Abstract
Thyroid function tests (TFTs) are amongst the most commonly requested laboratory investigations in both primary and secondary care. Fortunately, most TFTs are straightforward to interpret and confirm the clinical impression of euthyroidism, hypothyroidism or hyperthyroidism. However, in an important subgroup of patients the results of TFTs can seem confusing, either by virtue of being discordant with the clinical picture or because they appear incongruent with each other [e.g. raised thyroid hormones (TH), but with non-suppressed thyrotropin (TSH); raised TSH, but with normal TH]. In such cases, it is important first to revisit the clinical context, and to consider potential confounding factors, including alterations in normal physiology (e.g. pregnancy), intercurrent (non-thyroidal) illness, and medication usage (e.g. thyroxine, amiodarone, heparin). Once these have been excluded, laboratory artefacts in commonly used TSH or TH immunoassays should be screened for, thus avoiding unnecessary further investigation and/or treatment in cases where there is assay interference. In the remainder, consideration should be given to screening for rare genetic and acquired disorders of the hypothalamic–pituitary–thyroid (HPT) axis [e.g. resistance to thyroid hormone (RTH), thyrotropinoma (TSHoma)]. Here, we discuss the main pitfalls in the measurement and interpretation of TFTs, and propose a structured algorithm for the investigation and management of patients with anomalous/discordant TFTs.
Keywords: anomalous/discordant thyroid function tests (TFTs), assay interference, acquired and genetic disorders of hypothalamic–pituitary–thyroid axis
Introduction
Although thyroid disease in its most florid forms is easily recognised, minor perturbations of thyroid status can be more difficult to diagnose clinically, manifesting symptoms and/or signs that are non-specific (e.g. tiredness/lethargy; weight gain/loss; palpitations), and typically presenting to clinicians other than endocrinologists. Confirmation or exclusion of an underlying thyroid disorder therefore requires a high clinical index of suspicion, coupled with accurate measurement and interpretation of thyroid hormone (TH) and thyrotropin (TSH) concentrations. In the majority of cases, the results of thyroid function tests (TFTs) are straightforward, presenting a pattern that is readily recognised and consistent with the clinical impression of thyroid status. However, in a small, but significant subgroup of patients, the interpretation of TFTs is more challenging, either because the results appear discordant with the clinical picture (e.g. normal TSH in a patient with suspected thyrotoxicosis), or because different measurements appear to contradict each other (e.g. raised TH concentrations, but with non-suppressed TSH). In these patients, a structured approach to further investigation is required if resources are not to be wasted and inappropriate treatment recommended. In most instances, careful clinical reassessment of thyroid status, together with considering possible confounding factors [e.g. pregnancy, intercurrent (non-thyroidal) illness, drug therapy] readily identifies the cause of apparently anomalous/discordant TFTs. Where this is not the case, interference in one or other of TH (T4, thyroxine; T3, triiodothyronine) or TSH assays should be systematically screened for, and may require specialist laboratory work up. Thereafter, rare genetic and acquired disorders of hypothalamic–pituitary–thyroid (HPT) axis function should be considered, and referral to a specialist endocrine unit is advised. In this article we highlight the various pitfalls that can befall a clinician when faced with apparently anomalous or discordant TFTs, and show how a structured clinical approach, combined with judicious use of biochemical, radiological and genetic investigations, enables the cause of apparently confusing TFTs to be readily resolved in most cases.
General considerations when interpreting TFTs
A sound knowledge of hypothalamic–pituitary–thyroid axis physiology and the factors governing TH action at a tissue/cellular level, coupled with an understanding of the diverse array of congenital and acquired conditions that can manifest with different TFT patterns, is crucial to establishing the correct diagnosis in patients presenting with anomalous TFTs.
HPT axis physiology and TH action
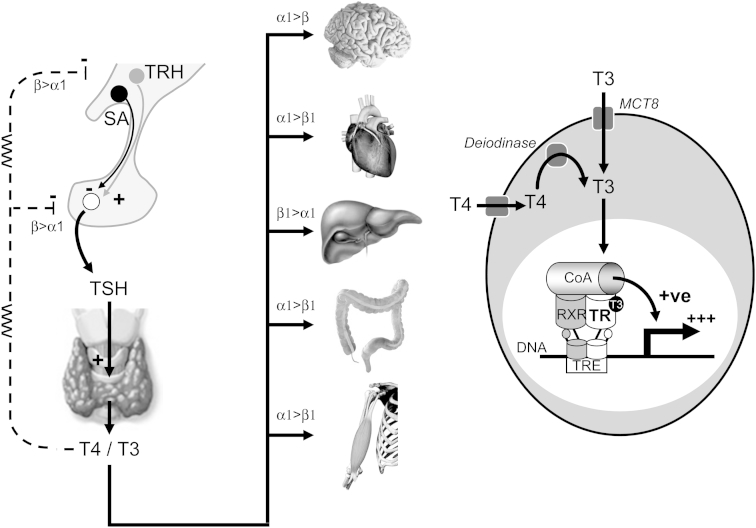
TH production is tightly regulated by hypothalamic thyrotropin releasing hormone (TRH) and pituitary TSH (Fig. 1). In any given individual T4 and T3 concentrations remain relatively constant throughout life, and reflect the ‘set-point’ of the hypothalamic–pituitary–thyroid (HPT) axis in that individual [1]. In the euthyroid state, the thyroid gland secretes 85–90% T4 and 10–15% T3, both of which are heavily (>99.5%) protein bound to thyroxine binding globulin (TBG), albumin and transthyretin (prealbumin). Cellular entry of TH in many tissues (e.g. the brain) is dependent on specific membrane proteins [e.g. monocarboxylate transporter 8 (MCT8)] [2] (Fig. 1). These transporters also appear to critically govern TH efflux from the thyroid gland (in particular the balance between secreted T4 and T3) [3]. A further tier of regulation of TH action is provided at a pre-receptor level by a family of intracellular deiodinases (DIOs): hepatic type 1 DIO mediates peripheral T4 to T3 conversion; DIO2 converts T4 to T3 in the hypothalamus and pituitary, thereby playing a central role in negative feedback regulation of the HPT axis; in contrast DIO3 converts T4 to reverse T3 (rT3) and T3 to T2, thereby limiting TH action [4] (Fig. 1). T3 is the principal bioactive hormone and although non-genomic effects of TH are recognised [5], its major actions are mediated by binding to a receptor (TR) in the nucleus of target cells (Fig. 1). Two thyroid hormone receptor genes (THRA, THRB) exist on chromosomes 17 and 3 respectively. Each gene undergoes alternate splicing to generate TRα1, TRα2, TRβ1 and TRβ2 isoforms, each with differing tissue distributions (e.g. TRα1 is the predominant isoform in the central nervous system, myocardium, colon and skeletal muscle; TRβ1 is highly expressed in the liver and kidney; TRβ2 plays a major role in negative feedback regulation at the level of the hypothalamus and pituitary) [6]. Interestingly, TRα2 is non-hormone binding and its role in normal physiology remains unclear, although it may serve as a natural antagonist of TRα1 action. TRs bind to specific recognition sequences (thyroid hormone response elements, TREs) in the promoter or other regulatory regions of TH target genes, and typically exert opposing effects depending on whether the receptor is occupied by ligand (T3) or not [7]. For example, on a positively regulated target gene (e.g. hepatic sex hormone binding globulin) unliganded TR recruits a repression complex [including corepressors such as NCoR (nuclear receptor corepressor) and SMRT (silencing mediator of retinoic acid and thyroid receptors)] that suppresses gene transcription. In contrast, following T3 binding, TR undergoes a conformational change that favours release of the corepressor complex and recruitment of an alternative coactivator complex, thus enhancing target gene transcription (Fig. 1). In this way TR is able to function as a molecular switch. Interestingly, T3 binding to TR has opposite effects on negatively regulated target genes (e.g. TRH, TSHα and TSHβ gene promoters) [7].
Fig. 1.
Schematic representation of the hypothalamic–pituitary–thyroid axis and the various factors governing thyroid hormone transport, metabolism and action at the tissue/cellular level. Key: α1, TRα1; β1, TRβ1; β2, TRβ2; CoA, coactivator; DNA, deoxyribonucleic acid; MCT8, monocarboxylate transporter 8; RXR, retinoid X receptor; SA, somatostatin; T3, triiodothyronine; T4, thyroxine; TR, thyroid receptor; TRE, thyroid response element; TRH, thyrotropin releasing hormone; TSH, thyroid stimulating hormone (thyrotropin).
Changes in thyroid status are normally associated with concordant changes in TH and TSH concentrations (e.g. raised T4 and T3 with suppressed TSH in thyrotoxicosis; low T4 and T3 with elevated TSH in hypothyroidism). However, the population reference ranges for TH are relatively broad – in contrast to the narrow individual variations of serum TH seen in normal subjects [1]. As a result, changes in TH concentrations sufficient to render a subject hypo- or hyper-thyroid may not necessarily be associated with numerically abnormal T4 or T3 concentrations (as occurs in so-called ‘subclinical’ hypo- or hyper-thyroidism). Accordingly, TSH has been recommended as a frontline screening test for thyroid dysfunction, as relatively modest changes in TH concentrations are associated with marked excursions in TSH. However, screening exclusively with TSH will result in misdiagnosis of some cases, whilst other conditions may be missed altogether (by virtue of returning a TSH result that falls within the reference range despite overt hypothalamic–pituitary–thyroid dysfunction) (Table 1). Accordingly, many laboratories now routinely offer combination screening with both T4 and TSH measurement. It is also important to bear in mind whether total (TT4, TT3) or free (FT4, FT3) TH are measured, as changes in circulating binding proteins (e.g. elevated TBG in pregnancy) can seriously confound interpretation of TT4 and TT3 concentrations (see below).
Table 1.
Conditions in which measurement of TSH alone may be misleading.
|
|
|
|
|
|
|
Key: TH, thyroid hormone; TSH, thyroid-stimulating hormone/thyrotropin.
Given the complexity of pathways that govern TH action at tissue and cellular levels, it is not surprising that some patients receiving exogenous thyroid hormone replacement therapy report on-going symptoms despite optimal thyroid function tests (e.g. normal T4 and T3 with TSH <2 mU/L in primary hypothyroidism). In some of these it is conceivable that restoring euthyroidism in the HPT axis does not equate with resolution of hypothyroidism in other target tissues. For these individuals, and for patients with central hypothyroidism where TSH is not a reliable indicator, better tissue markers of TH action are required to help guide TH replacement strategies.
TFT patterns and their aetiology
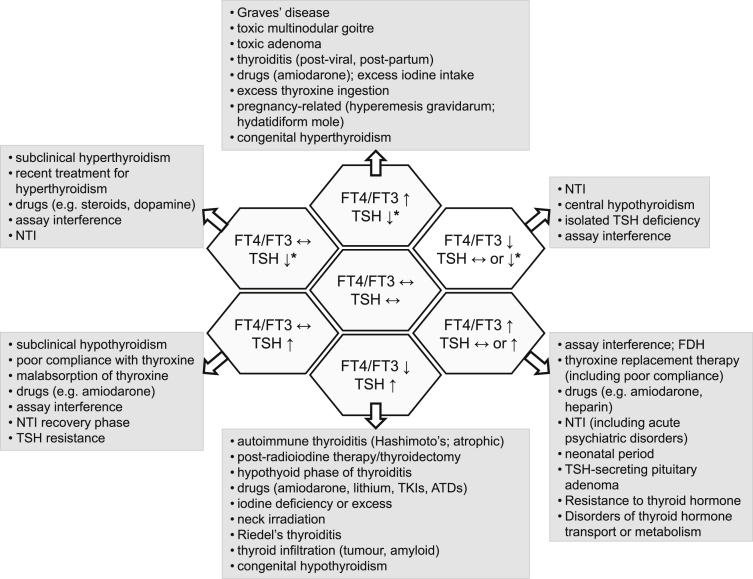
When interpreting TFTs it is helpful to keep in mind the seven major patterns that may be encountered and the conditions/circumstances in which these can arise (Fig. 2). Decisions on whether to initiate further investigations or the choice of laboratory, radiological and genetic tests to pursue, can then be focused with a view to confirming or excluding specific diagnoses.
Fig. 2.
Different patterns of thyroid function tests and their causes. Key: ATDs, antithyroid drugs; FDH, familial dysalbuminaemic hyperthyroxinaemia; FT4, free thyroxine; FT3, free triiodothyronine; NTI, non-thyroidal illness; TKIs, tyrosine kinase inhibitors; TSH, thyroid-stimulating hormone/thyrotropin [*signifies that TSH may be either fully suppressed (for example as seen in classical primary hyperthyroidism) or partially suppressed (i.e. measurable, but below the lower limit of normal)]. Reproduced with permission from: Koulouri O, Gurnell M. How to interpret thyroid function tests. Clin Med 2013; 13:282–6. Copyright © 2013 Royal College of Physicians.
Specific considerations when interpreting TFTs
Clinical context/thyroid status
As a general rule, thyroid function tests should only be requested when there are specific clinical features that require a primary disorder of hypothalamic–pituitary–thyroid function to be ascertained. Measuring TH and/or TSH concentrations when there is a low index of suspicion for HPT dysfunction risks the possibility of TFTs that confound, and a train of inappropriate investigations and management (e.g. in non-thyroidal illness). Accordingly, when apparently anomalous TFTs occur, the first step is to reappraise the patient's clinical status as this will help guide further management (see below). Importantly, many clinical laboratories provide generic reference ranges for T4, T3 and TSH, despite increasing evidence that this may not be appropriate, with, for example, ethnicity, iodine intake, gender, age, and body mass index influencing the reference range of serum TSH [8,9], while pregnancy is associated with major changes in both TH and TSH concentrations [10].
Non-thyroidal illness
A common pitfall in the interpretation of thyroid function tests is to overlook the confounding effects of ‘non-thyroidal illness’ (NTI). NTI (or sick euthyroid syndrome) is a relatively common finding following any acute or chronic illness, and is defined by the absence of an intrinsic abnormality of HPT function – rather it is considered a secondary adaptive change. Whether it is a beneficial response (e.g. to reduce metabolic rate) or a maladaptive response (with potential benefit from TH replacement therapy) has been much debated, but compelling evidence for the use of T3 or T4 therapy in the majority of patients with NTI is currently lacking [11].
Changes in TH (especially T3) and TSH may be seen as early as 24 h after the onset of non-thyroidal illness [12], and have been observed in subjects with poor nutrition/starvation, sepsis, burns, malignancy, myocardial infarction, post-surgery, and with chronic liver and renal disease [13,14]. NTI can be characterized by a variety of abnormal TFT patterns, which may evolve/change with progression or resolution of the underlying primary disorder (Fig. 2). Many commercial assays for free TH typically return low (or low-normal) FT4 and FT3, with normal or low (but rarely fully suppressed) TSH [15,16]. However, elevated FT4 may also be found, and it is not uncommon for the same sample to yield markedly discordant FT4 concentrations when run on different assay platforms, reflecting methodological differences/limitations [14,16]. Where total TH concentrations are measured, reductions in TT4, and in particular TT3, are common even in mild NTI, and are usually more marked than the corresponding decreases in free hormone concentrations (likely reflecting reduced serum TH binding capacity in acute and chronically ill patients, secondary to a fall in TH binding protein concentrations and/or impaired T4/T3 binding) [14]. The magnitude of T4 decrease has been reported to correlate with a less favourable outcome [17], and mortality can be as high as 80% when TT4 drops below 26 nmol/L [18]. When measured, reverse T3 (rT3) is usually raised.
In subjects with acute, major psychiatric illness, raised T4 with non-suppressed TSH is sometimes observed, but usually resolves spontaneously within a short time frame (<2 weeks); in others, TSH may be elevated or suppressed but without accompanying abnormalities of T4 or T3 [19].
There remains considerable debate regarding the precise mechanisms underpinning NTI, with changes noted at all levels in the pathway of TH synthesis/secretion, transport, cellular uptake and action [14]. These include, but are not restricted to: reduced hypothalamic TRH secretion from paraventricular nuclei [20]; impaired pituitary TSH secretion; decreased TH binding capacity in serum [21]; reduced tissue/cellular uptake of T4 and T3; altered deiodinase activity with reduced DIO1, but increased DIO2 and DIO3 (although findings in DIO3 knockout and DIO1/DIO2 knockout mice suggest altered deiodinase activity may be a consequence, rather than a cause, of the changes that occur in T4 and T3) [22,23]; and altered thyroid hormone receptor expression/signalling (e.g. reduced in skeletal muscle) [24].
The mediators of such changes are also much debated, but pro-inflammatory cytokines (including IL-1, IL-6, TNF-α) have been implicated in NTI in a variety of infectious, inflammatory and neoplastic states [14,25]. In addition, the reduction in leptin levels that accompanies malnutrition may directly impair hypothalamic TRH secretion [14]. A role for excess endogenous glucocorticoids has also been postulated, while the use of exogenous corticosteroid therapy and dopamine (see below) in critically ill patients may further suppress pituitary TSH release.
During recovery from intercurrent illness, TH and TSH concentrations return to normal, although in some patients TSH may become overtly elevated for a short period of time. This rise in TSH typically precedes the increase in T4 and T3 concentrations, suggesting that it is required for the restoration of euthyroidism [26]. It is important to be aware of this transient phenomenon in order to avoid inappropriate diagnosis and treatment.
Pregnancy
Pregnancy has a significant impact on HPT physiology and may be associated with marked changes in serum thyroid hormone and thyrotropin concentrations [27]. Under normal circumstances, about two thirds of circulating T4 is bound to TBG. During pregnancy TBG levels rise as a consequence of oestrogen-induced increased hepatic synthesis, together with reduced degradation. Accordingly, serum total T4 and T3 concentrations increase to approximately 150% of non-pregnant values – this occurs during the first half of pregnancy and is maintained thereafter until parturition. Free T4 concentrations also change during pregnancy: in the first trimester a transient rise is often observed and has been ascribed to the stimulatory effects of high circulating levels of human chorionic gonadotrophin (hCG) acting on the TSH receptor (TSH concentrations are correspondingly reduced, or even partially suppressed). In its most extreme form (hyperemesis gravidarum), affected women may become overtly thyrotoxic with a fully suppressed TSH. In this setting, it is important to distinguish gestational hyperthyroidism from other common causes of thyrotoxicosis (e.g. Graves' disease) as the management of these conditions differs significantly [28]. Measurement of anti-TSH receptor antibody (TRAb) titres can be particularly helpful.
As hCG levels decline, FT4 decreases and this has been shown to be a genuine effect rather than the result of analytical interference [29]. FT4 concentrations are often lower than those observed in the non-pregnant state, which may lead to concern regarding the possibility of central thyroid dysfunction if values are compared with non-pregnant reference ranges. Changes in FT3 broadly parallel those of FT4. TSH levels are restored as hCG levels fall in the second and third trimester.
In addition to the effects of fluctuating hCG levels and rising TBG, a number of other mechanisms have been proposed to contribute to the alterations in thyroid status observed in pregnancy, including an increased circulating plasma volume, enhanced DIO3 activity (placental origin: increased T4 & T3 degradation) and increased urinary iodine clearance (leading to goitre in iodine-deficient regions) [27].
Levothyroxine therapy
Although clinical and biochemical euthyroidism is readily restored in the majority of patients treated with levothyroxine (L-T4), an important subgroup manifests apparently anomalous TFTs, which can be a source of considerable frustration and confusion both for patient and clinician alike. [30] Table 2 summarizes the common causes and suggested approaches for investigation of anomalous TFTs in patients receiving L-T4 therapy.
Table 2.
Causes of anomalous TFTs in patients receiving levothyroxine therapy [30,63].
| TFT patterns/LT4 dosage requirements | Cause | Explanation |
|---|---|---|
| A. Normal TSH, mildly ↑FT4; (± higher than predicted L-T4 requirementsa) | Normal physiological variant | To abolish symptoms and normalise TSH concentrations, some individuals exhibit mildly elevated FT4 (possibly reflecting less efficient deiodination of T4 to T3); FT3 is typically normal |
| B. ↑TSH, low normal or ↓FT4; (Requirement for high L-T4 dosages to normalise TSHa) | (i) Maladministration | Patients should be advised to take L-T4 on an empty stomach; certain foodstuffs (e.g. fibre, espresso coffee) and some medications (e.g. iron, calcium, PPIs, sucralfate, aluminium hydroxide, cholestyramine, colestipol) may impair L-T4 absorption |
| (ii) Malabsorption syndromes | L-T4 malabsorption occurs with coeliac disease, achlorhydria, lactose intolerance (lactose is a constituent of some L-T4 preparations) | |
| (iii) Increased TH metabolism or excretion | Phenytoin, carbamazepine, phenobarbitone, rifampicin and some tyrosine kinase inhibitors (e.g. Imatinib) increase L-T4 requirements by enhancing hepatic metabolism of TH; occasional cases of increased urinary TH loss complicating nephrotic syndrome have also been reported | |
| (iv) Increased TH binding capacity | Oral oestrogen therapy or gonadotrophin-induced rise in oestrogen concentrations (e.g. IVF treatment) results in a marked increase in TBG and hence TH binding capacity, necessitating an increase in L-T4 therapy; similar effects are seen with SERMs and mitotane | |
| C. Unexpected change in L-T4 dosage requirements to maintain clinical and biochemical euthyroidism | Change in LT4 preparation | Not all L-T4 preparations are of comparable potency/bioavailability; changes in preparation are generally best avoided but, if necessary, should prompt more frequent TFT monitoringb |
| D. ↑TSH, normal FT4 | TSH assay interference | Heterophilic antibody interference in the TSH assay may yield falsely elevated results; FT3 is normal |
| E. Persistent ↑TSH, with ↓, ↑ or normal FT4, despite treatment with high L-T4 dosages | Poor compliance | Owing to their differing half-lives, intermittent thyroxine ingestion may result in normal or even elevated TH concentrations, but fails to normalize TSH |
| F. Supraphysiologic L-T4 required to normalise TSH, but with resultant ↑FT4 (and ↑FT3) | Resistance to thyroid hormone | Typically seen following inappropriate thyroid ablation or concomitant primary hypothyroidism in a patient harbouring a mutation in the human thyroid hormone receptor β (THRB) gene |
Key: FT4, free thyroxine; FT3, free triiodothyronine; L-T4, levothyroxine; PPI, proton pump inhibitor; SERMs, selective oestrogen receptor modulators; TFTs, thyroid function tests; TH, thyroid hormone; TSH, thyroid stimulating hormone/thyrotropin.
In athyreotic individuals total daily levothyroxine requirements can be estimated based on body weight and usually fall in the range 1.6–2.0 mcg/kg (NB: the elderly typically require lower dosages, and caution must be exercised when commencing treatment in those with confirmed/suspected ischaemic heart disease or arrhythmias).
The UK Medicines and Healthcare products Regulatory Agency (MHRA) have recently suspended one preparation of levothyroxine following discovery that it yielded variable control [64].
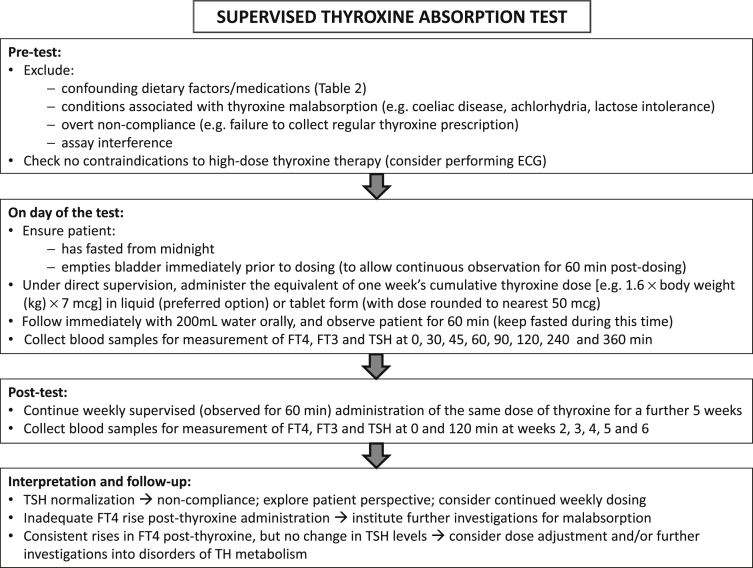
Even after careful consideration of all of these factors, discrimination between an as yet unidentified cause of true thyroxine malabsorption and poor L-T4 compliance can prove difficult. In these circumstances, a formal thyroxine absorption test may help resolve the issue [31]. A suggested protocol that combines both acute (6 h) and longer-term (6 week) supervised administration is shown in Fig. 3.
Fig. 3.
Protocol for supervised thyroxine absorption test, followed by weekly supervised thyroxine administration. Key: ECG, electrocardiogram; FT4, free thyroxine; FT3, free triiodothyronine; TSH, thyrotropin (thyroid stimulating hormone).
Drug therapy
An array of commonly prescribed drugs may result in altered thyroid function/status, either by modulating the HPT axis itself, or through downstream effects on thyroid hormone transport or metabolism [32,33].
HPT axis
Commonly prescribed medications that are capable of altering pituitary TSH or thyroidal T4 and T3 synthesis/secretion are shown in Table 3.
Table 3.
Examples of drugs directly affecting pituitary TSH or thyroidal T4 & T3 secretion in previously euthyroid subjects.
| TFT patterns | Comments | ||
|---|---|---|---|
| Drugs affecting thyroid hormone secretion | Iodide | 1. ↑TSH, ↓FT4 | Inorganic iodide (dietary/supplements) as well as iodine containing organic compounds (e.g. radiological contrast agents) can cause a transient disturbance of thyroid function; patients with diminished thyroid reserve (e.g. chronic autoimmune thyroiditis) are at risk of developing persistent hypothyroidism. |
| 2. ↓TSH, ↑FT4 | Individuals with latent/low-grade thyroid autonomy (e.g. in a pre-existing MNG) may develop overt hyperthyroidism following exposure to iodine-containing compounds (Jod-Basedow effect), which may persist for months | ||
| Amiodarone | 1. Transient ↑TSH; ↑FT4, normal FT3 | Short-lived rises in TSH are common during the first few months of treatment with amiodarone; inhibition of type 1 DIO leads to persistent elevation of FT4, but normal FT3 | |
| 2. ↓TSH, ↑FT4 | Two main types of thyrotoxicosis are recognised: type 1 (large iodine load precipitating latent thyroid autonomy); type 2 (destructive thyroiditis); amiodarone inhibits T4 → T3 conversion such that T4 is typically more markedly elevated than T3 | ||
| 3. ↑TSH, ↓FT4 | Hypothyroidism occurs in up to 15% of patients (particularly women and those with positive antithyroid antibodies); it may reflect failure to escape from the Wolff–Chaikoff effecta | ||
| Lithium | 1. ↑TSH, ↓ or normal FT4 | Overt or subclinical hypothyroidism | |
| 2. ↓TSH, ↑FT4 | Thyroiditis occurs in a small number of patients and is typically self-limiting | ||
| TKIs | 1. ↑TSH, ↓FT4 | Primary hypothyroidism (possibly due to a direct toxic effect on the thyroid gland) has been observed with some TKIs, e.g. Sunitinib, Sorafenib | |
| 2. ↓TSH, ↑FT4 | A prodromal thyrotoxic phase is occasionally seen in patients receiving Sunitinib | ||
| Immune modulators | 1. ↓TSH, ↑FT4 | Graves' disease has been reported in patients receiving: (i) Alemtuzumab (a humanised monoclonal antibody directed against CD52) for multiple sclerosis; (ii) HAART for HIV infection; (iii) INFα for chronic hepatitis C | |
| 2. ↑TSH, ↓FT4 | Hashimoto's thyroiditis may complicate INFα therapy (± prodromal thyrotoxic phase) | ||
| Drugs affecting TSH secretion | Dopamine agonists | ↓TSH (often transient), → (or mild ↓) FT4 | Intravenous infusion of dopamine or oral dopamine agonist therapy can suppress TSH secretion via activation of D2 receptors on pituitary thyrotrophs; however, clinically relevant central hypothyroidism does not usually occur, although some studies have suggested that dopamine infusion in a critically ill subject with concomitant NTI may result in genuine hypothyroidism |
| Glucocorticoids | ↓TSH (often transient), → (or mild ↓) FT4 | Glucocorticoid-mediated inhibition of hypothalamic TRH synthesis/release (acting via glucocorticoid receptors in the paraventricular nucleus) results in reduced pituitary TSH secretion; however, chronic hypercortisolism (endogenous or exogenous) is not generally associated with clinically significant central hypothyroidism | |
| Somatostatin analogues | ↓TSH (often transient), →(or mild ↓) FT4 | Both octreotide and lanreotide suppress pituitary TSH secretion through a direct inhibitory action on pituitary thyrotrophs; however, this effect is usually transient and not associated with clinically significant central hypothyroidism | |
| Rexinoids | ↓TSH, ↓FT4 | Bexarotene (Targretin®) has been linked to the development of biochemical and, in a significant proportion of patients, clinical hypothyroidism when used to treat patients with cutaneous T-cell lymphoma; inhibition of TSHβ transcription leads to decreased TSH production; additional effects on TH metabolism (deiodination, sulphation) have also been reported | |
| Metformin | ↓TSH, →FT4 | Several observational studies have reported an apparent TSH lowering effect of metformin therapy in patients with diabetes; however, whether this effect is limited to those with pre-existing disorders of the HPT axis, especially subjects with an elevated TSH (reflecting central activation of the axis) remains unclear, and further studies are required |
Key: D2, dopamine D2 receptors; DIO, deiodinase; FT4, free thyroxine; FT3, free triiodothyronine; HAART, highly active antiretroviral therapy; INFα, interferon alpha; NTI, non-thyroidal illness; T4, thyroxine; T3, triiodothyronine; TFTs, thyroid function tests; TKIs, tyrosine kinase inhibitors; TSH, thyroid stimulating hormone/thyrotropin; TSHβ, beta subunit of TSH. Adapted with permission from: Koulouri O, Gurnell M. How to interpret thyroid function tests. Clin Med 2013; 13:282–6. Copyright © 2013 Royal College of Physicians.
Wolff–Chaikoff effect = impairment of T4 and T3 synthesis by high intrathyroidal concentrations of iodine.
Altered TBG concentrations
In subjects with an intact HPT axis, drugs affecting TBG synthesis typically result in changes in total but not free serum thyroid hormone concentrations, although transient alterations in FT4 and FT3 have occasionally been observed [34]. Also, extreme changes in TBG concentrations can potentially affect some FT4 assays (Moran, Gurnell, Halsall & Chatterjee, unpublished observation). It is important to note that in patients on fixed dose L-T4 therapy, an increase or decrease in the total number of serum T4 and T3 binding sites necessitate an adjustment in levothyroxine dosage to maintain euthyroidism.
Drugs that are recognised to increase serum TBG concentrations include oral (but not transdermal) oestrogen, raloxifene, tamoxifen, mitotane, fluorouracil, methadone and heroin. In contrast, androgens, chronic glucocorticoid therapy and nicotinic acid have all been shown to inhibit TBG synthesis.
T4 and T3 displacement from TH binding proteins
In contrast to the situation described above, where quantitative changes in binding proteins bring about changes in total but not free TH concentrations, the presence of agents in serum that are capable of displacing T4 and T3 from their binding sites can alter hormone delivery and clearance and distort diagnostic tests for FT4 and FT3. A number of commonly prescribed drugs have been shown to bring about competition for TH binding sites on TBG, albumin and transthyretin, including furosemide (especially with doses >80 mg/day and when given intravenously) [35] aspirin, nonsteroidal anti-inflammatory agents, phenytoin and heparin [36].
The effect of heparin on serum free TH measurements merits particular consideration given the increasingly widespread use of low molecular weight heparin thromboprophylaxis in modern medical and surgical practice. The potential for heparin to raise free TH concentrations was first noticed by Schatz and colleagues in 12 patients undergoing haemodialysis [37]. To investigate this further, they administered intravenous heparin to nine healthy controls and five subjects with hypothyroidism, and were able to show a prompt (within 2–15 min) rise (up to five-fold) in FT4 concentrations, which could not be replicated by adding a similar concentration of heparin to the sample in vitro, thus confirming that this phenomenon is initiated in vivo [37].
Subsequent studies have shown that in heparin-treated subjects, serum non-esterified fatty acid (NEFA) concentrations may increase markedly as a consequence of heparin-induced activation of endothelial lipoprotein lipase in vivo, leading to increased NEFA generation in vitro during sample storage or incubation. In the presence of normal serum albumin concentrations, NEFA concentrations >2–3 mmol/L exceed normal serum binding capacity, resulting in direct competition for T4 and T3 binding sites on TBG either by NEFAs themselves or as a result of displacement of other ligands from the albumin sites that normally limit their free concentration [38,39]. Not surprisingly, this artefact is more pronounced when serum triglyceride concentrations are elevated, in the presence of hypoalbuminaemia, and with laboratory methods that require long incubation periods [40]. Indeed, in the presence of sufficient triglyceride substrate, even very low dose intravenous heparin (equivalent to that used to maintain the patency of an indwelling cannula), and subcutaneous low molecular weight heparin (LMWH) prophylaxis can lead to FT4 (and FT3) elevation. Moreover, the heparin effect has been observed with a variety of assay platforms including equilibrium dialysis, ultracentrifugation, and direct immunoassay [41].
Ideally therefore, measurement of FT4 and FT3 is best avoided in patients receiving heparin therapy. However, when indicated, taking a blood sample more than 10 h after the last injection of heparin, and analysing it without delay, can help reduce the risk of artifactual hyperthyroxinaemia, although clinicians should bear in mind that small rises in free TH may be inevitable in predisposed individuals [41]. Alternatively, measurement of total TH levels, together with TSH and TBG [36], can help confirm the patient's euthyroid status when displacement is suspected [42].
Assay interference
TSH measurement
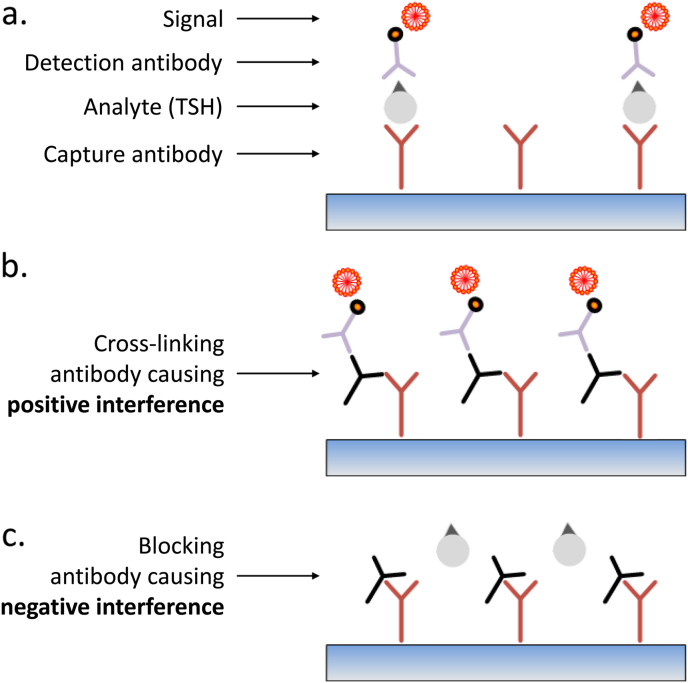
Most commercially available TSH assays are based on an immunometric two-site (or ‘sandwich’) format with capture and (labelled) detection antibodies directed against different epitopes on TSH, with the analyte essentially acting as a bridge between the two (Fig. 4a). The capture antibody is typically immobilized to a solid phase to ensure good separation between bound and unbound label, thus increasing sensitivity; often, several different detection antibodies are employed to further improve assay sensitivity. The presence of human anti-animal antibodies (HAAs) in a patient's serum can interfere with TSH measurement if directed against the same species as the assay antibodies: thus, a HAA that is capable of cross-linking the capture and detection antibodies may cause ‘positive interference’, leading to a falsely high TSH (Fig. 4b); conversely, a HAA that blocks TSH binding to either capture or detection antibodies will result in ‘negative interference’, causing a falsely low TSH readout (Fig. 4c). Many manufacturers now include panels of antigens or pre-immune serum from source animals in an attempt to ‘mop up’ HAAs. However, heterophile antibodies (which are weak, polyspecific antibodies that are similarly capable of causing negative or positive interference) can prove more difficult to remove. Such interference in the TSH assay may be seen in cases of Graves' disease and in patients with positive rheumatoid factor (RhF), although emerging evidence suggests that the finding of a raised RhF titre in this context may in itself reflect heterophilic antibody interference in the RhF assay (Chatterjee, Halsall & Gurnell, unpublished data). Interfering antibodies can also bind the analyte (TSH) rather than the assay antibodies. An extreme example of this type of interference is the ‘macro hormone’ complex, in which a specific anti-TSH immunoglobulin binds TSH and neutralizes its biological activity, but leaves epitopes exposed for interaction with the assay antibodies. The consequence is analogous to artefactually elevated prolactin concentrations seen in patients with macroprolactinaemia. If interference is suspected, it is best to seek the advice of the laboratory as there are several ways to confirm this, for example by demonstrating:
-
•
varying TSH results in assays that utilize different antibody pairs or incubation times,
-
•
a different TSH result following manoeuvres [using polyethylene glycol (PEG) or protein G/A treatment of sample] that remove the interfering antibody,
-
•
nonlinear TSH measurement following sample dilution: if either TSH or the assay reagents are weakly bound by interfering antibodies this interaction may be disrupted by dilution and a nonlinear dilution series will result.
Fig. 4.
Schematic representation of an immunoradiometric assay for measurement of serum TSH. a. TSH is bound by both capture (immobilised) and detection (labelled) antibodies. b. The presence of a human anti-animal (HAA) or heterophilic antibody that is capable of cross-linking the capture and detection antibodies even in the absence of analyte (TSH), results in positive assay interference. c. In contrast, an HAA or heterophilic antibody that binds either the capture or detection antibody to prevent crosslinking (even in the presence of TSH) results in negative assay interference.
Free T4/T3 measurement
Determination of FT4 (and FT3) is particularly challenging as the assay must detect very low concentrations of ‘free’ hormone relative to a vast excess of protein-bound analyte (>99.5%). The relatively small size of T4 (and T3) precludes use of a two-site assay format, so ‘competition assays’ are used; here, T4 in the serum competes with labelled T4 analogue for a fixed number of anti-T4 antibody binding sites or with an immobilized T4 analogue for a labelled anti-T4 antibody [43]. Free hormone assays are designed such that the equilibrium between T4 and its binding proteins is preserved during measurement, so that the amount of tracer displaced reflects the ‘free’ rather than ‘total’ hormone concentration. Clearly, the presence of factors in serum which affect this equilibrium will confound hormone measurement. Examples include:
-
•
fractionated or unfractionated heparin and other displacing agents (see above)
-
•
anti-iodothyronine antibodies, which can bind the tracer
-
•
HAAs or heterophile antibodies that block the assay antibody
-
•
variant thyroid hormone binding proteins [e.g. albumin in familial dysalbuminaemic hyperthyroxinaemia (FDH)] with altered affinity for T4
The use of a ‘two-step’ (‘back titration’) assay method, with a wash step prior to tracer addition, may reduce but not completely eliminate interference. If erroneous TH results are suspected with a ‘one-step’ assay, then re-measurement using a ‘two-step’ assay is a logical first step. If the problem persists, hormone measurement following equilibrium dialysis (ED) remains the gold-standard for eliminating FT4 assay interference, with the caveat that this method will not detect displacing agents, as discussed above.
Distinguishing resistance to thyroid hormone (TRβ RTH) from a TSH-secreting pituitary tumour (TSHoma)
Amongst the various discordant TFT patterns shown in Fig. 2, one of the most challenging distinctions to make is between resistance to thyroid hormone due to a loss-of-function mutation in the human THRB gene (TRβ RTH) [6] and a TSH-secreting pituitary adenoma (TSHoma/thyrotropinoma) [44].
Incidence/prevalence
TRβ RTH has an estimated incidence of 1 in 40–50,000 live births [6]. Thyrotropinomas are considered to be the rarest of the classical pituitary tumours, accounting for less than 2% of all adenomas, and with an estimated prevalence of 1 case per million population [44–46]. However, a recent report suggests that this historical figure may be changing (increasing 2–3 fold), as a consequence of increasing awareness among clinicians, coupled with the greater combination screening with T4 (T3) and ultrasensitive TSH assays, and increased availability of high resolution pituitary imaging [47].
Clinical features
The clinical phenotype of both disorders overlaps significantly, ranging from apparently asymptomatic individuals, through to those with more overt manifestations of thyrotoxicosis (in particular arrhythmias) and goitre. In TRβ RTH, a subgroup of patients with predominant central/pituitary resistance are particularly prone to developing thyrotoxic symptoms and signs [6]. In both contexts, a previous history of inappropriate attempts at thyroid ablation (on the basis of misdiagnosed primary hyperthyroidism) is a relatively frequent finding. Accordingly, it is not usually possible to distinguish between the two conditions based on clinical findings alone.
T4, T3 and TSH concentrations
The degree of hyperthyroxinaemia is comparable in both conditions [46], although there may be a tendency to higher (albeit overlapping) TSH concentrations in TSHomas (Koulouri, Moran, Halsall, Chatterjee & Gurnell, unpublished observation). Concomitant common thyroid dysfunction can modulate the degree of hyperthyroxinaemia in both disorders; thus, several cases of TRβ RTH or TSHoma with coexistent Hashimoto's thyroiditis have been reported, with lower than expected FT4 and FT3 concentrations (sometimes even within the reference range), with the underlying central diagnosis being suspected on the basis of inability to suppress TSH despite levothyroxine therapy in supraphysiologic dosage [48,49].
Other biochemical and dynamic tests
Raised serum concentrations of pituitary glycoprotein hormone α-subunit (ASU) are associated with TSHomas [44,46], but are also recorded in non-functioning and GH-secreting pituitary tumours. Furthermore, normal ASU concentrations, but an elevated ASU/TSH molar ratio (>1.0), are a recognized finding in TSH-secreting microadenomas; however, the latter needs careful interpretation with clinical context – elevated molar ratios are also seen in some normal subjects, especially postmenopausal women.
A number of peripheral tissue markers of thyroid hormone action have been proposed to aid discrimination between TRβ RTH and TSHoma, with serum SHBG (analysed using age- and gender-specific reference ranges) reported to be the most discriminatory [44]. However, the utility of such markers is limited by their lack of specificity, especially when measured in the basal state. For example, SHBG concentrations are known to be affected by several factors including exogenous oestrogen therapy, liver dysfunction, insulin resistance, obesity and increased growth hormone (which is co-secreted in about 18% of TSHomas [44]). Dynamic testing with measurement of tissue biomarkers and the TSH responsiveness to TRH, before and after administration of liothyronine (the ‘T3 suppression test’) may be more discriminatory [44–46], although there is a relative paucity of generalizable reference data for both normal controls and subjects with RTH and TSHoma. In addition, the need to limit its use to younger patients without coexistent cardiac disease further limits its utility.
In contrast, assessment of the TSH response to intravenous TRH (200 μg), with sampling at 20 and 60 min post-injection, is a relatively easy and safe test to perform, and may offer good discriminatory power in distinguishing TRβ RTH from TSHoma. A less than 1.5-fold rise in TSH is strongly suggestive of the presence of TSHoma [44] and occurs in the majority (80–90%) of macroTSHomas. Although a greater TSH rise may be seen in some microTSHomas, the response is still attenuated and readily distinguished from the more exuberant TSH response of TRβ RTH patients (typically greater than 5-fold, Koulouri, Moran, Halsall, Chatterjee and Gurnell, unpublished observation).
Pituitary imaging
The presence of an obvious lesion on pituitary imaging (especially a macroadenoma) favours the diagnosis of TSHoma, although the possibility of RTH with a concomitant incidentaloma must be borne in mind. In addition, an increasing proportion of microTSHomas, some of which are not readily visualised on conventional MRI, are being recognised, and dynamic MRI and/or functional imaging may be required to visualise these [44,45]. A further confounder is that persistently elevated TSH concentrations, either in the context of chronic non-compliance in primary hypothyroidism or following thyroid ablation in TRβ RTH, results in thyrotroph hyperplasia and pituitary enlargement which is reversible. [50]
Trial of somatostatin receptor ligand (SRL) therapy
Most TSHomas (90%) respond well to long-acting SRL therapy, with significant reductions in, and often normalization of, FT4 and FT3 concentrations after a two month trial [51]. In contrast, subjects with TRβ RTH do not show any significant change in FT4 and FT3 in response to depot SRL.
Genetics of THRB RTH
Approximately 80% of TRβ RTH cases are dominantly inherited, with the remainder occurring sporadically due to ‘de novo’ mutations [52]. Therefore, similarly abnormal TFTs in first-degree relatives strongly suggests RTH. THRB gene sequencing confirms the diagnosis in 85% of cases, but 15% are not associated with identifiable THRB mutations, such that absence of an abnormality in this gene does not exclude the diagnosis [52]. In addition, the recent identification of a non-pathological sequence variant in THRB in a kindred with primary autoimmune thyroid disease serves as a timely reminder that close correlation of clinical and genetic findings is mandatory in all cases of suspected RTH [53].
Other genetic disorders of TH transport, metabolism or action
MCT8 mutations
An X-linked disorder (Allan–Herndon–Dudley syndrome) of childhood-onset, with psychomotor retardation including speech and developmental delay and spastic quadriplegia, is caused by defects in the MCT8 (SLC16A2) gene, encoding a membrane transporter. In addition to neurological abnormalities, male patients exhibit a characteristic pattern of abnormal TFTs with elevated FT3, low FT4 and normal TSH concentrations [6,54,55].
Functional deiodinase deficiency
The deiodinase enzymes are part of a larger family of 25 human proteins containing selenocysteine. Recently, a multisystem selenoprotein deficiency disorder, manifesting with growth retardation in childhood or other features (male infertility, skeletal myopathy, photosensitivity, hearing loss) has been described and is associated with a characteristic thyroid hormone signature (raised FT4, normal/low FT3 and normal TSH concentrations), due to functional DIO deficiencies [6,56,57].
TRα RTH
In contrast to TRβ RTH, mutations in human TRα1 mediate RTH with features of hypothyroidism in a different subset of tissues (e.g. CNS, gastrointestinal tract, myocardium, skeletal muscle, skeleton), but are not associated with a markedly dysregulated pituitary–thyroid axis. To date only four individuals harbouring such mutations have been reported. Clinical features include growth and developmental retardation, skeletal dysplasia and constipation associated with low-normal T4 and high-normal T3 concentrations (resulting in a low T4:T3 ratio), together with subnormal reverse T3; TSH is normal [58–60].
Summary
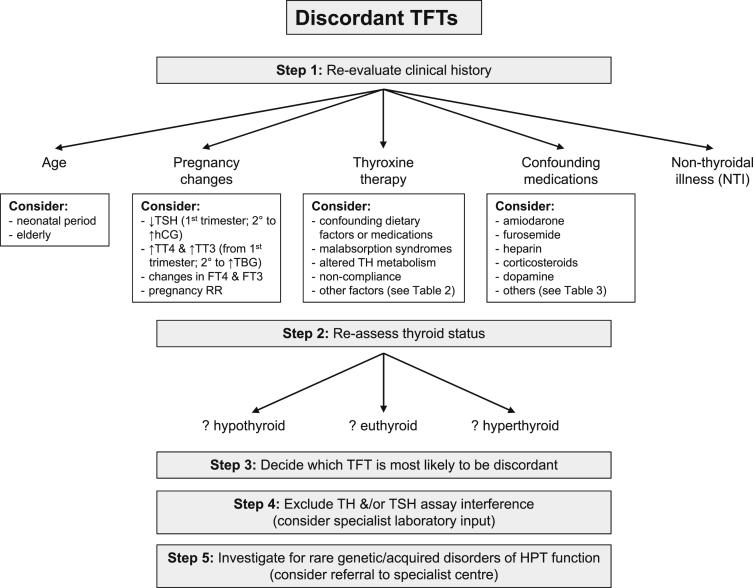
Although most thyroid function test patterns are easy to interpret, and concordant with clinical assessment of thyroid status, a small but important subset of patients exhibit results that are either discordant with the clinical picture or not congruent with each other. In such cases, a structured approach to further assessment is essential to avoid unnecessary/inappropriate investigation and treatment. Once confounding influences [e.g. physiological changes of age, pregnancy, intercurrent (non-thyroidal) illness or concomitant medication use] have been excluded, close liaison with the clinical biochemistry laboratory is required to systematically exclude thyroid hormone and TSH assay interference. Only then should further investigation for rare acquired and genetic causes of anomalous/discordant TFTs be considered. An algorithm summarizing this approach to investigation and differential diagnosis is shown in Fig. 5.
Practice points
-
•
Knowledge of hypothalamic–pituitary–thyroid axis physiology, the factors governing TH action at a tissue/cellular level, and the different patterns of TFTs that may be encountered in clinical practice, is central to establishing the correct diagnosis when clinical features and TFT results appear discordant/incongruous.
-
•
Reappraisal of the clinical context – in particular exclusion of confounding intercurrent illness or medication usage, coupled with reassessment of thyroid status – should be the first step to resolving such cases.
-
•
Targeted investigation to definitively exclude assay interference may require specialist laboratory input.
-
•
Genetic and acquired disorders of the HPT axis are rare, but should be considered if all other steps have failed to identify a cause for anomalous/discordant TFTs.
Research agenda
-
•
Age, gender and pregnancy-specific reference ranges for commonly used T4, T3 and TSH immunoassay platforms are urgently required. Also, harmonization of TFT assays in routine clinical practice will simplify data interpretation. [61]
-
•
The development and validation of mass spectrometry assays for free TH measurement should provide an additional useful laboratory tool to exclude immunoassay interference. [62]
-
•
The identification of better markers of TH action in different tissues is necessary to enable diagnosis of disorders with subtle HPT axis dysfunction and to guide treatment, especially in the context of central hypo- or hyper-thyroidism.
-
•
Increasing availability of next generation sequencing will likely expand the repertoire of genetic causes of abnormalities of the HPT axis and disorders of TH transport, metabolism and action.
Fig. 5.
Algorithm for the interpretation of discordant TFTs. Key: FT3, free triiodothyronine; FT4, free thyroxine; HPT, hypothalamic–pituitary–thyroid; RR, reference range; TFTs, thyroid function tests; TH, thyroid hormones; L-T4, levothyroxine; NTI, non-thyroidal illness, TT4, total thyroxine; TT3, total triiodothyronine; TSH, thyroid stimulating hormone (thyrotropin); TH, thyroid hormones; TBG, thyroxine binding globulin.
Disclosures
None of the authors have anything to disclose.
Acknowledgements
OK, CM, DJH, VKC and MG are supported by the UK National Institutes for Health Research comprehensive Cambridge Biomedical Research Centre. OK and MG are also supported by the Evelyn Trust. DJH is a NHS East of England Clinical Academic Senior Clinical Fellow and VKC is a Wellcome Trust Senior Investigator.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- [1].Andersen S., Pedersen K.M., Bruun N.H. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. Journal of Clinical Endocrinology and Metabolism. 2002;87:1068–1072. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 2.Visser W.E., Friesema E.C., Visser T.J. Minireview: thyroid hormone transporters: the knowns and the unknowns. Molecular Endocrinology. 2011;25:1–14. doi: 10.1210/me.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trajkovic-Arsic M., Müller J., Darras V.M. Impact of monocarboxylate transporter-8 deficiency on the hypothalamus-pituitary-thyroid axis in mice. Endocrinology. 2010;151:5053–5062. doi: 10.1210/en.2010-0593. [DOI] [PubMed] [Google Scholar]
- 4.Bianco A.C., Kim B.W. Deiodinases: implications of the local control of thyroid hormone action. Journal of Clinical Investigation. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis P.J., Davis F.B. Nongenomic actions of thyroid hormone on the heart. Thyroid. 2002;12:459–466. doi: 10.1089/105072502760143827. [DOI] [PubMed] [Google Scholar]
- 6.Gurnell M., Visser T., Beck-Peccoz P. Resistance to thyroid hormone. In: Jameson J.L., De Groot L.J., editors. Endocrinology. 6th ed. Saunders Elsevier; Philadelphia, PA: 2010. pp. 1745–1759. [Google Scholar]
- 7.Astapova I., Hollenberg A.N. The in vivo role of nuclear receptor corepressors in thyroid hormone action. Biochimica Biophysica Acta. 2013;1830:3876–3881. doi: 10.1016/j.bbagen.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vadiveloo T., Donnan P.T., Murphy M.J. Age- and gender-specific TSH reference intervals in people with no obvious thyroid disease in Tayside, Scotland: the thyroid epidemiology, audit, and research study (TEARS) Journal of Clinical Endocrinology and Metabolism. 2013;98:1147–1153. doi: 10.1210/jc.2012-3191. [DOI] [PubMed] [Google Scholar]
- 9.Biondi B. The normal TSH reference range: what has changed in the last decade? Journal of Clinical Endocrinology and Metabolism. 2013;98:3584–3587. doi: 10.1210/jc.2013-2760. [DOI] [PubMed] [Google Scholar]
- [10].Lazarus J.H., Soldin O.P., Evans C. Assessing thyroid function in pregnancy. In: Brent G.A., editor. Thyroid function testing. Springer; New York: 2010. pp. 209–233. [Google Scholar]
- [11].Farwell A.P. Thyroid hormone therapy is not indicated in the majority of patients with the sick euthyroid syndrome. Endocrine Practice. 2008;14:1180–1187. doi: 10.4158/EP.14.9.1180. [DOI] [PubMed] [Google Scholar]
- 12.Kaptein E.M. In: Thyroid hormone metabolism. Hennemann G., editor. Marcel Dekker; New York: 1986. pp. 297–334. [Google Scholar]
- 13.Docter R., Krenning E.P., de Jong M. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clinical Endocrinology. 1993;39:499–518. doi: 10.1111/j.1365-2265.1993.tb02401.x. [DOI] [PubMed] [Google Scholar]
- 14.Warner M.H., Beckett G.J. Mechanisms behind the non-thyroidal illness syndrome: an update. Journal of Endocrinology. 2010;205:1–13. doi: 10.1677/JOE-09-0412. [DOI] [PubMed] [Google Scholar]
- 15.Beckett G.J., Wilkinson E., Rae P.W. The clinical utility of a non-isotopic two-step assay (DELFIA) and an analogue radioimmunoassay (SimulTRAC) for free thyroxine compared. Annals of Clinical Biochemistry. 1991;28:335–344. doi: 10.1177/000456329102800404. [DOI] [PubMed] [Google Scholar]
- 16.Beckett G.J. Thyroid function and thyroid function tests in non-thyroidal illness. CPD Bulletin: Clinical Biochemistry. 2006;7:107–116. [Google Scholar]
- 17.Wartofsky L., Burman K.D. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome”. Endocrine Reviews. 1982;3:164–217. doi: 10.1210/edrv-3-2-164. [DOI] [PubMed] [Google Scholar]
- 18.De Groot L.J. Dangerous dogmas in medicine: the nonthyroidal illness syndrome. Journal of Clinical Endocrinology and Metabolism. 1999;84:151–164. doi: 10.1210/jcem.84.1.5364. [DOI] [PubMed] [Google Scholar]
- 19.Arem R., Cusi K. Thyroid function testing in psychiatric illness: usefulness and limitations. Trends in Endocrinology and Metabolism. 1997;8:282–287. doi: 10.1016/s1043-2760(97)00093-3. [DOI] [PubMed] [Google Scholar]
- 20.Fliers E., Guldenaar S.E., Wiersinga W.M. Decreased hypothalamic thyrotropin-releasing hormone gene expression in patients with nonthyroidal illness. Journal of Clinical Endocrinology and Metabolism. 1997;82:4032–4036. doi: 10.1210/jcem.82.12.4404. [DOI] [PubMed] [Google Scholar]
- 21.Jirasakuldech B., Schussler G.C., Yap M.G. A characteristic serpin cleavage product of thyroxine-binding globulin appears in sepsis sera. Journal of Clinical Endocrinology and Metabolism. 2000;85:3996–3999. doi: 10.1210/jcem.85.11.6966. [DOI] [PubMed] [Google Scholar]
- 22.Boelen A., Kwakkel J., Wieland C.W. Impaired bacterial clearance in type 3 deiodinase-deficient mice infected with Streptococcus pneumoniae. Endocrinology. 2009;150:1984–1990. doi: 10.1210/en.2008-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St Germain D.L., Galton V.A., Hernandez A. Minireview: defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology. 2009;150:1097–1107. doi: 10.1210/en.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lado-Abeal J., Romero A., Castro-Piedras I. Thyroid hormone receptors are down-regulated in skeletal muscle of patients with non-thyroidal illness syndrome secondary to non-septic shock. European Journal of Endocrinology. 2010;163:765–773. doi: 10.1530/EJE-10-0376. [DOI] [PubMed] [Google Scholar]
- 25.van der Poll T., Romijn J.A., Wiersinga W.M. Tumor necrosis factor: a putative mediator of the sick euthyroid syndrome in man. Journal of Clinical Endocrinology and Metabolism. 1990;71:1567–1572. doi: 10.1210/jcem-71-6-1567. [DOI] [PubMed] [Google Scholar]
- 26.Hamblin P.S., Dyer S.A., Mohr V.S. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. Journal of Clinical Endocrinology and Metabolism. 1986;62:717–722. doi: 10.1210/jcem-62-4-717. [DOI] [PubMed] [Google Scholar]
- 27.Brent G.A. Maternal thyroid function: interpretation of thyroid function tests in pregnancy. Clinical Obstetrics and Gynecology. 1997;40:3–15. doi: 10.1097/00003081-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Lazarus J.H. Thyroid function in pregnancy. British Medical Bulletin. 2011;97:137–148. doi: 10.1093/bmb/ldq039. [DOI] [PubMed] [Google Scholar]
- 29.Anckaert E., Poppe K., Van Uytfanghe K. FT4 immunoassays may display a pattern during pregnancy similar to the equilibrium dialysis ID-LC/tandem MS candidate reference measurement procedure in spite of susceptibility towards binding protein alterations. Clinica Chimica Acta. 2010;411:1348–1353. doi: 10.1016/j.cca.2010.05.032. [DOI] [PubMed] [Google Scholar]
- [30].Morris J.C. How do you approach the problem of TSH elevation in a patient on high-dose thyroid hormone replacement? Clinical Endocrinology. 2009;70:671–673. doi: 10.1111/j.1365-2265.2009.03536.x. [DOI] [PubMed] [Google Scholar]
- 31.Walker J.N., Shillo P., Ibbotson V. A thyroxine absorption test followed by weekly thyroxine administration: a method to assess non-adherence to treatment. European Journal of Endocrinology. 2013;168:913–917. doi: 10.1530/EJE-12-1035. [DOI] [PubMed] [Google Scholar]
- [32].Barbesino G. Drugs affecting thyroid function. Thyroid. 2010;20:763–770. doi: 10.1089/thy.2010.1635. [DOI] [PubMed] [Google Scholar]
- 33.Haugen B.R. Drugs that suppress TSH or cause central hypothyroidism. Best Practice & Research Clinical Endocrinology & Metabolism. 2009;23:793–800. doi: 10.1016/j.beem.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tahboub R., Arafah B.M. Sex steroids and the thyroid. Best Practice & Research Clinical Endocrinology & Metabolism. 2009;23:769–780. doi: 10.1016/j.beem.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Newnham H.H., Hamblin P.S., Long F. Effect of oral frusemide on diagnostic indices of thyroid function. Clinical Endocrinology. 1987;26:423–431. doi: 10.1111/j.1365-2265.1987.tb00799.x. [DOI] [PubMed] [Google Scholar]
- [36].Stockigt J.R., Lim C.F. Medications that distort in vitro tests of thyroid function, with particular reference to estimates of serum free thyroxine. Best Practice & Research Clinical Endocrinology & Metabolism. 2009;23:753–767. doi: 10.1016/j.beem.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Schatz D.L., Sheppard R.H., Steiner G. Influence of heparin on serum free thyroxine. Journal of Clinical Endocrinology and Metabolism. 1969;29:1015–1022. doi: 10.1210/jcem-29-8-1015. [DOI] [PubMed] [Google Scholar]
- 38.Mendel C.M., Frost P.H., Cavalieri R.R. Effect of free fatty acids on the concentration of free thyroxine in human serum: the role of albumin. Journal of Clinical Endocrinology and Metabolism. 1986;63:1394–1399. doi: 10.1210/jcem-63-6-1394. [DOI] [PubMed] [Google Scholar]
- 39.Mendel C.M., Frost P.H., Kunitake S.T. Mechanism of the heparin-induced increase in the concentration of free thyroxine in plasma. Journal of Clinical Endocrinology and Metabolism. 1987;65:1259–1264. doi: 10.1210/jcem-65-6-1259. [DOI] [PubMed] [Google Scholar]
- 40.Jaume J.C., Mendel C.M., Frost P.H. Extremely low doses of heparin release lipase activity into the plasma and can thereby cause artifactual elevations in the serum-free thyroxine concentration as measured by equilibrium dialysis. Thyroid. 1996;2:79–83. doi: 10.1089/thy.1996.6.79. [DOI] [PubMed] [Google Scholar]
- [41].Stevenson H.P., Archbold G.P., Johnston P. Misleading serum free thyroxine results during low molecular weight heparin treatment. Clinical Chemistry. 1998;44:1002–1007. [PubMed] [Google Scholar]
- 42.Gurnell M., Halsall D.J., Chatterjee V.K. What should be done when thyroid function tests do not make sense. Clinical Endocrinology. 2011;74:673–678. doi: 10.1111/j.1365-2265.2011.04023.x. [DOI] [PubMed] [Google Scholar]
- 43.Midgley J.E. Direct and indirect free thyroxine assay methods: theory and practice. Clin Chem. 2001;47:1353–1363. [PubMed] [Google Scholar]
- [44].Beck-Peccoz P., Persani L., Mannavola D. Pituitary tumours: TSH-secreting adenomas. Best Practice & Research Clinical Endocrinology & Metabolism. 2009;23:597–606. doi: 10.1016/j.beem.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Socin H.V., Chanson P., Delemer B. The changing spectrum of TSH-secreting pituitary adenomas: diagnosis and management in 43 patients. European Journal of Endocrinology. 2003;148:433–442. doi: 10.1530/eje.0.1480433. [DOI] [PubMed] [Google Scholar]
- 46.Beck-Peccoz P., Brucker-Davis F., Persani L. Thyrotropin-secreting pituitary tumors. Endocrine Reviews. 1996;17:610–638. doi: 10.1210/edrv-17-6-610. [DOI] [PubMed] [Google Scholar]
- 47.Ónnestam L., Berinder K., Burman P. National incidence and prevalence of TSH-secreting pituitary adenomas in Sweden. Journal of Clinical Endocrinology and Metabolism. 2013;98:626–635. doi: 10.1210/jc.2012-3362. [DOI] [PubMed] [Google Scholar]
- 48.Barkoff M.S., Kocherginsky M., Anselmo J. Autoimmunity in patients with resistance to thyroid hormone. Journal of Clinical Endocrinology and Metabolism. 2010;95:3189–3193. doi: 10.1210/jc.2009-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Idiculla J.M., Beckett G., Statham P.F. Autoimmune hypothyroidism coexisting with a pituitary adenoma secreting thyroid-stimulating hormone, prolactin and alpha-subunit. Annals of Clinical Biochemistry. 2001;38:566–571. doi: 10.1177/000456320103800518. [DOI] [PubMed] [Google Scholar]
- 50.Gurnell M., Rajanayagam O., Barbar I. Reversible pituitary enlargement in the syndrome of resistance to thyroid hormone. Thyroid. 1998;8:679–682. doi: 10.1089/thy.1998.8.679. [DOI] [PubMed] [Google Scholar]
- 51.Mannavola D., Persani L., Vannucchi G. Different responses to chronic somatostatin analogues in patients with central hyperthyroidism. Clinical Endocrinology. 2005;62:176–181. doi: 10.1111/j.1365-2265.2004.02192.x. [DOI] [PubMed] [Google Scholar]
- [52].Gurnell M., Chatterjee V.K. Oxford textbook of endocrinology and diabetes. Oxford University Press; New York, NY: 2011. Thyroid hormone resistance syndrome. [Google Scholar]
- 53.Larsen CC, Dumitrescu AM, Guerra Arguero L, et al. Incidental identification of a thyroid hormone receptor beta (THRB) gene variant in a family with autoimmune thyroid disease. Thyroid, published online before print. http://dx.doi.org/10.1089/thy.2013.0174. [DOI] [PMC free article] [PubMed]
- 54.Dumitrescu A.M., Liao X.H., Best T.B. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74:168–175. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friesema E.C., Grueters A., Biebermann H. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364:1435–1437. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- 56.Dumitrescu A.M., Liao X.H., Abdullah M.S. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nature Genetics. 2005;37:1247–1252. doi: 10.1038/ng1654. [DOI] [PubMed] [Google Scholar]
- 57.Schoenmakers E., Agostini M., Mitchell C. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. Journal of Clinical Investigation. 2010;120:4220–4235. doi: 10.1172/JCI43653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bochukova E., Schoenmakers N., Agostini M. A mutation in the thyroid hormone receptor alpha gene. New England Journal of Medicine. 2012;366:243–249. doi: 10.1056/NEJMoa1110296. [DOI] [PubMed] [Google Scholar]
- 59.van Mullem A.A., Chrysis D., Eythimiadou A. Clinical phenotype of a new type of thyroid hormone resistance caused by a mutation of the TRα1 receptor: consequences of LT4 treatment. Journal of Clinical Endocrinology and Metabolism. 2013;98:3029–3038. doi: 10.1210/jc.2013-1050. [DOI] [PubMed] [Google Scholar]
- 60.Moran C., Schoenmakers N., Agostini M. An adult female with resistance to thyroid hormone mediated by defective thyroid hormone receptor α. Journal of Clinical Endocrinology and Metabolism. 2013 doi: 10.1210/jc.2013-2215. Published online before print August 12. [DOI] [PubMed] [Google Scholar]
- 61.Klee G.G. Harmonization and standardization of thyroid function tests. Clinical Chemistry. 2010;56:879–880. doi: 10.1373/clinchem.2010.145540. [DOI] [PubMed] [Google Scholar]
- 62.Van Houcke S.K., Van Uytfanghe K., Shimizu E. IFCC international conventional reference procedure for the measurement of free thyroxine in serum: International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Working Group for Standardization of Thyroid Function Tests (WG-STFT)(1) Clinical Chemistry and Laboratory Medicine. 2011;49:1275–1281. doi: 10.1515/CCLM.2011.639. [DOI] [PubMed] [Google Scholar]
- 63.Liwanpo L., Hershman J.M. Conditions and drugs interfering with thyroxine absorption. Best Practice & Research Clinical Endocrinology & Metabolism. 2009;23:781–792. doi: 10.1016/j.beem.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Medicines and healthcare products regulatory agency. 2012. www.mhra.gov.uk/NewsCentre/Pressreleases/CON143688 [accessed 04.04.13] [Google Scholar]