The superfamily of G-protein coupled receptors (GPCRs) is one of the largest gene families in the mammalian genome. GPCRs represent >50% of all current drug targets for treating numerous diseases that affect both peripheral and central nervous systems. Identification of novel GPCR drug targets and their promise for treatment in psychiatric disease has been recently slowed by the dearth of selective targets and changes in the pharmaceutical landscape. However, several recent advances in GPCR crystallization and in vivo pharmacology offer potentially exciting opportunities for therapeutics that target GPCRs in unique ways to treat psychiatric diseases (Kenakin and Christopoulos, 2013).
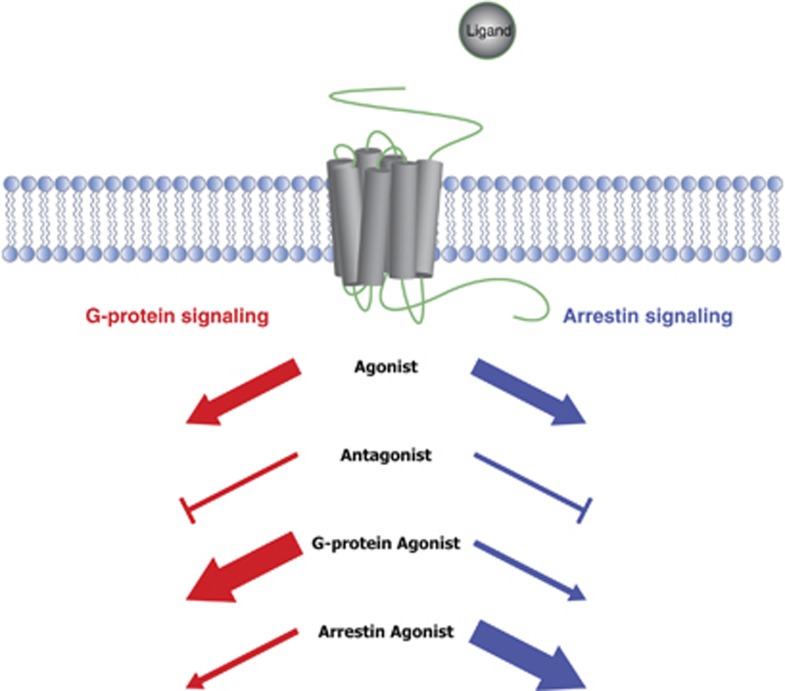
Current mathematical and pharmacological data support the conclusion that GPCRs are pluridimensional proteins that occupy numerous structural conformations and signaling states. Depending on the ligand, the GPCR has the ability to engage a G-protein and arrestin state, or favor one over the other (Figure 1). These conformations are determined by the type of ligand, the receptor type, as well as interacting accessory proteins. This concept termed ‘functional selectivity' (also called, biased signaling, ligand-directed signaling) is the ability of a ligand to direct a GPCR toward a conformation that selectively evokes a particular stimulus–response and is now a well-accepted concept in the field of GPCR research. Some GPCR classes that have been studied in the context of functional selectivity in vitro and in vivo include: serotonin, opioid, adrenergic, cannabinoid, muscarinic, and metabotropic glutamate receptors (Luttrell and Gesty-Palmer, 2010).
Figure 1.
Schematic of functional selectivity at GPCRs. Cartoon model depicting various states of functional selectivity. Depending on the ligand and the induced receptor conformation, GPCRs are capable of signaling through the G-protein-mediated signaling pathway or the arrestin-mediated signaling pathway independently or simultaneously. Wacker et al (2013) crystalized for the first time, both the G-protein and arrestin state of serotonin receptor. Similarly, ligands can be antagonists for both pathways, or only one pathway while agonizing the other (collateral agonists). This structure-based signaling complexity is thought to give rise to multiple different downstream outputs, and may involve additional, currently uncharacterized effectors. As we increase our understanding of the molecular architecture of these signaling states, the development of novel psychiatric treatments that target these states will become increasingly more possible.
A recent and elegant development in this field has the potential to greatly advance our understanding of functional selectivity and to open new doors for drug discovery. The study uncovered a ‘snapshot' of the serotonin 5HT2B receptor bound to ergotamine, an arrestin-biased ligand at this receptor (Wacker et al, 2013). For the first time, this paper and its complementary study, elucidated the molecular signature of the G-protein vs arrestin-biased conformation at serotonin receptors using an X-ray crystallography approach. Together, these reports suggest that for a GPCR to occupy an arrestin-biased (alternate-agonist) state, the ligand-receptor conformation sits in an intermediate state that includes both active and inactivate components, yet also interferes with G-protein signaling.
While the authors show convincing modeling for other GPCR types, whether this is indeed case for other biased ligands of other classes of GPCRs remains to be explored. However, this exciting result has the potential to accelerate our understanding of GPCR structure–function relationships, particularly as new ligands are developed for the treatment of psychiatric diseases. Having a clearer understanding of how various ligand types interact with and stabilize GPCR conformations will provide chemists with the necessary tools to rationally design more selective compounds with better delivery, higher affinity, and fewer unwanted side effects.
Complimentary to these exciting molecular advances, contemporary reports have shown promising in vivo data indicating that functionally selective ligands may offer novel strategies to achieve pain relief without the traditional abuse liability, dysphorigenic properties, or psychomimetic components of traditional opioid receptor ligands (Bruchas and Chavkin, 2010; Bruchas et al, 2011) by selectively targeting G-protein vs arrestin-biased signaling states. Furthermore, advances in dopamine and serotonin receptor behavioral pharmacology have revealed that engaging D2R arrestin-biased signaling to GSK3β or 5HT2A arrestin-biased Src/Akt signaling may offer selective treatments for schizophrenia, psychosis, and other mood disorders (Schmid and Bohn, 2010; Urs et al, 2012). The combined efforts of molecular pharmacologists, biochemists, and behavioral pharmacologists to decipher these complex relationships between the molecular signatures of GPCRs and how various GPCR conformations ultimately transduce cellular responses into behavioral output are an active area of study for both Academia and Industry. These new discoveries are likely to provide novel therapeutic strategies for treating psychiatric diseases.
FUNDING AND DISCLOSURE
The authors have no conflicts of interest or consulting relationships to disclose.
Acknowledgments
This work was supported by NIH/NIDA: R01DA033396, R00DA025182, R21DA035144 (to MRB), and T32DA007261 (SDC).
References
- Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, et al. Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12:205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62:305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2 A receptor via a ß-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 2010;30:13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs NM, Snyder JC, Jacobsen JPR, Peterson SM, Caron MG. Deletion of GSK3β in D2R-expressing neurons reveals distinct roles for β-arrestin signaling in antipsychotic and lithium action. Proc Natl Acad Sci USA. 2012;109:20732–20737. doi: 10.1073/pnas.1215489109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker D, Wang C, Katritch V, Han GW, Huang X-P, Vardy E, et al. Structural features for functional selectivity at serotonin receptors. Science. 2013;340:615–619. doi: 10.1126/science.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]