Abstract
The active form of vitamin D, 1,25-dihydroxyvitamin D [1,25(OH)2D3] is synthesized by the 1α-hydroxylase, which is encoded by the Cyp27B1 gene. Using transgenic mice that have replaced the Cyp27B1 gene with the bacterial lacZ reporter gene (β-galactosidase), the inflammatory conditions that induce Cyp27B1 in the immune system were probed. A variety of stimuli including lipopolysaccharide, anti-CD3 or PMA/ionomycin were used to stimulate splenocytes and bone marrow derived macrophage in vitro. Only anti-CD3 stimulation resulted in a low induction of β-galactosidase activity in the spleen, indicating that T cells might be a source of Cyp27B1. In vivo, challenge with lipopolysaccharide, α-galactosylceramide, and Listeria monocytogenes failed to induce β-galactosidase activity outside of the kidneys. During more prolonged and severe inflammation there was staining in both the lungs and the gastrointestinal tract for β-galactosidase. Furthermore, wild type reconstitution of the hematopoietic cell population in Cyp27B1 KO mice protected the mice from experimental colitis. T cell production of Cyp27B1 activity was shown to be from the CD8+ but not the CD4+ T cell population. CD8+ T cells expressed the reporter gene only after 48h of stimulation. The data is consistent with a model where CD8+ T cells are activated to produce Cyp27B1 and 1,25(OH)2D3 that serves to turn off the local immune response.
Keywords: Vitamin D, Cyp27B1, T cells, inflammation
Introduction
Vitamin D is a steroid hormone/nutrient that is found in the diet or synthesized in the skin after exposure to UVB light [1]. Vitamin D is inactive and hydroxylated in the liver to become 25-hydroxyvitamin D3 (25(OH)D3), which is the major circulating form of vitamin D. 25(OH)D3 is further converted into the active form of vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] by the 1α-hydroxylase enzyme (Cyp27B1 gene) [2]. 1,25(OH)2D3 binds to the nuclear vitamin D receptor (VDR) to regulate gene transcription crucial for calcium and phosphate homeostasis [3]. Since expression of the VDR was identified in immune cells [4, 5], accumulating evidence suggests an immuno-modulatory role of vitamin D particularly during inflammatory diseases.
The kidney is the major endocrine organ to express Cyp27B1 [6]. Experimentally, it has been shown that nephrectomized animals and humans prior to kidney transplantation failed to produce measurable 1,25(OH)2D3 [7, 8]. More recently, several different groups have reported mRNA for Cyp27B1 and/or polyclonal antibody staining of the 1α-hydroxylase in several extra-renal tissues such as the skin, gastrointestinal tract, and bone during normal physiological conditions [9–11]. Conversely, others have shown that Cyp27B1 is expressed only in the kidney of healthy animals and humans [8, 12–15] and the placenta of pregnant females [15, 16]. Thus, there is yet no strong evidence showing extra-renal Cyp27B1 activity under normal physiological conditions.
Renal Cyp27B1 production is mainly regulated by PTH, calcium, and phosphate homeostasis [17, 18]. When circulating calcium or phosphate levels decrease, PTH is secreted to induce Cyp27B1 production from the kidney proximal tubules [2]. 1,25(OH)2D3 then stimulates calcium or phosphate absorption from the intestine and promotes reabsorption of urinary calcium/phosphate and/or resorption of bone [19]. Elevated 1,25(OH)2D3 levels negatively regulates Cyp27B1 production [20]. In contrast to renal Cyp27B1, PTH stimulation or the levels of calcium and phosphate do not affect Cyp27B1 expression in immune cells [21–24]. In vitro, human monocytes/macrophages and dendritic cells have been reported to express 1α-hydroxylase mRNA and protein upon stimulation with pathogen-associated molecular patterns including toll-like receptor (TLR) 4 or TLR1/2 ligands [25–29]. Inflammatory stimuli including interferon (IFN)-γ and lipopolysaccharide (LPS) that converge through several signaling pathways (JAK/STAT, p38 MAPK, and NK-KB) have been shown to induce Cyp27B1 in monocytes [26]. Together, the studies suggest that extra-renal Cyp27B1 production may be regulated in immune cells in a manner that is independent of renal Cyp27B1 regulation.
In vivo, extra-renal production of Cy27B1 has been convincingly demonstrated in very sick sarcoidosis patients [30]. In 1981, an anephric sarcoidosis patient showed definitively that the immune system and macrophages in particular could produce the 1α-hydroxylase and 1,25(OH)2D3 [30]. Macrophages from the anephric sarcoidosis patient but not other patients with lung disease was identified as the source of the extra-renal 1α-hydroxylase ex vivo [31]. Granulomatous diseases of the lung and the gastrointestinal tract (sarcoidosis and Crohn’s disease) suggest that Cyp27B1 is expressed in the immune system during periods of severe illness [12, 32–34]. Hypercalcemia is associated with granulomatous disease even though vitamin D status is low [35]. The resolution of hypercalcemia occurs with the use of immune suppressants (corticosteroids) and the leads to normal serum calcium levels and improvement in the symptoms of the granulomatous disease [35].
Here, the inflammatory signals inducing Cyp27B1 activity in the immune system was investigated using the transgenic Cyp27B1 (Cyp) knockout (KO) mice with the bacterial LacZ reporter under the control of the Cyp27B1 promoter [15]. Mouse macrophages could not be stimulated in vitro to produce Cyp27B1 even when using LPS. In vitro, anti-CD3 stimulation significantly elevated IFN-γ production and Cyp27B1 promoter activity in splenocytes, suggesting the induction of Cyp27B1 in T cells. Purified CD8+ but not CD4+ T cells were shown to be the source of Cyp27B1. Whole mount staining of β-galactosidase in mice with inflammatory bowel disease (IBD) (dextran sodium sulfate (DSS) and IL-10/Cyp27B1 double knockout (DKO) mice showed spotty staining of the small intestine (SI) and large intestines, in 5–30% of the Cyp transgenic but not in wild-type (WT) or IL-10 KO control mice. Lungs and lymph nodes showed induction of β-galactosidase activity in a chronic lung inflammation model but only in 38% of DKO mice and not in any of the Cyp KO mice. In vivo evidence for an important role for Cyp27B1 in hematopoietic cells was demonstrated by the protection of Cyp KO mice from DSS colitis when they were reconstituted with WT bone marrow (BM) cells. The immune system does produce the 1α-hydroxylase and in the mouse CD8+ but not CD4+ T cells or macrophage are sources of Cyp27B1.
Methods and Materials
Mice and diet
Age and sex-match ed C57BL/6 WT, IL-10 KO, Cyp KO, and DKO mice were produced and housed at the Pennsylvania State University (University Park, PA). Cyp KO breeders were a gift from Dr. Hector DeLuca (University of Wisconsin, Madison, WI). For some experiments mice were ip injected with LPS from Escherichia coli 0111:B4 (16 mg/kg) (Sigma-Aldrich, St. Louis, MO) or 2μg of α-galactosylceramide (Sigma-Aldrich). All of the experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the Pennsylvania State University.
Antibodies
Cells were stained with PE CD8β (eBiosciences, San Diego, CA), PECy5 CD4, PE CD45.1, FITC CD45.2, and PECy5 TCRβ (BD Biosciences, San Jose, CA), analyzed on a FC500 bench top cytometer (Beckman Coulter, Brea, CA), and further evaluated with Flowjo 7.6.1 software (Tree Star, Inc., Ashland, OR).
Cell isolation and culture
Spleens were homogenized and lysed with red blood cell lysis buffer to obtain single-cell suspensions. For some experiments, CD4+ or CD8+ T cells from the spleen were purified using mouse CD4 or CD8 cell recovery column kits following the manufacturer’s instructions (Cedarlane Laboratories Ltd, Burlington, NC). Enrichment of CD4 and CD8 cells was only partial using the columns (>80% CD4 and >60% CD8). Additional purifications using CD4 and CD8 antibodies and a Cytopeia Influx cell sorter (BD Bioscience) achieved >99% CD4 and CD8 purities. BM cells were isolated from the femurs and cultured in DMEM supplemented with L929 conditioned media for 7 days as described [36]. Purity of the BM derived macrophage (BMDM) populations were >90% macrophages.
Cultures included the following concentrations of various stimuli: 0.5 μg/ml of LPS, or 10 ng/ml PMA and 2.5 μg/ml ionomycin, or 0.5 μg/ml anti-CD3 alone or 0.5 μg/ml anti-CD3 with 5 μg/ml anti-CD28 (BD Pharmingen, San Diego, CA) in RPMI 1640-C containing 10% FBS (Equitech-Bio, Inc, Kerrville, TX), 2 mM L-glutamine, 5 mM β-mercaptoethanol (Invitrogen, Carlsbad, CA), and 10 μg/ml gentamycin (Teknova, Hollister, CA). BMDM were stimulated with or without 0.1 μg/ml of LPS in DMEM containing 10% FBS, sodium pyruvate, nonessential amino acids (Mediatech, Inc, Manassas, VA), 2 mM L-glutamine, and 10 μg/ml gentamycin (Teknova) (DMEM). Supernatants were collected for cytokine detection, and cells were lysed for protein assays and β-galactosidase assays. Production of IL-1β, IL-6, IFN-γ, IL-4, and IL-17 (BD Biosciences) were measured by ELISAs following the manufacturer’s instructions.
β-galactosidase activity
Whole organ staining of kidney, lung, and intestinal tissues for β-galactosidase were done as follows. Heart perfusion with PBS and 4% paraformaldehyde was done. Fecal matter in gastrointestinal samples was thoroughly flushed with PBS. Tissues were cut longitudinally and stained with X-gal (Invitrogen) as previously described [15]. Briefly, tissues were fixed in 4% paraformaldehyde at 4 °C for 2 h and then rinsed with permeabilization buffer. Fixed tissues were stained in 1mg/ml of X-gal at 37 °C overnight. Tissues were rinsed with PBS and fixed with 4% paraformaldehyde for observation.
Whole kidney, lung, and liver tissues were homogenized and lysed for protein extraction. β-galactosidase activity was measured by β-galactosidase luciferase reporter system (Applied Biosystems, Foster City, CA). Protein assays were done to quantitate the amount of protein in the sample using BCA protein assay (Thermo Scientific, Rockford, IL). Luciferase activity was normalized to the amount of protein in the samples.
IBD models
Colitis was induced with DSS as previously described [37]. Briefly, mice were administrated 3.5% DSS in drinking water ad libitum for 5 days and resumed on water. Body weight (BW) of mice was tracked every day, and mice that had lost more than 20% of their BW were euthanized for humane reasons. Colonic length and blood score were measured following sacrifice. Blood score was assessed as follows: 0- no visible blood in the entire colon, 1- blood detected in less than 2/3 of the colon, 3- blood visible throughout the entire colon. Kidneys, SI, and colons were collected at day 5 post DSS for X-gal staining. Distal colons were fixed in 4% paraformaldehyde and sent for hematoxylin/eosin staining and scored blindly by two investigators as described previously [37].
IBD was monitored in the IL-10 KO as previously described [38]. Piroxicam (0.24 g/kg diet) (Sigma-Aldrich) was administered in the diet for 3 days to trigger the onset of colitis symptoms in IL-10 KO and DKO mice [39]. Kidneys, SI, and colons were collected for X-gal staining. Histopathogical sections of SI and distal colons were scored blindly by two investigators as described previously (49).
SR exposure
Saccharopolyspora rectivirgula (SR, ATCC 29024) was cultured and processed as described (31). SR was heat killed for 2 h at 90 °C. Heat killed SR was cultured and confirmed negative for group and β-galactosidase activity. Mice were intra-nasally inoculated under light anesthesia with 150 μg of heat killed SR or sterile PBS for 3 consecutive days per wk for 5 wks. Mice were sacrificed 24 h after the last inoculation (31). Lungs were fixed, stained and scored as previously [40].
BM transplantation
BM cells from CD45.1 WT mice were transferred into sublethally irradiated CD45.2 WT and CD45.2 Cyp KO recipient mice by iv injection. Reconstitution was assessed at 6 wks following reconstitution. DSS was induced in mice as described above and when the mice were 14 wks old. Intestinal intraepithelial lymphocytes were isolated from the SI as described [41] to assess the reconstitution of the gut.
Statistical analysis
For statistical analysis, one-way ANOVA and two-way ANOVA with Bonferroni post-tests were used. P < 0.05 was considered significant. Data were analyzed using Prism 6.0 statistical software (GraphPad Software, La Jolla, CA).
Results
CD3 stimulation but not LPS stimulation induces Cyp27B1 in vitro
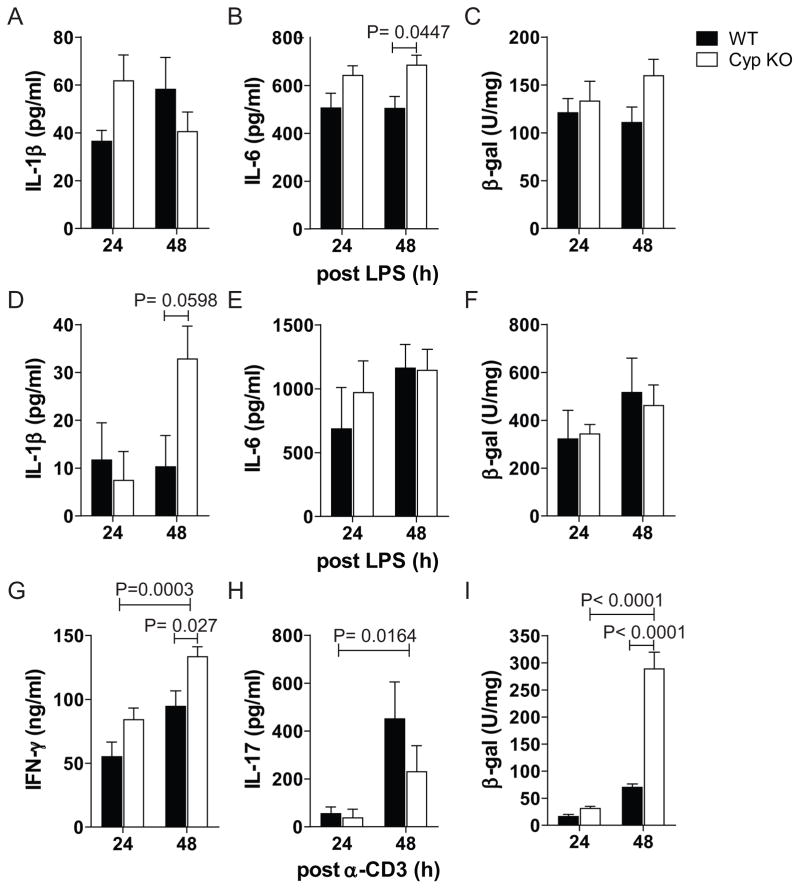
LPS induced splenocytes to produce IL-1β and IL-6 (Fig. 1A and B). The amount of IL-1β secreted was not different from cultures of WT or Cyp KO splenocytes while the amount of IL-6 was higher from the Cyp KO splenocytes than WT (Fig. 1A and 1B). There was no effect of LPS stimulation on the induction of β-galactosidase in the WT and Cyp KO splenocytes (Fig. 1C). BMDM from WT and Cyp KO mice were grown and both the WT and Cyp KO BMDM were 91% macrophage (F4/80+CD11b+). LPS stimulation of the BMDM showed induction of IL-1β, IL-6, and IL-12 (Fig. 1D, E and data not shown). IL-1β was higher but not significantly higher at 48h after LPS stimulation of Cyp KO BMDM compared to WT BMDM (Fig. 1D). Similar amounts of IL-6 and IL-12 were produced from LPS stimulated BMDM from WT and Cyp KO cultures (Fig. 1E and data not shown). β-galactosidase activity (Cyp27B1 induction) was not induced in the Cyp KO BMDM by LPS (Fig. 1F).
Figure 1. Stimulation with CD3 but not LPS induces Cyp27B1 in vitro.
Production of A) IL-1β, B) IL-6 and C) β-galactosidase (β-gal) activity in splenocytes 24h and 48h post-LPS stimulation. Production of D) IL-1β E) IL-6 and F) β-gal activity in BMDM 24h and 48h post LPS stimulation. Production of G) IFN-γ, H) IL-17 and I) β-gal activity of splenocytes from WT and Cyp KO mice 24h and 48h post anti-CD3 stimulation. Values are the mean ± SEM of n=3–4 per group. Representative data is shown from one of three independent experiments.
Production of IFN-γ and IL-17 were significantly higher at 48h compared to 24h after anti-CD3 stimulation (Fig. 1G and H). Cyp KO splenocytes stimulated with anti-CD3 produced significantly higher amounts of IFN-γ than WT splenocytes at 48h post-stimulation (Fig. 1G). Conversely, there was no difference in IL-17 production (Fig. 1H) and undetectable IL-4 production in the WT and Cyp KO stimulated cells (data not shown). β-galactosidase activity in Cyp KO splenocytes was low and not different than WT splenocytes 24h after anti-CD3 stimulation (Fig. 1I). β-galactosidase activity from Cyp KO splenocytes was higher at 48h than 24h of anti-CD3 stimulation, and there was a significant increase in β-galactosidase activity in the Cyp KO splenocytes compared to WT splenocytes (Fig. 1I). In vitro cultures that used PMA/ionomycin stimulation of Cyp KO and WT splenocytes showed induction of IL-4 and IFN-γ but no induction of β–galactosidase activity in the Cyp KO splenocytes (data not shown). In vitro stimulation with anti-CD3 stimulation but not LPS or PMA/ionomycin induces Cyp27B1 expression in the spleen.
Acute systemic inflammation and induction of Cyp27B1 promoter activity
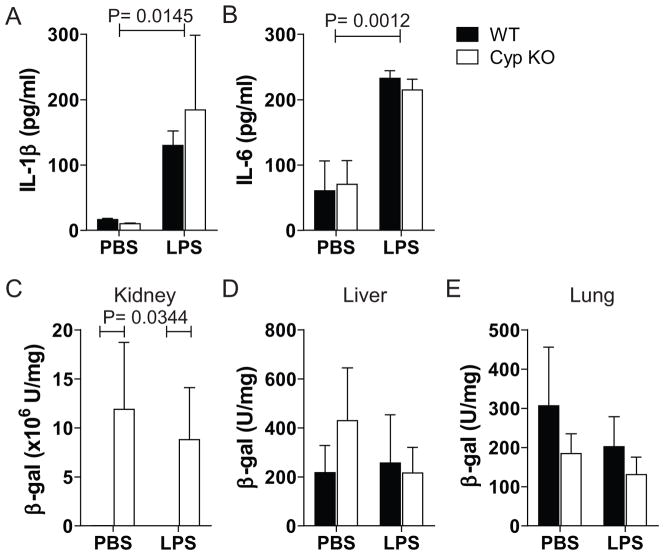
LPS injection (in vivo) significantly induced IL-1β and IL-6 production in the serum of WT and Cyp KO mice (Fig. 2A and B). There was no difference in the amounts of either IL-1β or IL-6 detectable in the serum of the WT and Cyp KO mice (Fig. 2A and B). Kidney expression of β-galactosidase was high and only detected in the Cyp KO mice regardless of whether they received a PBS or LPS challenge (Fig 2C). Low levels of β-galactosidase activity were detected in the liver and lung of both WT and Cyp KO mice (Fig. 2D and E). Expression of β-galactosidase was only found in the kidney of Cyp KO mice and the expression of β-galactosidase in the Cyp KO kidney was unaffected by LPS injection. IL-10/Cyp DKO mice produced significantly higher serum IL-1β and IL-6 levels than Cyp KO mice injected with LPS (data not shown). The DKO mice produced β-galactosidase activity in the kidney but not in other tissues (liver and lung, Table 1). The amount of β–galactosidase activity in the kidney of DKO mice was the same in LPS and PBS injected mice (data not shown).
Figure 2. Cyp27B1 was not induced during LPS-induced acute systemic inflammation.
Serum A) IL-1β and B) IL-6 and β-gal activity of C) kidney, D) lung, and E) liver in WT and Cyp KO 24h post stimulation with LPS or PBS. Values are the mean ± SEM of n=4 mice per group. Representative data is shown from one of three independent experiments.
Table 1.
In vivo Cyp27B1 induction during acute and chronic inflammation.
| Stimuli | Route of Administration | Genotype | Tissues | Cyp27B1 Induction† |
|---|---|---|---|---|
| LPS | Intraperitoneal | Cyp KO | Liver, lung | 0/9 (0%) |
| α-Galactosylceramide | Intraperitoneal | Cyp KO | Liver | 0/6 (0%) |
| L. monocytogenes | Intraperitoneal | Cyp KO | Spleen | 0/2 (0%) |
| DSS | Oral | Cyp KO | Intestines | 6/22 (27%) |
| S. rectivirgula | Intranasal | Cyp KO | Lung | 0/15 (0%) |
| S. rectivirgula | Intranasal | DKO | Lung | 3/8 (38%) |
| Piroxicam | Oral | DKO | Intestines | 3/9 (33%) |
1Cyp KO and IL-10/Cyp DKO mice were exposed to various stimuli to elicit inflammation in different tissues.
2 The ratio of the number of mice showing β-galactosidase staining in the tissue per numbers of mice tested.
WT and Cyp KO mice were infected with Listeria monocytogenes intraperitoneally. Only the kidney of the infected Cyp KO mice showed β–galactosidase activity (Table 1). NKT cell activation using injections of α-galactosylceramide in vivo increased IL-4 and IFN-γ secretion in the serum of both WT and Cyp KO mice (data not shown). The α-galactosylceramide injections did not induce β-galactosidase activity in the liver of WT and Cyp KO mice (Table 1). Extra-renal β–galactosidase activity is not detected following acute in vivo infection, α-galactosylceramide, or LPS induced inflammation (Table 1).
Acute intestinal inflammation and induction of Cyp27B1
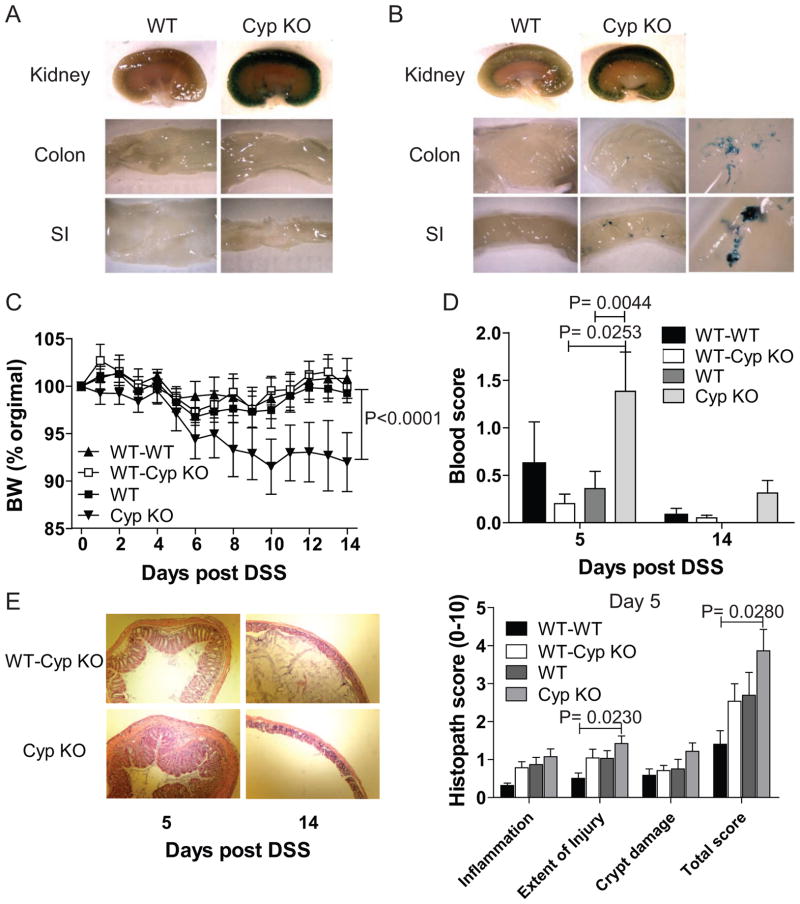
The kidney, colon and SI of WT mice were negative for β–galactosidase activity (Fig. 3A). Consistent with the literature [15] untreated healthy Cyp KO mice had β–galactosidase positive staining in the kidneys but not in the colon or SI (Fig. 3A). Five days after induction of intestinal inflammation with DSS the WT kidney, colon and SI were negative for β–galactosidase activity (Fig. 3B). As expected the kidney of all of the Cyp KO mice stained positive for β–galactosidase activity (Fig. 3B). In addition, after 5 days of DSS, Cyp KO mice had spotty staining in 6/22 SI and 3/22 colons evaluated (Fig. 3B and Table 1). Acute inflammation in the intestine induced Cyp27B1 expression in the SI and colon.
Figure 3. WT BM protects Cyp KO mice from DSS induced intestinal inflammation.
Whole mount staining for β-gal activity (blue) in the kidney, colon, and SI of WT and Cyp KO mice. A) Staining in a WT and Cyp KO mouse before and B) after DSS treatment for 5d. Cyp KO magnification (Mag) shows the 6-fold magnification of the staining of the Cyp KO colon and SI. Table 1 summarizes the staining results from all of the mice. C) Percentage of BW change, D) colonic blood scores and E) representative histopathological sections and scores of the distal colon from mice treated with DSS. Values are the mean ± SEM of n=3–19 mice per time-point and group. Representative data is shown from one of two independent experiments. BW change of Cyp KO mice is significantly different from BW change of WT-WT, WT-Cyp KO, and WT mice (P<0.0001). The Cyp KO blood scores were significantly different than WT-Cyp KO and WT. The WT-WT injury and total histopathology scores were found to be significantly different than Cyp KO. All other values were not different from other groups using one way ANOVA with Bonferroni post-hoc testing and P<0.05.
WT BM protects Cyp KO mice from DSS colitis
Cyp KO mice lost significantly more weight than WT mice following induction of DSS colitis (Fig. 3C). Cyp KO mice had significantly more blood in the colon than their WT counterparts at d5 post-DSS (Fig. 3D). The ability of WT BM to protect Cyp KO mice was tested. WT and Cyp KO mice were sub-lethally irradiated and reconstituted with WT BM (WT-WT, and WT-Cyp KO). Reconstitution of the blood mononuclear cells was over 90% (92–95%) while reconstitution of the gut was considerably less (from 43–45% of the mononuclear cells in the SI were of donor origin, Supplemental Fig. 1). WT BM protected Cyp KO mice from weight loss following induction of DSS colitis (Fig. 3C). In addition, histopathology of colons from Cyp KO and WT-Cyp KO mice at d5 post-DSS showed that there was less hyperplasia and crypt damage in the WT-Cyp KO mice than the Cyp KO mice (Fig. 3E). The reduced severity of DSS colitis was also evident in the lower blood scores at d5 in the WT-Cyp KO mice compared to the un-reconstituted Cyp KO mice. WT BM protects Cyp KO mice from DSS colitis.
SR induced lung inflammation induces β–galactosidase in DKO but not Cyp KO mice
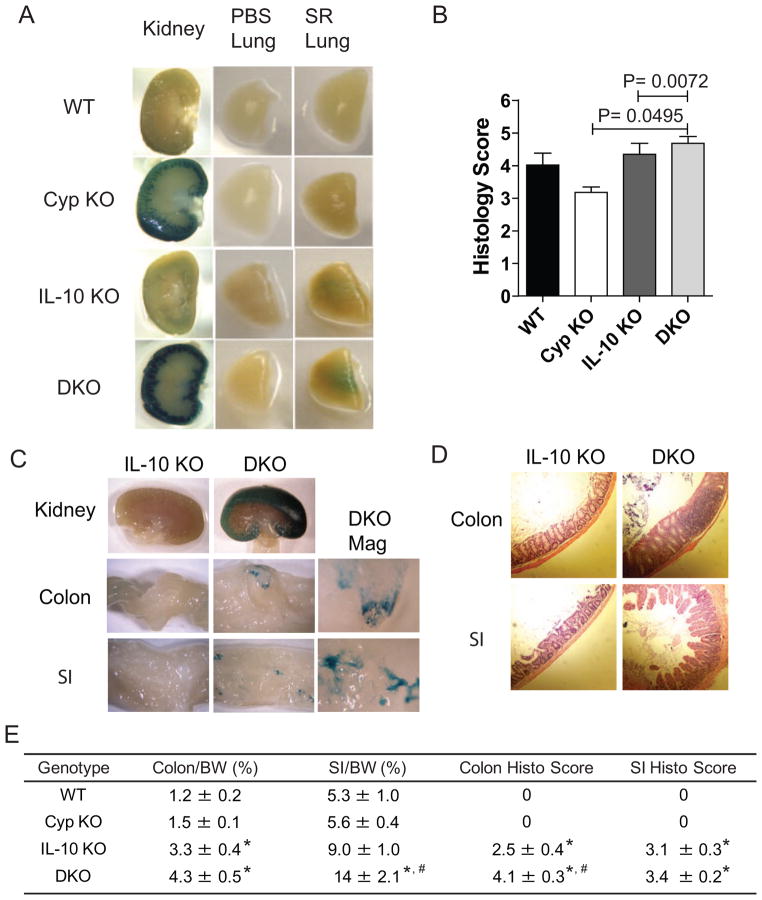
Chronic lung inflammation was induced using SR mediated hypersensitivity pneumonitis (5). Live SR produced β–galactosidase activity and therefore experiments were done using heat-killed and β–galactosidase activity negative SR. The kidneys from Cyp KO and DKO mice stained positive for β–galactosidase activity whether or not they came from mice exposed to SR (Fig. 4A and Supplemental Fig. 1B). Lungs from mice exposed to PBS were negative for β–galactosidase activity (Fig. 4A). In addition, lungs from Cyp KO mice exposed to SR were negative for β–galactosidase activity (Fig. 4A and Table 1). The lungs from the SR exposed DKO mice expressed low amounts of β–galactosidase activity (Fig. 4A). Evaluation of the severity of SR induced lung inflammation showed that WT and Cyp KO mice had significant inflammation but there was no difference in the severity of inflammation between WT and Cyp KO mice (Fig. 4B and Supplementary Fig. 1C). IL-10 KO and DKO mice had significantly higher histopathology scores in the lung following SR exposure compared to Cyp KO and WT mice but the IL-10 KO and DKO scores were not different from each other (Fig. 4B and Supplementary Fig. 1C). The low level β-galactosidase activity in the lungs of SR treated DKO mice was associated with higher inflammation in the DKO lung than in the Cyp KO lung.
Figure 4. Cyp27B1 induction during chronic inflammation of the lung and intestines.
Whole mount staining for β-gal activity (blue) in the kidney and lung of PBS or SR inoculated mice. A) Staining in a WT, Cyp KO, IL-10 KO and DKO mouse. Table 1 summarizes the staining results from all of the mice. B) Histopathological scores of lungs from WT, Cyp KO, IL-10 KO and DKO mice post SR inoculations (n=3/group). C) Whole mount staining for β-gal activity in the kidney, colon, and SI of IL-10 KO and DKO mice. DKO Mag shows the 6-fold magnification of the colon and SI. Table 1 summarizes the staining results from all of the mice. D) Representative histopathological sections of the SI and colon from IL-10 KO and DKO mice with IBD. E)Summary of the ratio of the colon or SI weight relative to the BW and histopathological scores of colon and SI (n=4–8/group). Values are the means ± SEMs of 3–8 mice per group. Representative data is shown from one of three independent experiments.
* Significant difference compared with WT and Cyp KO mice; # significant difference compared with IL-10 KO mice (P< 0.05).
Chronic colitis induces Cyp27B1 promoter activity in intestine
IL-10 KO and DKO mice were induced to develop colitis with piroxicam [39]. IL-10 KO kidney, SI and colon were negative for β-galactosidase activity (Fig. 4C). Conversely the kidneys from all of the DKO mice were positive for β-galactosidase activity (Fig. 4C). In addition, there was spotty staining of the SI and colon for β-galactosidase activity (Fig. 4C). Three of 9 SI and 2 of 9 colons were positive for β-galactosidase (Table 1). WT and Cyp KO mice had normal SI that were about 5.3–5.6% of their BW and colons that were 1.2–1.5 % of their BW (Fig. 4E). IL-10 KO and DKO mice showed significant signs of experimental IBD with enlarged SI and colons (Fig. 4E). The DKO mice with IBD had significantly larger SI/BW ratios and larger but not significantly larger colon/BW ratios than IL-10 KO mice (Fig. 4E). Histopathology sections and scores from DKO mice showed more severe inflammation in both the SI and colon than the IL-10 KO mice (Fig. 4D and E). DKO mice develop more severe experimental IBD that results in the expression of Cyp27B1 in the SI and colon.
Selective expression of Cyp27B1 in CD8+ T cells
Resting T cells were negative for β-galactosidase (data not shown). Enriched splenic CD4+ T cells (column purified 80%) and sorted CD4 + T cells (>99% pure) stimulated with CD3 and CD28 antibodies were negative for β-galactosidase activity (Fig. 5A and B). Enriched (65–70%) CD8+ T cells and later highly purified sorted (>99% pure) CD8+ T cells expressed β-galactosidase activity 48h after stimulation and the level of β-galactosidase was significantly higher compared to both WT cells and CD4+ Cyp KO T cells (Fig. 5A and B). Activated CD8+ T cells but not CD4+ T cells express the Cyp27B1 gene.
Figure 5. Cyp27B1 expression in CD8+ but not CD4+ T cells.
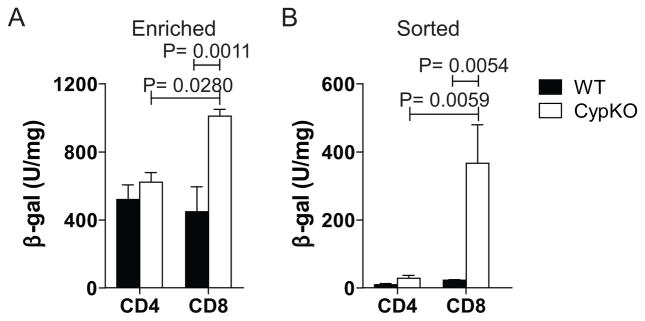
β-gal activity from A) column enriched CD4+ or CD8+ T cells and B) sorted CD4+ or CD8+ T cells stimulated with CD3 antibodies for 48h. Values are the means ± SEMs of 3–8 mice/group Representative data is shown from one of three independent experiments.
Discussion
T cell stimulation through the T cell receptor induced Cyp27B1 activity in vitro, suggesting that T cells are a source of Cyp27B1 in the murine immune system. This finding is consistent with a study indicating that activated T cells expressed Cyp27B1 mRNA and converted 25(OH)D3 into 1,25(OH)2D3 [42]. Our study further identified that activated CD8+ T cells but not CD4+ T cells were induced to express Cyp27B1. Unstimulated CD8+ T cells did not produce Cyp27B1. Our finding that T cells from Cyp KO mice overproduced IFN-γ suggests that the production of 1,25(OH)2D3 by CD8+ T cells is a mechanism by which IFN-γ production is inhibited. IFN-γ promotes the differentiation of CD8+ cytotoxic T cells, T helper-1 cells, and classic inflammatory macrophages [43, 44]. 1,25(OH)2D3 inhibits IFN-γ production directly and indirectly by inhibiting macrophage production of IL-12 [45, 46]. CD8+ T cell produced 1,25(OH)2D3 serves as a mechanism to inhibit T cell proliferation and IFN-γ production 48h after the initial antigen specific immune response has occurred.
The strongest evidence for an in vivo role of immune-derived Cyp27B1 is the protection of Cyp KO mice by WT BM. The only source of 1,25(OH)2D3 in the chimeric mice would be the BM derived cells. Vitamin D is known to regulate the balance between proinflammatory and regulatory T cells to control IBD [47]. It is not clear whether CD8+ T cells are the only cell type that produces the vitamin D 1α-hydroxylase in the mouse. Calcium ionophore or TLR stimulations via pattern recognition receptors has been suggested to induce Cyp27B1 expression in immune cells, particularly monocytes/macrophages and dendritic cells in vitro [24–28, 48]. LPS stimulated murine macrophage did not produce the β-galactosidase reporter in these experiments. Neither did acute and/or short term inflammation in vivo. Therefore the negative result using murine macrophage could reflect a species difference or a limitation with the Cyp27B1 reporter system used for these experiments. The Cyp KO mouse is missing exons 1–8 of the Cyp27B1 gene and all of the introns [15]. Regulation of gene expression can occur outside of the promoter region [49]. It is possible that although endocrine and CD8+ T cells Cyp27B1 gene expression is controlled through the promoter region, macrophage regulation of the Cyp27B1 gene relies on DNA sequences missing in the Cyp KO mice. At a minimum murine CD8+ T cells can produce 1,25(OH)2D3 and it seems likely that other immune cells would under certain circumstances produce the vitamin D 1α-hydroxylase. We have not measured Cyp27B1 enzymatic activity in purified CD8+ T cells but since the expression of the gene is as expected in the kidney we think it is likely that our reporter gene accurately identifies producers of the Cyp27B1.
IL-10 KO mice develop spontaneous chronic IBD and are susceptible to hypersensitivity pneumonitis [50, 51]. Our data show that Cyp27B1 deficiency resulted in significantly more severe IBD symptoms in the gut but no difference in susceptibility to SR mediated inflammation in the lung. Additionally, spotty Cyp27B1 activity was detected in inflamed intestine and lung tissues. Extra-renal production of Cyp27B1 has been suggested in chronic granulomatous diseases of the gut and lung like sarcoidosis and Crohn’s disease in humans [12, 32–34]. There is no mouse model for sarcoidosis and the animal models of IBD replicates several aspects of Crohn’s disease but not the granulomatous lesions common in human patients. Our data support the production of the vitamin D 1α-hydroxylase during chronic inflammation of both the lung and the gut.
For the first time the data clearly show an important biological role for BM as an in vivo extra-renal source of the vitamin D 1α-hydroxylase during disease. In addition, the data point to murine CD8+ T cells as a local producer of Cyp27B1 following activation of the T cells through the T cell receptor. The data do not support immune derived Cyp27B1 in resting immune cells in vitro and/or in healthy mice in vivo. Because of the kinetics of the Cyp27B1 induction and the requirement for activation, the data further suggest that local production of 1,25(OH)2D3 serves to shut the immune response down following antigen specific activation. Immune derived 1,25(OH)2D3 may be central for T cells and other immune cells to resolve and/or turn off an immune response.
Supplementary Material
A) Reconstitution rate of CD45.1 WT BM cells in the blood and SI of WT or Cyp KO recipient mice. Values are the means ± SEMs of 7 mice per group and one representative of two independent experiments. B) Representative histopathological sections of the lungs from WT, Cyp KO, IL-10 KO and DKO mice and C) β-gal activity in the kidneys following PBS or SR inoculation (n=4–10/group). Values are the means ± SEMs of 4–19 mice per group and one representative of three independent experiments.
Acknowledgments
GRANT SUPPORT: Supported by the National Institutes of Health/National Institute of Neurologic and Stroke Grant NS067563 and National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements AT005378.
We thank Dr. Hector DeLuca, University of Wisconsin, Madison WI for the Cyp27B1 KO mice. We thank Jing Chen for statistical help.
Abbreviations
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- 25(OH)D3
25-hydroxyvitamin D3
- BM
bone marrow
- BMDM
bone marrow derived macrophages
- BW
body weight
- Cyp
Cyp27B1
- DSS
dextran sodium sulfate
- DKO
double knockout
- gal
galactosidase
- IFN
interferon
- KO
knockout
- LPS
lipopolysaccharide
- MGN
magnification
- IBD
inflammatory bowel disease
- SI
small intestine
- TLR
toll-like receptor
- VDR
vitamin D receptor
- WT
wild-type
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211:590–3. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 2.Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997;277:1827–30. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- 3.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–49. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 4.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–10. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 5.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 6.Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970;228:764–6. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- 7.Eisman JA, Hamstra AJ, Kream BE, DeLuca HF. 1,25-Dihydroxyvitamin D in biological fluids: a simplified and sensitive assay. Science. 1976;193:1021–3. doi: 10.1126/science.1085035. [DOI] [PubMed] [Google Scholar]
- 8.Haussler MR, Baylink DJ, Hughes MR, Brumbaugh PF, Wergedal JE, Shen FH, et al. The assay of 1alpha, 25-dihydroxyvitamin D3: physiologic and pathologic modulation of circulating hormone levels. Clin Endocrinol (Oxf) 1976;5 (Suppl):151S–65S. doi: 10.1111/j.1365-2265.1976.tb03823.x. [DOI] [PubMed] [Google Scholar]
- 9.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–94. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 10.Liu N, Nguyen L, Chun RF, Lagishetty V, Ren S, Wu S, et al. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology. 2008;149:4799–808. doi: 10.1210/en.2008-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard GA, Turner RT, Sherrard DJ, Baylink DJ. Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3. J Biol Chem. 1981;256:7738–40. [PubMed] [Google Scholar]
- 12.Bosch X. Hypercalcemia due to endogenous overproduction of 1,25-dihydroxyvitamin D in Crohn’s disease. Gastroenterology. 1998;114:1061–5. doi: 10.1016/s0016-5085(98)70327-0. [DOI] [PubMed] [Google Scholar]
- 13.Shultz TD, Fox J, Heath H, 3rd, Kumar R. Do tissues other than the kidney produce 1,25-dihydroxyvitamin D3 in vivo? A reexamination. Proc Natl Acad Sci U S A. 1983;80:1746–50. doi: 10.1073/pnas.80.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeve L, Tanaka Y, DeLuca HF. Studies on the site of 1,25-dihydroxyvitamin D3 synthesis in vivo. J Biol Chem. 1983;258:3615–7. [PubMed] [Google Scholar]
- 15.Vanhooke JL, Prahl JM, Kimmel-Jehan C, Mendelsohn M, Danielson EW, Healy KD, et al. CYP27B1 null mice with LacZ reporter gene display no 25-hydroxyvitamin D3-1alpha-hydroxylase promoter activity in the skin. Proc Natl Acad Sci U S A. 2006;103:75–80. doi: 10.1073/pnas.0509734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisman Y, Harell A, Edelstein S, David M, Spirer Z, Golander A. 1alpha, 25-Dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in vitro synthesis by human decidua and placenta. Nature. 1979;281:317–9. doi: 10.1038/281317a0. [DOI] [PubMed] [Google Scholar]
- 17.Rost CR, Bikle DD, Kaplan RA. In vitro stimulation of 25-hydroxy cholecalciferol 1alpha-hydroxylation by parathyroid hormone in chick kidney slices: evidence for a role for adenosine 3′,5′-monophosphate. Endocrinology. 1981;108:1002–6. doi: 10.1210/endo-108-3-1002. [DOI] [PubMed] [Google Scholar]
- 18.Friedlander EJ, Henry HL, Norman AW. Studies on the mode of action of calciferol. Effects of dietary calcium and phosphorus on the relationship between the 25-hydroxyvitamin D3-1alpha-hydroxylase and production of chick intestinal calcium binding protein. J Biol Chem. 1977;252:8677–83. [PubMed] [Google Scholar]
- 19.Grant WB, Holick MF. Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev. 2005;10:94–111. [PubMed] [Google Scholar]
- 20.Henry HL. Regulation of the hydroxylation of 25-hydroxyvitamin D3 in vivo and in primary cultures of chick kidney cells. J Biol Chem. 1979;254:2722–9. [PubMed] [Google Scholar]
- 21.Adams JS, Ren SY, Arbelle JE, Horiuchi N, Gray RW, Clemens TL, et al. Regulated production and intracrine action of 1,25-dihydroxyvitamin D3 in the chick myelomonocytic cell line HD-11. Endocrinology. 1994;134:2567–73. doi: 10.1210/endo.134.6.8194484. [DOI] [PubMed] [Google Scholar]
- 22.Reichel H, Koeffler HP, Barbers R, Norman AW. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab. 1987;65:1201–9. doi: 10.1210/jcem-65-6-1201. [DOI] [PubMed] [Google Scholar]
- 23.Hakeda Y, Hiura K, Sato T, Okazaki R, Matsumoto T, Ogata E, et al. Existence of parathyroid hormone binding sites on murine hemopoietic blast cells. Biochem Biophys Res Commun. 1989;163:1481–6. doi: 10.1016/0006-291x(89)91146-7. [DOI] [PubMed] [Google Scholar]
- 24.Yuan JY, Freemont AJ, Mawer EB, Hayes ME. Regulation of 1alpha, 25-dihydroxyvitamin D3 synthesis in macrophages from arthritic joints by phorbol ester, dibutyryl-cAMP and calcium ionophore (A23187) FEBS Lett. 1992;311:71–4. doi: 10.1016/0014-5793(92)81370-2. [DOI] [PubMed] [Google Scholar]
- 25.Overbergh L, Decallonne B, Valckx D, Verstuyf A, Depovere J, Laureys J, et al. Identification and immune regulation of 25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages. Clin Exp Immunol. 2000;120:139–46. doi: 10.1046/j.1365-2249.2000.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 27.Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–90. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 28.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 29.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbour GL, Coburn JW, Slatopolsky E, Norman AW, Horst RL. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med. 1981;305:440–3. doi: 10.1056/NEJM198108203050807. [DOI] [PubMed] [Google Scholar]
- 31.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856–60. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell NH, Stern PH, Pantzer E, Sinha TK, De Luca HF. Evidence that increased circulating 1alpha, 25-dihydroxyvitamin D is the probable cause for abnormal calcium metabolism in sarcoidosis. J Clin Invest. 1979;64:218–25. doi: 10.1172/JCI109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papapoulos SE, Clemens TL, Fraher LJ, Lewin IG, Sandler LM, O’Riordan JL. 1, 25-dihydroxy cholecalciferol in the pathogenesis of the hypercalcaemia of sarcoidosis. Lancet. 1979;1:627–30. doi: 10.1016/s0140-6736(79)91076-6. [DOI] [PubMed] [Google Scholar]
- 34.Stern PH, De Olazabal J, Bell NH. Evidence for abnormal regulation of circulating 1alpha, 25-dihydroxyvitamin D in patients with sarcoidosis and normal calcium metabolism. J Clin Invest. 1980;66:852–5. doi: 10.1172/JCI109924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albright F, Carroll EL, Dempsey EF, Henneman PH. The cause of hypercalcuria in sarcoid and its treatment with cortisone and sodium phytate. J Clin Invest. 1956;35:1229–42. doi: 10.1172/JCI103378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicaise P, Gleizes A, Sandre C, Forestier F, Kergot R, Quero AM, et al. Influence of intestinal microflora on murine bone marrow and spleen macrophage precursors. Scand J Immunol. 1998;48:585–91. doi: 10.1046/j.1365-3083.1998.00487.x. [DOI] [PubMed] [Google Scholar]
- 37.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–92. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 39.Berg DJ, Zhang J, Weinstock JV, Ismail HF, Earle KA, Alila H, et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–42. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 40.Hwang SJ, Kim HS, Chung DH. Fas/Fas ligand-mediated apoptosis promotes hypersensitivity pneumonitis in mice by enhancing maturation of dendritic cells. Am J Respir Crit Care Med. 181:1250–61. doi: 10.1164/rccm.200909-1337OC. [DOI] [PubMed] [Google Scholar]
- 41.Bruce D, Cantorna MT. Intrinsic requirement for the vitamin D receptor in the development of CD8 alpha alpha-expressing T cells. J Immunol. 186:2819–25. doi: 10.4049/jimmunol.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–93. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 43.Noble A, Macary PA, Kemeny DM. IFN-gamma and IL-4 regulate the growth and differentiation of CD8+ T cells into subpopulations with distinct cytokine profiles. J Immunol. 1995;155:2928–37. [PubMed] [Google Scholar]
- 44.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–89. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cippitelli M, Santoni A. Vitamin D3: a transcriptional modulator of the interferon-gamma gene. Eur J Immunol. 1998;28:3017–30. doi: 10.1002/(SICI)1521-4141(199810)28:10<3017::AID-IMMU3017>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 46.D’Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–62. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ooi JH, Chen J, Cantorna MT. Vitamin D regulation of immune function in the gut: why do T cells have vitamin D receptors? Mol Aspects Med. 2011;33:77–82. doi: 10.1016/j.mam.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams JS, Gacad MA, Diz MM, Nadler JL. A role for endogenous arachidonate metabolites in the regulated expression of the 25-hydroxyvitamin D-1-hydroxylation reaction in cultured alveolar macrophages from patients with sarcoidosis. J Clin Endocrinol Metab. 1990;70:595–600. doi: 10.1210/jcem-70-3-595. [DOI] [PubMed] [Google Scholar]
- 49.Pike JW, Meyer MB. Regulation of mouse Cyp24a1 expression via promoter-proximal and downstream-distal enhancers highlights new concepts of 1,25-dihydroxyvitamin D(3) action. Arch Biochem Biophys. 523:2–8. doi: 10.1016/j.abb.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enter ocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 51.Gudmundsson G, Bosch A, Davidson BL, Berg DJ, Hunninghake GW. Interleukin-10 modulates the severity of hypersensitivity pneumonitis in mice. Am J Respir Cell Mol Biol. 1998;19:812–8. doi: 10.1165/ajrcmb.19.5.3153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Reconstitution rate of CD45.1 WT BM cells in the blood and SI of WT or Cyp KO recipient mice. Values are the means ± SEMs of 7 mice per group and one representative of two independent experiments. B) Representative histopathological sections of the lungs from WT, Cyp KO, IL-10 KO and DKO mice and C) β-gal activity in the kidneys following PBS or SR inoculation (n=4–10/group). Values are the means ± SEMs of 4–19 mice per group and one representative of three independent experiments.