Abstract
Low socioeconomic status (SES) background has been identified as a risk for several mental disorders. However evidence regarding SES and the developmental course of personality disorder (PD) has not been addressed. Nor is it clear whether an SES relationship to PD symptom course may be attributable to known associated risks. Further, specificity of such relationships to a particular PD diagnostic pattern independent of comorbidity with other PD or with depression has not been investigated. Data are from a general population studied longitudinally between ages 10 and 36 in four assessment waves. Effects of SES-associated risks on the level of symptoms of schizotypal and borderline disorders are estimated and compared to effects on depressive symptoms. Low family SES had robust modest independent effects on both PDs over the entire age span despite substantial cumulative effects of trauma history, stressful recent life events, IQ, poor parenting, and comorbid symptoms. SES effects on depressive symptoms were generally absent, but a small “protective” effect of low SES appeared when comorbidity with PD symptoms was taken into account. Cumulatively, these risks account for developmental failures of substantial magnitude and consequence, marking the importance of understanding the remaining mechanisms of SES effects and programmatic implications for minimizing associated risk.
There is a growing appreciation that maladaptive personality traits and symptoms reflect a long-term risk for dysfunction (Caspi, Roberts, & Shiner, 2005) including, at the extreme, personality problems sufficient to be defined as psychiatric disorders. Current evidence suggests that these disorders may account for a substantial fraction of psychiatric disability, including that often attributed to other comorbid disorders (Shea, Glass, Pilkonis, Watkins, & Docherty, 1987; Zanarini, Frankenburg, Hennen, & Silk, 2003). Two personality disorders (PDs) with serious negative long-term prognoses are schizotypal PD and borderline PD (Carpenter & Gunderson, 1977; Johnson et al., 2000; Lenzenweger & Cicchetti, 2005). Thus, these syndromes have relative prominence in the clinical and research literature.
Schizotypal PD is a spectrum aspect of schizophrenia defined by symptoms prevalent in relatives of schizophrenia patients, including interpersonal wariness and paranoia and odd beliefs or experiences. Borderline PD is a problematic syndrome of affective dyscontrol and impulsivity, well known to clinicians because of high levels of treatment seeking often characterized by self-harm threats and behaviors. Relatively few studies of these disorders employ prospectively assessed childhood risks other than sexual abuse or other maltreatment (Johnson, Bromley, & McGeoch, 2005). The widespread co-occurrence of symptoms, both among the 10 systematically defined PDs and between the PDs and Axis I disorders such as depression and anxiety disorders, further complicates our understanding of the development of these disorders.
Family of Origin Socioeconomic Status (SES)
Past research has shown that low socioeconomic class (low income, low education level, low status occupation) is a risk for a whole range of physical and mental disorders in adults (Adler, Marmot, McEwan, & Stewart, 1999; Brown & Harris, 1978; Dohrenwend & Dohrenwend, 1969; Hollingshead & Redlich, 1958) and in their offspring (Black & Krishnakumar, 1998; Bradley & Corwyn, 2002; Miech, Caspi, Moffitt, Wright, & Silva, 1999; Rank, 2000; Timms, 1996). Costello, Compton, Keeler, and Angold (2003) have shown that increased family income not attributable to individual or family characteristics were associated with decline in psychiatric problems in offspring in poor families. Torgersen, Kringlen, and Cramer (2001) show that low personal SES is associated with increased risk of PD in adults. Data from the Children in the Community (CIC) study demonstrated that parental low SES was a risk for adolescent offspring PD (Johnson, Cohen, Dohrenwend, Link, & Brook, 1999), which in turn, tended to lead to poorer adult attainment. A long history of research showing the elevated risk of adult antisocial PD among the offspring of families in poverty has established this relationship (Brandt, 2006; Pagani, Boulerice, Vitaro, & Tremblay, 1999; Petras et al, 2004). The current study examined the developmental course of disorders from early adolescence to adulthood. Because antisocial PD is not defined until adulthood, we did not include it in our analyses.
The general consistency of these findings of apparent environmental effects on offspring mental disorder is in stark contrast to past small to nil estimated influence of shared environment (including parental SES) in twin studies of disorders and personality problems. Fortunately, recent appreciation of the limits of the usual analytic models for these studies has revived the scientific respectability of studies of environmental effects (Moffitt, Caspi, & Rutter, 2006) and promoted an interest in further knowledge of the environmental conditions in which psychopathology-related genes may or may not be expressed (Boomsma, de Geus, van Ball, & Koopmans, 1999; Button, Scourfield, Martin, Purcell, & McGuffin, 2005; Caspi et al., 2003; Jang, Dick, Wolf, Livesley, Paris, 2005; Jang, Wolf, Larstone, 2006; Moffitt, 2005). Thus, it is increasingly appreciated that apparent environmental and genetic risks often show relationships with negative outcomes such as mental disorders that are mutually conditional (Caspi et al., 2002; Eley et al., 2004; Rutter, 2005). Some environmental risks have little or no association with disorder outcomes in the absence of genetic risk (or, as may be appropriately equivalently interpreted, apparent genetic risk has little or no negative impact in the absence of relevant environmental risk).
Potential SES Mediators
Mechanisms of the parent SES/offspring psychopathology relationship are likely to include general risks that characterize low SES families more frequently, even in developed countries (Entwisle, Alexander, & Olson, 1997; Paris, 1996; Rutter & Maughan, 1997). Some of these risks may be traumatic in that they involve extreme negative exposures including violence, death, other loss of close family members, or extreme parental problems such as arrest or alcohol or drug dependence (e.g., Parke & Clarke-Stewart, 2003). Parental neglect or other maltreatment and otherwise seriously problematic parenting such as lack of adequate monitoring and supervision also are more frequent in low SES families (Serbin & Karp, 2004). Low-income children may also be exposed to a range of environmental toxins that may affect behavior directly or indirectly (Schell, 1997).
Increased risk of maladaptive personality patterns may also be attributable to a whole range of lesser stressful experiences or fewer positive experiences that characterize the lives of both parents and offspring in lower SES families (Rank, 2000). These include a higher than average level of stressful life events (SLEs) experienced over any given time interval. These events may not have as long-lasting effects as more extreme trauma, but may nevertheless lead to temporary increases in symptoms. There are yet additional candidates: the inadequacy of school or other community resources for the development of skills, social attachments, and positive world frames may partly account for these associations with family SES, the mechanisms of which are likely to vary over time and place (Link & Phelan, 1995).
Unlike studies of the relationship between socioeconomic status and mental disorder in adults (Dohrenwend et al., 1992), the issue of direction of causation does not arise in children: parental SES is not generally attributable to offspring problems. Nevertheless associations between parental SES and offspring mental disorder may be because of shared causes. These would include partially heritable traits that influence SES such as intelligence (Gottfredson, 2004), aspects of personality (Caspi et al., 2005; Johnson et al., 2005; Kohn, 1995), or parental psychopathology (Serbin & Karp, 2004). The major parental disorders that have been studied with regard to both SES and offspring problems have been depression and antisocial PD. With regard to these disorders, a frequent focus has been on parenting as a potential mediator of intergenerational transmission, with less attention to the potential role of parental SES per se (McLeod & Shanahan, 1993).
Investigation of Impact on the Trajectories of PD Symptoms
In the analyses reported here we examine the direct effects of family SES on the level of schizotypal and borderline PD symptoms as they change over four assessments beginning as young as age 9 and ending as old as age 38. Effects of SES mediated by offspring IQ, cumulative trauma, problematic parenting, and recent SLEs are also reported. Thus, available data permit estimation of the potentially changing impact of these risks on the developmental course of symptoms over this segment of the life course. A recent review suggested that socioeconomic position, reflected in income and assets, may be a more critical influence on health and mental health than SES, at least in adulthood (Muntaner, Eaton, Miech, & O’Campo, 2004). To check on this question we also examined the effects of family poverty on offspring symptoms. Of course, family poverty and family SES are substantially correlated (r = .52 in our sample). Therefore, the central analyses report the stronger associations of SES with symptoms. However, the discussion reviews the consequences of including a consideration of family poverty during the offspring’s childhood in these models.
To clarify the meaning of these findings we compare the associations of these risks for elevated symptoms of two PDs, schizotypal and borderline PD, with their associations with depressive symptoms. In addition, we determine the extent to which any similarity in associations with these contextual risks may be because of comorbidity among symptoms of these different disorders. Prior work (e.g., Kessler, Davis, & Kendler, 1997) makes it clear that unambiguous attribution of adversities as risks for psychiatric disorders need to take comorbidity into account: adversity may operate via an increased risk of one disorder, which in turn, influences the probability of another.
Given this context of prior literature and theory, we hypothesize that SES of family of origin will be a risk for both PD and depressive symptoms. There is accumulated evidence that personality problems have a negative effect on attainment of higher educational levels, occupational status, and income (Caspi, Wright, Moffitt, & Silva, 1998; Judge, Higgins, Thoreson, & Barrick, 1999). These personality traits of lower SES parents may be passed on to offspring by modeling, genetic, or epigenetic mechanisms. As noted, lower SES parents may practice less optimal parenting practices, or may have a greater vulnerability to, or less ability to avoid or overcome other environmental risks for their offspring.
We also hypothesize that regardless of disorder, family of origin SES effects will diminish somewhat as the cohort moves from adolescence to the transition to adulthood, when effects of youths’ own evolving attainment may be much more salient. Thus, we predict an interaction of parental SES with offspring symptomatic trajectory, reflecting this diminishing influence.
Other potential mediators of the parental SES effect will be added to the model. One time-varying potential mediator is exposure to serious trauma, including childhood neglect or abuse or parental loss. Previous research has shown that these experiences may lead to long-lasting distortion of the individual’s understanding and expectations of the world around him/her and consequent maladaptive behavior patterns. Recent SLEs have consistently been shown to relate to higher levels of depressive symptoms. There is also evidence that SLEs “can trigger the behavioral expression of vulnerability” (to schizophrenia or schizophrenia-spectrum problems; Walker, Kestler, Bollini, & Hochman, 2004, p. 401). In large part, PDs reflect relatively stable personality vulnerabilities despite symptomatic fluctuation over time and general decline in symptoms in young people as they mature (Johnson, Cohen, Kasen, Skodol, & Brook, 2000; Lenzenweger & Castro). In keeping with this perspective we hypothesize that SLE effects on PD symptom levels will be less marked than those on depressive symptoms, particularly for schizotypal symptoms that have not been traditionally viewed as particularly vulnerable to SLEs. For both PDs, effects of SLEs may be entirely attributable to co-morbidity of PD with depression. SLE effects on depression may increase with age, as failure to avoid or escape such problems leads to loss of hope and diminished ability to cope (agency), both symptoms of depression. Early SLEs include family problems for which the adolescent may assume less responsibility and identify less, and be less likely to generalize to expectations for the future. If so, we anticipate an increasing association between time-varying (current) SLEs and symptoms.
As noted earlier, problematic parenting has also been shown to be more prevalent in lower SES families, and is therefore another potential mediator of SES effects. Finally, the mediating or risk modifying effects of offspring verbal IQ will also be examined as potentially accounting for parental SES effects on offspring symptoms.
Current Study
In sum, the questions to be addressed in these analyses include the following:
What is the magnitude and duration of the impact of family of origin SES on the level and trajectory of symptoms of borderline and schizotypal personality disorder? It is hypothesized that the SES impact will decline as offspring enter adulthood.
How do these estimates of magnitude and duration of SES compare with those on depressive symptoms of offspring?
Are these associations of SES with PD or depressive symptom trajectories attributable to known risks associated with SES, including history of child abuse or cumulative trauma, problematic parenting, or recent negative life events?
Are these associations of SES with PD or depressive symptom trajectories attributable to low IQ?
Are the associations of SES with symptoms attributable to comorbidity with symptoms of the other disorders?
Method
Sample
CIC study participants were members of a cohort of children born between 1965 and 1974 and first assessed for mental disorders in 1983. The sample was based on a random residence-based cohort of children between the ages of 1 and 10 originally drawn from 100 neighborhoods in two upstate New York counties in 1975. In the first follow-up in 1983 the located sample was supplemented with a newly drawn sample in urban poverty areas in the same counties to replace those lost to follow-up because of neighborhood obliteration following urban renewal. The original sample was drawn approximately 50% from urban areas, including a substantial number from a central city, about 25% suburban, and about 25% rural or small town. Families were closely representative of the sampled area and generally of families in the northeastern United States with regard to socioeconomic status and most demographic variables. Of course, substantial geographic mobility characterized this sample as is generally true of American families with young children. Young adult participants currently live in more than 35 different states and foreign countries.
As in the sampled region, 54% were Catholic and more than 40% were Protestant by maternal report. Ninety-one percent were Caucasian and 8% had at least one African American parent. The sample included a small number of Native American and Asian background children. Mothers and children were interviewed in their homes by trained lay interviewers in 1983 (778 families), 1986 (776 families, including 34 newly located families from the 1975 cohort), and 1991–1994 (776 families), at mean offspring ages 13.7 (SD = 2.6), 16.1 (SD = 2.8), and 22.0 (SD = 2.7), respectively. Young adults were interviewed again between 2002 and 2005 (n = 680, mean age 33.2, SD = 2.9). The sole significant predictor of participation failure other than offspring death (n = 13), refusal for any further participation (n = 4), or failure to locate (n = 26 in the most recent assessment), is male gender. This report is based on all 787 participants for whom there were self-reported data on personality disorder from at least two assessments. Written informed consent was obtained from all participants prior to each assessment. The study procedures were carried out in accordance with appropriate institutional guidelines and have been approved by the institutional review board of the New York State Psychiatric Institute.
Measures
Symptoms
Personality disorder symptoms in adolescence were assessed using information from both parent and child, and in adulthood from the offspring only. In these analyses, which use repeated measures of symptoms assessed over a 20-year period as the dependent variables, constancy of measurement is required. For this reason, measures of symptoms of borderline and schizotypal PDs as reported by the offspring only employ the same questions for each of the four assessments. There were multiple questions asked for each symptom; thus, these measures tend to avoid the extreme distribution problems characterizing dichotomous single items. These self-report measures correlate substantially with the combined informant assessments, for which considerable evidence regarding validity is available (Cohen, Crawford, Johnson, & Kasen, 2005; Crawford et al., 2005). In the most recent adult assessment these symptom scales also correlated fairly well with the screening measures for the Structured Clinical Interview for Diagnosis—II (First, Gibbon, Spitzer, Williams, & Benjamin, 1997) for schizotypal (.49) and borderline disorders (.74). Depressive symptoms were assessed with items covering most DSM depression criteria adapted from the System Checklist—90 (Derogotis, Lipman, Rickels, Uhlenhuth, & Covi, 1974). Responses to these questions were on a Lickert scale of occurrence frequency over the preceding 2 years. This measure of depressive symptoms was used in preference to a measure based on the diagnostic assessment because the wording of the latter changed as necessary to the changing ages of the youth and the employment of a clinical diagnosis in the most recent assessment. The scale reflects symptoms of dysthymia rather than major depressive disorder, which would require a definable depressive episode. Internal consistency reliability was .68 in early adolescence and increased steadily with age to .85 in the most recent assessment.
See Table 1 for means and standard deviations of symptom and other measures at each assessment. Of particular note is the decline with age of symptom means of both personality disorders as well as the relative stability in their standard deviations. In contrast, depressive symptoms were slightly higher at mean age 22, with a substantial increase in standard deviation by mean age 33.
Table 1.
Means of time-varying study measures
| Wave 1983 Mean (SD) |
Wave 1986 Mean (SD) |
Wave 1992 Mean (SD) |
Wave 2003 Mean (SD) |
|
|---|---|---|---|---|
| Age | 13.74 (2.56) | 16.14 (2.76) | 22.04 (2.72) | 33.14 (2.90) |
| Cumulative trauma | 0.71 (1.24) | 0.90 (1.42) | 1.07 (1.55) | 1.28 (1.72) |
| Schizotypal symptoms | 19.60 (9.19) | 16.80 (7.67) | 15.74 (7.69) | 14.66 (8.47) |
| Borderline symptoms | 26.02 (11.89) | 24.96 (10.52) | 23.45 (11.11) | 18.82 (11.22) |
| Depressive symptoms | 5.44 (3.39) | 5.23 (3.26) | 5.47 (3.57) | 5.11 (5.93) |
Risks
Family SES was measured as a standardized sum of standardized measures of father’s educational level, mother’s educational level, family income, father’s occupational status, and mother’s occupational status (if employed). Although many studies use only one or two of these measures, a history of research on SES indicates that the best measure combines these components (Hauser, 1994; White, 1982). All were mother reported and extremely stable over the adolescent assessments. Occupational status was scored in keeping with the Hollingshead and Redlich (1958) status ranking of American occupations.
Cumulative trauma included reported history of child abuse or neglect, parental alcohol or substance abuse or dependence, parental arrest/imprisonment, parental death, death of a spouse, death of a child, army combat experience, close personal exposure to violent death, or family suicide. These reports were culled from a number of sources for each participant, including official records, maternal report, family history interview of mother, and participant report. As implied by the variable name, these are the experiences that previous literature has identified as most likely to have long-lasting negative impact. Incidence was accumulated over the assessed years and employed as a time-varying covariate. Thus, if an individual experienced the death of a parent in early childhood and no other defined traumatic event until adult combat experience, his or her trauma score would be 1 at each assessment point until the age of combat experience, when it would rise to 2.
SLEs were reported by the youth for the period (usually 1 year) prior to each assessment. The exception was the initial (mean age 13) assessment in which a life event (LE) schedule was not employed. Relevant items from this assessment were combined as reported by mother and/or youth, including parental fighting, family loss of income, separation from a parent, loss of a close friend, suspension or expulsion from school, and death of a family member. In subsequent youth assessments an adaptation of Coddington’s (1972) life event schedule for children was used. The adult assessment employed the SLE measure developed for the National Comorbidity Study (Kessler et al., 1997). To make these somewhat different measures comparable we have standardized them at each assessment point so that effects are measured for stressful event levels compared to age peers.
IQ was assessed by the Quick Test Picture Vocabulary Measure (Ammons & Ammons, 1962), for which Forms A and B were administered in 1983 and 1986, respectively. Scores were averaged to maximize long-term validity.
Measures of poor parenting by mothers and by fathers were created from the extensive measures reported by participants and their mothers in the first two assessments. To maximize the discrimination of “stylistic” differences in parent behavior from poor parenting as such, extreme responses to scale items were counted in these measures (e.g., mother is never easy to talk to, never wants me to tell her just how I feel about things, or almost never talks with child for as long as 15 min). Maternal poor parenting was a count of extremes on punishment, inconsistent discipline, little supervision, verbal abuse, high possessiveness, control through guilt, maternal anger/hostility, poor nurturing/ support, and little communication. Poor parenting by father summed extremes on low contact, little availability, little communication, fights with mother, does not help mother with family, little affection toward child, not seen as an admirable father by offspring. When the biological father did not live with the child, father was defined as stepfather or other man acting as a father figure. In the absence of an alternative father figure the biological father was the subject of these questions although not living with the child.
Analysis
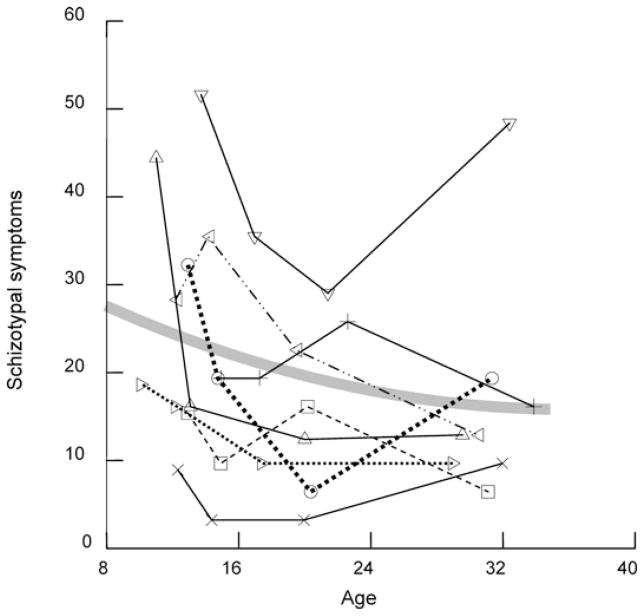
The multilevel developmental change analyses were carried out using a SAS PROC MIXED program that permits assessment of the variance of individual differences in symptom means (estimated at a constant age for all participants), linear age change (slopes), and individual variation around their own means and slopes. The basic “random” model estimates these variance components across the study participants and these constitute the dependent variables in the subsequent analyses. A clear idea of the nature of the dependent variables in these data is most easily obtained by an examination of the raw data over the four assessments of a subsample of this cohort (Figure 1). Each segmented line in this figure connects schizotypal symptom level at each of the four specific assessment ages of an individual over this 20-year period. The curved line graphs the model for the whole sample. As can be seen, although the symptoms declined with age for the cohort as a whole (Table 1), individual trajectories (average annual change) varied tremendously. Just as the sample mean in an ordinary regression analysis is a poor description of scores for many individuals, the average developmental course is unlikely to be well approximated by any single study participant.
Figure 1.
The trajectories of schizotypal symptoms for eight study participants and the estimated growth curve for the entire sample.
To this basic model in which average symptoms decline with age, the subsequent multilevel regression models add predictors of average symptoms (estimated at age 22 for all participants) and age trajectories (linear and quadratic functions). Added predictors begin with participant gender and family SES, continuing with other predictors (IQ and poor parenting) that are represented by single values for each participant, followed by predictors that may change at each assessment (cumulative trauma, SLEs, and eventually symptoms of other disorders). Interactions among these variables were tested as a check on the assumption of the absence of such effects, as was conditionality of the magnitude of these effects on the age of the participant. The predictor assessing curvilinearity in age trajectories was retained to permit examination of conditionality of curvilinear age changes on other predictors. Interactions not specifically hypothesized that were statistically significant (p <.01) were retained in subsequent models and the remainder are considered noninformative and are not reported here. However, the Δχ2 test of improvement of model fit to the data includes the degrees of freedom associated with these assumption tests.
The second set of predictors includes time-varying cumulative trauma exposure, recent SLEs, and the conditionality of these effects on age (interactions with age). Again, interactions of these with the first set of fixed predictors (gender, SES) were examined to determine whether the assumption of nonconditionality of effects was warranted. The third model added IQ, poor mothering, and poor fathering to the predictors, again checking for changes in effects as participants aged. The final set of predictors included the symptoms of the other disorders (PD and depression). Again, interactions with prior independent variables were tested to ensure that the assumption of nonconditionality was warranted.
Findings
Schizotypal PD symptoms
Findings from the model including only age, gender, and SES as fixed predictors are shown in the Model 1 column of Table 2 where random effects indicating the significance of between-participant differences in mean symptoms and in the age associated change in symptom level are also provided. The random model shows that individual differences in linear age changes in schizotypal symptom level did not vary significantly beyond differences attributable to gender and SES effects. However, mean differences and variations around those means (residuals) varied significantly among study participants.
Table 2.
Developmental models of age changes in schizotypal symptoms
| Predictors | Model 1 Estimate (SE) |
Model 2 Estimate (SE) |
Model 3 Estimate (SE) |
Model 4 Estimate (SE) |
|---|---|---|---|---|
| Random Effects | ||||
|
| ||||
| Mean variation | 17.222 (1.627)** | 14.004 (2.335)** | 13.658 (1.449)** | 4.272 (0.996)** |
| Age changes | 0 | 0 | 0 | 0 |
| Residual | 48.720 (1.522)** | 47.981 (1.525)** | 48.156 (1.506)** | 45.654 (1.464)** |
|
| ||||
| Fixed Effects | ||||
|
| ||||
| Intercept (age 20) | 17.325 (0.307)** | 16.210 (0.335)** | 15.412 (0.359)** | 8.305 (0.416)** |
| Age | −0.485 (0.024)** | −0.520 (0.024)** | −0.513 (0.024)** | −0.393 (0.024)** |
| Age2 | 0.015 (0.002)** | 0.017 (0.002)** | 0.016 (0.002)** | 0.018 (0.002)** |
| Male (vs. female) | 1.853 (0.394)** | 2.204 (0.382)** | 2.305 (0.376)** | 2.615 (0.303)** |
| Family SES | −1.670 (0.198)** | −1.291 (0.199)** | −0.845 (0.213)** | −0.682 (0.173)** |
| SES×Age | 0.015 (0.017) | 0.022 (0.018) | 0.021 (0.017) | 0.016 (0.018) |
| Cumulative trauma | 0.787 (0.132)** | 0.606 (0.136)** | 0.307 (0.112)** | |
| SLE | 0.832 (0.163)** | 0.779 (0.162)** | 0.036 (0.160) | |
| Age×SLE | 0.055 (0.018)** | 0.065 (0.018)** | 0.025 (0.019) | |
| IQ | −0.055 (0.016)** | −0.048 (0.013)** | ||
| Poor mothering | 0.539 (0.157)** | 0.130 (0.127) | ||
| Poor fathering | 0.302 (0.131)* | 0.121 (0.106) | ||
| Borderline symptoms | 0.268 (0.017)** | |||
| Depressive symptoms | 0.292 (0.051)** | |||
| Δχ2 | 488.7 (5 df)** | 85.3 (3 df)** | 75.9 (3 df)** | 118.8 (2 df)** |
Note: SES, socioeconomic status; SLE, stressful life events.
p <.05;
p <.01.
The “fixed effect” estimates of age clearly show the decline in average reported symptoms over this age span also noted (over a shorter age span) in an earlier report (Johnson, Cohen, Kasen, et al., 2000). Schizotypal symptoms declined about 0.50 units per year, decelerating in the older years (significant quadratic effect of age). Males had mean scores over 2 units higher than females. There was a decline of nearly 2 units (B = 1.670) for each standard deviation increase in family SES. Not noted in the table, the gender difference was consistent over the age span (did not show a significant interaction with age).
Model 2 added cumulative trauma, SLEs, and the interaction of SLEs with age to the fixed predictors. A potential change in the effect of cumulative trauma with age was not statistically significant. The relationship of SLEs with schizotypal symptoms increased linearly over the age span. Together, cumulative trauma and SLEs accounted for about .25 of the effect of family SES (=1 − 1.291/1.670).
Model 3 added IQ, poor mothering, and poor fathering to the model. Each of these variables was significantly associated with higher symptoms of schizotypal PD (poor fathering only at the α < .05 level). When these predictors were included the estimated effects of family SES were reduced by 35% (=1 − 0.845/ 1.291), but retained a significant independent effect.
The final Model 4 added time-varying co-morbid symptoms of borderline PD and depression. These comorbid symptoms accounted for over two-thirds of the remaining individual differences (random effects) in mean symptoms of schizotypal PD estimated at age 20 (=1 − 4.272/13.658). The estimated average linear age decrease declined by about 20% (=1 − 0.393/0.513) when age-varying comorbidity effects were taken into account, but the greater mean symptoms of schizotypal PD in men was independent of comorbidity. The association of SLEs with schizotypal symptoms was entirely accounted for by these comorbid symptoms, although about one-half of the association of cumulative trauma was not (=1 − .307/.606). The small independent effect of lower IQ was predominantly independent of potential comorbidity mediation; however, parenting effects were not. Finally, although the effect of family SES declined to less than one-half its effect in Model 1 (=0.682/1.670), this association remained robust even when all other predictors were included.
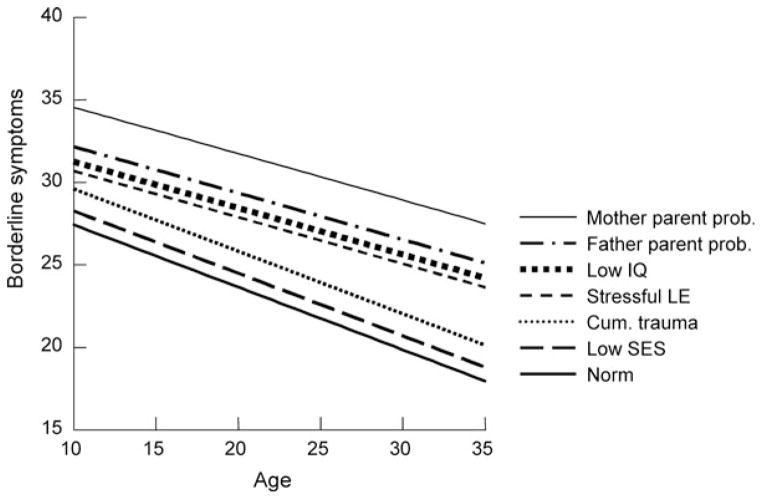
The Model 3 effects are graphed in Figure 2 where the basic trajectory is seen to substantially decline from age 10 to age 36. Associated symptom levels are graphed for males, for SES at 1 SD below the norm, for those experiencing two traumatic events above the average of 1, and at 1 SD above the average SLE. High risk is estimated at 1 SD below the IQ mean and at three domains of parenting “errors” of fathers and of mothers (compared to the average of about 1).
Figure 2.
The estimated average and high-risk developmental trajectories of schizotypal symptoms. Note: Each ascending line adds the named risk to the previous risks.
As can be seen, for the hypothetical male above the average risk on these variables, the level of symptoms at age 35 is estimated to be as substantial as was the norm (overall average) at age 15. Similarly, for that fortunate girl who was similarly below the average risk at age 15, the estimated schizotypal PD symptom level would be as low as the average 35-year-old.
Borderline PD symptoms
Findings from the initial model including only age, gender, and SES as predictors are shown in the first column of Table 3 where random effects are also provided. For borderline symptoms there was significant random variation in both means and linear age changes. The “fixed” or average age changes show the decline in reported symptoms over this age span that was strictly linear: the nonsignificant quadratic age term was included in this model to be comparable to the models for other symptoms. Females had higher average borderline symptoms than did males, and each SD increase in family SES was associated with 2.039 units lower symptom level. Again, gender and SES effects did not change significantly with age.
Table 3.
Developmental models of age changes in borderline symptoms
| Predictors | Model 1 Estimate (SE) |
Model 2 Estimate (SE) |
Model 3 Estimate (SE) |
Model 4 Estimate (SE) |
|---|---|---|---|---|
| Random Effects | ||||
|
| ||||
| Mean variation | 49.731 (3.524)** | 41.795 (3.123)** | 37.551 (2.919)** | 11.785 (1.473)** |
| Age changes | 0.074 (0.023)** | 0.049 (0.021)** | 0.050 (0.022)** | 0 |
| Residual | 64.971 (2.454)** | 64.119 (2.435)** | 64.096 (2.432)** | 49.392 (1.602)** |
|
| ||||
| Fixed Effects | ||||
|
| ||||
| Intercept (age 20) | 24.550 (0.442)** | 22.799 (0.472)** | 21.231 (0.503)** | 8.784 (0.485)** |
| Age | −0.353 (0.031)** | −0.415 (0.031)** | −0.402 (0.031)** | −0.205 (0.027)** |
| Age2 | −0.003 (0.003)** | 0.001 (0.003) | 0.001 (0.003) | 0.002 (0.003) |
| Male (vs. female) | −1.045 (0.586)** | −0.501 (0.554) | −0.470 (0.539) | −0.053 (0.374) |
| Family SES | −2.039 (0.294)** | −1.450 (0.287)** | −0.865 (0.304)** | −0.827 (0.210)** |
| Age×SES | 0.038 (0.023) | 0.044 (0.022) | 0.042 (0.022) | 0.031 (0.019) |
| Cumulative trauma | 1.088 (0.187)** | 0.736 (0.190)** | 0.362 (0.135)** | |
| SLE | 2.117 (0.200)** | 2.048 (0.200)** | 0.939 (0.172)** | |
| Age×SLE | 0.091 (0.023)** | 0.102 (0.023)** | 0.013 (0.021) | |
| IQ | −0.044 (0.023) | −0.045 (0.015)** | ||
| Poor mothering | 1.224 (0.225)** | 0.715 (0.155)** | ||
| Poor fathering | 0.505 (0.188)** | 0.174 (0.129) | ||
| Schizotypal symptoms | 0.301 (0.020)** | |||
| Depressive symptoms | 1.512 (0.048)** | |||
| Δχ2 | 280.5 (5 df)** | 175.8 (3 df)** | 93.5 (3 df)** | 189.1 (2 df)** |
p <.01.
Model 2 added time-varying cumulative trauma and SLEs to the fixed predictors, accounting for about 16% of the variance in mean symptoms estimated at age 20 (=1 − 41.795/49.731) and nearly a third of the “random” variation in linear age changes in symptoms (=1 − 0.0490/0.074). Over half of the (nonsignificant) gender difference in symptoms was also accounted for by trauma and SLEs (=1 − 0.501/1.045), as was nearly 30% of the SES effect (=1 − 1.450/2.039). The effects of SLEs increased with age.
Model 3 added IQ, poor mothering, and poor fathering to the fixed predictors. Despite the robust elevation of borderline symptoms associated with poor parenting these potential mediators had little effect on the random model estimates or most fixed predictors. However, these parenting variables did account for over 40% of the family SES effect (=1 − 0.865/ 1.450).
In the final Model 4 the comorbid time-varying symptoms of schizotypal PD and depression were added to the predictors. The magnitude of the association of schizotypal symptoms with simultaneous borderline symptoms was about equivalent to the estimated association of borderline symptoms with schizotypal symptoms (0.301 compared to 0.268; Table 2). However, the estimated independent association of depressive symptoms with comorbid symptoms of borderline PD (B = 1.512) was very much larger than the depression association with symptoms of schizotypal PD (B = 0.292; Table 2), reflecting the substantial role of emotional dysregulation in borderline disorder. In this model the individual “random” variation in means was reduced to under a quarter of its original value (=11.785/49.731), the individual differences in linear age changes were completely accounted for, and even the residual individual variation in symptom level over timewas reduced nearly a quarter (=1 − 49.392/64.971). Age effects were also reduced by half, and gender differences were entirely accounted for by comorbid symptoms. The effects of cumulative trauma and SLEs were reduced by half but remained statistically significant. A modest effect of low IQ and a more robust effect of poor mothering persisted. However, the effect of family SES, B = −.827, was only modestly lower than in the previous model and retained over 40% of its magnitude in the original model (=1 − 0.827/ 2.039).
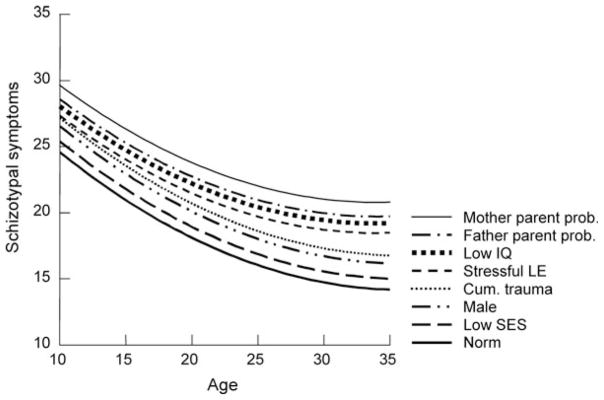
In Figure 3 we graph the cumulative effects of Model 3 risks on the course of symptoms of borderline PD. As shown here, over this age span borderline symptoms normatively declined by about one-third. The symptom difference between males and females was entirely accounted for by the differential impact of SLEs on women. Other than SLEs, the largest risk was problematic mothering. For the unfortunate person with elevation on all risks the estimated symptom level at age 35 was about equivalent to the normative 10-year-old. Equivalently, for the hypothetical boy whose life avoided all risks, the estimated borderline symptoms at age 10 would be at the same level as the norm for those 25 years older.
Figure 3.
The estimated average and high-risk developmental trajectories of borderline symptoms. Note: Each ascending line adds the named risk to the previous risks. The nonsignificant effect of gender is omitted here.
Depressive symptoms
In the initial model of depressive symptoms (Table 4) we see that there was significant but modest variation in linear change in depressive symptoms (random model), but that the overall linear change in symptoms with age over these two decades was not statistically significant. Rather, symptoms of depression rose from early adolescence to early 20s and fell (modestly) thereafter (Bagesq = −.005). Symptoms were, on average, higher in females: the overall age pattern did not differ by gender. Family SES was not significantly related to depressive symptoms.
Table 4.
Developmental models of age changes in depressive symptoms
| Predictors | Model 1 Estimate (SE) |
Model 2 Estimate (SE) |
Model 3 Estimate (SE) |
Model 4 Estimate (SE) |
|---|---|---|---|---|
| Random Effects | ||||
|
| ||||
| Mean variation | 3.584 (0.305)** | 3.167 (0.283)** | 2.964 (0.272)** | 1.000 (0.148)** |
| Age changes | 0.010 (0.003)** | 0.005 (0.003) | 0.005 (0.003) | 0 |
| Residual | 7.769 (0.289)** | 7.740 (0.288)** | 7.719 (0.287)** | 5.906 (0.189)** |
|
| ||||
| Fixed Effects | ||||
|
| ||||
| Intercept (age 20) | 5.858 (0.132)** | 5.521 (0.145)** | 5.199 (0.156)** | 0.811 (0.169)** |
| Age | −0.016 (0.011) | −0.041 (0.012)** | −0.039 (0.012)** | 0.050 (0.010)** |
| Age2 | −0.005 (0.001)** | −0.005 (0.001)** | −0.005 (0.001)** | −0.005 (0.001)** |
| Male (vs. female) | −0.777 (0.172)** | −0.627 (0.166)** | −0.675 (0.165)** | −0.698 (0.120)** |
| Family SES | −0.012 (0.086) | 0.091 (0.086) | 0.086 (0.093) | 0.286 (0.068)** |
| Age×SES | −0.007 (0.008) | 0.002 (0.008) | 0.001 (0.008) | −0.005 (0.007) |
| Cumulative trauma | 0.191 (0.058)** | 0.099 (0.060) | −0.054 (0.045) | |
| Age×Cumul. Trauma | 0.016 (0.006)** | 0.017 (0.006)** | 0.015 (0.005)** | |
| SLE | 0.795 (0.092)** | 0.771 (0.092)** | 0.450 (0.082)** | |
| Age×SLE | 0.035 (0.009)** | 0.037 (0.009)** | 0.022 (0.007)** | |
| Sex×SLE | −0.456 (0.126)** | −0.451 (0.125)** | −0.382 (0.112)** | |
| IQ | 0.014 (0.007) | 0.023 (0.005)** | ||
| Poor mothering | 0.231 (0.069)** | −0.009 (0.050) | ||
| Poor fathering | 0.157 (0.057)** | 0.055 (0.042) | ||
| Schizotypal symptoms | 0.039 (0.007)** | |||
| Borderline symptoms | 0.176 (0.006)** | |||
| Δχ2 | 75.8 (5 df)** | 143.0 (5 df)** | 53.4 (3 df)** | 134.3 (2 df)** |
p <.01.
The next model included several robust correlates of depression. Cumulative trauma was a reliable predictor, and its effects increased with age. SLEs were also an independent powerful predictor, especially in women. (When the gender interaction is in the model the main effect is that of women and the interaction reflects the gender difference.) The increase in depressive symptoms per SLE standard deviation was less than half as large for men (0.795 − 0.456 = 0.339) as for women (0.795). For both men and women the effect of SLEs on depressive symptoms tended to increase with age. Family SES effects remained nil.
The third model included IQ, which was not a significant correlate of depression, as well as poor mothering, and poor fathering; both were associated with higher depressive symptoms. These variables accounted for about one half of the effect of cumulative trauma (=0.099/0.191). Other estimated effects were little affected.
The final model of depressive symptoms included time-varying symptoms of schizotypal and borderline PDs, each of which was independently positively related to depressive symptoms in both men and women. The remaining individual variation in mean depression symptoms was much reduced in this model; to about a third of the variance in the previous random model (1.000 compared to 2.964). Thus symptoms of these two PDs accounted for about two-thirds of the variance in depressive symptoms not associated with the other model predictors and brought the estimated mean of depressive symptoms close (0.811) to the minimum possible score (0) if all risks were absent, especially low symptoms of PD.
Including PD symptoms as time-varying covariates affected the effect magnitude of several other predictors. Effects of SLEs and the increase in these effects with age were both reduced. These comorbid symptoms entirely accounted for the previously significant associations of poor parenting with depressive symptoms. In addition, taking these PD symptoms into account, more depressive symptoms characterized those with higher IQ. Finally, taking into account the level of schizotypal and borderline symptoms, depressive symptoms were significantly higher in offspring of higher SES families.
Discussion
Necessarily, there are limitations of these data. All of the measures employed in these analyses, including symptom assessment, were fully structured and administered by trained lay interviewers. Predictive variables except child abuse records are reported by mother and/or offspring and not based on observations or administered tests except for the measure of IQ. Causal conclusions regarding comorbidity effects must be inhibited by the simultaneity of measures of all disorder symptoms.
Strengths of the study include the stability of sample participation, its diversity in terms of SES and urban, suburban, and rural residence, as well as the extensive period over which these repeated assessments were made.
The influence of parental SES
In these analyses we have shown that there was an association between parental socioeconomic status and offspring symptoms of both borderline and schizotypal PDs that persisted without decline in magnitude from early teenage years over at least the next 25 years. Thus, our expectation of decline in SES impact over this substantial interval was not supported. Each standardized unit decrease in SES predicted a 0.20 SD increase in schizotypal PD symptoms and 0.18 SD increase in borderline PD symptoms. A fraction of these relationships were accounted for by greater exposure to trauma, by ongoing increased levels of stressful life experiences, problematic parenting, and by somewhat lower mean verbal intelligence. Nevertheless, 40 to 50% of the SES effect remained net of these potential mediators (51% on schizotypal PD symptoms, 42% on borderline PD symptoms). We also examined interactive/ conditional effects of risks that were elevated among offspring from lower SES homes as suggested by Paris (1996), but failed to identify any strong enough to emerge in these analyses. However, in analyses not noted above, the effect of stressful life experiences on symptoms of schizotypal disorder were less powerful in youth with higher measured verbal intelligence. Nevertheless, inclusion of this conditional effect in the model had no more than a trivial effect on the estimate of the SES association with schizotypal symptoms. We also examined the effect of inclusion of family poverty as a fixed predictor. As anticipated, in some analyses the correlation between this variable and SES resulted in a nonsignificant estimated effect for each. However, other analyses of PD symptoms showed an interactive effect such that the highest mean symptoms were manifest in offspring from families with both low SES and having income below the poverty level.
The SES effects on PD symptoms were robust and consistent in magnitude over this large age range even when comorbid symptoms of other PD and depression were taken into account. Although the estimated SES effect on schizotypal symptoms was reduced by 18%, the SES association with borderline symptoms remained surprisingly stable. In contrast, when symptoms of these personality disorders were entered in the analysis of depressive symptoms the SES effect went from nil to significantly positive. Thus, these findings suggest that depressive symptoms would be more prevalent among offspring of higher SES families if they had as many personality problems and other risks as offspring of lower SES families. Analyses in which we included family poverty as a predictor of offspring depressive symptoms did not show significant effects of poverty on offspring depression or substantive changes in the estimated effects of other predictors.
To a degree we may view these differences in the developmental associations of these correlated symptom clusters with SES of family background as an “experiment of nature” (Cicchetti, 2003). What might be the reasons that the relationship between chronic depressive symptoms and lower SES background might, in some sense, be entirely attributable to comorbid symptoms of these personality disorders? As measured here, depressive symptoms reflect persistent problems reflecting dysthymia rather than the episodic symptoms defining major depressive disorder. It may be that such chronic symptoms represent a violation of one’s expectations either of own achievements or of environmental approval and reward, and a loss of hope regarding the future. In the absence of personality problems, offspring from lower SES families may tend to get implicit training that such hope and high expectations are against the norm, perhaps even foolish. Comorbid PD symptoms also accounted for the relationship between maladaptive parenting and depressive symptoms, reminding us that part or even much of the correlation between parenting and problematic symptoms may be an effect of the symptoms on the quality of parenting.
To what additional mechanisms may these SES effects on personality disorder symptoms into adulthood be attributed? There is a range of what might be viewed as sociocultural adaptations to low SES that may be both maladaptive in the sense of promoting symptom expression and passed on to offspring. Among these is the probable tendency for low SES parents to exhibit higher levels of “expressed emotion,” that is, negative evaluative comments about their offspring that promote ongoing emotional and behavioral problems (Caspi et al., 2004). Patterns of misbehavior management are also often less effective and themselves a risk in such families (McCord, 1995).
This study has as yet no direct measure of genetic risk. Thus, these analyses cannot determine the extent to which some of the impact of SES net of potential mediators may be attributable to genetic risk as a common cause of parental low SES and offspring disorder. However, earlier analyses of adolescent disorder groups in this study showed that parental psychopathology did not fully eliminate the significant effect of family SES on adolescent psychopathology, even when combined with other relevant covariates (Johnson et al., 1999). Nor is it clear the extent to which measures of parental psychopathology reflect partially shared genetic risk, reflected in part in SES, or parental reactions to SES-associated environmental risks. Although poor parenting fully accounted for associations of parental disorders with offspring disorders in another study based on this cohort (Johnson, Cohen, Kasen, Smailes, & Brook, 2001), SES-related parental problems may themselves be transmitted to offspring as epigenetic inheritance (Harper, 2005). That is, phenotypic responses to low SES may be transmitted from parent to offspring in the absence of offspring actual experience of low SES-associated risk. Such responses might reasonably include wariness of unfamiliar persons and the tolerance of relative isolation that characterizes schizotypy. It is perhaps more challenging to identify candidates for this kind of transmission of a risk for borderline disorder symptoms, although an anxious attachment style is one possibility. Other potential mediators of an ongoing influence of parental SES may be low confidence in one’s ability to master environmental stressors (Shanahan & Bauer, 2004) or other aspects of low resilience (Masten et al., 2004).
Such influences may be mediated through general personality dimensions. Nor can we rule out SES-associated cultural factors as direct mediators or as variables that potentiate other unmeasured SES correlates, such as poor educational opportunities and facilities. As Link and Phelan (1995) have argued, poverty and lower SES may operate to make a wide range of negative outcomes more probable in ways that vary substantially over time, place, and kind of outcome. In this sense, low SES or poverty may be thought of as a “fundamental cause,” the mechanisms of which reflect lower access to a wide range of promoters of health and well-being, and vulnerability to a similarly broad range of risks. These influences are likely to be particularly strong in settings and countries where the range of education, work status, and income is broad. For this reason, the increasing Gini index of the degree of income inequality in a country like the United States (Jones & Weinberg, 2000; Sen, 1973) is especially troubling.
SES-associated risks
Cumulative trauma and SLEs had consistent associations with higher symptoms of all three disorders. As we anticipated, the association of (standardized) SLEs with borderline symptoms increased with age, possibly because of the greater impact of ongoing symptoms on the likelihood of such events as study participants aged. However, a caution needs to be made in this interpretation. Because of the necessarily different measures of SLE suitable across this wide age range, we standardized the scores obtained at each assessment. Doing so may have removed a real increase in such events as participants aged into more responsible roles and less familial sheltering from stress.
SLEs had a greater impact on depressive symptoms of women than for those of men, as found previously. Poor parenting had symptom elevating effects on all three disorders, with higher estimates per mothering problem than per fathering problem and the fathering problem effect on schizotypal symptoms significant at only p < .05. Although it is tempting to interpret changes in parenting effects in the final models as reflecting mediation via comorbidity, it is important to keep in mind that the symptoms of each disorder were measured at the same four occasions, spaced at varying intervals. Thus, priority of onset cannot be estimated in these data. Indeed, it is now widely assumed that parenting will have been influenced by exposure to offspring symptoms, as well as having influence on such symptoms.
The developmental course of personality disorder symptoms from age 10 to age 36
In these analyses we show that the cumulative effect of these measured risks may account for very substantial failure to “develop out of” maladaptive symptoms of these personality disorders. One way to view these developmental barriers is to express each coefficient as a fraction of the average annual decline in symptoms expected over this developmental period of 25 years. For example, there is an expected (average) decline of nearly .50 symptom unit per year for schizotypal symptoms as measured here. Therefore, the coefficient for family SES suggests that for a child in a family 1 SD below the sample mean (below which nearly one-third of the sample falls), we may expect a developmental delay in symptom decline of about 3.4 years. For the approximately 5% of the population who were 2 SD below mean SES this estimated delay would double to nearly 7 years.
For symptoms of borderline disorder the estimated developmental delay associated with low SES is even greater: 5.8 estimated years of developmental delay per standard deviation below the SES mean. Of course, in both cases more than half of this estimated effect was mediated by correlated risks, so that to the extent that these risks were avoided (or increased) these delays may be less or more, respectively.
Finally, the very different findings for the dysthymia-like symptoms measured here cannot be ignored. Despite their correlation with virtually all personality disorders as well as many Axis I disorders, depressive symptoms did not follow the usual SES risk pattern. Of course, these problems have a very different developmental pattern: depressive symptoms increased in prevalence from early adolescence to late adolescence to young adulthood and gradually declined thereafter. Thus, one cannot view depression as a “developmental disorder” in the sense of a graded symptomatic decline with maturity. Nor was low IQ a risk factor for depression: on the contrary, when all other risks and comorbidity were taken into account the persons with higher verbal IQ had more symptoms. In addition, as shown elsewhere, the associations of depression with poor parenting were entirely accounted for by comorbid personality disorder symptoms (Johnson, Quigley, & Sherman, 1997).
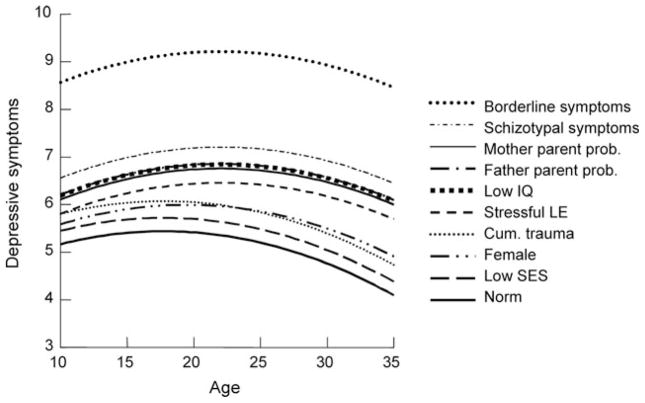
Perhaps the most dramatic findings with regard to depression were the enormous impact of comorbid symptoms of schizotypal and, especially, borderline personality disorders. These findings are reproduced in Figure 4. Indeed, if these personality disorders could be appropriately viewed as causative, based on these data in their absence the expected depressive symptoms would be near or at zero. This finding is consistent with clinical findings of a much more positive prognosis in major depression patients without personality disorder (Pilkonis & Frank, 1988; Shea, et al., 1987).
Figure 4.
The estimated average and high-risk trajectories of depressive symptoms including comorbid PD symptoms. Note: Each ascending line adds the named risk to the previous risks.
Acknowledgments
This study was supported by the New York State Office of Mental Hygiene, National Institute of Mental Health (R01-MH36971, MH38916, MH49191, MH60911, P.C., Principal Investigator), and National Institute on Drug Abuse (R01-DA03188, J.B., Principal Investigator).
References
- Adler NE, Marmot M, McEwen BS, Stewart J, editors. Socioeconomic status and health in industrialized nations. New York: Academic Science; 1999. [Google Scholar]
- Ammons RS, Ammons CH. The Quick Test: Provisional manual. Psychological Reports. 1962;11:11–161. [Google Scholar]
- Black MM, Krishnakumar A. Children in low-income, urban settings. American Psychologist. 1998;53:635–646. doi: 10.1037//0003-066x.53.6.635. [DOI] [PubMed] [Google Scholar]
- Boomsma D, de Geus EJ, van Baal GC, Koopmans JR. A religious upbringing reduces the influence of genetic factors on disinhibition: Evidence for interaction between genotype and environment on personality. Twin Research. 1999;2:115–125. doi: 10.1375/136905299320565988. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annual Review of Psychology. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Brandt DE. Delinquency, development, and social policy. New Haven, CT: Yale University Press; 2006. [Google Scholar]
- Brown GW, Harris T. Social origins of depression: A study of psychiatric disorder in women. New York: Free Press; 1978. [Google Scholar]
- Button TMM, Scourfield J, Martin N, Purcell S, McGuffin P. Family dysfunction interacts with genes in the causation of antisocial symptoms. Behavior Genetics. 2005;35:115–120. doi: 10.1007/s10519-004-0826-y. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Gunderson JG. Five year follow-up comparison of borderline and schizophrenic patients. Comprehensive Psychiatry. 1977;18:567–571. doi: 10.1016/s0010-440x(97)90007-9. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Morgan J, Rutter M, Taylor A, Arseneault L, et al. Maternal expressed emotion predicts children’s externalizing behavior problems: Using MZ-twin differences to identify environmental effects on behavioural development. Developmental Psychology. 2004;40:149–161. doi: 10.1037/0012-1649.40.2.149. [DOI] [PubMed] [Google Scholar]
- Caspi A, Roberts BW, Shiner RL. Personality development: Stability and change. Annual Review of Psychology. 2005;56:453–486. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington HL, et al. Influence of life stress on depression, Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:291–293. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Caspi A, Wright BRE, Moffitt TE, Silva PA. Early failure in the labor market: Childhood and adolescent predictors of unemployment in the transition to adulthood. American Sociological Review. 1998;63:424–451. [Google Scholar]
- Cicchetti D. Experiments of nature: Contributions to developmental theory. Development and Psychopathology. 2003;15:833–835. doi: 10.1017/s0954579403000397. [DOI] [PubMed] [Google Scholar]
- Coddington RD. The significance of life events as etiological factors in the diseases of children: II. A study of a normal population. Journal of Psychosomatic Research. 1972;16:205–213. doi: 10.1016/0022-3999(72)90045-1. [DOI] [PubMed] [Google Scholar]
- Cohen P, Crawford T, Johnson JG, Kasen S. The Children in the Community study of the development of personality disorders. Journal of Personality Disorders. 2005;19:466–486. doi: 10.1521/pedi.2005.19.5.466. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Compton SN, Keeler G, Angold A. Relationships between poverty and psychopathology. Journal of the American Medical Association. 2003;290:2023–2029. doi: 10.1001/jama.290.15.2023. [DOI] [PubMed] [Google Scholar]
- Crawford TN, Cohen P, Johnson JG, Kasen S, First MB, Gordon K, et al. Self-reported personality disorder in the children in the community sample: Convergent and prospective validity in late adolescence and adulthood. Journal of Personality Disorders. 2005;10:30–52. doi: 10.1521/pedi.19.1.30.62179. [DOI] [PubMed] [Google Scholar]
- Derogotis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): A self-report symptom inventory. Behavioral Science. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP, Dohrenwend BS. Social status and psychological disorder. New York: Wiley; 1969. [Google Scholar]
- Dohrenwend BP, Levav I, Shrout PE, Schwartz S, Naveh G, Link BG, et al. Socioeconomic status and psychiatric disorders: The causation-selection issue. Science. 1992;255:946–952. doi: 10.1126/science.1546291. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, et al. Gene–environment interaction analysis of serotonin system markers with adolescent depression. Molecular Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Entwisle DR, Alexander KL, Olson LS. Children, schools, and inequality. New York: Westview Press; 1997. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. User’ guide for the Structured Clinical Interview for DSM IV Personality Disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Gottfredson L. Intelligence: Is it the epidemiologists’ elusive “fundamental cause” of social class inequalities in health? Journal of Social & Personality Psychology. 2004;86:174–199. doi: 10.1037/0022-3514.86.1.174. [DOI] [PubMed] [Google Scholar]
- Harper LV. Epigenetic inheritance and the inter-generational transfer of experience. Psychological Bulletin. 2005;131:340–360. doi: 10.1037/0033-2909.131.3.340. [DOI] [PubMed] [Google Scholar]
- Hauser RM. Measuring socioeconomic status in studies of child development. Child Development. 1994;65:1541–1545. doi: 10.1111/j.1467-8624.1994.tb00834.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social class and mental illness: A community study. New York: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang KL, Dick DM, Wolf H, Livesley WJ, Paris J. Psychosocial adversity and emotional instability: An application of gene–environment interaction models. European Journal of Personality. 2005;19:359–372. [Google Scholar]
- Jang KL, Wolf H, Larstone R. What is the role of personality in psychopathology?: A view from behavior genetics. In: Krueger RF, Tackett JL, editors. Personality and psychopathology. New York: Guilford Press; 2006. pp. 153–173. [Google Scholar]
- Johnson JG, Bromley E, McGeoch PG. Role of childhood experiences in the development of maladaptive and adaptive personality traits. In: Oldham JM, Skodol AE, Bender DS, editors. Textbook of personality disorders. Washington, DC: American Psychiatric Press; 2005. [Google Scholar]
- Johnson JG, Cohen P, Dohrenwend BP, Link BG, Brook JS. A longitudinal investigation of social causation and social selection processes involved in the association between socioeconomic status and psychiatric disorders. Journal of Abnormal Psychology. 1999;108:490–499. doi: 10.1037//0021-843x.108.3.490. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, Skodol A, Brook J. Age-related change in personality disorder symptom levels between early adolescence and adulthood. Acta Psychiatrica Scandinavica. 2000;102:265–275. doi: 10.1034/j.1600-0447.2000.102004265.x. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, Smailes E, Brook JS. Association of maladaptive parental behavior with psychiatric disorder among parents and their offspring. Archives of General Psychiatry. 2001;58:453–460. doi: 10.1001/archpsyc.58.5.453. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Smailes E, Kasen S, Oldham JM, Skodol AE. Adolescent personality disorders associated with violence and criminal behavior during adolescence and early adulthood. American Journal of Psychiatry. 2000;157:1406–1412. doi: 10.1176/appi.ajp.157.9.1406. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Quigley JF, Sherman MF. Adolescent personality disorder symptoms mediate the relationship between perceived parental behavior and Axis I symptomatology. Journal of Personality Disorders. 1997;11:381–390. doi: 10.1521/pedi.1997.11.4.381. [DOI] [PubMed] [Google Scholar]
- Jones AF, Weinberg DH. Current population reports (series P 60-201) Bureau of the Census; 2000. The changing shape of the nation’s income distribution: 1947–1998. Retrieved from http://www.census.gov/prod/2000pubs/p60-204.pdf. [Google Scholar]
- Judge TA, Higgins CA, Thoreson CJ, Barrick MR. The big five personality traits, general mental ability, and career success across the life span. Personnel Psychology. 1999;52:621–652. [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychological Medicine. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kohn M. Social structure and personality through time and space. In: Moen P, Elder GH, Luscher K, editors. Examining lives in context: Perspectives on the ecology of human development. Washington, DC: American Psychological Association; 1995. pp. 141–168. [Google Scholar]
- Lenzenweger MF, Castro DD. Predicting change in borderline personality: Using neurobehavioral systems indicators within an individual growth curve framework. Development and Psychopathology. 2005;17:1207–1237. doi: 10.1017/s0954579405050571. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Cicchetti D. Toward a developmental psychopathology approach to borderline personality disorder. Development and Psychopathology. 2005;17:893–898. doi: 10.1017/s095457940505042x. [DOI] [PubMed] [Google Scholar]
- Link BG, Phelan JC. Social conditions as fundamental causes of disease. Journal of Health & Social Behavior. 1995;56(Suppl):80–94. [PubMed] [Google Scholar]
- Masten AS, Burt KB, Roisman GJ, Obradovic H, Long JD, Tellegan A. Resources and resilience in the transition to adulthood: Continuity and change. Development and Psychopathology. 2004;16:1071–1094. doi: 10.1017/s0954579404040143. [DOI] [PubMed] [Google Scholar]
- McCord J, editor. Coercion and punishment in long-term perspectives. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- McLeod J, Shanahan M. Poverty, parenting, and children’s mental health. American Sociological Review. 1993;58:351–366. [Google Scholar]
- Miech RA, Caspi A, Moffitt TE, Wright BRE, Silva PA. Low socioeconomic status and mental disorders: A longitudinal study of selection and causation during young adulthood. American Journal of Sociology. 1999;104:1096–1131. [Google Scholar]
- Moffitt TE. The new look of behavioral genetics in developmental psychopathology. Psychological Bulletin. 2005;131:533–554. doi: 10.1037/0033-2909.131.4.533. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured gene–environment interactions in psychopathology: Concepts, research strategies, and implications for research, intervention, and public understanding of genetics. Perspectives on Psychological Science. 2006;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Muntaner C, Eaton WW, Misch R, O’Campo P. Socioeconomic position and major mental disorders. Epidemiologic Reviews. 2004;26:53–62. doi: 10.1093/epirev/mxh001. [DOI] [PubMed] [Google Scholar]
- Pagani L, Boulerice B, Vitaro F, Tremblay RE. Effects of poverty on academic failure and delinquency in boys: A change and process model approach. Journal of Child Psychology and Psychiatry. 1999;40:1209–1219. [PubMed] [Google Scholar]
- Paris J. Social factors in the personality disorders. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Parke RS, Clarke-Stewart KA. Effects of parental incarceration on children: Perspectives, promises and policies. In: Travis J, Waul M, Solomon A, editors. From prison to home. Washington, DC: Urban Institute Press; 2003. pp. 189–232. [Google Scholar]
- Petras H, Schaeffer CM, Ialongo N, Hubbard S, Muthen B, Lambert SF, et al. When the course of aggressive behavior in childhood does not predict antisocial outcomes in adolescence and young adulthood: An examination of potential explanatory variables. Development and Psychopathology. 2004;16:919–941. doi: 10.1017/s0954579404040076. [DOI] [PubMed] [Google Scholar]
- Pilkonis PA, Frank E. Personality pathology in recurrent depression: Nature, prevalence, and relationship to treatment response. American Journal of Psychiatry. 1988;145:435–441. doi: 10.1176/ajp.145.4.435. [DOI] [PubMed] [Google Scholar]
- Rank MR. Socialization of socioeconomic status. In: Nichols WC, editor. Handbook of family development and intervention. New York: Wiley; 2000. pp. 129–142. [Google Scholar]
- Rutter M. Environmentally mediated risks for psychopathology: Research strategies and findings. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:3–18. doi: 10.1097/01.chi.0000145374.45992.c9. [DOI] [PubMed] [Google Scholar]
- Rutter M, Maughan B. Psychosocial adversities in childhood and adult psychopathology. Journal of Personality Disorders. 1997;11:4–18. doi: 10.1521/pedi.1997.11.1.4. [DOI] [PubMed] [Google Scholar]
- Schell LM. Culture as stressor: A revised model of biocultural interaction. American Journal of Physical Anthropology. 1997;102:67–77. doi: 10.1002/(SICI)1096-8644(199701)102:1<67::AID-AJPA6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Sen A. On economic inequality. Oxford: Oxford University Press; 1973. [Google Scholar]
- Serbin LA, Karp J. The intergenerational transfer of psychosocial risk: Mediators of vulnerability and resilience. Annual Review of Psychology. 2004;55:333–363. doi: 10.1146/annurev.psych.54.101601.145228. [DOI] [PubMed] [Google Scholar]
- Shanahan MJ, Bauer DJ. Developmental properties of transactional models: The case of life events and mastery from adolescence to young adulthood. Development and Psychopathology. 2004;16:1095–1117. doi: 10.1017/s0954579404040155. [DOI] [PubMed] [Google Scholar]
- Shea MT, Glass DR, Pilkonis PA, Watkins J, Docherty JP. Frequency and implications of personality disorders in a sample of depressed out-patients. Journal of Personality Disorders. 1987;1:27–42. [Google Scholar]
- Timms DWG. Social mobility and mental health in a Swedish cohort. Social Psychiatry. 1996;31:38–48. doi: 10.1007/BF00789121. [DOI] [PubMed] [Google Scholar]
- Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Archives of General Psychiatry. 2001;58:590–596. doi: 10.1001/archpsyc.58.6.590. [DOI] [PubMed] [Google Scholar]
- Walker E, Kestler L, Bollini A, Hochman KM. Schizophrenia: Etiology and course. Annual Review of Psychology. 2004;55:401–430. doi: 10.1146/annurev.psych.55.090902.141950. [DOI] [PubMed] [Google Scholar]
- White KR. The relation between socioeconomic status and academic achievement. Psychological Bulletin. 1982;91:461–481. [Google Scholar]
- Zanarini M, Frankenburg FR, Hennen J, Silk KR. The longitudinal course of borderline psychopathology: 6-year prospective follow-up of the phenomenology of borderline personality disorder. American Journal of Psychiatry. 2003;160:274–283. doi: 10.1176/appi.ajp.160.2.274. [DOI] [PubMed] [Google Scholar]