Abstract
Apoptosis is programmed cell death triggered by activation of death receptors or cellular stress. Activation of caspases is the hallmark of apoptosis. Arrestins are best known for their role in homologous desensitization of G protein-coupled receptors (GPCRs). Arrestins quench G protein activation by binding to activated phosphorylated GPCRs. Recently, arrestins have been shown to regulate multiple signalling pathways in G protein-independent manner via scaffolding signalling proteins. Here we demonstrate that arrestin-2 isoform is cleaved by caspases during apoptosis induced via death receptor activation or by DNA damage at evolutionarily conserved sites in the C-terminus. Caspase-generated arrestin-2-(1-380) fragment translocates to mitochondria increasing cytochrome C release, which is the key checkpoint in cell death. Cells lacking arrestin-2 are significantly more resistant to apoptosis. The expression of wild-type arrestin-2 or its cleavage product arrestin-2-(1-380), but not of its caspase-resistant mutant, restores cell sensitivity to apoptotic stimuli. Arrestin-2-(1-380) action depends on tBID: at physiological concentrations, arrestin-2-(1-380) directly binds tBID and doubles tBID-induced cytochrome C release from isolated mitochondria. Arrestin-2-(1-380) does not facilitate apoptosis in BID knockout cells, whereas its ability to increase caspase-3 activity and facilitate cytochrome C release is rescued when BID expression is restored. Thus, arrestin-2-(1-380) cooperates with another product of caspase activity, tBID, and their concerted action significantly contributes to cell death.
Keywords: apoptosis, arrestin, BID, caspases, cytochrome C
Apoptosis involves activation of caspases concentrating on key pathways and producing stereotypic morphological and biochemical changes.1 The release of cytochrome C from mitochondria that promotes massive activation of executioner caspases-3/7 is critical checkpoint in cell commitment to death.2, 3 Effectors BAK and BAX oligomerize and form pores in the outer mitochondrial membrane allowing cytochrome C to escape to the cytoplasm.4, 5 The composition of this pore and the interplay of pro- and anti-apoptotic BCL proteins regulating cytochrome C release remain unclear.2, 6, 7 The involvement of non-BCL players is an emerging idea.6, 8 Although proteomic surveys suggest that caspase cleavage might supply regulators of apoptosis,9, 10 very few caspase products were shown to have a direct role. Activated caspases are generated by caspase cleavage of inactive pro-enzymes.11 Another example is BID: caspase-generated tBID (caspase-cleaved BID) translocates to mitochondria and promotes cytochrome C release. No other caspase cleavage product has been reported to affect this critical step.
Here we show that during apoptosis induced by various stimuli in several cell types from different species, caspases cleave arrestin-2 generating arrestin-2-(1-380) fragment (1–380). In contrast to predominantly cytoplasmic arrestin-2, 1-380 translocates to mitochondria, increasing cytochrome C release induced by another caspase product, tBID. 1-380 doubles tBID-induced cytochrome C release and selectively facilitates death of BID-expressing cells. The cooperation of arrestin-2-(1-380) with tBID in cytochrome C release is an earlier unappreciated element of the core mechanism of apoptosis.
Results
Arrestin-2 is a caspase substrate
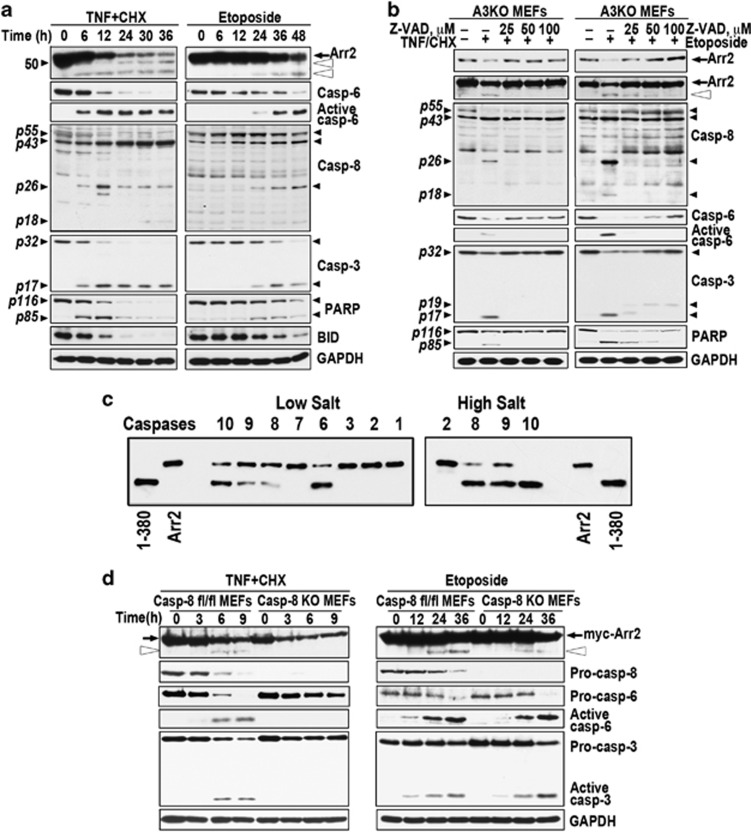
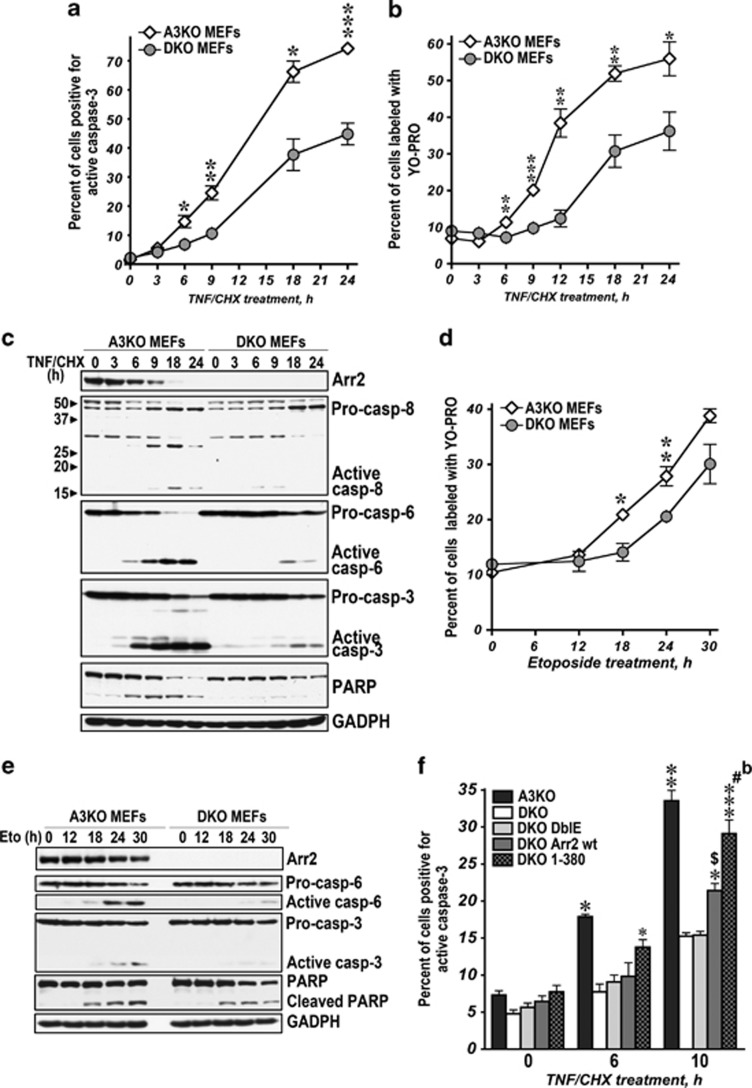
Mammalian cells express arrestin-2 (We use systematic names of arrestin proteins: arrestin-1 (historic names S-antigen, 48 kDa protein, visual or rod arrestin), arrestin-2 (β-arrestin or β-arrestin1), arrestin-3 (β-arrestin2 or hTHY-ARRX), and arrestin-4 (cone or X-arrestin); for unclear reasons its gene is called ‘arrestin 3' in HUGO database).12 Therefore, to determine the role of arrestin-2 we used arrestin-3 knockout (A3KO) mouse embryonic fibroblasts (MEFs). The treatment of A3KO MEFs with tumor necrosis factor α (TNFα) acting via TNFR113 resulted in the activation of multiple caspases and cleavage of arrestin-2, poly-(ADP-ribose) polymerase (PARP), and BID (Figure 1a). Caspase-8 is directly activated by TNFR1.14 Treatment with topoisomerase II inhibitor etoposide resulted in the activation of multiple caspases. Progressive loss of endogenous full-length (FL) arrestin-2 was mirrored by the accumulation of smaller fragments, which parallels the cleavage of known caspase substrates15 PARP and BID (Figure 1a). TNFα induced much faster activation of caspase-8 than etoposide and faster cleavage of arrestin-2 and known caspase-8 substrate BID (Figure 1a). Thus, activation of both pathways results in arrestin-2 cleavage, which occurs faster upon TNFα treatment.
Figure 1.
Arrestin-2 is uniformly cleaved by caspases during apoptosis induced by TNFα or etoposide. (a) A3KO MEFs exposed to 10 ng/ml of TNFα with 10 μg of CHX (left) or 100 μM etoposide (right) for the indicated times were lysed and analyzed by western blotting. Black arrow, FL arrestin-2; open arrowheads, 1-408 and 1-380 fragments. Small black arrowheads on the left show the positions of Mr standards (kDa) or characteristic cleavage fragments. Arrestin-2 cleavage parallels the activation of caspase-3, -6, and -8, and the cleavage of caspase substrates PARP and BID. Here and in other figures, GAPDH serves as loading control. (b) A3KO MEFs were pre-treated for 2 h with indicated concentrations of pan-caspase inhibitor z-VAD-fmk and then exposed to TNFα/CHX for 12 h (left) or etoposide for 36 h (right). Cell lysates were analyzed by western blotting with F4C1 monoclonal antibody: short exposure (upper blot) shows WT arrestin-2 levels (black arrow) and longer exposure (lower blot) reveals cleavage products (open arrowhead, 1-380). The activation of caspase-3, -6, and -8 and PARP cleavage was determined in parallel. (c) Purified arrestin-2 (75 ng) was incubated for 3 h at 37 °C in 50 μl of caspase cleavage buffer (low salt) in the presence of 1 unit of indicated active human recombinant caspases (Millipore). Initiator caspases-2, -8, -9 and -10 were also assayed in the presence of 1.0 M ammonium citrate (high salt). The products were analyzed by western blotting with F4C1 antibody. Indicated forms of purified arrestin-2 were run as standards. (d) Caspase-8 fl/fl and caspase-8 knockout (KO) MEFs were treated with TNFα/CHX (left) or etoposide (right) for the indicated times. TNFα induces robust activation of caspases-8, -6, and -3 in caspase-8 fl/fl MEFs and appearance of arrestin-2-(1-380) (open arrowhead) after 6–9 h. Caspase-8 knockout MEFs do not show caspase activation or generation of 1-380. On etoposide treatment, both caspase-8 fl/fl and knockout MEFs show similar activation of caspase-3 and -6 and generation of 1-380. See also Supplementary Figure S1
Increasing concentrations of pan-caspase inhibitor Z-VAD-fmk16 progressively reduced activation of caspases-3, -6, and -8 in A3KO MEFs and cleavage of arrestin-2 and PARP upon TNFα or etoposide treatment (Figure 1b). Full inhibition of the TNFα-induced cleavage was achieved at 25 μM Z-VAD in parallel with full inhibition of caspase activation and PARP cleavage, whereas caspase activity, PARP cleavage, and cleavage of arrestin-2 persisted in etoposide-treated cells up to 100 μM Z-VAD (Figure 1b). Thus, arrestin-2 is targeted by caspases, rather than other proteases activated during apoptosis. Arrestin-2 contains several potential caspase cleavage sites D/VxxD17 accessible in its basal conformation.18 We tested the cleavage of arrestin-2 mutants carrying single Asp→Glu substitutions in etoposide-treated Rat-1 cells and in vitro. The data excluded most potential sites, identified Asp380 and Asp408 as targets, demonstrated caspase resistance of arrestin-2-D380E,D408E (DblE), and identified 1-380 as the main product (Supplementary Figures S1A,B). Intracellular concentrations of 1-380 generated from endogenous arrestin-2 in cells treated with etoposide or TNFα reached 55 or 90 nM, respectively (Supplementary Figure S1C).
Purified arrestin-2 was cleaved by recombinant active human caspases-6, -8, -9, and -10 in isotonic buffer (Figure 1c). At high salt-promoting dimerization of upstream caspases,19, 20 initiator caspases -8, -9, and -10, but not -2, effectively cleaved arrestin-2 (Figure 1c). Caspases-6, -8, -9, and -10 in vitro generate a fragment of the same size as purified 1-380. Thus, multiple caspases cleave arrestin-2 at the same sites in vitro and in apoptotic cells (Figures 1a–c). To test the role of caspase-8 directly activated by death receptors, we generated caspase-8-deficient MEFs expressing Cre in cells with floxed caspase-8 alleles.21 In contrast to control MEFs, where TNFα induced arrestin-2 cleavage (Figure 1d), 1-380 fragment was not observed in caspase-8 knockout cells. Although cells lacking caspase-8 die and the levels of all proteins decrease, TNFα-induced death in this case was non-apoptotic, as evidenced by the absence of active caspase-3 and -6 (Figure 1d). Upon inhibition or in the absence of capsase-8, TNFα induces necroptosis22, 23, 24, 25 that ultimately results in cell lysis and loss of cellular content. Correspondingly, we observed reduction of the arrestin-2 concentration without the appearance of the cleavage product, as well as the reductions in the levels of pro-caspases 6 and 3 without accumulation of active caspases (Figure 1d). In contrast, etoposide treatment of control and caspase-8 knockout MEFs induced apoptosis with characteristic activation of caspase-3 and -6 and appearance of 1-380 in both cell types (Figure 1d). Thus, arrestin-2 is only cleaved in cells with active caspases, all of which target Asp380 and yield 1-380 fragment. Importantly, Asp380 is conserved in arrestins of vertebrates and urochordate Ciona intestinalis, but not in nematode Caenorhabditis elegans (Supplementary Figure S1D).
Caspase-generated 1-380 translocates to mitochondria and facilitates cytochome C release
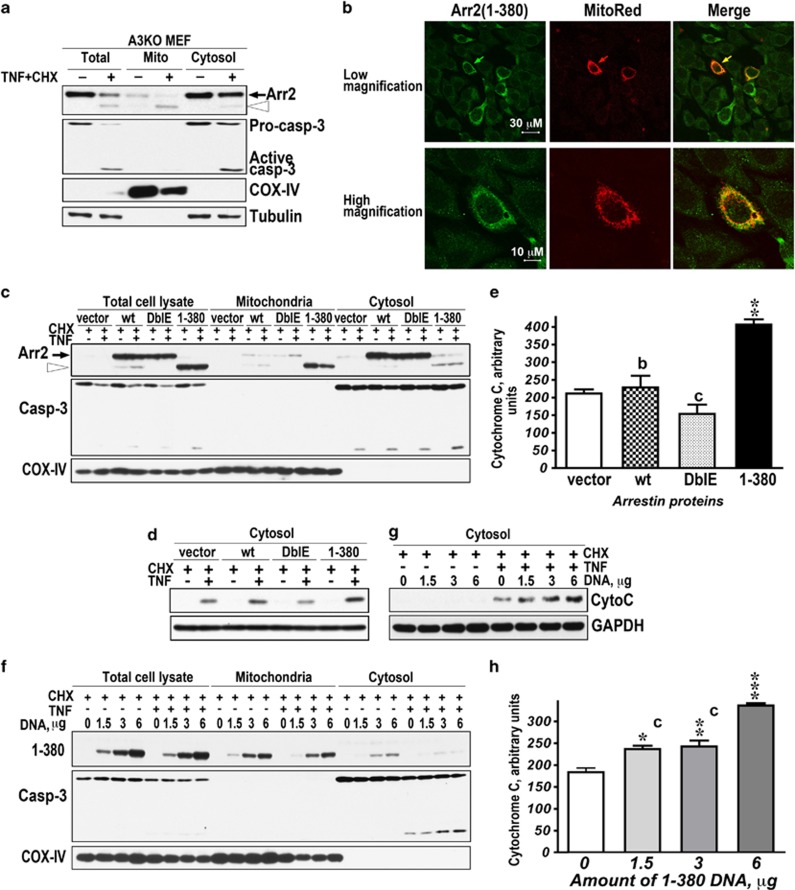
Arrestin-2 is predominantly cytoplasmic in most cells.26 In control and TNFα-treated A3KO MEFs, FL arrestin-2 co-fractionated with cytosolic markers tubulin and caspase-3, whereas the majority of 1-380 co-fractionated with mitochondrial marker COX-IV (subunit IV of cytochrome C oxidase; Figure 2a). To confirm this finding by an independent method, we visualized expressed 1-380 with anti-arrestin antibody in arrestin-2/3 double knockout (DKO) MEFs, where the mitochondria contained expressed Mito-DsRed (Figure 2b), and found that 1-380 fragment largely co-localized with mitochondria in control and apoptotic cells (Figure 2b). Thus, unlike FL arrestin-2, 1-380 spontaneously localizes to mitochondria.
Figure 2.
Caspase-generated 1-380 translocates to mitochondria and facilitates cytochrome C release in TNFα-treated cells. (a) Control (−) and TNFα/CHX-treated (+) A3KO MEFs were fractionated, as described in the Materials and Methods. 1-380 generated from endogenous arrestin-2 localizes to mitochondria. Black arrow, FL arrestin-2; open arrowhead, 1-380. Western blots for mitochodrial (subunit IV of cytochrome C oxidase, COX-IV) and cytoplasmic (tubulin and caspase-3) markers are shown. (b) DKO MEFs expressing 1-380 and pDS-Red2-Mito were fixed, and 1-380 was detected with arrestin polyclonal primary (F431, 1 : 500) and green secondary antibody. (c) Intracellular distribution of expressed WT arrestin-2 (wt), DblE, and 1-380 in A3KO MEFs treated with CHX or TNFα/CHX for 6 h. Cell lysates and fractions were resolved by SDS-PAGE and immunoblotted for arrestin-2, caspase-3, or COX-IV. Black arrow, FL arrestin-2; open arrowhead, 1-380. (d) Cytosolic cytochrome C in the same cells was detected by western blotting. (e) Quantification of the data in panel (d), shown as normalized optical density (means±S.E.M.). The results of four experiments were analyzed by one-way ANOVA with Protein as the main factor. The effect of Protein was highly significant (F(3,8)=22.2, P=0.0003). **P<0.01 to empty vector (vector); cP<0.001; and bP<0.01 to 1-380 according to Bonferroni/Dunn post hoc test. (f) Subcellular fractionation of CHX or TNFα/CHX-treated A3KO MEFs expressing increasing amounts of 1-380 (1-380 DNA per 60 mm dish is shown). (g) Cytosolic cytochrome C in these cells was measured by western blotting. (h) Quantification of the data in (g), shown as normalized optical density (means±S.E.M.). The results of four experiments were analyzed by one-way ANOVA with Concentration as the main factor. The effect of Concentration was highly significant (F(3,12)=44, P<0.0001). ***P<0.001, **P<0.01, *P<0.05 to 0 μg of 1-380 DNA; and cP<0.001 to 6 μg of 1-380 DNA, according to Bonferroni/Dunn post hoc test
Massive activation of caspase-3 via the apoptosome organized by cytochrome C is the key step in apoptosis.3 Therefore, we tested whether mitochondrial 1-380 facilitates cytochrome C release by comparing the effects of WT arrestin-2 (cleaved by caspases like endogenous protein), caspase-resistant DblE, and 1-380 fragment transfected into A3KO MEFs (Figures 2c–e). In all cases, TNFα induced caspase-3 activation. Cell fractionation confirmed cytoplasmic localization of WT arrestin-2 and DblE and preferential mitochondrial localization of 1-380, both expressed or generated by caspases from WT arrestin-2 (Figure 2c). In contrast to DblE, 1-380 significantly increased cytochrome C release upon TNFα treatment (Figures 2d and e). In apoptotic cells, cytoplasmic cytochrome C progressively increased with the expression level of 1-380 (Figures 2f–h). Cytoplasmic cytochrome C was only detected in apoptotic cells. Thus, 1-380 likely cooperates with another protein generated during apoptosis.
The functional role of 1-380 is conserved in different cells undergoing apoptosis initiated by different stimuli
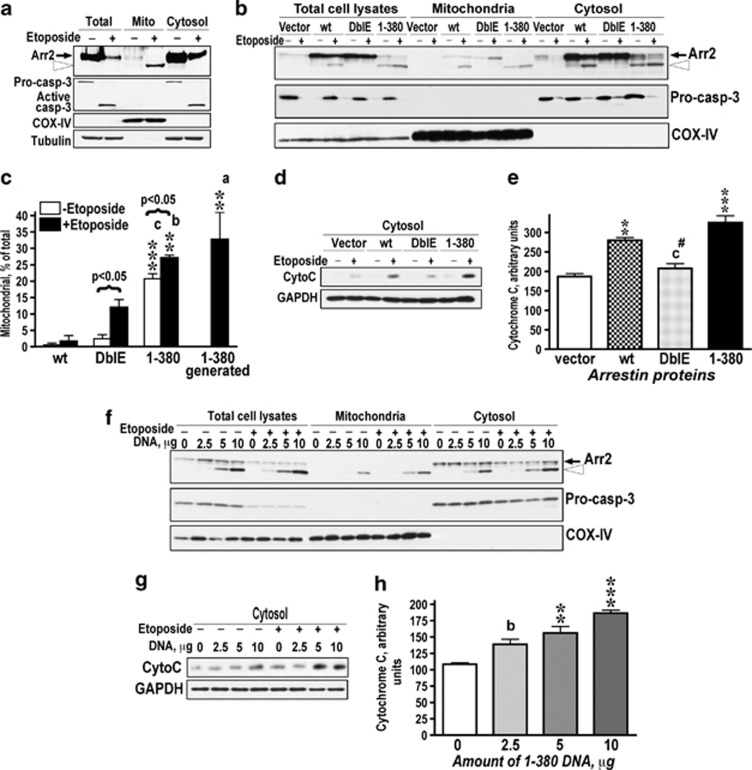
Arrestin-2 is similarly cleaved by caspases in cells treated with TNFα or etoposide that initiate apoptosis via distinct pathways (Figure 1). Therefore, we determined the localization and function of 1-380 in etoposide-treated Rat-1 cells (Figure 3). In these cells, 1-380 generated from endogenous or expressed WT arrestin-2 also largely localized to mitochondria (Figures 3a–c). Cytoplasmic cytochrome C was dramatically increased upon etoposide treatment (Figure 3d). Overexpression of WT arrestin-2 increased the amount of 1-380 in mitochondrial fraction (Figures 3b and c) and enhanced cytochrome C release (Figures 3d and e). Expressed 1-380 also associated with mitochondria (Figures 3b and c) and promoted cytochrome C release in a dose-dependent manner (Figures 3f–h), whereas DblE was ineffective (Figures 3d and e). Thus 1-380 in different cells undergoing apoptosis induced via extrinsic or intrinsic pathway invariably localizes to mitochondria, where it facilitates cytochrome C release (Figures 2 and 3).
Figure 3.
Arrestin-2-(1-380) facilitates cytochrome C release from mitochondria in etoposide-treated Rat1 cells. (a) Control (−) and etoposide-treated (40 μM) (+) Rat-1 cells were fractionated, as described in the Materials and Methods. 1-380 (open arrowhead) generated from endogenous arrestin-2 localizes to mitochondria, whereas WT arrestin-2 (black arrow) remains in the cytosol. Western blots for mitochondrial (COX IV) and cytoplasmic (tubulin and caspase-3) markers are shown. (b) Intracellular distribution of expressed FL arrestin-2 (wt), DblE, and 1-380 in control (−) and etoposide-treated (+) Rat-1 cells. Black arrow, FL arrestin-2; open arrowhead, 1-380. (c) Indicated forms of arrestin-2 in the mitochondrial fraction were quantified by western blotting and expressed as the percentage of the total. (1-380) generated—the fragment generated from expressed WT arrestin-2 during apoptosis and present only in etoposide-treated cells. Its accumulation in the mitochondria is quantitatively similar to that of the expressed 1-380 fragment. The data were analyzed by ANOVA with Protein and Drug (+/− etoposide) as main factors. Both factors were highly significant: Protein, F(2.12)=120.9, P<0.0001; Drug, F(1,12)=21.1, P=0.0006. As the Protein × Drug interaction was significant (F(2,12)=3.9, P=0.049), the amount of arrestins in mitochondria with or without etoposide treatment was analyzed separately. **P<0.01, ***P<0.001 to wild type; aP<0.05, bP<0.01, cP<0.001 to DblE. Comparison of the effect of etoposide treatment for each protein was made by Student's t-test (results shown in brackets). (d) Cytosolic cytochrome C in the same cells was measured by western blotting. (e) Quantification of cytosolic cytochrome C in etoposide-treated cells. Normalized optical density of western blot bands is shown (means±S.E.M.). The results of four experiments were analyzed by one-way ANOVA with Protein as the main factor. The effect of Protein was significant (F(3,6)=29.5, P=0.0001). ***P<0.001; **P<0.01, to empty vector; #P<0.05 to wt; cP<0.001 to 1-380. (f) Intracellular distribution of 1-380 (open arrowhead) in control (−) and etoposide-treated (+) cells transfected with indicated amounts of 1-380 DNA. (g) Western blotting of cytosolic cytochrome C in these cells. (h) Quantification of cytosolic cytochrome C in etoposide-treated cells as normalized optic density (means±S.E.M.). The results of four experiments were analyzed by one-way ANOVA with Concentration as the main factor. The effect of the factor was significant (F(3,8)=23.1, P=0.0003); ***P<0.001; **P<0.01 to 0 μg; bP<0.01 to the 10 μg
Arrestin-2-(1-380) facilitates tBID-induced cytochrome C release from isolated mitochondria
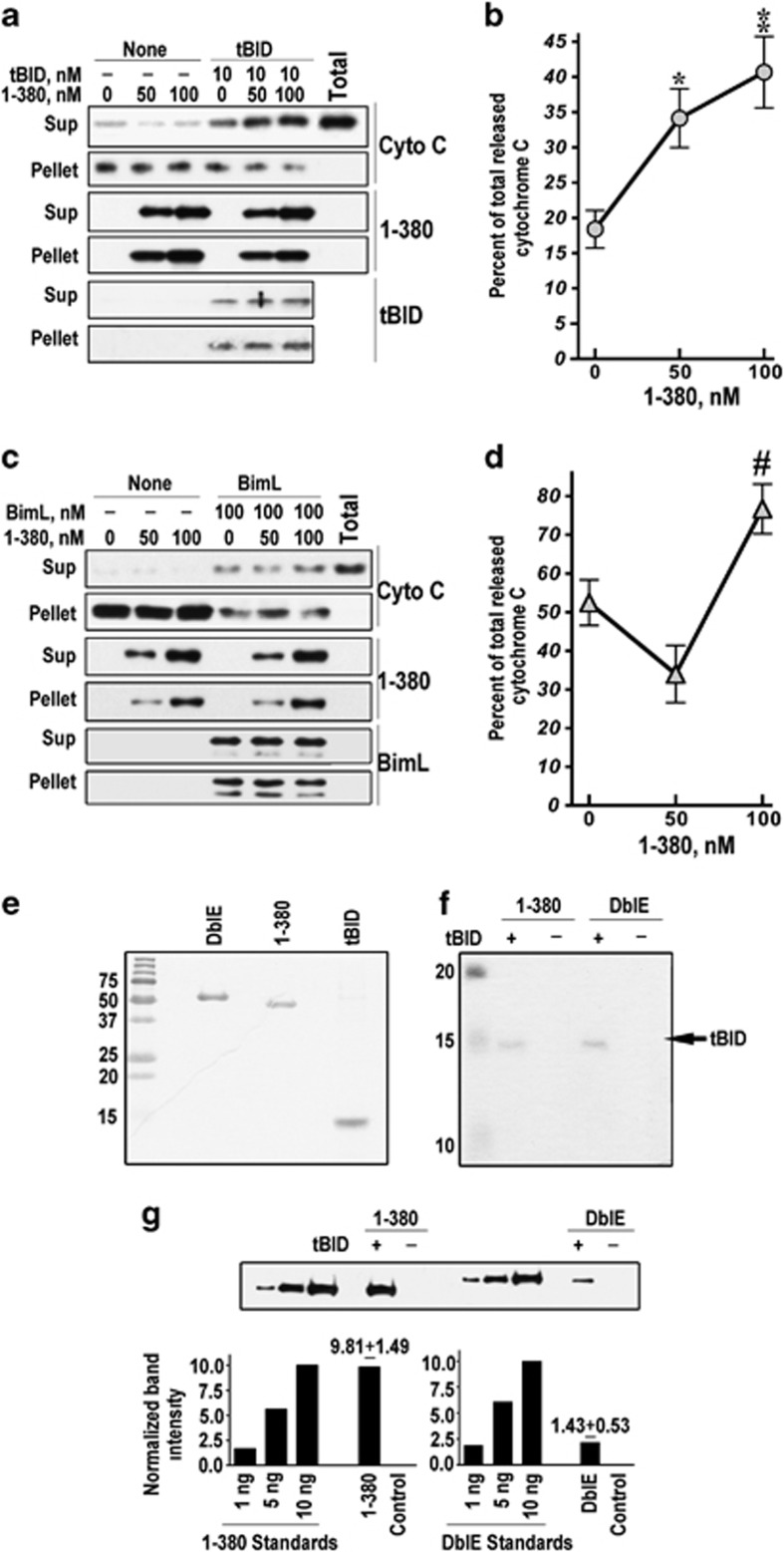
Pro-apoptotic BCL proteins tBID, BIM, and PUMA6 translocate to mitochondria and induce cytochrome C release. tBID is one of the best-studied pro-apoptotic BH3-only proteins that directly binds BAX, promoting its oligomerization and the formation of pores in the outer mitochondrial membrane.5, 7, 27, 28 Purified 1-380 readily bound isolated mitochondria but did not induce cytochrome C release (Figure 4a). Mitochondria incubated with 10 nM tBID released ∼20% of total cytochrome C. In the presence of 50–100 nM 1-380, the fraction of cytochrome C released by tBID increased up to 2-fold (Figures 4a and b). Arrestin-2 in cells reaches 200 nM;29 caspase cleavage of endogenous arrestin-2 yields 55–90 nM 1-380 (Supplementary Figure S1C), which significantly increased tBID-induced cytochrome C release (Figure 4b). 1-380 associated with mitochondria in vitro (Figure 4a) and in intact cells (Figures 2 and 3), but did not affect the amount of tBID bound to mitochondria (Figure 4a). DblE did not bind mitochondria in vitro and in cells (Figures 2 and 3 and Supplementary Figure S2A) and did not affect cytochrome C release in the presence or absence of tBID (Supplementary Figure S2B). BIM is another BH3-only protein that releases cytochrome C.30 At 100 nM, BIM released ∼50% of cytochrome C, and 50–100 nM arrestin-2-(1-380), which progressively increased the effect of tBID (Figures 4a and b), did not show consistent effect on BIM-induced cytochrome C release (Figures 4c and d), suggesting that 1-380 action is specific for tBID. Although it is conceivable that 1-380 cooperates with tBID without interacting with it, direct binding of 1-380 to tBID would provide the simplest explanation. Therefore, we incubated purified 1-380 and DblE with Ni column with and without bound pure His-tagged tBID (Figures 4e and f), which was fully functional (Supplementary Figure S2C), and measured retained arrestins (Figure 4g). The amount of 1-380 bound to tBID was ∼5-fold greater than that of the DblE protein (Figure 4g). Thus, 1-380 likely promotes cytochrome C release via direct interaction with tBID. Caspase cleavage facilitates arrestin-2 association with mitochondria (Figures 2 and 3) and dramatically increases its affinity for tBID (Figure 4g).
Figure 4.
Arrestin-2-(1-380) directly binds tBID and isolated mitochondria, facilitating tBID-induced cytochrome C release. (a) Isolated mouse liver mitochondria (20 μg) were incubated with or without 10 nM tBID and 0, 50, or 100 nM purified 1-380 for 20 min at room temperature. Mitochondria were pelleted by centrifugation at 16 000 × g for 10 min at 4 °C. The distribution of cytochrome C (Cyto C), 1-380, and tBID in the pellet and supernatant is shown. (b) The fraction of cytochrome C released by 10 nM tBID without (0 nM) or with 1-380 (50 and 100 nM) is shown as the percentage of the total cytochrome C (released by Triton X-100) (means±S.E.M. of four experiments). **P<0.01; *P<0.05 as compared with 10 nM tBID alone by one-way ANOVA with Concentration as the main factor (F(2,18)=10.1, P=0.0011) followed by Bonferroni/Dunn post hoc comparison. (c) Isolated mouse liver mitochondria (20 μg) were incubated with or without 100 nM BimL and 0, 50, or 100 nM purified 1-380 for 1 h at 37 °C. Mitochondria were pelleted by centrifugation at 16 000 × g for 10 min at 4 °C. The distribution of cytochrome C (Cyto C), 1-380, and BimL in the pellet and supernatant is shown. (d) The fraction of cytochrome C released by 100 nM BimL without (0 nM) or with 1-380 (50 and 100 nM) is shown as the percentage of the total cytochrome C (released by Triton X-100) (means±S.E.M. of four experiments). #P<0.05 as compared with 100 BimL plus 50 nM 1-380 by one-way ANOVA with Bonferroni/Dunn post hoc comparison. (e–g) In vitro binding of purified recombinant DblE or 1-380 to His6-tagged tBID, which retains full biological activity (Supplementary Figure S2C). (e) Coomassie gel showing the purity of DblE, 1-380, and tBID. (f) Bound His6-tagged tBID (20% of total eluate) was analyzed by SDS-PAGE and Coomassie staining. Similar amounts of tBID were bound to Ni-beads used for pull-down. (g) Bound DblE and 1-380 were measured by western blotting, with known amounts of purified recombinant proteins used as standards. Quantification of DblE and 1-380 bound to tBID (numbers above bars are means±S.E.M.; n=4) is shown. See also Supplementary Figure S2
Arrestin-2-(1-380) facilitates apoptotic cell death
The release of cytochrome C and subsequent massive activation of caspase-3 via apoptosome commits the cell to suicide.3 Direct participation of 1-380 in cytochrome C release in vitro and in vivo (Figures 2, 3, 4) suggests that arrestin-2 cleavage can have an important role in cell death. Therefore, we compared the time course of TNFα-induced apoptosis in A3KO MEFs endogenously expressing arrestin-2 and DKO MEFs. DKO MEFs were significantly less vulnerable to TNFα-induced death (Figure 5a, Supplementary Figure S4A). Caspase-3 activation in A3KO MEFs was much faster than in DKO MEFs: the fraction of caspase-3-positive cells expressing endogenous arrestin-2 was consistently 2–3-fold greater (Figure 5a). Because of the biological importance of this finding, we quantified the fraction of apoptotic cells by an alternative method, using selective YO-PRO-1 dye.31 Upon TNFα treatment, the proportion of apoptotic A3KO MEFs was also 2-3-fold higher than DKO MEFs (Figure 5b). The most striking difference was observed at 6–12 h, when A3KO MEFs demonstrate rapid caspase-3 activation and apoptotic death, whereas DKO MEFs lag behind (Figures 5a–c). The activation of caspase-6 and -8 and PARP cleavage, other hallmarks of TNFα-induced apoptosis, also proceeded much faster in A3KO MEFs (Figure 5c). Reduced caspase-8 activity in DKO MEFs was likely due to weaker positive feedback activation downstream of mitochondria32, 33, 34, 35 as a result of loss of arrestin-2. We tested whether the difference in apoptosis-related proteins contributes to greater sensitivity of A3KO MEFs to TNFα and found higher levels of pro-apoptotic TNF1R, TRADD, and especially BAK (∼3-fold) in DKO cells, with lower level of pro-survival cFLIP-s (Supplementary Figure S3). This is the opposite of what one would expect in more resistant cells. We also found similar levels of anti-apoptotic BCL2, BCL-XL, and MCL1 in both cell lines, with slightly lower BCL-XL in more resistant DKO MEFs (Supplementary Figure S3). Thus, the presence of arrestin-2, rather than any other player, underlies greater sensitivity of A3KO MEFs to TNFα. Cell death and caspase activation induced by etoposide via intrinsic pathway were also significantly faster in A3KO than in DKO MEFs (Figures 5d and e), albeit the difference was smaller, reflecting secondary role of BID-mediated signalling in apoptosis induced by genotoxic drugs, such as etoposide.36, 37, 38 Thus, 1-380 generated from endogenous arrestin-2 facilitates apoptosis induced by different stimuli.
Figure 5.
The generation of 1-380 facilitates apoptotic cell death. (a–c) A3KO and DKO MEFs were treated with TNFα/CHX (10 ng/ml and 10 μg/ml) for the indicated times. Apoptosis was assessed by the percentage of cells positive for active caspase-3 (a) or stained with YO-PRO-1 (b) using FACS in four independent experiments. The data were analyzed by two-way ANOVA with MEF and Time as main factors. In both caspases-3 and YO-PRO experiments, the effects of MEF were highly significant (P<0.0001). ***P<0.001; **P<0.01; *P<0.05 to DKO MEFs according to unpaired Student's t-test for each time point. (c) The activation of caspases in A3KO and DKO MEFs treated with TNFα/CHX analyzed by western blotting. (d) A3KO and DKO MEFs were treated with etoposide (100 μM) for the indicated times. Apoptosis was assessed by the percentage of cells stained with YO-PRO-1. The two-way ANOVA analysis with MEF and Time as main factors yielded highly significant effect of MEF (P=0.0001). **P<0.001; *P<0.005 to DKO MEFs according to unpaired Student's t-test for each time point. (e) The activation of caspase-6 and -3 in A3KO and DKO MEFs treated with etoposide analyzed by western blotting. (f) A3KO or DKO MEFs expressing GFP, wt Arr2+GFP, DblE+GFP, or 1-380+GFP were exposed to TNFα/CHX for the indicated times. Apoptosis was determined as the percentage of cells positive for active caspase-3 in GFP-positive subsets measured by FACS (means±S.E.M. of four experiments). The data were analyzed by one-way ANOVA for each time point with MEF Type as the main factor. The MEF effect was highly significant for 6 and 10 h TNFα/CHX treatment time points. **P<0.001, *P<0.01 to DKO, DKO DblE, and DKO Arr2 wt; ***P<0.001; *P<0.05 to DKO MEFs; $P<0.05, #P<0.001 to DblE-GFP; bP<0.01 to Arr2-GFP according to Bonferroni/Dunn post hoc test. See also Supplementary Figure S3
If the presence of arrestin-2-(1–380) determines cell sensitivity to TNFα, the expression of WT arrestin-2 or 1-380 should rescue apoptosis in DKO MEFs. Therefore, we co-cistronically expressed WT arrestin-2, 1-380, or DblE with GFP (to identify transfected cells), or GFP only (control) in DKO MEFs (Supplementary Figure S4B and C). Cells stained for active caspase-3 were sorted to evaluate apoptosis only in transfected cells (Figure 5f, Supplementary Figure S4C). DKO MEFs expressing 1-380 had higher caspase-3 activity after 6 and 10 h of TNFα treatment and were not significantly different from A3KO MEFs (Figure 5f). WT arrestin-2 significantly increased caspase-3 activity at 10 h, whereas uncleavable DblE that cannot yield 1-380 fragment did not affect cell viability (Figure 5f). The rescue of DKO MEFs sensitivity to TNFα by arrestin-2 rules out potential role of other players, indicating that the presence or absence of arrestin-2 determines the difference. Thus, caspase-generated arrestin-2-(1-380) significantly contributes to the progression of apoptosis in vivo.
The absence of BID abrogates pro-apoptotic action of 1-380
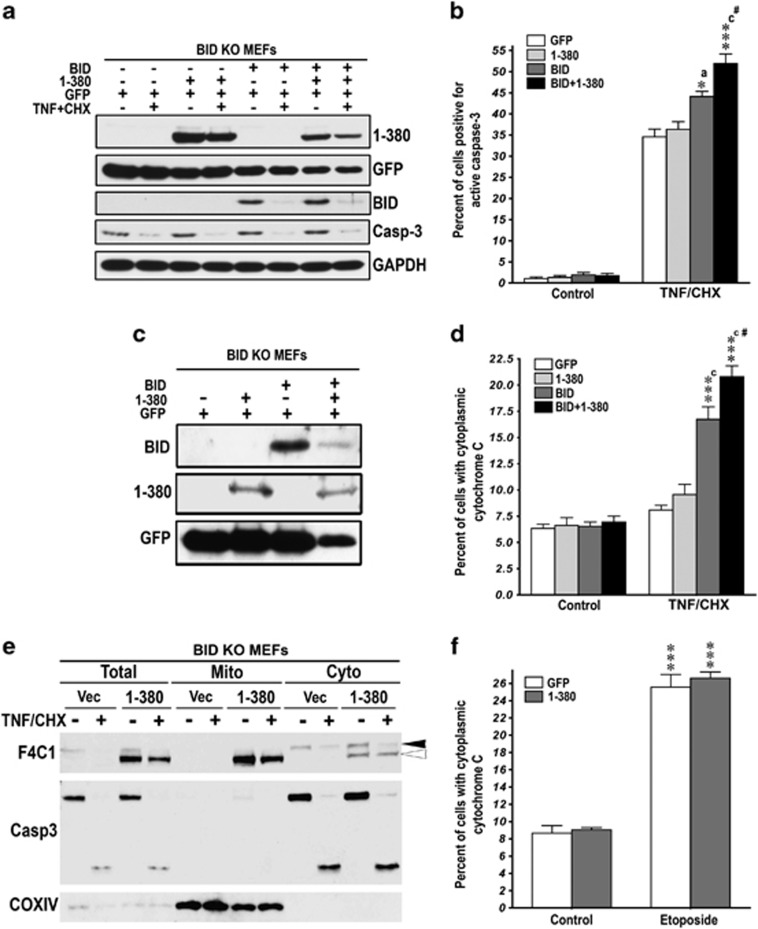
1-380 facilitates cytochrome C release from isolated mouse liver mitochondria induced by tBID but not BIM (Figure 4). As other BH3-only BCL proteins can induce cytochrome C release, we tested whether the action of 1-380 is strictly dependent on BID in vivo using BID knockout MEFs infected with retroviruses co-expressing 1-380 and GFP; BID and GFP; 1-380, BID, and GFP; or GFP only (control) (Figure 6a, Supplementary Figure S5A). We used cell sorting method to determine the rate of caspase-3 activation exclusively in infected cells (Figure 6b, Supplementary Figure S5B). TNFα treatment for 6 h dramatically increased the fraction of caspase-3-positive BID-deficient cells, but 1-380 in the absence of BID had no effect (Figure 6b). The restoration of BID increased vulnerability of these cells to TNFα. In BID-expressing MEFs, co-expression of 1-380 significantly increased the fraction of caspase-3-positive cells (Figure 6b), even though BID level in cells co-expressing 1-380 was much lower (Figure 6c). As both tBID and 1-380 participate in cytochrome C release (Figure 4), we determined their effect on cytoplasmic cytochrome C. TNFα did not increase cytoplasmic cytochrome C in BID-deficient cells (Figure 6d). BID expression rescued cytochrome C release, and co-expression of 1-380 with BID further increased cytoplasmic cytochrome C (Figure 6d). Importantly, although expressed 1-380 localized to mitochondria in the absence of BID (Figure 6e), it did not affect cytoplasmic cytochrome C in BID-deficient cells treated with TNFα (Figure 6d). In contrast to TNFα, etoposide induced robust cytochrome C release in BID-deficient MEFs (Figure 6f). These data reflect the fact that etoposide-induced apoptosis is p53-dependent and cytochrome C release is mediated by PUMA and, to lesser degree, NOXA39, 40 that are intact in BID-deficient MEFs. However, expression of 1-380 had no effect on the etoposide-induced cytochrome C release in the absence of BID (Figure 6f). Thus, 1-380 increases cytochrome C release and facilitates apoptosis only in the presence of BID. As BID-deficient MEFs have normal levels of other BCL proteins, these data indicate that 1-380 selectively cooperates with tBID.
Figure 6.
The action of 1-380 depends on the expression of BID. (a) BID KO MEFs were transduced with virus expressing GFP (control); GFP+1-380; GFP+BID; or GFP+1-380+BID (Supplementary Figure S4A). Expression of arrestin (F4C1), GFP, and BID in BID KO MEFs was determined by western blotting in lysates of control and TNFα/CHX-treated cells. Western blotting for procaspase-3 (Casp-3) shows the cleavage of caspase-3 induced by TNFα/CHX (10 ng/ml and 10 μg/ml). (b) BID KO MEFs expressing GFP, GFP+1-380, GFP+BID, or GFP+1-380+BID were exposed to TNFα/CHX for 6 h. Apoptosis was measured as a fraction of GFP-expressing cells positive for active caspase-3 by FACS (means±S.E.M. of four experiments). The data were analyzed by one-way ANOVA with protein as the main factor. The effect of Protein was highly significant (F(3,16)=19.7, P<0.001). ***P<0.001; *P<0.05 to GFP control, aP<0.05, cP<0.001 to 1-380; and #P<0.05 to BID according to Bonferroni/Dunn post hoc comparison. (c) BID KO MEFs were transduced with virus expressing GFP (control), GFP+1-380, GFP+BID, or GFP+1-380+BID. Cells were pre-sorted for GFP and re-plated before treatment with TNFα/CHX for 6 h. Expression of arrestin (F4C1), GFP, and BID in BID KO MEFs was determined by western blotting. (d) BID KO MEFs expressing GFP, GFP+1-380, GFP+BID, or GFP+1-380+BID were exposed to TNFα/CHX for 6 h. Apoptosis was measured as a fraction of cells with cytosolic cytochrome C among GFP-positive cells by FACS (means±S.E.M. of four experiments). The data were analyzed by one-way ANOVA with protein as the main factor. The effect of Protein was highly significant (F(3,12)=27.9, P<0.001). ***P<0.001 to GFP control; cP<0.001 to 1-380; #P<0.05 to BID according to Bonferroni/Dunn post hoc comparison. (e) Subcellular localization of 1-380 in BID KO MEFs transduced with retrovirus expressing 1-380 with and without TNFα/CHX treatment for 6 h. (f) BID KO MEFs were transduced with virus expressing GFP (control) or GFP+1-380. Cells were pre-sorted for GFP and re-plated before treatment with etoposide (100 μM) for 36 h. Apoptosis was measured as a fraction of cells with cytosolic cytochrome C among GFP-positive cells by FACS (means±S.E.M. of four experiments). Two-way ANOVA with Protein (GFP versus 1-380) and Treatment (control versus etoposide) yielded significant effect of Treatment (P<0.0001) but no effect of Protein or Protein × Treatment interaction (P>0.05). ***P<0.001 to respective control values according to Student's t-test. See also Supplementary Figure S4
Discussion
Arrestin-2 is cleaved by caspases during apoptosis induced via extrinsic or intrinsic pathway. Caspase-generated 1-380 has an active role: it translocates to mitochondria and increases cytochrome C release induced by tBID, doubling it at concentrations produced from endogenous arrestin-2, and dramatically increasing apoptotic cell death. This is the first case where two caspase-generated protein fragments directly cooperate in inducing cytochrome C release, which is a critical point in committing vertebrate cells to apoptosis.
Signalling proteins Akt, c-Raf1, Src, MEK, and PKC are caspase substrates.17 However, caspase cleavage of proteins regulating G protein-coupled receptors (GPCRs) has never been reported. GPCRs are the largest receptor family with 800–3400 members in mammals. G-protein activation by GPCRs is terminated by receptor phosphorylation and arrestin binding.41 Ubiquitous arrestin-2 and -3 regulate the majority of GPCRs and bind numerous non-receptor partners involved in ‘life-or-death' decisions in the cell.12, 42 Arrestins can promote cell death: tight association of visual arrestin with activated rhodopsin induces apoptosis of Drosophila43, 44 and mammalian45 photoreceptors; the binding of arrestin-3 to ubiquitin ligase Mdm2 enhances p53-mediated apoptosis;46 and arrestin-3 suppresses anti-apoptotic signalling of NFκB.47 In other cases, arrestins are cytoprotective: arrestin-2 stimulates transcription of BCL-2 promoting survival of CD4+ T cells;48 arrestin-3 suppresses apoptosis via regulation of BAD phosphorylation;49 and arrestins mediate pro-survival signaling of NK150 and insulin-like growth factor-1 receptors.51 GPCR activation in arrestin-2/3 DKO MEFs induces apoptosis, whereas the expression of either arrestin is protective.52 Thus arrestins can affect cell survival via signalling, but direct pro-apoptotic action of arrestin-2 at the core of cell death machinery has never been reported.
Arrestin-2 is cleaved by multiple caspases at Asp380, yielding 1-380, and DblE mutant is caspase-resistant in all cell types. Asp380 is present in homologous positions in arrestin-2 in multiple species, suggesting that this mechanism is conserved in vertebrates. Unlike many substrates, arrestin-2 is not just an ‘innocent victim' of caspases. Generated 1-380 fragment translocates to mitochondria and enhances cytochrome C release by ‘assisting' another caspase product, tBID. Increase of cytochrome C release by 1-380 has an important biological role, significantly accelerating apoptosis. The rate of caspase activation and cell death of A3KO MEFs is 2–3-fold higher than that of DKO MEFs lacking it (Figure 5). Dramatically, higher sensitivity of A3KO than of DKO MEFs to pro-apoptotic insults cannot be explained by differential expression of other proteins. Rescue experiments support a critical role of arrestin-2 in higher vulnerability of A3KO MEFs: expression of 1-380 in DKO MEFs facilitates apoptosis to the level observed in A3KO MEFs. WT arrestin-2, but not caspase-resistant DblE, also increases sensitivity of DKO MEFs to TNFα. Two conservative Asp→Glu substitutions in the C-tail of DblE prevent caspase cleavage but preserve signaling properties (Supplementary Figure S1B). DblE has no pro-apoptotic effect, demonstrating that arrestin-2 does not affect cell death via signaling, but acts via novel mechanism requiring caspase cleavage.
Residential mitochondrial protein MITCH2 enhances tBID action by recruiting tBID to mitochondria.53, 54 Arrestin-2 does not have mitochondrial localization signal, and mitochondria contain little FL arrestin-2. However, large proportion of 1-380 localizes to mitochondria. Direct binding of purified 1-380 to isolated mitochondria and mitochondrial localization of expressed 1-380 even in non-apoptotic cells show that it has higher affinity for protein(s) residing in this compartment than FL arrestin-2. Importantly, 1-380 does not facilitate tBID recruitment to mitochondria or induce cytochrome C release by itself in cells or isolated mitochondria. Instead, it directly interacts with tBID and specifically facilitates tBID-induced cytochrome C release. The absence of BID completely abrogates biological effect of 1-380. Thus, caspase cleavage of arrestin-2 is a gain-of-function event enhancing its interaction with tBID and the ability to facilitate tBID-induced cytochrome C release.
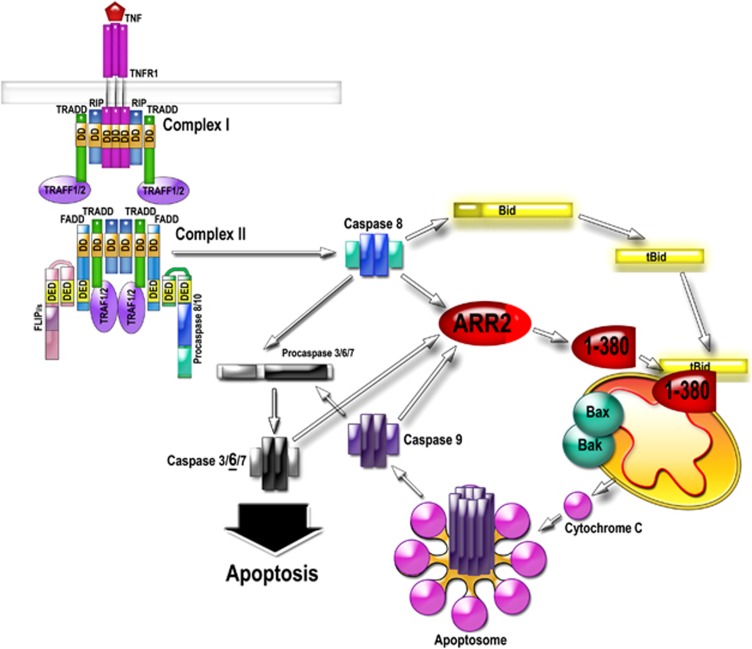
Both 1-380 and tBID are effectively generated by caspase-8, suggesting that their convergence at mitochondria has crucial role in apoptosis. However, apoptosis, like most cellular processes, has multiple backup mechanisms.1, 38 Cleavage of arrestin-2 and BID are no exceptions: in the absence of caspase-8, 1-380 and tBID are generated by other caspases. Caspase activity in the cell is greatly increased by released cytochrome C via apoptosome.3 Thus, cooperation of 1-380 and tBID creates a potent positive feedback, tipping the balance towards cell death (see Figure 7). This mechanism also sets a threshold for an irreversible cell ‘decision' to die: simultaneous generation of both fragments is necessary to maximize the death signal. Importantly, the magnitude of 1-380 effect on isolated mitochondria and intact cells (Figures 4, 5, 6) was comparable with that of established pro-apoptotic player tBID.
Figure 7.
Caspase-cleaved arrestin-2 and BID cooperatively enhance cytochrome C release and cell death. The activation of the death receptor by TNFα results in the assembly of the multi-protein complex II that directly activates caspase-8. Caspase-8 cleaves arrestin-2 and BID, generating 1-380 and tBID. 1-380 directly binds tBID and greatly enhances its ability to induce cytochrome C release from mitochondria. Cytochrome C organizes apoptosome, activating caspase-9, which then activates massive amounts of caspase-3. Therefore, cytochrome C release considered ‘the point of no return' in cell commitment to death is facilitated via interaction of tBID with caspase-generated arrestin-2 fragment 1-380.TNFR, TNFα receptor; RIP, receptor-interacting serine/threonine-protein kinase 1; FADD, Fas-associated death domain protein; TRADD, TNF receptor-associated death domain (TRADD); TRAF, TNF receptor-associated factor; FLIP, FLICE-like inhibitory protein (a.k.a. CFLAR, CASP8, and FADD-like apoptosis regulator); DD, death domain; DED, death effector domain
Studies of interactions of pro- and anti-apoptotic BCL proteins with each other and pore-forming BAK and BAX suggest that BID, BIM, and PUMA are direct activators.27, 28, 55 However, many molecular details of pore formation are missing.2, 8 Our data support the idea that direct involvement of additional players explains inconsistencies between in vitro studies with BCL proteins and in vivo apoptosis.6, 8 Our results suggest that tBID in complex with 1-380, rather than tBID alone, is the biologically relevant inducer of cytochrome C release. Although direct binding of MITCH2 and tBID was recently demonstrated,53 it is possible that in cells MITCH2 facilitates tBID recruitment to mitochondria54 by interacting with tBID–(1-380) complex. It is also tempting to speculate that BIM and PUMA might also have their specific ‘helpers' in cytochrome C release, possibly generated by caspases. Our finding suggests that non-BCL proteins must be taken into account for comprehensive understanding of the molecular mechanisms of apoptosis.
In particular, discrete protein fragments generated by caspases might serve as effectors.9, 10 The functional consequences of the cleavage of most of the 777 caspase substrates in CASBAH database17 remain unknown. Caspase cleavage of several kinases unleashes or abrogates their pro-apoptotic or pro-survival functions, respectively, via changes in activity, localization, or substrate preferences.56 Caspase cleavage products of diverse proteins contribute to the progression of apoptosis due to loss or gain of function or via dominant-negative action.1 Our experiments revealed a direct role of arrestin-2-(1–380) in cytochrome C release, identifying it as an earlier unappreciated active participant in the core mechanism of apoptosis. This is the first example of direct cooperation of two caspase products, 1-380 and tBID, at the point where the cell makes a fateful decision to live or die. This cooperation likely contributes to making this decision irreversible and also effectively sets a threshold for cell commitment to apoptotic death.
Materials and Methods
Reagents and antibodies
Antibodies against caspase-3, caspase-6, cleaved caspase-6, TRADD, FLIP, COX IV, PARP, BCL-2, BCL-XL, and MCL-1 were from Cell Signaling Technology (Beverly, MA, USA); TNFR1 antibody, recombinant mouse TNFα and caspase-8-cleaved recombinant mouse BID (tBID) were from R&D systems (Minneapolis, MN, USA); anti-FADD antibody and anti-BID antibody were from Santa Cruz (Santa Cruz, CA, USA); caspase-8 mouse monoclonal antibody (1G12) was from Enzo Life Sciences (Plymouth Meeting, PA, USA); antibody against glyceraldehyde-3-phosphate dehygrogenase (GAPDH) and rabbit anti-caspase-8 antibody were from Millipore (Billerica, MA, USA); and anti-GFP monoclonal antibody and anti-cytochrome C antibody were from BD Bioscience (Palo Alto, CA, USA). Mouse monoclonal pan-arrestin F4C1 antibody recognizing epitope DGVVLVD (residues 43-47) in the N-domain was from Dr. LA Donoso (Wills Eye Institute, Philadelphia, PA, USA). Rabbit polyclonal antibodies against residues 357–418 of arrestin-2 (No. 178) were described previously.29 Active recombinant caspases 1 through 10 were from Millipore or BioMol (Plymouth Meeting, PA, USA), and caspase inhibitors were from BioMol. All restriction enzymes were from New England Biolabs (Ipswich, MA, USA). Tissue culture media and reagents were from Invitrogen (Carlsbad, CA, USA). Mutations in bovine arrestin-2 in pGEM2-based plasmid were introduced by PCR, as described.57 C-terminally GFP-tagged mutants were constructed by subcloning fragments carrying the mutation into appropriately digested pEGFP-N1-arrestin2. All constructs were verified by dideoxy sequencing.
Cell culture and transfection
Arrestin-3 knockout and DKO MEFs58 (a gift from Dr. RJ Lefkowitz, Duke University), COS7, BID KO MEFs (a gift of Dr. SS Zinkel, Vanderbilt University, Nashville, TN, USA), caspases-8 fl/fl MEFs (a gift of Dr. SM Hedrick, University of California, San Diego, CA, USA), Rat-1 cells, and HEK293T cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin (P/S) at 37 °C and 5% CO2. Lipofectamine 2000 (1 : 3, DNA:lipid) was used to trasfect cells. For apoptosis assays, cells were stimulated with the indicated dose of TNFα with cychloheximide (CHX) or etoposide for the indicated time periods at 37 °C with 5% CO2. Total DNA was balanced with empty vector in all experiments.
Protein preparation and western blotting
Cells were lysed in Lysis solution (Ambion, Grand Island, NY, USA) and incubated for 5 min at 95 °C. Protein concentration was measured with Bradford reagent (Bio-Rad, Hercules, CA, USA). The protein was precipitated with nine volumes of methanol, pelleted by centrifugation (10 000 × g, 10 min at RT), washed with 90% methanol, dried, and dissolved in SDS sample buffer at 0.5 mg/ml. Equal amounts of protein were analyzed by reducing SDS-PAGE and western blotting onto Immobilon-P (Millipore, Bedford, MA, USA). The membrane was blocked with 5% non-fat dry milk in TBS with 0.1% Triton X-100 (TBST) and incubated with appropriate primary and then secondary antibodies coupled with horseradish peroxidase (Jackson Immunoresearch Laboratories, West Grove, PA, USA) in TBST with 1% BSA. Bands were visualized with SuperSignal enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA) and detected by exposure to X-ray film (Fujifilm, Stamford, CT, USA).
In vitro transcription and translation
pGEM2-based plasmids with the bovine arrestin-2 coding sequence equipped with the ‘idealized' 5'-untranslated region under the control of the SP6 promoter were linearized with Hind III downstream of the coding sequence. In vitro transcription and translation were performed as described.59 Arrestin proteins were labeled by the incorporation of [3H]leucine and [14C]leucine with the specific activity of the mix between 1.9 and 2.5 Ci/mmol, resulting in the specific activity of arrestins within the range of 66–85 Ci/mmol (146–190 d.p.m./fmol). The translation of every mutant used in this study produced a single-labeled protein band with the expected mobility on SDS-PAGE.
In vitro caspase cleavage assays
With in vitro translated arrestins
To identify the caspase cleavage sites in arrestin-2, 50 fmol of in vitro-translated wild-type or mutant arrestin2 was incubated for 90 min at 30 °C with 100 units of indicated caspase (Biomol). Biomol defines one unit of caspase activity as the amount of enzyme that at 30 °C cleaves 1 pmol/min of caspase substrate Ac-IETD-pNA (Biomol catalog No. P-431; present at 200 μM). The reactions were stopped by SDS sample buffer, and the proteins were analyzed on 10% polyacrylamide gel. The products were visualized by autoradiography.
With purified arrestins
For the caspase cleavage experiments in vitro, 75 ng of purified recombinant arrestin-2 was incubated for 3 h at 37 °C in 50 μl of caspase cleavage buffer in the presence or absence of 1 unit of individual purified human recombinant active caspases (Millipore). The standard assay buffer was 50 mM HEPES, pH7.4, 100 mM NaCl, 0.1% CHAPS, 1 mM EDTA, and 10 mM DTT. Assay in high salt buffer was routinely done using 1.0 M ammonium citrate in standard assay for caspases-2, -8, -9, and -10.19, 20 APO-10 was added in caspase-10 reaction to give a final concentration of 0.09%. Reactions were terminated by SDS sample buffer and boiling for 5 min and analyzed by western blotting with F4C1. Note that caspases used with purified arrestin-2 were from Millipore (Calbiochem, La Jolla, CA, USA), which defines one unit of the recombinant caspase as the enzyme activity that cleaves 1 nmol of the caspase substrate IETD-pNA per hour at 37 °C.
Subcellular fractionation
Cells were harvested by centrifugation at 600 × g for 10 min at 4 °C, washed in phosphate-buffered saline (PBS), resuspended in five volumes of hypotonic buffer (10 mM NaCl, 1.5 mM MgCl2, 10 mM Tris-HCl, pH 7.5, protease inhibitors) and incubated 5 min on ice. Cells were homogenized by 10 passes through a 26G 5/8 needle fitted on 1-ml syringe, and sucrose concentration was adjusted to 250 mM with 2 M sucrose. Unbroken cells and nuclei were removed by centrifugation at 1300 × g for 10 min at 4 °C. The resulting supernatant was centrifuged at 15 000 × g for 15 min at 4 °C to obtain heavy membrane fraction. The supernatant was centrifuged at 100 000 × g for 1 h at 4 °C to obtain cytosolic fraction (S100). Heavy membrane fraction was washed with 10 mM Tris-HCl, pH 7.5, 1 mM EDTA (T10E buffer) containing 250 mM sucrose and solubilized in Lysis solution (Ambion).
Expression and purification of recombinant proteins
Arrestin-2-(1-380) and arrestin-2-(D380/408E) were expressed in Escherichia coli and purified as described.59 Recombinant mouse His-tagged tBID was expressed in E. coli strain BL21-codon plus(DE3)-RIL using pET15b-Bidp15 (Addgene plasmid 8782; Addgene, Cambridge, MA, USA). Bacteria were cultured at 30 °C in LB with ampicillin. The induction of expression was started at A600=0.8–1.0 by the addition of 0.4 mM isopropyl β-D-thiogalactoside at 30 °C for 3 h. The bacteria were pelleted by centrifugation and resuspended in the buffer containing 5 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH7.9, 2 mM benzamidine, and 1 mM PMSF. In all, 1.5 ml of fresh 3 mg/ml lysozyme (dissolved in lysis buffer) was added to cell suspension from 1.1 l of original culture and incubated for 40 min on ice. Then, 1 ml of 3 mg/ml DNase II (Sigma, St. Louis, MO, USA) was added and the suspension was sonicated (three times for 15 s at 95% amplitude) and centrifuged at 12 000 r.p.m. for 90 min (GSA rotor, Sorvall, Lafayette, CO, USA). The supernatants were passed through a His-Bind Nickel-agarose affinity column (Qiagen, Valencia, CA, USA). The column was washed with buffer containing 60 mM imidazole, 500 mM NaCl, and 20 mM Tris-HCl, pH7.9 (CB). The proteins were eluted with buffer containing with a 400-ml linear gradient (CB/0.05 M imidazole→CB/0.25 M imidazole), and 10-ml fractions were collected. The major tBID peak elutes in 8–12 fractions between 90 mM and 240 mM imidazole. The tBid-containing fractions were pooled and concentrated to 0.5–3 mg/ml and passed through a 0.8-μm Millipore filter. The purity was determined by 15% SDS-PAGE followed by Commassie Blue staining. Protein was measured by Bradford method (Bio-Rad). The activity of purified Hig6-tagged tBid was tested using isolated mouse liver mitochondria (Supplementary Figure S2C).
Immunocytochemistry
DKO MEFs cells transfected with pDS-Red2-Mito (Clontech, Mountain View, CA, USA) and arrestin-2-(1-380) for 48 h were fixed by incubation with 4% paraformaldehyde for 10 min at RT. Cells were blocked in PBS/0.3% Triton/3% bovine serum albumin for 1 h at RT. Polyclonal antibody to arrestin (F431, 1 : 500) was used to label arrestin-2-(1-380). Primary antibodies were visualized with Alexa 488 labeled-chicken anti-rabbit secondary antibody (Invitrogen; 1 : 200) for 1 h at RT. Immunofluorescence was imaged with a Zeiss LSM510 inverted confocal microscope equipped with a 60 × lens with numerical aperture (NA) of 1.40 (Vanderbilt University Medical Center Cell Imaging Shared Resource). All images shown are from single confocal sections processed using Adobe Photoshop (Adobe, San Jose, CA, USA).
In vitro arrestin–tBID binding assay
Binding of DblE and arrestin-2-(1-380) to purified His-tagged tBID was assayed by His-tag pull-down using Ni-NTA resin (Qiagen), according to the manufacturer's instructions. Briefly, 50 μl purified His-tBID (10 μg) were incubated with 25 μl Ni-NTA resin (50% slurry) in binding buffer (50 mM Hepes, pH 7.3, 150 mM NaCl) at 4 °C with gentle rotation for 1 h. Subsequently, 50 μl of arrestin (10 μg) solution containing 50 mM imidazole were added to the Ni-NTA-tBID mixture and incubated at 4 °C with gentle rotation for 1 h. The suspensions were transferred to centrifuge filters (Ultrafree, Millipore) and washed three times with 200 μl wash buffer (50 mM imidazole, 50 mM Hepes, pH 7.3, 150 mM NaCl). The proteins were eluted from Ni-NTA by 100 μl elution buffer (250 mM imidazole, 50 mM Hepes, pH 7.3, 150 mM NaCl). The eluates were analyzed by SDS-PAGE and western blotting. Samples obtained in the absence of His-tBID served as controls for non-specific binding.
Retroviral constructs and infection
pFB-IRES2-EGFP, pFB-380-IRES2-EGFP, pFB-IRES2-EGFP-SV40-BID, and pFB-380-IRES2-EGFP-SV40-BID were generated as follows: arrestin-2-(1-380) in pIRES2-EGFP (Clontech) was generated by excising the coding sequence from pGEM2-based plasmid and subcloning it into the appropriately digested pIRES2-EGFP. IRES2-EGFP or arrestin-2-(1-380)-IRES2-EGFP from pIRES2-EGFP vector were excised with EcoRI and Not I and ligated into the retroviral vector pFB to create pFB-IRES2-EGFP or pFB-(1-380)-IRES2-EGFP, respectively. Alleles encoding FL BID from pcDNA3.0-BID were inserted under SV40 promotor of pCMS-EGFP (Clontech) and used as template for PCR. SV40-BID cDNA was amplified by PCR (with forward primer 5′-ATAAGAATGCGGCCGCTCACACCGCATACGC-3′ and reverse primer 5′-TAAAGCGGCCGCGGATCCTCAGTCCATCTC-3′), digested with Not I, and subcloned into the pFB-IRES2-EGFP or pFB-380-IRES2-EGFP digested with Not I. All constructs were verified by dideoxy sequencing.
To generate retrovirus, 6 μg of appropriate pFB construct and 6 μg pVpack-GP (Stratagene, Cedar Creek, TX, USA; 217566) and pVpack-VsvG (Stratagene, 217567) retroviral packaging plasmids were co-transfeced into HEK293T using MBS mammalian transfection kit from Agilent Technologies (Wilmington, DE, USA). For the next 4 days after transfection, the media containing virus was collected and replaced with fresh complete DMEM. Virus-containing medium filtered through 0.45-μm filter (5 ml), and 6 μg/ml polybrene was used daily per 100-mm dish. After 48 h of infection, cells were trypsinized and re-plated in two of 60-mm dishes for flow cytometry and two wells of six-well plate for western blotting. After additional 48 h of infection, cells were harvested, washed, and stained with anti-active caspase-3 antibody for flow cytometry.
Quantification of apoptosis
Apoptotic cells were identified by staining using AlexaFlour-647 rabbit anti-active caspase-3 antibody kit (BD Pharmingen, San Jose, CA, USA) or YO-PRO-131 (Invitrogen). MEFs were plated and treated with 10 ng/ml TNFα with 10 μg/ml cycloheximide (CHX) or etoposide (40 μM for Rat1 cells or 100 μM for MEFs) for the indicated times. Cells were trypsinized, pelleted, washed with ice-cold PBS, fixed in 4% paraformaldehyde for 10 min at room temperature, and washed with 1% FBS in PBS. Cells were permeabilized in 0.1% Triton X-100 for 5 min at RT, stained with AlexaFluor-647 anti-active caspase-3 antibody for 1 h, and washed with 1% FBS in PBS. Cells positive for the caspase-3 were quantified using LSRII flow cytometer (Becton Dickinson, San Jose, CA, USA). Cells were gated for analysis by a combination of forward and side scatter channels, which were identical for all samples. Experiments were repeated at least three times in triplicate. Flowjo software (Tree Star Inc., Ashland, OR, USA) was used for analysis. Cells used for quantification expressed GFP along with constructs of interest to identify transfected/infected cells. The fraction of apoptotic cells was independently measured using YO-PRO-1 staining, according to the manufacturer's protocol (Invitrogen). YO-PRO-1 specifically labels apoptotic cells without affecting cell viability.31 For YO-PRO-1 staining, MEFs were treated with 10 ng/ml TNF-α and 10 μg/ml CHX for the indicated times, harvested, washed, and resuspended in cold PBS with suggested dilutions of YO-PRO-1. Cells were incubated for 30 min on ice and then analyzed in an LSRII flow cytometer (Becton Dickinson) using 488 nm excitation and measuring fluorescence emission at 530 nm.
Additionally, apoptosis in BID KO MEFs was analyzed by the release of cytochrome C from the mitochondria essentially as described60 with some modifications. BID KO MEFs were infected with retrovirus to express GFP, 1-380+GFP, BID+GFP, or 1-380+BID+GFP. GFP-positive cells were isolated by cell sorting using an Aria III (BD Biosciences, San Jose, CA, USA), re-plated into 60-mm dish (1 × 105/dish), and grown overnight. Cells were then treated with of TNFα/CHX (10 ng/ml/10 μg/ml) or 10 μg/ml CHX alone for 6 or 10 h, harvested, and treated with digitonin (50 μg/ml in PBS with 100 mM KCl) for 5 min on ice (until >95% were permeabilized as assessed by trypan blue exclusion). The cells were then fixed in paraformaldehyde (4% in PBS) for 20 min at room temperature, washed twice with PBS, blocked in blocking buffer (3% BSA, 005% saponin in PBS) for 1 h at room temperature, and centrifuged. Mouse monoclonal anti-cytochrome C (clone 6H2.B4; BD Pharmingen) was added to the cell pellet, and the cells were incubated at 4 °C overnight. Cells were washed once with blocking buffer, and the cell pellet was incubated with Alexa Fluor 647 goat anti-mouse IgG (H+L) (Invitrogen) for 1 h at room temperature. The cells were washed once with blocking buffer, resuspended in PBS, and analyzed by flow cytometry.
Cytochrome C release from isolated mitochondria
The guidelines in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health was strictly followed for the animal procedures. The protocol was approved by the Institutional Animal Care and Use Committee of the Vanderbilt University. Mice were euthanized by isoflurane overdose, and the livers were rapidly removed. All subsequent steps were performed at 4 °C. One mouse liver was rinsed twice with PBS, then 0.5 g of liver tissue was homogenized in 5 ml of Buffer A (225 mM mannitol, 75 mM sucrose, 0.1 mM EGTA, 1 mg/ml fatty acid-free BSA, 10 mM HEPES-K, pH 7.4) with four strokes in a 7-ml tenbroeck ground glass homogenizer, then with three strokes of a loose-fitting pestle in a Dounce homogenizer, and diluted with an additional 5 ml of Buffer A. Homogenate was centrifuged at 1100 × g for 10 min to remove cell debris. Mitochondria from the supernatant were pelleted at 10 000 × g and washed once with Buffer A. Final pellet was resuspended in Buffer C (125 mM KCl, 0.5 mM MgCl2, 3.0 mM succinic acid, 3.0 mM glutamic acid, 10 mM HEPES-K pH 7.4, 25 mg/ml leupeptin, 3 mg/ml aprotinin, 100 mM PMSF, and 1 mM benzamidine) and kept on ice. Before the experiments, freshly isolated mitochondria were resuspended in Buffer C supplemented with 2 μM rotenone. Samples containing 20 μg of mitochondria in 50 μl were incubated with purified proteins at room temperature for 20 min. Mitochondria were pelleted by centrifugation at 16 000 × g for 10 min at 4 °C. Pellets and supernatants were analyzed on 15% gel. Untreated mitochondria served as a negative control for spontaneous cytochrome C release. Maximum cytochrome C release was measured in the presence of 0.5% Triton X-100. The intensity of the cytochrome C band in the supernatant was quantified to calculate the fraction of released cytochrome C.
Generation of caspase-8 knockout MEFs
Primary MEFs from day 13.5 Casp8 fl/fl mouse embryos21 were provided by Dr. SM Hedrick, UCSD. Immortalized caspase-8 fl/fl MEFs were infected with Cre-expressing retrovirus (pHR-MMPCreGFP from DF/HCC DNA Resource Core, Harvard, MA, USA). For single-cell cloning, MEFs were trypsinized, diluted, and seeded in 96-well plates. Clones were amplified to confluency on 60 mm dishes. The deletion of caspase-8 in several independent clones was confirmed by western blotting with caspase-8 antibody (1G12; Enzo Bio, Plymouth Meeting, PA, USA).
Statistical analysis was performed using ANOVA with Bonferroni/Dunn post hoc test with correction for multiple comparisons, or two-tailed Student's t-test, where appropriate.
Acknowledgments
We thank Dr. RJ Lefkowitz, Dr. LA Donoso, Dr. AL George, Dr. SS Zinkel, and Dr. SM Hedrick for A3KO and DKO MEFs, F4C1 antibody, pCMS vector, BID-deficient MEFs, and Caspase-8 fl/fl MEFs, respectively. Supported in part by NIH grants NS045117 and NS065868 (EVG), GM077561, GM081756, and EY011500 (VVG), GM047417 (JLB), Vanderbilt University Discovery Grant 1040659012 (VVG), and NARSAD Young Investigator Award (EVG). VUMC Cell Imaging Core was supported by S10 RR015682, and VUMC Flow Cytometry Shared Resource was supported by P30 CA68485 and DK058404.
Glossary
- 1-380
arrestin-2-(1-380) fragment generated by caspase cleavage
- A3KO
arrestin-3 knockout
- CHX
cycloheximide
- COX-IV
subunit IV of cytochrome C oxidase
- DblE
arrestin-2-D380E, D408E caspase-resistant mutant
- DKO
arrestin-2/3 double knockout
- FL
full-length
- GPCR
G protein-coupled receptor
- MEF
mouse embryonic fibroblast
- PARP
poly-(ADP-ribose) polymerase
- tBID
caspase-cleaved BID
- TNFα
tumor necrosis factor α
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by B Zhivotovsky
Supplementary Material
References
- Crawford ED, Wells JA. Caspase substrates and cellular remodeling. Annu Rev Biochem. 2011;80:1055–1087. doi: 10.1146/annurev-biochem-061809-121639. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13:780–788. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnareva Y, Andreyev AY, Kuwana T, Newmeyer DD. Bax activation initiates the assembly of a multimeric catalyst that facilitates Bax pore formation in mitochondrial outer membranes. PLoS Biol. 2012;10:e1001394. doi: 10.1371/journal.pbio.1001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization. Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix MM, Simon GM, Cravatt BF. Global mapping of the topography and magnitude of proteolytic events in apoptosis. Cell. 2008;134:679–691. doi: 10.1016/j.cell.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrus S, Trinidad JC, Barkan DT, Sali A, Burlingame AL, Wells JA. Global sequencing of proteolytic cleavage sites in apoptosis by specific labeling of protein N termini. Cell. 2008;134:866–876. doi: 10.1016/j.cell.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolan DW, Zorn JA, Gray DC, Wells JA. Small-molecule activators of a proenzyme. Science. 2009;326:853–858. doi: 10.1126/science.1177585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, Gurevich VV. Arrestins are ubiquitous regulators of cellular signaling pathways. Genome Biol. 2006;7:236. doi: 10.1186/gb-2006-7-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantari C, Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta. 2011;1813:558–563. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Wang L, Du F, Wang X. TNF-α induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Timmer JC, Salvesen GS. Caspase substrates. Cell Death Differ. 2007;14:66–72. doi: 10.1038/sj.cdd.4402059. [DOI] [PubMed] [Google Scholar]
- Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-medicated and chemical-induced apoptosis. J Biol Chem. 1999;274:5053–5060. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- Lüthi AU, Martin SJ. The CASBAH: a searchable database of caspase substrates. Cell Death Differ. 2007;14:641–650. doi: 10.1038/sj.cdd.4402103. [DOI] [PubMed] [Google Scholar]
- Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C. Crystal structure of beta-arrestin at 1.9 A: possible mechanism of receptor binding and membrane translocation. Structure. 2001;9:869–880. doi: 10.1016/s0969-2126(01)00644-x. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, et al. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS. Activation of caspases-8 and -10 by FLIP(L) Biochem J. 2004;382:651–657. doi: 10.1042/BJ20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner DR, Ch'en IL, Kolla RV, Hoffmann A, Hedrick SM. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol. 2005;175:3469–3473. doi: 10.4049/jimmunol.175.6.3469. [DOI] [PubMed] [Google Scholar]
- Bohgaki T, Mozo J, Salmena L, Matysiak-Zablocki E, Bohgaki M, Sanchez O, et al. Caspase-8 inactivation in T cells increases necroptosis and suppresses autoimmunity in Bim-/- mice. J Cell Biol. 2011;195:277–291. doi: 10.1083/jcb.201103053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- Pobezinskaya YL, Kim YS, Choksi S, Morgan MJ, Li T, Liu C, et al. The function of TRADD in signaling through tumor necrosis factor receptor 1 and TRIF-dependent Toll-like receptors. Nat Immunol. 2008;9:1047–1054. doi: 10.1038/ni.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Herreweghe F, Festjens N, Declercq W, Vandenabeele P. Tumor necrosis factor-mediated cell death: to break or to burst, that's the question. Cell Mol Life Sci. 2010;67:1567–1579. doi: 10.1007/s00018-010-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Raman D, Gurevich EV, Vishnivetskiy SA, Gurevich VV. Visual and both non-visual arrestins in their ‘inactive' conformation bind JNK3 and Mdm2 and relocalize them from the nucleus to the cytoplasm. J Biol Chem. 2006;281:21491–21499. doi: 10.1074/jbc.M603659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, Benovic JL, Gurevich VV. Arrestin2 expression selectively increases during neural differentiation. J Neurochem. 2004;91:1404–1416. doi: 10.1111/j.1471-4159.2004.02830.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Idziorek T, Estaquier J, De Bels F, Ameisen JC. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J Immunol Methods. 1995;185:249–258. doi: 10.1016/0022-1759(95)00172-7. [DOI] [PubMed] [Google Scholar]
- Wesselborg S, Engels IH, Rossmann E, Los M, Schulze-Osthoff K. Anticancer drugs induce caspase-8/FLICE activation and apoptosis in the absence of CD95 receptor/ligand interaction. Blood. 1999;93:3053–3063. [PubMed] [Google Scholar]
- de Vries JF, Wammes LJ, Jedema I, van Dreunen L, Nijmeijer BA, Heemskerk MH, et al. Involvement of caspase-8 in chemotherapy-induced apoptosis of patient derived leukemia cell lines independent of the death receptor pathway and downstream from mitochondria. Apoptosis. 2007;12:181–193. doi: 10.1007/s10495-006-0526-6. [DOI] [PubMed] [Google Scholar]
- Wieder T, Essmann F, Prokop A, Schmelz K, Schulze-Osthoff K, Beyaert R, et al. Activation of caspase-8 in drug-induced apoptosis of B-lymphoid cells is independent of CD95/Fas receptor-ligand interaction and occurs downstream of caspase-3. Blood. 2001;97:1378–1387. doi: 10.1182/blood.v97.5.1378. [DOI] [PubMed] [Google Scholar]
- von Haefen C, Wieder T, Essmann F, Schulze-Osthoff K, Dörken B, Daniel PT. Paclitaxel-induced apoptosis in BJAB cells proceeds via a death receptor-independent, caspases-3/-8-driven mitochondrial amplification loop. Oncogene. 2003;22:2236–2247. doi: 10.1038/sj.onc.1206280. [DOI] [PubMed] [Google Scholar]
- Shelton SN, Shawgo ME, Robertson JD. Cleavage of Bid by executioner caspases mediates feed forward amplification of mitochondrial outer membrane permeabilization during genotoxic stress-induced apoptosis in Jurkat cells. J Biol Chem. 2009;284:11247–11255. doi: 10.1074/jbc.M809392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler B, Anguissola S, Concannon CG, Rehm M, Kögel D, Prehn JH. Bid participates in genotoxic drug-induced apoptosis of HeLa cells and is essential for death receptor ligands' apoptotic and synergistic effects. PLoS One. 2008;3:e2844. doi: 10.1371/journal.pone.0002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee EA, Keogh SA, Martin SJ. Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ. 2000;7:556–565. doi: 10.1038/sj.cdd.4400689. [DOI] [PubMed] [Google Scholar]
- Michalak EM, Villunger A, Adams JM, Strasser A. In several cell types tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ. 2008;15:1019–1029. doi: 10.1038/cdd.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G protein-coupled receptors. Pharm Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Kiselev A, Socolich M, Vinos J, Hardy RW, Zuker CS, Ranganathan R. A molecular pathway for light-dependent photoreceptor apoptosis in Drosophila. Neuron. 2000;28:139–152. doi: 10.1016/s0896-6273(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Alloway PG, Howard L, Dolph PJ. The formation of stable rhodopsin-arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron. 2000;28:129–138. doi: 10.1016/s0896-6273(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Chen J, Shi G, Concepcion FA, Xie G, Oprian D, Chen J. Stable rhodopsin/arrestin complex leads to retinal degeneration in a transgenic mouse model of autosomal dominant retinitis pigmentosa. J Neurosci. 2006;26:11929–11937. doi: 10.1523/JNEUROSCI.3212-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Gao H, Ni Y, Wang B, Wu Y, Ji L, et al. Beta-arrestin 2 functions as a G-protein-coupled receptor-activated regulator of oncoprotein Mdm2. J Biol Chem. 2003;278:6363–6370. doi: 10.1074/jbc.M210350200. [DOI] [PubMed] [Google Scholar]
- Luan B, Zhang Z, Wu Y, Kang J, Pei G. Beta-arrestin2 functions as a phosphorylation-regulated suppressor of UV-induced NF-kappaB activation. EMBO J. 2005;24:4237–4246. doi: 10.1038/sj.emboj.7600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Feng Y, Kang J, Liu C, Li Z, Li D, et al. Critical regulation of CD4(+) T cell survival and autoimmunity by beta-arrestin 1. Nat Immunol. 2007;8:817–824. doi: 10.1038/ni1489. [DOI] [PubMed] [Google Scholar]
- Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. {beta}-Arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J Biol Chem. 2009;284:8855–8865. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Dery O, Bunnett NW. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc Natl Acad Sci USA. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povsic TJ, Kohout TA, Lefkowitz RJ. Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem. 2003;278:51334–51339. doi: 10.1074/jbc.M309968200. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Vines CM, Cimino DF, Prossnitz ER. Arrestins block G protein-coupled receptor-mediated apoptosis. J Biol Chem. 2004;279:24578–24584. doi: 10.1074/jbc.M402121200. [DOI] [PubMed] [Google Scholar]
- Katz C, Zaltsman-Amir Y, Mostizky Y, Kollet N, Gross A, Friedler A. Molecular basis of the interaction between proapoptotic truncated BID (tBID) protein and mitochondrial carrier homologue 2 (MTCH2) protein: key players in mitochondrial death pathway. J Biol Chem. 2012;287:15016–15023. doi: 10.1074/jbc.M111.328377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaltsman Y, Shachnai L, Yivgi-Ohana N, Schwarz M, Maryanovich M, Houtkooper RH, et al. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat Cell Biol. 2010;12:553–562. doi: 10.1038/ncb2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa M, Kornbluth S. Caspases and kinases in a death grip. Cell. 2009;138:838–854. doi: 10.1016/j.cell.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Celver J, Abdryashitov RI, Chavkin C, Gurevich VV. Targeted construction of phosphorylation-independent b-arrestin mutants with constitutive activity in cells. J Biol Chem. 1999;274:6831–6834. doi: 10.1074/jbc.274.11.6831. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Nat Acad Sci USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Benovic JL. Arrestin: mutagenesis, expression, purification, and functional characterization. Methods Enzymol. 2000;315:422–437. doi: 10.1016/s0076-6879(00)15859-8. [DOI] [PubMed] [Google Scholar]
- Waterhouse NJ, Trapani JA. A new quantitative assay for cytochrome c release in apoptotic cells. Cell Death Differ. 2003;10:853–855. doi: 10.1038/sj.cdd.4401263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.