biomass production. Trends in Biotechnology. 2011;29:9–17. doi: 10.1016/j.tibtech.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Harsch MA, Hulme PE, McGlone MS, Duncan RP. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecology Letters. 2009;12:1040–1049. doi: 10.1111/j.1461-0248.2009.01355.x. [DOI] [PubMed] [Google Scholar]
- Hartmann H. Will a 385 million year-struggle for light become a struggle for water and for carbon? – How trees may cope with more frequent climate change-type drought events. Global Change Biology. 2011;17:642–655. [Google Scholar]
- Hegerl GC, Hanlon H, Beierkuhnlein C. Climate science: Elusive extremes. Nature Geosciences. 2011;4:142–143. [Google Scholar]
- Heide OM. Dormancy release in beech buds (Fagus sylvatica) requires both chilling and long days. Physiologia Plantarum. 1993;89:187–191. [Google Scholar]
- Holmgren M, Scheffer M, Huston MA. The Interplay of Facilitation and Competition in Plant Communities. Ecology. 1997;78:1966–1975. [Google Scholar]
- Holtum J, Winter K. Elevated CO2 and forest vegetation: more a water issue than a carbon issue? Functional Plant Biology. 2010;37:694–702. [Google Scholar]
- Hubert B, Francois L, Warnant P, Strivay D. Stochastic generation of meteorological variables and effects on global models of water and carbon cycles in vegetation and soils. Journal of Hydrology. 1998;212:318–334. [Google Scholar]
- IPCC . In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt K, Tignor M, Miller H, editors. Cambridge University Press; Cambridge, UK and New York, USA: 2007a. p. 996. [Google Scholar]
- IPCC . In: Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Parry M, Canziani O, Palutikof J, Van Der Linden P, Hanson C, editors. Cambridge University Press; Cambridge, UK: 2007b. p. 976. [Google Scholar]
- Jackson M, Drew M. Effect of flooding on herbaceous plants. In: Kozlowski T, editor. Flooding and plant growth. Academic Press; London: 1984. pp. 47–128. [Google Scholar]
- Jentsch A, Beierkuhnlein C. Research frontiers in climate change: Effects of extreme meteorological events on ecosystems. Comptes Rendus Geoscience. 2008;340:621–628. [Google Scholar]
- Jentsch A, Kreyling J, Boettcher-Treschkow J, Beierkuhnlein C. Beyond gradual warming: extreme weather events alter flower phenology of European grassland and heath species. Global Change Biology. 2009;15:837–849. [Google Scholar]
- Jochner SC, Beck I, Behrendt H, Traidl-Hoffmann C, Menzel A. Effects of extreme spring temperatures on urban phenology and pollen production: a case study in Munich and Ingolstadt. Climate Research. 2011;49:101–112. [Google Scholar]
- Jonas T, Rixen C, Sturm M, Stoeckli V. How alpine plant growth is linked to snow cover and climate variability. Journal of Geophysical Research. 2008;113:G03013. [Google Scholar]
- Kelly A, Goulden M. Rapid shifts in plant distribution with recent climate change. Proceedings of the National Academy of Sciences. 2008;105:11823–11826. doi: 10.1073/pnas.0802891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T, Di Matteo G, Rotenberg E, Cohen S, Yakir D. Existing traits’ plasticity will sustain growth and survival of a major pine species under predicted climate change in the Mediterranean. (submitted)
- Knapp A, Beier C, Briske D, et al. Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience. 2008;58:811–821. [Google Scholar]
- Knapp A, Fay PA, Blair JM, et al. Rainfall Variability, Carbon Cycling, and Plant Species Diversity in a Mesic Grassland. Science. 2002;298:2202–2205. doi: 10.1126/science.1076347. [DOI] [PubMed] [Google Scholar]
- Körner C. Alpine plants: stressed or adapted? In: Scholes Jd, Barker Mg., editors. Physiological Plant Ecology. Blackwell Science; Oxford, UK: 1998. pp. 297–311. [Google Scholar]
- Körner C. Limitation and stress - always or never? Journal of Vegetation Science. 2003;14:141–143. [Google Scholar]
- Körner C, Basler D. Phenology Under Global Warming. Science. 2010;327:1461–1462. doi: 10.1126/science.1186473. [DOI] [PubMed] [Google Scholar]
- Kozlowski T. Responses of woody plants to flooding and salinity. Tree Physiology Monograph. 1997;1:1–29. [Google Scholar]
- Kramer K, Degen B, Buschbom J, Hickler T, Thuiller W, Sykes MT, De Winter W. Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change--Range, abundance, genetic diversity and adaptive response. Forest Ecology and Management. 2010;259:2213–2222. [Google Scholar]
- Kramer P. Causes of injury to plants resulting from flooding of the soil. Plant Physiology. 1951;26:722–736. doi: 10.1104/pp.26.4.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer P, Boyer J. Water relations of plant and soils. Academic Press; San Diego: 1995. p. 495. [Google Scholar]
- Kuster TM, Arend M, Bleuler P, Günthardt-Goerg MS, Schulin R. Water regime and growth of young oak stands subjected to air-warming and drought on two different forest soils in a model ecosystem experiment. Plant Biology. 2012 doi: 10.1111/j.1438-8677.2011.00552.x. [DOI] [PubMed] [Google Scholar]
- Langley JA, Megonigal JP. Ecosystem response to elevated CO2 levels limited by nitrogen-induced plant species shift. Nature. 2010;466:96–99. doi: 10.1038/nature09176. [DOI] [PubMed] [Google Scholar]
- Laurent J, Francois L, Bar-Hen A, Bel L, Cheddadi R. European bioclimatic affinity groups: Data-model comparisons. Global and Planetary Change. 2008;61:28–40. [Google Scholar]
- Lenoir J, Gégout J, Marquet P, De Ruffray P, Brisse H. A Significant Upward Shift in Plant Species Optimum Elevation During the 20th Century. Science. 2008;320:1768–1771. doi: 10.1126/science.1156831. [DOI] [PubMed] [Google Scholar]
- Leuzinger S, Hartmann A, Körner C. Water relations of climbing ivy in a temperate forest. Planta. 2011a;233:1087–1096. doi: 10.1007/s00425-011-1363-6. [DOI] [PubMed] [Google Scholar]
- Leuzinger S, Körner C. Rainfall distribution is the main driver of runoff under future CO2-concentration in a temperate deciduous forest. Global Change Biology. 2010;16:246–254. [Google Scholar]
- Leuzinger S, Luo Y, Beier C, Dieleman W, Vicca S, Körner C. Do global change experiments overestimate impacts on terrestrial ecosystems? Trends In Ecology & Evolution. 2011b;26:236–241. doi: 10.1016/j.tree.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Leuzinger S, Thomas R. How do we improve Earth system models? Integrating Earth system models, ecosystem models, experiments and long-term data. New Phytologist. 2011;191:15–18. doi: 10.1111/j.1469-8137.2011.03778.x. [DOI] [PubMed] [Google Scholar]
- Leuzinger S, Zotz G, Asshoff R, Körner C. Responses of deciduous forest trees to severe drought in Central Europe. Tree Physiology. 2005;25:641–650. doi: 10.1093/treephys/25.6.641. [DOI] [PubMed] [Google Scholar]
- Levine J, Rees M. Effects of Temporal Variability on Rare Plant Persistence in Annual Systems. The American Naturalist. 2004;164:350–363. doi: 10.1086/422859. [DOI] [PubMed] [Google Scholar]
- Levine JM, Mceachern AK, Cowan C. Rainfall effects on rare annual plants. Journal of Ecology. 2008;96:795–806. [Google Scholar]
- Lindner M, Maroschek M, Netherer S, et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. Forest Ecology and Management. 2010;259:698–709. [Google Scholar]
- Lloyd J, Taylor J. On the temperature dependence of soil respiration. Functional Ecology. 1994;8:315–323. [Google Scholar]
- Lortie CJ, Brooker RW, Kikvidze Z, Callaway RM. The value of stress and limitation in an imperfect world: A reply to Körner. Journal of Vegetation Science. 2004;15:577–580. [Google Scholar]
- Luo Y, Melillo J, Niu S, et al. Coordinated approaches to quantify long-term ecosystem dynamics in response to global change. Global Change Biology. 2011;17:843–854. [Google Scholar]
- Luterbacher J, Liniger MA, Menzel A, Estrella N, Della-Marta PM, Pfister C, Rutishauser T, Xoplaki E. Exceptional European warmth of autumn 2006 and winter 2007: Historical context, the underlying dynamics, and its phenological impacts. Geophysical Research Letters. 2007;34:L12704. [Google Scholar]
- Macmillan R, Pettapiece W, Nolan S, Goddard T. Generic procedure for automatically segmenting landforms into landform elements using DEMS, heuristic rules and fuzzy logic. Fuzzy Sets and Systems. 2000;113:81–109. [Google Scholar]
- Martins M, Neto C, Costa J. The meaning of mainland Portugal beaches and dunes’ psammophilic plant communities: a contribution to tourism management and nature conservation. Journal of Coastal Conservation. (in press) [Google Scholar]
- Matyas C. Climatic adaptation of trees: rediscovering provenance tests. Euphytica. 1996;92:45–54. [Google Scholar]
- Matyssek R, Wieser G, Ceulemans R, et al. Enhanced ozone strongly reduces carbon sink strength of adult beech (Fagus sylvatica) - Resume from the free-air fumigation study at Kranzberg Forest. Environmental Pollution. 2010;158:2527–2532. doi: 10.1016/j.envpol.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Mcdowell N, Pockman WT, Allen CD, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- Menzel A, Fabian P. Growing season extended in Europe. Nature. 1999;397:659–659. [Google Scholar]
- Menzel A, Jakobi G, Ahas R, Scheifinger H, Estrella N. Variations of the climatological growing season (1951–2000) in Germany compared with other countries. International Journal of Climatology. 2003;23:793–812. [Google Scholar]
- Menzel A, Seifert H, Estrella N. Effects of recent warm and cold spells on European plant phenology. International Journal of Biometeorology. 2011;55:921–932. doi: 10.1007/s00484-011-0466-x. [DOI] [PubMed] [Google Scholar]
- Menzel A, Sparks TH, Estrella N, et al. European phenological response to climate change matches the warming pattern. Global Change Biology. 2006;12:1969–1976. [Google Scholar]
- Midgley GF, Hughes GO, Thuiller W, Rebelo AG. Migration rate limitations on climate change-induced range shifts in Cape Proteaceae. Diversity And Distributions. 2006;12:555–562. [Google Scholar]
- Miller JR, Turner MG, Smithwick EAH, Dent CL, Stanley EH. Spatial Extrapolation: The Science of Predicting Ecological Patterns and Processes. Bioscience. 2004;54:310–320. [Google Scholar]
- Minacapilli M, Agnese G, Blanda F, et al. Estimation of actual evapotranspiration of Mediterranean perennial crops by means of remote-sending based surface energy balance models. Hydrology and Earth System Sciences. 2009;13:1061–1074. [Google Scholar]
- Misson L, Degueldre D, Collin C, Rodriguez R, Rocheteau A, Ourcival J-M, Rambal S. Phenological responses to extreme droughts in a Mediterranean forest. Global Change Biology. 2011;17:1036–1048. [Google Scholar]
- Morgan JA, Pataki DE, Körner C, et al. Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia. 2004;140:11–25. doi: 10.1007/s00442-004-1550-2. [DOI] [PubMed] [Google Scholar]
- Moriana O, Orgaz F, Fereres E, Pastor M. Yield responses of a mature olive orchard to water deficits. Journal of the American Society of Horticultural Science. 2003;128:425–431. [Google Scholar]
- Neppel L, Desbordes M, Masson J. Spatial extension of extreme rainfall events: return period of isohyets area and influence of rain gauges network evolution. Atmospheric resaearch. 1997;45:183–199. [Google Scholar]
- Niinemets U, Valladares F. Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecological Monographs. 2006;76:521–547. [Google Scholar]
- Orlowsky B, Seneviratne S. Global changes in extreme events: regional and seasonal dimension. Climatic Change. 2012;110:669–696. [Google Scholar]
- Orlowsky B, Seneviratne S. Investigating spatial climate relations using CARTs: An application to persistent hot days in a multimodel ensemble. Journal of Geophysical Research. 2011;116:D14106. [Google Scholar]
- Otto D, Rasse D, Kaplan J, Warnant P, Francois L. Biospheric carbon stocks reconstructed at the Last Glacial Maximum: comparison between general circulation models using prescribed and computed sea surface temperatures. Global Planetary Change. 2002;33:117–138. [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Parolin P, Wittmann F. Struggle in the flood: tree responses to flooding stress in four tropical floodplain systems. AoB Plants. 2010:plq003. doi: 10.1093/aobpla/plq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasolli L, Notarnicola C, Bruzzone L, et al. Estimation of Soil Moisture in an Alpine Catchment with RADARSAT2 Images. Applied and Environmental Soil Science. 2011 Article ID 175473. [Google Scholar]
- Peters D, Herrick J. Strategies for ecological extrapolation. Oikos. 2004;106:627–636. [Google Scholar]
- Polgar CA, Primack RB. Leaf-out phenology of temperate woody plants: from trees to ecosystems. New Phytologist. 2011;191:926–941. doi: 10.1111/j.1469-8137.2011.03803.x. [DOI] [PubMed] [Google Scholar]
- Porporato A, Daly E, Rodriguez-Iturbe I. Soil water balance and ecosystem response to climate change. American Naturalist. 2004;164:625–632. doi: 10.1086/424970. [DOI] [PubMed] [Google Scholar]
- Pretzsch H, Dieler J. The dependency of the size-growth relationship of Norway spruce (Picea abies [L.] Karst.) and European beech (Fagus sylvatica [L.]) in forest stands on long-term site conditions, drought events, and ozone stress. Trees – Structure and Function. 2011;25:355–369. [Google Scholar]
- Rahmstorf S, Coumou D. Increase of extreme events in a warming world. Proceedings of the National Academy of Sciences. 2011;108:17905–17909. doi: 10.1073/pnas.1101766108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan T. Ecophysiology of salt excretion in the xero-halophyte Reaumuria hirtella. New Phytologist. 1998;139:273–281. [Google Scholar]
- Raz-Yaseef N, Yakir D, Rotenberg E, Schiller G, Cohen S. Ecohydrology of a semi-arid forest: partitioning among water balance components and its implications for predicted precipitation changes. Ecohydrology. 2010;3:143–154. [Google Scholar]
- Reddy A, Chaitanya K, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. Journal of Plant Physiology. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Rehfeldt G, Wykoff W, Ying C. Physiologic Plasticity, Evolution, and Impacts of a Changing Climate on Pinus Contorta. Climatic Change. 2001;50:355–376. [Google Scholar]
- Rengifo E, Tezara W, Herrera A. Water relations, chlorophyll a fluorescence, and contents of saccharides in tree species of a tropical forest in response to flood. Photosynthetica. 2005;43:203–210. [Google Scholar]
- Riccardi M, Bonfante A, Mencuccini M, Di Tommasi P, De Lorenzi F, Menenti M. Assessing the adaptive capacity of some olive cultivars to future climate. Geophysical Research Abstracts. 2011;13:10688. [Google Scholar]
- Rigon R, Bertoldi G, Over T. GEOtop: a distributed hydrological model with coupled water and energy budgets. Journal of Hydrometeorology. 2006;7:371–388. [Google Scholar]
- Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- Rummukainen M. Changes in climate and weather extremes in the 21st century. Wiley Interdisciplinary Reviews: Climate Change. 2012;3:115–129. [Google Scholar]
- Rustad LE. The response of terrestrial ecosystems to global climate change: Towards an integrated approach. Science of The Total Environment. 2008;404:222–235. doi: 10.1016/j.scitotenv.2008.04.050. [DOI] [PubMed] [Google Scholar]
- Sala A, Piper F, Hoch G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytologist. 2010;186:274–281. doi: 10.1111/j.1469-8137.2009.03167.x. [DOI] [PubMed] [Google Scholar]
- Saxe H, Cannell MGR, Johnsen Ø , Ryan MG, Vourlitis G. Tree and forest functioning in response to global warming. New Phytologist. 2001;149:369–399. doi: 10.1046/j.1469-8137.2001.00057.x. [DOI] [PubMed] [Google Scholar]
- Schär C, Vidale P, Lüthi D, Frei C, Häberli C, Liniger M, Appenzeller C. The role of increasing temperature variability in European summer heatwaves. Nature. 2004;427:332–336. doi: 10.1038/nature02300. [DOI] [PubMed] [Google Scholar]
- Scheifinger H, Menzel A, Koch E, Peter C. Trends of spring time frost events and phenological dates in Central Europe. Theoretical and Applied Climatology. 2003;74:41–51. [Google Scholar]
- Schiller G, Atzmon N. Performance of Aleppo pine (Pinus halepensis) provenances grown at the edge of the Negev desert: A review. Journal of Arid Environments. 2009;73:1051–1057. [Google Scholar]
- Schimper A. Plant-geography upon a physiological basis. Clarendon Press; Oxford: 1903. p. 839. [Google Scholar]
- Schwalm CR, Ek AR. Climate change and site: relevant mechanisms and modeling techniques. Forest Ecology and Management. 2001;150:241–257. [Google Scholar]
- Seneviratne SI, Luthi D, Litschi M, Schar C. Land-atmosphere coupling and climate change in Europe. Nature. 2006;443:205–209. doi: 10.1038/nature05095. [DOI] [PubMed] [Google Scholar]
- Seneviratne SI, Nicholls N, Easterling D, et al. Changes in climate extremes and their impacts on the natural physical environment. In: Field CB, Barros V, Stocker TF, Qin D, Dokken DJ, Ebi KL, Mastrandrea MD, Mach KJ, Plattner GAK, Allen SK, Tignor M, Midgley PM, editors. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change (IPCC) Cambridge University Press; Cambridge, UK, and New York, USA: 2012. pp. 109–230. [Google Scholar]
- Silvertown J, Dodd M, Gowing D, Mountford J. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature. 1999;400:61–63. [Google Scholar]
- Smith MD. An ecological perspective on extreme climatic events: a synthetic definition and framework to guide future research. Journal of Ecology. 2011;99:656–663. [Google Scholar]
- Smith T, Huston M. A theory of the spatial and temporal dynamics of plant communities. Plant Ecology. 1989;83:49–69. [Google Scholar]
- Stratton T, Price DT, Gajewski K. Impacts of daily weather variability on simulations of the Canadian boreal forest. Ecological Modelling. 2012;222:3250–3260. [Google Scholar]
- Suding KN, Lavorel S, Chapin FS, et al. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Global Change Biology. 2008;14:1125–1140. [Google Scholar]
- Taylor K. Summarizing multiple aspects of model performance in a single diagram. Journal of Geophysical Research. 2001;106:7183–7192. [Google Scholar]
- Templer PH, Reinmann AB. Multi-factor global change experiments: what have we learned about terrestrial carbon storage and exchange? New Phytologist. 2011;192:797–800. doi: 10.1111/j.1469-8137.2011.03959.x. [DOI] [PubMed] [Google Scholar]
- Teuling AJ, Seneviratne SI, Stockli R, et al. Contrasting response of European forest and grassland energy exchange to heatwaves. Nature Geosciences. 2010;3:722–727. [Google Scholar]
- Thorpe A, Aschehoug E, Atwater D, Callaway R. Interactions among plants and evolution. Journal of Ecology. 2011;99:729–740. [Google Scholar]
- Tognetti R, D’andria R, Lavini A, Morelli G. The effect of deficit irrigation on crop yield and vegetative development of Olea europaea L. (cvs. Frantoio and Leccino) European Journal of Agronomy. 2006;25:356–364. [Google Scholar]
- Tomozeiu R, Cacciamani C, Pavan V, Morgillo A, Busuioc A. Climate change scenarios for surface temperature in Emilia-Romagna (Italy) obtained using statistical downscaling models. Theoretical and Applied Climatology. 2007;90:25–47. [Google Scholar]
- Van Dam JC, Groenendijk P, Hendriks RFA, Kroes JG. Advances of modeling water flow in variably saturated soils with SWAP. Vadose Zone Journal. 2008;7:640–653. [Google Scholar]
- Van Peer L, Nijs I, Reheul D, De Cauwer B. Species richness and susceptibility to heat and drought extremes in synthesized grassland ecosystems: compositional vs physiological effects. Functional Ecology. 2004;18:769–778. [Google Scholar]
- Vitasse Y, Porté A, Kremer A, Michalet R, Delzon S. Responses of canopy duration to temperature changes in four temperate tree species: relative contributions of spring and autumn leaf phenology. Oecologia. 2009;161:187–198. doi: 10.1007/s00442-009-1363-4. [DOI] [PubMed] [Google Scholar]
- Waisel Y, Eshel A, Agami M. Salt balance of leaves of the mangrove Avicennia marina. Physiologia Plantarum. 1986;67:67–72. [Google Scholar]
- Walther G-R, Post E, Convey P, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Weltzin JF, Loik ME, Schwinning S, et al. Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience. 2003;53:941–952. [Google Scholar]
- Wolkovich EM, Cook BI, Allen JM, et al. Warming experiments underpredict plant phenological responses to climate change. Nature. 2012 doi: 10.1038/nature11014. [DOI] [PubMed] [Google Scholar]
- Zeppel MJB, Adams HD, Anderegg WRL. Mechanistic causes of tree drought mortality: recent results, unresolved questions and future research needs. New Phytologist. 2011;192:800–803. doi: 10.1111/j.1469-8137.2011.03960.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann NE, Yoccoz NG, Edwards TC, Meier ES, Thuiller W, Guisan A, Schmatz DR, Pearman PB. Climatic extremes improve predictions of spatial patterns of tree species. Proceedings of the National Academy of Sciences. 2009;106:19723–19728. doi: 10.1073/pnas.0901643106. [DOI] [PMC free article] [PubMed] [Google Scholar]