Abstract
Etch-and-rinse adhesive systems are the oldest of the multi-generation evolution of resin bonding systems. In the 3-step version, they involve acid-etching, priming and application of a separate adhesive. Each step can accomplish multiple goals. This review explores the therapeutic opportunities of each separate step. Acid-etching, using 32-37% phosphoric acid (pH 0.1-0.4) not only simultaneously etches enamel and dentin, but the low pH kills many residual bacteria. Some etchants include anti-microbial compounds such as benzalkonium chloride that also inhibits matrix metalloproteinases (MMPs) in dentin. Primers are usually water and HEMA-rich solutions that ensure complete expansion of the collagen fibril meshwork and wet the collagen with hydrophilic monomers. However, water alone can re-expand dried dentin and can also serve as a vehicle for protease inhibitors or protein cross-linking agents that may increase the durability of resin-dentin bonds. In the future, ethanol or other water-free solvents may serve as dehydrating primers that may also contain antibacterial quaternary ammonium methacrylates to inhibit dentin MMPs and increase the durability of resin-dentin bonds. The complete evaporation of solvents is nearly impossible. Manufacturers may need to optimize solvent concentrations. Solvent-free adhesives can seal resin-dentin interfaces with hydrophobic resins that may also contain fluoride and antimicrobial compounds. Etch-and-rinse adhesives produce higher resin-dentin bonds that are more durable than most 1 and 2-step adhesives. Incorporation of protease inhibitors in etchants and/or cross-linking agents in primers may increase the durability of resin-dentin bonds. The therapeutic potential of etch-and-rinse adhesives has yet to be fully exploited.
Keywords: Acid-etchants, Primers, Adhesives, Durability, MMPs
INTRODUCTION TO STATE OF THE ART ETCH-AND-RINSE ADHESIVES
Buonocore [1] was the first to demonstrate that acid-etching enamel with phosphoric acid increased resin-enamel bond strengths. He believed that acid-etching simply increased the microscopic surface area available for resin retention. However, one of his students, John Gwinnett, who was a trained electron microscopist, looked at the interface more closely. He reported that adhesive resins could penetrate into acid-etched enamel prisms where they could actually envelop apatite crystallites [2] rendering them acid-resistant. This was the first true hybrid layer, although that term had not yet been introduced. Resin-treatment of acid-etched enamel created a new structure that was neither enamel nor resin but a hybridization of the two materials. It was the first example of in situ dental tissue engineering.
Nakabayashi et al. [3] were the first to demonstrate true hybrid layer formation in acid-etched dentin. This was best observed by transmission electron microscopy but was later demonstrated by scanning electron microscopy following argon ion beam etching [4]. Nakabayashi’s group was the first to demonstrate that resins could infiltrate into acid-etched dentin to form a new structure composed of a resin-matrix reinforced by collagen fibrils. He named this new biocomposite the hybrid layer (Fig. 1).
Figure 1.
Schematic of a hybrid layer (HL) created by an etch-and-rinse adhesive. Note that the depth of the hybrid layer (green) is about four acid-etched tubule diameters (i.e. ca. 8 μm). The collagen fibrils in the HL are continuous with the underlying mineralized matrix. A single dentinal tubule is shown devoid of a resin tag to illustrate its presence.
Evolution of etch-and-rinse adhesives
When Fusayama [5] introduced the revolutionary concept of total-etching of cavities (i.e. simultaneous etching of enamel and dentin), the technique was resisted by U.S. and European dentists. They thought that 40% phosphoric acid would induce adverse pulpal reactions when allowed to etch dentin. Later work revealed that acid-etching dentin more than 0.5 mm thick produced no adverse pulpal reactions if the etched dentin could be sealed from oral bacteria. The adverse pulpal reactions seen in the U.S. and Europe were due to bacterial leakage, not acids per se [6]. The lack of pulpal reactions to total-etching in Japan was due to the fact that they only excavated carious dentin. As part of their minimal invasive restorative philosophy, Japanese dentists did not extend outline forms into normal dentin as was the practice in the U.S. and Europe. Excavated caries-affected dentin, unlike normal dentin, is almost impermeable to all solutes and solvents [7], thereby protecting the pulp from irritants.
The introduction of dry bonding
The first marketed etch-and-rinse adhesive was Clearfil Bond System-F (Kuraray Co., Ltd., Tokyo, Japan) in 1978. It utilized 40% phosphoric acid used in the total-etch manner. Adverse pulpal reactions continued to be reported in the U.S. following acid-etching of dentin with phosphoric acid because clinicians were performing “dry bonding”. That is, after total-etching, they would dry the cavity walls to confirm that the enamel margins were “frosty” or had a chalk-like color. This meant that the enamel was properly etched. What was not realized at that time was that drying the cavity caused the acid-etched dentin to collapse. Such collapsed demineralized dentin had lost the interfibrillar spaces between exposed collagen fibrils [8] that serve as inward diffusion channels for monomer infiltration. Consequently, resin-enamel bond strengths were high (ca. 20 MPa) but resin-dentin bond strengths were very low (ca. 5 MPa). Such low resin-dentin bond strengths were not sufficient to resist the forces of polymerization shrinkage (about 24 MPa in class I cavities) [9]. Thus, during polymerization of resin composites, one or more of the bonded walls would debond, creating bacterial leakage through normal permeable dentin that could irritate the pulp.
The introduction of wet-bonding
The low resin-dentin bond strengths associated with “dry” bonding created dentin sensitivity, microleakage, secondary caries and loss of bonded restorations. Kanca found that water was an excellent rewetting agent and this led to him [10] to introduce the concept of “wet-bonding”. This technique increased the strength of resin-dentin bonds, allowing good sealing of dentin and much less post-operative pain. At this point resin-dentin bonds equaled or exceeded resin-enamel bonds and the era of safe, reproducible resin-dentin bonding began.
Dentin bonding as a form of tissue engineering
In most tissue engineering applications, one uses a 3-D scaffold (often made of collagen) that is designed to be resorbed over several weeks to months, to provide replacement by regenerating tissues of the host [11,12]. Unlike classical tissue engineered constructs, where the scaffold is designed to be resorbed and replaced by normal tissue, in erupted teeth there are no tissues available for regeneration of occlusal hard tissue surfaces while teeth are in function. Instead, biocomposites must be engineered within minutes, in situ, with the expectation that they will last for decades! Because progress in adhesive dentistry has been incremental, we fail to recognize how far we have come in 55 years since Buonocore [1] first acid-etched enamel. Each day, practitioners bond relatively hydrophobic resins to enamel and dentin within a few minutes and in doing so have completely transformed the surface chemistry of these hard tissues from wet, crystalline, hydrophilic surfaces that are acid-labile, to softer but tougher, hydrophobic, drier dentin surfaces that are chemically compatible with resin composites. These tooth colored biocomposites are acid-resistant. They can be made to be antibacterial [13,14]. Such bonding begins by acid-etching to increase the permeability of resins to enamel [2] and dentin [8]. In dentin, this is a unique form of tissue engineering. Acid-etching with 37 wt% phosphoric acid completely demineralizes the surface 5-8 μm of the intertubular dentin matrix to create nanometer-sized porosities (Fig. 2) within the underlying collagen fibrillar matrix. This permits infiltration of solvated comonomers into and around collagen fibrils to gain retention for tooth colored resin-composite fillings [14]. Even more amazing is the contrast between the porosities of most bioengineered scaffolds (5-20 μm) compared to the porosity of interfibrillar spaces between resin-infiltrated collagen fibrils in hybrid layers that are only 10-30 nm wide. Thus, the dental biocomposites that are made by dentists in situ are created at a nanometer scale over a distance of 5-8 μm!
Figure 2.
Scanning electron micrograph of acid-etched dentin showing two dentinal tubules containing remnants of peritubular dentin matrix. INSERT: High magnification of branching collagen fibrils (ca. 75 nm in diameter) separated by interfibrillar spaces that serve as channels for resin infiltrations during bonding.
Composition of mineralized vs. demineralized dentin vs. hybrid layers
Mineralized dentin is composed of approximately 50 vol% mineral phase, 30 vol% collagen and 20 vol% water [15] (Table 1). During the acid-etching process in etch-and-rinse adhesives, the entire 50 vol% of surface and subsurface mineral is solubilized, extracted and is replaced by rinse-water which, when combined with the intrinsic 20 vol% of water yields a new water content of 70 vol% surrounding the 30 vol% collagen fibrils that remain anchored into the underlying mineralized dentin. During the subsequent comonomer infiltration phase of resin bonding, this 70 vol% of water should ideally be completely replaced by 70 vol% of resin comonomers that polymerize in situ to produce a hybridized biocomposite of resin, reinforced with collagen fibrils known as the hybrid layer or interdiffusion zone (Table 1). However, due to the presence of residual solvent, and due to fluid movement out of dentinal tubules (Fig. 3) into the hypertonic comonomer mixtures [16,17] water replacement by resin is never ideal [8]. This results in incomplete infiltration of resin into water-filled collagen fibril matrices. Additional water may enter the solvated comonomer-infiltrated demineralized matrix from dentinal tubules during the solvate-evaporation phase of bonding [18]. This creates small local regions within the polymerized hybrid layer that are water-rich and resin-poor. These can be identified using water-soluble tracers [19]. The distribution of these tracers in bonded interfaces has been called nanoleakage. It reveals the water distribution in nanoscale porosities within bonded interfaces. Although the water-rich zones are generally sparse in freshly created bonds, they increase in size with aging [19]. Some nanoleakage occurs in adhesive layers. When nanoleakage occurs in freshly made resin-bonded (Fig. 4A) hybrid layers it can be seen under high magnification TEM as linear arrays of individual silver grains that we believe represents intrafibrillar water (Fig. 4B) or as single silver grains that represent absorbed water. The larger 200 × 1000 nm reticular silver deposits are thought to represent residual water-filled terminal branches of dentinal tubules.
Table 1.
Theoretical composition of demineralized dentin before and after bonding procedures.
| Mineralized dentin |
Etched/rinsed dentin |
Primed/infiltrated dentin |
Aged/degradation | |
|---|---|---|---|---|
| Mineral | 50 | 0 | 0 | 0 |
| Collagen | 30 | 30 | 30 | 0-30b |
| Water | 20 | 70 | 0a | 0-30b |
| Resin | 0 | 0 | 70a | 20-50c |
Perfect hybrid layer;
water replaces destroyed collagen fibrils;
loss of collagen fibrils often leads to loss of interfibrillar resin.
Figure 3.
Transmission electron micrograph of an adhesive layer containing a fluid-filled droplet of dentinal fluid that exuded from a dentinal tubule before the adhesive polymerized. H = hybrid layer; T = dentinal tubule; D: = underlying mineralized dentin that was demineralized during laboratory processing, exposing cross-banded collagen fibrils.
Figures 4.
A&B – Unstained transmission electron micrograph (TEM) of resin-dentin bond created by an acid-etched adhesive. After immersion in 50 wt% ammoniacal silver nitrate for 24 h to reveal the distribution of water in the hybrid and adhesive layers, the specimens were processed for TEM. A: Lower power TEM showing a water tree (black arrow) in the adhesive (A) layer. The region between the opposing open arrows is the hybrid layer. Large silver grains in the hybrid layer are due to the terminations of lateral branches of dentinal tubules. C = composite; D = underlying dentin. B: High magnification of Fig. 4A showing silver grains in terminal branches of tubules in the hybrid (H) layer. The black arrow points to a linear array of tiny silver grains thought to reside in intrafibrillar water inside collagen fibrils.
Direct evidence of degradation of hybrid layers
Many authors examine hybrid layers by transmission electron microscopy because the structures of interest such as collagen fibrils are only 50-100 nm wide. Normal, freshly created hybrid layers show collagen fibrils that take up heavy metals to form alternating dark and light “banding” with a periodicity of 67 nm due to the unique quarter-overlapping aggregation of collagen fibrils (Fig. 5). There are also thin (20 nm wide) interfibrillar spaces between these collagen fibrils that are filled with adhesive resin that are electron-lucent [20].
Figure 5.

A very high magnification TEM of the top of a hybrid layer (H). A: adhesive layer. Note the 67 nm cross-banding (open arrowhead) of collagen fibrils. These fibrils are about 75 nm in diameter. Note that the interfibrillar spaces are only 20 nm wide. They are filed with electron-lucent resin.
Over time, both in vitro [21] and in vivo [22], the hybrid layers created by etch-and-rinse adhesives undergo degradation within 6 months to 3-5 years (Figs. 6A and 6B). This is manifested by a loss of cross-banding of collagen fibrils, a loss of heavy metal staining and a large increase in water uptake.
Figure 6.

A&B – Stained transmission electron micrographs of resin-dentin bonds made by Optibond FL to acid-etched dentin. A: After storage in water for 48 hr, the specimens were processed. FA = filled adhesive; H – hybrid layer; D = laboratory demineralized dentin. Black arrow heads = bottom of hybrid layer. Note well-stained collagen fibrils filling the hybrid layer. B: Similar bonded specimen after incubation in water for 44 months. More than half the collagen fibrils in the hybrid layer have lost their ability to pick up stain (*). Endogenous dentin MMPs are thought to give broken collagen fibrils to gelatin. (Reproduced from Armstrong et al., Oper Dent 2004;29:705-712, with permission)
The 30 vol% fraction of the hybrid layer that was previously occupied by collagen in hybrid layers is now largely occupied by water due to the loss of insoluble collagen (Fig. 7). It is thought that collagen is broken down to ¾ and ¼ fragments by MMP-8, a true collagenase and that further degradation is done by MMP-2 and -9 that are gelatinases[23]. The conversion of insoluble collagen fibrils to soluble gelatin peptides causes a loss of continuity between the hybrid layers and anchored mineralized collagen fibrils in the underlying dentin. This results in a loss of retention of resin composites to dentin [22] that can be measured as a significant decrease in microtensile bond strength.
Figure 7.

Unstained transmission electron micrograph of a resin-dentin bond made with Single Bond Plus after 12 months of function in vivo. The specimen was immersed in 50 wt% ammoniacal silver nitrate to stain water-filled voids in the hybrid layer. Normally there are only a few water-filled voids in hybrid layers (Fig. 4A). Here, huge amounts of silver (*) have been taken up to stain the water uptake that replaced the hydrolyzed collagen. A = adhesive; H = hybrid layer; D = underlying mineralized dentin.
The task of materials scientists is to determine why resin-dentin bonds do not last as long as resin-enamel bonds [24]. Those problems need to be solved so that resin-dentin bonds can become as durable as amalgam restorations. We need to identify which therapeutic approaches are possible. Not all approaches will be clinically practical, but enough will be useful so that resin-dentin bonds in the future will be far more durable than they are today.
STABILITY OF ETCH-AND-RINSE ADHESIVES
Decrease in bond strength over time
Although many studies of the durability of resin-dentin bonds are done by reducing resin-bonded crowns to tiny resin-dentin sticks [25], that technique is designed to accelerate aging effects. The clinically-relevant model is to incubated intact resin-bonded crowns in water at 37°C for varying lengths of time, and only reduce them to sticks just prior to microtensile testing [26]. DeMunck et al. [26] found that 4 year water storage of the 3-step etch-and-rinse adhesive (Optibond FL, Kerr, Orange, CA, USA) gave the highest microtensile bond strength, and that it did not change over 4 yr, regardless of whether they were stored intact or as sticks. The other 3-step system they studied (Scotchbond Multi-Purpose, 3M ESPE, St. Paul, MN, USA) also was insensitive to 4 yr of storage regardless of whether it was storage as sticks or intact crowns. Both 2-step adhesives used in that study, Optibond Solo and Scotchbond 1 (Single Bond in North America) showed significant reductions in bond strength over 4 yr when stored as sticks. As the transmission electron micrographs of the authors of that study were unstained and were done on failed bonds, it is not clear whether the collagen fibrils in the hybrid layer had degraded.
Transmission electron microscopy evidence of collagen degradation
Armstrong and his colleagues [21] did a similar study by bonding acid-etched dentin with Optibond FL and then reducing the teeth to small specimens to accelerate aging for up to 5 years. They found that the microtensile bond strength fell from 46.7 MPa to 17.7 MPa over the 5 year period. The most important observation of that study was the transmission electron micrographs (TEMs) of nonstressed resin-dentin bonds that showed no degradation of the hybrid layers of the 48 h specimens (Fig. 6A) but extensive degradation of the 44 month specimens (Fig. 6B). About half of the hybrid layer volume would not take up heavy metal stains that collagen fibrils typically bind. This was the first indication that collagen fibrils in hybrid layers could degrade. That observation was confirmed by Pashley et al. [27] and later by Hebling et al. [28].
Importance of peripheral enamel seal
The stability of Optibond FL and Scotchbond Multi-Purpose (SBMP) over 4 yr when the bonded teeth were stored intact, and their instability when stored as individual “sticks”, suggests that the presence of the peripheral enamel acid-etched resin seal contributed to the long-term durability of resin bonds. It is well known that resin-enamel bonds are more durable [24] than resin-dentin bonds. This is due, in part, to the absence of collagen in enamel and to its dryness relative to dentin. Enamel can be dried with an air stream unlike dentin, where such procedures usually draw additional water from underlying tubules to the dentin surface [18]. Thus, acid-etched enamel is a better substrate for resin bonding that dentin.
The importance of the bonded enamel border on the durability of resin-dentin bonds was confirmed by Gamgorgi et al. [29]. Using SBMP and Single Bond (SB), extracted teeth were bonded with or without outer enamel seals, and then stored in water for 6 m before being prepared into sticks for microtensile bond strength testing (μTBS). No significant reduction in μTBS was observed for SBMP bonds with or without enamel borders after 6 m. However, SB bonds made to specimens without enamel borders were significantly lower than those made to specimens with enamel borders, especially in peripheral dentin.
Similar results were obtained by Abdalla and Feilzer [30] who compared the 4 yr durability of an etch-and-rinse adhesive (Admira Bond, Voco) with two self-etching adhesives Clearfil SE Bond (Kuraray) vs. Hybrid Bond (Sun Medical) when the periphery of the bond was or was not bonded to enamel. In the bonds that were not protected by enamel, all μTBS fell significantly over 4 yr while none of the bonds made to enamel-protected dentin fell significantly over the same time.
Importance of functional stress
Clearly, resin-dentin bonds protected by peripheral enamel are more durable than unprotected resin-dentin bonds in in vitro studies that did not involve any simulations of functional stressing. This may not be the case in vivo. Three different groups of investigators have placed etch-and-rinse adhesives in class I cavities in which the peripheral enamel was acid-etched with 37% phosphoric acid for 15 s prior to bonding [22,28,31,32]. In all four studies, there was evidence of severe hybrid layer degradation with 6-14 months even though all cavosurface margins of these class I restorations were in acid-etched enamel. Thus, the protective effect of a peripheral enamel seal seems to be lost when the bonded teeth are in function. It is likely that teeth under function undergo sufficient flexing and compression to induce cyclic strain in the adhesive and hybrid layers that somehow accelerate degradation of resin-dentin hyrid layers.
ENDOGENOUS DENTIN PROTEASES DEGRADE COLLAGEN FIBRILS
Demineralized dentin contains bound matrix metalloproteinases -2, -3, -8, -9 and -20 (MMPs) and cathepsins [23,33] that, once activated by acid-etching, can slowly degrade the collagen fibrils of resin-infiltrated hybrid layers [22,28,31,32]. How can these enzymes be inactivated or inhibited?
Chelation of calcium and zinc from acid-etched dentin
Matrix metalloproteinases (MMPs) require calcium to maintain their tertiary structure and zinc ions for their catalytic hydrolase activity [34]. It is possible to inactivate these MMPs by treating acid-etched dentin with divalent cation chelators such as EDTA. While some have advocated the use of 0.5 M EDTA (pH 7) to condition the surface of enamel and dentin [35,36], a procedure that would also inactivate any exposed MMPs, the “etching” effect of EDTA is so weak that it takes several minutes to etch 1-2 μm into dentin. Most clinicians would prefer to etch with 32-37% phosphoric acid gel for 15 sec, and then treat the acid-etched dentin with anti-MMP chelators. There are other possible chelators such as 1,10-phenanthroline or ethylene diamine tetraphosphonic acid, etc. that might inactivate MMPs in etched dentin.
Protein cross-linking agents
Another approach to inactivating the endogenous MMPs of dentin is to cross-link their peptide chains immediately after acid-etching. This causes them to lose molecular mobility that is essential for their enzyme activity. Glutaraldehyde has been used as a cross-linking agent in vitro for decades [37]. However, residual glutaraldehyde can leach from treated tissue and has been shown to be cytotoxic [38]. Five percent glutaraldehyde, 35% HEMA, 60% water mixtures (Gluma Desensitizer, Heraeus Kulzer) continue to be used as dentin desensitizers [39]. One wonders if the glutaraldehyde in Gluma can cross-link MMPs in acid-etched dentin. Newer cross-linking agents such as proanthocyanidins have been shown to inhibit MMPs [40], although those authors cross-linked for 2 hrs. Newer, nontoxic cross-linkers like carbodiimide maybe more appropriate for inactivating endogenous proteases of dentin. Although many authors have shown that cross-linking increases resin-dentin bond strength, the treatment times of 10, 30 min or hours are not clinically relevant [41]. However, we have found that 1 min treatments with carbodiimide completely inactivated the proteolytic activity of soluble rhMMPs and the proteases of dentin matrices [Pashley, Tay and Tezvergil-Mutluay unpublished observations].
Specific versus nonspecific inhibitors of proteases
Primers can also be used to delivery specific MMP-inhibitors such as SB-3CT [42] or Galardin, also known as GM6001 or Illomastat [43] to etched dentin. Nonspecific MMP inhibitors can also be added to primers. For instance, many authors have shown the efficacy of incorporating chlorhexidine (CHX) in adhesive primers at preventing degradation of hybrid layers [22,28,31,32,44,45]. Chlorhexidine may also inhibit some cysteine cathepsins, another class of collagenolytic enzymes found in dentin [33].
THE ROLE OF PROTEOGLYCAN HYDROGELS IN INTERFIBRILLAR SPACES
Interfibrillar spaces in acid-etched dentin contain more than water. They also contain highly hydrated negatively-charged proteoglycans that form a hydrogel within that space [46,47]. Removal of these molecules by prolonged enzymatic treatment has been shown to produce large increases (49 and 63%, respectively) in bond strength of Scotchbond Multi-Purpose and Prime & Bond NT [48]. Those author’s used chondroitinase ABC to remove interfibrillar chondroitin sulfate-containing glycosaminoglycans (GAGs) that allowed better resin infiltration. Unfortunately, the time necessary for enzymatic removal (24 h) of GAGs is not clinically relevant.
Molecular sieving within proteoglycan hydrogels
If the GAG “hydrogels” remain hydrated in interfibrillar spaces, they may be responsible for “molecular sieving” of larger dimethacrylates like BisGMA, that cross-link polymers, from smaller 2-hydroxyethylmethacrylate (HEMA), that do not cross-link polymers, restricting BisGMA to the upper half of hybrid layers [49,50]. HEMA-rich lower halves of hybrid layers might be undergo large positive and negative strains during function that could lead to fatigue failure of collagen fibrils.
Scott and Thomlinson [47] showed that organic solvents (e.g. ethanol, acetone) cause a collapse of anionic glycosamineglycans gels in connective tissues by removing their water content.
Manufacturers of 2-step, etch-and-rinse adhesives dissolve their comonomers in ethanol or acetone, not water. This is because the dimethacrylates they use to toughen their polymers by cross-linking are not miscible with water but are soluble in ethanol (Table 2). Two-step etch-and-rinse adhesive blends contain both primer (i.e. HEMA) and adhesive (i.e. BisGMA) monomers in solvents containing low concentrations of water in the same bottle. They are applied in two layers; the first layer serves as a primer, while the second layer serves as an adhesive layer. However, this formulation compromise prevents the therapeutic opportunity provided by a separate water-rich primer in 3-step adhesives. Since comonomer blends are dissolved in ethanol, can acid-etched dentin be saturated with ethanol to enhance monomer penetration?
Table 2.
Relative solubility (g comonomers/mL solvent) of comonomer blends and selected monomers in water vs. ethanol.
| Water | Ethanol | Ethanol/water | |
|---|---|---|---|
| 70% ethoxy BisGMA/28% TEGDMA | 0.0027 | 0.1735 | 64.3 |
| 70% BisGMA/28% TEGDMA | 0.0038 | 0.1632 | 42.8 |
| 70% BisGMA/28% HEMA | 0.0063 | 0.1268 | 20.1 |
| 40% BisGMA/30% TCDM/28% HEMA | 0.0118 | 0.1296 | 11.0 |
| 40% BisGMA/30% BisMP/28% HEMA | 0.0422 | 0.1515 | 3.6 |
| 100% HEMA | 0.1120 | 0.1451 | 1.3 |
| 100% TEGDMA | 0.0095 | 0.1692 | 17.8 |
| 100% BisGMA | 0.0 | 0.1688 | -- |
| 100% Bis-MP | 0.1282 | 0.1890 | 1.5 |
Use of an ethanol primer
When ethanol-solvated blends are applied to water-saturated dentin, they often undergo nanoscopic phase changes [51]. However, if after acid-etching and rinsing, the water is replaced with an ethanol primer, the matrix becomes saturated with ethanol, the same solvent the comonomers are suspended in, and there can be no phase changes [52].
Ethanol wet bonding [52] was developed to enhance the durability of etch-and-rinse adhesives. In this technique, ethanol is used to chemically dehydrate acid-etched demineralized dentin matrices [53]. This results in a lateral shrinkage of collagen fibrils, causing an increase in the width of their fibrillar spacer and a reduction in the hydrophilicity of the collagen matrix. Tay et al. [54] solvated BisGMA in ethanol and applied it to ethanol-saturated acid-etched dentin. They showed that excellent, high bond strengths could be achieved between BisGMA and dentin. We do not recommend bonding BisGMA to dentin but we did demonstrate the proof-of-concept of ethanol wet-bonding using this water-insoluble monomer.
Clinical implications of ethanol wet-bonding
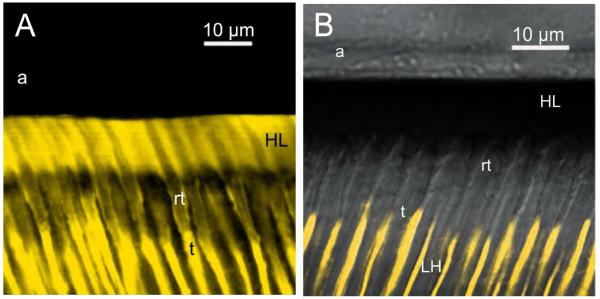
We speculate that bonding contemporary etch-and-rinse adhesives to water-saturated dentin leaves a very thin layer of water or a hydrogel between the infiltrated adhesive and the collagen fibrils of the matrix. This layer seems to provide a fluid-filled continuum from resin tags into the base of the hybrid layer through lateral branches of dentinal tubules (Fig. 8) throughout the full thickness of the hybrid layer. What evidence is there that this is true? We have prepared resin-bonded crown segments containing the top of the pulp chamber from extracted teeth, mounted on a plastic plate penetrated by an 18 ga. tube (Fig. 9). The pulp chamber was filled with a water-soluble fluorescent dye under a physiologic pressure (20 cm H2O) for 3 h after bonding with etch-and-rinse adhesives [55]. During this time, an aqueous solution of fluorescent dye slowly seeped out the dentinal tubules, permeated around poorly hybridized resin tags, and continued to diffuse fluorescent dye outward via lateral branches of dentinal tubules that ramify into smaller branches that finally become continuous with interfibrillar spaces around collagen fibrils in the hybrid layer. The end result in specimens that were bonded while moist with water, is that the entire hybrid layer becomes fluorescent, regardless of the adhesive resin (Fig. 10A). When the same adhesives are applied to ethanol-saturated dentin (Fig. 10B), the hybrid layers were not fluorescent. This means that fluorescent dye can not permeate around resin tags to reach the hybrid layer when resins were bonded to ethanol-saturated dentin. Rather, the resin tags have hybridized with the surround collagen fibrils and have sealed the tubules in ethanol-saturated dentin much better than in water-saturated dentin [56].
Figure 8.
A&B – Schematic of micropermeability of A: poorly hybridized resin tags in etched dentin saturated with water prior to resin infiltration versus B: perfectly hybridized resin tags in etched dentin saturated with ethanol prior to resin infiltration. Yellow fluorescent tracer was forced from the pulp chamber, out the tubules toward the hybrid layer. A: the resin could not displace water-filled lateral branches of tubules in the hybrid layer. This allowed yellow tracer to diffuse throughout the hybrid layer. B: the resin easily dissolved in the ethanol-filled lateral branches sealing the hybrid layer from dentinal fluid. (Reproduced from Sauro et al., J Biomed Mater Res Part B: Appl Biomater 2009;90B:327-337, with permission)
Figure 9.
Schematic of the simple apparatus used to infuse fluorescent tracer from the pulp chamber, through dentinal tubules, around resin tags into hybrid layers under physiologic pressure. (Reproduced from Sauro et al., J Biomed Mater Res Part B: Appl Biomater 2009;90B:327-337, with permission)
Figure 10.
A&B – A: Confocal laser scanning microscropy images of resin-dentin bonds made to crown segments. After polymerizing the resin, the pulp chamber was filled with lucifer yellow and place under 20 cm H2O pressure to allow the fluorescent tracer to seep wherever there were water-filled submicron channels from the pulp to the hybrid layer. A: resin-dentin bond made to acid-etched water-saturated dentin. Note that there is a fluorescence continuum from dentinal tubules (t), around resin tags (rt), into the hybrid layer. Note that the entire hybrid layer was fluorescent B: When the same resin was bonded to etched dentin saturated with ethanol, the lucifer yellow in the dentinal tubules (t) stopped when it encountered the 10 μm thick zone of well-hybridized resin tags (rt), just below the hybrid layer. No lucifer yellow passed around any resin tags, leaving the hybrid layer free of fluorescence.
The use of fluorescent dye under pressure to trace the permeation pathways from the pulp, out dentinal tubules to the hybrid layer has been called “micropermeability” [57]. The large differences in micropermeability of resin bonds five experimental resins of increasing hydrophilicity and Single Bond Plus made to water-versus ethanol-saturated dentin were highly significant (p<0.05, Table 3).
Table 3.
Change between the micropermeability of resin-bonded dentin interfaces created with the ethanol-saturated and water-wet technique.
| Micropermeability of Resin-Bonded Dentin Interfaces | |||
|---|---|---|---|
| Adhesive Systems | Ethanol-Saturated Technique |
Water-Wet Technique | % Change |
| Resin 1 | 218 ± 43cB | 1875 ± 43aA | 88.4 |
| Resin 2 | 98 ± 43cB | 1762 ± 43aA | 94.4 |
| Resin 3 | 100 ± 43cB | 1168 ± 43bA | 91.4 |
| Resin 4 | 95 ± 43cB | 1038 ± 43bA | 90.8 |
| Resin 5 | 91 ± 43cB | 989 ± 43bA | 90.8 |
| Single Bond Plus | 100 ± 43cB | 1084 ± 43bA | 90.8 |
Different lower case letters indicate statistically significantly differences between the adhesive systems applied on dentin with the same technique (p<0.05). Different upper case letters indicates statistically significant differences between the ethanol-saturated dentin vs. water-wet dentin technique used for each adhesive system (p<0.05). (Reproduced from Sauro et al., J Biomed Mater Res Part B: Appl Biomater 2009;90B:327-337, with permission)
Effects of ethanol-wet bonding to contemporary dental adhesives
When we used ethanol wet-bonding to bond Scotchbond Multi-Purpose, Single Bond, and All Bond-2 to acid-etched dentin, the microtensile bond strengths all increased about 17% [Carrilho et al., unpublished observations] compared to water wet bonding (Table 4). Scotchbond Multi-Purpose bonds made to water-wet dentin fell 28% over 18 months of water storage; the same adhesive bonded to ethanol-saturated dentin did not change over 6 months (Table 4). Bonds made with Single Bond (SB) to water-wet dentin fell 52% in 18 months while SB bonds made to ethanol-saturated dentin only fell 27%. Bonds made with All Bond-2 to water-saturated dentin fell 35% in 18 months; the same adhesive bonded to ethanol-saturated dentin fell 27% in 18 months. In these same experiments, we compared changes in bond strength over time in groups treated with 2% chlorhexidine (CHX) in water, 2% CHX in ethanol or no CHX treatment. Scotchbond MP-bonded to dentin primed with 2% CHX in water, fell 16% over 6 months. Priming dentin with 2% CHX in ethanol prevented significant decreasing SBMP bonds over 6 months (Table 4). However, because SBMP bonded to ethanol-saturated dentin also did not fall over 6 months, the excellent results obtained with 2% CHX in ethanol may have been due to ethanol alone.
Table 4.
Bond strength of commercial adhesives bonded to water vs. ethanol-saturated dentin.
| Acid-etched Dentin Saturation | |||||
|---|---|---|---|---|---|
| Adhesive | Testing-time | WSD (control) | ESD | CHX/WSD | CHX/ESD |
| SBMP | Baseline | 37.8 ± 4.9bA | 43.5 ± 4.5abA | 35.8 ± SBMP 5.8bA | 46.1 ± 4.5aA |
| 18 months | 27.4 ± 5.4bBC | 43.6 ± 3.4aA | 30.1 ± 5.0bAB | 44.4 ± 3.0aAB | |
| SB | Baseline | 35.7 ± 5.7aAB | 42.0 ± 5.6aAB | 34.4 ± 6.0aAB | 40.4 ± 6.1aAB |
| 18 months | 17.1 ± 4.3cC | 30.6 ± 4.7abB | 26.2 ± 5.3bB | 36.3 ± 5.9aB | |
| AL-2 | Baseline | 34.4 ± 6.1aAB | 41.3 ± 7.3abAB | 32.5 ± 4.9bAB | 42.9 ± 4.9aA |
| 18 months | 22.3 ± 4.6bC | 30.1 ± 5.2abB | 26.1 ± 5.6abAB | 38.7 ± 6.5aAB | |
Values are mean ± SD. N = 7 teeth/group, from 15-25 beams/tooth. Analysis per row = differences between groups are identified with different lower case letters. Analysis per column = differences between groups are identified with different upper case letters (p<0.05). Abbreviations: Scotchbond Multi-Purpose (SBMP); Single Bond (SB); All-Bond 2 (AB-2); Applied on dentin saturated with water (WSD); Ethanol-saturated dentin (ESD); Chlorhexidine in water base (CHX/WSD); Chlorhexidine in ethanol base (CHX/ESD).
Not only did ethanol wet-bonding reduce nanoleakage [58] and micropermeability [55], several recent publications indicate that ethanol-wet bonding alone seems to prevent the long-term degradation of resin-dentin bonds [58] that is commonly seen in contemporary adhesive systems, without the use of any extrinsic inhibitor. In a recent 1 yr bond durability study, Scotchbond Multi-Purpose (SBMP) was bonded to water-wet dentin, while a more hydrophobic blend of 70% BisGMA, 28% TEGDMA was bonded to ethanol-wet dentin [58]. After one year of accelerated aging, SBMP bonds created on water-saturated dentin fell from 40.6 ± 2.5 to 27.5 ± 3.3 MPa, a 32% decrease compared to 24 h controls. In contrast, bonds made with the more hydrophobic BisGMA/TEGDMA resin in ethanol-saturated resin only fell from 43.7 ± 7.4 to 39.8 ± 2.7 MPa, an insignificant change.
Can ethanol wet-bonding inhibit dentin proteases?
Figure 11A shows a TEM of an etch-and-rinse adhesive bonded using the ethanol-wet bonding technique. This is associated with an increase in the width of the interfibrillar spaces. Theoretically, this should allow more resin to coat each collagen fibril and protect it from water uptake. This is analogous to the use of ABC enzymes to remove GAGs from interfibrillar spaces but can be accomplished in 1 min of ethanol rinsing instead of 24 hrs of enzyme incubation. In addition, stained transmission electron microscopy (TEM) of these specimens revealed that 1-year-old hybrid layers created by BisGMA/TEGDMA resin in ethanol-saturated dentin looked just like the 24 h controls (Fig. 11A). The collagen fibrils took up heavy metal stain and exhibited normal ultramorphology [58]. In contrast, when water-saturated specimens bonded with SBMP were examined after 1 yr of accelerated aging, about 80% of the hybrid layer had degraded so much that few collagen fibrils could be found and there was little heavy metal staining that is typical for demineralized normal hybrid layers [58]. Normal hybridized dentin was seen in the top 0.5 μm of the hybrid layer and around dentinal tubules but the rest of the hybrid layer had been degraded to gelatin (Fig. 11B). Unfortunately, gelatin is soluble. When the ethanol-wet bonded specimens were immersed in 50 wt% annoniacal silver nitrate to measure their water content (i.e. silver nanoleakage), the silver uptake was limited to the top 0.5 μm of the hybrid layer (Fig. 12A). Water-saturated specimens were bonded with SBMP and incubated in water for 1 yr. When these specimens were evaluated for their water content by immersion in 50% ammoniacal silver nitrate, that portion of the hybrid layer that was shown to be free of heavy metal staining was filled with silver [57], indicating that most of the hybrid layer was filled with water or a gelatin hydrogel (Fig. 12B).
Figure 11.
A&B – A: Stained TEM of acid-etched specimen bonded with BisGMA/TEGDMA resin under ethanolwet bonding conditions. A = adhesive resin; H = hybrid layer occupies space above the two open arrow heads. Appearance of bond after 1 yr of water storage. B: Stained TEM of Scotchbond MultiPurpose Plus bond made to acid-etched dentin saturated with water, after 1 yr of storage in water. C = hybrid composite; A = adhesive; Hδ = hybrid layer; D = underlying mineralized dentin that was demineralized during TEM processing; T = resin tags in tubules. Note how much stain was taken up by laboratory demineralized dentin and how little was taken up by hybrid layer due to degradation of collagen by endogenous MMPs. (Reproduced from Sadek et al., Dent Mater 2010;26:380-386, with permission)
Figure 12.
A&B – A: Unstained TEM of 1 yr old resin-dentin bond made with BisGMA/TEGDMA resin to acid-etched, ethanol-saturated dentin. After immersion of the specimen in silver nitrate for 24 h, the hybrid layer (H) was seen to take up very little silver grains. B: Unstained TEM of 1 yr old resin dentin bond made with Scotchbond MultiPurpose to water-saturated dentin. After immersion in silver nitrate, the hybrid layer (Hδ) took up massive amounts of silver indicating that the collagen fibrils with the hybrid layer had been completely destroyed by endogenous MMPs and replaced with water. (Reproduced from Sadek et al., Dent Mater 2010;26:380-386, with permission)
The fact that there was no decrease in the bond strength of BisGMA/TEGDMA bonded to ethanol-saturated dentin over a 1 yr period deserves more discussion. Either ethanol can denature matrix-bound MMPs or there is a more intimate association between adhesive resins and collagen fibrils (and their bound MMPs) than can exist in resin-infiltrated into water-saturated dentin. If resins can truly infiltrate collagen by they not only inactivate proteolytic enzymes bound to collagen, they may also provide nanomechanical coupling between resin and collagen.
Theoretical advantage of resin-collagen interpenetration
Nakabayashi and Pashley [15] speculated that there are several ways that adhesive resins might interact with collagen fibrils during creation of hybrid layers (Fig. 13). If there was too much water in interfibrillar spaces so that resins did not polymerize very well [59,60], then there would be a poor association between the adhesive resin and collagen. If there was residual water on the collagen, the polymer chains of the resin may not have interpenetrated with collagen peptides. However, if there was a layer of ethanol on the collagen, the adhesive monomers may dissolve into that alcohol layer and create an interpenetrating network of polymer and peptide chains. These two different associations between resin and collagen could theoretically lead to different mechanical properties when loaded in tension. In water-saturated dentin loaded in tension (interface model, Fig. 13A), we speculate that there is no association between resin and collagen and the collagen might elongate more than the resin, placing most of the strain on the resin that would fail before the collagen. In ethanol-saturated dentin (interphase model, Fig. 13B), there is an intimate association between resin and collagen. Both resin and collagen would share the stress as they elongated, thereby lowering local stress concentrations. These ideas must be regarded as speculations until new nanotechnologies can be developed to test these concepts. If resins can develop intimate molecular associations with ethanol-saturated collagen peptides, they should be able to develop similar molecular level associations with MMP peptides bound to collagen. This is not a new concept. For more than a decade, scientists have been taking “molecular-level” impressions of substrates and even enzymes, using methacrylate monomers similar to those used in adhesive dentistry. After free radical polymerization, the impressions are separated from the substrate. These impressions reproduce the 3-D structure of the substrate indicating that these materials “wet” substrate molecules. This technique is called “molecular impringing” [61,62]. The phenomenon has been called “nano-interdigitation” [63] of polymer interpenetration. We speculate that ethanol-saturated dentin allows adhesive resins to flow into the catalytic sites of MMPs and polymerize, thereby inactivating the enzymes. This is another example of how therapeutic primers can increase the durability of resin-dentin bonds.
Figure 13.
A and B – Schematic of theoretical relationships between resin in interfibrillar spaces and collagen fibrils. A: Interface model, the presence of a layer of water on the collagen limits the molecular level interaction between resin and collagen to a sharp interface. When strained, the collagen stains more than the resin, carrying most of the stress. B: Interphase model, the presence of ethanol in and around the collagen allows comonomers to dissolve into intimate contact with collagen creating 3-D interphase zone rather than a 2-D interface. When strained, both collagen and resin share stress, thereby improving stress distribution. (Modified from Nakabayashi and Pashley, Hybridization of Dental Hard Tissues, Quintessence Publishing Co., with permission)
IS THERE A ROLE FOR THERAPEUTICS IN DENTIN BONDING?
Etch-and-rinse adhesives that utilize 3-steps are more durable than 2-step etch-and-rinse adhesives [64]. By maintaining separate acid-etching, priming and bonding steps in etch-and-rinse adhesives, one can accomplish multiple therapeutic goals in each step. By looking at 3-step etch-and-rinse adhesive systems as a form of tissue engineering, one discovers many opportunities for added value therapies. One advantage of a three-step bonding procedure is the opportunity to use each step for multiple purposes.
Acid etching
For instance, 35-37% phosphoric acid etchant can simultaneously etch enamel and dentin [5,10]. When acid-etching enamel with 32-37% phosphoric acid, both the intra- and interprismatic enamel can be well-etched at pH 0.4 that is not possible with self-etching primers or all-in-one adhesives that typically have pHs of 2-2.8. Studies have shown that 32-37% phosphoric acid decimates residual bacteria in caries-affected dentin [65]. Phosphoric acid also inactivates MMP activity in dentin by 65 to 95% [27,66].
If that phosphoric acid also contains 1 wt% benzalkonium chloride (BAC) such as ETCH-37 w/BAC, marketed by Bisco, Inc. (Schaumburg, IL, USA), then one can bind BAC to etched collagen as it is being etched. We have recently found that BAC is a good matrix metalloprotease (MMP) inhibitor [67] that can withstand the low pH of phosphoric acid and does not lower enamel or dentin bond strengths [68].
When using etchants that contain benzalkonium chloride, a potent antimicrobial agent, one is also acid-etching enamel and dentin, killing most of the residual bacterial in the dentin and allowing BAC to bind to the demineralized matrix where it exerts both an anti-microbial effect as well as an anti-MMP effect [67]. This is a fine example of multi-role therapeutics. Others have added 2% chlorhexidine to 37% phosphoric acid for its anti-MMP effect [69].
It is important that acid-etched enamel and dentin by thoroughly rinsed to remove all reaction products. This water also insures that the demineralized dentin matrix is fully expanded [70]. If the excess water is not removed by blotting or a half second air blast just prior to bonding, the residual water may induce phase changes in etch-and-rinse adhesives that contain BisGMA [51,71].
Application of primers
The use of a primer was originally designed to re-expand dried collapsed dentin and to coat the wet collagen fibrils with a hydrophilic monomer like HEMA. However, primers in etch-and-rinse adhesive systems offer additional therapeutic opportunities. For instance, some clinicians treat acid-etched dentin with 0.2-2 wt% chlorhexidine in water or ethanol. Both ethanol and CHX tend to kill any bacteria that survive acid-etching and then CHX binds to acid-etched dentin [72] where it inhibits dentin MMPs and prolong the durability of resin-dentin bonds [28]. Acid-etched water-rinsed intertubular dentin matrix is almost 70% water and 30% organic matrix (Table 1). Thus, application of almost any therapeutic agent will diffuse into that unbound water and then bind to the organic matrix. Such binding has recently been shown for chlorhexidine [72], benzalkonium chloride [67] and polyvinylphosphonic acid, another potent MMP inhibitor [73]. Both chlorhexidine and benzalkonuim chloride (BAC) are positively charged molecules that bind to negatively charged demineralized dentin matrix.
Interactions of acids and primers with matrix MMPs
In a recent paper from the Van Meerbeek’s group [42], the authors measured whether matrix-bound MMPs could be released from acid-etched dentin matrices by phosphoric acid-etching and/or etch-and-rinse primers. They showed that some MMP-2 could be extracted from unetched dentin powder but that more could be extracted from 35% phosphoric acid treated dentin. Treatment of soluble MMP-2 by primers inactivated the enzyme. Matrix-bound MMPs may be more resistant to the primers since most adhesive bonds degrade over time when they are treated with both primers and adhesives.
MMPs are relatively large enzymes having molecular weights in the range of 60-90,000 daltons. In the dentin matrix, almost all of the MMPs are bound to collagen fibrils. The binding is so strong that extreme measures are required to extract them such as the use of 4 moles/L quanidine HCl or 4 moles/L urea for 24 hrs. When these extractions are repeated, more and more MMPs can be extracted, indicating that extraction of these tightly bound MMPs is an inefficient process and is not a practical method of stabilizing resin-dentin bonds.
We have considered trying to extract MMPs from acid-etched dentin just prior to bonding to see if the bonds are more durable over time. Such attempts have not been successful. While some MMPs can be extracted from the dentin matrix by bonding procedures [42,74], it is likely that the largest fraction of MMPs remain bound to the matrix, and are activated by acid-etching. That is why we have concentrated our work on the inhibition of endogenous MMPs of dentin in their natural bound form. Their active or catalytic site is adjacent to their collagen binding site. We believe that endogenous dentin MMPs attack the same collagen fibrils to which they are bound. In our view of how MMPs degrade hybrid layers, to increase the durability of resin-dentin bonds we must inhibit the bound MMPs in situ.
Recent work has shown that the demineralized matrix of mineralized tissues permit molecules smaller than 6 kDa to diffuse in and out of the matrix water [75]. However, molecules larger than 40 kDa can not diffuse in or out of the matrix. This means that even if test agents are able to debind MMPs with molecular weights between 60-90 kDa from the collagen matrix, most of those MMPs will not be able to be extracted. Only MMPs on the surface of the collagen fibrils may be extractable.
One advantage of having MMPs bound to the matrix is that the collagen matrix can serve as a binding “sink” for MMP-inhibitors. When we compared the uptake of chlorhexidine digluconate to demineralized versus mineralized dentin powder, the uptake of demineralized dentin was 8-10 × higher than to mineralized dentin [72].
Although there are many specific MMP inhibitors [42,43], these inhibitors may also bind nonspecifically to collagen fibrils that can serve as a reservoir of bound inhibitors. This arrangement may keep the bound MMPs saturated with the inhibitors. This is true for chlorhexidine [72] and benzalkonium chloride [67]. Whether this is true of all MMP inhibitors remains to be determined.
SOLVATED VERSUS NEAT ADHESIVES
The problem of residual solvents
Careful studies of the amount of evaporation of solvents from adhesive resin blends indicates that the 3-5 sec advocated by manufacturers is too little to even remove half of the solvent [76]. Both OptiBond FL primer and Clear SE primer contain 45-47% water. When the primers were air-dried with an air syringe for 30 s, only 38.2% and 31.8%, respectively of the water content of the primers was evaporated for OptiBond FL and Clear SE primers [77]. Similar results were obtained by Cadenaro et al. [17] in ethanol-solvated resin blends. Thus, when one adds solvent to resin comonomer blends to decrease their viscosity and increase the wetting characteristics, one is unlikely to remove more than half of the solvent by evaporation. After polymerization the residual solvent will be replaced by water [78] that will likely plasticize the polymer and reduce its mechanical properties.
Use of solvent-free adhesives
When three-step etch-and-rinse adhesive systems are used properly, the primed dentin should be rich in methacrylates and relatively free of solvents. Due to Raoult’s law, as the volatile solvents in the primer are evaporated, the concentration of the nonvolatile monomers rises rapidly. This causes changes in the colligative properties of the residual solvent such as lowering its vapor pressure, the driving force for solvent evaporation [17,79]. Thus, there is a tendency for primed surfaces to contain residual solvent that can weaken the strength of the primer [77] as well as decrease its degree of conversion [17].
The therapeutic use of adhesives
In 3-step etch-and-rinse adhesive systems, the solvent is in the primer, not the adhesive. Most 3-step etch-and-rinse adhesives (OptiBond FL, Scotchbond Multi-Purpose) use solvent-free adhesives. These adhesives wet the solvent-rich primers, but cover them with a more hydrophobic, dense adhesive that seals the primed dentin. They can be used to seal bonds made with all-in-one adhesives [80,81], thereby increasing bond strength and reducing nanoleakage. Such treatment of all-in-one adhesives with a solvent-free adhesive also increases the durability of resin-dentin bonds [82]. Solvent-free adhesive blends generally have water sorption and solubility values that are less than half that of solvated resin blends [82,83]. This means they will take up less water over time and plasticize less than solvated resin blends [84-86]. The solvent-free layers show very little nanoleakage compared to solvated resin blends [81].
Attempts to add “therapeutic reagents” to adhesive blends is more problematic because once polymerized, reagents dissolved in adhesives can not diffuse out of the polymerized resin. Polymerized dental resins are known to leach monomers such as methacrylic acid (86 daltons), HEMA (130 daltons), TEGDMA (286.3 daltons) and BisGMA (512.6 daltons). They do not leach oligomers. Thus, it appears that dental polymers are unable to release molecules larger than about 500 daltons. Small molecules like fluoride, calcium, hydroxyl ions, etc. can diffuse out, but larger molecules like chlorhexidine have difficulty exiting polymers. The release of fluoride from adhesives is regarded as being therapeutically useful [87,88].
SUMMARY
Thus, although 3-step etch-and-rinse adhesive systems are the oldest of the marketed adhesives, their separation of key ingredients offers more therapeutic flexibility than “simpler” combination adhesives. Each of the 3-steps can accomplish multiple tasks ending with sealing the bonded interface with a relatively hydrophobic adhesive layer.
It remains to be seen whether manufacturers will utilize the full potential of each of the 3-steps for maximum therapeutic effect. The concept of therapeutic use of acidic conditioners, primers and adhesives is a new exciting idea whose time has come.
ACKNOWLEDGEMENTS
This study was supported, in part, by grants R01 DE 015306-06 (PI. David H. Pashley) and R21 DE 019213-01 (PI. Franklin R. Tay) from the National Institute of Dental and Craniofacial Research, grant #8126472 (PI. Arzu Tezvergil-Mutluay) from Academy of Finland and grant FAPESP #07/54618-4 and CNPq #300615/2007-8 (PI. Marcela Carrilho). This study was performed during the Young Investigator Program (FAPESP) of Dr. Marcela Carrilho at the Oral Biology Research Center of the University of São Paulo/SP. The authors are grateful to Mrs. Michelle Barnes for her secretarial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Buoncore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955;34:849–853. doi: 10.1177/00220345550340060801. [DOI] [PubMed] [Google Scholar]
- 2.Gwinnett AJ, Matsui A. A study of enamel adhesives. The physical relationship between enamel and adhesives. Arch Oral Biol. 1967;12:1615–1619. doi: 10.1016/0003-9969(67)90195-1. [DOI] [PubMed] [Google Scholar]
- 3.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Sci. 1982;16:265–273. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 4.Inokoshi S, Hosoda H, Harnirattisai C, Shimida Y, Tatsumi T. A study on the resin-impregnated layer of dentin. Part I. A comparative study on the decalcified and undecalcified sections and the application of argon ion beam etching to disclose the resin-impregnated layer of dentin. Jpn J Conserv Dent. 1990;33:427–442. [Google Scholar]
- 5.Fusayama T. New Concepts in Operative Dentistry. Quitessence Publishing Co., Inc.; Tokyo: 1980. pp. 61–156. [Google Scholar]
- 6.Pashley DH. The effects of acid-etching on the pulpodentin complex. Buonocore Memorial Lecture. 1992;17:229–242. Oper Dent, 1992. [PubMed] [Google Scholar]
- 7.Tagami J, Hosoda H, Burrow MF, Nakajima M. Effect of aging and caries on dentin permeability. Proc Finn Dent Soc. 1992;88(Suppl 1):149–154. [PubMed] [Google Scholar]
- 8.Pashley DH, Ciucchi B, Sano H. Permeability of dentin to adhesive agents. Quintessence Int. 1993;24:618–631. [PubMed] [Google Scholar]
- 9.Asmussen E, Munksgaard EC. Adhesion of restorative resins to dentinal tissues. In: Vanherle G, Smith DC, editors. Posterior Composite Resin Dental Restorative Materials. Peter Szule Publishing Co.; 1985. p. 228. Fig. 25. [Google Scholar]
- 10.Kanca J. Improved bond strength through acid-etching of dentin and bonding to wet dentin surfaces. J Am Dent Assoc. 1992;123:35–43. doi: 10.14219/jada.archive.1992.0248. [DOI] [PubMed] [Google Scholar]
- 11.Baum BJ, Mooney DJ. The impact of tissue engineering on dentistry. J Am Dent Assoc. 2000;131:309–318. doi: 10.14219/jada.archive.2000.0174. [DOI] [PubMed] [Google Scholar]
- 12.Gotlieb EL, Murray PE, Namerow KN, Kuttler S, Garcia-Godoy F. An ultrastructural investigation of tissue-engineered pulp constructs implanted within endodontically treated teeth. J Am Dent Assoc. 2008;139:457–465. doi: 10.14219/jada.archive.2008.0189. [DOI] [PubMed] [Google Scholar]
- 13.Kenawy E-R, Abdel-Hay FI, El-Raheem A, El-Shansboury R, El-Newehy MH. Biologically active polymers: synthesis and antimicrobial activity of modified glycidyl methacrylate polymers having a quaternary ammonium and phosphorium groups. J Control Release. 1998;50:145–152. doi: 10.1016/s0168-3659(97)00126-0. [DOI] [PubMed] [Google Scholar]
- 14.Vaidyanathan M, Sheehy EC, Gilbert SC, Beighton D. Antimicrobial properties of dentine bonding agents determined using in vitro and ex vivo methods. J Dent. 2009;37:514–521. doi: 10.1016/j.jdent.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Nakabayashi N, Pashley DH. Hybridization of Dental Hard Tissues. Quintessence Publishing; Chicago: 1998. pp. 65–67. [Google Scholar]
- 16.Pashley DH, Horner JA, Brewer PO. Interactions of conditioners on the dentin surfaces. Oper Dent. 1992;17(Suppl 5):127–150. [PubMed] [Google Scholar]
- 17.Cadenaro M, Breschi L, Rueggeberg FA, Suchko M, Grodin E, Agee KA, Di Lenarda R, Tay FR, Pashley DH. Effects of residual ethanol on the rate and degree of conversion of five experimental resins. Dent Mater. 2009;25:621–628. doi: 10.1016/j.dental.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto M, Ito S, Tay FR, Svizero NR, Sano H, Kaga M, Pashley DH. Fluid movement across the resin-dentin interface during and after bonding. J Dent Res. 2004;11:843–848. doi: 10.1177/154405910408301104. [DOI] [PubMed] [Google Scholar]
- 19.Tay FR, Hashimoto M, Pashley DH, Peters MC, Lai SCN, Yiu CKY, Cheong C. Aging affects two modes of nanoleakage expression in bonded dentin. J Dent Res. 2003;82:537–541. doi: 10.1177/154405910308200710. [DOI] [PubMed] [Google Scholar]
- 20.Van Meerbeek B, Yoshida K, Lambrechts P, Vanherle G, Duke ES, Eick JD, Robinson SI. A TEM study of two water-based adhesive systems bonded to dry and wet dentin. J Dent Res. 1998;77:50–59. doi: 10.1177/00220345980770010501. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong SR, Vargas MA, Chung I, Pashley DH, Campbell JA, Laffoon JE, Qian F. Resin-dentin interfacial and micortensile bond strength after five-year water storage. Oper Dent. 2004;29:705–712. [PubMed] [Google Scholar]
- 22.Carrilho MRO, Geraldeli S, Tay FR, de Goes MF, Carvalho RM, Tjäderhane L, Reis AF, Hebling J, Mazzoni A, Breschi L, Pashley DH. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86:529–533. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S-C, Kern M. The role of host-derived dentinal matrix metalloproteinases in reducing dentin bonding of resin adhesives. Int J Oral Sci. 2009;1:163–176. doi: 10.4248/IJOS.09044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loguercio AD, Moura SK, Pellizzaro A, Del-Bianco K, Patzlaff RT, Grande RHM, Rees A. Durability of enamel bonding using two-step self-etch systems on ground and unground enamel. Oper Dent. 2008;33(1):79–88. doi: 10.2341/07-42. [DOI] [PubMed] [Google Scholar]
- 25.Shono Y, Terashita M, Shimada J, Kozono Y, Carvalho RM, Russell CM, Pashley DH. Durability of resin-dentin bonds. J Adhes Dent. 1999;1:211–218. [PubMed] [Google Scholar]
- 26.DeMunck J, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K. Four-year water degradation of total-etch adhesives bonded to dentin. J Dent Res. 2003;82:136–140. doi: 10.1177/154405910308200212. [DOI] [PubMed] [Google Scholar]
- 27.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 28.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–746. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 29.Gamborgi GP, Loguercio A, Reis A. Influence of enamel border and regional variability on durability of resin-dentin bonds. J Dent. 2007;35:371–376. doi: 10.1016/j.jdent.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Abdalla AI, Feilzer AJ. Four year water storage of a total-etch and two self etching adhesives bonded to dentin. J Dent. 2008;26:611–617. doi: 10.1016/j.jdent.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Brackett WW, Tay FR, Brackett MG, Dip A, Sword RJ, Pashley DH. The effect of chlorhexidine on dentin hybrid layers in vivo. Oper Dent. 2007;32(2):107–111. doi: 10.2341/06-55. [DOI] [PubMed] [Google Scholar]
- 32.Brackett MG, Tay FR, Brackett WW, Dip A, Dipp FA, Mai S, Pashley DH. In vivo chlorhexidine stabilization of an acetone-based dentin adhesives. Oper Dent. 2009;34(4):381–385. doi: 10.2341/08-103. [DOI] [PubMed] [Google Scholar]
- 33.Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, Carrilho MR, Pashley DH, Tay FR, Salo T, Tjäderhane L. Cysteine cathepsins in human dentin-pulp complex. J Endod. 2010;36:475–481. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 34.Visse R, Nagase H. Matrix metalloproteases and tissue inhibitors of metalloproteinases. Cir Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 35.Osorio R, Erhardt MCG, Pimenta LAF, Osorio F, Toledano M. EDTA treatment improves resin-dentin bonds resistance to degradation. J Dent Res. 2005;85:736–740. doi: 10.1177/154405910508400810. [DOI] [PubMed] [Google Scholar]
- 36.Sauro S, Mannocci F, Toledano M, Osorio R, Pashley DH, Watson TF. EDTA or H3PO4/NaOCl dentin treatments may increase hybrid layer resistance to degradation: A microtensile bond strength and confocal micropermeability study. J Dent. 2009;37:279–288. doi: 10.1016/j.jdent.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Cheung DT, Tong D, Perelman N, Ertl D, Nimhi ME. Mechanism of cross-linking proteins by glutaraldehyde. IV. In vitro and in vivo stability of a cross-linked collagen matrix. Conn Tiss Res. 1990;25:27–34. doi: 10.3109/03008209009009810. [DOI] [PubMed] [Google Scholar]
- 38.Huang LL, Cheung DT, Nimni ME. Biochemical changes and cytotoxicity associated with the degradation of polymeric glutaraldehyde derived cross-links. J Biomed Mater Res. 1990;24:1185–201. doi: 10.1002/jbm.820240905. [DOI] [PubMed] [Google Scholar]
- 39.Pashley DH, Tay FR, Haywood VB, Collins MA, Drisko CL. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. Compend Cont Edu Dent. 2008;29(Spec Ill 8):1–35. [Google Scholar]
- 40.La VD, Howell AB, Grenier D. Cranberry proanthocyanidins inhibit MMP production and activity. J Dent Res. 2009;88:627–632. doi: 10.1177/0022034509339487. [DOI] [PubMed] [Google Scholar]
- 41.Bedran-Russo AKB, Vidal CMP, Santos PHD, Castellan CS. Long-term effect of carbodiimide on dentin matri and resin-dentin bonds. J Biomed Mater Res B Appl Biomater. 2010;94B:250–255. doi: 10.1002/jbm.b.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeMunck J, Vander Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdenakker G, Van Meerbeek B. Inhibition of enzymatic degradation of adhesive-dentin interfaces. J Dent Res. 2009;88:1101–1106. doi: 10.1177/0022034509346952. [DOI] [PubMed] [Google Scholar]
- 43.Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, Visintini E, Cadenaro M, Tay FR, Dorigo EDS, Pashley DH. Use of a specific MMP inhibitor (Galardin) for preservation of hybrid layer. Dent Mater. 2010 doi: 10.1016/j.dental.2010.02.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breschi L, Cammelli F, Visiniti E, Mazzoni A, Vita F, Carrilho M, Cadenaro M, Foulger S, Tay FR, Pashley DH, Di Lenarda R. Influence of chlorhexidine concentration on the durability of etch-and-rinse dentin bonds: a 12-month study. J Adhes Dent. 2009;11:191–198. [PMC free article] [PubMed] [Google Scholar]
- 45.Loguercio AD, Stanislawczuk R, Polli LG, Costa JA, Michel MD, Reis A. Influence of chlorhexidine digluconate concentration and application time on resin-dentin bond strength durability. Eur J Oral Sci. 2009;117:587–596. doi: 10.1111/j.1600-0722.2009.00663.x. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg M, Takaji M. Dentine proteoglycans: composition, ultrastructure and functions. Histochem J. 1993;25:781–806. [PubMed] [Google Scholar]
- 47.Scott JE, Thomlinson AM. The structure of interfibrillar proteoglycan bridges (‘shape modules’) in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. J Anat. 1998;192:391–405. doi: 10.1046/j.1469-7580.1998.19230391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazzoni A, Pashley DH, Ruggeri A, Vita F, Falconi M, Di Lenarda R, Breschi L. Adhesion to chondroitinase ABC treated dentin. J Biomed Mater Res B: Appl Biomater. 2007;86B:228–236. doi: 10.1002/jbm.b.31010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 50.Shin TP, Yao X, Huenergardt R, Walker MP, Wang Y. Morphological and chemical characterization of bonding hydrophobic adhesive to dentin using ethanol wet bonding technique. Dent Mater. 2009;25:1050–1057. doi: 10.1016/j.dental.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye Q, Wang Y, Spencer P. Nanophase separation of polymers exposed to simulated bonding conditions. J Biomed Mater Res Part B: Appl Biomater. 2009;88B:339–348. doi: 10.1002/jbm.b.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, Garcia-Godoy F. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007;20:7–21. [PubMed] [Google Scholar]
- 53.Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, Pashley DH. Effects of resin hydrophilicity on dentin bond strength. J Dent Res. 2006;85:1016–1021. doi: 10.1177/154405910608501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tay FR, Pashley DH, Kapur RR, Carrilho MRO, Hur YB, Garrett LV, Tay KCY. Bonding BisGMA to dentin – a proof of concept. J Dent Res. 2007;86:1034–1039. doi: 10.1177/154405910708601103. [DOI] [PubMed] [Google Scholar]
- 55.Sauro S, Watson TF, Mannocci F, Tay FR, Pashley DH. Two-photon laser confocal microscopy study of resin-dentin interfaces created with water or ethanol wet-bonding technique: qualitative and quantitative micropermeability assessment. J Biomed Mater Res: Part B Appl Biomater. 2009;90B:327–337. doi: 10.1002/jbm.b.31290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carrilho MR, Tay FR, Sword J, Donnelly AM, Agee KA, Nishitani Y, Sadek FT, Carvalho RM, Pashley DH. Dentine sealing provided by smear layer/smear plugs versus adhesive resins/resin tags. Eur J Oral Sci. 2007;115:321–329. doi: 10.1111/j.1600-0722.2007.00465.x. [DOI] [PubMed] [Google Scholar]
- 57.Griffiths BM, Watson TF, Sheriff M. The influence of dentin bonding systems and their handling characteristics on the morphology and micropermeability of the dentine adhesive interface. J Dent. 1999;27:63–71. doi: 10.1016/s0300-5712(97)90022-1. [DOI] [PubMed] [Google Scholar]
- 58.Sadek FT, Castillan CS, Braga RR, Mai S, Tjäderhane L, Pashley DH, Tay FR. One-year stability of resin-dentin bonds created with a hydrophobic ethanol-wet bonding technique. Dent Mater. 2010;26:380–386. doi: 10.1016/j.dental.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Jacobson T, Söderholm K-J. Some effects of water on dentin bonding. Dent Mater. 1995;11:132–136. doi: 10.1016/0109-5641(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 60.Paul SJ, Leach M, Rueggeberg FA, Pashley DH. Effect of water content on the physical properties of model dentine primer and bonding resin. J Dent. 1999;27:209–214. doi: 10.1016/s0300-5712(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 61.Baggiani C, Anfossi L, Giovannoli C. Molecular imprinted polymers: useful tools for pharmaceutical analysis. Curr Pharm Anal. 2006;2:219–247. [Google Scholar]
- 62.Pradhan S, Boopathi M, Kumar O, Baghel A, Pandey P, Mahato TH, Singh B, Vijayaraghavan R. Molecularly imprinted nanopatterns for the recognition of biological warfare agent ricin. Biosensors and Bioelectronics. 2009;25:592–598. doi: 10.1016/j.bios.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 63.Marshall SJ, Bayne SC, Baier R, Tomsia AP, Marshall GW. A review of adhesion science. Dent Mater. 2010;26:e11–e16. doi: 10.1016/j.dental.2009.11.157. [DOI] [PubMed] [Google Scholar]
- 64.DeMunck J, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84:118–132. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 65.Mertz-Fairhurst EJ, Curtis JW, Ergle JW, et al. Ultraconservative and cariostatic sealed restorations: Results at year 10. J Am Dent Assoc. 1998;129:55–66. doi: 10.14219/jada.archive.1998.0022. [DOI] [PubMed] [Google Scholar]
- 66.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, Toledano M, Pashley EL, Tay FR. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentin by etch-and-rinse adhesives. Biomaterials. 2006;27:4470–4476. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 67.Tezvergil-Mutluay A, Mutluay MM, Gu L-S, Agee KA, Carvalho RM, Manso A, Carrilho M, Tay FR, Breschi L, Suh BI, Pashley DH. The anti-MMP activity of benzalkonium chloride. J Dent. 2010a doi: 10.1016/j.jdent.2010.10.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanca J. One Step bond strength to enamel and dentin. Am J Dent. 1977;9:5–8. [PubMed] [Google Scholar]
- 69.Stanislawczuk R, Amaral RC, Grande CZ, Gagler D, Reis A, Loguercio AD. Chlorhexidine containing acid conditioner preserves the longevity of resin-dentin bonds. Oper Dent. 2009;34:483–492. doi: 10.2341/08-016-L. [DOI] [PubMed] [Google Scholar]
- 70.Becker TD, Agee KA, Joyce AP, Rueggeberg FA, Borke JL, Waller JL, Tay FR, Pashley DH. Infiltration/evaporation-induced shrinkage of demineralized dentin by solvated model adhesives. J Biomed Mater Res Part B (Appl Biomat) 2007;80B:156–165. doi: 10.1002/jbm.b.30580. [DOI] [PubMed] [Google Scholar]
- 71.Van Landuyt KL, DeMunck J, Snauwaert J, Coutinho E, Poitevin A, Yoshida Y, Inoue S, Peumans M, Suzuki K, Lambrechts P, Van Meerbeek B. Monomer-solvent phase separation ine one-step self-etch adhesives. J Dent Res. 2005;84:183–188. doi: 10.1177/154405910508400214. [DOI] [PubMed] [Google Scholar]
- 72.Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L, Carvalho RM, Tjäderhane L, Looney S, Wimmer C, Tezvergil-Mutluay A, Tay FR, Pashley DH. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent Mater. 2010;26:771–778. doi: 10.1016/j.dental.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tezvergil-Mutluay A, Agee KA, Hoskika T, Tay FR, Pashley DH. The inhibitory effect of polyvinylphosphoric acid on functional MMP activities in human demineralized dentin. Acta Biomaterialia. 2010 doi: 10.1016/j.actbio.2010.05.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, Ruggeri A, Tay FR, Dorigo EDS, Pashley DH. Chlorhexidine stabilizes the adhesive inteface: A 2-year in vitro study. Dent Mater. 2010;26:320–325. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toroian D, Lim EL, Price PA. The size exclusion characteristics of type I collagen: implications for the role of noncollagenous bone constituents in mineralization. J Biol Chem. 2007;282:22437–22447. doi: 10.1074/jbc.M700591200. [DOI] [PubMed] [Google Scholar]
- 76.Yiu CKY, Pashley EL, Hiraishi N, King NM, Goracci S, Ferrari M, Carvalho RM, Pashley DH, Tay FR. Solvent and water retention in dental adhesive blends after evaporation. Biomater. 2005;26:6863–6872. doi: 10.1016/j.biomaterials.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 77.Ikeda T, DeMunck J, Shirai K, Hikita K, Inoue S, Sano H, Lambrechts P, Van Meerbeek B. Effect of evaporation of primer components on ultimate tensile strengths of primer-adhesive mixtures. Dent Mater. 2005;21:1051–1058. doi: 10.1016/j.dental.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 78.Malacarne-Zanon J, Pashley DH, Agee KA, Foulger S, Alves MC, Breschi L, Cadenaro M, Garcia FP, Carrilho MR. Effects of ethanol addition on water sorption/solubility and percent conversion of monomers in model dental adhesives. Dent Mater. 2009;25:1275–1284. doi: 10.1016/j.dental.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pashley EL, Zhang Y, Lockwood P, Rueggeberg F, Pashley DH. Effects of HEMA on water evaporation from water-HEMA mixtures. Dent Mater. 1998;14:6–10. doi: 10.1016/s0109-5641(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 80.King NM, Tay FR, Pashley DH, Hashimoto M, Ito S, Brackett WW, Garcia-Godoy F, Sunico M. Conversion of one-step to two-step self-etch adhesives for improving efficacy and extended application. Am J Dent. 2005;18:126–134. [PubMed] [Google Scholar]
- 81.Brackett WW, Ito S, Tay FR, Haisch LD, Pashley DH. Microtensile dentin bond strength of self-etching resins: Effect of a hydrophobic layer. Oper Dent. 2005;30(6):733–738. [PubMed] [Google Scholar]
- 82.Reis A, Albuquerque M, Pegoraro M. Mattei G, Bauer JRO, Grande RHM, Klein-Junior CA, Baumhardt-Neto R, Loguercio AD. Can the durability of one-step self-etch adhesives be improved by double application or by an extra layer of hydrophobic resin? J Dent. 2008;36:309–315. doi: 10.1016/j.jdent.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 83.Malacarne J, Carvalho RM, De Goes MF, Svizero N, Pashley DH, Tay FR, Yiu CK, Carrilho MRO. Water sorption/solubility of dental adhesive resins. Dent Mater. 2006;22:973–980. doi: 10.1016/j.dental.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 84.Fabre HSC, Fabre S, Cefaly DFG, Carrilho MRO, Garcia FCP, Wang L. Water sorption and solubility of dentin bonding agents light-cured with different light sources. J Dent. 2007;35:253–258. doi: 10.1016/j.jdent.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 85.Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, Rueggeberg F, Foulger S, Sato T, Nishitani Y, Yoshiyama M, Tay FR, Pashley DH. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26:6449–6459. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 86.Hosaka K, Nakajima M, Takahashi M, Itoh S, Ikeda M, Tagami J, Pashley DH. Relationship between mechanical properties of one-step, self-etch adhesives and water sorption. Dent Mater. 2010;26:360–367. doi: 10.1016/j.dental.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 87.Nakajima M, Okuda M, Ogata M, Pereira PNR, Tagami J, Pashley DH. The durability of a fluoride-releasing resin adhesive system to dentin. Oper Dent. 2003;28:186–192. [PubMed] [Google Scholar]
- 88.Shinohara MS, De Goes MF, Schneider LFJ, Ferracane JL, Pereira PNR, Di Hipolito V, Nikaido T. Fluoride-containing adhesive: Durability on dentin bonding. Dent Mater. 2009;25:1383–1391. doi: 10.1016/j.dental.2009.06.011. [DOI] [PubMed] [Google Scholar]