Abstract
The basement membrane complex (BMC) is a critical component of the extracellular matrix (ECM) that supports and facilitates the growth of cells. This study investigates four detergents commonly used in the process of tissue decellularization and their effect upon the BMC. The BMC of porcine urinary bladder was subjected to either 3% Triton-X 100, 8 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 4% sodium deoxycholate, or 1% sodium dodecyl sulfate (SDS) for 24 hours. The BMC structure for each treatment group was assessed by immunolabeling, scanning electron microscopy (SEM), and second harmonic generation (SHG) imaging of the fiber network. The composition was assessed by quantification of dsDNA, glycosaminoglycans (GAGs), and collagen content. Results showed that collagen fibers within samples treated with 1% SDS and 8 mM CHAPS were denatured and the ECM contained less GAGs compared to samples treated with 3% Triton X-100 or 4% sodium deoxycholate. Human microvascular endothelial cells (HMECs) were seeded onto each BMC and cultured for 7 days. Cell-ECM interactions were investigated by immunolabeling for integrin β-1, SEM imaging, and semi-quantitative assessment of cellular infiltration, phenotype, and confluence. HMECs cultured on a BMC treated with 3% Triton X-100 were more confluent and had a normal phenotype compared to HMECs cultured on a BMC treated with 4% sodium deoxycholate, 8 mM CHAPS, and 1% SDS. Both 8 mM CHAPS and 1% SDS damaged the BMC to the extent that seeded HMECs were able to infiltrate the damaged sub-basement membrane tissue, showed decreased confluence, and an atypical phenotype. The choice of detergents used for tissue decellularization can have a marked effect upon the integrity of the BMC of the resultant bioscaffold.
Keywords: Re-endothelization, Organ engineering, Extracellular matrix, Biologic scaffold, Regenerative medicine, Decellularization
1. Introduction
The decellularization of tissues for the purpose of utilizing the extracellular matrix (ECM) as a bioscaffold for reconstructive surgical procedures or whole organ engineering involves the use of various enzymes, detergents and mechanical/physical methods[1–3]. During the process of decellularization, parenchymal cells within the source tissues and organs such as the dermis, small intestine, urinary bladder, liver and lung are destroyed and/or removed[1, 2, 4–7]. However, the less abundant but equally important non-parenchymal cells are also removed in the process. Such cells include the endothelial cells of the resident vascular network structures and any site appropriate epithelial cell populations. The remaining vascular network, devoid of endothelial cells, has been proposed as a potential guide and substrate for revascularization[8–11]. Therefore, the effects of decellularization methods upon the structure and composition of the basement membrane complex (BMC) are critical for subsequent in-vitro or in-vivo recellularization.
There have been several published methods for decellularizing tissues and generating biologic scaffolds composed of ECM, each of which describes a unique and specific recipe of enzymes and detergents. Commonly used detergents include Triton X-100[11, 12], 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)[18], sodium deoxycholate[13], and sodium dodecyl sulfate (SDS)[8, 14–17]. Detergents are able to solubilize cell membranes and dissociate DNA from proteins, making such agents attractive for the decellularization process. Studies have shown that ionic detergents can be more effective for cellular removal than non-ionic and zwitterionic detergents[18]. However, subjecting tissue to harsh detergents, such as SDS, can disrupt the ECM structure[19], eliminate growth factors[20], and/or denature essential proteins[21].
The present study compared the effects of four commonly used decellularization agents upon the BMC and its ability to support endothelial cells in vitro. The findings have relevance for decellularization strategies used in the production of ECM derived biologic scaffolds and whole organ engineering.
2. Materials and Methods
2.1. Scaffold Preparation and Decellularization
Porcine urinary bladders were obtained from animals (~120 kg) at a local abattoir (Thoma's Meat Market, Saxonburg, PA).
Bladders were frozen (>16 h at −80 °C) and thawed completely before use. The BMC and underlying lamina propria were isolated and harvested from the bladders as previously described [7, 22, 23]. The tissue was then placed in 0.02% Trypsin/0.05% EGTA solution for two hours at 37°C with physical agitation to detach cells from the extracellular matrix. Tissue samples were then subjected to either, 3% Triton-X 100 (Sigma-Aldrich), 8 mM CHAPS (Sigma-Aldrich), 4% sodium deoxycholate (Sigma-Aldrich), 1% SDS (Bio-Rad), or Type I water (non-detergent control) for 24 hours with physical agitation (300 rpm on an orbital shaker). Scaffolds were next rinsed with 1X PBS for 15 min followed by water for 15 min and each repeated. A 24 hour 1X PBS wash followed. Scaffolds were subsequently rinsed with 1X PBS followed by water for 15 min each and repeated. Lastly, scaffolds were sterilized via gamma irradiation at a dose of 2 × 106 RADS.
2.2. dsDNA Quantification
Scaffolds were digested in 0.6% Proteinase K solution for at least 24 hours at 50°C until no visible tissue remained. Phenol/Chloroform/Isoamyl alcohol was added and samples were centrifuged at 10,000xg for 10 min at 4°C. The top aqueous phase containing the DNA was transferred into a new tube. Sodium acetate and ethanol was added to each sample and the solution was mixed and placed at −80°C overnight. While still frozen, the samples were centrifuged at 4°C for 10 min at 10,000×g. Supernatant was discarded and all residual alcohol was removed. Pellet was suspended in TE buffer. Double stranded DNA was quantified using Quant-iT PicoGreen Reagent (Invitrogen Corp., Carlsbad, CA, USA) according to the manufacturer’s instructions. The dsDNA assay was performed in duplicate, and was performed two times.
2.3. Preparation of Urea-Heparin Extracts for Growth Factor Assays
Three hundred (300) mg of ECM powder was suspended in 4.5 ml of urea-heparin extraction buffer. The extraction buffer consisted of 2 M urea and 5 mg/ml heparin in 50 mM Tris with protease inhibitors [1mM Phenylmethylsulfonyl Fluoride (PMSF), 5 mM Benzamidine, and 10 mM N-Ethylmaleimide (NEM)] at pH 7.4. The extraction mixture was rocked at 4°C for 24 hours and then centrifuged at 3,000 g for 30 minutes at 4°C. Supernatants were collected and 4.5 ml of freshly prepared urea-heparin extraction buffer was added to each pellet. Pellets with extraction buffer were again rocked at 4°C for 24 hours, centrifuged at 3,000 g for 30 minutes at 4°C, and supernatants were collected. Supernatants from first and second extractions were dialyzed against Barnstead filtered water (three changes, 80 to 100 volumes per change) in Slide-A-Lyzer Dialysis Cassettes, 3500 MWCO (Pierce, Rockford, IL). The concentration of total protein in each dialyzed extract was determined by the bicinchoninic acid (BCA) Protein Assay (Pierce, Rockford, IL) following the manufacturer’s protocol, and extracts were frozen in aliquots until time of assay.
2.4 Growth Factor Assays
Concentrations of basic fibroblast growth factor (bFGF),and vascular endothelial growth factor (VEGF) in urea-heparin extracts of dermis samples were determined with the Quantikine Human FGF basic Immunoassay (R&D Systems, Minneapolis, MN), and the Quantikine Human VEGF Immunoassay (R&D Systems). Manufacturer’s instructions were followed for both growth factor assays. Each assay for bFGF and VEGF was performed in duplicate, and each growth factor assay was performed two times. Results are reported as mean ± standard error. It should be noted that growth factor assays measured the concentration of each growth factor and did not measure growth factor activity.
2.5. Soluble Collagen and Sulfated GAG Quantification
10 mg ECM/ml (dry weight) were enzymatically digested in a solution of 1 mg/ml porcine pepsin (SigmaeAldrich, St. Louis, MO) in 0.01 N HCl under a constant stir rate for 72 h at room temperature. The pH neutralized pepsin digests were diluted and assayed for soluble, triple helical collagen content using the Sircol Collagen Assay (Biocolor Ltd., Carrickfergus, United Kingdom) per the manufacturer’s instructions. The pH neutralized pepsin digest were also analyzed for total protein recovered using the BCA protein assay (Pierce). A pepsin buffer solution was used as the negative control and subtracted from the signal. Similarly, 50 mg/ml of powdered ECM in 100 mM Tris (pH 7.5) was digested with 0.1 mg/ml proteinase K (Sigma) at 50 °C for 24 h with gentle agitation. The proteinase K digests were then assayed for sulfated GAG concentration using the Blyscan Sulfated Glycosaminoglycan Assay (Biocolor Ltd.) per the manufacturer’s instructions. All results were normalized to dry weight tissue. Assays were performed in duplicate on three independent samples for each treatment group.
2.6. Histologic Staining and Immunolabeling of the BMC
Fixed scaffolds were embedded in paraffin and cut into 5µm sections. Sections were either stained with Hematoxylin and Eosin (H&E), Movat’s Pentachrome, or used for immunolabeling. For immunolabeling, slides were manually deparaffinized, placed in Citrate Antigen Retrieval Buffer (10 mM, pH 6), and heated to 95°C for 20 min. Slides were then cooled to room temperature, rinsed in 1X PBS three times for 3 min, placed in humidity chamber to incubate for 1 hr with blocking solution (2% Goat Serum, 1% BSA 0.1% Triton X-100 0.1% Tween) at room temperature, then incubated overnight at 4°C with anti-collagen I antibody (Sigma-Aldrich, C2456, 1:1000) in blocking solution. Slides were then rinsed with 1X PBS as above, treated with 3% hydrogen peroxide in methanol solution for 30 min, and re-rinsed. Biotinylated secondary antibody Horse Anti-Mouse IgG (Vector Labs, 1:100) was then applied for 30 min. Slides were rinsed as above, ABC solution applied for 30 min in humidity chamber at 37°C, re-rinsed, and 3,3'-diaminobenzidine (DAB, Vector Labs) was applied under microscope. To stain collagen IV (ab6586, Abcam, 1:500), laminin (L9393, Sigma-Aldrich, 1:100), and Collagen VII (C6805, Sigma-Aldrich, 1:10) the same protocol as used for collagen I was applied with an added 0.05% pepsin in 0.01 mM hydrochloric acid for 15 minutes in humidity chamber at 37°C following citrate acid buffer antigen retrieval. Staining for collagen VII also used a blocking solution that contained 4% goat serum and 2% BSA, and a 1 hour hydrogen peroxide incubation time. After DAB staining, all slides were counterstained with hematoxylin, dehydrated and manually coverslipped using standard mounting medium. Images were taken at the luminal interface of the tissue.
2.7. Analysis of the ECM Fiber Network of the BMC Luminal Surface
A complete set of fiber network descriptors was collected from SEM images of each BMC including: pore size distribution, node density (number of fibers intersections per µm2), and fiber diameter. Porosity was described by the mean of the pore size (µm2) histogram. Automated extraction of these fiber architectural features was achieved with an algorithm, which has been previously described in detail [24]. Briefly, the SEM image was digitally processed by a cascade of steps including equalization with a 3×3 median filter, local thresholding through the Otsu method, thinning, smoothing, morphological operators, skeletonization, binary filtering for Delaunay network refinement, and ultimately the detection of fiber network architecture and its descriptors. For each treatment group ten images were analyzed.
2.8. Quantification of Collagen Fiber Denaturation via SHG
To both visualize and quantify the integrity of the collagen fiber network of the basement membrane, intact samples were imaged enface from the surface of the BMC with an Olympus FV1000 multiphoton system (MPM). The Olympus FV1000 MPM system was operated with Olympus Fluroview software, and was equipped with a Chameleon ultra diode-pumped laser, and a 25× XL Plan N objective with a N.A. of 1.05 and a field of view of 500 um. The excitation wavelength was chosen at 800 nm at a 5% laser transmissivity. The photomultiplier voltage was maintained at 400 V across all samples for subsequent signal intensity analysis. The emission wavelength was received by a filter set to 400±100nm for second harmonic generation signal of collagen. Image scans were performed at a depth of 25 µm, 50 µm, 75 µm, and 100 µm to encompass the BMC with a sampling speed set to 2 µs/pixel with a 2 line Kalman filter. Image sections were then imported into ImageJ for intensity analysis through a background subtraction, and then applying the integrated density function whereby area*intensity. This parameter provides a relative measurement of the SHG signal. It has previously been found that denaturation of collagen fibers results in the destruction of the SHG due to the loss of the noncentrosymmetric crystalline structure at the molecular level[25]. Additional image stacks were acquired for select samples with an incremental z-step of 0.5 µm to a depth of 100 µm for 3D reconstruction and visualization using Imaris software.
2.9. Endothelial Cell Seeding and Culture
Sterilized scaffolds were placed with the BMC luminal surface facing up in a 6 well plate. HMECs (a gift from Francisco Candal, Center for Disease Control and Prevention, Atlanta, GA) were cultivated in MCDB-131 medium containing 10% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin and 100 ug/mL streptomycin. MCDB-131 medium was from Invitrogen (Carlsbad, CA); all other reagents for cell growth were from Thermo Fisher Hyclone (Logan, Utah). Cells were grown at 37°C in 5% CO2 and were harvested for seeding when they were approximately 100% confluent. HMECs were seeded on the BMC surface of each treatment group in triplicate. A total of 1 × 106 cells were cultured on each scaffold within a 2cm diameter stainless steel culture ring containing 5 ml of culture medium. Scaffolds were then placed in an incubator at 37°C in 5% CO2 for 24 hrs of culture, at which time the culture rings were removed and the seeded scaffolds were transferred to a new 6 well plate with fresh media. Culture media was then replaced on day 2 and day 5. After 7 days of culture, seeded scaffolds were fixed in 10% neutral buffered formalin, gluteraldehyde, or liquid nitrogen for subsequent analysis.
2.10. Immunolabeling of Seeded HMECs
After 7 days of culture samples were fixed in formalin for at least 24 hours, embedded in paraffin and cut into 5 µm transverse sections. Sections were either stained with Hematoxylin and Eosin (H&E), or used for Ki67 and integrin β-1 immunolabeling. Slides for immunolabeling were deparaffinized and rehydrated with decreasing concentration of alcohol and water. Antigen retrieval was performed with Citrate Antigen Retrieval Buffer (10mM, pH6). Retrieval buffer was heated until a boiling point was reached, slides were immersed, removed from heat, and cooled for 20 min. Slides were washed with 1X PBS 3× for 3 min each. 0.05% Pepsin digest was applied to samples for 15 min minutes in humidity chamber at 37°C. Blocking solution was applied (2% Goat serum 1% BSA 0.1% Triton 0.1% Tween) for 1hr at room temp. Slides were washed with 1X PBS as above. Rabbit anti-integrin β-1 (Abcam, AB52971, 1:1000) in blocking buffer was applied to each sample. Rabbit anti-Ki67 (Abcam, AB15580, 1:100) in blocking was applied to each sample on a separate slide. The samples were then incubated at 4°C overnight. Slides were washed with 1X PBS as above. Alexa-Flour 594 goat anti rabbit (Invitrogen, 1:200) was applied for 1 hr at room temperature for the anti-integrin β-1sample. Alexa-Flour 488 goat anti rabbit (Invitrogen, 1:200) was applied for 1 hr at room temperature for the anti-Ki67 samples. Slides were washed with 1X PBS as above. Coverslips were added with anti-FADE containing DAPI (Invitrogen, P36931). Analysis of apoptosis in tissue sections was performed with a DeadEnd™ Colorimetric TUNEL System (Promega Corp. PR-G7130) according to the manufacturer’s specifications.
2.11. Semi-Quantitative Scoring of HMECs
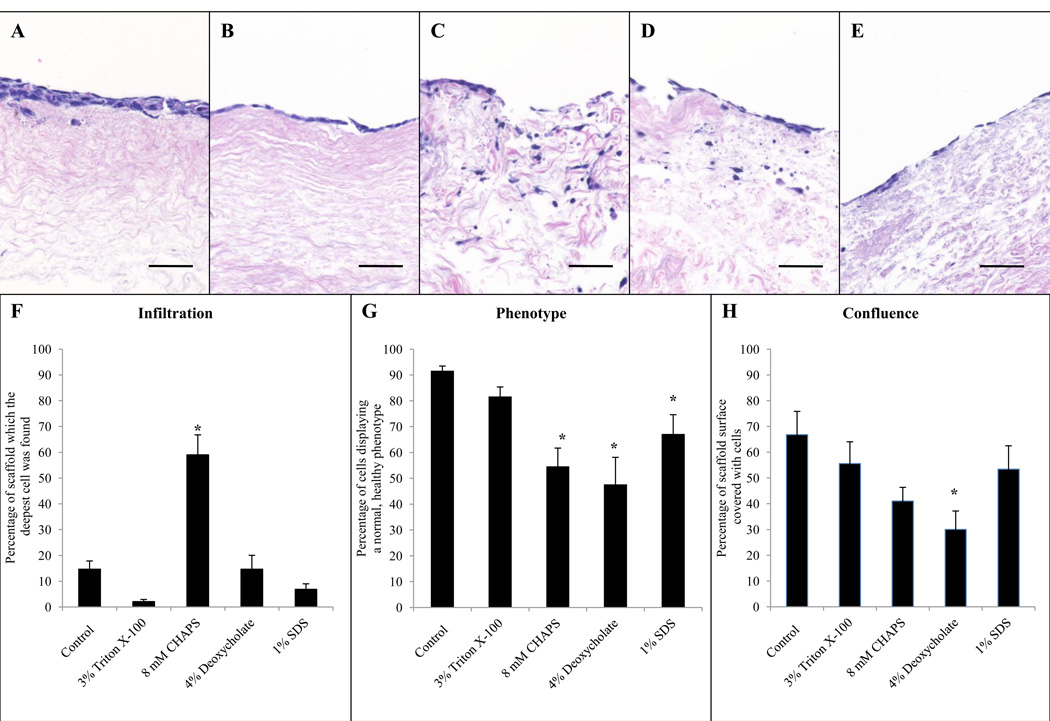
Fixed seeded scaffolds were embedded in paraffin and cut into 5µm sections. Sections were stained with H&E and images were taken of the HMECs. The images were then evaluated by five blinded investigators using a standardized system as previously described [20]. Criteria included cellular infiltration, confluence, and cell phenotype. Associated descriptions of these metrics can be found in Table 1 and graphical examples in supplementary Fig. 3 All aspects were evaluated on a scale of 0 to 100.
Table 1.
Definitions and descriptions of the metrics used to semi-quantitative score the HMECs.
| Confluence (%) | The confluence score is defined as the percentage of the BMC surface covered with cells. A score of 100 would indicate a fully coated surface with adjoining cells and no gaps. |
| Phenotype (%) | They phenotype score is defined as the percentage of healthy appearing cells. A healthy cell is flat and fully adhered to surrounding tissue and other cells. An unhealthy is round and not adhered to the surrounding tissue or other cells. |
| Infiltration (%) | The infiltration score is defined as the percentage of the total depth in which cells have migrated within the tissue. For example, if cells are found halfway into the tissue, this would correspond to an infiltration score of 50. |
2.12. Scanning Electron Microscopy
SEM was used to examine the surface topology of urinary bladders treated with each detergent. Scanning electron micrographs were also taken of the HMEC seeded scaffolds after 7 days of culture on each sample. Samples were fixed in 2.5% glutaraldehyde in 1X PBS, cut into blocks of approximately 8mm3and washed thoroughly in 1X PBS for three times at 15 minutes each. Samples were then fixed in 1% OsO4 in 1X PBS for 15 minutes each, dehydrated in graded series of alcohol (30%–100%) baths for 15 minutes each. Samples were then critically point dried with hexamethyldisiloxane mounted on studs, sputter coated, and stored in a desiccator until imaged. SEM images were captured using a JEOL 6335F Field Emission SEM with backscatter detector.
2.13. Statistical Analysis
Results are shown as averages ± standard error. A one-way analysis of variance was performed to determine whether a particular detergent group was significantly different, followed by a post-hoc Dunnets test to determine whether any detergent treatment was different from the non-detergent control group (p<0.05).
3. Results
3.1. dsDNA Content
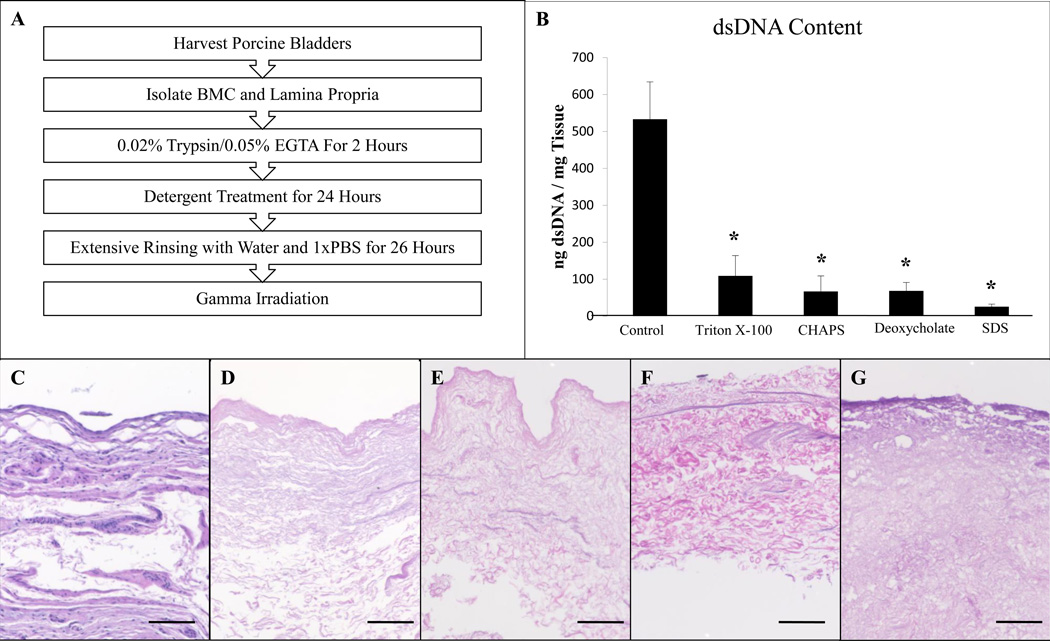
No visible nuclei were observed by imaging of Hematoxylin and Eosin stained sections for any of the detergent groups (Figure 1C–G). Double stranded DNA quantification of the scaffolds showed that each detergent caused markedly greater removal of the dsDNA compared to treatment with Type I water (Figure 1B). Scaffolds treated with 1% SDS contained less dsDNA than those treated with 8 mM CHAPS (P<0.05) or 4% sodium deoxycholate (P<0.05). 1% SDS was the only detergent able to meet a previously established decellularization criterion of 50 ng dsDNA/mg tissue (Figure 1F) [1].
Figure 1.
Overview of the process used to prepare the BMC scaffolds (A). Double stranded DNA quantification of the scaffolds treated with each detergent showed significant removal compared to those treated with Type I water (B). Hematoxylin and eosin stained sections of BMC scaffolds prepared with no detergent (C), Triton X-100 (D), CHAPS (E), sodium deoxycholate (F), and SDS (G). No signs of nuclear material are visible for any of the detergent groups. Scale bar represents 200µm.
3.2. Collagen and sulfated GAG Content
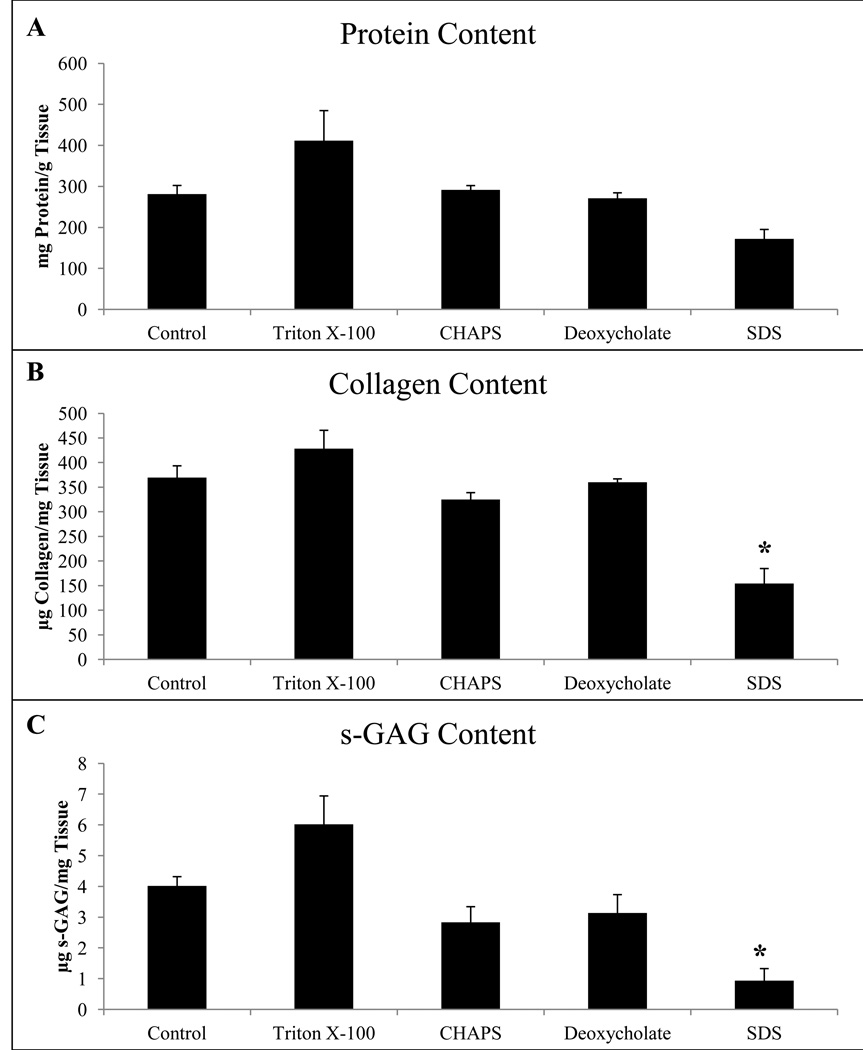
While scaffolds treated with 3% Triton X-100, 8 mM CHAPS, and 4% sodium deoxycholate retained a soluble collagen content similar to that of the water control, treatment with 1% SDS resulted in a significant loss of detectable soluble collagen (Figure 2B). The assay used detected only soluble collagen, therefore non-soluble remnant collagen may still be present. This finding suggests that detergent treatment with SDS resulted in either a decrease in soluble collagen present or modification of the molecular structure of this collagen to the point of insolubility. The greater amount of soluble collagen for Triton X-100 compared to the water control is an artifact of the normalization to dry weight. More specifically, the relative density of ECM to total weight is increased after decellularization for Triton X-100 after removal of cellular content compared to the water control. Scaffolds treated with 3% Triton X-100, 4% sodium deoxycholate, and 8mM CHAPS retained GAGs similar to that of the water control, while scaffolds treated with 1% SDS retained a lesser amount of detectable GAGs than the water control (Figure 2C).
Figure 2.
Biochemical assays to quantify soluble protein from pepsin extract (A), collagen (B), and sulfated GAGs (C) normalized to dry weight tissue. Graph shows mean ± standard error, and * indicates significance at p<0.05 when compared to the water control group.
3.3. Immunolabeling
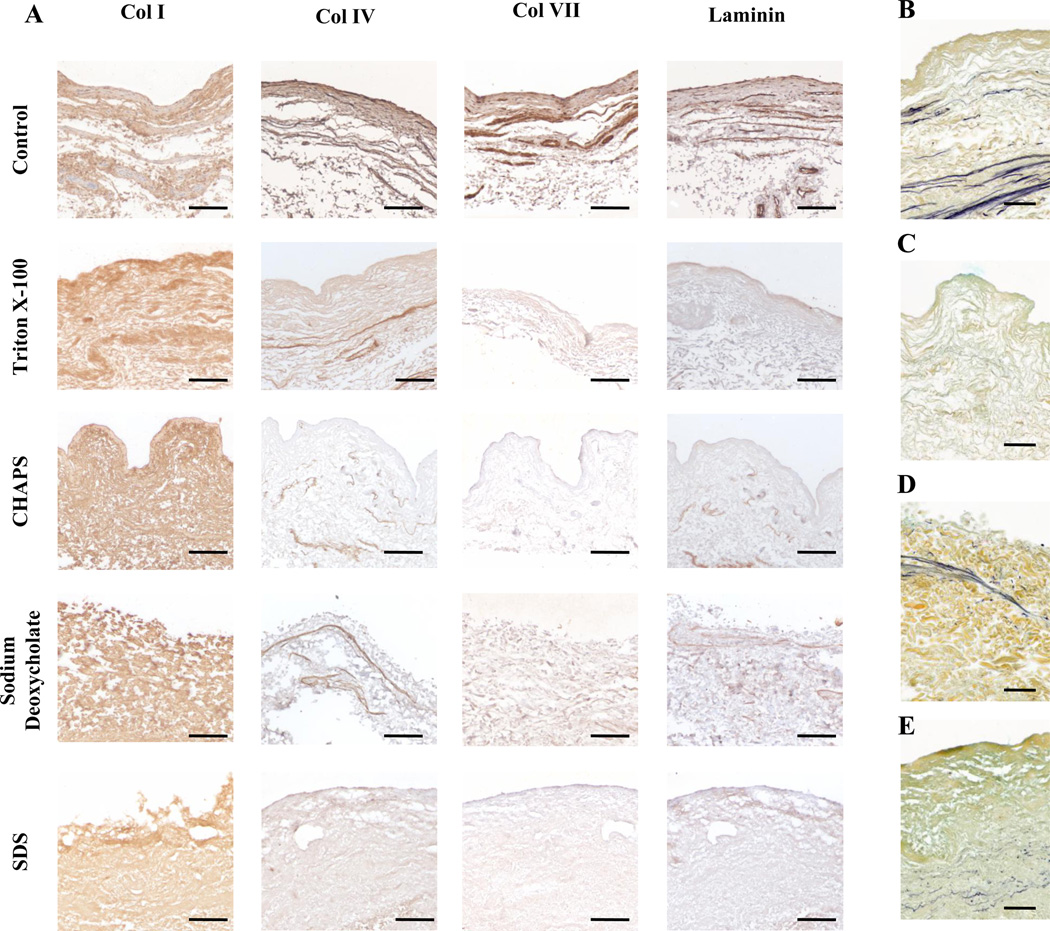
The no detergent control showed positive staining at the basement membrane surface of collagen I, collagen IV, collagen VII, and laminin (Figure 3A) as previously reported[26]. All scaffold treatments were positive for collagen I staining (Figure 3A). No treated scaffolds stained positive for collagen IV, VII, or laminin except for Triton X-100 and sodium deoxycholate treated scaffolds, both of which had positive expression of collagen IV (Figure 3A). However, this positive staining was not localized to the surface as would be expected for an intact basement membrane.
Figure 3.
Immunolabeling of Collagen I, Collagen IV, Collagen VII, and laminin for BMC scaffolds prepared with water, Triton X-100, CHAPS, sodium deoxycholate, and SDS (A). Movats Pentachrome stains for Triton X-100 (B), sodium deoxycholate (C), CHAPS (D), and SDS (E) where yellow, blue, and purple represents collagen, proteoglycans and GAGs, and elastin, respectively. Scale bar represents 100µm.
3.4. Movats Stain
Scaffolds treated with Triton X-100 and sodium deoxycholate retained elastin fibers, whereas CHAPS had no visible elastin fibers and SDS had only a small amount of thin fragmented fibers. GAGs were visible in both Triton X-100 and CHAPS while not visible for sodium deoxycholate and SDS confirming the observations from sulfated GAG quantification (Figure 3B).
3.5. Analysis of the BMC Fiber Network
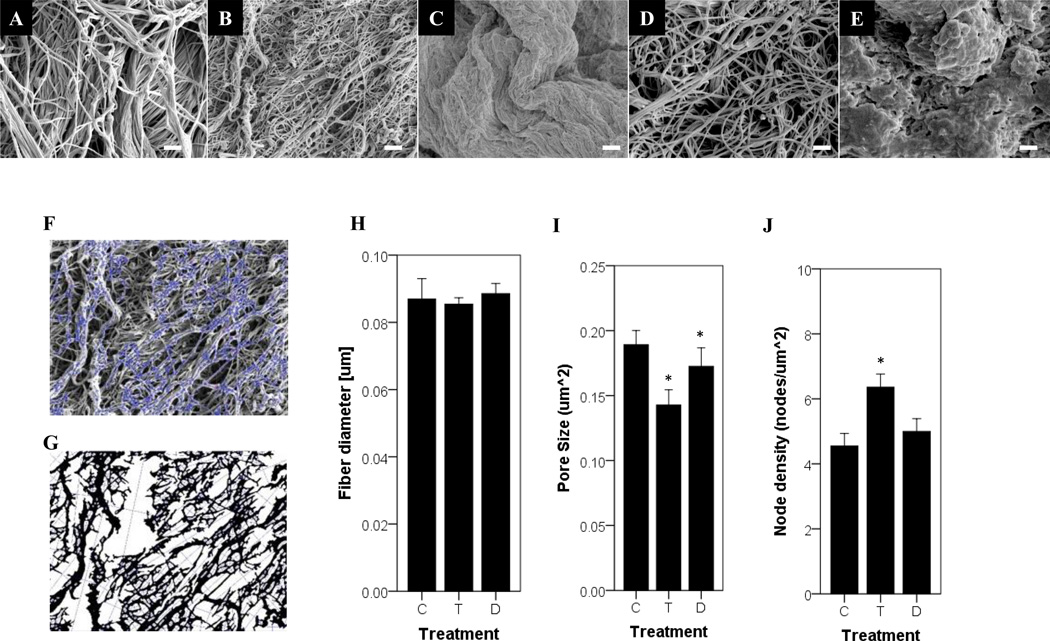
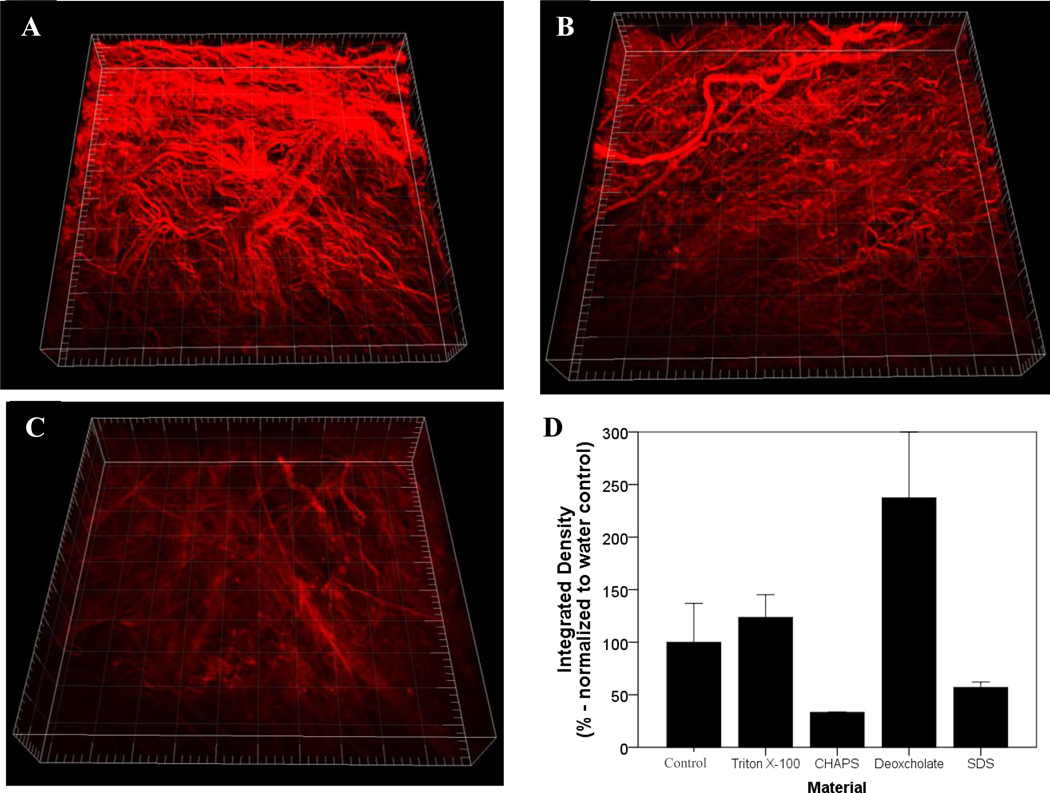
Quantitative assessment of the SEM of the BMC luminal surface showed that treatment without a detergent, with 3% Triton X-100, or with 4% sodium deoxycholate retained an intricate fiber network (Figure 4 B, C& E). However, treatment with 8 mM CHAPS and 1% SDS resulted in an amorphous structure lacking distinct fibers (Figure 4 D&F). The fiber diameter was not different with treatment of Triton X-100 or sodium deoxycholate compared to the no detergent control (Figure 4I). While there was a slightly smaller pore size for Triton X-100 and sodium deoxycholate compared to the no detergent control(Figure 4J), and a higher node density for Triton X-100 these changes were small compared to previously published variations(Figure 4K) [4, 24]. Thus, treatment with Triton X-100 and sodium deoxycholate were able to retain the original configuration of the fiber network. Multiphoton imaging confirmed a loss of a distinct fiber network for SDS compared to Triton X-100 beneath the surface of the sample (Figure 5A–C). The lower collagen signal intensity for SDS indicates fiber denaturation (Figure 5D). The higher signal intensity value for triton x-100 and sodium deoxycholate compared to the water control may be due an increase in the density of ECM constituents due to loss of cellular material. These values provide a relative comparison of the effects of detergent treatments that are consistent in finding with visual observations of both SHG volumes and SEM images.
Figure 4.
SEM of the BMC fiber network for scaffolds prepared with water as a no detergent control (A), Triton X-100 (B), CHAPS (C), sodium deoxycholate (D), and SDS (E). Scare bar represents 1µm. An automated algorithm (F,G) was applied to quantify fiber network parameters of fiber diameter (H), pore size (I), and node density (J)[24]. Graph shows mean ± standard error, and * indicates significance at p<0.05 when compared to the water control group. C, T, and D denote the water control, Triton X-100, and sodium deoxycholate, respectively.
Figure 5.
3 dimensional rendering of collagen fiber network from second harmonic generation (SHG) signal with two photon microscopy (TPM) for BMC scaffolds prepared with water (A), Triton X-100 (B), and SDS (C). Major tick represents 50 µm, whereby the total length and depth is 500 µm and 100 µm, respectively. An integrated density integrity was applied and normalized to the no detergent control (D). Graph shows mean ± standard error, and * indicates significance at p<0.05.
3.6. Semi-quantitative HMEC scoring
HMECs cultured on the BMC prepared with 3% Triton X-100 had a similar level of confluence, infiltration depth, and phenotype compared to cells cultured on scaffolds treated with type I water (control). These HMECs were characterized by a flat morphology (Figure 6B). HMECs cultured on the BMC prepared with 8 mM CHAPS were less confluent, had a greater infiltration depth, and an atypical phenotype compared to HMECs cultured on the control (Figure 6). HMECs cultured on scaffolds prepared with 4% sodium deoxycholate were less confluent, had a similar infiltration depth, and an atypical phenotype compared to cells cultured on a no detergent control (Figure 6). HMECs cultured on scaffolds prepared with 1% SDS had a similar percentage of confluence, similar infiltration depth, but a less normal phenotype compared to cell cultured on a no detergent control (Figure 6).
Figure 6.
Hemoxylin and eosin stained sections of endothelial cells cultured on the BMC of porcine urinary bladders subjected to water (A), Triton X-100 (B), CHAPS (C), sodium deoxycholate (D), and SDS (E) for 24hrs. Semi-quantitative analysis of cellular infiltration (F), phenotype (G), and level of confluence (H) was performed by five blinded scores. Scale bar represents 50µm. Graph shows mean ± standard error, and * indicates significance at p<0.05.
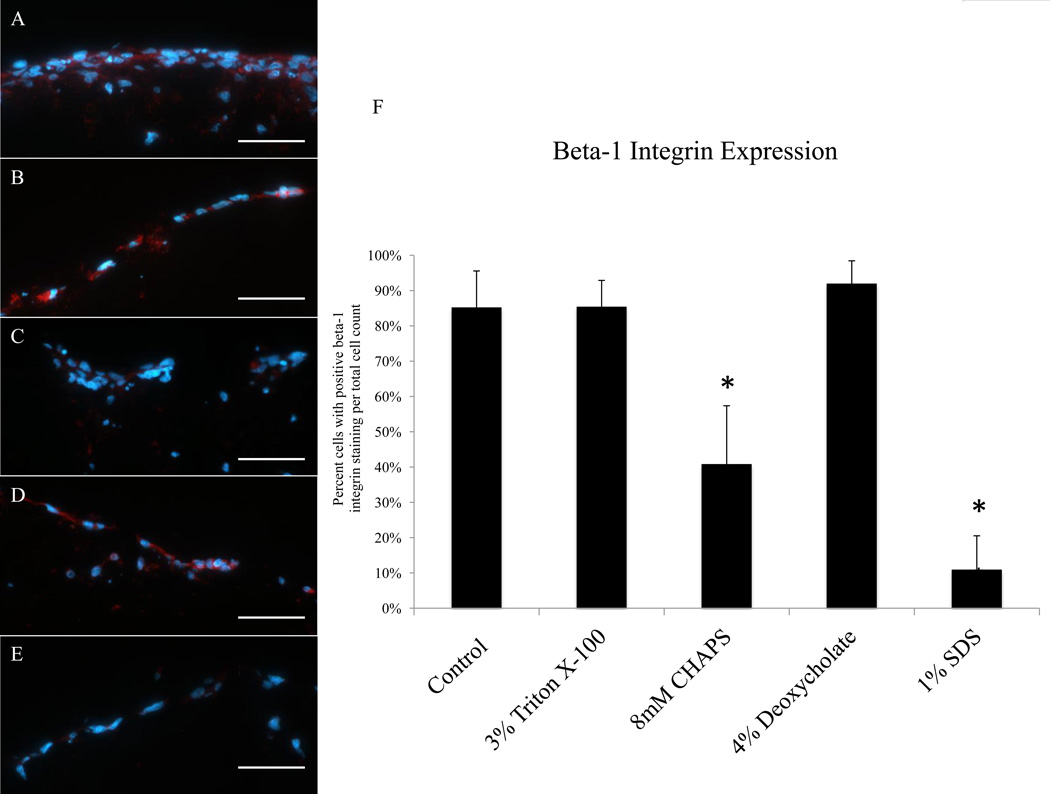
3.7. Integrin β-1 Expression, Ki67, and TUNEL
HMECs cultured on the BMC prepared with 8 mM CHAPS and 1% SDS had a lower number of cells stain positive for integrin β-1 compared to HMECs cultured on the BMC not subjected to a detergent (Figure 7). HMECs cultured on the BMC prepared with 3% Triton X-100 and 4% sodium deoxycholate had a similar percentage of cells expressing integrin β-1 compared to cells cultured on the no detergent control tissue (Figure 7). The percent of cells positive for Ki67 was below 3% for all groups and no significant differences were seen when comparing to the control (Supplemental Figure 1). Minimal TUNEL-positive cells were found on the BMC prepared with 3% Triton X-100 (Supplemental Figure 5).
Figure 7.
Immunofluorescent images of integrin β-1 (red) and DAPI (blue) of HMECs cultured on the BMC of porcine urinary bladders exposed to water (A), Triton X-100 (B), CHAPS (C), sodium deoxycholate (D), and SDS (E) for 24hrs. Percentage of cells positive for integrin β-1 was determined for each group (F). Scale bar represents 50 µm. Graph shows mean ± standard error, and * indicates significance at p<0.05.
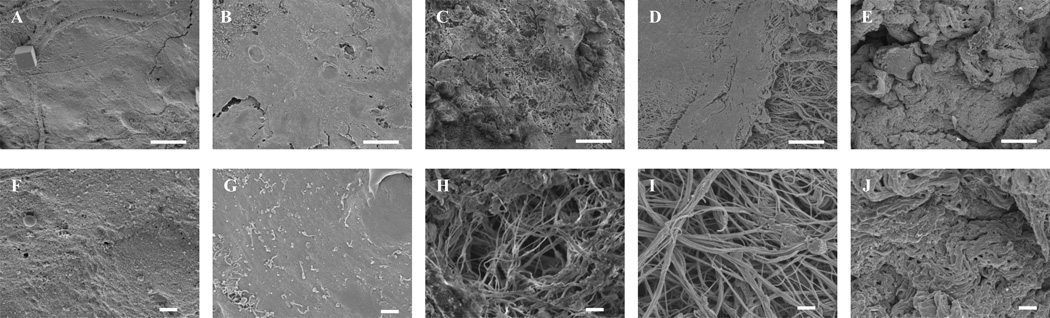
3.8. SEM of Seeded Endothelial Cells
SEM images of HMECs cultured on the BMC prepared with 3% Triton X-100 are similar to the no detergent control in terms of cell morphology and coverage of the BMC. SEM images of seeded scaffolds prepared with 4% sodium deoxycholate showed areas of endothelial cell coverage as well as exposed ECM. 8 mM CHAPS and 1% SDS, however, showed greater area of exposed ECM and less endothelial cell coverage (Figure 8).
Figure 8.
SEM images (2,000×) of HMECs cultured for 7 days BMC scaffolds prepared with water (A), Triton X-100 (B), CHAPS (C), sodium deoxycholate (D), and SDS (E). Scale bar represents 10 µm. SEM images (10,000×) of HMECs cultured for 7 days BMC scaffolds prepared with water (F), Triton X-100 (G), CHAPS (H), sodium deoxycholate (I), and SDS (J). Scale bar represents 1 µm.
4. Discussion
Thorough decellularization of tissues and organs is essential for promoting a constructive remodeling host response when such decellularized structures are used as therapeutic bioscaffolds [27]. If a tissue is not thoroughly decellularized and residual cellular material is present, the in-vivo remodeling response is characterized by chronic inflammation, fibrotic encapsulation, and scar tissue formation [27–29]. The basement membrane is one of the first extracellular matrix structures made by the developing embryo with its major constituent laminin-111 synthesized at the eight cell stage[30]. This basement membrane is the first matrix structure with which embryonic stem cells interact and represents a key biosignal for separating endoderm from ectoderm; thus, it is logical that the BMC can represent an important structure in a bioscaffold composed of ECM. Scaffolds containing a BMC are used in a variety of pre-clinical and clinical applications[31–40]. Some of these scaffolds are seeded with cells before use[41–43]. Examples of ECM scaffolds with a BMC structure include several dermal ECM products such as Alloderm™ and Strattice®, urinary bladder matrix such as MatriStem™, and virtually all three dimensional whole organ scaffolds such as liver[10, 12, 14, 44–46], lung[6, 15, 17] and kidney[16, 47–50]. Therefore, the results of the present study have relevance for a variety of biomaterial applications involving the use of ECM scaffold materials.
Four detergents commonly used for decellularization of tissues and organs were systematically evaluated and compared for their effect on the BMC and the ability of the resulting BMC to support human microvascular endothelial cells in vitro. The detergents investigated were 3% Triton X-100, 4% sodium deoxycholate, 8 mM CHAPS, and 1% SDS. The detergents and their respective concentrations were selected because of their frequent use as decellularization agents and their different chemical characteristics [1]. All detergents facilitate cell lysis and solubilize the released hydrophobic proteins through the formation of micelles. Triton X-100 is non-ionic containing an uncharged hydrophilic head group and disrupts lipid–lipid and lipid–protein interactions, while leaving protein–protein interactions intact. Non-ionic detergents are considered a non-denaturant and are widely used in the proteomics field for isolating membrane proteins in their biologically active form [51–53]. In contrast, sodium deoxycholate and SDS are anionic detergents containing a net negatively charged hydrophilic head group that can solubilize cytoplasmic and nuclear membranes, denature ECM proteins, and disrupt native tissue structure. SDS contains a straight hydrocarbon chain whereas sodium deoxycholate contains a more complicated rigid steroidal structure. CHAPS is zwitterionic, contains a rigid steroid ring structure, and has properties of both non-ionic and anionic detergents while containing a net charge of zero. Therefore, it is not surprising that these detergents each have distinctly different effects on the BMC. Results of the present study show that these detergent specific effects change not only the ultrastructure and composition of the BMC, but also the behavior of seeded endothelial cells.
In its native state, the BMC defines the spatial relationships among various populations of cells, and influences cell behavior. For ECM scaffold materials that have a BMC on one surface but not the opposite surface (i.e., the material has a “sidedness”), it has been shown HMECs seeded on the non-BMC side invade below the surface of the material and populate the underlying connective tissues. In contrast, HMECs seeded on the BMC will form confluent layers on, but will not invade, the intact surface of the BMC[22]. Results of the present study are consistent with these previous findings. Of note however, the present study also shows that tissue exposed to SDS and CHAPS as part of the decellularization process is left with a BMC upon which the HMECs are less confluent, can migrate through the BMC into the subjacent tissue, and show an atypical phenotype compared to those seeded on an undamaged BMC. These findings, combined with the SEM results, altered collagen fiber organization, and loss of GAGs lead to the unavoidable conclusion that the ultrastructure and composition of the BMC are negatively affected when exposed to SDS and CHAPS. This conclusion, however, must be limited to the specific concentrations and exposure times investigated in the present study. These timeframes and concentrations were chosen because of their relatively common use. It is also unknown whether these findings will apply to tissues with a BMC other than the urinary bladder.
The compositional and structural complexity of the BMC is noteworthy [22]. The BMC contains laminin-111, collagen IV, heparan sulfate proteoglycan, entactin/nidogen, and several growth factors arranged in a three dimensional ultrastructure which promotes cell adhesion, growth, migration, and invasion. This complexity provides a rational explanation for the potent biological activity of the BMC, and a plausible explanation, in fact expectation, for the finding that decellularization processes such as detergent exposure affect cell:matrix interactions. It is likely that cells interact with multiple components within the matrix. Components such as laminin-111, collagen IV, heparan sulfate proteoglycan, and entactin interact with adjacent cells via integrin receptors and in particular with integrins containing the β1 subunit. Exposure of the BMC to 8 mM CHAPS and 1% SDS decreased the number of cells staining positive for integrins containing the β1 subunit. These receptors regulate the cellular cytoskeleton and cell behavior. Furthermore, many of the major components, such as laminin-111, have multiple active sites for binding to cell surface receptors or other ECM components. Integrins are critical for cellular adhesion to the matrix and can induce either proliferative or differentiation responses. These factors emphasize the importance of understanding the effects of variables such as detergent exposure upon the subsequent biologic activity of materials composed of ECM derived by decellularization of source tissues, particularly when the resultant ECM has a BMC component.
Differences in scaffold surface fiber organization and evidence of collagen fiber denaturation were apparent from both SEM inspection and the results of automated image algorithms. SDS and CHAPS caused marked alterations of collagen fiber architecture while Triton X-100 and sodium deoxycholate were better tolerated and showed the surface of the BMC maintained an appearance that more closely resembled that of the no detergent control. These structural changes and the associated changes in the ligand landscape provide insight into the results of the cell seeding experiments. When HMECs were cultured on porcine urinary bladder basement membrane exposed to the chosen detergents, clear differences were seen in cell morphology, confluence, infiltration depth, and integrin β-1 expression. Findings of the present study provide useful information for the rational design of decellularization protocols for various tissues and organs.
5. Conclusions
The choice of detergent used for the decellularization of a tissue or organ is an important factor in the preparation of an ECM scaffold for therapeutic applications. Each detergent, depending on its chemical characteristics, has unique and distinct effects on ECM composition and structure. Less disruptive detergents, such as Triton X-100 or other non-ionic detergents are preferred for maintaining the native BMC structure and composition compared to more harsh detergents, such as SDS, which can denature essential ligands and proteins within the BMC. The disruption or denaturing of the native BMC architecture can negatively impact the interaction of cells with the scaffold. The results of this study can aid in the formulation of tissue and organ decellularization protocols such that the native biological activity of the resulting extracellular matrix scaffold is maximally preserved.
Supplementary Material
Acknowledgements
Denver Faulk was partially supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (NIH 1F31AA021324-01). Christopher Carruthers was partially supported by the National Science Foundation (NSF) Graduate Research Fellowship. The authors would like to thank Deanna Rhoads and the McGowan Histology Center for histologic section preparation and the center for Biologic Imaging at the University of Pittsburgh for access to imaging facilities. The authors would also like to thank Francisco Candal from the Center for Disease Control and Prevention, Atlanta, GA for providing the HMECs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Disclosures:
None
References
- 1.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Vorotnikova E, et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29(8):690–700. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Wolf MT, et al. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 33(29):7028–7038. doi: 10.1016/j.biomaterials.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sellaro TL, et al. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A. 16(3):1075–1082. doi: 10.1089/ten.tea.2008.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen TH, et al. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs. 195(3):222–231. doi: 10.1159/000324896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freytes DO, Stoner RM, Badylak SF. Uniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffolds. J Biomed Mater Res B Appl Biomater. 2008;84(2):408–414. doi: 10.1002/jbm.b.30885. [DOI] [PubMed] [Google Scholar]
- 8.Ott HC, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 9.Ott HC, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 16(8):927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 10.Uygun BE, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 16(7):814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baptista PM, et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 53(2):604–617. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 12.Soto-Gutierrez A, et al. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng Part C Methods. 17(6):677–686. doi: 10.1089/ten.tec.2010.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wainwright JM, et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 16(3):525–532. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao J, et al. Construction of a portal implantable functional tissue-engineered liver using perfusion-decellularized matrix and hepatocytes in rats. Cell Transplant. 20(5):753–766. doi: 10.3727/096368910X536572. [DOI] [PubMed] [Google Scholar]
- 15.Song JJ, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 92(3):998–1005. doi: 10.1016/j.athoracsur.2011.05.018. discussion 1005-6. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan DC, et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 33(31):7756–7764. doi: 10.1016/j.biomaterials.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Petersen TH, et al. Tissue-engineered lungs for in vivo implantation. Science. 329(5991):538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudson TW, Liu SY, Schmidt CE. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10(9–10):1346–1358. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 19.Courtman DW, et al. Development of a pericardial acellular matrix biomaterial: biochemical and mechanical effects of cell extraction. J Biomed Mater Res. 1994;28(6):655–666. doi: 10.1002/jbm.820280602. [DOI] [PubMed] [Google Scholar]
- 20.Reing JE, et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials. 31(33):8626–8633. doi: 10.1016/j.biomaterials.2010.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasimir MT, et al. Comparison of different decellularization procedures of porcine heart valves. Int J Artif Organs. 2003;26(5):421–427. doi: 10.1177/039139880302600508. [DOI] [PubMed] [Google Scholar]
- 22.Brown BN, et al. Surface characterization of extracellular matrix scaffolds. Biomaterials. 31(3):428–437. doi: 10.1016/j.biomaterials.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freytes DO, et al. Biaxial strength of multilaminated extracellular matrix scaffolds. Biomaterials. 2004;25(12):2353–2361. doi: 10.1016/j.biomaterials.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 24.D'Amore A, et al. Characterization of the complete fiber network topology of planar fibrous tissues and scaffolds. Biomaterials. 31(20):5345–5354. doi: 10.1016/j.biomaterials.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theodossiou TA, et al. Second harmonic generation confocal microscopy of collagen type I from rat tendon cryosections. Biophys J. 2006;91(12):4665–4677. doi: 10.1529/biophysj.106.093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown B, et al. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12(3):519–526. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 27.Keane TJ, et al. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33(6):1771–1781. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 28.Brown BN, et al. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badylak SF, et al. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14(11):1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 30.Wu TC, et al. Immunohistochemical localization of entactin and laminin in mouse embryos and fetuses. Dev Biol. 1983;100(2):496–505. doi: 10.1016/0012-1606(83)90242-7. [DOI] [PubMed] [Google Scholar]
- 31.Parekh A, et al. Repair of the tympanic membrane with urinary bladder matrix. Laryngoscope. 2009;119(6):1206–1213. doi: 10.1002/lary.20233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecheminant J, Field C. Porcine urinary bladder matrix: a retrospective study and establishment of protocol. J Wound Care. 21(10):476, 478–480, 482. doi: 10.12968/jowc.2012.21.10.476. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, et al. Effect of an Inductive Hydrogel composed of Urinary Bladder Matrix upon Functional Recovery Following Traumatic Brain Injury. Tissue Eng Part A. doi: 10.1089/ten.tea.2012.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L, et al. Porcine urinary bladder matrix-polypropylene mesh: a novel scaffold material reduces immunorejection in rat pelvic surgery. Int Urogynecol J. 23(9):1271–1278. doi: 10.1007/s00192-012-1745-8. [DOI] [PubMed] [Google Scholar]
- 35.Badylak SF, et al. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant. 2006;15(Suppl 1):S29–S40. doi: 10.3727/000000006783982368. [DOI] [PubMed] [Google Scholar]
- 36.Robinson KA, et al. Extracellular matrix scaffold for cardiac repair. Circulation. 2005;112(9 Suppl):I135–I143. doi: 10.1161/CIRCULATIONAHA.104.525436. [DOI] [PubMed] [Google Scholar]
- 37.Nieponice A, Gilbert TW, Badylak SF. Reinforcement of esophageal anastomoses with an extracellular matrix scaffold in a canine model. Ann Thorac Surg. 2006;82(6):2050–2058. doi: 10.1016/j.athoracsur.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 38.Eberli D, et al. Tunica repair with acellular bladder matrix maintains corporal tissue function. Int J Impot Res. 2007;19(6):602–609. doi: 10.1038/sj.ijir.3901587. [DOI] [PubMed] [Google Scholar]
- 39.Boruch AV, et al. Constructive remodeling of biologic scaffolds is dependent on early exposure to physiologic bladder filling in a canine partial cystectomy model. J Surg Res. 161(2):217–225. doi: 10.1016/j.jss.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Kelly DJ, et al. Increased myocyte content and mechanical function within a tissue-engineered myocardial patch following implantation. Tissue Eng Part A. 2009;15(8):2189–2201. doi: 10.1089/ten.tea.2008.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosario DJ, et al. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen Med. 2008;3(2):145–156. doi: 10.2217/17460751.3.2.145. [DOI] [PubMed] [Google Scholar]
- 42.Shah U, Bien H, Entcheva E. Microtopographical effects of natural scaffolding on cardiomyocyte function and arrhythmogenesis. Acta Biomater. 6(8):3029–3034. doi: 10.1016/j.actbio.2010.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eweida A, et al. Cultured keratinocytes on urinary bladder matrix scaffolds increase angiogenesis and help in rapid healing of wounds. Adv Skin Wound Care. 24(6):268–273. doi: 10.1097/01.ASW.0000398665.51283.44. [DOI] [PubMed] [Google Scholar]
- 44.Soto-Gutierrez A, et al. Engineering of an hepatic organoid to develop liver assist devices. Cell Transplant. 19(6):815–822. doi: 10.3727/096368910X508933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baeck C, Streetz K. The recellularized liver matrix: a novel way of transplantation? Hepatology. 52(4):1509–1511. doi: 10.1002/hep.23956. [DOI] [PubMed] [Google Scholar]
- 46.Shupe T, et al. Method for the decellularization of intact rat liver. Organogenesis. 6(2):134–136. doi: 10.4161/org.6.2.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badylak SF, et al. Engineered whole organs and complex tissues. Lancet. 379(9819):943–952. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 48.Baptista PM, et al. Whole organ decellularization - a tool for bioscaffold fabrication and organ bioengineering. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6526–6529. doi: 10.1109/IEMBS.2009.5333145. [DOI] [PubMed] [Google Scholar]
- 49.Arenas-Herrera JE, et al. Decellularization for whole organ bioengineering. Biomed Mater. 8(1):014106. doi: 10.1088/1748-6041/8/1/014106. [DOI] [PubMed] [Google Scholar]
- 50.Song JJ, et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 19(5):646–651. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnold T, Linke D. The use of detergents to purify membrane proteins. Curr Protoc Protein Sci. 2008;4(4):1–4. doi: 10.1002/0471140864.ps0408s53. [DOI] [PubMed] [Google Scholar]
- 52.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta. 2004;3:1–2. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Prive GG. Detergents for the stabilization and crystallization of membrane proteins. Methods. 2007;41(4):388–397. doi: 10.1016/j.ymeth.2007.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.