Highlights
-
•
Health inequalities reduce in adolescence compared to childhood in South Africa.
-
•
Biological & socio-economic status factors should be considered to understand change in inequality.
-
•
Obesity prevention in South Africa should target girls whose mothers have a high BMI.
Keywords: Health inequalities, Adolescence, Body composition, Neighbourhood, South Africa
Abstract
Despite the strongly established link between socio-economic status (SES) and health across most stages of the life-course, the evidence for a socio-economic gradient in adolescent health outcomes is less consistent. This paper examines associations between household, school, and neighbourhood SES measures with body composition outcomes in 16 year old South African Black urban adolescents from the 1990 born Birth to Twenty (Bt20) cohort. Multivariable regression analyses were applied to data from a sub-sample of the Bt20 cohort (n = 346, 53% male) with measures taken at birth and 16 years of age to establish socio-economic, biological, and demographic predictors of fat mass, lean mass, and body mass index (BMI). Results were compared with earlier published evidence of health inequality at ages 9–10 years in Bt20. Consistent predictors of higher fat mass and BMI in fully adjusted models were being female, born post term, having a mother with post secondary school education, and having an obese mother. Most measures of SES were only weakly associated with body composition, with an inconsistent direction of association. This is in contrast to earlier findings with Bt20 9–10 year olds where SES inequalities in body composition were observed. Findings suggest targeting obesity interventions at females in households where a mother has a high BMI.
1. Introduction
Despite the strong established link between socioeconomic status (SES) and health outcomes across the life-course (Marmot, 2005), the evidence for a social gradient in adolescent health outcomes is less consistent (West, 1997). Based on evidence from developed countries, West (1997) suggests that adolescence may be a period in which health inequalities (defined by SES) may become more equal. He suggests that severe chronic diseases are the exception to this rule because they have their origins in early life when inequalities are stronger.
Adolescence is defined as the period of somatic, psychological, social, and sexual development that occurs in the 5–10 years after the onset of the adolescent growth spurt in height/and or weight and the maturation of the hypothalamic-pituitary-gonadal axis. These biological changes are accompanied by increasing engagement in adult social and sexual activities (Bogin, 2013). The explanation for more equality in health outcomes in adolescence has been related to this increasing engagement in adult social activities using a sociological framework, whereby peer or youth culture or sub-culture and school influences become more important during the secondary school years of adolescent life compared to social class or household SES (West, 1997). This suggests that the SES environment external to the household becomes increasingly more important as adolescents spend less time in the home and more time with peers in the school and neighbourhood environments. This is a logical explanation given that adolescence marks the onset of increasing independence from the family, with more time being spent in the community (Allison et al., 1999). Further, West (1997) argues that the school and peer environment could be more important than even the neighbourhood SES environment because adolescents tend to identify more with their peer group culture than with other external forces such as aspects of the household and neighbourhood not relevant to that culture. This means that assessing relevant measures of the school, neighbourhood, and peer environment become more important for assessing inequalities in adolescence. Critics of West's hypothesis argue that more equal health outcomes in adolescence may be an artefact of the data because, for instance, studies use poor measures of adolescent SES (e.g. adult occupation measures have been used as proxies for adolescent SES) (Judge and Benzeval, 1993) and/or use self-report health outcomes, which may themselves be influenced by an individual's SES (Davey Smith et al., 1994). Poorly measured SES and/or health outcome variables have the potential to mask SES effects that may otherwise exist.
Recently, a number of review studies have explored the relationship between SES and adolescent health outcomes (Holstein et al., 2009; Starfield et al., 2002) and found mixed evidence for a relationship, although the balance of the evidence is more strongly towards inequalities existing rather than not existing. More specifically focussing on the relationship between SES and obesity/adiposity, a review by Shrewsbury and Wardle (2008) of 45 child and adolescent studies (9 of which include only adolescents) from developed countries between 1990 and 2005 found an inverse association in 6 out of 9 studies for adolescent boys and 1 out of the 9 adolescent studies for girls (Shrewsbury and Wardle, 2008). Thus, this provides mixed evidence for a link between SES and obesity/adiposity in adolescence. A separate review paper of 15 studies from developed countries (7 adolescent) specifically studied the association between overweight/obesity and factors in the physical environment, such as built environment and population density (Holstein et al., 2009). Whilst this review found evidence of associations between neighbourhood environments and overweight/obesity in childhood, no such relationship was observed for adolescents. There is much less evidence examining inequalities in adolescent obesity within developing country contexts than in developed countries. The limited existing evidence relating SES to adolescent obesity in South Africa suggests no association or a weak link in boys at the household level (Kruger et al., 2006) and no evidence exists to test the association at the neighbourhood or school level.
Previous researchers studying the link between SES and health outcomes in adolescents have called for a need to use more longitudinal approaches in order to establish how inequalities might develop or become more equal over the early life-course (Starfield et al., 2002). The Bt20 cohort provides a unique opportunity to test West's hypothesis and to study adolescent inequalities in body composition outcomes taking into account the early life course in a transitioning economy because inequalities have already been studied in childhood. It is therefore possible to compare findings from the earlier childhood analysis with analyses that focus on the adolescent years in the cohort. This earlier childhood work with the Bt20 cohort revealed a positive association between household SES measures taken in infancy and later childhood (ages 9–10 years) with body composition outcomes at ages 9–10 years (Griffiths et al., 2008). The findings showed that the infancy SES environment was more strongly associated with lean mass than fat mass and that age 9–10 year household SES measures were more strongly associated with fat mass than lean mass. At ages 9–10 years, there was a low prevalence of overweight and obesity in Bt20, with a higher prevalence of malnutrition, thus indicating that the high SES children were advantaged.
The Bt20 cohort were born in 1990, the year that Nelson Mandela was released from prison. In the four decades preceding Mandela's release, the combined impact of Apartheid and the economic sanctions placed on the South African government by the international community led to extreme inequality (Cameron, 2003). By the late 1990s, inequality in South Africa remained only second to Brazil (May, 2000). Despite these high levels of inequality, South Africa is currently one of the more developed countries in sub-Saharan Africa (UNDP, 2011) with a higher prevalence of chronic disease than most other sub-Saharan African countries. For instance, World Health Organisation (WHO, 2011) data show that it ranks second in sub-Saharan Africa for its male and female adulthood (aged 15+) prevalence of combined overweight and obesity (68.5% females and 41.3% males with a BMI > 25 kg/m2) (WHO, 2011).
The Bt20 cohort present a unique opportunity to test West's hypothesis within a developing country context because of the comprehensive set of SES measures at the household, neighbourhood, and school level, which address some of the earlier concerns of critics of West's argument about the lack of robust measures of SES. These data also offer the opportunity to test West's idea that aspects of the school SES environment might more strongly measure inequalities at this age than more traditional neighbourhood SES measures. Using objectively measured outcomes of body mass, lean mass, and fat mass also overcomes earlier problems of adolescent studies using self report health outcomes. The period of adolescence studied in this paper is limited to age 16 years, which is when participant body composition and community SES assessments were undertaken in the cohort. To test West's hypothesis in an urban South African context, this paper will examine the association between household/neighbourhood/school SES measures in infancy/at age 16 years and body composition outcomes at age 16 years controlling for a range of potentially confounding factors.
2. Materials and methods
2.1. Participants
Birth to Twenty (Bt20) is a longitudinal cohort study of 3273 singleton births occurring in 1990 to permanently resident mothers in Johannesburg–Soweto, South Africa (Richter et al., 2004, 2007). At ages 9–10 years, a sub-sample from the cohort (n = 429) was enrolled into a longitudinal study assessing factors influencing bone health and body composition. Because the sub-cohort are predominantly African Black this paper focuses on the African Black group because the African White group were not present in sufficient numbers to analyse as a separate group. Bone health participants had more detailed health and SES assessments than the Bt20 cohort. For instance neighbourhood and school measures of SES were not collected on the main cohort at age 16 years. Those African Black participants with data on household/neighbourhood/school SES and anthropometric and dual-energy X-ray absorptiometry (DXA) data at 16 years were included in current analyses (n = 346, 53% male).
Ethical approval was granted by the ethics committees of the University of the Witwatersrand, South Africa for the primary data collection and Loughborough University, UK for the secondary analyses of the data. The primary caregiver gave written informed consent for their adolescent to participate and the adolescent provided written ascent to participate at 16 years.
2.2. Socio-economic status measures
During infancy and at age 16 years household SES measures were caregiver assessed using a questionnaire that was based on standard measures used by the Demographic and Health Surveys (www.measuredhs.com). Measures included caregiver's education and occupation, home ownership and type, and household consumer durable ownership. These are measures that have been commonly used to assess the association between SES and child health or fertility in developing countries according to a review of 67 studies (Bollen et al., 2001). In addition to the most commonly used measures of SES, marital status and water and sanitation provision were also tested in this study. Those not marrying or cohabiting are likely to live in households that have less adults and which are more likely to be female headed. Having less adults in the household results in a higher dependency ratio and potentially reduced resources for expenditure on children. Female headed households and higher dependency ratios have been shown to be associated with higher risk of chronic poverty in the South African context (Roberts, 2001). Bt20 collected information on the type of water and toilet facility and whether it was shared, sole, or other type of access. In infancy, information was only available on whether the household had inside provision of water and toilet facilities, outside facilities, or a mixture of both. At age 16 years, more detailed information was available to allow households to be split into whether they had hot or cold tap water or another source of water as well as whether this source was sole or shared. At age 16 years more detailed information was also available regarding the toilet facilities so that households could be distinguished by whether they had access to a flush versus other type of toilet and whether that access was sole or shared. Sole use of water and toilet facilities is preferable and would indicate a higher SES in this context. Similarly hot water and a flush toilet would be considered the optimal water and sanitation provision. Measures of water and sanitation provision not only capture the SES environment but also disease risk. Where facilities are shared or less optimal (i.e. no availability of hot water) the risk for morbidity will be higher and this may have direct influences on body composition outcomes that are separate from SES effects.
At 16 years of age neighbourhood and school SES were self-assessed using a culturally relevant questionnaire, which was developed by consulting community leaders and Bt20 adolescents/caregivers using focus group discussions and in-depth interviews in 2005/2006 (Sheppard et al., 2010). Based on the findings of this earlier formative work, neighbourhood was defined for all participants as an area approximately 20 min walk or 2 km from home in any direction.
The neighbourhood SES questionnaire included questions relating to: (1) economic aspects of neighbourhoods including neighbourhood wealth, perceived inequalities in wealth, type, condition, and spacing of housing, infrastructure and service provision type, condition of roads, and neighbourhood problems (e.g. traffic congestion, illegal dumping), (2) social aspects of neighbourhoods including safety, crime, activities for young people, neighbourhood friends, peer pressure, noise, and religious involvement, and (3) school environment with questions on school type, facilities, class sizes, out of school activities, and problems (e.g. poor academic standards, alcohol and drug consumption, weapons).
To enable a more parsimonious analysis of SES measures and to avoid problems of multicollinearity, principal components analysis (PCA) was used to construct neighbourhood SES indices. A theory based approach was used to develop seven neighbourhood SES indices as well as two household SES indices and PCA confirmed the appropriateness of grouping these variables together. In each case, the first component scores were extracted and the statistical assumption that all Eigenvalues be greater than 1 was met. The first components explained between 27 and 91% of variation. In most cases only one Eigenvalue was greater than 1. However, where there were two Eigenvalues greater than 1, the scree plots showed a clear inflection between the first and second component in all cases meaning that 1 component was extracted for the analysis. Furthermore, the second components for any of the indices were hard to interpret in a theory driven approach to construction because they included both positive and negative values and there was no clear substantive reason that could be found for the variables that were assigned negative values versus positive values in any of the indices. Further details about the composition and fit of the neighbourhood indices have previously been reported (Griffiths et al., 2012). Neighbourhood indices created were; (1) neighbourhood economic index, (2) neighbourhood need for more services and facilities index, (3) neighbourhood problem index, (4) neighbourhood crime prevention index, and (5) neighbourhood social support and happiness index. Two variables (How safe do you feel in the neighbourhood and how much crime is there in the neighbourhood?) did not load well onto any index and were thus retained as individual variables. There were also two school indices identified; (6) school environment index and (7) school problems index. In addition to these neighbourhood and school measures, household questionnaire data were used to construct two indices that measured ownership of consumer durables, the first during infancy and the second at age 16 years. Regression factor scores were extracted for each index and tertiles for each index were created. A variable was created to identify adolescents who transitioned from one index of consumer durables tertile to another between infancy and 16 years.
2.3. Anthropometric and DXA-derived body composition measures
Birth weight and weight and height at 16 years were assessed using standard techniques (Lohman et al., 1991). Weight was measured using digital scales and height using a stadiometer (Holtain, UK). Low birthweight was defined as a birthweight less than 2.5 kg. Body mass index was calculated as weight (Kg)/height (m)2 and adolescents were classified as normal weight, overweight, or obese using Cole et al.’s international age specific cut-off points.
At 16 years of age a fan-beam densitometer (model QDR 4500A; Hologic Inc, Bedford, Massachusetts) was used to obtain DXA readings of body composition. Total body fat mass (FM) and lean mass (LM) were assessed using the adult software version 8.21 (Hologic Inc) to enable longitudinal follow up with comparable software into adulthood. DXA scans used recommended standardised patient positioning and scan analysis.
2.4. Other variables used in the analysis
Caregivers reported the ethnicity of the adolescent as recorded on the official birth notification. Individuals born before 37 weeks gestation were classified as preterm and after 41 weeks as post term. Adolescent's parity and mother's marital status and age were self reported during infancy. Adolescent's reported smoking status (current, previous smoker, or never smoked) at age 16 years and assessed their own pubertal development with the use of picture cards detailing the different stages of the Tanner scales for breasts and genitalia or pubic hair development (Tanner, 1962). Maternal weight and height were available from data that were collected between 2002 and 2004 when the cohort were aged 12–14 years. Maternal BMI was calculated in the same way as for adolescents, but overweight and obesity were defined using internationally accepted cut-offs of >25 kg/m2 and >30 kg/m2, respectively.
2.5. Statistical analyses
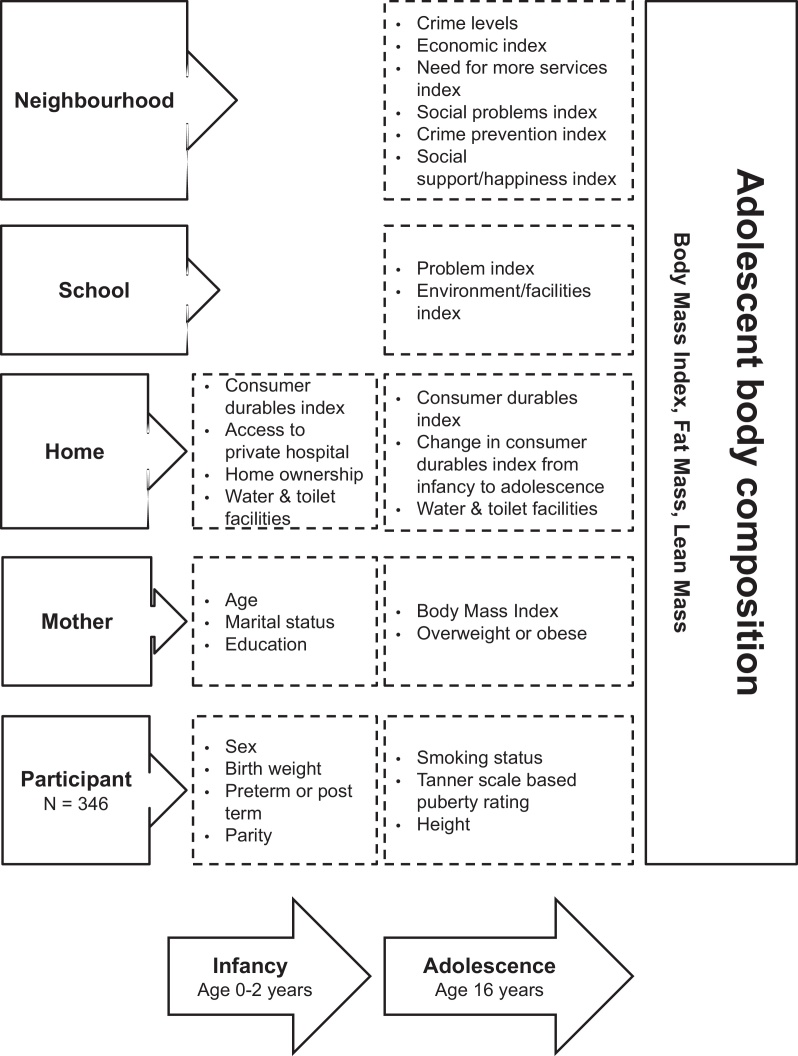
General linear regression was used with outcomes of BMI, FM, and LM, as well as binary logistic regression models that dichotomised adolescents into those who were overweight and obese compared to those who were not. Fig. 1 shows the independent variables included in the analysis of body composition outcomes at age 16 years broken down by characteristics of the child, mother, household, neighbourhood, or school (displayed on the vertical axis) by age of measurement (horizontal axis). For a number of variables used in the analyses there was missing information for some cases because of incomplete responses at a data assessment period where the variables were recorded. This was especially problematic for variables like maternal BMI measured at ages 12–14 years where 61 cases did not have this information recorded. To exclude cases that had missing information on any one of the predictor variables in Fig. 1 would have reduced the sample size by close to a third. To exclude variables with large numbers of missing cases would have excluded important information available for testing the papers key hypotheses. A decision was therefore made to incorporate this missing information into analyses by creating a missing category where relevant for predictor variables that were included in the analysis. Where a missing category shows a significant association with the outcome, this suggests that the missing group are different to the reference group for that variable. In adjusted models, of all of the missing categories entered, only the missing category for term birth was significantly different to the reference group in all three models. This group had a very small sample size. The models were re-estimated without the missing cases for this variable and no changes to substantive findings were observed. The models with all missing cases included are therefore presented in the paper. Multicolinearity checks were undertaken for all variables including for missing cases in the adjusted models using VIF statistics. This ensured that including missing cases did not introduce problems of multicolinearity to the analyses if the same cases were missing for a number of variables.
Fig. 1.
Variables measured in infancy and age 16 years that were tested for an association with body composition outcomes at age 16 years in the urban South African Birth to Twenty cohort.
Initial unadjusted regression analyses explored relationships between each of the measures shown in Fig. 1 and each of the outcomes (results not shown). Subsequently multivariable regression analyses adjusted for all variables that had shown a relationship (p < 0.1) with the relevant outcome in the unadjusted analysis. Height was included as a covariate in all FM and LM models to correct for body size. This approach does not have the same flaws as percentage body fat or lean tissue or fat and lean mass indices, which include the fat or lean mass component in both the numerator and denominator and therefore over adjusts for weight (Cole et al., 2008). In addition, sex was included as a covariate in all adjusted models. This means that an initial step of the modelling process (labelled step 0) models sex and height (for FM and LM only) as a predictor of the outcomes. Regression models were then built in steps that added to this initial step 0; (1) significant infancy variables from the initial unadjusted analysis, (2) significant year 16 household and neighbourhood and school SES variables, (3) significant infancy and year 16 variables, and (4) model 3 plus significant other variables. This approach allowed for the effects of the infancy variables and the year 16 variables to be interpreted separately, and subsequently for any mediating effect of the year 16 variables on the association between the infancy variables and the outcomes as well as any mediating effect of the other variables on the association between infancy and year 16 SES variables and the outcomes to be investigated. Where both maternal BMI and weight status measured when the cohort were aged 12–14 years (i.e. normal, overweight, obese) were significantly associated with an outcome only weight status was taken forward to the multivariable analysis. In the FM model, there was a problem of multicolinearity between the missing category of the maternal BMI variable and the pubertal status variable. Because the pubertal status variable was not significant in adjusted analysis and the maternal BMI variable was, pubertal status was not included in the adjusted regression model. The VIF statistics confirmed that there were no further problems with multicolinearity between the predictor variables included in any of the models (results not shown). All analyses were conducted using SPSS 16.0 (Chicago, IL).
3. Results
Descriptive statistics revealed an overweight/obesity prevalence at 16 years of 18.8% (30.4% in girls, 8.6% in boys (χ2 = 26.78, df = 1, p < 0.001)) (Table 1). Cohort mothers were predominantly aged below 30 years at the time of the participant's birth, were giving birth to their first or second child, had not graduated from high school, and were overweight or obese when the cohort member was 16 years of age (Table 2). At birth, 49% of the cohort lived in rented local authority housing and 48% had outside water and toilet facilities. At age 16 years, approximately 18% only had shared access to water and 50% had outside access to sole or shared use of toilet facilities (Table 2). There was fluidity in relative household SES between birth and 16 years with only 38% households remaining in the same tertile of the household consumer durable SES index (Table 2).
Table 1.
Mean (SD) birthweight, weight, height, body mass index (BMI), fat mass (FM), lean mass (LM); and percent low birth weight, overweight, and obese by sex for South African Black children aged 16 years.
| Total n = 346 | ||
|---|---|---|
| Sex (n) | Male (185) |
Female (161) |
| Mean (SD) birthweight (g)b |
3162.8 (515.7) |
3008.5 (493.2)a |
| Percent (n) LBW (birthweight < 2500 g)b |
8.6 (16) |
13.7 (22) |
| Age 16 mean (SD) weight (kg) |
58.3 (10.6) |
57.9 (11.6) |
| Age 16 mean (SD) height (cm) |
168.9 (7.5) |
158.2 (5.8)a |
| Age 16 mean (SD) BMI (kg/m2) |
20.4 (3.6) |
23.1 (4.3)a |
| Age 16 mean (SD) FM (kg) |
9.7 (6.7) |
19.2 (7.6)a |
| Age 16 mean (SD) LM (kg) |
47.0 (6.1) |
37.3 (4.9)a |
| Age 16 percent (n) overweightc |
5.4 (10) |
22.4 (36)a |
| Age 16 percent (n) obeseb |
3.2 (6) |
8.1 (13)a |
| Age 16 percent (n) overweight or obese |
8.6 (16) |
30.4 (49)a |
Indicates a significant (p < 0.05) sex difference in this variable. Continuous variables were tested using an independent samples t-test and categorical variables using a multidimensional Chi-square test.
10 cases did not have birthweight, and therefore LBW, recorded.
Overweight and obesity are defined using Cole et al.’s age appropriate international cut-offs for children and adolescents.
Table 2.
Descriptive Statistics for predictors of body mass index (BMI), fat mass (FM (kg)), and lean mass (LM (kg)) used in regression analyses for African Black Bt20 16 year olds.a
| Total n = 346 | Percent or mean (SD) |
|---|---|
| Infancy measures | |
| Female | 46.5 |
| Male | 53.5 |
| Term | 84.6 |
| Preterm | 13.6 |
| Post term | 1.2 |
| Missing | 0.6 |
| Parity 1 | 40.9 |
| Parity 2 | 28.9 |
| Parity 3 | 16.5 |
| Parity 4 plus | 12.7 |
| Missing parity | 0.9 |
| Mother's age 15–19 yrs | 19.7 |
| Mother's age 20–24 yrs | 29.8 |
| Mother's age 25–29 yrs | 25.7 |
| Mother's age 30–34 yrs | 17.6 |
| Mother's age 35–39 yrs | 7.2 |
| Married/cohabiting | 24.5 |
| Previously married | 0.6 |
| Single | 74.3 |
| Missing marital status | 0.6 |
| Mother's education ≤grade 10 | 55.2 |
| Mother's education grades 11–12 | 35.3 |
| Mother's education post school | 8.1 |
| Mother's education missing | 1.4 |
| Public hospital | 95.3 |
| Private hospital | 3.8 |
| Missing hospital | 0.9 |
| Owns Property | 28.6 |
| Rented private | 17.1 |
| Rented local authority | 49.4 |
| Provided by employer | 2.0 |
| Missing home ownership | 2.9 |
| Indoor water/toilet | 21.9 |
| Inside & outside water/toilet | 24.3 |
| Outside water/toilet | 48.3 |
| Missing water/toilet | 5.5 |
| Sole use water/toilet | 76.8 |
| Sole & shared toilet/water | 3.8 |
| Shared toilet/water | 13.9 |
| Missing sole/shared water | 5.5 |
| Age 16 measures | |
| Indoor sole use hot/cold water | 26.9 |
| Indoor share use hot/cold water | 9.8 |
| Indoor sole use cold water | 26.9 |
| Indoor share use cold water | 8.4 |
| Other | 25.7 |
| Missing | 2.3 |
| Sole use indoor flush toilet | 35.3 |
| Shared use indoor flush toilet | 10.1 |
| Sole use outdoor flush toilet | 37.6 |
| Shared use outdoor flush toilet | 12.1 |
| Other/missing | 4.9 |
| Increase SES tertile 0–16 yrs | 22.2 |
| Stay same SES tertile 0–16yrs | 37.6 |
| Reduce SES tertile 0–16 yrs | 31.2 |
| Missing SES tertile change | 9.0 |
| Currently smoke | 22.2 |
| Previously smoked | 50.0 |
| Never smoked | 8.7 |
| Missing smoking data | 19.1 |
| Tanner = 5 on either scale | 20.6 |
| Tanner = 4 on either scale | 30.6 |
| Tanner ≤3 on either scale | 44.8 |
| Missing Tanner scale | 4.0 |
| Maternal BMI (kg/m2)b | 28.8 (5.9) |
| Maternal overweightc | 30.6 |
| Maternal obese | 28.3 |
| Maternal not overweight/obese | 23.4 |
| Missing maternal weight | 17.7 |
This table does not include the household/neighbourhood SES indices included in the anlaysis because by definition approximately a third of the sample fits into each of the index tertiles.
Maternal BMI was assessed between 2002 and 2004 when the cohort were aged between 12 and 14 years of age.
Maternal overweight and obesity defined using internationally accepted cut-offs of >25 kg/m2 and >30 kg/m2, respectively.
3.1. Body mass index
As results for the fully adjusted logistic regression models were equivalent to those for the linear model of BMI, only the linear regression results are presented for brevity. The variables shown in Table 3 were found to be significant in initial unadjusted linear regression models of BMI at 16 years and were included into the adjusted regression analysis for BMI. In step one of the BMI adjusted regression model, all infancy variables entered into the model apart from the SES index of infancy consumer durables were significant (Table 3). None of the year 16 variables in step two were significantly associated with BMI. When both infancy and year 16 variables were adjusted for in step three, all significant variables in the previous steps retained significance and direction of association, and indoor sole use of running cold water at 16 years became significant. When the model was also adjusted for maternal weight status measured when the cohort were aged 12–14 years, maternal post secondary school education became insignificant. The only significant SES measures associated with BMI in the fully adjusted model were having poorer access to water and toilet facilities in infancy compared to the reference category, which was associated with increased BMI, and having poorer access to water facilities at age 16 years compared to the reference category, which was associated with a lower BMI. This adjusted model explained 24% of the variance in BMI at age 16 years compared to 10% of the variance in BMI explained by the model with just sex entered as a predictor (step 0).
Table 3.
Adjusted parameter estimates from a linear regression model of body mass index (BMI) at 16 years for African Black Bt20 participants using predictor variables that had a previous significant bivariate association with BMI.
| n (total = 346) | Step 0a adjusted parameter estimate (SE) | Step 1 adjusted parameter estimate (SE) | Step 2 adjusted parameter estimate (SE) | Step 3 adjusted parameter estimate (SE) | Step 4 adjusted parameter estimate (SE) | |
|---|---|---|---|---|---|---|
| Constant | 346 | 17.73 (0.66) | 17.52 (0.80) | 19.30 (1.18) | 18.99 (1.219) | 17.71 (1.24) |
| Sex (refb male) | 185 | |||||
| Female | 161 | 2.69 (0.43)*** | 2.82 (0.41)*** | 2.52 (0.44)*** | 2.68 (0.43)*** | 2.67 (0.41)*** |
| Infancy variables | ||||||
| Term birth (ref term) | 293 | |||||
| Preterm | 47 | −0.83 (0.61) | −0.73 (0.64) | −0.48 (0.62) | ||
| Post term | 4 | 5.88 (1.93)** | 6.35 (1.97)** | 5.36 (1.92)** | ||
| Missing | 2 | 10.00 (3.46)** | 9.18 (3.51)** | 9.05 (3.40)** | ||
| Maternal education (ref up to grade 10) | 191 | |||||
| Grades 11–12 | 122 | 0.12 (0.45) | 0.18 (0.48) | 0.25 (0.47) | ||
| Post school | 28 | 1.80 (0.77)* | 1.74 (0.78)* | 1.11 (0.76) | ||
| Missing | 5 | 0.53 (2.50) | 0.36 (2.57) | 0.89 (2.49) | ||
| Water & toilet facilities (ref sole use) | 266 | |||||
| Both sole and shared use | 13 | 2.22 (1.08)* | 2.43 (1.10)* | 3.07 (1.07)** | ||
| Shared use | 48 | 0.38 (0.60) | 0.44 (0.63) | 0.29 (0.61) | ||
| Missing | 19 | 1.76 (1.29) | 1.78 (1.32) | 1.91 (1.29) | ||
| Index of consumer durables (ref highest tertile) | 84 | |||||
| Middle tertile | 137 | −0.53 (0.52) | −0.49 (0.53) | −0.12 (0.52) | ||
| Lowest tertile | 107 | −0.54 (0.57) | −0.37 (0.59) | −0.15 (0.58) | ||
| Missing | 18 | −1.01 (1.38) | −0.89 (1.43) | −1.01 (1.39) | ||
| Year 16 variables | ||||||
| Water facilities (ref indoor sole use hot/cold) | 93 | |||||
| Indoor shared use hot/cold water | 34 | −0.23 (0.80) | −0.59 (0.79) | −0.45 (0.76) | ||
| Indoor sole use cold water | 93 | −1.08 (0.60) | −1.17 (0.59)* | −1.15 (0.57)* | ||
| Indoor share use cold water | 29 | 0.26 (0.85) | 0.20 (0.83) | 0.01 (0.80) | ||
| Other water source | 89 | −0.44 (0.61) | −0.54 (0.61) | −0.55 (0.59) | ||
| Missing | 8 | −2.85 (1.48) | −2.91 (1.44)* | −2.78 (1.39) | ||
| Neighbourhood safety (ref very unsafe/unsafe) | 39 | |||||
| Average safety | 82 | −0.06 (0.81) | −0.19 (0.78) | −0.06 (0.75) | ||
| Safe | 167 | −0.57 (0.75) | −0.55 (0.73) | −0.43 (0.71) | ||
| Very safe | 58 | −0.81 (0.87) | −0.95 (0.84) | −0.68 (0.81) | ||
| Neighbourhood crime (ref a lot) | 71 | |||||
| Some crime | 103 | −1.00 (0.64) | −1.00 (0.63) | −0.83 (0.61) | ||
| Average crime | 81 | 0.53 (0.67) | 0.35 (0.66) | 0.58 (0.64) | ||
| Not much crime | 80 | 0.01 (0.68) | −0.17 (0.67) | −0.16 (0.65) | ||
| No crime | 11 | −0.73 (1.32) | −0.56 (1.29) | −0.50 (1.25) | ||
| Index of school environment (ref highest tertile) | 112 | |||||
| Middle tertile | 116 | −0.38 (0.53) | −0.15 (0.52) | −0.04 (0.50) | ||
| Lowest tertile | 113 | −0.24 (0.56) | −0.01 (0.55) | 0.06 (0.53) | ||
| Missing | 5 | −1.25 (1.85) | −0.98 (1.80) | −0.39 (1.77) | ||
| Other variables | ||||||
| Maternal weight status (ref normal weight)c | 81 | |||||
| Overweight | 106 | 0.02 (0.56) | ||||
| Obese | 98 | 2.40 (0.57)*** | ||||
| Missing | 61 | 0.41 (0.66) | ||||
| Adjusted R2 | 346 | 0.10 | 0.19 | 0.11 | 0.19 | 0.24 |
p < 0.05.
p < 0.01.
p < 0.001 (two tailed).
Multivariable regression models were built in five steps: (0) sex, (1) significant infancy variables from initial analysis, (2) significant year 16 variables from initial analysis, (3) significant infancy and year 16 variables from initial analysis and (4) added significant other variables from initial analysis.
Ref = reference category.
Maternal overweight and obesity defined using internationally accepted cut-offs of >25 kg/m2 and >30 kg/m2, respectively.
3.2. Fat mass
The variables shown in Table 4 were found to be significant in initial unadjusted linear regression models of FM at 16 years and are similar to the list of variables identified for their association with BMI in unadjusted analyses. In step one of the adjusted FM regression model, all infancy variables tested apart from home ownership were significant (Table 4). All of the significant variables in step one were associated with significantly higher FM in all subsequent steps. Of the year 16 variables, only being in the lowest tertile of the index of school environment was significant and only in step two where there was no adjustment for infancy and other variables. The only significant SES measure in the fully adjusted model was having poorer access to water and toilet facilities in infancy and having a mother with post secondary school education, which were both associated with increased FM. The final adjusted model (step 4) explained 43% of the variance in FM at age 16 years compared to 31% of the variance in FM explained by the model with height and sex only as predictors (step 0).
Table 4.
Adjusted parameter estimates from a linear regression model of fat mass (FM) at 16 years for African Black Bt20 participants using predictor variables that had a previous significant association with FM in models adjusting for height only.
| n | Step 0a adjusted parameter estimate (SE) | Step 1 adjusted parameter estimate (SE) | Step 2 adjusted parameter estimate (SE) | Step 3 adjusted parameter estimate (SE) | Step 4 adjusted parameter estimate (SE) | |
|---|---|---|---|---|---|---|
| Constant | 346 | −8.17 (10.32) | −6.30 (9.91) | −6.24 (10.50) | −5.10 (10.09) | −8.58 (9.84) |
| Height (cm) | 346 | 0.05 (0.06) | 0.03 (0.06) | 0.05 (0.06) | 0.03 (0.06) | 0.04 (0.05) |
| Sex (refb male) | 185 | |||||
| Female | 161 | 10.01 (0.98)*** | 10.28 (0.94)*** | 9.66 (1.00)*** | 10.06 (0.96)*** | 9.99 (0.96)*** |
| Infancy variables | ||||||
| Term birth (ref term) | 293 | |||||
| Preterm | 47 | −1.75 (1.10) | −1.65 (1.12) | −1.29 (1.10) | ||
| Post term | 4 | 10.57 (3.43)** | 10.97 (3.48)** | 8.54 (3.43)* | ||
| Missing | 2 | 22.50 (6.17)*** | 22.81 (6.12)*** | 20.77 (6.09)** | ||
| Maternal education (ref up to grade 10) | 191 | |||||
| Grades 11–12 | 122 | 0.69 (0.80) | 0.63 (0.84) | 0.84 (0.82) | ||
| Post school | 28 | 4.24 (1.39)** | 4.12 (1.41)** | 2.83 (1.40)* | ||
| Missing | 5 | 0.46 (4.98) | −0.70 (5.04) | −0.91 (4.91) | ||
| Home ownership (ref owns property) | 99 | |||||
| Rented private | 59 | 0.18 (1.14) | 0.47 (1.15) | 0.01 (1.13) | ||
| Rented local authority | 171 | 0.23 (0.87) | 0.38 (0.88) | 0.07 (0.86) | ||
| Provided by employer | 7 | −2.38 (2.66) | −1.95 (2.71) | −2.76 (2.66) | ||
| Missing | 10 | −5.26 (3.87) | −4.95 (3.93) | −3.28 (3.91) | ||
| Water and toilet facilities (ref sole use) | 266 | |||||
| Both sole and shared use | 13 | 5.90 (1.93)** | 5.50 (1.95)** | 6.38 (1.91)** | ||
| Shared use | 48 | 0.30 (1.10) | 0.10 (1.12) | −0.03 (1.09) | ||
| Missing | 19 | 4.90 (2.33)* | 5.03 (2.38)* | 3.88 (2.33) | ||
| Year 16 variables | ||||||
| Toilet facilities (ref sole use indoor flush) | 122 | |||||
| Share use indoor flush toilet | 35 | 1.43 (1.39) | 0.63 (1.33) | 0.29 (1.30) | ||
| Sole use outdoor flush toilet | 130 | 0.33 (0.93) | 0.024 (0.90) | −0.06 (0.88) | ||
| Share use outdoor flush toilet | 42 | 2.06 (1.31) | 1.70 (1.25) | 2. 24 (1.23) | ||
| Other toilet type/missing | 17 | −1.46 (1.87) | −2.19 (1.80) | −1.55 (1.75) | ||
| Neighbourhood safety (ref very unsafe/unsafe) | 39 | |||||
| Average safety | 82 | −1.54 (1.43) | −1.63 (1.37) | −1.35 (1.34) | ||
| Safe | 167 | −1.44 (1.31) | −1.32 (1.26) | −0.57 (1.23) | ||
| Very safe | 58 | −2.03 (1.53) | −2.16 (1.45) | −1.61 (1.42) | ||
| Index of school environment (ref highest tertile) | 112 | |||||
| Middle tertile | 116 | −1.42 (0.97) | −0.99 (0.92) | −0.93 (0.90) | ||
| Lowest tertile | 113 | −2.10 (1.00)* | −1.51 (0.98) | −1.69 (0.96) | ||
| Missing | 5 | −3.77 (3.34) | −3.77 (3.18) | −2.74 (3.14) | ||
| Other variables | ||||||
| Smoking status (ref currently smokes) | 275 | |||||
| Previously smoked | 106 | −0.19 (1.04) | ||||
| Never smoked | 155 | 0.34 (0.99) | ||||
| Missing | 14 | 3.37 (1.99) | ||||
| Maternal weight status (ref normal weight)c | 81 | |||||
| Overweight | 106 | 0.21 (0.99) | ||||
| Obese | 98 | 4.34 (1.03)*** | ||||
| Missing | 61 | 1.79 (1.18) | ||||
| Adjusted R2 | 0.31 | 0.39 | 0.31 | 0.39 | 0.43 | |
p < 0.05.
p < 0.01.
p < 0.001 (two tailed).
Multivariable regression models were built in five steps: (0) sex and height, (1) significant infancy variables from initial analysis, (2) significant year 16 variables from initial analysis, (3) significant infancy and year 16 variables from initial analysis and (4) added significant other variables from initial analysis.
Ref = reference category.
Maternal overweight and obesity defined using internationally accepted cut-offs of >25 kg/m2 and >30 kg/m2, respectively.
3.3. Lean mass
The variables shown in Table 5 were found to be significant in initial unadjusted linear regression models of FM at 16 years and were entered into the adjusted analyses. All infancy variables entered into the adjusted analyses remained significant in all steps of the model building, although none of the year 16 variables were significant in any step (Table 5). In the fully adjusted model, none of the socio-economic measures were significant, maternal obesity measured when the cohort were aged 12–14 years was associated with significantly higher LM. 66% of the variance in LM at 16 years was explained by the fully adjusted model (step 4) compared to 61% of the variance explained by the model that only included sex and height (step 0).
Table 5.
Adjusted parameter estimates from a linear regression model of lean mass (LM) at 16 years for African Black Birth to Twenty participants for predictor variables that had a previous significant association with LM in models adjusting for height only.
| n | Step 0a adjusted parameter estimate (SE) | Step 1 adjusted parameter estimate (SE) | Step 2 adjusted parameter estimate (SE) | Step 3 adjusted parameter estimate (SE) | Step 4 adjusted parameter estimate (SE) | |
|---|---|---|---|---|---|---|
| Constant | 346 | −22.07 (6.62) | −25.73 (6.35) | −28.582 (6.697) | −28.046 (6.430) | −30.039 (6.428) |
| Height (cm) | 346 | 0.47 (0.04)*** | 0.42 (0.04)*** | 0.469 (0.037)*** | 0.425 (0.036)*** | 0.438 (0.036)*** |
| Sex (refb male) | 185 | |||||
| Female | 161 | −4.67 (0.63)*** | −4.81 (0.60)*** | −4.582(0.637)*** | −4.740 (0.607)*** | −4.690 (0.624)*** |
| Infancy variables | ||||||
| Birthweight (g) | 342 | 0.002 (0.001)*** | 0.002 (0.001)*** | 0.002 (0.001)** | ||
| Term birth (ref term) | 293 | |||||
| Preterm | 47 | 0.59 (0.74) | 0.804 (0.747) | 0.888 (0.753) | ||
| Post term | 4 | 5.75 (2.20)** | 5.942 (2.205)** | 5.007 (2.231)* | ||
| Missing | 2 | 13.52 (3.10)*** | 13.204 (3.103)*** | 13.144 (3.185)*** | ||
| Year 16 variables | ||||||
| Neighbourhood safety (ref very unsafe/unsafe) | 39 | |||||
| Average safety | 82 | 1.18 (0.90) | 1.46 (0.86) | 1.51 (0.86) | ||
| Safe | 167 | 0.16 (0.83) | 0.36 (0.79) | 0.40 (0.79) | ||
| Very safe | 58 | 0.07 (0.96) | 0.20 (0.92) | 0.31 (0.92) | ||
| Index of crime prevention (ref highest tertile) | 114 | |||||
| Middle tertile | 117 | 0.40 (0.61) | 0.66 (0.58) | 0.76 (0.58) | ||
| Lowest tertile | 115 | 1.02 (0.61) | 0.86 (0.58) | 0.76 (0.58) | ||
| Other variables | ||||||
| Smoking status (ref currently smokes) | 275 | |||||
| Previously smoked | 106 | −0.09 (0.69) | ||||
| Never smoked | 155 | −0.10 (0.65) | ||||
| Missing | 14 | 1.35 (1.35) | ||||
| Maternal weight status (ref normal weight)c | 81 | |||||
| Overweight | 106 | 0.70 (0.64) | ||||
| Obese | 98 | 2.03 (0.67)** | ||||
| Missing | 61 | 0.31 (0.76) | ||||
| Adjusted R2 | 0.61 | 0.65 | 0.61 | 0.65 | 0.66 | |
p < 0.05.
p < 0.01.
p < 0.001 (two tailed).
Multivariable regression models were adjusted for height and sex and were built in four steps: (1) significant infancy variables from initial analysis, (2) significant year 16 variables from initial analysis, (3) significant infancy and year 16 variables from initial analysis and (4) added significant other variables from initial analysis.
Ref = reference category.
Maternal overweight and obesity defined using internationally accepted cut-offs of >25 kg/m2 and >30 kg/m2, respectively.
4. Discussion
Findings show little evidence of a relationship of body composition at age 16 with SES both when looking at the significance of the parameter estimates and the change in adjusted R-squared values between unadjusted and adjusted models. Associations have been tested using an extensive range of household, school, and neighbourhood SES measures. For all three outcomes, the largest change in the R-squared value between step 0 (sex and height only model) and step 2 (age 16 predictor variables plus sex and height) is 1%. The changes in the R-squared are slightly larger, although still modest, for the difference between step 0 (sex and height only model) and step 1 (infancy predictor variables plus sex and height) with a 9% difference for BMI, 8% difference for FM, and a 4% difference for LM. This study's findings have shown that there does appear to be evidence supporting West's hypothesis of more equal health in adolescence (assessed using body composition outcomes) in these 16 year old urban South Africans at least based on the SES measures assessed at age 16 years. This is because observed significant differences by SES in body composition outcomes at 9–10 years (Griffiths et al., 2008) are not seen in the analyses presented in this paper at age 16.
SES variables that were found to be significantly associated with the FM and BMI outcomes were measures taken in the infancy period; access to sanitation and water facilities and maternal education. This suggests that if inequalities do exist at this age they endure from the infancy period rather than being created at age 16 years. This is different to what was observed at ages 9–10 years when infancy SES measures were only associated with LM but not FM and BMI. In that earlier work, SES measures taken at 9–10 years were more strongly associated with FM and BMI than infancy SES measures (Griffiths et al., 2008). At age 16 years, those born to mothers with post secondary school education compared to lower levels of education were associated with higher values of FM and BMI (for BMI this was only before controlling for maternal BMI). Eight percent of the cohort had mothers with post secondary school education. For many decades it has been well established that maternal education is associated with improved income and access to resources (Cleland and van Ginneken, 1988). Maternal education is also normally associated with improved knowledge and subsequently improved child health (e.g. Cleland and van Ginneken, 1988; Boyle et al., 2006). However, in South Africa where the Bt20 cohort were born into a climate of a high prevalence of under nutrition and low prevalence of over nutrition it is likely that health messages would have targeted preventing child under rather than over nutrition. Thus public knowledge available regarding obesity was likely limited even for educated individuals. The extra resources available to more educated mothers may therefore have provided a more calorie dense diet, resulting in increased FM and BMI for their offspring before health messages and knowledge regarding obesity were available. There is mounting evidence that lean mass is programmed by growth in utero and the first six months of life (Wells et al., 2005; Singhal et al., 2003), meaning that LM is less likely to be related to current environmental conditions when assessing adolescent outcomes. This may explain why maternal education has been found to be associated with FM and BMI in this study but not LM as environmental conditions are less likely to influence LM by age 16.
Although some household measures of sanitation and water facilities showed some association with BMI and FM, the direction of significance changed between different measures in infancy and at age 16 years. In infancy, being in one of the middle ranking SES groups (i.e. sole and shared use of water and toilet facilities) resulted in higher predicted BMI/FM than those who had sole access to water and toilet facilities. In contrast, the age 16 SES measure that was significant identified the middle ranking group in terms of SES (having indoor running cold water) to have predicted lower BMI scores than the high ranking group who had both indoor cold and hot running water. This means that the significance was not linear within the variable at either age (i.e. the middle SES group showed significance rather than the category representing the lower end of the SES measure when comparing to the high SES group). There is therefore not a consistent pattern of association with this measure of SES and BMI and FM either within or between the age groups assessed.
Adolescent reported neighbourhood and school SES variables at age 16 years showed some association with BMI and FM in unadjusted models whereas caregiver assessed household consumer durable measures did not. This gives some support to West's idea that there is a need to measure relevant aspects of an adolescent's SES environment, which includes their wider social environment. The Bt20 school and neighbourhood SES questionnaire should be relevant to the adolescents as it was developed using input from information gained from Bt20 participants at age 15 years in focus group discussions about relevant aspects of their neighbourhood and school environment (Sheppard et al., 2010). However, even these adolescent assessed neighbourhood and school measures were not significantly associated with body composition in adjusted models. West's hypothesis was built upon evidence relating to adolescent health inequalities in high income countries and this is the first formal test of the hypothesis within a low and middle income country setting using cohort data.
The findings of this paper are important because they suggest that for body composition outcomes, SES is not strongly driving differences within the adolescent period. It is not yet known whether SES inequalities previously observed in the Bt20 cohort in infancy (Willey et al., 2009) and childhood (Griffiths et al., 2008), but not during adolescence in the present paper, will emerge in adulthood. We have also not fully assessed the ‘peer culture’ measure that West proposed may be important in adolescence. This aspect would be difficult to assess in the cohort without a qualitative study to fully understand adolescent ‘peer culture’ in the urban South African context to develop quantitative measures of peer culture. It is also possible that, besides the original social reasons proposed by West (1997) for more equal health in adolescents, biological factors also complicate the assessment of health inequalities at this age in this cohort. Such biological factors combined with the rapid social change that has been taking place in South Africa in the post-Apartheid period could influence the findings observed at age 16 years. Body composition is very heavily influenced by pubertal status. During puberty both boys and girls experience the adolescent growth spurt, but sexually dimorphic increases occur in fat mass in girls and lean mass in boys under the influence of the sex hormones oestrogen and testosterone, respectively (Tanner, 1989; Roemmich and Rogol, 1999). Pubertal status is itself associated with SES, with low SES being associated with later entry into puberty (Tanner, 1962; Adair, 2001). In the South African transitioning context, this would likely reduce the BMI and fat mass of the low SES group acquired by age 16, compared to those from the higher SES groups who enter puberty earlier. This is because pubertal development, at least in girls, is associated with increasing fat mass (Bogin, 1999) and body mass index (Kaplowitz, 2008). At the same time, for low SES adolescents (compared to high SES) the transitioning environment could be starting to result in changes to lifestyle behaviours such as increasing access to high fat diets and less physical activity because a significant increase in overweight was observed in the cohort between late childhood and age 16 years. Low SES groups tend to be more vulnerable to such changing lifestyle factors associated with nutrition transition during periods of transition in middle income countries (Popkin, 2001, Monteiro et al., 2001). The high SES group could also have been provided with more protection from becoming overweight from environmental factors associated with the rapidly changing environment such as being the first to be exposed to knowledge about how to prevent obesity. This would result in the high SES group's experience of earlier puberty cancelling out the effects of the broader SES environment on body composition and making the SES groups appear more equal at this age. This assertion needs further testing in this South African context because the findings of this analysis show those with mothers with post secondary school education to have predicted higher FM and BMI, suggesting that this community may not yet have transitioned to the usual pattern of increased risk for lower SES groups observed in middle income countries in previous research. Nevertheless, there is a need for researchers to consider the biological context as well as the social context when studying inequalities in adolescent health.
Overweight and obesity are an emerging health problem in South Africa and also in the Bt20 cohort. 18.8% of the adolescents in the present study were overweight or obese, with 30% of females being in this category. This is a 20% increase compared to the documented prevalence at ages 9–10 years in African Black girls in the same cohort (Griffiths et al., 2008). The higher prevalence of obesity in females observed in this study is supported by similar work in another township location in South Africa of adults where female prevalence of obesity was 5.7 times higher than males (Case and Menendez, 2009). The rising prevalence of obesity, especially in females, means that it is important to understand the factors leading to increased risk for overweight to reduce risk for later chronic diseases in this context. Although we do not find strong associations between SES and the body composition outcomes in this paper, we do show other factors to be important. These include maternal BMI, measured when the cohort were aged 12–14 years, as well as variables assessed in infancy, including for FM and BMI whether the infant was born post term (although this finding is based on a very small sample size and needs further exploration with larger sample sizes) and the infant's birth weight (LM). Maternal BMI explains 5% of the variance in BMI, 4% for FM, and 1% for LM. Maternal BMI is potentially a marker of genetic potential for obesity, it is also an indicator of a shared obesogenic environment as well as a potential marker in itself of household SES. If we cross-tabulate maternal BMI grouped into normal, overweight, obese, and missing with the household SES tertile index assessed in infancy and the household SES tertile index assessed at age 16 years of age, the Chi-squared statistics produced show no statistically significant association. However, maternal BMI is associated with maternal education measured in infancy (χ2 = 16.41, p = 0.012) and the adjusted standardised residuals show that this significance comes from the post secondary school education group being significantly less likely to be normal weight and significantly more likely to be obese. This is the same pattern of association that is shown with FM, BMI and LM in adolescents. This suggests that as well as potential genetic drivers of associations between maternal BMI and adolescent body composition outcomes, that there may also be factors related to the resources associated with maternal education that influence increased maternal BMI as well as adolescent body composition outcomes, especially in a society like South Africa that has undergone such rapid nutrition transition.
5. Limitations
Previous findings related to attrition in the cohort show no bias in attrition by SES in the first sixteen years of the overall cohort's life (Norris et al., 2007). However, the specific sub-sample used here for this analysis has a significantly higher SES on some measures compared to the original Bt20 children, thus under-representing the poor (results not shown). It is possible that with further inclusion of the poor group into the analysis that there would have been more of a spread in SES, which may have resulted in more significant findings being observed. Despite differences between this sub-sample and the main cohort, no such differences exist between this sub-sample and the one used in the 9–10 year analysis where SES differences were observed. With alpha set at 0.05 and power at 0.80, the sample size analysed has the statistical power to detect medium to large effect sizes, but is not adequate to detect small effect sizes (Cohen, 1992). Nevertheless the same bone health sub-sample was used to perform the analysis on the 9–10 year old children thus making the findings of the two papers comparable in power for studying change in the effect of the SES measures over the early life-course.
This study also lacks environmental SES measures during infancy outside of the household, which means that it only studies the effect of environmental measures of SES in adolescence. It has not been possible to fully test West's idea that peer SES may be a stronger influence on health outcomes in adolescence compared to other SES measures because this study's school measures did not encompass measures of the social status of peers. When modelling neighbourhood health effects, researchers often use a multilevel approach to control for the clustering of SES characteristics within neighbourhoods (Pickett and Pearl, 2001). However, the definition of neighbourhood used here (within 20 min of home) meant that no two households exactly shared a neighbourhood, although they will share some aspect of their neighbourhood with other participants. Not being able to correct for this partial lack of independence could lead to slightly biased standard error estimates. Finally there were a reasonable number of missing cases across the variables used in these analyses, which have been included in the analysis as missing categories. It is possible that results would have changed if information had been available for all of these cases on all variables. However, the only missing group to show significance in the fully adjusted models was for the variable measuring whether the birth was a term birth or not. The significance of model estimates did not change when including or excluding this small number of missing cases (results not shown for the models where the cases were excluded).
6. Conclusion
This study shows evidence of a weaker association between SES and body composition outcomes at age 16 years in this urban South African context compared to studies of the same cohort in infancy and childhood. There was some association between two infancy measures of SES (maternal education and sanitation) and BMI and FM, although the direction of this association was not consistent. Those with the highest levels of maternal education had adolescents with the highest predicted BMI and FM, whilst those with a middle level of water and sanitation provision had significantly different BMI and FM to those with the highest level of water and sanitation provision. The direction of the association for the water and sanitation variables changed between infancy and age 16 years. Taken together this evidence reveals more equal health in adolescents for the first time in a transitioning setting using longitudinal data. It supports West's hypothesis of more equal health outcomes in adolescence, although we observe limited evidence for the importance of the school context in shaping inequalities in this South African urban environment. There are both biological and wider societal level social factors that were occurring in South Africa at this time that could go some way to explaining a more equal profile in body composition outcomes at this age in this context. These factors, in addition to the original social reasons for less inequality proposed by West (1997), show that adolescence is a complex period to study in relation to SES health inequalities because of the challenge of adequately measuring the social and biological context in which health outcomes occur at this stage in the life course.
There is a need to assess whether inequalities evolve again in early adulthood in this cohort to more fully understand the pattern of inequality beyond adolescence. Furthermore, studying change in BMI over the period from late childhood to adolescence and relating it to SES status could add further understanding to how inequalities in BMI evolve through adolescence in transitioning contexts. With the range of variables tested within this study at the mother, household, school, and neighbourhood level, findings suggest that targeting obesity interventions at females in households with a mother who already has a high BMI, and especially to those of higher educational status, would target the most at risk for obesity in adolescence within this transitioning context.
Financial support
Bt20 receives financial and logistic support from the University of the Witwatersrand. The Bone Health study was financially supported by the Wellcome Trust (UK) and Birth to Twenty continues to be supported by the Wellcome Trust (UK) (reference number 092097/Z/10/Z). The neighbourhood and school socioeconomic measures data were funded by the Medical Research Council (UK) through grant id 70363. PG's time in writing the paper was also supported by a British Academy Mid-career Fellowship (Ref: MD120048). The funders had no influence on study design or in the collection, analysis and interpretation of data or in writing the report. The authors take responsibility for all of these aspects of the study.
Author contributions
PG conceived the research question with the help of SN, JP and NC. PG and WJ carried out the data analysis, PG led the writing of the manuscript with the help of WJ, and all authors took responsibility for final editing, reviewing and approval of the document.
Acknowledgements
The authors would like to thank the Bt20 participants and research team, and also Gretchen Hanke for her help in cleaning the age-16 socioeconomic data. The authors would also like to thank Dr Jennifer Van Hook for her perceptive comments on an earlier draft of the paper presented at the 2011 Population Association of America annual meeting.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Paula L. Griffiths, Email: P.griffiths@lboro.ac.uk.
William Johnson, Email: w.johnson@nshd.mrc.ac.uk.
Noël Cameron, Email: n.cameron@lboro.ac.uk.
John M. Pettifor, Email: John.Pettifor@wits.ac.za.
Shane A. Norris, Email: san@global.co.za.
References
- Adair L. Size at birth predicts age at menarche. Pediatrics. 2001;107:E59. doi: 10.1542/peds.107.4.e59. [DOI] [PubMed] [Google Scholar]
- Allison K.W., Burton L., Marshall S., Perez-Febles A., Yarrington J., Kirsh L.B., Merriwether-DeVries C. Life experiences among urban adolescents: examining the role of context. Child Development. 1999;70:1017–1029. doi: 10.1111/1467-8624.00074. [DOI] [PubMed] [Google Scholar]
- Bogin B. Childhood, adolescence and longevity. In: Hewlett B.L, editor. Adolescent Identity. Evolutionary, Cultural and Developmental Perspectives. 1st ed. Routledge; New York: 2013. pp. 23–39. [Google Scholar]
- Bogin B. 2nd ed. Cambridge University Press; Cambridge: 1999. Patterns of Human Growth. [Google Scholar]
- Bollen K., Glanville J.L., Stecklov G. Socioeconomic status and class in studies of fertility and health in developing countries. Annual Review of Sociology. 2001;27:153–185. [Google Scholar]
- Boyle M.H., Racine Y., Georgiades K., Snelling D., Hong S., Omariba W., Hurley P., Rao-Melacini P. The influence of economic development level, household wealth and maternal education on child health in the developing world. Social Science and Medicine. 2006;63:2242–2254. doi: 10.1016/j.socscimed.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Cameron N. Physical growth in a transitional economy: the aftermath of South African apartheid. Economics and Human Biology. 2003;1:29–42. doi: 10.1016/s1570-677x(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Case A., Menendez A. Sex differences in obesity rates in poor countries: evidence from South Africa. Economics and Human Biology. 2009;7:271–282. doi: 10.1016/j.ehb.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland J.G., van Ginneken J.K. Maternal education and child survival in developing Countries: the search for pathways of influence. Social Science and Medicine. 1988;27:1357–1368. doi: 10.1016/0277-9536(88)90201-8. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cole T.J., Bellizzi M.C., Flegal K.M., Dietz W.H. Establishing a standard definition for child overweight and obesity worldwide: international survey. British Medical Journal. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T.J., Fewtrell M.S., Prentice A. The fallacy of using percentage body fat as a measure of adiposity. American Journal of Clinical Nutrition. 2008;87:1959. doi: 10.1093/ajcn/87.6.1959. (author reply 1959–60) [DOI] [PubMed] [Google Scholar]
- Davey Smith G., Blane D., Bartley M. Explanations for socio-economic differentials in mortality. European Journal of Public Health. 1994;4:131–144. [Google Scholar]
- Griffiths P.L., Sheppard Z.A., Johnson W., Cameron N., Pettifor J.M., Norris S.A. Associations between household and neighbourhood socio-economic status and systolic blood pressure among urban South African adolescents. Journal of Biosocial Science. 2012;44:433–458. doi: 10.1017/S0021932012000107. [DOI] [PubMed] [Google Scholar]
- Griffiths P.L., Rousham E.K., Norris S.A., Pettifor J.M., Cameron N. Socio-economic status and body composition outcomes in urban South African children. Archives of Disease in Childhood. 2008;93:862–867. doi: 10.1136/adc.2006.112649. [DOI] [PubMed] [Google Scholar]
- Holstein B.E., Currie C., Boyce W., Damsgaard M.T., Gobina I., Kökönyei G., Hetland J., de Looze M., Richter M., Due P., The HBSC Social Inequalities Focus Group Socio-economic inequality in multiple health complaints among adolescents: international comparative study in 37 countries. International Journal of Public Health. 2009;54:S260–S270. doi: 10.1007/s00038-009-5418-4. [DOI] [PubMed] [Google Scholar]
- Judge K., Benzeval M. Health inequalities: new concerns about the children of single mothers. British Medical Journal. 1993;306:677. doi: 10.1136/bmj.306.6879.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz P.B. Link between body fat and the timing of puberty. Pediatrics. 2008;121:s208–s217. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- Kruger R., Kruger H.S., Macintyre U.E. The determinants of overweight and obesity among 10- to 15-year-old schoolchildren in the North West Province, South Africa – the THUSA BANA (Transition and Health during Urbanisation of South Africans; BANA, children) study. Public Health Nutrition. 2006;9:351–358. doi: 10.1079/phn2006849. [DOI] [PubMed] [Google Scholar]
- Lohman T.G., Roche A.F., Martorell R. Human Kinetics Books; Champaign: 1991. Anthropometric Standardization Reference Manual. [Google Scholar]
- Marmot M. Social determinants of health inequalities. The Lancet. 2005;365:1099–1104. doi: 10.1016/S0140-6736(05)71146-6. [DOI] [PubMed] [Google Scholar]
- May J. David Phillip Publishers; Cape Town: 2000. Inequality in South Africa. Meeting the Challenge. [Google Scholar]
- Monteiro C.A., Condey W.L., Popkin B.M. Independent effects of income and education on the risk of obesity in the Brazillian adult population. Journal of Nutrition. 2001;131:881S–886S. doi: 10.1093/jn/131.3.881S. [DOI] [PubMed] [Google Scholar]
- Norris S.A., Richter L.M., Fleetwood S.A. Panel studies in developing countries: case analysis of sample attrition over the past 16 years within the Birth to Twenty cohort in Johannesburg, South Africa. Journal of International Development. 2007;19:1143–1150. [Google Scholar]
- Pickett K.E., Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. Journal of Epidemiology and Community Health. 2001;55:111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin B.M. The nutrition transition and obesity in the developing world. Journal of Nutrition. 2001;131:871S–873S. doi: 10.1093/jn/131.3.871S. [DOI] [PubMed] [Google Scholar]
- Richter L.M., Norris S.A., De Wet T. Transition from Birth to Ten to Birth to Twenty: the South African cohort reaches 13 years of age. Paediatric and Perinatal Epidemiology. 2004;18:290–301. doi: 10.1111/j.1365-3016.2004.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter L., Norris S., Pettifor J., Yach D., Cameron N. Cohort Profile: Mandela's children: the 1990 Birth to Twenty study in South Africa. International Journal of Epidemiology. 2007;36:504–511. doi: 10.1093/ije/dym016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B.J. Chronic and transitory poverty in post-Apartheid South Africa: evidence from KwaZulu-Natal. Journal of Poverty. 2001;5:1–27. [Google Scholar]
- Roemmich J.N., Rogol A.D. Hormonal changes during puberty and their relationship to fat distribution. American Journal of Human Biology. 1999;11:209–224. doi: 10.1002/(SICI)1520-6300(1999)11:2<209::AID-AJHB9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Sheppard Z.A., Norris S.A., Pettifor J.M., Cameron N., Griffiths P.L. How can we learn about community socio-economic status and poverty in a developing country urban environment? An example from Johannesburg–Soweto, South Africa. African Population Studies. 2010;24:53–70. [Google Scholar]
- Shrewsbury V., Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross-sectional studies 1990–2005. Obesity. 2008;16:275–284. doi: 10.1038/oby.2007.35. [DOI] [PubMed] [Google Scholar]
- Singhal A., Wells J.C.K., Cole T.J., Fewtrell M., Lucas A. Programming of lean body mass: a link between birth weight, obesity and cardiovascular disease. American Journal of Clinical Nutrition. 2003;77:726–730. doi: 10.1093/ajcn/77.3.726. [DOI] [PubMed] [Google Scholar]
- Starfield B., Riley A.W., Witt W.P., Robertson J. Social class gradients in health during adolescence. Journal of Epidemiology and Community Health. 2002;56:354–361. doi: 10.1136/jech.56.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J.M. 2nd ed. Harvard University Press; Cambridge, MA, USA: 1989. Foetus into Man: Physical Growth from Conception to Maturity. [Google Scholar]
- Tanner J.M. Blackwell; Oxford, UK: 1962. Growth at Adolescence. [Google Scholar]
- UNDP . 2011. Human Development Reports. [Google Scholar]
- Wells J.C.K., Hallal P.C., Wright A., Singhal A., Victora C.G. Fetal, infant and childhood growth: relationships with body composition in Brazilian boys aged 9 years. International Journal of Obesity. 2005;29:1192–1198. doi: 10.1038/sj.ijo.0803054. [DOI] [PubMed] [Google Scholar]
- West P. Health inequalities in the early years: is there equalisation in youth? Social Science & Medicine. 1997;44:833–858. doi: 10.1016/s0277-9536(96)00188-8. [DOI] [PubMed] [Google Scholar]
- WHO . 2011. Global Infobase. [Google Scholar]
- Willey B.A., Cameron N., Norris S.A., Pettifor J.M., Griffiths P.L. Socio-economic predictors of stunting in preschool children – a population-based study from Johannesburg and Soweto. South African Medical Journal. 2009;99:450–456. [PubMed] [Google Scholar]