Abstract
The pharmacological modification of dopamine transmission has long been employed as a therapeutic tool in the treatment of many mental health disorders. However, as many of the pharmacotherapies today are not without significant side effects, or they alleviate only a particular subset of symptoms, the identification of novel therapeutic targets is imperative. In light of these challenges, the recognition that dopamine receptors can form heteromers has significantly expanded the range of physiologically relevant signaling complexes as well as potential drug targets. Furthermore, as the physiology and disease relevance of these receptor heteromers is further understood, their ability to exhibit pharmacological and functional properties distinct from their constituent receptors, or modulate the function of endogenous homomeric receptor complexes, may allow for the development of alternate therapeutic strategies and provide new avenues for drug design. In this review, we describe the emerging neurobiology of the known dopamine receptor heteromers, their physiological relevance in brain, and discuss the potential role of these receptor complexes in neuropsychiatric disease. We highlight their value as targets for future drug development and discuss innovative research strategies designed to selectively target these dopamine receptor heteromers in the search for novel and clinically efficacious pharmacotherapies.
Keywords: dopamine receptor heteromers, heterooligomerization, receptor dimerization, receptor complexes, G protein-coupled receptor, neuropsychiatric disorders
INTRODUCTION
Alterations in dopaminergic signaling have been linked to a number of mental health disorders, including schizophrenia, drug addiction, depression, and attention-deficit hyperactivity disorder (ADHD) (Beaulieu and Gainetdinov, 2011; Faraone and Biederman, 1998; Porcelli et al, 2011; Seeman, 2009; Volkow et al, 2004), and the pharmacological modification of dopamine transmission has long been employed as a therapeutic tool in the treatment of many dopamine-related disorders. The physiological effects of dopamine are mediated by five dopamine receptor subtypes, divided into two major subclasses: the D1-like (D1, D5) and the D2-like (D2, D3, D4) receptors, which are typically coupled to the stimulatory Gs/olf and inhibitory Gi/o proteins, respectively. Although traditionally G protein-coupled receptors (GPCRs), such as the dopamine receptors, have been depicted as monomeric entities, it is now widely accepted that GPCRs exist as oligomeric complexes (George et al, 2002; Milligan, 2004; Terrillon and Bouvier, 2004), and homomerization has been repeatedly shown to have a critical involvement in important cellular processes, such as receptor translocation to the plasma membrane (Hague et al, 2004; Karpa et al, 2000; Kong et al, 2006; López-Giménez et al, 2007; Salahpour et al, 2004; White et al, 1998). In addition, the discovery that dopamine receptors could form heteromeric complexes (Baragli et al, 2007; Ferrada et al, 2008, 2009; Ginés et al, 2000; Hillion et al, 2002; Lee et al, 2004; Marcellino et al, 2008a; Marcellino et al, 2008b; Scarselli et al, 2001; Torvinen et al, 2005) has opened up novel avenues of research for drug discovery, as many of these receptor heteromers may exhibit discrete distributions in brain with pharmacological and functional properties distinct from their constituent receptors. For example, the dopamine D1-D2 receptor heteromer was first identified in rat striatum (Lee et al, 2004) and shown to couple to the Gq/11 protein, a finding that effectively linked dopamine directly to calcium signaling in brain (Rashid et al, 2007a). Dopamine had been linked to calcium signalling previously in older literature, with some suggesting that the D1 receptor (D1R) itself or a ‘D1-like' receptor was responsible, but the interpretation of those studies in light of what we now know suggests that much of the calcium signal in striatum would have been attributable to the D1-D2 receptor heteromer, with the calcium signal in cortex or other brain regions possibly to the D5 receptor (D5R) or the D2-D5 receptor heteromer.
Given the extensive involvement of dopamine receptors in the etiology and therapeutic management of mental health disorders, and the remarkable potential of dopamine receptor heteromers to access diverse signaling cascades or to modulate the nature of the transduced signal, these heteromeric complexes represent likely candidates in the search for new drug therapies. Indeed, as many of the pharmacotherapies today are not without significant side effects, or they alleviate only a particular subset of symptoms, the identification of novel therapeutic targets is imperative. This review will discuss the known dopamine receptor heteromers that have been reported to have a potential link to neuropsychiatric disorders and will review the recent advances that have contributed to the understanding of how these receptor heteromers may be important to the pathophysiology and therapeutic management of schizophrenia, addiction, depression, and ADHD.
THE RECEPTOR INTERFACE: RECEPTOR HOMOMERS VERSUS RECEPTOR HETEROMERS
Although GPCR heteromerization between class C GPCRs such as the GABAB receptor is recognized as obligatory and has been accepted for many years (Jones et al, 1998; Kaupmann et al, 1998), oligomerization between class A GPCRs is still the subject of much debate and, as such, the structural mechanism by which these receptors physically interact has been the focus of a number of research studies. What has become increasingly evident is that the interface(s) between the receptors in homomeric complexes likely involves residues located in transmembrane domains (TM), such as TM4 and TM5, as has been shown for the D2 receptor (D2R) (Guo et al, 2003; Lee et al, 2003), the alpha(1b)-adrenoceptor (López-Giménez et al, 2007), the 5HT1A receptor (Gorinski et al, 2012), and the 5HT2C receptor (Mancia et al, 2008). These observations have also been supported by the recent crystal structure reported for the beta1-adrenergic receptor dimer (Huang et al, 2013) in a lipid membrane-like environment which showed two dimer interfaces, one involving TM1, TM2, helix 8 and extracellular loop 1, and the second involving TM4, TM5, intracellular loop 2, and extracellular loop 2. The analysis of the crystal structure of the chemokine CXCR4 receptor dimer (Wu et al, 2010) reported receptor interfaces at TM5 and TM6. In contrast to homomeric receptor complexes, the receptor interface(s) involving class A GPCR heteromers does not appear to rely predominantly on TM interactions, resulting in concern as to whether the binding energy between the receptors in these heteromers is actually sufficient to result in stable long-lasting physical interactions (Gurevich and Gurevich, 2008). However, it has been demonstrated in several examples that certain amino-acid residues, specifically two or more adjacent arginines on one protomer and two or more adjacent glutamic acids, or aspartic acids, or a phosphorylated residue on the other protomer, is sufficient to induce the formation of stable non-covalent complexes (Jackson et al, 2006; Woods and Ferré, 2005). Indeed, this mechanism of interaction has been reported for both the D1-D2 receptor heteromer (O'Dowd et al, 2012) and the D2-D5 receptor heteromer (O'Dowd et al, 2013), whereby adjacent glutamic acid residues in the carboxyl tail of the D1R or D5R interacted with two different sets of adjacent arginine residues in intracellular loop 3 (IC3) of the D2R. Similarly, it has been shown for the A2-D2 receptor heteromer that there exists an arginine–phosphate electrostatic interaction between the C-terminal tail of the A2 receptor (A2R) and the IC3 of the D2R that is of high energy strength (Ciruela et al, 2004) and which possesses covalent-like stability (Woods and Ferré, 2005). Indeed it has been proposed that this arginine–phosphate interaction may represent a common mechanism in receptor heteromerization (Fuxe et al, 2010), including GPCR heteromerization with non-GPCRs such as has been suggested for the D1-NMDA receptor heteromer (Woods et al, 2005; Woods and Ferré, 2005).
Clearly more research is required to identify the underlying mechanisms by which class A GPCRs heteromerize, as they do not all involve electrostatic amino-acid interactions, and TM domain interactions may not have such a crucial role in the formation of at least some of these receptor complexes. Nonetheless, the mechanism of the interaction mediating receptor heteromerization does not appear to be necessary for the classification of a receptor heteromer as recommended by the International Union of Basic and Clinical Pharmacology (Pin et al, 2007), whereby receptor heteromers can be accepted by the scientific community provided their existence in native tissue has been firmly demonstrated. In line with this, at least two of the following criteria should be met: (1) There is evidence for physical association in native tissues or primary cells, preferably through the use of energy transfer technologies or antibodies selective for specific receptor oligomers (Wager-Miller et al, 2002), (2) A specific functional property for the receptor heteromer is known so receptors in native tissue can be identified, and (3) the existence of the heteromer was confirmed in vivo through the use of knockout animals or RNAi technology. The recommendations for the recognition and nomenclature of GPCR oligomers was adopted in a Web-based information system, the G Protein-Coupled Receptor-Oligomerization Knowledge Base (GPCR-OKB) (http://www.gpcr-okb.org), in which all available information on GPCR oligomers was included. It is important to note that although the present review will focus on dopamine receptor heteromers that have met the above criteria, it will additionally address some putative heteromer receptor species that have not yet been demonstrated to exist in vivo. A comprehensive characterization of the dopamine receptor heteromers discussed herein is presented in Table 1.
Table 1. Physical and Functional Evidence for Dopamine Receptor Heteromers.
| Heteromer |
Physicial interaction |
Functional evidence | Relevance | References | |
|---|---|---|---|---|---|
| In vitro | In vivo | ||||
| D1-D2 | Co-IP, NLS FRET rat striatal neurons radioligand binding | Co-IP rat STR, PFC FRET in situ rat CP, NAc, GP | Novel Gq-coupling resulting in intracellular calcium release and BDNF expression, signaling blocked by D1R and D2R antagonists, GSK-3β inactivation | Addiction Schizophrenia | Lee et al (2004), Rashid et al (2007a), Hasbi et al (2009), Pei et al (2010), Perreault et al (2010, 2011; 2013), O'Dowd et al (2012) |
| D2-D4 | BRET D2R and D4.4, no heteromer between D2R and D4.7 variant | Colocalization in mouse STR | Potentiation of ERK activation when D2R and D4R coexpressed but not with D4.7 variant, knock-in mice expressing D4.7 variant show no synergistic increase in striatal ERK activation | ADHD | Borroto-Escuela et al (2011), González et al (2012) |
| D1-D3 | BRET, FRET | Co-IP rat STR | Agonist-induced D1R cytoplasmic sequestration abolished by D3R coexpression, D3R stimulation enhanced D1R agonist affinity and potentiated D1R-mediated behaviors | Addiction | Fiorentini et al (2008), Marcellino et al (2008b) |
| D2-D3 | Co-IP | Colocalization STR | In the presence of excess D3R, the properties of partial D2R agonists transformed to antagonists | Schizophrenia | Scarselli et al (2001), Novi et al (2007), Maggio and Millan, (2010) |
| D2-D5 | FRET, NLS | Colocalization, rat cortex, VP, CP | Gq-coupling resulting in intracellular calcium release followed by extracellular calcium influx | So et al (2009), Hasbi et al (2010), O'Dowd et al (2013) | |
| A1-D1 | Co-IP | Co-IP rat NAc | A1R promoted D1R G protein uncoupling and dampened receptor signaling | Addiction | Ginés et al (2000), Toda et al (2003) |
| A2-D2 | Co-IP, FRET, BRET | Colocalization in STR | A2R promoted D2R G protein uncoupling and dampened receptor signaling | Addiction Schizophrenia Parkinson's disease | Hillion et al (2002), Canals et al (2003), Fuxe et al (2005), Azdad et al (2009), Marcellino et al (2010) |
| A2-D2-mGlu5 | Biomolecular fluorescence complementation, BRET, sequential BRET-FRET | Co-IP rat STR | - | Schizophrenia | Cabello et al (2009), Fuxe et al (2010) |
| D1-NMDA | BRET | Co-IP rat HIP, STR PSD, PFC, pull-down assay rat HIP | Uncoupling the heteromer with a disrupting peptide upregulated NMDA-mediated LTP in rat HIP and promoted working memory | Schizophrenia | Lee et al (2002), Fiorentini et al (2003), Pei et al (2004), Kruse et al (2009), Nai et al (2010) |
| D2-NMDA | Co-IP rat STR PSD, pull-down assay STR | Heteromer formation induced by cocaine disrupted the CaMKII/NR2B interation and reduced NMDA receptor-mediated currents | Addiction | Liu et al (2006) | |
| D2-5HT2A | Co-IP, FRET, BRET | Colocalization in STR | 5HT2AR-mediated PLC activation was synergistically enhanced by D2R activation, D2R-mediated AC inhibition was attenuated by 5HT2AR activation | Schizophrenia | Łukasiewicz et al (2010), Borroto-Escuela et al (2011), Albizu et al (2011) |
| D1-H3 | BRET | Co-IP rat STR | D1R mandatory for H3R-induced ERK activation, D1R- and H3R-induced ERK activation blocked by antagonists for either receptor | Addiction ADHD Schizophrenia | Ferrada et al (2009), Moreno et al (2011) |
| D2-H3 | BRET | Co-IP rat STR | H3R agonists dampened D2R receptor function and D2R-induced locomotor activity | Addiction ADHD Schizophrenia | Ferrada et al (2008), Moreno et al (2007) |
Abbreviations: 5HT2AR, 5HT2A receptor; A1R, adenosine A1 receptor; A2R, adenosine A2 receptor; AC, adenylyl cyclase; ADHD, attention-deficit hyperactivity disorder; BDNF, brain-derived neurotrophic factor; BRET, bioluminescent resonance energy transfer; CaMKII, calcium calmodulin kinase II; Co-IP, coimmunoprecipitation; CP, caudate putamen; D1R, dopamine D1 receptor; D2R, dopamine D2 receptor; D3R, dopamine D3 receptor; D4R, dopamine D4 receptor; ERK, extracellular signal-related kinase; FRET, fluorescent resonance energy transfer; GP, globus pallidus; GSK-3β, glycogen synthase kinase 3β; H3R, histamine H3 receptor; HIP, hippocampus; LTP, long-term potentiation; mGlu5, metabotropic glutamate receptor 5; NAc, nucleus accumbens; NR2B, NMDA receptor subunit 2B; PLC, phospholipase C; PFC, prefrontal cortex; PSD, postsynaptic density; STR, striatum; VP, ventral pallidum.
THE DOPAMINE D1-D2 RECEPTOR HETEROMER
Dopamine D1R and D2R can form heteromeric receptor complexes that occur via electrostatic interactions between specific glutamic acid residues in the carboxyl-tail of the D1R and arginine residues in the third intracellular loop of the D2R, residues present in both the long and short isoforms of the D2R (O'Dowd et al, 2012). The D1-D2 receptor heteromer was first identified in vivo by coimmunoprecipitation from rat striatum (Lee et al, 2004) and soon thereafter confirmed in heterologous cells (Dziedzicka-Wasylewska et al, 2006; So et al, 2005) and subsequently in primary striatal neuronal culture by confocal fluorescence resonance energy transfer (FRET) studies (Hasbi et al, 2009), using the endogenously expressed dopamine receptors for the identification of receptor–receptor interactions. Indeed, a physical interaction between the endogenous GPCRs in vivo was first shown between the endogenously expressed D1R and D2R using quantitative confocal FRET in brain sections in situ (Hasbi et al, 2009), and expression of the D1-D2 heteromer has now been shown in regions of the rat mesolimbic and basal ganglia circuitry (Perreault et al, 2010; Perreault et al, 2011). Coexpression of D1R and D2R within a medium spiny neuron (MSN) does not necessarily indicate heteromerization per se, but the D1-D2 receptor–receptor distance determined to be <100 Å documented by the FRET analyses is indicative of heteromer formation. Specifically, the D1-D2 heteromer was found to be selectively expressed in MSNs with a unique phenotype, in that these neurons also expressed both dynorphin (DYN) and enkephalin (ENK) (Perreault et al, 2010), as well as GABA and glutamate (Perreault et al, 2012), and to have representation along both the direct striatonigral and indirect striatopallidal pathways (Perreault et al, 2010). For example, while a relatively low number of D1R-containing MSNs expressed the D2R (∼6%) in caudate putamen (CP), higher coexpression levels were evident in ventral pallidum and entopeduncular nucleus, with the highest levels documented in the nucleus accumbens (NAc) shell (∼17–34%) and globus pallidus (∼60%) (Bertran-Gonzalez et al, 2008; Perreault et al, 2010). In addition, as D1-D2 heteromer expression has been reported to occur selectively at presynaptic but not at postsynaptic terminals of MSNs (Perreault et al, 2010), together these findings suggest that MSNs that express the D1-D2 receptor heteromer may have a unique physiological function at a local level as well as distal effects through their efferent projections. Together, these findings indicate that MSNs coexpressing the D1R and D2R in the basal ganglia embody a physiologically relevant subset of neurons and thus may represent a third major dopamine receptor neuronal pathway, in addition to the D1R/DYN-expressing striatonigral and D2R/ENK-expressing striatopallidal MSNs, which is involved in the regulation of thalamic output (Perreault et al, 2011).
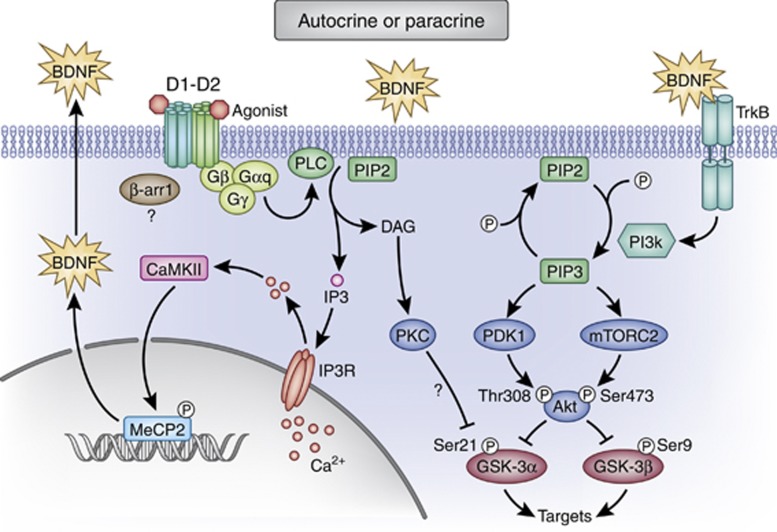
The D1-D2 heteromer has been shown to exhibit pharmacological and cell signaling properties distinct from its constituent receptors (Hasbi et al, 2009; Lee et al, 2004; Rashid et al, 2007a; So et al, 2009; Verma et al, 2010) and the expression of dopamine D1-D2 receptor heteromers in the mesocorticolimbic system and basal ganglia nuclei suggest this receptor complex may have etiological significance in disorders characterized by abnormal dopamine signaling. More specifically, calcium signaling elicited by the D1-D2 heteromer, through activation of Gq/11 and phospholipase C (PLC), resulted in the activation of calcium calmodulin kinase IIα (CaMKII) (Ng et al, 2010; Perreault et al, 2012; Rashid et al, 2007a) and consequently increased expression of brain-derived neurotrophic factor (BDNF) in NAc and ventral tegmental area (VTA) (Hasbi et al, 2009; Perreault et al, 2012) (Figure 1), both of which have significant roles in the pathological processes underlying drug addiction. For instance, CaMKII in NAc shell has been shown to be critical to cocaine seeking, serving as a biochemical link between dopamine and glutamate (Anderson et al, 2008). In addition, whereas BDNF signaling in NAc and VTA has been shown to mediate the magnitude of the reward responses to cocaine (Bahi et al, 2008; Graham et al, 2007; Graham et al, 2009), BDNF in VTA suppressed the ability of morphine to increase dopamine neuron excitability and promote reward (Koo et al, 2012) and was a negative modulator. These findings indicate that BDNF in VTA may exert opposing effects on the reward circuitry of the brain that are specific to the psychostimulant being tested. Thus, although evidence suggests a potential role for the D1-D2 heteromer in mediating addictive processes, its exact impact on the brain reward circuitry and the mechanisms underlying addiction requires further investigation.
Figure 1.
Signaling pathways activated by the dopamine D1-D2 receptor heteromer. Activation of the Gq-coupled D1-D2 heteromer results in PLC-dependent intracellular calcium release, the activation of CaMKII, and increased expression of BDNF potentially via phosphorylation of MeCP2. Dopamine D1-D2 heteromer activation can additionally lead to the phosphorylation, and inactivation, of GSK-3. The phosphorylation state of GSK-3 can potentially be regulated by BDNF-induced activation of TrkB and the subsequent phosphorylation and activation of Akt. Akt then phosphorylates GSK-3α, and GSK-3β resulted in their inactivation. β-Arrestin1 may also inhibit GSK-3α and GSK-3β activation. GSK-3α and GSK-3β can also be phosphorylated by PKC. BDNF, brain-derived neurotrophic factor; β-arr1/2, β-arrestin1 or β-arrestin2; CaMKII, calcium calmodulin kinase II; DAR, dopamine receptor; DAG, diacylglycerol; GSK-3, glycogen synthase kinase-3; IP3, inositol trisphosphate; IP3R, inositol trisphosphate receptor; MeCP2; methyl CpG-binding protein 2; mTORC2, mTOR complex 2; PIP2, phosphatidylinositol (4,5)-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PDK1, phosphoinositide-dependent kinase-1; PLC, phospholipase C; PKC, protein kinase C; TrkB, tropomyosin receptor kinase B.
The dopamine hypothesis of schizophrenia postulates a hyperactivity of subcortical dopamine transmission; however, it has also been suggested that dysregulated calcium signaling may have a central role in generating the psychopathology of schizophrenia (Lidow, 2003). Although neither of the most abundant dopamine receptors (D1R or D2R) was known to directly regulate calcium signaling, it has been shown that coactivation of both receptors within the dopamine D1-D2 receptor heteromer led to a novel Gq-linked increase in intracellular calcium (Lee et al, 2004; Rashid et al, 2007a). Furthermore, D1-D2 heteromer-mediated signaling could be attenuated by D2R antagonists (as well as D1R antagonists) (Hasbi et al, 2009; Rashid et al, 2007a), indicating the D1-D2 heteromer as being a pharmacological target for antipsychotics in vivo. Thus, clinical administration of most antipsychotics would result in blockade of D2 receptor function, as well as D1-D2 receptor heteromer function. The antipsychotic clozapine, for instance, has been shown to uncouple the subset of D1-D2 receptor heteromers that were in an agonist-detected high-affinity state (Dziedzicka-Wasylewska et al, 2008; Faron-Górecka et al, 2008), a finding that may be of particular relevance given reports of enhanced D1-D2 receptor heteromer expression and activation in cells (Dziedzicka-Wasylewska et al, 2006) and striatum (Perreault et al, 2010) under conditions of persistent dopamine stimulation. Specifically, using FRET techniques it has been shown that the concomitant activation of D1R and D2R by subtype-specific agonists in HEK cells promoted the formation of D1-D2 heteromers (Dziedzicka-Wasylewska et al, 2006). Similarly in vivo, under conditions of hyperdopaminergia, such as occurs with repeated amphetamine administration, enhanced D1-D2 receptor interactions in rat striatum, as indicated by FRET, were apparent as was an increased proportion of the D1-D2 heteromer in the agonist-detected high-affinity state, suggestive of an increase in the functional activity of the receptor complex (Perreault et al, 2010). These findings indicate that there is dynamic regulation of the D1-D2 heteromer that responds to endogenous dopamine levels, such as the high levels that may occur with repeated amphetamine administration, and the increased high-affinity state of the heteromer detected under such circumstances may represent a biomarker of the high dopamine-sensitized state. Postmortem analysis of globus pallidus samples from antipsychotic-treated and untreated schizophrenia patients also revealed a comparable increase in the high-affinity state of the D1-D2 heteromer (Perreault et al, 2010), a finding possibly reflective of increased dopamine transmission in this region. As a physiologically relevant fraction of MSNs within the NAc and globus pallidus express the D1-D2 receptor heteromer (Perreault et al, 2010), together these findings strongly implicate this receptor complex as being significant to dopamine transmission and should be investigated as a therapeutic target for schizophrenia. Further support for a potential role for the D1-D2 heteromer in schizophrenia comes from a recent finding demonstrating that activation of the D1-D2 receptor heteromer could inactivate glycogen synthase kinase-3β (GSK-3β) in rodent prefrontal cortex (PFC) (Perreault et al, 2013) (Figure 1). In schizophrenia, cortical GSK-3β activation is upregulated (Emamian et al, 2004) and has been implicated as contributing to cognitive dysfunction in the disorder (Freyberg et al, 2010; Karam et al, 2010). This suggests potential for D1-D2 heteromer activation as a therapeutic intervention to normalize cortical GSK-3β levels in schizophrenia patients, with potential consequential improvements in cognitive performance. However, given that D2R activation has been associated with increased GSK-3β levels via a non-canonical mechanism involving β-arrestin2 signaling (Beaulieu et al, 2007), and antipsychotics do not invariably improve cognitive deficits in schizophrenia, more research is clearly required to elucidate a role for dopamine-mediated changes in GSK-3β activation in cognitive dysfunction in this disorder and the potential relative importance of β-arrestin-biased signaling.
A potential involvement of the D1-D2 receptor heteromer in depression came to light when it was demonstrated in the postmortem striatum of depressed patients that there was an increased interaction between D1R and D2R (Pei et al, 2010). Using a disrupting peptide in rats, the authors further showed that disruption of the D1-D2 heteromer in the PFC, but not in the NAc or hippocampus, resulted in anti-depressant-like effects in the forced swim test. Similarly, in a learned helplessness paradigm, enhanced association of the D1R and D2R was reported in the PFC and striatum of rats following inescapable foot shock, an effect diminished in the presence of the antidepressant imipramine (Pei et al, 2010). Although the distribution of the D1-D2 heteromer in PFC has not been characterized, approximately 15–25% of the pyramidal neurons in rodent medial PFC coexpress the D1R and D2R (Zhang et al, 2010), implicating these neurons in the anti-depressant-like effects of D1-D2 heteromer disruption.
THE DOPAMINE D2-D4 RECEPTOR HETEROMER
Dopamine D4 receptor (D4R) expression in brain is the lowest among the types of dopamine receptors (Missale et al, 1998; Rondou et al, 2010). Unlike the rat D4R, the gene encoding the human D4R has a number of polymorphic variants (Van Tol et al, 1991), due to repeats of a 16 amino-acid sequence in the third intracellular loop, numbering between 2 and 11. Three of the variants were identified to be the most abundant, D4.2R, D4.4R, and D4.7R (Borroto-Escuela et al, 2011).
Interactions among D4R variants, forming homomer and heteromer species, were shown to occur in transfected cell models. All three of the common variants noted above were able to form homomers. Furthermore, the D4.2R and the D4.4R variants were shown to form heteromers with each other, whereas the D4.7R variant was shown to be refractory to forming heteromer complexes with the other two variants (Borroto-Escuela et al, 2011; González et al, 2012).
Some degree of co-distribution of dopamine D2R and D4R was observed, notably in the dorsal striatum, and therefore it was investigated and confirmed that dopamine D4R variants were able to form heteromeric complexes with both the long and short forms of D2R, although with some differences (Borroto-Escuela et al, 2011; González et al, 2012). Using BRET and in situ Proximity Ligation Assay techniques in cotransfected cells, it was shown that the long form of human D2R (D2LR) was able to interact and form heteromers with the three human D4R isoforms (Borroto-Escuela et al, 2011), with the D4.7R variant being the least effective. Interestingly, allosteric modulations of receptor activity were observed using MAPK assays for the various receptor heteromers. Thus, in cells cotransfected with D2LR and each of the D4R variants, D2 agonist-induced extracellular signal-related kinase (ERK) phosphorylation was enhanced upon coactivation by a D4R agonist, PD168077, in cells coexpressing D2R with D4.2R and D4.4R but not in cells coexpressing D2LR with D4.7R. This may indicate an enhanced allosteric receptor–receptor interaction between certain protomers forming the heteromer complexes. In contrast, the D4.7R variant showed reduced ability to form a heteromer with D2LR, in keeping with the failure to observe any additive effect after combined treatment with D2R and D4R agonists on MAPK activity when these receptors were expressed together. This may suggest that the number of repeats in D4R variants may be a determinant for the formation of heteromers. The D4.7R variant showed higher propensity for homomer formation compared with the other variants tested, while the opposite was observed for heteromer formation (Borroto-Escuela et al, 2011).
Similar to D2LR, the short form of D2R (D2SR) was also shown to form heteromer complexes with D4.2R and D4.4R while the D4.7R failed to interact with D2SR in BRET studies (González et al, 2012). Biochemical crosstalk between the D2SR and cotransfected D4R variants was observed and consisted of ability to potentiate D4R-mediated ERK phosphorylation. Consistent with the failure of D4.7R to form heteromers with D2SR, this biochemical fingerprint of ERK activity potentiation was not observed in cells cotransfected with D2SR and D4.7R. Subsequently, the biochemical fingerprint (potentiation of D4R-mediated MAPK activation by D2R stimulation and not the inverse) was used in mouse striatal slices to show that D2SR was able to form heteromers with the mouse D4R (the equivalent of human D4.2R). Furthermore, when the MAPK study was performed in striatal slices taken from gene knock-in mice carrying the human D4.7R, no synergistic effect was observed, confirming that D2SR was not able to form heteromers with the D4.7R variant.
Interestingly, dopamine-induced decrease of K+-induced glutamate release was shown to involve both D2R and D4R, as the respective antagonists, L-741626 and L-745870, were not only able to partially inhibit dopamine effect individually but were also able to completely abolish dopamine effect when co-applied (González et al, 2012). Moreover, striatal D4R was shown to selectively and locally modulate glutamate release, and the D2R agonist quinelorane synergistically potentiated the D4R-mediated effect, but not the inverse, suggesting a specific qualitative D2S-D4 heteromer-mediated effect in the brain similar to the biochemical fingerprint seen with MAPK activation in transfected cells. It was then postulated that the failure of D2SR to form heteromers with D4.7R may impair dopamine-induced modulation of corticostriatal glutamatergic neurotransmission, which may be linked to ADHD (González et al, 2012). The presence of the 7-repeat allele of D4R seems to affect neuropsychological functioning depending on age and ADHD status (Altink et al, 2012), and there are many genetic association studies linking this repeat allele (D4.7R) and other candidate genes (Kebir and Joober, 2011) to the development of ADHD. The role of D2R in ADHD is still not particularly clear, and the discovery of a role for the dopamine D2-D4 receptor heteromer may represent a new research target for ADHD.
ADENOSINE-DOPAMINE RECEPTOR HETEROMERS: A1-D1, A2-D2
The existence of the A1-D1 receptor heteromer was reported over a decade ago following the demonstration of coimmunoprecipitation of adenosine A1 receptor (A1R) and D1R in fibroblast cells (Ginés et al, 2000), and shortly thereafter, its expression was shown by the same method in rat NAc (Toda et al, 2003). The A2-D2 heteromer, also identified by coimmunoprecipitation, was first shown in neuroblastoma cells (Hillion et al, 2002) and has since been shown to exist in living cells by FRET and BRET analysis (Canals et al, 2003; Kamiya et al, 2003). It has been suggested that the A1-D1 and A2-D2 heteromers have a discrete distribution in the basal ganglia, with selective expression along the striatonigral and stratopallidal pathways, respectively (Ferré et al, 2007; Franco et al, 2007; Fuxe et al, 2008), and functional studies indicate that these receptor complexes may be the molecular entities responsible, at least in part, for the antagonistic interactions between adenosine and dopamine receptors, functioning to uncouple the dopamine receptors from their respective G-proteins and dampen receptor signaling (Azdad et al, 2009; Franco et al, 2007; Fuxe et al, 2005; Fuxe et al, 2008).
The ability of adenosine-dopamine receptor heteromerization to attenuate dopamine receptor function indicates that these receptor complexes are of relevance to dopamine transmission in the basal ganglia, and thus have a potential role in dopamine disorders. For example, following cocaine withdrawal the coimmunoprecipitation of A1R and D1R was reduced in rat NAc, indicating a reduction in heteromer formation (Toda et al, 2003). Similarly, using BRET methodology, cocaine was shown, through direct actions on D2R, to induce a conformational change in the A2-D2 complex, resulting in reduced BRETmax, potentially indicative of a reduction in heteromer expression (Marcellino et al, 2010). In schizophrenia animal models, evidence of antipsychotic effects of the A2 receptor (A2R) agonist CGS 21680 have been demonstrated (Andersen et al, 2002; Rimondini et al, 1997), and adenosine augmentation has been shown to ameliorate both psychotic and cognitive schizophrenia-like symptoms in mice (Shen et al, 2012), potentially by acting at the A2R within the A2-D2 heteromer, and thus reducing the proportion of D2R in the agonist-induced high-affinity state and D2R signaling (Fuxe et al, 2005; Fuxe et al, 2010). It has been further suggested that an imbalance in adenosine signaling, a neurotransmitter which modulates both dopamine and glutamate transmission, may be a central factor in the susceptibility to develop schizophrenia (Boison et al, 2012). In support of this hypothesis, it has been repeatedly shown that enhancing NMDA receptor function improves the negative and cognitive symptoms of schizophrenia (Coyle, 2012), and interestingly, heteromerization of the D2R with the A2R negatively regulated D2R-induced suppression of NMDA-mediated depolarization plateau potential (Azdad et al, 2009).
Thus far, studies have strongly suggested independent roles for the A1-D1 and A2-D2 receptor heteromers in regulating striatonigral and striatopallidal dopamine transmission, respectively. However, given the identification of neurons that coexpress the D1R and D2R in both the direct and indirect pathways, the expression of A1-D1-D2 or D1-D2-A2 heteromers in these neurons is a possibility, an intriguing prospect given that the sites of interaction between the D2R and D1R or A2R have been reported and are distinct (Ciruela et al, 2004; O'Dowd et al, 2012). Heterotrimeric receptor complexes involving dopamine receptors have already been identified, including the higher-order adenosine-dopamine receptor heteromer, the A2-D2-mGlu5 heteromer (Cabello et al, 2009), which has been suggested as a therapeutic target for schizophrenia by counteracting exaggerated D2R signaling in the ventral striatopallidal pathway (Fuxe et al, 2010).
THE DOPAMINE D1-D3, D2-D3 AND D2-D5 RECEPTOR HETEROMERS
The D1R and D3 receptors (D3R) show prominent colocalization in certain neurons of the direct striatonigral pathway (Ridray et al, 1998) and have been shown to form D1-D3 heteromers both in cells by BRET and FRET and to coimmunopecipitate from rat striatum (Fiorentini et al, 2008; Marcellino et al, 2008b). The physiological effect of this interaction was a D3R-stimulated increase in D1R-mediated responses in neurons that coexpressed both receptors (Fiorentini et al, 2008; Marcellino et al, 2008b). Although this heteromer was suggested to have potential therapeutic value as a drug target in Parkinson's disease (Ferré et al, 2010), these findings may also have significant future implications for disorders involving striatal D1R transmission, the most notable being drug addiction, where D1R signaling has such a critical role in drug reward.
The existence of a functional D2-D3 receptor heteromer was first shown in cells using chimeras generated from receptor fragments of the D2R and D3R in combination with coimmunoprecipitation (Scarselli et al, 2001). More recently, the D2-D3 heteromer was demonstrated to coexist with D2R and D3R homomers in cells at the plasma membrane using the newly developed SNAP and CLIP tag reagents (Pou et al, 2012). A putative role for the D2-D3 heteromer as a target for antipsychotics and, in particular, for antipsychotics with partial D2R agonism has been suggested (Maggio and Millan, 2010), an idea based on findings in cells that in the presence of excess D3R, the properties of partial D2R agonists, such as the antipsychotic aripiprazole, were transformed to antagonist actions (Novi et al, 2007). Thus the actions of these drugs would be postulated to have differing brain region-dependent effects, dependent on the density of expression of D3R. As a result, in the medial ventral striatum where the preponderance of D2-D3 heteromers may occur (based on D3R localization), ‘partial D2R agonists' could function as D2R antagonists, whereas in the dorsal striatum, where D2-D3 heteromers would be few in number, they would exhibit partial D2R agonism (Maggio and Millan, 2010).
Expression of the dopamine D2-D5 receptor heteromer has been demonstrated in living cells (O'Dowd et al, 2013; So et al, 2009) and the two receptors have been shown to interact via electrostatic interactions between residues in the C-tail of the D5R and the third intracellular loop of the D2R (O'Dowd et al, 2013). Similar to the D1-D2 heteromer, the D2-D5 heteromer has been linked to increased intracellular calcium accumulation (So et al, 2009); however the signaling mechanisms underlying these increases in calcium mobilization are completely distinct (Hasbi et al, 2010). For example, in contrast to the D1-D2 receptor heteromer, which induces increased calcium release solely from intracellular stores (Lee et al, 2004; Rashid et al, 2007a), calcium mobilization induced by the D2-D5 heteromer was shown in cells to involve a small rise in intracellular calcium mediated by Gq and PLC, followed by a large influx of extracellular calcium through store-operated calcium channels (So et al, 2009). In addition, unlike the D1R, activation of the D5R triggered a robust calcium signal, an effect that was attenuated when the D5R heteromerized with coexpressed D2R and disinhibited when both receptors were activated (So et al, 2009). Therefore, together these findings suggest that in regions where the D2R and D5R are coexpressed, such as in rat cortex and ventral pallidum, and to a much lesser degree in CP (So et al, 2009), the D2-D5 heteromer may have a role in regulating signaling events linked to calcium, one consequence of which may be the activation of CaMKII, a protein kinase previously discussed herein to be involved in both drug addiction and schizophrenia.
DOPAMINE-NMDA RECEPTOR HETEROMERS: D1-NR1, D2-NR2B
It was shown in rat hippocampus that the NR1 subunit of the N-methyl-𝒟-aspartate (NMDA) receptor could coimmunoprecipitate with the D1R from rat hippocampal tissue (Lee et al, 2002). Similarly, in striatal postsynaptic density (PSD) preparations the C-terminal tail of the D1R, but not the D5R, coimmunoprecipitated with the NR1 subunit of the NMDA receptor (Fiorentini et al, 2003). The formation of the D1-NR1 heteromer was shown to occur through a strong and stable arginine–phosphate electrostatic interaction (Woods et al, 2005; Woods and Ferré, 2005) and reported to be enhanced by ligand occupancy of the NMDA/glutamate-binding site of the NMDA receptor, to slow down lateral diffusion, and stabilize D1R localization in the synapse (Scott et al, 2006). Presumably, increased synaptic localization would make these receptors more susceptible to activation by released dopamine, culminating in enhanced signal transduction and neuronal responsiveness. Indeed, it was shown in cells and hippocampal neurons that activation of the NDMA receptor promoted D1R translocation to the plasma membrane and enhanced D1R-mediated cyclic AMP accumulation (Pei et al, 2004), thus resulting in an overall increase in D1R activation and function. Conversely, direct D1R interactions with the NR1 subunit decreased NMDA currents and NMDA-mediated excitotoxicity (Lee et al, 2002). In addition, the D1-NMDA receptor complex may be of relevance for disorders involving cognitive dysfunction such as schizophrenia, as activation of the D1R upregulated NMDA receptor-mediated LTP in hippocampus in a CaMKII-dependent manner and promoted working memory, whereas uncoupling the D1-NMDA complex abolished the D1R-induced upregulation of NMDA-mediated LTP and impaired working memory in mice (Nai et al, 2010). Interestingly, an interaction between the D1R and NMDA receptor was also demonstrated in rat PFC (Kruse et al, 2009), a region critically involved in the cognitive impairments inherent in schizophrenia (Lewis, 2012), and in which NMDA hypofunction has been implicated (Jentsch et al, 1997; Mohn et al, 1999). It has thus been suggested that reduced D1R and NMDA function may contribute to the etiology of schizophrenia, and additionally, that the prefrontal cortical hypodopaminergia inherent in the disorder may be secondary to NMDA receptor dysfunction (Nai et al, 2010).
A direct interaction between the D2R and the NR2B subunit of the NMDA receptor was demonstrated in the PSD of excitatory synapses in striatum, the physiological function of which was to disrupt the interaction of CaMKII with the NR2B subunit, reduce CaMKII-mediated NR2B phosphorylation, and inhibit NMDA receptor-mediated currents (Liu et al, 2006). In the same study, Liu et al (2006) also showed that while acute cocaine administration to mice increased the physical association of the two receptors, disruption of the D2R-NR2B complex significantly reduced cocaine-induced locomotor activation and stereotypy, a finding which directly linked the D2-NR2B heteromeric complex to behavioural responses evoked by cocaine.
OTHER DOPAMINE RECEPTOR HETEROMERS
Despite the D2R and the serotonin 5HT2A receptor (5HT2AR) having distinct cell signaling properties, being linked to the Gi/o and Gq/11 proteins, respectively, they are both receptor targets for antipsychotic drugs in schizophrenia. Although the existence of the D2-5HT2A heteromer has not been definitively demonstrated in vivo, functional crosstalk between the two receptors has been shown at both the level of receptor pharmacology, cell signaling properties, as well as in a behavioural assay of locomotor activity (Albizu et al, 2011). This crosstalk was suggested by the authors to be potentially mediated by the D2-5HT2A receptor heteromer as this heteromeric complex has been reported to occur in cells using FRET, BRET, and coimmunoprecipitation techniques (Albizu et al, 2011; Borroto-Escuela et al, 2010; Łukasiewicz et al, 2010), and the site of interaction reported to occur between the C-tail of the 5HT2AR and the third intracellular loop of the D2R (Łukasiewicz et al, 2010). Interestingly, ligands for the 5HT2AR or the D2R were shown to directly influence the heterodimerization process in cells, with agonists reducing the FRET value between the 5HT2AR and the D2R in the D2-5HT2A heteromer and antagonists increasing the FRET values (Łukasiewicz et al, 2010). The authors therefore posited that the agonists and antagonists may have promoted the formation of homomeric or heteromeric receptor entities, respectively, although these results could represent conformational changes induced by the drugs. Given that heteromeric receptor complexes often exhibit unique functional characteristics compared with their constituent receptors, the ability of pharmacological agents to selectively target the signaling pathways being initiated in favor of homomeric or heteromeric complexes may have significant future therapeutic implications in any number of human diseases.
The histamine H3 receptor (H3R) has also been shown by BRET to form a heteromeric complex with the D1R and D2R in cells (Ferrada et al, 2008; Ferrada et al, 2009) and to coimmunoprecipitate with the D1R or D2R in the striatum (Moreno et al, 2011). At a functional level, it was demonstrated that H3R-induced activation of ERK phosphorylation occurred only in striatal slices of mice expressing the D1R but not in mice gene-deleted for the D1R. Conversely, both D1R and H3R antagonists attenuated D1R- or H3R-induced ERK activation (Moreno et al, 2011). As the H3R has been implicated in a number of psychiatric disorders, including schizophrenia, addiction, and ADHD (Vohora and Bhowmik, 2012), these findings suggest a potential contribution of the D1-H3 heteromer in mediating some of the effects attributed solely to the H3R, and thus further investigation into the role of the D1-H3 heteromer in the etiology of these mental health disorders is warranted.
FUTURE RESEARCH DIRECTIONS
The involvement of the dopaminergic system in a wide array of mental disorders has resulted in the pharmacological targeting of dopamine receptors as the mainstay for many of the treatments currently available. Most of the ligands developed and clinically used have a single dopamine receptor type or subtype as a target. The successive demonstration of the presence of more complex physical and functional interactions among the dopamine receptors and between these receptors and other receptors, including other GPCRs and ion channels, should add a new dimension to rational drug design that may lead to the development of new approaches taking into account the presence and physiological relevance of these heteromers (George et al, 2002).
Bivalent Ligands
One approach based on the notion of receptor dimerization is the development of bivalent ligands. Such compounds are formed of two ligand moieties linked through a spacer capable of binding to both protomers of a dimer (Guixà-González et al, 2012). Some of these bivalent ligands were described in the literature for different homo- and heteromers, such as bivalent ligands for D2-D2 homodimers (Kühhorn et al, 2011), for D2-A2 heterodimers (Soriano et al, 2009), or opioid receptor heterodimers (Balboni et al, 2010; 2011; Zhang et al, 2009). Physicochemical limitations due notably to their large molecular sizes (Morphy and Rankovic, 2006; Guixà-González et al, 2012) may represent, however, a big challenge against their pharmaceutical development and clinical use.
In many cases, receptor heteromerization has been shown to confer novel pharmacological profiles as well as signaling properties different from those of the protomers that constitute these receptor complexes (George and O'Dowd, 2007; Maggio et al, 2009; Smith and Milligan, 2010). Interestingly, these receptor heteromer complexes are mostly confined to some brain regions, as is the case for the dopamine D1-D2 heteromer (Hasbi et al, 2009; Perreault et al, 2010), which makes targeting GPCR heteromers a pharmacological alternative that offers the advantage of a higher brain region specificity and a better targeting of receptor signals.
Allosterism
Receptor oligomerization through protein–protein interactions can be considered as a form of allosterism (Maggio et al, 2009), in that the binding of a compound to one protomer of the heteromer may positively or negatively modulate the drug occupancy of the other protomer, or in some cases, some heteromers may display a selectivity to ligands not observed in the case of individual receptors (George and O'Dowd, 2007; Maggio et al, 2009; Smith and Milligan, 2010). In the first type of scenario, some examples were cited for the GABAB receptor complex (Galvez et al, 2001), for A2-D2 receptor heterodimers (Franco et al, 2000), D2-D3 heteromers (Maggio et al, 2009), delta-kappa opioid heterodimers (Jordan and Devi, 1999), as well as for somatostatin-dopamine sSST5-D2 receptors (Rocheville et al, 2000) (reviewed in Maggio et al, 2009). For the second case, which relates to the differing specificity of certain ligands to either the heteromer or a constituent homomer, some examples were described such as for the dopamine D1-D2 heteromer (Rashid et al, 2007b) and dopamine D2-D3 heteromer (Maggio and Millan, 2010; Maggio et al, 2009). For example, two D1R-like agonists, SKF 83959 and SKF 83822, although showing high radioligand-binding affinities for the D1R in D1-D2 or D1-D1 receptor complexes, showed very specific functional effects, with SKF 83959 robustly stimulating the D1-D2 heteromer-mediated calcium signal and not activating adenylyl cyclase by the D1-D1 homomer, whereas SKF 83822 robustly stimulated adenylyl cyclase by the D1-D1 homomer with no effect on calcium release through the D1-D2 heteromer complexes (George and O'Dowd, 2007; Rashid et al, 2007a; Hasbi et al, 2009). Furthermore, examining the binding pockets within each receptor in the D1-D2 heteromer complex revealed that SKF 83959 occupied both binding pockets and acted as a full agonist at the D1R and a partial agonist at the D2R within the D1-D2 receptor heteromer (Rashid et al, 2007a; 2007b; George and O'Dowd, 2007). Another D1R agonist, SKF 81297, showed no specificity for the signal and robustly stimulated both Gs-mediated adenylyl cyclase activity through D1-D1 homomer complexes as well as Gq-mediated intracellular calcium release through D1-D2 heteromer complexes. Thus, these agonists show selective actions to activate either the D1-D2 heteromer (SKF 83959) or the D1-D1 homomer (SKF 83822), or both at the same time (SKF 81297), suggesting there are significant differences within the binding pockets of the receptors depending on whether they are within a homomeric/heteromeric complex (Rashid et al, 2007b; George and O'Dowd, 2007; Hasbi et al, 2009; 2010; Verma et al, 2010). These differences in the binding pockets of the receptors induced by heteromerization may be an aspect that can be capitalized upon to develop heteromer-specific compounds.
Selective Modulation of Signaling Pathways
In numerous cases of receptor complex formation, such as with the D1-D2 receptor heteromer, the heteromerization confers to the receptor complex a different signaling mechanism than that activated by the two individual protomers (George and O'Dowd, 2007). The modification of the signaling properties by receptor oligomerization may constitute another manifestation of the effects of allosterism, whether these changes are minor resulting in differences in efficacy or are major with a complete signal switching (reviewed in Smith and Milligan, 2010), as is the case for the D1-D2 heteromer. The stimulation of the dopamine D1-D2 receptor heteromer, for instance, triggers a Gq-mediated intracellular mobilization of calcium that neither D1R or D2R individually are associated with, and whose specificity was shown using D1R−/−, D2R−/−, and D5R−/− gene-deleted mice (George and O'Dowd, 2007; Rashid et al, 2007a, Hasbi et al, 2009). One approach to better study the pathophysiologies linked to receptor heteromerization would be to generate ligands more specific to either heteromeric or homomeric complexes, capable of activating one signaling pathway or the other. Another approach would be to target a particular signaling pathway or even a component of a signaling pathway specific to the receptor oligomer in question. However, although targeting the signaling pathway may be an interesting path to investigate, the results may not be definitive as these signaling pathways are not specific to a particular homo- or heteromer receptor complex.
Biased Agonism
Another approach that is under scrutiny in drug development is based on a ligand's preference for one signaling pathway over another, a phenomenon also known as ‘biased agonism' (Beaulieu and Gainetdinov, 2011). Of notable interest was the discovery of the ability of some GPCR ligands to activate G-protein-independent but β-arrestin-dependent signaling (Beaulieu and Gainetdinov, 2011). This biased agonism may represent another interesting path in drug development, although there are not yet many compounds with a clear demonstration of potential clinical use, notably in the case of the dopamine receptor heteromers.
Disrupting the Heteromers
Identification of the specific receptor–receptor interaction interfaces involved in the formation/stabilization of receptor heteromers may yield another approach to antagonize heteromer function, based essentially on disrupting the receptor complexes. The parts of a receptor that are involved in the interaction with another protomer may be used as a target to disrupt the interaction, using a peptide that mimics the interaction interface. For the D1-D2 receptor heteromer, it has been shown that specific amino acids in the D1R carboxyl tail (O'Dowd et al, 2011; 2012; Łukasiewicz et al, 2009) are important in D1-D2 heteromer formation. These specific amino acids in the D1R carboxyl tail interacted with a region in the third intracellular loop of D2R, common to both D2LR and D2SR (O'Dowd et al, 2011; 2012; Łukasiewicz et al, 2009). A peptide generated based on these findings from the D1R was found to disrupt the D1-D2 receptor heteromer physically in brain and to inhibit its calcium-mediated signaling pathway with behavioral consequences (Hasbi et al, under review). Another peptide generated from the region of D2LR that is lacking in the D2SR was also reported to disrupt the D1-D2 receptor heteromer and showed antidepressant-like effects in mice (Pei et al, 2010). In the absence of specific antagonists for the heteromers, this disrupting peptide strategy may be very useful in studying the biology of receptor heteromers as well as their link to disease pathophysiologies in animal models. The specificity of the peptide(s) used to target a specific heteromer should, however, be tested to avoid disrupting other receptor complexes.
In conclusion, dopamine receptors participate in homomeric and heteromeric complexes with significant implications for the deeper understanding of the complex physiological roles of these receptors in brain. The emerging significance of these receptor–receptor interactions has added an enormous degree of complexity in our attempts to understand dopamine receptor function in brain. However, these novel signaling complexes provide fascinating new possibilities and novel perspectives on physiological mechanisms and models of neuropsychiatric disease. Further, these complexes provide novel targets for drug discovery, as besides the classical tools to target a specific type or subtype of dopamine receptor, investigators now have the opportunity to open new fields of research to generate compounds that may take into account that these receptors exist as heteromeric complexes, often with distinct anatomical localization as well as signaling and functional properties. Contemporary drug discovery strategies have not incorporated the issue of receptor heteromers into the discovery process, and this presents a new challenge that must be surmounted. Different approaches are possible in the search for ligands specifically targeting receptor heteromers without affecting homomers or vice versa, such as the development of bivalent ligands, the targeting of a particular signaling pathway or one of its components, specifically probing binding pocket differences, or the use of peptides to specifically disrupt these receptor complexes. In other words, the potential for true ‘designer drugs' targeting dopamine receptor heteromers may be within reach, aiming for selective activation or inactivation of these receptor complexes.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This work was supported by a grant from the National Institute on Drug Abuse (to SRG) and a Canadian Institute of Health Research Postdoctoral Fellowship (to MLP). SRG holds a Canada Research Chair in Molecular Neuroscience.
References
- Albizu L, Holloway T, González-Maeso J, Sealfon SC. Functional crosstalk and heteromerization of serotonin 5-HT2A and dopamine D2 receptors. Neuropharmacology. 2011;61:770–777. doi: 10.1016/j.neuropharm.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altink ME, Rommelse NN, Slaats-Willemse DI, Vásquez AA, Franke B, Buschgens CJ, et al. The dopamine receptor D4 7-repeat allele influences neurocognitive functioning, but this effect is moderated by age and ADHD status: an exploratory study. World J Biol Psychiatry. 2012;13:293–305. doi: 10.3109/15622975.2011.595822. [DOI] [PubMed] [Google Scholar]
- Andersen MB, Fuxe K, Werge T, Gerlach J. The adenosine A2A receptor agonist CGS 21680 exhibits antipsychotic-like activity in Cebus apella monkeys. Behav Pharmacol. 2002;13:639–644. doi: 10.1097/01.fbp.0000047148.28986.67. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, et al. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Azdad K, Gall D, Woods AS, Ledent C, Ferré S, Schiffmann SN. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology. 2009;34:972–986. doi: 10.1038/npp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A, Boyer F, Chandrasekar V, Dreyer JL. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacology (Berl) 2008;199:169–182. doi: 10.1007/s00213-008-1164-1. [DOI] [PubMed] [Google Scholar]
- Balboni G, Salvadori S, Marczak ED, Knapp BI, Bidlack JM, Lazarus LH, et al. Opioid bifunctional ligands from morphine and the opioid pharmacophore Dmt-Tic. Eur J Med Chem. 2011;46:799–803. doi: 10.1016/j.ejmech.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni G, Salvadori S, Trapella C, Knapp BI, Bidlack JM, Lazarus LH, et al. Evolution of the bifunctional lead μ agonist/ δ antagonist containing the Dmt-Tic opioid pharmacophore. ACS Chem Neurosci. 2010;1:155–164. doi: 10.1021/cn900025j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baragli A, Alturaihi H, Watt HL, Abdallah A, Kumar U. Heterooligomerization of human dopamine receptor 2 and somatostatin receptor 2 Co-immunoprecipitation and fluorescence resonance energy transfer analysis. Cell Signal. 2007;19:2304–2316. doi: 10.1016/j.cellsig.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR.2011The physiology, signaling, and pharmacology of dopamine receptors Pharmacol Rev 63182–217.(A clear synopsis of the biology of dopamine receptors and their role in disease.). [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Singer P, Shen HY, Feldon J, Yee BK. Adenosine hypothesis of schizophrenia—opportunities for pharmacotherapy. Neuropharmacology. 2012;62:1527–1543. doi: 10.1016/j.neuropharm.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Romero-Fernandez W, Tarakanov AO, Marcellino D, Ciruela F, Agnati LF, et al. Dopamine D2 and 5-hydroxytryptamine 5-HT((2)A) receptors assemble into functionally interacting heteromers. Biochem Biophys Res Commun. 2010;401:605–610. doi: 10.1016/j.bbrc.2010.09.110. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Van Craenenbroeck K, Romero-Fernandez W, Guidolin D, Woods AS, Rivera A, et al. Dopamine D2 and D4 receptor heteromerization and its allosteric receptor-receptor interactions. Biochem Biophys Res Commun. 2011;404:928–934. doi: 10.1016/j.bbrc.2010.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello N, Gandia J, Bertarelli DC, Watanabe M, Lluís C, Franco R, et al. 2009Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher-order oligomers in living cells J Neurochem 1091497–1507.(The only demonstration thus far of a higher order receptor heteromer, A2-D2-mGlu5, as seen in living cells.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, et al. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Burgueño J, Casadó V, Canals M, Marcellino D, Goldberg SR, et al. Combining mass spectrometry and pull-down techniques for the study of receptor heteromerization. Direct epitope-epitope electrostatic interactions between adenosine A2A and dopamine D2 receptors. Anal Chem. 2004;76:5354–5363. doi: 10.1021/ac049295f. [DOI] [PubMed] [Google Scholar]
- Coyle JT. NMDA receptor and schizophrenia: a brief history. Schizophr Bull. 2012;38:920–926. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M, Faron-Górecka A, Andrecka J, Polit A, Kuśmider M, Wasylewski Z. Fluorescence studies reveal heterodimerization of dopamine D1 and D2 receptors in the plasma membrane. Biochemistry. 2006;45:8751–8759. doi: 10.1021/bi060702m. [DOI] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M, Faron-Górecka A, Górecki A, Kuśmider M. Mechanism of action of clozapine in the context of dopamine D1-D2 receptor hetero-dimerization—a working hypothesis. Pharmacol Rep. 2008;60:581–587. [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J. Neurobiology of attention-deficit hyperactivity disorder. Biol Psychiatry. 1998;44:951–958. doi: 10.1016/s0006-3223(98)00240-6. [DOI] [PubMed] [Google Scholar]
- Faron-Górecka A, Górecki A, Kuśmider M, Wasylewski Z, Dziedzicka-Wasylewska M. The role of D1-D2 receptor hetero-dimerization in the mechanism of action of clozapine. Eur Neuropsychopharmacol. 2008;18:682–691. doi: 10.1016/j.euroneuro.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Ferrada C, Ferré S, Casadó V, Cortés A, Justinova Z, Barnes C, et al. Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology. 2008;55:190–197. doi: 10.1016/j.neuropharm.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrada C, Moreno E, Casadó V, Bongers G, Cortés A, Mallol J, et al. Marked changes in signal transduction upon heteromerization of dopamine D1 and histamine H3 receptors. Br J Pharmacol. 2009;157:64–75. doi: 10.1111/j.1476-5381.2009.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Agnati LF, Ciruela F, Lluís C, Woods AS, Fuxe K, et al. Neurotransmitter receptor heteromers and their integrative role in ‘local modules': the striatal spine module. Brain Res Rev. 2007;55:55–67. doi: 10.1016/j.brainresrev.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Lluís C, Lanciego JL, Franco R. Prime time for G-protein-coupled receptor heteromers as therapeutic targets for CNS disorders: the dopamine D(1)-D(3) receptor heteromer. CNS Neurol Disord Drug Targets. 2010;9:596–600. doi: 10.2174/187152710793361603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol. 2008;74:59–69. doi: 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Gardoni F, Spano P, Di Luca M, Missale C. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J Biol Chem. 2003;278:20196–20202. doi: 10.1074/jbc.M213140200. [DOI] [PubMed] [Google Scholar]
- Franco R, Ferré S, Agnati L, Torvinen M, Ginés S, Hillion J, et al. Evidence for adenosine/dopamine receptor interactions: indications for heterodimerization. Neuropsychopharmacology. 2000;23:S50–S59. doi: 10.1016/S0893-133X(00)00144-5. [DOI] [PubMed] [Google Scholar]
- Franco R, Lluís C, Canela EI, Mallol J, Agnati L, Casadó V, et al. Receptor-receptor interactions involving adenosine A1 or dopamine D1 receptors and accessory proteins. J Neural Transm. 2007;114:93–104. doi: 10.1007/s00702-006-0566-7. [DOI] [PubMed] [Google Scholar]
- Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167:388–396. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Ferré S, Canals M, Torvinen M, Terasmaa A, Marcellino D, et al. 2005Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function J Mol Neurosci 26209–220.(Concise summary of the role of A2-D2 receptor heteromers in Parkinson's disease and schizophrenia.). [DOI] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Leo G, Agnati LF. Molecular integration via allosteric interactions in receptor heteromers. A working hypothesis. Curr Opin Pharmacol. 2010;10:14–22. doi: 10.1016/j.coph.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Rivera A, Díaz-Cabiale Z, Filip M, Gago B, et al. Receptor-receptor interactions within receptor mosaics. Impact on neuropsychopharmacology. Brain Res Rev. 2008;58:415–452. doi: 10.1016/j.brainresrev.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Galvez T, Duthey B, Kniazeff J, Blahos J, Rovelli G, Bettler B, et al. Allosteric interactions between GB1 and GB2 subunits are required for optimal GABA(B) receptor function. EMBO J. 2001;20:2152–2159. doi: 10.1093/emboj/20.9.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, O'Dowd BF. A novel dopamine receptor signaling unit in brain: heterooligomers of D1 and D2 dopamine receptors. ScientificWorldJournal. 2007;7:58–63. doi: 10.1100/tsw.2007.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, O'Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov. 2002;1:808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- Ginés S, Hillion J, Torvinen M, Le Crom S, Casadó V, Canela EI, et al. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci USA. 2000;97:8606–8611. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González S, Rangel-Barajas C, Peper M, Lorenzo R, Moreno E, Ciruela F, et al. 2012Dopamine D4 receptor, but not the ADHD-associated D4.7 variant, forms functional heteromers with the dopamine D2S receptor in the brain Mol Psychiatry 17650–662.(The first evidence demonstrating a lack of heteromer formation between the dopamine D2 short receptor and the ADHD-associated D4.7 variant in brain.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorinski N, Kowalsman N, Renner U, Wirth A, Reinartz MT, Seifert R, et al. Computational and experimental analysis of the transmembrane domain 4/5 dimerization interface of the serotonin 5-HT(1A) receptor. Mol Pharmacol. 2012;82:448–463. doi: 10.1124/mol.112.079137. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, et al. Tropomyosin-related kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biol Psychiatry. 2009;65:696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guixà-González R, Bruno A, Marti-Solano M, Selent J. Crosstalk within GPCR heteromers in schizophrenia and Parkinson's disease: physical or just functional. Curr Med Chem. 2012;19:1119–1134. doi: 10.2174/092986712799320574. [DOI] [PubMed] [Google Scholar]
- Guo W, Shi L, Javitch JA. The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J Biol Chem. 2003;278:4385–4388. doi: 10.1074/jbc.C200679200. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. How and why do GPCRs dimerize. Trends Pharmacol Sci. 2008;29:234–240. doi: 10.1016/j.tips.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague C, Uberti MA, Chen Z, Hall RA, Minneman KP. Cell surface expression of alpha1D-adrenergic receptors is controlled by heterodimerization with alpha1B-adrenergic receptors. J Biol Chem. 2004;279:15541–15549. doi: 10.1074/jbc.M314014200. [DOI] [PubMed] [Google Scholar]
- Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O'Dowd BF, et al. 2009Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth Proc Natl Acad Sci USA 10621377–21382.(The first demonstration of endogenous GPCR heteromers in situ in brain and first evidence linking the dopamine D1-D2 receptor heteromer to BDNF expression and neuronal maturation.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, O'Dowd BF, George SR.2010Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms Curr Opin Pharmacol 1093–99.(A review of the functional differences between dopamine D1-D2 and D2-D5 receptor heteromers in the regulation of intracellular calcium signaling.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casadó V, Scott R, Terasmaa A, et al. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- Huang J, Chen S, Zhang JJ, Huang XY. Crystal structure of oligomeric beta1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat Struct Mol Biol. 2013;20:419–425. doi: 10.1038/nsmb.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SN, Wang HY, Yergey A, Woods AS. Phosphate stabilization of intermolecular interactions. J Proteome Res. 2006;5:122–126. doi: 10.1021/pr0503578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Redmond DE, Jr, Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T, Saitoh O, Yoshioka K, Nakata H. Oligomerization of adenosine A2A and dopamine D2 receptors in living cells. Biochem Biophys Res Commun. 2003;306:544–549. doi: 10.1016/s0006-291x(03)00991-4. [DOI] [PubMed] [Google Scholar]
- Karam CS, Ballon JS, Bivens NM, Freyberg Z, Girgis RR, Lizardi-Ortiz JE, et al. Signaling pathways in schizophrenia: emerging targets and therapeutic strategies. Trends Pharmacol Sci. 2010;31:381–390. doi: 10.1016/j.tips.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpa KD, Lin R, Kabbani N, Levenson R. The dopamine D3 receptor interacts with itself and the truncated D3 splice variant d3nf: D3-D3nf interaction causes mislocalization of D3 receptors. Mol Pharmacol. 2000;58:677–683. doi: 10.1124/mol.58.4.677. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–768. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- Kebir O, Joober R. Neuropsychological endophenotypes in attention-deficit/hyperactivity disorder: a review of genetic association studies. Eur Arch Psychiatry Clin Neurosci. 2011;261:583–594. doi: 10.1007/s00406-011-0207-5. [DOI] [PubMed] [Google Scholar]
- Kong MM, Fan T, Varghese G, O'Dowd BF, George SR. Agonist-induced cell surface trafficking of an intracellularly sequestered D1 dopamine receptor homo-oligomer. Mol Pharmacol. 2006;70:78–89. doi: 10.1124/mol.105.021246. [DOI] [PubMed] [Google Scholar]
- Koo JW, Mazei-Robison MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, et al. BDNF is a negative modulator of morphine action. Science. 2012;338:124–128. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse MS, Premont J, Krebs MO, Jay TM. Interaction of dopamine D1 with NMDA NR1 receptors in rat prefrontal cortex. Eur Neuropsychopharmacol. 2009;19:296–304. doi: 10.1016/j.euroneuro.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kühhorn J, Götz A, Hübner H, Thompson D, Whistler J, Gmeiner P. Development of a bivalent dopamine D2 receptor agonist. J Med Chem. 2011;54:7911–7919. doi: 10.1021/jm2009919. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, et al. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Lee SP, O'Dowd BF, Rajaram RD, Nguyen T, George SR. D2 dopamine receptor homodimerization is mediated by multiple sites of interaction, including an intermolecular interaction involving transmembrane domain 4. Biochemistry. 2003;42:11023–11031. doi: 10.1021/bi0345539. [DOI] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, et al. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Cortical circuit dysfunction and cognitive deficits in schizophrenia--implications for preemptive interventions. Eur J Neurosci. 2012;35:1871–1878. doi: 10.1111/j.1460-9568.2012.08156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res Brain Res Rev. 2003;43:70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]
- Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, et al. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- López-Giménez JF, Canals M, Pediani JD, Milligan G. The alpha1b-adrenoceptor exists as a higher-order oligomer: effective oligomerization is required for receptor maturation, surface delivery, and function. Mol Pharmacol. 2007;71:1015–1029. doi: 10.1124/mol.106.033035. [DOI] [PubMed] [Google Scholar]
- Maggio R, Aloisi G, Silvano E, Rossi M, Millan MJ. Heterodimerization of dopamine receptors: new insights into functional and therapeutic significance. Parkinsonism Relat Disord. 2009;(Suppl 4):S2–S7. doi: 10.1016/S1353-8020(09)70826-0. [DOI] [PubMed] [Google Scholar]
- Maggio R, Millan MJ. Dopamine D2-D3 receptor heteromers: pharmacological properties and therapeutic significance. Curr Opin Pharmacol. 2010;10:100–107. doi: 10.1016/j.coph.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Mancia F, Assur Z, Herman AG, Siegel R, Hendrickson WA. Ligand sensitivity in dimeric associations of the serotonin 5HT2c receptor. EMBO Rep. 2008;9:363–369. doi: 10.1038/embor.2008.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I, et al. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology. 2008a;54:815–823. doi: 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Ferré S, Casadó V, Cortés A, Le Foll B, Mazzola C, et al. Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J Biol Chem. 2008b;283:26016–26025. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Navarro G, Sahlholm K, Nilsson J, Agnati LF, Canela EI, et al. Cocaine produces D2R-mediated conformational changes in the adenosine A(2A)R-dopamine D2R heteromer. Biochem Biophys Res Commun. 2010;394:988–992. doi: 10.1016/j.bbrc.2010.03.104. [DOI] [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptor dimerization: function and ligand pharmacology. Mol Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Moreno E, Hoffmann H, Gonzalez-Sepúlveda M, Navarro G, Casadó V, Cortés A, et al. 2011Dopamine D1-histamine H3 receptor heteromers provide a selective link to MAPK signaling in GABAergic neurons of the direct striatal pathway J Biol Chem 2865846–5854.(Evidence that D1-H3 receptor heteromers integrate dopamine- and histamine-related signals involved in controlling the function of striatal neurons of the direct striatal pathway.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morphy R, Rankovic Z. The physicochemical challenges of designing multiple ligands. J Med Chem. 2006;49:4961–4970. doi: 10.1021/jm0603015. [DOI] [PubMed] [Google Scholar]
- Nai Q, Li S, Wang SH, Liu J, Lee FJ, Frankland PW, et al. Uncoupling the D1-N-methyl-D-aspartate (NMDA) receptor complex promotes NMDA-dependent long-term potentiation and working memory. Biol Psychiatry. 2010;67:246–254. doi: 10.1016/j.biopsych.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Ng J, Rashid AJ, So CH, O'Dowd BF, George SR. Activation of calcium/calmodulin-dependent protein kinase IIalpha in the striatum by the heteromeric D1-D2 dopamine receptor complex. Neuroscience. 2010;165:535–541. doi: 10.1016/j.neuroscience.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novi F, Millan MJ, Corsini GU, Maggio R. Partial agonist actions of aripiprazole and the candidate antipsychotics S33592, bifeprunox, N-desmethylclozapine and preclamol at dopamine D(2L) receptors are modified by co-transfection of D(3) receptors: potential role of heterodimer formation. J Neurochem. 2007;102:1410–1424. doi: 10.1111/j.1471-4159.2007.04660.x. [DOI] [PubMed] [Google Scholar]
- O'Dowd BF, Ji X, Alijaniaram M, Nguyen T, George SR. Separation and reformation of cell surface dopamine receptor oligomers visualized in cells. Eur J Pharmacol. 2011;658:74–83. doi: 10.1016/j.ejphar.2011.02.030. [DOI] [PubMed] [Google Scholar]
- O'Dowd BF, Ji X, Nguyen T, George SR.2012Two amino acids in each of D1 and D2 dopamine receptor cytoplasmic regions are involved in D1-D2 heteromer formation Biochem Biophys Res Commun 41723–28.(Identification of the sites of interaction between the D1 and D2 receptors within the D1-D2 receptor heteromer.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd BF, Nguyen T, Ji X, George SR. D(5) dopamine receptor carboxyl tail involved in D(5)-D(2) heteromer formation. Biochem Biophys Res Commun. 2013;431:586–589. doi: 10.1016/j.bbrc.2012.12.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Lee FJ, Moszczynska A, Vukusic B, Liu F. Regulation of dopamine D1 receptor function by physical interaction with the NMDA receptors. J Neurosci. 2004;24:1149–1158. doi: 10.1523/JNEUROSCI.3922-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Li S, Wang M, Diwan M, Anisman H, Fletcher PJ, et al. 2010Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects Nat Med 161393–1395.(Evidence linking disruption of the D1-D2 receptor heteromer in the prefrontal cortex to anti-depressant-like activity in rats.). [DOI] [PubMed] [Google Scholar]
- Perreault ML, Fan T, Alijaniaram M, O'Dowd BF, George SR. Dopamine D1-D2 receptor heteromer in dual phenotype GABA/glutamate-coexpressing striatal medium spiny neurons: Regulation of BDNF, GAD67 and VGLUT1/2. PLoS One. 2012;7:e33348. doi: 10.1371/journal.pone.0033348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, et al. 2010The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia J Biol Chem 28536625–36634.(The first direct evidence showing the neuroanatomical localization of the D1-D2 heteromer to both the striatonigral and striatopallidal pathways and increased functional activity of the D1-D2 heteromer under conditions of hyperdopaminergia.).20864528 [Google Scholar]
- Perreault ML, Hasbi A, O'Dowd BF, George SR. The dopamine D1-D2 receptor heteromer in striatal medium spiny neurons: evidence for a third distinct neuronal pathway in basal ganglia. Front Neuroanat. 2011;5:31. doi: 10.3389/fnana.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Jones-Tabah J, O'Dowd BF, George SR. A physiological role for the dopamine D5 receptor as a regulator of BDNF and Akt signalling in rodent prefrontal cortex. Int J Neuropsychopharmacol. 2013;25:1–7. doi: 10.1017/S1461145712000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Neubig R, Bouvier M, Devi L, Filizola M, Javitch JA, et al. International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol Rev. 2007;59:5–13. doi: 10.1124/pr.59.1.5. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Drago A, Fabbri C, Serretti A. Mechanisms of antidepressant action: an integrated dopaminergic perspective. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1532–1543. doi: 10.1016/j.pnpbp.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Pou C, Mannoury la Cour C, Stoddart LA, Millan MJ, Milligan G. Functional homomers and heteromers of dopamine D2L and D3 receptors co-exist at the cell surface. J Biol Chem. 2012;287:8864–8878. doi: 10.1074/jbc.M111.326678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, O'Dowd BF, Verma V, George SR. Neuronal Gq/11-coupled dopamine receptors: an uncharted role for dopamine. Trends Pharmacol Sci. 2007b;28:551–555. doi: 10.1016/j.tips.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, et al. 2007aD1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum Proc Natl Acad Sci USA 104654–659.(First detailed pharmacological characterization of the D1-D2 receptor heteromer, its calcium signal generation and demonstration of linkage to Gq in striatum.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridray S, Griffon N, Mignon V, Souil E, Carboni S, Diaz J, et al. Coexpression of dopamine D1 and D3 receptors in islands of Calleja and shell of nucleus accumbens of the rat: opposite and synergistic functional interactions. Eur J Neurosci. 1998;10:1676–1686. doi: 10.1046/j.1460-9568.1998.00173.x. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Ferré S, Ogren SO, Fuxe K. Adenosine A2A agonists: a potential new type of atypical antipsychotic. Neuropsychopharmacology. 1997;17:82–91. doi: 10.1016/S0893-133X(97)00033-X. [DOI] [PubMed] [Google Scholar]
- Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC.2000Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity Science 288154–157.(Identification of a novel dopamine/somatostatin heteromer, D2-SSTR5, that exhibits enhanced functional activity.). [DOI] [PubMed] [Google Scholar]
- Rondou P, Haegeman G, Van Craenenbroeck K. The dopamine D4 receptor: biochemical and signalling properties. Cell Mol Life Sci. 2010;67:1971–1986. doi: 10.1007/s00018-010-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A, Angers S, Mercier JF, Lagacé M, Marullo S, Bouvier M. Homodimerization of the beta2-adrenergic receptor as a prerequisite for cell surface targeting. J Biol Chem. 2004;279:33390–33397. doi: 10.1074/jbc.M403363200. [DOI] [PubMed] [Google Scholar]
- Scarselli M, Novi F, Schallmach E, Lin R, Baragli A, Colzi A, et al. D2/D3 dopamine receptor heterodimers exhibit unique functional properties. J Biol Chem. 2001;276:30308–30314. doi: 10.1074/jbc.M102297200. [DOI] [PubMed] [Google Scholar]
- Scott L, Zelenin S, Malmersjo S, Kowalewski JM, Markus EZ, Nairn AC, et al. Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc Natl Acad Sci USA. 2006;103:762–767. doi: 10.1073/pnas.0505557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Glutamate and dopamine components in schizophrenia. J Psychiatry Neurosci. 2009;34:143–149. [PMC free article] [PubMed] [Google Scholar]
- Shen HY, Singer P, Lytle N, Wei CJ, Lan JQ, Williams-Karnesky RL, et al. Adenosine augmentation ameliorates psychotic and cognitive endophenotypes of schizophrenia. J Clin Invest. 2012;122:2567–2577. doi: 10.1172/JCI62378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ, Milligan G. Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev. 2010;62:701–725. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So CH, Varghese G, Curley KJ, Kong MM, Alijaniaram M, Ji X, et al. D1 and D2 dopamine receptors form heterooligomers and cointernalize after selective activation of either receptor. Mol Pharmacol. 2005;68:568–578. doi: 10.1124/mol.105.012229. [DOI] [PubMed] [Google Scholar]
- So CH, Verma V, Alijaniaram M, Cheng R, Rashid AJ, O'Dowd BF, et al. Calcium signaling by dopamine D5 receptor and D5-D2 receptor hetero-oligomers occurs by a mechanism distinct from that for dopamine D1-D2 receptor hetero-oligomers. Mol Pharmacol. 2009;75:843–854. doi: 10.1124/mol.108.051805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano A, Ventura R, Molero A, Hoen R, Casadó V, Cortés A, et al. Adenosine A2A receptor-antagonist/dopamine D2 receptor-agonist bivalent ligands as pharmacological tools to detect A2A-D2 receptor heteromers. J Med Chem. 2009;52:5590–5602. doi: 10.1021/jm900298c. [DOI] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO Rep. 2004;5:30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S, Alguacil LF, Kalivas PW.2003Repeated cocaine administration changes the function and subcellular distribution of adenosine A1 receptor in the rat nucleus accumbens J Neurochem 871478–1484.(The first evidence showing A1-D1 receptor heteromers in rat nucleus accumbens and their functional regulation by cocaine.). [DOI] [PubMed] [Google Scholar]
- Torvinen M, Marcellino D, Canals M, Agnati LF, Lluís C, Franco R, et al. Adenosine A2A receptor and dopamine D3 receptor interactions: evidence of functional A2A/D3 heteromeric complexes. Mol Pharmacol. 2005;67:400–407. doi: 10.1124/mol.104.003376. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, et al. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- Verma V, Hasbi A, O'Dowd BF, George SR. Dopamine D1-D2 receptor Heteromer-mediated calcium release is desensitized by D1 receptor occupancy with or without signal activation: dual functional regulation by G protein-coupled receptor kinase 2. J Biol Chem. 2010;285:35092–35103. doi: 10.1074/jbc.M109.088625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohora D, Bhowmik M. Histamine H3 receptor antagonists/inverse agonists on cognitive and motor processes: relevance to Alzheimer's disease, ADHD, schizophrenia, and drug abuse. Front Syst Neurosci. 2012;6:72. doi: 10.3389/fnsys.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Wager-Miller J, Westenbroek R, Mackie K. Dimerization of G protein-coupled receptors: CB1 cannabinoid receptors as an example. Chem Phys Lipids. 2002;121:83–89. doi: 10.1016/s0009-3084(02)00151-2. [DOI] [PubMed] [Google Scholar]
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- Woods AS, Ciruela F, Fuxe K, Agnati LF, Lluís C, Franco R, et al. Role of electrostatic interaction in receptor-receptor heteromerization. J Mol Neurosci. 2005;26:125–132. doi: 10.1385/JMN:26:2-3:125. [DOI] [PubMed] [Google Scholar]
- Woods AS, Ferré S. Amazing stability of the arginine-phosphate electrostatic interaction. J Proteome Res. 2005;4:1397–1402. doi: 10.1021/pr050077s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Yekkirala A, Tang Y, Portoghese PS. A bivalent ligand (KMN-21) antagonist for mu/kappa heterodimeric opioid receptors. Bioorg Med Chem Lett. 2009;19:6978–6980. doi: 10.1016/j.bmcl.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Burke MW, Calakos N, Beaulieu JM, Vaucher E. Confocal analysis of cholinergic and dopaminergic inputs onto pyramidal cells in the prefrontal cortex of rodents. Front Neuroanat. 2010;4:21. doi: 10.3389/fnana.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łukasiewicz S, Faron-Górecka A, Dobrucki J, Polit A, Dziedzicka-Wasylewska M. Studies on the role of the receptor protein motifs possibly involved in electrostatic interactions on the dopamine D1 and D2 receptor oligomerization. FEBS J. 2009;276:760–775. doi: 10.1111/j.1742-4658.2008.06822.x. [DOI] [PubMed] [Google Scholar]
- Łukasiewicz S, Polit A, Kędracka-Krok S, Wędzony K, Maćkowiak M, Dziedzicka-Wasylewska M. Hetero-dimerization of serotonin 5-HT(2A) and dopamine D(2) receptors. Biochim Biophys Acta. 2010;1803:1347–1358. doi: 10.1016/j.bbamcr.2010.08.010. [DOI] [PubMed] [Google Scholar]