Abstract
Telomeres mediate biologic aging in organisms as diverse as plants, yeast, and mammals. We propose a telomere theory of reproductive aging that posits telomere shortening in the female germ line as the primary driver of reproductive aging in women. Experimental shortening of telomeres in mice, which normally do not exhibit appreciable oocyte aging, and which have exceptionally long telomeres, recapitulates the aging phenotype of human oocytes. Telomere shortening in mice reduces synapsis and chiasmata, increases embryo fragmentation, cell cycle arrest, apoptosis, spindle dysmorphologies, and chromosome abnormalities. Telomeres are shorter in the oocytes from women undergoing in vitro fertilization, who then produce fragmented, aneuploid embryos that fail to implant. In contrast, the testes are replete with spermatogonia that can rejuvenate telomere reserves throughout the life of the man by expressing telomerase. Differences in telomere dynamics across the life span of men and women may have evolved because of the difference in the inherent risks of aging on reproduction between men and women. Additionally, growing evidence links altered telomere biology to endometriosis and gynecologic cancers, thus future studies should examine the role of telomeres in pathologies of the reproductive tract.
Keywords: Reproductive aging, telomeres
Telomeres are short, tandem repeats of DNA that cap linear chromosome ends by binding members of the shelterin protein complex to form protective telomere loops (Fig. 1). An insufficient number of telomere repeats leads to chromosome uncapping, cell senescence, and death. Telomere length decreases with age, provides a surrogate marker for biological age, and predicts a number of age-related conditions, including diabetes mellitus (1), cardiovascular disease (2), liver disorders (3, 4), cancer (5), and death from all causes (6). Conservation of telomere length predicts exceptional longevity and health in old age (7). Generalized longevity is correlated with reproductive longevity in women (8–11), so common mechanisms may underlie both.
FIGURE 1.
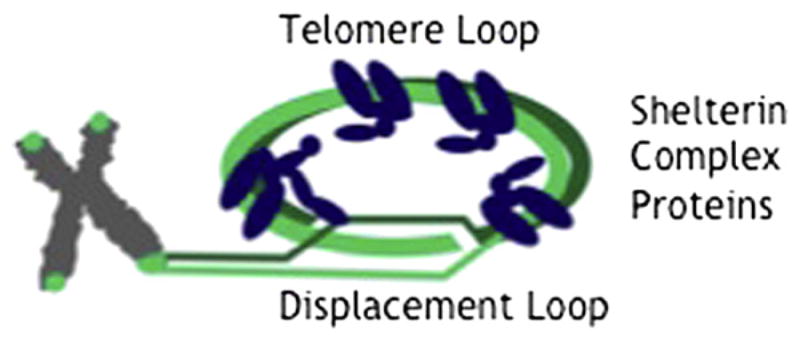
Schematic diagram of telomere loop (T loop) and shelterin proteins. Telomere repeat-binding factor 1 and 2 (TRF1, TRF2) associate with double-stranded DNA and serve as binding substrate for TIN2, TPP1, and Rap1. A third DNA-binding protein, Pot1, associates with the single-stranded DNA of the displacement loop (D loop).
Kalmbach. Telomeres and human reproduction. Fertil Steril 2013.
Aging of the reproductive system in women poses a paradox: the somatic tissues of the uterus remain receptive throughout a woman’s life, but the germ line in women, unlike in men, exhibits precocious and profound aging. As women increasingly delay attempts at childbearing, oocyte aging poses the major challenge to reproductive medicine. By their late 30s, while the rest of their organs function at near peak capacity, otherwise healthy women experience markedly decreased implantation rates and increased rates of miscarriage and aneuploid offspring. By their 40s, most women experience overt infertility. The effects of oocyte aging on human reproduction are so ubiquitous and profound that they risk blinding us to the intriguing possibility that oocyte aging may be comprehensible by translating findings from the rapidly evolving science of cellular aging. We now know that fundamental mechanisms conserved from plants to humans drive cellular aging. We have proposed a telomere theory of female reproductive aging that posits telomere shortening as the major driver of oocyte aging. Here, we argue that differences in telomere length dynamics across the life span between men and women represent an evolutionary conserved strategy to meet the special reproductive needs of Homo sapiens, a long-lived, reproductively altruistic species, which secured its dominant niche on earth by prolonging gestation and childrearing. We also review the role of telomere length in pathologies of the reproductive tract.
THE BIOLOGY OF TELOMERES
Telomere shortening results from normal cell division, reactive oxygen species, genotoxic insults, and genetic predisposition. With every round of cell division, the process of DNA synthesis fails to replicate a small amount of DNA at the chromosome end. Telomeres serve as a disposable buffer that protects the gene-rich DNA on the interior of the chromosome from this end-replication problem. Reactive oxygen species produced from normal cellular metabolism and exogenous genotoxic insults also shorten telomeres by oxidizing the guanine-rich telomeric DNA and triggering a DNA damage response, which leads to excision of telomere repeats. Replication-linked telomere loss takes place only in dividing cells, but oxidative stress depletes telomeres even in nondividing cells such as oocytes (12).
Telomere length is a highly heritable trait, and transmission of telomere length across generations arises from both genetic and epigenetic mechanisms. Genetic variation in loci involved in telomere length regulation, such as TERT and TERC, is associated with precocious aging and cancer (7). Exonic mutations in genes encoding critical components of shelterin and/or telomerase produce severe premature aging phenotypes, including dyskeratosis congenita, idiopathic pulmonary fibrosis, and acquired aplastic anemia (13).
Even without deleterious mutations, human cells demonstrate progressive loss of telomere reserve over their life span. Critically short telomeres activate the senescence pathway, which results in p53-dependent cell cycle arrest and apoptosis (14).
Rare cells compensate for telomere shortening by expressing telomerase, a reverse-transcriptase that restores a modest number of repeats to telomere ends during each S-phase of the cell cycle. In humans, however, telomerase is expressed only in male germ cells, stem cells, and cancer cells. Notably, oocytes, eggs, zygotes, and cleavage- and morula-stage embryos do not express appreciable levels of telomerase activity until the blastocyst stage. Moreover, because of the relative inefficiency of telomerase, even stem cells can eventually lose sufficient telomere reserve to lose their “stemness”—the stem cell theory of aging proposes that the body’s declining regenerative capacity arises from progressive shortening of telomeres and increasing cell senescence and death of stem cells (15).
TELOMERE LENGTH AND FEMALE REPRODUCTIVE AGING
Women universally experience marked loss of reproductive capacity well before other organ systems experience similar decline. The phenotype of oocyte aging in women includes decreased synapsis and chiasmata, increased meiotic and mitotic nondisjunction, spindle dysmorphologies, miscarriage, and embryo arrest, fragmentation, and apoptosis. Meiotic nondisjunction occurs at high rates throughout the life of women and further accelerates over the decade before menopause. Fertility in women begins to decline by their mid-30s, and conceptions that do occur carry increased risk of miscarriage and karyotypic abnormalities. Eggs donated from younger women completely abrogate the effects of reproductive aging, which highlights the central role of oocytes in reproductive aging. We have proposed a telomere theory of reproductive aging (16, 17), which posits that age-related oocyte dysfunction in women results from progressive telomere shortening. Telomeres in oocytes begin to shorten during fetal oogenesis, when oocytes destined to ovulate late in life progress through more cell cycles (18), and continue to shorten in the adult ovary from the chronic effects of oxidative and genotoxic stress. Telomere shortening in eggs promotes genomic instability, apoptosis, and cell cycle arrest, the hallmarks of telomere-mediated cellular senescence.
Telomere function is essential for meiosis. During the early prophase of meiosis in organisms as diverse as plants, yeast, mice, and humans, telomeres tether chromosomes to the nuclear membrane to facilitate homologous pairing and initiate synapsis to form chiasmata (19, 20). Normal segregation of chromosomes during later meiosis in the adult depends on the chiasmata formed during fetal life. Chiasmata provide countertraction against spindle-pulling forces to allow all kinetochores to secure attachments to spindle fibers before the cell can progress to anaphase. Telomere shortening via genetic manipulation reduces synapsis and recombination in mice (21).
We have tested the telomere theory of reproductive aging by experimentally shortening telomeres in mice, which normally do not exhibit significant age-related oocyte dysfunction, and have discovered that it recapitulates the phenotype of reproductive aging in women (21, 22). As telomeres approach the length characteristic of telomeres in human oocytes, murine oocytes exhibit asymmetric spindles and abnormal chromosome congression. Subsequent embryos exhibit chromosome abnormalities, stop dividing, and tend to fragment. For more in-depth treatment of studies of animal models of reproductive senescence, we refer readers to our previous reviews (16, 17).
We also have demonstrated shorter telomeres in oocytes from women who did not conceive after in vitro fertilization (IVF) compared with those who did (23) and in oocytes from cycles that produced fragmented embryos (24). A more recent study demonstrated a direct relationship between short telomere length in polar bodies and aneuploid embryos from patients undergoing IVF (25). Another study found that women with diminished ovarian reserve had shorter telomeres and lower telomerase activity in their granulosa cells (26). Intriguingly, a number of studies report a relationship between reproductive aging and leukocyte telomere length, suggesting a relationship between germ line and somatic telomere lengths. Older mothers who give birth to children with Down syndrome have significantly shorter average leukocyte telomere length than age-matched mothers who gave birth to karyotypically normal children (27), although this does not seem to be the case for younger mothers of Down syndrome babies (27, 28). Further, women with unexplained recurrent pregnancy loss also have shorter leukocyte telomere length compared with age-matched controls (29).
TELOMERES AND MALE REPRODUCTION
The adult human ovary does not contain oogonia (30), so girls are born with a fixed cohort of oocytes, whereas testes in the adult male maintain spermatogonia capable of replicating germ cells throughout adult life (31). In differentiated spermatozoa, telomeres are anchored to the nuclear membrane where they play a fundamental role in the organization of the sperm nucleus (32). After fertilization, sperm telomeres are the first site in the sperm genome to respond to oocyte signals for pro-nucleus formation and microtubule-guided movement (33). The importance of telomeres during fertilization has been demonstrated in telomerase knockout mice, which exhibit significantly shortened telomeres as well as disrupted reproductive function in both sexes (34, 35).
Unlike telomere length in oocytes, which decreases with the age of the female, telomere length in sperm increases with age (31, 36–38). Telomere lengthening in human sperm presumably arises from the continued action of telomerase, which is expressed at high levels in spermatogonia. Though sperm maintain longer average telomere length, sperm telomere length does vary among individual men and individual spermatozoa (39, 40). Variation in sperm telomere length could result from variable activity of telomerase and/or from the effects of oxidative stress, which shorten telomeres. Compromised telomere length in sperm may contribute to segregation errors, apoptosis with reduced sperm count, and reduced fertility (33).
TELOMERES AND THE DIFFERING REPRODUCTIVE STRATEGIES OF MEN AND WOMEN
Advancing paternal age is associated with increasing telomere length in offspring (31, 36, 41). Advanced paternal grandfather’s age at the father’s birth also predicts longer telomere length in grandchildren, suggesting that telomere lengthening in sperm may have a cumulative, transgenerational, epigenetic effect (31, 37, 42). Longer telomeres in offspring of older fathers translates into increased survival to old age for the offspring (43). This paternal age effect on the offspring’s telomere length may provide an adaptive strategy to enhance the life span of an older male’s offspring (44) and/or to compensate for short telomere length in the oocyte.
In contrast to men, the pool of germ cells in women is established during early fetal life, and the resulting oocytes live for decades without appreciable telomerase or other stem cell activity. Consistently, telomeres are shorter in oocytes than in somatic or sperm cells (45–47). Telomeres in oocytes further shorten with aging and, when critically short, contribute to infertility and miscarriage. Why such sexually dimorphic strategies in germ line telomere dynamics? Women bear the risks of the prolonged human pregnancy and risky parturition. Humans have evolved an exceptionally long gestation period, and human babies are born with large heads relative to the maternal pelvis. The human placenta, which evolved to support this energy-intensive fetal growth, is highly invasive. Childbirth in grand multiparas poses a substantial risk of maternal death from postpartum hemorrhage, placenta accreta, and eclampsia. Effective treatment for these conditions did not exist until recent history. Maternal death deprives extant offspring of the beneficial effects of mothers and grandmothers. Oocyte aging in women, mediated by telomere shortening, may have provided a natural and protective contraceptive effect across the hundreds of thousands of years of human and millions of years of protohuman evolution, when no other contraceptive methods were available and the costs of unfettered reproduction to mothers, grandmothers, and their offspring were vast. Telomere shortening in the female germ line, therefore, provides an evolutionarily conserved reproductive strategy that no longer provides adaptive advantage (48).
Abundant evidence suggests a paternal aging effect and minimal or no maternal age effect on telomere length of offspring (37), but these studies measure telomere length in leukocytes rather than in the germ line itself, where telomere dynamics may differ. Consistent with a maternal age effect on telomere length in offspring are reports that maternal age at conception is inversely related to life span of offspring (49). The implications of a maternal germ line effect on telomere length in offspring are vast because millions of women across the world now are delaying childbearing to ages when oocyte telomeres are exceedingly short.
TELOMERES IN EARLY DEVELOPMENT AND PREGNANCY
During the earliest stages of preimplantation embryo development, telomeres undergo extensive lengthening, both from telomerase-dependent and telomerase-independent mechanisms. In mouse embryos, the most dramatic telomere lengthening takes place before implantation (47). Later, proliferating embryonic stem cells with robust telomerase activity contribute to the growth of the embryo, chorion, and placenta (50). Shortened telomeres are associated with recurrent miscarriage (29). Near the end of gestation, the placenta enters a state of senescence (51). Because telomerase activity decreases during maturation of the placenta (50), telomere shortening during placental development may provide one mechanism to explain post dates reduction in placental mass and impaired fetal growth.
Complications of gestation, such as intrauterine growth restriction due to placental insufficiency and preeclampsia, have been hypothesized to result from the effects of hypoxia/reperfusion on oxidative stress and the consequent telomere length in placental tissue. In support of this hypothesis, telomeres are shorter in placental trophoblasts from pregnancies complicated by intrauterine growth restriction (52), which agrees with another study that found weak telomerase activity in the placentas of intrauterine growth restriction (53). Telomeres are also shorter in trophoblasts from pregnancies with preeclampsia, and in preeclampsia combined with intrauterine growth restriction (54). A history of infertility has been associated with complications in pregnancy. Because telomere shortening produces both infertility and pregnancy complications, it may provide a common mechanism to explain the association between these two conditions.
TELOMERES AND ENDOMETRIOSIS
Endometriosis, defined as the presence of endometrial cells outside the uterine cavity (55), affects 10% to 15% of women of reproductive age (56, 57) and up to 40% of infertile women (56). Except in rare cases when endometriosis completely occludes both fallopian tubes, the mechanisms underlying the link between endometriosis and fertility remain unclear although telomere biology may provide this link. Interestingly, the endometrium is one of the few adult tissues that expresses high levels of telomerase (58). Endometrium shed into the peritoneal cavity during retrograde menstruation could therefore retain enhanced replicative capacity due to its robust telomerase activity, which would facilitate implantation of ectopic endometrium (59). Some evidence supports the hypothesis that endometrial stem cells with robust telomerase contribute to the pathogenesis of endometriosis (59). Hematopoietic stem cells also may contribute to endometriosis (60). Alterations in pathways involved in telomere-length homeostasis could influence the survival of ectopic endometrial and hematopoietic stem cells while also affecting fertility.
TELOMERES AND CANCERS OF THE REPRODUCTIVE TRACK
Cancers of the gynecologic tract affect over 80,000 women annually (61), and several of the most common gynecologic cancers and/or their treatments have been linked to the risk of infertility. Early studies showing high telomerase activity in cancers compared with healthy tissue led to the assumption that long telomeres would distinguish cancers (62, 63). However, a consensus has emerged that progressive telomere shortening, from both age-related replicative attrition and oxidative stress, precedes the genomic instability that leads to transformation. Cancer patients generally appear to have shorter telomeres; a recent meta-analysis of 27 studies showed an association between short telomere length in leukocytes and the development of a variety of cancers (62), including ovarian cancer. Although this association in leukocytes is not consistent with all cancers studied (62, 64), telomere length is consistently shorter when assayed in the tumor cells directly (65, 66).
The study of telomeres may be particularly relevant to gynecologic cancers due to the link between estrogen and telomerase activity. The human telomerase catalytic subunit (hTERT) is up-regulated by estrogen via direct and indirect effects on its promoter (67, 68). Telomerase is not typically expressed in ovarian surface epithelium, although it is up-regulated in 95% of ovarian cancers (69). Similarly, advanced ovarian cancer staging typically correlates with an increased level of telomerase activity (63). Thus, hTERT appears to play a critical role in telomere length maintenance in cancerous ovarian tissue (70).
As telomere length attrition occurs with age and cancer risk increases with age, it has been difficult to ascertain the causal role of telomere length in cancer pathogenesis. An elegant study in mother-daughter pairs affected by hereditary breast cancer recently demonstrated earlier disease onset and more aggressive disease coinciding with dramatically shorter telomere lengths in affected daughters (71). This relationship has not been explored in hereditary cancers affecting reproductive tissues. Because telomere shortening contributes both to female infertility and malignancy, a generalized predisposition to telomere shortening with age may explain the association between female infertility and some gynecologic cancers.
MEASUREMENT OF TELOMERE LENGTH
The gold standard method to measure telomere length, telomere restriction fragment (TRF), is based on the Southern blot assay, and remains the only method to determine absolute telomere length (72, 73). This method, however, is severely limiting in studies of telomeres in eggs and embryos because it employs gel electrophoresis, hybridization with a radiolabeled probe, and therefore a large amount of DNA (0.5–5.0 μg). Quantitative polymerase chain reaction (qPCR) substantially reduces the requirement of starting DNA material to 35 ng/reaction (74). The qPCR assay has been scaled to measure telomere length in single polar bodies and blastomeres (25). Quantitative PCR measures relative rather than absolute telomere length by generating a ratio between total telomere DNA (T) and DNA from amplification of a single copy gene (S), thereby providing a relative T/S ratio. With the advent of Sybr-green PCR technology, the time to measure telomere length by qPCR has been reduced from days to hours, thus facilitating application to large-scale human studies.
Both TRF and qPCR methods have the limitation of measuring only average telomere length. Alternatively, quantitative fluorescence in situ hybridization (qFISH) of metaphase spreads reveals telomere length at the level of the individual telomere (Fig. 3). Although qFISH returns a relative telomere length, it remains the most sensitive method for determining telomere length in samples limited to a small number of cells. Any of these methods may be complemented by a highly sensitive telomerase activity assay (47, 75), which reveals the extent to which telomerase may or may not be contributing to telomere length maintenance in the tissue of interest.
FIGURE 3.
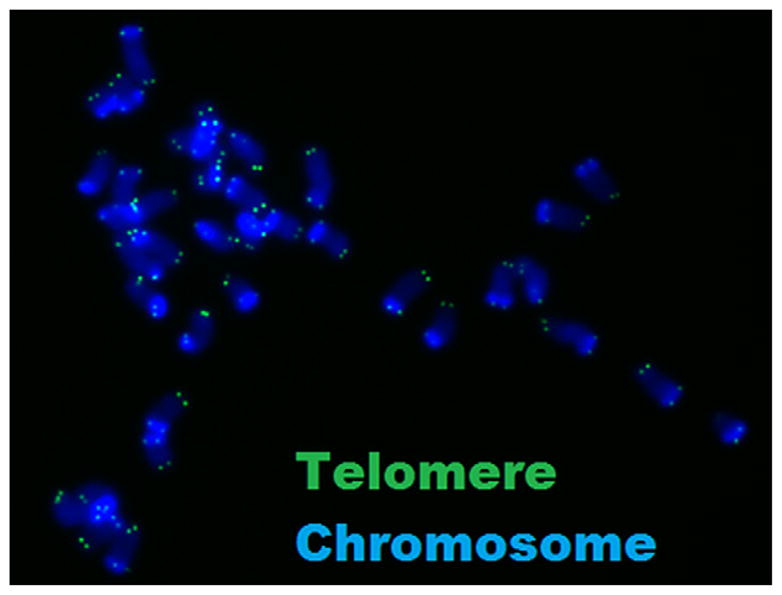
Representative metaphase spread from preimplantation murine blastomere. Double-stranded DNA (blue) is bound by nonspecific DNA-binding dye, and telomeres are bound by a PNA probe specific to telomere DNA repeats.
Kalmbach. Telomeres and human reproduction. Fertil Steril 2013.
CONCLUSION
Telomeres, which are essential components of linear chromosomes, evolutionarily conserved from plants to man, protect against chromosome end-fusions and genomic instability. Telomeres erode with aging, both in dividing and nondividing cells, which triggers cell cycle arrest, senescence, and eventual cell death. The male germ line, replete with spermatogonia, maintains and even lengthens telomeres with age. In contrast, women’s ovaries lack germ line stem cells, so oocytes in women undergo profound aging that culminates in genomic instability, abnormal spindles, and in resulting abnormal embryos with cell cycle arrest, fragmentation, and apoptosis. Indeed, age provides the most robust predictor of fertility in women, regardless of other diagnoses, and the locus of reproductive aging rests almost exclusively in the oocyte.
We propose that telomeres provide a timer of reproductive aging in the female. Experimental telomere shortening in female mice, which normally show almost no appreciable age-related oocyte dysfunction, recapitulates the reproductive-aging phenotype of women as the mouse’s telomere length approaches that of human telomeres. Experimental shortening of telomeres reduces synapsis and chiasmata, increases embryo fragmentation and arrest, disrupts meiotic spindles, and contributes to genomic instability. Telomeres are shorter in oocytes from women who fail IVF, and in oocytes that go on to produce fragmented (24) and aneuploid embryos (25). Telomere length in peripheral leukocytes also influences the risk of unexplained recurrent pregnancy loss and trisomic offspring. The telomere theory of reproductive aging, therefore, provides a parsimonious explanation for the otherwise disparate pathophysiologic changes in the eggs and embryos of older women.
Germ line telomere shortening in women provides a striking contrast to the telomere lengthening in the male germ line. These differences in telomere dynamics across the reproductive life span may reflect the fundamentally different reproductive strategies followed by men and women. Men gain selective advantage whenever they reproduce, regardless of age. Late reproducing women, on the other hand, risk maternal death and rendering their heirs motherless and grand-motherless, thereby reducing their offspring’s reproductive fitness. Telomere-mediated oocyte aging may have protected women across the millions of years of protohuman evolution when grand multiparity and advanced maternal age made a catastrophic combination.
Growing evidence also links endometriosis and gynecologic cancers, two pathologic conditions affecting the female reproductive system, with altered telomere biology. Cells from both endometriosis and cancer gain longevity by expressing telomerase and augmenting telomere reserve. Because infertility, endometriosis, and gynecologic cancers all involve alterations in telomeres, and because infertility has been associated with both endometriosis and gynecologic cancers, further studies should examine a common role for telomeres in these pathologies of the reproductive tract.
FIGURE 2.
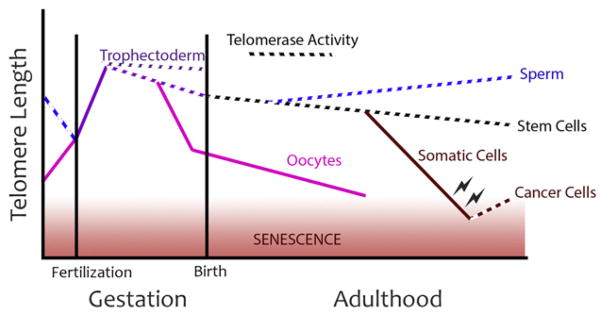
Schematic representation of telomere length relationships over mammalian development. Preimplantation development is associated with the greatest increase in telomere length in the course of development, owing to the telomerase-independent alternative lengthening of telomeres (ALT) pathway. Embryonic development follows with a period of telomere reserve maintained by telomerase activity in virtually every embryonic tissue. Through adulthood, telomerase expression is limited to stem cells, the male germ line, and cancer. Paradoxically, oocytes exhibit marked loss of telomere reserve that fails to recover over a mammalian life span.
Kalmbach. Telomeres and human reproduction. Fertil Steril 2013.
Acknowledgments
Supported by the Department of Obstetrics and Gynecology and the Clinical and Translational Sciences Institute, New York University (National Institutes of Health, Grant 1UL1RR029893).
Footnotes
K.H.K. has nothing to disclose. D.M.F.A. received a grant from the CAPES Foundation, the Ministry of Education of Brazil, and travel support from the New York University Department of Obstetrics and Gynecology. R.C.D. received a grant from Sao Paolo University. T.W.K. has nothing to disclose. M.L.S.-S. has nothing to disclose. F.W. has nothing to disclose. L.L. has nothing to disclose. D.L.K. has received honoraria and travel support from professional societies to speak on the topic of this paper, and is on the scientific advisory board of Cooper Surgical (unrelated to this work).
References
- 1.Murillo-Ortiz B, Albarran-Tamayo F, Arenas-Aranda D, Benitez-Bribiesca L, Malacara-Hernandez J, Martinez-Garza S, et al. Telomere length and type 2 diabetes in males, a premature aging syndrome. Aging Male. 2011;15:54–8. doi: 10.3109/13685538.2011.593658. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, et al. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66:421–9. doi: 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann D, Srivastava U, Thaler M, Kleinhans KN, N’Kontchou G, Scheffold A, et al. Telomerase gene mutations are associated with cirrhosis formation. Hepatology. 2011;53:1608–17. doi: 10.1002/hep.24217. [DOI] [PubMed] [Google Scholar]
- 4.Calado RT, Brudno J, Mehta P, Kovacs JJ, Wu C, Zago MA, et al. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology. 2011;53:1600–7. doi: 10.1002/hep.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Delgado B, Yanowsky K, Inglada-Perez L, Domingo S, Urioste M, Osorio A, et al. Genetic anticipation is associated with telomere shortening in hereditary breast cancer. PLoS Genet. 2011;7:e1002182. doi: 10.1371/journal.pgen.1002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011;66:312–9. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atzmon G, Cho M, Cawthon RM, Budagov T, Katz M, Yang X, et al. Evolution in health and medicine Sackler colloquium: genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc Natl Acad Sci USA. 2010;107(Suppl 1):1710–7. doi: 10.1073/pnas.0906191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snowdon DA, Kane RL, Beeson WL, Burke GL, Sprafka JM, Potter J, et al. Is early natural menopause a biologic marker of health and aging? Am J Pub Health. 1989;79:709–14. doi: 10.2105/ajph.79.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann Epidemiol. 1998;8:229–35. doi: 10.1016/s1047-2797(97)00207-x. [DOI] [PubMed] [Google Scholar]
- 10.Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005;162:1089–97. doi: 10.1093/aje/kwi324. [DOI] [PubMed] [Google Scholar]
- 11.Smith KR, Gagnon A, Cawthon RM, Mineau GP, Mazan R, Desjardins B. Familial aggregation of survival and late female reproduction. J Gerontol A Biol Sci Med Sci. 2009;64:740–4. doi: 10.1093/gerona/glp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Trimarchi JR, Smith PJ, Keefe DL. Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell. 2002;1:40–6. doi: 10.1046/j.1474-9728.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 13.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–65. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Lange T. How telomeres solve the end-protection problem. Science. 2009;326:948–52. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores I, Blasco MA. The role of telomeres and telomerase in stem cell aging. FEBS Lett. 2010;584:3826–30. doi: 10.1016/j.febslet.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 16.Keefe DL, Marquard K, Liu L. The telomere theory of reproductive senescence in women. Curr Opin Obstet Gynecol. 2006;18:280–5. doi: 10.1097/01.gco.0000193019.05686.49. [DOI] [PubMed] [Google Scholar]
- 17.Keefe DL, Liu L. Telomeres and reproductive aging. Reprod Fertil Dev. 2009;21:10–4. doi: 10.1071/rd08229. [DOI] [PubMed] [Google Scholar]
- 18.Henderson SA, Edwards RG. Chiasma frequency and maternal age in mammals. Nature. 1968;218:22–8. doi: 10.1038/218022a0. [DOI] [PubMed] [Google Scholar]
- 19.Bass HW, Riera-Lizarazu O, Ananiev EV, Bordoli SJ, Rines HW, Phillips RL, et al. Evidence for the coincident initiation of homolog pairing and synapsis during the telomere-clustering (bouquet) stage of meiotic prophase. J Cell Sci. 2000;113:1033–42. doi: 10.1242/jcs.113.6.1033. [DOI] [PubMed] [Google Scholar]
- 20.Scherthan H. Telomere attachment and clustering during meiosis. Cell Mol Life Sci. 2007;64:117–24. doi: 10.1007/s00018-006-6463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Franco S, Spyropoulos B, Moens PB, Blasco MA, Keefe DL. Irregular telomeres impair meiotic synapsis and recombination in mice. Proc Natl Acad Sci USA. 2004;101:6496–501. doi: 10.1073/pnas.0400755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Blasco MA, Keefe DL. Requirement of functional telomeres for meta-phase chromosome alignments and integrity of meiotic spindles. EMBO Rep. 2002;3:230–4. doi: 10.1093/embo-reports/kvf055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keefe DL, Liu L, Marquard K. Telomeres and aging-related meiotic dysfunction in women. Cell Mol Life Sci. 2007;64:139–43. doi: 10.1007/s00018-006-6466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keefe DL, Franco S, Liu L, Trimarchi J, Cao B, Weitzen S, et al. Telomere length predicts embryo fragmentation after in vitro fertilization in women—toward a telomere theory of reproductive aging in women. Am J Obstet Gynecol. 2005;192:1256–61. doi: 10.1016/j.ajog.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 25.Treff NR, Su J, Taylor D, Scott RT., Jr Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011;7:e1002161. doi: 10.1371/journal.pgen.1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butts S, Riethman H, Ratcliffe S, Shaunik A, Coutifaris C, Barnhart K. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J Clin Endocrinol Metab. 2009;94:4835–43. doi: 10.1210/jc.2008-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh S, Feingold E, Chakraborty S, Dey SK. Telomere length is associated with types of chromosome 21 nondisjunction: a new insight into the maternal age effect on Down syndrome birth. Hum Genet. 2010;127:403–9. doi: 10.1007/s00439-009-0785-8. [DOI] [PubMed] [Google Scholar]
- 28.Dorland M, van Montfrans JM, van Kooij RJ, Lambalk CB, te Velde ER. Normal telomere lengths in young mothers of children with Down’s syndrome. Lancet. 1998;352:961–2. doi: 10.1016/s0140-6736(05)61516-4. [DOI] [PubMed] [Google Scholar]
- 29.Hanna CW, Bretherick KL, Gair JL, Fluker MR, Stephenson MD, Robinson WP. Telomere length and reproductive aging. Hum Reprod. 2009;24:1206–11. doi: 10.1093/humrep/dep007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Wu C, Lyu Q, Yang D, Albertini DF, Keefe DL, et al. Germline stem cells and neo-oogenesis in the adult human ovary. Dev Biol. 2007;306:112–20. doi: 10.1016/j.ydbio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Kimura M, Cherkas LF, Kato BS, Demissie S, Hjelmborg JB, Brimacombe M, et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4:e37. doi: 10.1371/journal.pgen.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moskovtsev SI, Willis J, White J, Mullen JB. Disruption of telomere-telomere interactions associated with DNA damage in human spermatozoa: systems biology in reproductive medicine. 2010;56:407–12. doi: 10.3109/19396368.2010.502587. [DOI] [PubMed] [Google Scholar]
- 33.Thilagavathi J, Venkatesh S, Dada R. Telomere length in reproduction. Andrologia. doi: 10.1111/and.12008. Published online August 29, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–74. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 35.Ioannou D, Griffin DK. Male fertility, chromosome abnormalities, and nuclear organization. Cytogenet Genome Res. 2011;133:269–79. doi: 10.1159/000322060. [DOI] [PubMed] [Google Scholar]
- 36.Gibbons A. American Association of Physical Anthropologists. Older dads have healthier kids than you think. Science. 2012;336:539. doi: 10.1126/science.336.6081.539. [DOI] [PubMed] [Google Scholar]
- 37.Eisenberg DT, Hayes MG, Kuzawa CW. Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc Natl Acad Sci USA. 2012;109:10251–6. doi: 10.1073/pnas.1202092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prescott J, Du M, Wong JY, Han J, De Vivo I. Paternal age at birth is associated with offspring leukocyte telomere length in the nurses’ health study. Hum Reprod. 2012;27:3622–31. doi: 10.1093/humrep/des314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santiso R, Tamayo M, Gosalvez J, Meseguer M, Garrido N, Fernandez JL. Swim-up procedure selects spermatozoa with longer telomere length. Mutat Res. 2010;688:88–90. doi: 10.1016/j.mrfmmm.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Baird DM, Britt-Compton B, Rowson J, Amso NN, Gregory L, Kipling D. Telomere instability in the male germline. Hum Mol Genet. 2006;15:45–51. doi: 10.1093/hmg/ddi424. [DOI] [PubMed] [Google Scholar]
- 41.Sartorius GA, Nieschlag E. Paternal age and reproduction. Hum Reprod Update. 2010;16:65–79. doi: 10.1093/humupd/dmp027. [DOI] [PubMed] [Google Scholar]
- 42.Arbeev KG, Hunt SC, Kimura M, Aviv A, Yashin AI. Leukocyte telomere length, breast cancer risk in the offspring: the relations with father’s age at birth. Mech Ageing Dev. 2011;132:149–53. doi: 10.1016/j.mad.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res. 2012;730:68–74. doi: 10.1016/j.mrfmmm.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eisenberg DT. An evolutionary review of human telomere biology: the thrifty telomere hypothesis and notes on potential adaptive paternal effects. Am J Hum Biol. 2011;23:149–67. doi: 10.1002/ajhb.21127. [DOI] [PubMed] [Google Scholar]
- 45.Wright DL, Jones EL, Mayer JF, Oehninger S, Gibbons WE, Lanzendorf SE. Characterization of telomerase activity in the human oocyte and preimplantation embryo. Mol Hum Reprod. 2001;7:947–55. doi: 10.1093/molehr/7.10.947. [DOI] [PubMed] [Google Scholar]
- 46.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–9. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 47.Liu L, Bailey SM, Okuka M, Munoz P, Li C, Zhou L, et al. Telomere lengthening early in development. Nat Cell Biol. 2007;9:1436–41. doi: 10.1038/ncb1664. [DOI] [PubMed] [Google Scholar]
- 48.Keefe DL. Reproductive aging is an evolutionarily programmed strategy that no longer provides adaptive value. Fertil Steril. 1998;70:204–6. doi: 10.1016/s0015-0282(98)00161-7. [DOI] [PubMed] [Google Scholar]
- 49.Gavrilov LA, Gavrilova NS. Biodemography of exceptional longevity: early-life and mid-life predictors of human longevity. Biodemography Soc Biol. 2012;58:14–39. doi: 10.1080/19485565.2012.666121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kyo S, Takakura M, Tanaka M, Kanaya T, Sagawa T, Kohama T, et al. Expression of telomerase activity in human chorion. Biochem Biophys Res Commun. 1997;241:498–503. doi: 10.1006/bbrc.1997.7767. [DOI] [PubMed] [Google Scholar]
- 51.Rosso P. Placenta as an aging organ. Curr Concepts Nutr. 1976;4:23–41. [PubMed] [Google Scholar]
- 52.Biron-Shental T, Sukenik Halevy R, Goldberg-Bittman L, Kidron D, Fejgin MD, Amiel A. Telomeres are shorter in placental trophoblasts of pregnancies complicated with intrauterine growth restriction (IUGR) Early Hum Dev. 2010;86:451–6. doi: 10.1016/j.earlhumdev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Izutsu T, Kudo T, Sato T, Nishiya I, Ohyashiki K, Mori M, et al. Telomerase activity in human chorionic villi and placenta determined by TRAP and in situ TRAP assay. Placenta. 1998;19:613–8. doi: 10.1016/s0143-4004(98)90022-4. [DOI] [PubMed] [Google Scholar]
- 54.Biron-Shental T, Sukenik-Halevy R, Sharon Y, Goldberg-Bittman L, Kidron D, Fejgin MD, et al. Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2010;202:381, e1–7. doi: 10.1016/j.ajog.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 55.Metzger DA, Haney AF. Etiology of endometriosis. Obstet Gynecol Clin North Am. 1989;16:1–14. [PubMed] [Google Scholar]
- 56.Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–58. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 57.Lapp T. ACOG issues recommendations for the management of endometriosis. American College of Obstetricians and Gynecologists. Am Fam Physician. 2000;62:1431–4. [Google Scholar]
- 58.Yokoyama Y, Takahashi Y, Morishita S, Hashimoto M, Niwa K, Tamaya T. Telomerase activity in the human endometrium throughout the menstrual cycle. Mol Hum Reprod. 1998;4:173–7. doi: 10.1093/molehr/4.2.173. [DOI] [PubMed] [Google Scholar]
- 59.Hapangama DK, Turner MA, Drury JA, Quenby S, Saretzki G, Martin-Ruiz C, et al. Endometriosis is associated with aberrant endometrial expression of telomerase and increased telomere length. Hum Reprod. 2008;23:1511–9. doi: 10.1093/humrep/den172. [DOI] [PubMed] [Google Scholar]
- 60.Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann NY Acad Sci. 2008;1127:106–15. doi: 10.1196/annals.1434.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.U.S. Centers for Disease Control and Prevention. United States cancer statistics: 1999–2008 incidence and mortality Web-based report. Available at: http://apps.nccd.cdc.gov/uscs/
- 62.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:1238–50. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng PS, Iwasaka T, Yamasaki F, Ouchida M, Yokoyama M, Nakao Y, et al. Telomerase activity in gynecologic tumors. Gynecol Oncol. 1997;64:171–5. doi: 10.1006/gyno.1996.4523. [DOI] [PubMed] [Google Scholar]
- 64.Prescott J, McGrath M, Lee IM, Buring JE, De Vivo I. Telomere length and genetic analyses in population-based studies of endometrial cancer risk. Cancer. 2010;116:4275–82. doi: 10.1002/cncr.25328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akbay EA, Contreras CM, Perera SA, Sullivan JP, Broaddus RR, Schorge JO, et al. Differential roles of telomere attrition in type I and II endometrial carcinogenesis. Am J Pathol. 2008;173:536–44. doi: 10.2353/ajpath.2008.071179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meeker AK, Hicks JL, Iacobuzio-Donahue CA, Montgomery EA, Westra WH, Chan TY, et al. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin Cancer Res. 2004;10:3317–26. doi: 10.1158/1078-0432.CCR-0984-03. [DOI] [PubMed] [Google Scholar]
- 67.Misiti S, Nanni S, Fontemaggi G, Cong YS, Wen J, Hirte HW, et al. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol Cell Biol. 2000;20:3764–71. doi: 10.1128/mcb.20.11.3764-3771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, et al. Estrogen activates telomerase. Cancer Res. 1999;59:5917–21. [PubMed] [Google Scholar]
- 69.Li H, Simpson ER, Liu JP. Oestrogen, telomerase, ovarian ageing and cancer. Clin Exp Pharmacol Physiol. 2010;37:78–82. doi: 10.1111/j.1440-1681.2009.05238.x. [DOI] [PubMed] [Google Scholar]
- 70.Terry KL, Tworoger SS, Vitonis AF, Wong J, Titus-Ernstoff L, De Vivo I, et al. Telomere length and genetic variation in telomere maintenance genes in relation to ovarian cancer risk. Cancer Epidemiol Biomarkers Prev. 2012;21:504–12. doi: 10.1158/1055-9965.EPI-11-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mirabello L, Yu K, Kraft P, De Vivo I, Hunter DJ, Prescott J, et al. The association of telomere length and genetic variation in telomere biology genes. Hum Mutat. 2010;31:1050–8. doi: 10.1002/humu.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Allshire RC, Dempster M, Hastie ND. Human telomeres contain at least three types of G-rich repeat distributed non-randomly. Nucleic Acids Res. 1989;17:4611–27. doi: 10.1093/nar/17.12.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kimura M, Stone RC, Hunt SC, Skurnick J, Lu X, Cao X, et al. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5:1596–607. doi: 10.1038/nprot.2010.124. [DOI] [PubMed] [Google Scholar]
- 74.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]


