We recently reported that damaging de novo mutations in persons with schizophrenia from otherwise healthy families disrupt genes that orchestrate neurogenesis in fetal prefrontal cortex (Gulsuner et al, 2013). By sequencing genomic DNA from entire families, we identified point mutations and copy number variants that appeared de novo in 105 persons with schizophrenia and in 84 of their healthy siblings. Patients were more likely than unaffected siblings to harbor damaging de novo mutations (47/105 vs 30/84, OR=1.91, X2=4.45; p=0.035). The proportion of schizophrenia attributable to damaging de novo mutations in this sample was 21%.
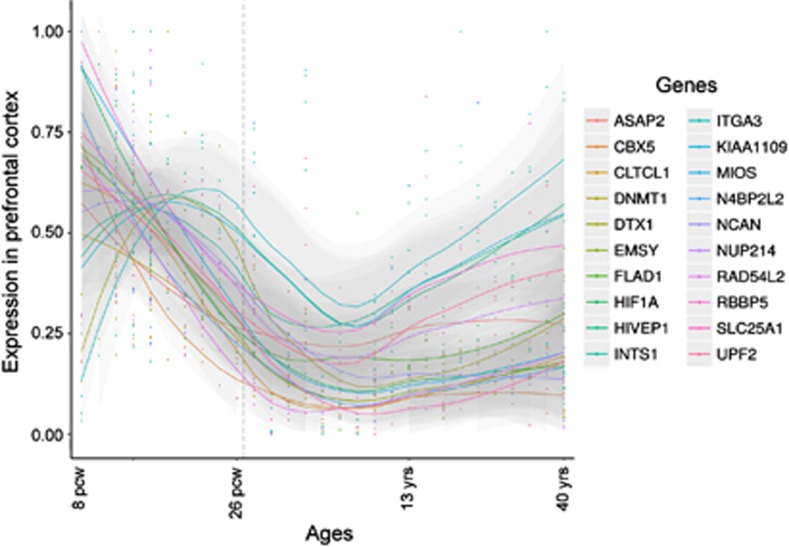
Even more striking than the difference in mutation frequencies was the distinctive functional relationship among the genes harboring mutations. The genes disrupted by damaging de novo mutations in patients formed a network defined by protein interaction (Mostafavi et al, 2008) and by transcriptional co-expression (BrainSpan: Atlas of the Developing Human Brain, 2013) in the dorsolateral and ventrolateral prefrontal cortex during fetal development. Interactions among these genes were not significantly enriched in other brain regions or during other developmental periods, nor were interactions enriched among genes harboring benign de novo events. Of the 54 genes disrupted by damaging de novo mutations in patients, 50 mapped to this network. These genes are active in pathways critical to neurogenesis, including neuronal migration, synaptic transmission, signaling, transcriptional regulation, and transport. Many network genes share a pattern of high expression in prefrontal cortex during the early fetal development, with transcription levels declining in the late fetal development and childhood only to rise again during young adulthood (Figure 1), coinciding with the typical age of onset of the illness.
Figure 1.
Expression levels of selected genes with damaging de novo mutations in dorsolateral prefrontal cortex throughout development (8 post-conception weeks to 40 years). Many network genes were highly expressed during early fetal prefrontal cortical development, with expression declining in late fetal development and childhood, and rising again during adulthood, coinciding with the typical age of onset of schizophrenia. Data were obtained from BrainSpan: Atlas of the Developing Human Brain (2013). RPKM values were max-min normalized and Loess smoothed across time points.
The results suggest that aberrant prefrontal cortical development is critical to the pathogenesis of schizophrenia. The prefrontal cortex is highly interconnected with other brain regions, and organizes information to coordinate executive skills, including working memory, attention span, problem solving, and self-regulation (Catts et al, 2013). Given the clearinghouse role of prefrontal cortex in brain function, disruptions in this region during early development likely result in the overall loss of neocortical integrity and connectivity. Impairments in executive functions are characteristic of schizophrenia, suggesting that higher-order cognitive deficits are early manifestations of the syndrome, with psychosis emerging as critical brain regions mature.
Some genes with de novo mutations in patients function in neurotransmitter pathways that suggest possible avenues for treatment, including GLS (glutamate synthesis), ADCY9 (glutamate and GABA signaling), and SLC18A2 (serotonin, dopamine, norepinephrine, epinephrine, and histamine transport). CACNA1I, a brain-specific, T-type calcium channel that regulates neuronal firing in the thalamus, striatum, nucleus accumbens, and prefrontal cortex, was the only gene disrupted in two unrelated probands, each harboring a different de novo event. Some antipsychotic medications, including clozapine, inhibit T-type calcium channels (Choi and Rhim, 2010).
Key mechanisms underlying complex psychiatric disorders can be identified by characterizing brain pathways disrupted by mutant genes in affected persons. By integrating genomic analyses with brain mapping strategies, we were able to define possible disease-related processes and to identify potential targets for treatment. Applying the same approach in large-scale sequencing studies will help elucidate basic neurobiological mechanisms underlying normal brain circuitry and neuropsychiatric disease.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This work was supported by NIMH grants R01MH083989, R01MH083849, R01MH083756, R01MH084071, and R03TW008696.
References
- BrainSpan: Atlas of the Developing Human Brain (2013) ). www.brainspan.org .
- Catts VS, Fung SJ, Long LE, Joshi D, Vercammen A, Allen KM, et al. Rethinking schizophrenia in the context of normal neurodevelopment. Front Cell Neurosci. 2013;7:60. doi: 10.3389/fncel.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Rhim H. Inhibition of recombinant Ca(v)3.1 (alpha1G) T-type calcium channels by the antipsychotic drug clozapine. Eur J Pharmacol. 2010;626:123–130. doi: 10.1016/j.ejphar.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, Casadei S, et al. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell. 2013;154:518–529. doi: 10.1016/j.cell.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi S, Ray D, Warde-Farley D, Grouios C, Morris Q. GeneMANIA: a real-time multiple association network integration algorithm for predicting gene function. Genome Biol. 2008;9 (Suppl 1:S4. doi: 10.1186/gb-2008-9-s1-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]