Abstract
There has been significant progress in personalized drug development. In large part, this has taken place in the oncology field and been due to the ability of researchers/clinicians to discover and develop novel drug development tools (DDTs), such as biomarkers. In cancer treatment research, biomarkers have permitted a more accurate pathophysiological characterization of an individual patient, and have enabled practitioners to target mechanistically the right drug, to the right patient, at the right time. Similar to cancer, patients with substance use disorders (SUDs) present clinically with heterogeneous symptomatology and respond variably to therapeutic interventions. If comparable biomarkers could be identified and developed for SUDs, significant diagnostic and therapeutic advances could be made. In this review, we highlight current opportunities and difficulties pertaining to the identification and development of biomarkers for SUDs. We focus on cocaine dependence as an example. Putative diagnostic, pharmacodynamic (PD), and predictive biomarkers for cocaine dependence are discussed across a range of methodological approaches. A possible cocaine-dependent clinical outcome assessment (COA)—another type of defined DDT—is also discussed. At present, biomarkers for cocaine dependence are in their infancy. Much additional research will be needed to identify, validate, and qualify these putative tools prior to their potential use for medications development and/or application to clinical practice. However, with a large unmet medical need and an estimated market size of several hundred million dollars per year, if developed, biomarkers for cocaine dependence will hold tremendous value to both industry and public health.
Keywords: biomarkers, cocaine dependence, drug development tools, addiction
INTRODUCTION
Substance use disorders (SUDs) not only have dire consequences on an individual's health, but also significantly on our society, economy, healthcare, and criminal justice systems. It has been estimated that SUDs cost the United States nearly $500 billion per year (Volkow and Skolnick, 2012). There remain surprisingly few FDA-approved medications for SUDs, despite an improved understanding of the biological underpinnings of these disorders, as well as the cost to both an individual, and society at large. Whereas some medications exist for SUDs like tobacco-, and opiate-dependence, there are no FDA-approved medications for cannabis-, methamphetamine-, or cocaine dependence.
SUDs are characterized by drug abuse and dependence. As an individual becomes increasingly dependent on a drug, discontinuation of the abused drug leads to withdrawal symptoms, such as dysphoria. The development of dependence has been conceptualized as a neurobehavioral disorder that advances from impulsive drug use to compulsive drug abuse. This transition moves through four distinct stages: (1) experimental use, (2) regular use, (3) daily preoccupation, and finally, (4) dependence. In clinical trials, patients are routinely screened using Diagnostic and Statistical Manual of Mental Disorders Version-IV (DSM-IV) criteria for cocaine dependence (Association AP, 2000). As this review is largely focused on highlighting the means to enhance translational drug development, we will use the term ‘cocaine dependence' instead of ‘cocaine addiction'.
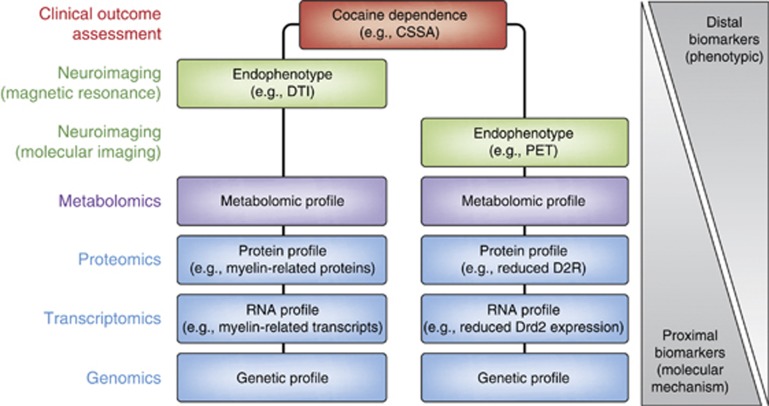
For this review, the authors have taken a narrative approach based primarily on the authors' own research, with an attempt to include work of other researchers in the field in order to provide a more balanced review of the literature. This paper focuses on several broad areas of potential biomarkers for cocaine dependence, beginning with an overview of different types of biomarkers that can be assessed and the challenges and potential utility for biomarkers for cocaine dependence. Specific biomarkers and clinical outcome assessments are then discussed, based on the types of drug development tools (DDTs) presented in Figure 1. Some of these biomarkers are more amenable to cross-species translational work. Others (such as clinical outcome assessments and brain proteomics) can only be used in humans or animal models. Overall, the field is in its infancy and much work remains to be done. Toward this, perspectives from industry and the FDA are included as part of future considerations. As scientific understanding continues to expand rapidly, the development of DDTs such as biomarkers are likely to increase the efficiency by which new therapeutics for SUDs are developed. The advent of new, more effective therapeutics for SUDs aids the treatment of co-morbid psychiatric disorders, and ultimately improves public health.
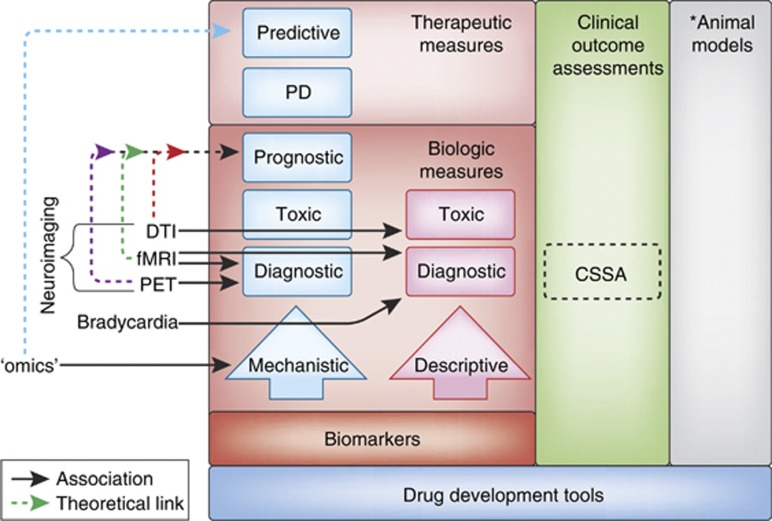
Figure 1.
Types of biomarkers. Biomarkers are presented hierarchically within the context of the FDA's set of Drug Development Tools (DDTs) (see ‘Introduction' for additional details). Biomarkers are classified into two primary tracts—‘descriptive' and ‘mechanistic'. Descriptive biomarkers are indirect, consequential correlates of the underlying pathophysiological processes. Mechanistic biomarkers represent a direct measure of the pathophysiological underpinnings of the disease process (see also ‘Future Directions'). Mechanistic biomarkers provide a foundation from which other types of biomarkers can be developed and are hence more ‘actionable'. Actionable biomarkers depicted have several possible clinical applications, and these types of biomarkers are hierarchically arranged. Predictive and pharmacodynamic (PD) biomarkers are measures of a therapeutic response (ie, light red box), whereas prognostic, toxic, and diagnostic markers are biological measures alone (ie, dark red box) (see also Text Box). As an example, a prognostic biomarker might provide a prodromal measure enabling physicians an opportunity to identify and medically manage individuals prior to the potential development of a substance use disorder (SUD). Otherwise, optimally, a predictive biomarker could help tailor the right drug to the right patient, at the right time by predicting disease progression (right patient), providing pharmacodynamic (PD) information to facilitate a therapeutic assessment of safety/efficacy (right drug), and predicting which individuals might respond to a particular therapy (right time). Ultimately, it is these biomarkers that are on the mechanistic pathway that will offer the greatest value for enhanced drug development and personalized medicine. Boxes in the left column refer to those methodologies as described within the text; solid lines refer to those approaches with some preliminary associations for a specific type of biomarker (see text); dotted lines refer to theoretical links across the various approaches (boxes). Within the context of the DDTs, the Cocaine Selective Severity Assessment (CSSA) is most appropriately described as an observer- or clinical-reported Clinical Outcome Assessment (COA) (see text). *Although they are one type of FDA-defined DDT, animal models (right column) are not discussed herein and this portion is therefore grayed out.
Developmental Challenges Surrounding Medications for Substance Dependence
Multiple issues likely contribute to the lack of novel treatments for substance dependence. First, there is the prospect of a low return on investment for any new medication. It was recently reported that as increasing numbers of patents expire, large pharmaceutical companies will only be able to recover 26 cents per dollar invested, or <0.3% return on new product revenues (Esserman and Woodcock, 2011; Paul et al, 2010). This is perhaps best illustrated by the recent discontinuation notices from several psychotherapeutic drug development programs in industry (eg, GlaxoSmithKline, Astra-Zeneca, and Cephalon) (Skolnick and Volkow, 2012). Second, there are the overall costs associated with developing a safe and effective medication, which have traditionally been estimated to be $1–1.8 billion, whereas more recent estimates put the cost between $4–11 billion per new drug approval, and requiring an average of 13.5 years from start to finish (Paul et al, 2010). These ever-increasing expenses limit drug development to fewer and fewer large-cap pharmaceutical companies. A third obstacle is the perceived market size. There is a market, as well as a large unmet medical need for medications to effectively treat SUDs. Given that market sales for Suboxone (a treatment for opioid dependence) was greater than $1.2 billion in 2011, the development of novel medications for other SUDs such as cocaine dependence where no treatments currently exist is clearly warranted (Skolnick and Volkow, 2012). In 2010, nearly one million Americans aged ⩾12 met the DSM-IV criteria for cocaine dependence. Moreover, out of the four million drug-related emergency-department visits made in the US in 2010 by patients aged ⩾21, 1.9 million involved drug misuse or abuse. In 39.4% of those visits cocaine was the most commonly involved illicit drug (SAMHSA, The DAWN report, 2012). There is clearly a market and a significant health need for pharmaceutical companies to move past the purported ‘negative connotation' that is commonly associated with the development of a treatment for the dependence on an illicit substance such as cocaine. Finally, among all the external challenges the industry faces in bringing a medication to market, the lack of developmental efficiency is of paramount importance (Paul et al, 2010). At present, only 6–8% of CNS compounds that make it to clinical development are ultimately approved. For SUDs, this is further complicated by the vast biological heterogeneity associated with disorders of the central nervous system, as well as the diverse assessments various stakeholders might have for a potential treatment (ie, patients, caregivers, physicians, insurance payers, and pharmaceutical companies). In addition, it is noteworthy that the National Institutes of Health established the National Center for Advancing Translational Sciences, to ‘transform the translational science process so that new treatments and cures for disease can be delivered to patients faster' in December 2011.
Role for Biomarkers in Translational Drug Development
The clinical diagnosis of SUDs such cocaine dependence currently relies on medical history, physical examination, self-report questionnaires, and/or urine-drug screens (UDS). However, UDS are limited to only being able to assess relatively acute drug intake (Neumann and Spies, 2003). For example, a UDS measures the presence of benzoylecgonine, the primary metabolite of cocaine directly. Yet, even in the same individual, UDS results can vary considerably depending on when and how the test is performed. Benzoylecgonine concentrations will be the highest if the sample is collected as part of the first urination after waking, whereas testing at other times of the day will yield benzoylecgonine concentrations that can vary widely. Also, UDS measures the results of drug use but not the underlying neurobiology that is the target for medications for SUDs.
One way for significant advances in basic science to be translated into new opportunities for medication development is through the identification of DDTs, biomarkers being a prime example. As defined by the FDA (Figure 1), a biomarker is a ‘characteristic that is objectively measured and is an indicator of normal biological processes, pathogenic processes, or biological responses to a therapeutic intervention.' There are several types of biomarkers including: mechanistic, diagnostic, prognostic, pharmacodynamic, and predictive (Figure 1 and Text Box). Diagnostic biomarkers can be used to stratify patient populations into personalized treatment regimens, whereas predictive biomarkers can forecast a pharmacotherapeutic response (positive or negative). A diagnostic biomarker could be used to minimize decision-making ambiguity in clinical trials, allow for improved patient prognosis, and a greater understanding of a drug's mechanism(s) of action. More specifically, improved biomarkers could help to: (1) more objectively diagnose the heterogeneous diseases, such as cocaine dependence, (2) improve signal detection to assess more efficiently small treatment effects, and (3) better characterize patient (sub)populations to more efficiently test (stratify) and optimize subsequent treatment regimens. Tailoring therapies to the appropriate patient population may be the best way to evaluate a medication earlier in the phases of drug development, before the putative medication is advanced to later and more costly stages (ie, Phase II/III), where there has traditionally been a low likelihood of success (Paul et al, 2010).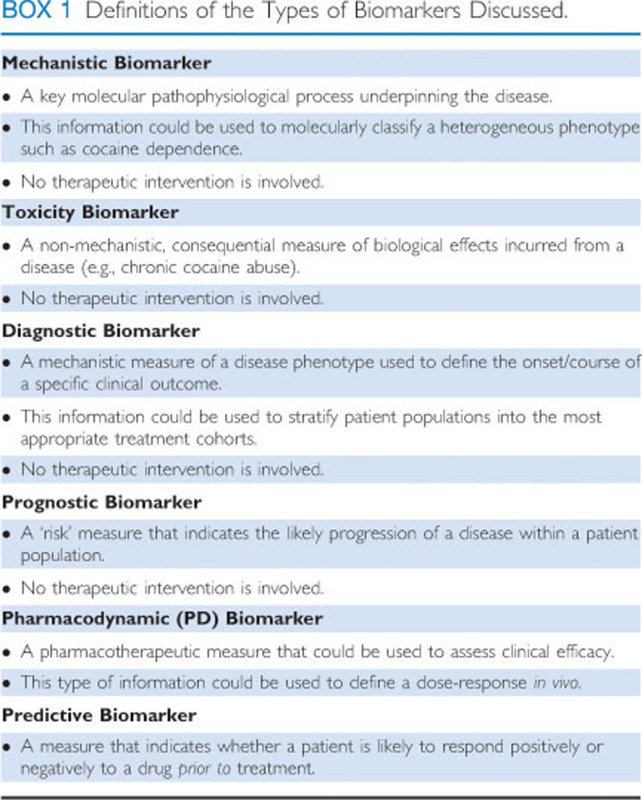
CLINICAL OUTCOME ASSESSMENT: THE CSSA
As defined by the FDA, a Clinical Outcome Assessment (COA) is an evaluative tool that ‘directly or indirectly measures how patients feel or function and can be used to determine whether or not a drug has been demonstrated to provide a treatment benefit. COAs can also measure a safety benefit (eg, fewer side effects) compared with other treatments' (FDA Guidance for Industry: Qualification Process for Drug Development Tools, 2010). COAs are distinguished according to who is doing the reporting of the outcome: (1) patient-reported assessments, (2) observer-reported assessments, or (3) clinician-reported assessments. In this regard, the Cocaine Selective Severity Assessment (CSSA) may hold promise as an indicator of treatment benefit.
A cocaine withdrawal syndrome was first described in 1986 (Gawin and Kleber, 1986). The CSSA was developed as an observer-based assessment to measure symptom severity by investigators at the University of Pennsylvania. It is an 18-item interviewer-administered questionnaire. Items include, for example: (1) lethargy, (2) sleep changes, (3) appetite changes, (4) irritability, (5) depressed mood, (6) anxiety, (7) cocaine craving, (8) inattention, (9) paranoia, and (10) HR changes (eg, bradycardia). Items are measured on a 7-point scale, making the maximum CSSA score 112. The CSSA has been found to be a valid and reliable indicator of cocaine withdrawal syndrome, and is highly correlated with cocaine dependence severity when measured by the addiction severity index (ASI) composite drug score (Kampman et al, 1998).
CSSA scores have also been shown to predict treatment outcome. Data from multiple trials have shown that patients who enter treatment with more severe cocaine withdrawal symptoms as measured by the CSSA scores do poorly (Kampman et al, 2001; Kampman et al, 2002; Mulvaney et al, 1999). For example, CSSA scores may predict response to treatment with the anticonvulsant drug topiramate. In a 13-week trial, 170 cocaine-dependent patients were randomly assigned to topiramate or placebo. The percent of patients abstinent during the last 3 weeks of the trial was significantly different between the two groups, where 20% of the topiramate-treated patients were cocaine abstinent, compared with 7% of placebo-treated patients. Cocaine withdrawal symptoms were determined to be among the best predictors of a positive treatment response. The patients more likely to respond to topiramate had higher CSSA scores, and thus predicted to have more severe cocaine withdrawal symptoms. Patients who had CSSA scores in the highest percentile who were being treated with topiramate had twice as many cocaine-negative UDSs, and 29% remained abstinent during the last three weeks of the trial. Comparatively, only 3% of the placebo-treated subjects remained abstinent during the same timeframe (Kampman et al, 2011). Also during this trial of cocaine-dependent patients, topiramate treatment was associated with reductions in three individual items from the CSSA: energy level, activity level, and attention. On average, patients treated with topiramate were more likely than placebo-treated patients to report greater energy, more activity, and a greater ability to focus.
At the University of Pennsylvania Treatment Research Center, the CSSA is used in all the clinical trials, making it possible to evaluate the CSSA, as well as individual items included in the CSSA, as predictors of outcome in outpatient cocaine pharmacotherapy trials (Table 1). Data from seven clinical trials, each of them between 7–12 weeks in duration, were included. We limited the trials to those that involved only cocaine-dependent patients, patients with co-morbidities were not allowed. We included patients assigned to either placebo or active medications as none of the medications evaluated turned out to be significantly better than placebo. For predictor variables we used items from the baseline ASI, the baseline UDS and CSSA scores obtained at baseline. For outcome variables we used either 3 weeks of continuous abstinence, defined by all negative and no missing UDSs, a 50% reduction in the ASI composite drug score, or no self-reported cocaine use measured by timeline follow back (TLFB) in the last 4 weeks of the trial.
Table 1. Baseline Predictors of Outcome in Cocaine Pharmacotherapy Trials Entered Individually into Three Separate Logistic Regression Models with Three Different Dependent Variables.
|
3 Weeks of continuous cocaine abstinence |
50% reduction in ASI composite drug scores |
Self-reported abstinence, last 4 weeks of a trial |
||||
|---|---|---|---|---|---|---|
| Model χ2 | (p) | Model χ2 | (p) | Model χ2 | (p) | |
| ASI Composite Drug score | 8.8 | 0.003 | 0.30 | 0.58 | 7.4 | 0.007 |
| ASI Composite Alcohol score | 0.75 | 0.38 | 6.4 | 0.01 | 1.6 | 0.21 |
| ASI Composite Psychiatric score | 0.86 | 0.35 | 1.7 | 0.20 | 0.46 | 0.50 |
| ASI Composite Medical Score | 1.5 | 0.22 | 3.3 | 0.07 | 0.17 | 0.68 |
| ASI Composite Family/Social score | 0.49 | 0.48 | 2.3 | 0.13 | 0.06 | 0.80 |
| ASI Composite Legal Score | .27 | 0.60 | 0.98 | 0.32 | 0.01 | 0.90 |
| ASI Composite Employment score | 10.8 | 0.001 | 2.6 | 0.10 | 1.7 | 0.19 |
| Age | 4.1 | 0.04 | 2.3 | 0.13 | 0.60 | 0.44 |
| Gender | 0.80 | 0.37 | <.01 | 0.99 | 0.01 | 0.91 |
| Years of education | 0.89 | 0.35 | 4.2 | 0.04 | 0.77 | 0.38 |
| Days cocaine use in past 30 days | 24.6 | <.001 | 3.4 | 0.06 | 21.5 | <.001 |
| Years of cocaine use, lifetime | 1.2 | 0.27 | 0.07 | 0.79 | 2.1 | 0.14 |
| Days of alcohol use in past 30 days | 0.05 | 0.82 | 3.2 | 0.07 | <.01 | 0.99 |
| Years of alcohol use, lifetime | 3.3 | 0.07 | 6.3 | 0.01 | 4.1 | 0.04 |
| Initial UDS | 23.9 | <.001 | 8.1 | 0.004 | 14.0 | <0.001 |
| Initial CSSA score | 16.5 | <.001 | 8.4 | 0.004 | 15.5 | <0.001 |
Only the initial urine-drug screen (UDS) and the initial Cocaine Selective Severity Assessment (CSSA) score were significant predictors for all three models.
Four-hundred and two cocaine-dependent subjects were included. The average age of the subjects was 39. They were mostly African-American men (81%) who smoked crack cocaine (87%). They used cocaine about 13 days in a month and spent about $600 a month for cocaine. They had about 10 years of regular cocaine use. The average ASI composite drug score at baseline was 0.230. The average baseline CSSA score was 27.
We evaluated our predictors using logistic regression with the three outcome measures: 3 weeks clean at any point in the trial determined by UDS results, a 50% reduction in ASI composite drug scores from baseline to the end of trial, and self-reported abstinence during the last 4 weeks of the trial.
Results of the logistic regression showed that the 7 ASI composite scores obtained at baseline were not consistent predictors of outcome. We then looked at demographic and drug use variables from the ASI, the initial UDS and baseline CSSA scores. The strongest and most consistent predictors of outcome were the CSSA scores and the results of the initial UDS (Table 1).
We then put all the predictor variables into a backward logistic regression. The final model for predicting 3 weeks of continuous abstinence included: age, days of cocaine use in the prior 30 days, CSSA scores, and initial UDS. The model correctly predicted outcome in 79% of cases. Overall, the sensitivity (96%) and positive predictive (81%) value were high such that patients with frequent use, high CSSA scores, and a positive UDS were unlikely to achieve 3 weeks of abstinence. However the model is less effective at identifying patients likely to do well (specificity 18% and negative predictive value 58%). For predicting 50% reduction in ASI composite drug scores, the items included in the final model were a bit different and included: age, years of education, years of alcohol use, CSSA scores, and ASI employment composite scores. For this model sensitivity was 81% and specificity 39%. The model correctly predicted outcome in 64% of cases. For prediction of self-reported abstinence at the end of the trial, predictors in the final model included: days of cocaine use, UDS results, CSSA score, and the ASI composite family social score. Sensitivity was 91%, specificity 36%, and positive predictive value 75%. The model correctly predicted outcome in 74% of cases. For all three outcomes the baseline CSSA score was a significant predictor of outcome. In subsequent analysis we looked at the 18 individual CSSA items as predictors on their own. Few were consistent predictors of outcome. However, there were three items that were predictors, the two craving measures: (1) craving intensity (p<0.001), (2) craving frequency (p=0.003), and one withdrawal physical sign, (3) bradycardia (p=0.003) (Table 2). Patients with low heart rates did more poorly in treatment, suggesting that bradycardia itself could be a potential biomarker as discussed further below.
Table 2. All 18 Individual Items from the Baseline CSSA Entered Individually into Three Separate Logistic Regression Models with Three Different Dependent Variables: (1) Craving Intensity, (2) Craving Frequency, and (3) Bradycardia Were Significant Predictors of Outcome in all Three Regression Models.
|
3 Weeks of continuous cocaine abstinence |
50% reduction in ASI composite drug scores |
Self-reported abstinence, last 4 weeks of a trial |
||||
|---|---|---|---|---|---|---|
| Model χ2 | (p) | Model χ2 | (p) | Model χ2 | (p) | |
| Energy level | 1.7 | 0.20 | 2.0 | 0.15 | 6.9 | 0.009 |
| Activity level | 3.3 | 0.07 | 1.7 | 0.19 | 2.7 | 0.10 |
| Tension | 0.45 | 0.50 | 5.2 | 0.02 | 4.2 | 0.04 |
| Attention | 5.1 | 0.02 | 2.5 | 0.11 | 1.2 | 0.28 |
| Paranoid ideation | 1.0 | 0.32 | 0.12 | 0.73 | 0.59 | 0.44 |
| Anhedonia | 2.6 | 0.82 | 2.2 | 0.14 | 7.9 | 0.005 |
| Depression | 2.4 | 0.12 | 1.8 | 0.18 | 8.7 | 0.003 |
| Suicidality | 0.97 | 0.32 | 0.76 | 0.38 | 1.4 | 0.29 |
| Irritability | 8.7 | 0.003 | 3.1 | 0.07 | 3.7 | 0.06 |
| Hyperphagia | 0.18 | 0.66 | 0.27 | 0.59 | 0.17 | 0.68 |
| Hypophagia | 2.1 | 0.14 | 1.3 | 0.25 | 3.6 | 0.06 |
| Carbohydrate craving | 5.6 | 0.02 | 0.01 | 0.93 | 0.40 | 0.52 |
| Cocaine-craving intensity | 22.0 | <0.001 | 6.7 | 0.009 | 17.6 | <0.001 |
| Cocaine-craving frequency | 17.0 | <0.001 | 4.7 | 0.03 | 9.1 | 0.003 |
| Bradycardia | 11.6 | 0.001 | 3.5 | 0.04 | 9.0 | 0.003 |
| Decreased sleep | 1.8 | 0.18 | 0.48 | 0.48 | 4.4 | 0.04 |
| Increased sleep | 5.3 | 0.02 | 0.67 | 0.41 | 4.3 | 0.03 |
| Anxiety | 0.03 | 0.83 | 4.0 | 0.04 | 0.19 | 0.66 |
Three variables indicated in bold were significant predictors of outcome in the three regression models.
CARDIOVASCULAR BIOMARKERS
The acute effects of cocaine on the cardiovascular system are well established. Cardiovascular function is acutely impacted by cocaine in two main ways (Schwartz et al, 2010). First, cocaine acts as a stimulant in the sympathetic nervous system, increasing catecholamine levels, heart rate (HR), blood pressure, and myocardial contractility. These actions collectively result in an increase in oxygen demand. At the same time, these actions also produce vasoconstriction, which limits oxygen supply and subsequently increases the risk of ischemia or infarction. Second, cocaine acts as an anesthetic, blocking both sodium and potassium channels in myocardial tissue to inhibit normal contractile function. These effects are capable of inducing arrhythmias that appear on the electrocardiogram (ECG) as a prolongation of the QRS and QT intervals (Haigney et al, 2006; Magnano et al, 2006).
The chronic effects of cocaine on the cardiovascular system are more complex, and not as well defined. It has been well established that long-term cocaine abuse is associated with the development of various cardiac pathological conditions, including accelerated hypertension, coronary atherosclerosis, left ventricular hypertrophy, and myocarditis. These conditions appear to be correlated with the prevalence of cocaine-associated chest pain reported in emergency department patients (Hollander et al, 1995; Maraj et al, 2010; Phillips et al, 2009; Qureshi et al, 2001; Schwartz et al, 2010). In view of these cocaine-related effects, it seems reasonable to consider how cardiovascular measures may be utilized as a putative biomarker for cocaine dependence.
Bradycardia as a Potential Diagnostic Marker of Cocaine Dependence
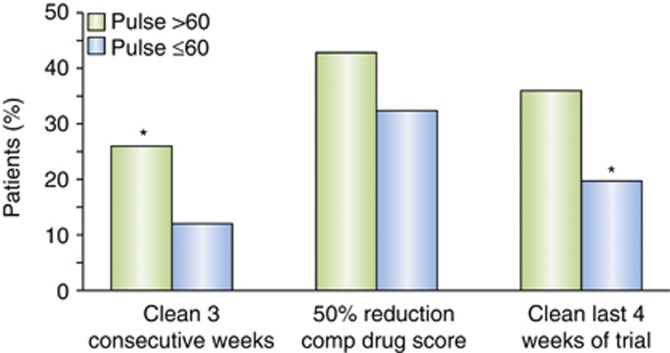
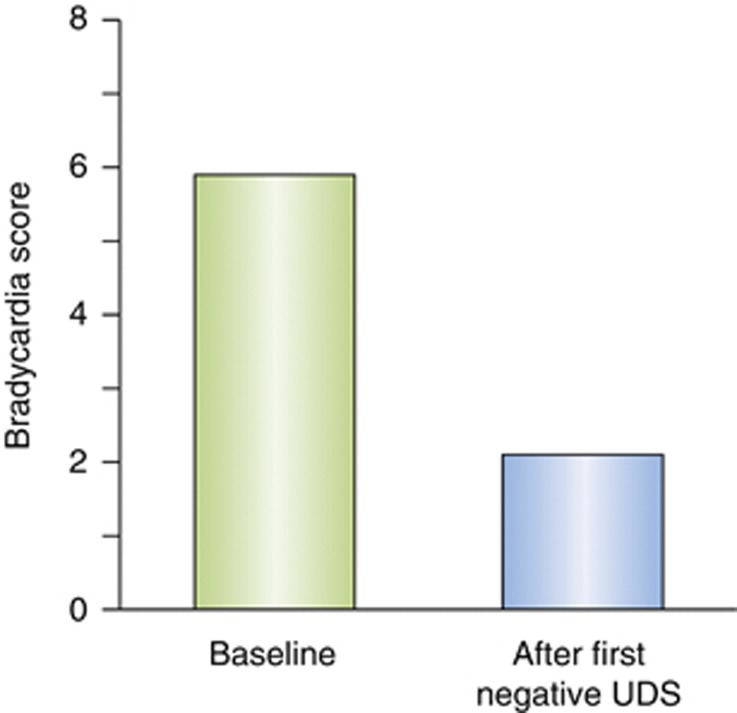
Bradycardia is measured in the CSSA by a 7-point scale: 0 (>64 bpm), 1 (64-63 bpm), 2 (62-61 bpm), 3 (60-59 bpm), 4 (58-57 bpm), 5 (56-55 bpm), 6 (54-53 bpm), and 7 (<53 bpm). In the analysis of the 402 cocaine-dependent subjects who completed the CSSA at the Penn Addiction Research Program, the percent of patients achieving a successful outcome was compared by χ2 with bradycardia dichotomized at the 75th percentile (score of ⩾3 vs <3). A heart rate of ⩽60 was a significant predictor of the inability to achieve ⩾3 weeks of continuous abstinence (p=0.01), and a significant predictor of not achieving 4 weeks of self-reported abstinence at the end of the trial (p=0.003) (Figure 2). In order to determine whether bradycardia at the start of treatment was a cocaine withdrawal effect or a stable trait of poor performers, we evaluated patients who had the lowest heart rates and looked at their heart rate after they had achieved 3–5 days of cocaine abstinence verified by a negative UDS. We found that heart rates increased with abstinence in this case shown by a lower bradycardia score (Figure 3), suggesting that bradycardia may be related to cocaine withdrawal.
Figure 2.
A heart rate of ⩽60 beats per minute (bpm) was a significant predictor of the inability to achieve ⩾3 weeks of continuous abstinence (p=0.01), and a significant predictor of not achieving 4 weeks of self-reported abstinence at the end of the trial (p=0.003).
Figure 3.
Among subjects with the lowest heart rates at the start of treatment, heart rates increased significantly (lower CSSA bradycardia score) after 3–5 days of abstinence verified by a negative urine-drug screen (UDS). Bradycardia acts like a withdrawal sign.
To further assess the relationship between cocaine withdrawal and bradycardia, we examined ability of bradycardia to predict 3 weeks of continuous abstinence from cocaine, controlling for baseline UDS results and days of cocaine use in the 30 days prior to treatment. In the final regression model, days of cocaine use had a Wald coefficient of 12.9 (p<0.001), initial UDS had a Wald coefficient of 13.8 (p<0.001), and bradycardia had a Wald coefficient of 6.3 (p=0.012). Bradycardia remained a significant predictor controlling for ‘recency-of-use'. Therefore, bradycardia appears to be an important part of the cocaine withdrawal syndrome and predictive of outcome on its own.
At the University of Texas—Houston Center for Neurobehavioral Research on Addictions, the role of electrocardiograms (ECG) parameters has also been diagnostically characterized in cocaine-dependent subjects (Sharma et al, 2011, 2012). Based on evidence of a relationship between repeated exposure to cocaine and eventual myocardial β-adrenergic receptor downregulation, it was hypothesized that chronic cocaine users would present with a higher incidence of ECG-measured sinus bradycardia compared with non-drug-using controls. This study used a retrospective chart review methodology to examine ECG recordings taken from 195 cocaine-dependent subjects. All patients meeting criteria for current DSM-IV-defined cocaine dependence over the age of 18 were eligible for inclusion; those with concomitant illicit opiate, benzodiazepine, amphetamine or methamphetamine use, as verified by serial urine toxicology testing were excluded. For additional controls, de-identified ECG data from 1296 gender- and race-matched subjects were reviewed using the publically available data set from the NIH/National Heart Lung and Blood Institute Atherosclerosis Risk in Communities (ARIC) study. Standard 12-lead ECGs were reviewed by two independent and experienced cardiologists. Sinus bradycardia was defined as a heart rate (HR) <60 beats per minute (bpm); severe bradycardia was defined as HR <50 bpm. Cocaine-dependent subjects provided information regarding duration of cocaine use (years) and frequency of recent use (positive days in past 30 as measured by a UDS for benzoylecgonine).
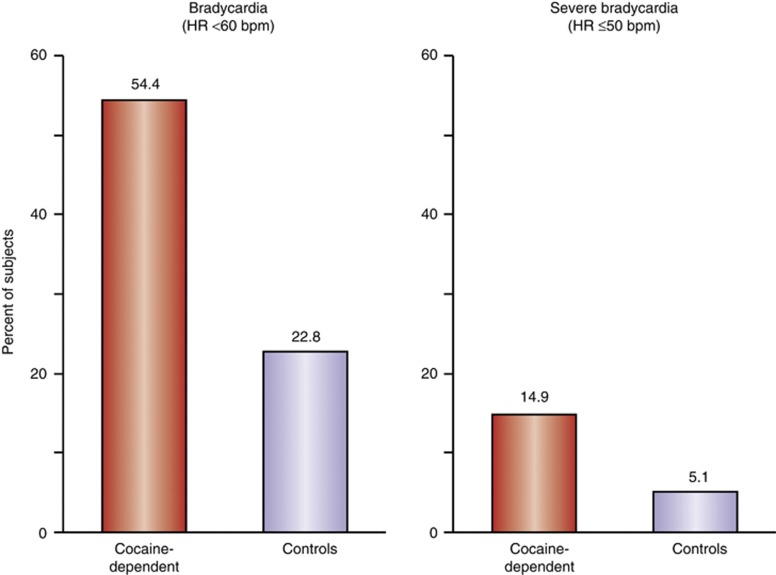
Analysis of variance with age as a covariate showed that cocaine-dependent subjects exhibited significantly lower HRs (M=60.5±10.0 bpm; range 38–95 bpm) compared with controls (M=66.1±9.8 bpm; range 36–120 bpm; p<0.001). As shown in Figure 4, the proportion of subjects with HRs <60 bpm was higher for cocaine-dependent subjects, where bradycardia was present in 54.4% of cocaine-dependent subjects compared with only 22.8% of controls. Similarly, the proportion of cocaine-dependent subjects with severe bradycardia was significantly higher in cocaine-dependent subjects (14.9%) compared with controls (5.1% p<0.001).
Figure 4.
The proportion of subjects with bradycardia (heart rate<60 bpm) and severe bradycardia (heart rate<50 bpm) in cocaine-dependent subjects and matched controls.
These data support an association between chronic cocaine use and severe bradycardia. One potential cause of this association is a physiological response to the desensitization and downregulation of myocardial β-adrenergic receptors after repeated and prolonged stimulation. Dysregulation of β-adrenergic receptors has been identified as an important variable in cardiac failure (Muthumala et al, 2008). It is possible that bradycardia may be a candidate for a physiologically descriptive (see Figure 1) biomarker for cocaine dependence.
The data presented here provides evidence to support further research on the development of cardiovascular biomarkers for cocaine dependence. It is clear that both acute and chronic exposure to cocaine produces measurable changes in both heart rate and other ECG parameters. As cardiovascular measures are non-invasive, inexpensive, and part of a routine medical evaluation, they would be easily adapted for use as a biomarker. Further research is required in order to determine which cardiovascular measures could become biomarkers for cocaine dependence. Although heart rate and bradycardia have been the focus of our discussion here, other ECG-derived parameters (eg, systolic/diastolic function, LVH, QTc interval, QRS complex, early repolarization) and/or non-ECG parameters (eg, cardiac stress testing) await future exploration.
NEUROIMAGING BIOMARKERS
As with many types of biomarkers for cocaine dependence, brain imaging is primarily used as a mechanistic biomarker. However, there is emerging evidence from neuroimaging studies that suggest these measures may have utility as biomarkers for toxicity, prognosis, or pharmacodynamics.
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is used clinically to analyze brain structure to assess pathology associated with brain disease processes. However, MRI can also be used as a measure of health benefits during treatment. As discussed by Winchell et al (2012), ‘The aim of treatment is often expressed as an effort to modifying patients' drug use behavior, but the desired effect is improvement in physical and psychosocial consequences.' Documentation of benefits of pharmacotherapy for cocaine dependence on the structure and function of the brain remain in the early stages of research, but they may become critical in the justification of its use as a potential therapeutic.
Utilizing fMRI as a Biomarker for Cocaine Dependence
Functional MRI (fMRI) provides information about brain function in cocaine-dependent individuals during performance of specific tasks that are related to drug dependence or during a resting state. There are a number of fMRI studies examining basic brain mechanisms associated with cocaine dependence that would fall under the mechanistic biomarker category (Breiter et al, 1997; Hester and Garavan, 2009; Li et al, 2000; Maas et al, 1998; Tomasi et al, 2007). More recently, several studies have begun to use fMRI as a potential predictive biomarker of treatment response.
Brewer et al (2008) scanned 20 treatment-seeking, cocaine-dependent subjects prior to initiating treatment with behavioral and pharmacotherapy. Subjects performed a Stroop task in the fMRI scanner at baseline prior to treatment. The Stroop task is a measure of cognitive control, which is impaired in cocaine users. Subjects were then randomized into cohorts receiving behavioral therapy or the pharmacotherapeutic disulfiram (Antabuse). Results of this study showed that the percentage of drug-free UDSs during treatment correlated with striatal activation at baseline; the cocaine-dependent subjects who had a higher striatal activation level at baseline showed the highest percentage of negative UDSs. In addition, a longer duration of self-reported abstinence correlated with activation of ventromedial prefrontal cortex, left posterior cingulate cortex, and right striatum. Finally, treatment retention was correlated with diminished activation of the dorsolateral prefrontal cortex at baseline. The authors concluded that treatment outcome was correlated with brain activation patterns of circuitry known to be important for cognitive control (Brewer et al, 2008).
Moeller et al (2010) studied predictors of treatment response to medications by having cocaine-dependent subjects undergo an fMRI scan while performing a working memory task at baseline prior to treatment. A working memory task was chosen because it is known that dopamine is related to working memory and dopamine function is affected by chronic cocaine use (discussed in detail in the Molecular Imaging section below). Nineteen treatment-seeking, cocaine-dependent subjects were compared with 14 non-drug-using controls. After undergoing the fMRI scans, cocaine-dependent subjects were randomized to treatments that included cognitive behavioral therapy or several different medications. Results of the study showed that cocaine-dependent subjects had significantly lower brain activation in the caudate/putamen, cingulate gyrus, inferior, middle and superior frontal gyri, thalamus, and subthalamic nuclei compared to non-drug-using controls. Within cocaine-dependent subjects, activation in the thalamus significantly correlated with subsequent treatment response as measured by a treatment effectiveness score (TES) (Spearman r=0.642, uncorrected p=0.003, Bonferroni corrected p=0.037). The conclusions of the study were that cocaine-dependent subjects exhibit an alteration of brain function in the frontal, striatal, and thalamic brain regions in a circuit associated with motor control, reward, and cognition. Subjects with pretreatment thalamic deactivation showed the poorest subsequent treatment response. The thalamus has substantial dopaminergic innervation from the striatum, and molecular imaging studies (described below) have shown that cocaine users have reduced dopamine release and D2 receptor binding in the striatum. Thus, the fMRI findings in the thalamus could be related to thalamic involvement in mesocortical and mesolimbic dopamine projections. However, this remains to be determined.
A third study by Moeller et al (2012b) used fMRI to evaluate 15 treatment-seeking cocaine-dependent individuals at baseline and 6-month follow-up. Subjects underwent fMRI scans using a drug Stroop task at baseline and follow-up. Results of that study found that brain activation in the midbrain and thalamus were higher (and more positively correlated) at 6-month follow-up than baseline. In addition, there were correlations between increased midbrain activity and reduced simulated cocaine choice, and normalization of midbrain activation at 6-month follow-up was suggested by an exploratory analysis. Most recently, researchers have examined whether baseline fMRI of cocaine-dependent subjects performing a cocaine Stroop task was predictive of treatment outcome at 3 months (Marhe et al, 2013). A final sample of 26 cocaine-dependent subjects underwent baseline scans during cocaine detoxification and then was seen after 3 months for the assessment of drug use. Patients underwent various outpatient treatments during the 3-month time period between scans and the follow-up assessment. Results of this study showed that there were two significant predictors of treatment outcome on a step-wise regression: (1) cocaine craving and (2) brain activation on fMRI in the right dorsal anterior cingulate. When combined, both predictors accounted for 45% of the variance of cocaine use. These data collectively support the potential of fMRI brain activation as a predictive biomarker for cocaine dependence.
Utilizing fMRI as a Potential Pharmacodynamic Biomarker
fMRI has also been used as a pharmacodynamic biomarker to determine the mechanisms of treatment response of medications in the brain. Ersche et al (2010) administered a dopamine antagonist (amisulpride) and a dopamine agonist (pramipexole) to stimulant dependent subjects undergoing fMRI while performing a drug word Stroop paradigm. Results showed that drug users had an ‘attentional bias' for drug-related words, which was correlated with greater activation of prefrontal and cerebellar cortices. Dopaminergic medications affected brain activation differentially in subjects with higher vs lower compulsivity as measured by the Obsessive Compulsive Drug Use Scale. Subjects with high compulsivity showed increased brain activation after pramipexole, whereas those subjects with lower compulsivity had reduced brain activation after pramipexole.
An fMRI study of the effects of the wakefulness-enhancing medication modafinil on brain activation while performing a cocaine cue task in cocaine users was carried out by Goudriaan et al (2013). In that study, cocaine users showed higher brain activation in prefrontal cortical regions and striatum than controls while watching cocaine-related pictures. After modafinil administration, there were no significant differences between cocaine users and controls in brain activation, suggesting a ‘normalization' of brain function by modafinil. Another study of the effects of a novel adenosine A2A antagonist (SYN115) on fMRI brain activation while subjects performed a working memory task was carried out by Moeller et al (2012a). In that study, there was significantly greater brain activation after SYN115 in several brain regions, including the orbitofrontal cortex and insula, suggesting that SYN115 produced brain changes consistent with enhancement of dopamine function via adenosine A2A receptor blockade. These studies provide evidence that fMRI can be used as a pharmacodynamic biomarker to aid in medication development for cocaine dependence.
Overall, there is an expanding literature showing a relationship between fMRI brain activation and treatment outcome in cocaine dependence. Emerging data support a potential role for fMRI as a pharmacodynamic and a predictive biomarker. These studies also point to brain mechanisms that may be important for treatment outcome, and thus could be useful in the medications development process, despite the current limitations. Only one study to date has examined the predictive validity of fMRI brain activation beyond the standard measures that may enhance clinical decision-making and data collection methods. Nevertheless, bearing these limitations in mind there is now some evidence that fMRI may be a useful predictive biomarker for cocaine dependence in the future.
Utilizing Diffusion Tensor Imaging as a Potential Toxicity Biomarker
Neural tissue consists of tightly packed and coherently aligned axons surrounded by glial cells that are often organized into bundles. Diffusion anisotropy is hindered perpendicularly to the bundles; cell membranes are mainly responsible for anisotropy. As measured by diffusion tensor imaging (DTI), fractional anisotropy (FA) is a percentile of reduction in random unrestrained motion of water molecules. A decrease in FA can be interpreted as less restraint of water molecules, and an alteration of underlying white matter structure (ie, pathology).
As a potential toxicity biomarker, several studies have used DTI to study white matter pathology in cocaine-dependent subjects. Lim et al (2002) performed DTI on 12 cocaine-dependent subjects and 13 age-similar control subjects. Results of that study showed that the cocaine-dependent subjects had significantly lower FA in the frontal white matter compared with the controls. This finding was replicated in a second study performed by the same group (Lim et al, 2008).
Moeller et al (2005) performed DTI in 18 cocaine-dependent subjects and 18 healthy controls, focusing on the corpus callosum. Findings of that study were that cocaine-dependent subjects had lower FA in the anterior corpus callosum, and that within cocaine-dependent subjects there was a significant correlation between FA and impulsivity as measured by commission errors on a continuous performance test. This study lends support to the theory that at least some of the behavioral problems cocaine-dependent subjects exhibit are related to white matter dysfunction. Further support comes from a study evaluating a different group of 15 cocaine-dependent subjects and 18 control subjects. This study examined the relationship between DTI metrics and performance on the Iowa Gambling Task (IGT), a measure of decision making. Results showed that cocaine-dependent subjects displayed impaired IGT performance, and poorer decision making (Lane et al, 2010). Whole-brain, voxelwise analysis of FA showed that cocaine-dependent subjects had lower FA and higher radial diffusivity in both frontal and parietal white-matter regions, as well as the corpus callosum. There was also a significant relationship between FA, radial diffusivity, and impaired decision making, such that subjects with evidence of impaired IGT performance exhibited lower FA and higher radial diffusivity. Other studies in cocaine-dependent subjects have shown increased radial diffusivity in cocaine users compared with controls, suggesting that altered myelin may be responsible for the DTI findings in cocaine users (Ma et al, 2009; Moeller et al, 2007). At least one animal study also showed that chronic cocaine administration altered white matter myelin by reducing myelin basic protein (Narayana et al, 2009). These studies suggest collectively that DTI could be a potential toxicity biomarker for cocaine dependence.
Utilizing DTI as a Potential Predictive Biomarker
Most recently, studies have examined the relationship between DTI metrics and treatment outcome or abstinence in cocaine-dependent subjects as a predictive biomarker. A study by Xu et al (2010) examined the relationship between white-matter integrity as measured by DTI and treatment outcome in 16 cocaine-dependent subjects. DTI was performed at baseline prior to 8 weeks of therapy. Results of that study showed that self-reported abstinence and percent cocaine-negative UDS correlated significantly with baseline FA and radial diffusivity in several brain regions including the corpus callosum, frontal and parietal white matter. These results suggest that white-matter pathology as measured by DTI is predictive of treatment outcome in cocaine dependence. Bell et al (2011) compared DTI findings between 43 cocaine-dependent subjects with various lengths of abstinence with 43 non-drug-using controls. Results of that study showed that cocaine-dependent subjects had lower FA in several brain regions including the corpus callosum and superior longitudinal fasciculus. When separated into short-term, mid-term and long-term abstinent groups, cocaine users showed differences in white-matter FA across these groups in several brain regions. These results suggest that at least some of the DTI-measured white-matter pathology in cocaine-dependent subjects may be altered by abstinence.
MOLECULAR IMAGING
As with MRI, there is substantial literature on the use of molecular imaging as a mechanistic biomarker for cocaine dependence. Alterations in neurochemistry that occur in drug dependence in the human brain can be imaged with positron emission tomography (PET) or single photon emission tomography. These modalities use a radioactive marker attached to a ligand specific for brain receptors or transporters, which allow the measurement of these biomarkers of brain neurochemistry. Imaging studies of cocaine dependence have largely focused on the dopamine receptors of the striatum, such as the dopamine D2 family of receptors (referred to as D2). Overall, these studies show that dependence is associated with a reduction in D2 receptor binding. Receptor-binding levels are decreased in several SUDs, including cocaine-, alcohol-, methamphetamine-, and opiate dependence. The reduction in binding is relatively consistent, with decreases of ∼15–20% compared with matched controls. The behavioral significance of the decrease in D2 receptor binding has been investigated in studies of cocaine abuse in addition to imaging studies in non-human primates and non-addicted human volunteers.
Utilizing PET to Elucidate Mechanistic Biomarkers
Imaging studies in cocaine dependence show that low D2 receptor binding in the striatum, imaged during early or protracted withdrawal, is associated with decreases in activity in frontal brain regions that have been shown to have a key role in salience attribution (orbitofrontal cortex) and inhibitory control (anterior cingulate gyrus) (Volkow et al, 1998). In rhesus monkeys, social stress is associated with lower striatal D2 binding in subordinate compared with that in dominant animals, and serves as a risk factor for increased cocaine self-administration (Morgan et al, 2002). Meanwhile, rodents selected for impulsive behavior have lower D2 receptor binding, compared with non-impulsive rodents, and show an increased propensity to self-administer cocaine (Dalley et al, 2007). Importantly, both stress and impulsive behavior are associated with an increased risk of dependence in humans (Chambers et al., 2003; Poling et al, 2007).
PET studies in healthy controls show that low D2 receptor binding is predictive of a pleasurable experience following the psychostimulant administration (Volkow et al, 1998). Additionally, studies in healthy controls with a family history of dependence (compared with controls without a family history) show that family history is associated with higher D2 receptor binding, suggesting that this may be a biomarker for resilience (Volkow et al, 2006). Together, these studies suggest that low D2 receptor binding may serve as a risk factor for the development of dependence, although other studies have shown that cocaine self-administration itself also produces a decrease in D2 receptor binding (Nader et al, 2006). These studies do indicate that low D2 receptor binding may be associated with a greater severity of disease and a propensity to initiate cocaine self-administration.
In addition to imaging the D2 receptor, PET can also be used to image dopamine release within the striatum. The D2 receptors are imaged before and after the administration of a stimulant, which causes the dopamine neurons to release dopamine (Breier et al, 1997; Laruelle et al, 1997; Volkow et al, 1994). In drug and alcohol dependence, pre-synaptic dopamine release in the striatum is blunted compared with controls (Martinez et al, 2007a). In healthy controls, a recent PET imaging study showed that increasing striatal dopamine with a stimulant challenge (amphetamine) resulted in a greater willingness to expend effort to obtain a reward (Treadway et al, 2012), which is consistent with studies in rodents (Salamone et al, 2003).
Imaging studies involving cocaine abuse show an inverse relationship to the one detailed above. Blunted pre-synaptic dopamine release, specifically in the ventral striatum, has been associated with increased cocaine-seeking behavior (Martinez et al, 2007b). In this study, cocaine abusers underwent PET imaging scans to measure pre-synaptic dopamine release, followed by laboratory sessions in which the choice was presented between cocaine and an alternative reinforcer. The choices were weighted towards the money (which had a higher value than the dose of cocaine). The results showed that subjects with the lowest dopamine release in the ventral striatum were more likely to choose the cocaine. These results suggest that blunted pre-synaptic dopamine release in the ventral striatum is associated with an impaired ability to shift behavior in the setting of competing rewards in cocaine abuse. Notably, although studies have shown that other types of dependence are also associated with low dopamine release, a recent study in heroin-dependent subjects did not show a correlation between dopamine transmission and heroin self-administration (Martinez et al, 2012). Thus, even though both cocaine- and heroin dependence share the same striatal biomarker, low dopamine release in heroin abusers was not predictive of drug-seeking behavior, unlike the study of cocaine abusers.
Utilizing PET as a Potential Predictive Biomarker
Recent studies have begun to explore the use of molecular imaging as a predictive biomarker for cocaine dependence. An imaging study of treatment-seeking cocaine abusers used PET to image dopamine D2 receptors and dopamine release prior to 6 months of behavioral treatment (Martinez et al, 2011). The behavioral treatment consisted of contingency management (CM) combined with community reinforcement approach (CRA). Cocaine-abusing participants were given vouchers (money) for abstinence as they attended clinic visits. Over time, the voucher became more and more valuable. The results of this study showed that subjects who responded to treatment had higher values of D2 receptor binding, and greater values of pre-synaptic dopamine release compared with the subjects who did not respond to treatment, and who relapsed within 2 weeks. Similar results have been shown in a study of methamphetamine abusers, where subjects who responded to treatment had both higher D2 receptor binding and dopamine release compared with subjects who did not respond (Wang et al, 2012). Furthermore, in both the studies, the cocaine and methamphetamine abusers who responded to treatment did not differ from the control group with respect to measures of D2 receptor-binding and dopamine release.
Thus, these studies now show that there is a subgroup of addicted individuals who do not express the reduced D2 receptor binding seen previously with PET imaging. This subgroup is distinguished by the fact that they respond to behavioral treatments for drug dependence. In addicted individuals, intact dopamine signaling at the D2 receptor in the striatum appears to serve as a predictive biomarker for treatment response. When presented with alternatives to drug use, addicted individuals with intact dopamine release and D2 receptor binding mechanisms are capable of shifting their behavior away from taking drugs toward other reinforced behaviors. The unique contribution of dopamine function in risk for development and maintenance of cocaine dependence remains to be determined. More research is needed on the specific advantages of molecular imaging over other less costly measures as a potential predictive biomarker for cocaine dependence.
Potential Limitations of Neuroimaging Biomarkers
Brain imaging is becoming a commonly used tool in medication development for other central nervous system disorders, and there is increasing evidence of the utility of brain imaging in medication development for drug dependence (Wong et al, 2009). However, there are several issues that need to be addressed before neuroimaging biomarkers can be more widely applied. One obvious disadvantage of neuroimaging is its current relative costs. The high costs associated with both MRI and molecular imaging may limit this technology from being used in large-scale clinical trials. In addition, further research will need to assess whether these measures (eg, fMRI) have predictive validity beyond more easily obtained assessments from other tissues (eg, blood). There is a need for further research on the reliability of these methods as a robust biomarker for cocaine dependence. Finally, the creation of neuroimaging standards for each of these types of biomarkers is vital. Studies to date have used a variety of behavioral tasks and imaging methods, making it difficult to compare results across studies. Having standardized methods will allow for easy comparison of results across studies and the appropriate development of biomarkers and their application to future drug development.
BIOMARKER DISCOVERY
Metabolomics
Metabolomics can be defined as a comprehensive analysis of the chemical products from a specific tissue. ‘The analysis of the chemical fingerprints left by metabolic processes has already started to play a crucial part in personalized medicine, particularly cancer therapy' (Nature, 2010). Analytical samples can be taken from a variety of tissue types or media including brain tissue, CSF, blood, plasma, or urine to provide a map of the physiological processes and provide metabolic ‘signature' associated with a clinical outcome. The most immediate and significant benefits of metabolomics are its ability to uncover underlying mechanisms of cocaine dependence (or other SUDs), identify potential biomarkers, and discover new therapeutic targets.
To date, very few studies have examined differences in metabolites between cocaine-dependent and control subjects. In an early metabolomic study of cocaine dependence, a targeted profile was created for 18 cocaine-dependent subjects (confirmed to be abstinent for 2 weeks prior to study) and 10 healthy controls (Patkar et al, 2009). Specifically, blood plasma metabolite differences related to tryptophan (serotonin and kynurenine), tyrosine (dopamine and catecholamines) and purine (adenosine, guanine, and xanthine) were analyzed. Relationships between observed metabolic changes and clinical outcomes of addiction were also assessed. Results of this study were that a consistent elevation in n-methylserotonin, a byproduct of serotonin, was positively correlated to chronic cocaine exposure. Together with xanthine, n-methylserotonin accounted for 73% of the variance in the severity of cocaine use, when measured by the ASI. Metabolomics data such as these can be used to characterize the mechanisms cocaine dependence or the reinforcing effects of cocaine dependence; however, as with other potential biomarkers for cocaine dependence, metabolomics is at an early stage of development.
Proteomics
Similar to metabolomics, proteomics provides a global biological assessment. This approach is designed for the analysis of coordinately expressed proteins, enabling an unbiased biological view of the proteome in order to delineate the multitude of neurobiological effects of drugs of abuse (Hemby, 2010; Matsumoto et al, 2007). Quantitative protein expression data can offer valuable insight into underlying genomic, transcriptional, and post-transcriptional regulatory activity, as well as a more representative view of the disease phenotype. Given the heterogeneous nature of SUDs and our limited knowledge of the molecular pathology of this complex illness, these data will be particularly important for linking causal molecular mechanisms to a clinical outcome.
To be of maximum value, identified biomarkers need to demonstrate consistent responses across multiple species including humans. If a biomarker (or panel of biomarkers) can be identified across an array of animal species, these descriptors could offer insight into the underlying mechanisms of dependence and be utilized as an important tool for drug development. The use of brain tissue for proteomic studies necessitates the use of animal models for addictions. It is important to note that some animal models may be more closely related to human drug use behavior than others. Bearing these limitations in mind, proteomics have a potential to provide mechanistic biomarkers for cocaine dependence.
Rodent models
Studies have utilized proteomic technologies to examine protein changes in rodent models. Various aspects of cocaine dependence have been investigated including reward, rates of extinction, and reinstatement. In one recent study, Guan and Guan (2013) used conditioned place preference (CPP) to evaluate repeated-exposure, cocaine-induced reward effect in rats. Following pre-conditioning, rats were randomly divided into either a cocaine- (10 mg/kg/day) or saline-treated group, while CPP training was run for days 1–8. Protein expression profiles were assembled from tissue taken from rat medial prefrontal cortex (mPFC). Pathway analysis revealed the involvement of proteins in metabolism (27.5%), actin-cytoskeleton regulation (27.5%), and signal transduction (25%). These findings are in agreement with previous proteomics studies, suggesting both metabolic and mitochondrial dysfunction following chronic cocaine administration.
In 2009, del Castillo et al (2009) analyzed proteomes taken from the nucleus accumbens (NAc) of rats undergoing different rates of extinction following cocaine-induced place conditioning. Rats were divided into four main groups: (1) those that did not extinguish and were administered cocaine (NEC), (2) those that did not extinguish, but were administered saline (NE SAL), (3) those that did extinguish and were administered cocaine (EC), and (4) those that did extinguish, but were administered saline (E SAL). Analysis revealed 18 proteins that were differentially expressed across the four groups, while comparison of E SAL and NE SAL revealed five proteins including four mitochondrial proteins and a nucleoside kinase. These changes were thought to be associated with extinction to cocaine-associated environmental cues.
A proteomic approach has also been utilized to identify proteins regulating reinstatement (Reissner et al, 2011). There has been growing evidence in the literature reporting the involvement of cAMP and PKA as mediators of cocaine reinstatement. Following proteomic analysis, 42 proteins were identified in a postsynaptic density-enriched sub-fraction of the NAc.
Among these, AKAP79/150, a PSD scaffolding protein that localizes signaling molecules such as PKA and GluR1 to the synapse was shown to be increased following cocaine self-administration. As cocaine-seeking is thought to require upregulation of AMPA receptors, this proteomic study helped to identify proteins that may contribute to relapse vulnerability within a specific sub-cellular proteome of the NAc. It also represents one of the more advanced studies that utilized proteomics in addition to other methodologies in order to uncover a specific mechanistic pathway thought to be involved in cocaine dependence.
Non-human primate and human studies
Olausson et al used multiple proteomic approaches in order to characterize putative changes in the proteome of the orbitofrontal cortex (OFC) tissue of juvenile Vervet monkeys that were thought to be correlated with observed cognitive deficits following cocaine administration (Olausson et al, 2007). After behavioral analysis, differential expression of OFC proteins was compared with controls 3–4 weeks following the last cocaine administration. Synaptoneurosomes were isolated from the OFC of each monkey in order to reduce the complexity of the proteome and target a more specific cellular domain. Results revealed significant differences in proteins that are involved in cellular metabolic processes such as mitochondrial function, signal transduction, and cytoskeleton regulation. The effects of chronic intravenous cocaine self-administration on protein abundance and phosphorylation in the NAc of rhesus monkeys has also been investigated (Tannu et al, 2010). In this study, 18 proteins were found to be differentially expressed in NAc tissue. A significant number of these proteins were either directly or indirectly related to the hyperglutamatergic state that was identified in both rhesus monkeys self-administering cocaine and cocaine overdose victims (Tang et al, 2003; Hemby and Tannu, 2009). Interestingly, the study identified several proteins and pathways that were identical or similar to those identified in cocaine overdose victims. Similar proteins included those involved in cell structure, synaptic plasticity, signal transduction, metabolism, and mitochondrial function. More specifically, glial fibrillary acidic protein, syntaxin binding protein 3, protein kinase C isoform, adenylate kinase isoenzyme 5, and mitochondrial-related proteins were increased in monkeys self-administering cocaine, whereas beta-soluble N-ethylmaleimide-sensitive factor attachment protein, neural, and non-neural enolase were decreased. As mentioned previously, the study also explored the phosphorylated proteome of the accumbens. Fifteen proteins were found to be differentially expressed between the groups. Protein levels that increased included GABA-A receptor-associated protein 1, 14-3-3 gamma protein, glutathione S-transferase, and brain type aldolase. Protein levels that decreased were beta-actin, Rab GDP dissociation inhibitor, guanine deaminase, peroxiredoxin 2 isoform b, and several mitochondrial proteins. Together, these findings imply that there is a coordinate dysregulation of proteins that are related to cell structure, signaling, metabolism, and mitochondrial function which underlie long-term compromised cellular function in the frontal cortex and NAc following chronic cocaine exposure.
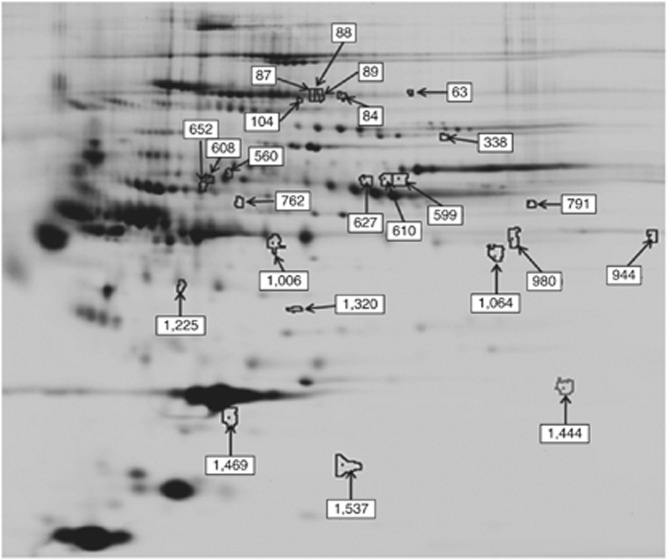
In order to identify potential candidates for biomarkers, the Hemby lab undertook a preliminary assessment of the plasma proteome in rhesus monkeys 15 days following the last self-administration session. Following plasma depletion of abundant proteins, fractions from each subject were compared using the 2D-DIGE proteomics platform to determine differences in the abundance levels of proteins in a pH range of 3–10 to elucidate possible biomarkers for cocaine withdrawal (Figure 5 and Table 3). Note that the majority of the 20 differentially expressed proteins are low abundance compared with the total protein content.
Figure 5.
The plasma proteome of cocaine withdrawal. The identified protein spots exhibited differential intensity between acute cocaine withdrawal and control groups and are detailed below. Note that the majority of differentially expressed proteins are low abundance compared with the total protein content. (see also Table 3).
Table 3. Differentially Regulated Proteins in Plasma During Cocaine Withdrawal.
| Gene name | Type | Gel spot no | Fold change | t-test (p-value) |
|---|---|---|---|---|
| Apolipoprotein A-ll | Transporter | 1537 | −1.2 | 0.012 |
| Coiled-coil-helix-coiled-coil-helix domain 1 | Other | 63 | 1.42 | 0.014 |
| Clusterin | Apoptosis | 1469 | −2.42 | 0.049 |
| Fibronectin1 isoform 6 preproprotein | Enzyme | 87 | 1.59 | 0.0029 |
| Fibronectin1 isoform4 preproprotein | Enzyme | 88 | 1.75 | 0.018 |
| Fibronectin1 isoform 1 preproprotein | Enzyme | 89 | 1.68 | 0.0028 |
| Vitamin D-binding protein | Transporter | 1320 | −1.59 | 0.054 |
| GDP dissociation inhibitor 1 | Brain: GTPase activation | 84 | 1.54 | 0.0032 |
| GDNF-inducible zinc finger protein 1 | Transcriptional regulation | 599 | −1.3 | 0.024 |
| Heat shock 70 kDa protein 9 (mortalin) | Protein exportfrom nucleus | 610 | −1.28 | 0.044 |
| Interleukin 13 | Cytokine | 652 | −3.83 | 0.048 |
| Kininogen 1 | Receptor binding | 608 | −1.56 | 0.016 |
| Phospholipase D1, phosphatidylcholine-specific | Enzyme | 560 | 1.12 | 0.077 |
| Peroxisome proliferator-activated receptor α | Ligand-dependent nuclear receptor | 762 | −2.14 | 0.014 |
| Serine peptidase inhibitor, clade B,member 6b | Peptidase activity | 1444 | 3.3 | 0.076 |
| S1 RNA binding domain 1 | Other | 791 | −1.41 | 0.052 |
| TBC1 domain family, member 8B | Regulation of Rab GTPase activity | 104 | 1.57 | 0.091 |
| Vacuolar protein sorting 28 homolog | Transporter | 859 | 2.41 | 0.056 |
| Vitronectin | Other | 627 | −1.34 | 0.026 |
| 21 nc finger protein 161 homolog | Transcriptional regulation | 338 | 1.19 | 0.08 |
Gel spot numbers correspond with numbered spots identified/highlighted in Figure 5.
There is growing evidence of the potential for proteomics to have a role in biomarker development for cocaine dependence. However, there are several issues that will need to be addressed in future research. In order to develop a panel of biomarkers for cocaine dependence, one must first determine the stage of dependence of interest. Clearly defined and agreed upon standards for sample collection, preservation, depletion of abundant proteins, separation, and mass spectrometry procedures need to be delineated (eg, those initiated by the Human Proteomics Organization) in order to ensure reproducibility and provide confidence in the candidate biomarkers that are identified.
Transcriptomics
Over the past decade, numerous studies have utilized high-throughput approaches to examine cocaine-induced changes in the brain. Transcriptomic technologies have been conducted to examine large-scale changes in all transcribed RNA following cocaine exposure. Genomics-based studies have revealed novel mechanisms of drug-induced neuronal- and non-neuronal dysregulation in both human and rodent postmortem brain tissue (Ahmed et al, 2005; Ang et al, 2001; Backes and Hemby, 2003; Hemby, 2004; Yuferov et al, 2005).
Two groups of studies are highlighted here to exemplify the application of this approach. In the first example, mRNA-Seq libraries from the NAc were prepared from cocaine-, withdrawal-, and control groups of mice (Eipper-Mains et al, 2013). Expression of several GABA, glutamate, neuropeptides, and endocannabinoid receptors were altered after cocaine exposure. Drd2 expression in NAc increased significantly during withdrawal, which is consistent with the observations seen in the PET studies mentioned earlier. Additionally, there was a sustained upregulation of many cadherin genes, but a sustained downregulation of many protocadherins. These data were consistent with human postmortem analysis of cocaine overdose tissue, albeit from different regions of the brain (Lehrmann et al, 2006; Mash et al, 2007).
In the second example, ∼39 000 gene transcripts from the postmortem brains of human cocaine abusers were examined. These studies found that the expression of a group of myelin-related genes, including myelin basic protein, proteolipid protein, and myelin-associated oligodentrocyte basic protein was substantially decreased in cocaine abusers in the NAc (Albertson et al, 2004). However, a follow-up study did not find changes in myelin-related gene expression in the NAc, but did find decreased expression of the transcript encoding PLP-1 in the caudate, putamen, and internal capsule (Kristiansen et al, 2009). These findings are consistent with the neuroimaging (ie, DTI) findings of altered white-matter structure in cocaine users discussed above, thus indicating that myelin-related proteins may be a useful toxicity biomarker for future consideration, and illustrate the potential for combining types of biomarkers to aid in mechanistic biomarker discovery.
Genomics
Genetic factors represent ∼40–60% of the risk of developing a SUD (Kreek et al, 2005). Accordingly, genetic variants are likely to be useful in understanding inter-individual differences that are relevant to risks for development of cocaine dependence, and improving our understanding of underlying differential responses to cocaine. Genetic biomarkers may also help in identifying a subpopulation of cocaine-dependent patients who are likely to respond to novel therapies. Whereas both preliminary pharmacogenomic genome-wide association studies and pharmacogenetic candidate-gene association studies show promise for elucidating potential mechanistic biomarkers, few predictive or prognostic biomarker studies have been completed in this area (Yuferov et al, 2010).
A recent example of a study of a predictive genetic biomarker in cocaine dependence examined the relationship between genetic variation of the dopamine beta hydroxylase (DBH) gene and the response to the medication disulfiram. Several clinical trials have shown that disulfiram (Antabuse) reduces cocaine positive urines in cocaine-dependent subjects (Carroll et al, 2004; Carroll et al, 1998; Petrakis et al, 2000). In addition to blocking the effect of acetaldehyde dehydrogenase (through which disulfiram produces the toxic reaction to alcohol), disulfiram has copper-chelating effects on several other enzymes, including DBH, which is the enzyme that converts dopamine to norepinephrine (Barth and Malcolm, 2010). Brain imaging studies in cocaine users described previously showed that dopamine D2 receptor binding, and amphetamine-induced dopamine release, was reduced in the striatum, potentially leading to a ‘reward deficient' state (Martinez et al, 2007b). It was theorized that the dopamine-enhancing effect of disulfiram is the mechanism through which it has its treatment effect for cocaine dependence (Barth and Malcolm, 2010).
A polymorphism of the DBH gene (variant C-1021T) has been shown to be associated with altered plasma levels of DBH (Zabetian et al, 2001). In a recently published pharmacogenetic clinical trial of disulfiram for cocaine dependence, subjects with the CC DBH allele genotype (which is associated with normal levels of DBH) who were treated with disulfiram showed a significant reduction in cocaine-positive urines compared with CC DBH allele subjects treated with placebo. However, subjects with CT/TT alleles (associated with lower levels of DBH) showed no difference in cocaine-positive urines, whether or not they were treated with disulfiram. Collectively, these data suggest that disulfiram treatment response is greater in cocaine-dependent subjects who have the normal level of DBH activity due to their particular genotype (ie, CC genotype) (Kosten et al, 2013).
FUTURE RESEARCH DIRECTIONS
Cocaine dependence remains a disorder without an FDA-approved pharmacologic treatment. An effective drug product is needed to address this unmet medical need. As described in this paper, DDTs, CSSAs, animal models, and biomarkers hold great promise in facilitating drug development and enhancing clinical decision making. Yet, despite their potential, the field is in its infancy.
A Proposed Path Forward—Moving Proximally
Most putative biomarkers that have been identified to date can be classified as ‘descriptive' (Figure 1), are largely symptomatic or ‘distal' in nature (Figure 6), and yet to be validated. Neuroimaging biomarkers such as molecular resonance imaging (eg, DTI) describe more of the overarching endophenotype associated with cocaine dependence, and are therefore better suited for characterizing symptomatic toxicity and/or efficacy once a SUD has developed.
Figure 6.
Distal vs proximal biomarkers for cocaine dependence.
Whereas ‘distal' markers hold great future value, molecular imaging techniques such as PET or molecular ‘-omics' will be able to establish more directly the underlying causal mechanisms of molecular interaction. The identification and validation of these ‘proximal' biomarkers (ie, mechanistic biomarkers; Figures 1 and 6) will be pivotal for better defined decision-making points to enhance drug development as well as improve medical management of SUDs such as cocaine dependence.
A mechanistic biomarker will be of greatest potential benefit for pharmaceutical development if it can encompass at least three essential criteria (Figure 1). First, a mechanistic biomarker should be able to be measured both pre-clinically and clinically. Second, the putative biomarker should demonstrate involvement in the CNS. Third, pharmacokinetic–pharmacodynamic (PK–PD) responses for the putative biomarker should be scalable between pre-clinical and clinical models (Soares, 2010). From there, the subsequent identification of potential ‘actionable' biomarkers (eg diagnostic) can be developed (see Figure 1) (Robinson et al, 2013). The application of one or more of these types of ‘actionable' biomarkers will subsequently enhance the technical probability of success for an effective pharmacotherapeutic drug (or biologic) for cocaine dependence. Molecular diagnostic biomarkers could be used for patient selection to identify subpopulations with specific cocaine dependence endophenotypes. Prognostic biomarkers could be employed to improve clinical trial design (eg, stratification, patient selection, or enrichment) and optimize dosing. Pharmacodynamic biomarkers could be used for identifying target engagement, activity response, efficacy response, and safety-related response(s). Ideally, predictive biomarkers help tailor medical treatment to the individual by finding the patients who are likely to respond to a particular pharmacotherapy.
In summary, there is a growing body of evidence that DDTs such as biomarkers derived from cardiovascular, neuroimaging, and molecular techniques will enhance the efficiency of medication development for cocaine dependence. The major challenge of future research will be the considerable undertaking to systematically identify and evaluate potential mechanistic biomarkers and their potential role in medication development via well-defined preclinical and clinical studies. Most biomarkers to date are descriptive in nature, although studies continue to define potential mechanistic biomarkers. Validated mechanistic biomarkers will serve as the foundation for the identification of other ‘actionable' diagnostic, toxic, prognostic, pharmacodynamic, and predictive biomarkers in order to enhance drug development. ‘Basic biomedical science will [continue to] churn out candidate biomarkers with tantalizing potential to improve [drug development], whereas methods to use them effectively…will evolve more slowly. The balance between these forces may well determine the success or failure of the drug development enterprise over the next decade' (Woodcock, 2010).
FUNDING AND DISCLOSURE
All listed authors contributed to the composition of this review. Dr Moeller is a consultant for Boehringer Ingelheim. Dr Merchant is an employee of Eli Lilly. All other authors declare no conflict of interest.
Acknowledgments
We wish to thank Ms Dena Procaccini for her excellent technical assistance in the preparation of this manuscript.
Disclaimer
The views expressed in this presentation are those of the authors and may not necessarily reflect the position of the US FDA (SA) or NIH (KJB).
References
- 2020 visions. Nature. 2010;463:26–32. doi: 10.1038/463026a. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, Morales M, et al. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci USA. 2005;102:11533–11538. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang E, Chen J, Zagouras P, Magna H, Holland J, Schaeffer E, et al. Induction of nuclear factor-kappaB in nucleus accumbens by chronic cocaine administration. J Neurochem. 2001;79:221–224. doi: 10.1046/j.1471-4159.2001.00563.x. [DOI] [PubMed] [Google Scholar]
- Association AP 2000Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TRFourth ednText RevisionAmerican Psychiatric Publishing: Washington, DC, USA; 943 [Google Scholar]
- Backes E, Hemby SE. Discrete cell gene profiling of ventral tegmental dopamine neurons after acute and chronic cocaine self-administration. J Pharmacol Exp Ther. 2003;307:450–459. doi: 10.1124/jpet.103.054965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth KS, Malcolm RJ. Disulfiram: an old therapeutic with new applications. CNS Neurol Disord Drug Targets. 2010;9:5–12. doi: 10.2174/187152710790966678. [DOI] [PubMed] [Google Scholar]
- Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H. Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend. 2011;114:159–168. doi: 10.1016/j.drugalcdep.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, deBartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: Evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, et al. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Castillo C, Morales L, Alguacil LF, Salas E, Garrido E, Alonso E, et al. Proteomic analysis of the nucleus accumbens of rats with different vulnerability to cocaine addiction. Neuropharmacology. 2009;57:41–48. doi: 10.1016/j.neuropharm.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Eipper-Mains JE, Kiraly DD, Duff MO, Horowitz MJ, McManus CJ, Eipper BA, et al. Effects of cocaine and withdrawal on the mouse nucleus accumbens transcriptome. Genes Brain Behav. 2013;12:21–33. doi: 10.1111/j.1601-183X.2012.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Bullmore ET, Craig KJ, Shabbir SS, Abbott S, Muller U, et al. Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Arch Gen Psychiatry. 2010;67:632–644. doi: 10.1001/archgenpsychiatry.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esserman LJ, Woodcock J. Accelerating identification and regulatory approval of investigational cancer drugs. JAMA. 2011;306:2608–2609. doi: 10.1001/jama.2011.1837. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration . Guidance for Industry: Qualifications Process for Drug Development Tools. U.S. Department of Health and Human Services. Center for Drug Evaluation Research. Silver Spring. Maryland; 2010. [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Veltman DJ, van den Brink W, Dom G, Schmaal L. Neurophysiological effects of modafinil on cue-exposure in cocaine dependence: a randomized placebo-controlled cross-over study using pharmacological fMRI. Addict Behav. 2013;38:1509–1517. doi: 10.1016/j.addbeh.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Guan X, Guan Y. Proteomic profile of differentially expressed proteins in the medial prefrontal cortex after repeated cocaine exposure. Neuroscience. 2013;236:262–270. doi: 10.1016/j.neuroscience.2013.01.039. [DOI] [PubMed] [Google Scholar]
- Haigney MC, Alam S, Tebo S, Marhefka G, Elkashef A, Kahn R, et al. Intravenous cocaine and QT variability. J Cardiovasc Electrophysiol. 2006;17:610–616. doi: 10.1111/j.1540-8167.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- Hemby SE. Morphine-induced alterations in gene expression of calbindin immunopositive neurons in nucleus accumbens shell and core. Neuroscience. 2004;126:689–703. doi: 10.1016/j.neuroscience.2004.01.056. [DOI] [PubMed] [Google Scholar]
- Hemby SE. Cocainomics: new insights into the molecular basis of cocaine addiction. J Neuroimmun Pharmacol. 2010;5:70–82. doi: 10.1007/s11481-009-9189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Tannu N. Modeling substance abuse for applications in proteomics. Methods Mol Biol. 2009;566:69–83. doi: 10.1007/978-1-59745-562-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Neural mechanisms underlying drug-related cue distraction in active cocaine users. Pharmacol Biochem Behav. 2009;93:270–277. doi: 10.1016/j.pbb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Hollander JE, Burstein JL, Hoffman RS, Shih RD, Wilson LD. Cocaine-associated myocardial infarction. Clinical safety of thrombolytic therapy. Cocaine Associated Myocardial Infarction (CAMI) Study Group. Chest. 1995;107:1237–1241. doi: 10.1378/chest.107.5.1237. [DOI] [PubMed] [Google Scholar]
- Kampman K, Pettinati H, Lynch K, Roth T, Liebman R, Paulsen B, et al. (eds) (2011Topiramate for the treatment of comorbid alcohol and cocaine dependence College on Problems of Drug Dependence Annual Meeting20 June 2011, Hollywood, FL, USA [Google Scholar]
- Kampman KM, Alterman AI, Volpicelli JR, Maany I, Muller ES, Luce DD, et al. Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychol Addictive Behav. 2001;15:52–59. doi: 10.1037/0893-164x.15.1.52. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinieb RM, D'Angelo LD, et al. 1998Reliability and validity of the cocaine selective severity assessment Addictive Behav 2349–461.This paper is the first description of the Cocaine Selective Severity Assessment (CSSA). CSSA severity scores declined consistently over time for subjects who continued in treatment and remained abstinent, and were highly correlated with the Addiction Severity Index (ASI) outcomes, suggesting this interviewer-reported outcome measure holds promise as a potential FDA-defined Clinical Outcome Assessment tool. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney FD, Rukstalis M, Alterman AI, Pettinati H, et al. Cocaine withdrawal severity and urine toxicology results from treatment entry predict outcome in medication trials for cocaine dependence. Addictive Behaviors. 2002;27:251–260. doi: 10.1016/s0306-4603(01)00171-x. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, et al. 2013Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine beta-hydroxylase Biol Psychiatry 73219–224.This study is one of the few pharmacogenetic studies linking specific genotypes with treatment outcome in cocaine dependence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Bannon MJ, Meador-Woodruff JH. Expression of transcripts for myelin related genes in postmortem brain from cocaine abusers. Neurochem Res. 2009;34:46–54. doi: 10.1007/s11064-008-9655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Steinberg JL, Ma L, Hasan KM, Kramer LA, Zuniga EA, et al. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One. 2010;5:e11591. doi: 10.1371/journal.pone.0011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Iyer RN, Al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, et al. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse. 1997;25:1–14. doi: 10.1002/(SICI)1098-2396(199701)25:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Colantuoni C, Deep-Soboslay A, Becker KG, Lowe R, Huestis MA, et al. Transcriptional changes common to human cocaine, cannabis and phencyclidine abuse. PLoS One. 2006;1:e114. doi: 10.1371/journal.pone.0000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, et al. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med. 2000;43:45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, et al. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Hasan KM, Steinberg JL, Narayana PA, Lane SD, Zuniga EA, et al. Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend. 2009;104:262–267. doi: 10.1016/j.drugalcdep.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, et al. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Magnano AR, Talathoti NB, Hallur R, Jurus DT, Dizon J, Holleran S, et al. Effect of acute cocaine administration on the QTc interval of habitual users. Am J Cardiol. 2006;97:1244–1246. doi: 10.1016/j.amjcard.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Maraj S, Figueredo VM, Lynn Morris D. Cocaine and the heart. Clin Cardiol. 2010;33:264–269. doi: 10.1002/clc.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R, Luijten M, van de Wetering BJ, Smits M, Franken IH. Individual differences in anterior cingulate activation associated with attentional bias predict cocaine use after treatment. Neuropsychopharmacology. 2013;38:1085–1093. doi: 10.1038/npp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. 2011Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment Am J Psychiatry 168634–641.This paper is one of the few imaging studies showing PET scans could be potential biomarkers of treatment response in cocaine dependence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Kim JH, Krystal J, Abi-Dargham A. Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin N Am. 2007a;17:539–555. doi: 10.1016/j.nic.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007b;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A, et al. Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry. 2012;71:192–198. doi: 10.1016/j.biopsych.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, ffrench-Mullen J, Adi N, Qin Y, Buck A, Pablo J. Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. PLoS One. 2007;2:e1187. doi: 10.1371/journal.pone.0001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto I, Alexander-Kaufman K, Iwazaki T, Kashem MA, Matsuda-Matsumoto H. CNS proteomes in alcohol and drug abuse and dependence. Expert Rev Proteom. 2007;4:539–552. doi: 10.1586/14789450.4.4.539. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Valdes I, Lai LY, et al. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Res. 2007;154:253–258. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Lane SD, Kjome KL, Ma L, Ferre S, et al. Increased orbitofrontal brain activation after administration of a selective adenosine A(2A) antagonist in cocaine dependent subjects. Front Psychiatry. 2012a;3:44. doi: 10.3389/fpsyt.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, et al. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Res. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Tomasi D, Woicik PA, Maloney T, Alia-Klein N, Honorio J, et al. Enhanced midbrain response at 6-month follow-up in cocaine addiction, association with reduced drug-related choice. Addict Biol. 2012b;17:1013–1025. doi: 10.1111/j.1369-1600.2012.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Mulvaney FD, Alterman AI, Boardman CR, Kampman K. Cocaine abstinence symptomatology and treatment attrition. J Substance Abuse Treat. 1999;16:129–135. doi: 10.1016/s0740-5472(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Muthumala A, Drenos F, Elliott PM, Humphries SE. Role of beta adrenergic receptor polymorphisms in heart failure: systematic review and meta-analysis. Eur J Heart Fail. 2008;10:3–13. doi: 10.1016/j.ejheart.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Narayana PA, Ahobila-Vajjula P, Ramu J, Herrera J, Steinberg JL, Moeller FG. Diffusion tensor imaging of cocaine-treated rodents. Psychiatry Res. 2009;171:242–251. doi: 10.1016/j.pscychresns.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann T, Spies C. Use of biomarkers for alcohol use disorders in clinical practice. Addiction. 2003;98 (Suppl 2:81–91. doi: 10.1046/j.1359-6357.2003.00587.x. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Krueger DD, Tronson NC, Nairn AC, Taylor JR. Orbitofrontal cortex and cognitive-motivational impairments in psychostimulant addiction: evidence from experiments in the non-human primate. Ann N Y Acad Sci. 2007;1121:610–638. doi: 10.1196/annals.1401.016. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Rozen S, Mannelli P, Matson W, Pae CU, Krishnan KR, et al. Alterations in tryptophan and purine metabolism in cocaine addiction: a metabolomic study. Psychopharmacology (Berl) 2009;206:479–489. doi: 10.1007/s00213-009-1625-1. [DOI] [PubMed] [Google Scholar]
- Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. 2010How to improve R&D productivity: the pharmaceutical industry's grand challenge Nat Rev Drug Discov 9203–214.This review importantly outlines the function of key compoents of R&D productivity such as value, cost and the probability of technical success. The authors propose several specific strategies by which future work can appreciably enhance overall R&D efficiency. This significantly includes the identification of biomarkers, which can be used to optimize portfolio decision making earlier in the R&D process. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, et al. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95:219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- Phillips K, Luk A, Soor GS, Abraham JR, Leong S, Butany J. Cocaine cardiotoxicity: a review of the pathophysiology, pathology, and treatment options. Am J Cardiovasc Drugs. 2009;9:177–196. doi: 10.2165/00129784-200909030-00005. [DOI] [PubMed] [Google Scholar]
- Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suri MF, Guterman LR, Hopkins LN. Cocaine use and the likelihood of nonfatal myocardial infarction and stroke: data from the Third National Health and Nutrition Examination Survey. Circulation. 2001;103:502–506. doi: 10.1161/01.cir.103.4.502. [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Uys JD, Schwacke JH, Comte-Walters S, Rutherford-Bethard JL, Dunn TE, et al. AKAP signaling in reinstated cocaine seeking revealed by iTRAQ proteomic analysis. J Neurosci. 2011;31:5648–5658. doi: 10.1523/JNEUROSCI.3452-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson WH, Lindstrom TM, Cheung RK, Sokolove J.2013Mechanistic biomarkers for clinical decision making in rheumatic diseases Nat Rev Rheumatol 9267–276.This review importantly outlines various different types of biomarkers, and assigns value to 'actionable' vs. 'descriptive' biomarkers. 'Actionable' biomarkers are markers rooted in mechanism of disease, can be used to guide clinical decision making and are therefore of greater value, whereas 'descriptive' biomarkers are more likely to be consequential markers outside the pathological underpinnings of disease, not as useful for guiding clinical decision making, and, therefore, not as valuable. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122:2558–2569. doi: 10.1161/CIRCULATIONAHA.110.940569. [DOI] [PubMed] [Google Scholar]
- Sharma J, Dougherty AH, Schmitz JM, Moeller FG. National Institute on Drug Abuse (NIDA) P50 Grant Review Meeting, Rockville, MD, USA; 2011. Cardiovascular effects of cocaine use as a biomarker of treatment outcome in cocaine dependence. [Google Scholar]
- Sharma J, Moeller FG, Schmitz JM, Rathnayaka N, Shoham D, Dougherty AH.(eds) (2012Chronic cocaine abuse is associated with substantial bradycardia American College of Cariology: Dallas, TX, USA [Google Scholar]
- Skolnick P, Volkow ND. Addiction therapeutics: obstacles and opportunities. Biol Psychiatry. 2012;72:890–891. doi: 10.1016/j.biopsych.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares HD. The use of mechanistic biomarkers for evaluating investigational CNS compounds in early drug development. Curr Opin Investig Drugs. 2010;11:795–801. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Statistics and Quality . The DAWN Report: Outcomes of Drug-Related Emergency Department Visits Associated with Polydrug Use. Rockville, MD; 2012. [Google Scholar]
- Tang WX, Fasulo WH, Mash DC, Hemby SE. Molecular profiling of midbrain dopamine regions in cocaine overdose victims. J Neurochem. 2003;85:911–924. doi: 10.1046/j.1471-4159.2003.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannu NS, Howell LL, Hemby SE. Integrative proteomic analysis of the nucleus accumbens in rhesus monkeys following cocaine self-administration. Mol Psychiatry. 2010;15:185–203. doi: 10.1038/mp.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, et al. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 2007;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Skolnick P.2012New medications for substance use disorders: challenges and opportunities Neuropsychopharmacology 37290–292.This review importantly highlights the opportunities for (e.g., unmet medical need for several SUDs, substantial market for several SUDs, advancing formulation technology) as well as hurdles that must be dealt with (e.g., resources required for development, regulatory obsticles, inconsistent medical care) in order to bring novel therapies for substance use disorders to the marketplace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Logan J, Schlyer D, Hitzemann R, et al. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16:255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2012;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchell C, Rappaport BA, Roca R, Rosebraugh CJ.2012Reanalysis of methamphetamine dependence treatment trial CNS Neurosci Ther 18367–368.This paper discusses the potential pitfalls of statistical significance for urine drug screen outcomes as opposed to clinical significance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Tauscher J, Grunder G.2009The role of imaging in proof of concept for CNS drug discovery and development Neuropsychopharmacology 34187–203.This publication highlights the potential role of neuroimaging in medication development. [DOI] [PubMed] [Google Scholar]
- Woodcock J. Assessing the clinical utility of diagnostics used in drug therapy. Clin Pharmacol Ther. 2010;88:765–773. doi: 10.1038/clpt.2010.230. [DOI] [PubMed] [Google Scholar]
- Xu J, DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35:1541–1549. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Levran O, Proudnikov D, Nielsen DA, Kreek MJ. Search for genetic markers and functional variants involved in the development of opiate and cocaine addiction and treatment. Ann NY Acad Sci. 2010;1187:184–207. doi: 10.1111/j.1749-6632.2009.05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Nielsen D, Butelman E, Kreek MJ. Microarray studies of psychostimulant-induced changes in gene expression. Addict Biol. 2005;10:101–118. doi: 10.1080/13556210412331308976. [DOI] [PubMed] [Google Scholar]
- Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T, et al. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet. 2001;68:515–522. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]