Abstract
Successful reproduction in mammals depends on solicitational behaviors that enhance the probability of encountering potential mates. In female Syrian hamsters, one such behavior is vaginal scent marking. Recent evidence suggests that the neuropeptide oxytocin (OT) may be critical for regulating this behavior. Blockade of OT receptors in the bed nucleus of the stria terminalis (BNST) or the medial preoptic area (MPOA) decreases vaginal marking responses to male odors; lesion data suggest that BNST, rather than MPOA, mediates this effect. However, how OT interacts with sexual odor processing to drive preferential solicitation is not known. To address this issue, intact female Syrian hamsters were exposed to male or female odors and their brains processed for immunohistochemistry for Fos, a marker of recent neuronal activation, and OT. Additional females were injected intracerebroventricularly (ICV) with an oxytocin receptor antagonist (OTA) or vehicle, and then tested for vaginal marking and Fos responses to sexual odors. Colocalization of OT and Fos in the paraventricular nucleus of the hypothalamus was unchanged following exposure to male odors, but decreased following exposure to female odors. Following injections of OTA, Fos expression to male odors was decreased in BNST, but not in MPOA or the medial amygdala (MA). Fos expression in BNST may be functionally relevant for vaginal marking, given that there was a positive correlation between Fos expression and vaginal marking for BNST, but not MPOA or MA. Together, these data suggest that OT facilitation of neuronal activity in BNST underlies the facilitative effects of OT on solicitational responses to male odors.
Keywords: Olfaction, Chemosensory, Precopulatory, Sexual motivation, Appetitive
Introduction
Proceptive behaviors that facilitate the identification and attraction of potential mating partners are an important component of sexual behavior for most mammals (Beach, 1976). For species wherein the sexes do not typically cohabitate as adults, such as Syrian hamsters (Mesocricetus auratus), these behaviors are particularly critical for mating success (Gattermann et al., 2001; Pfaff et al., 2008). Female Syrian hamsters engage in a number of different proceptive responses, including vaginal scent marking (Petrulis, 2009). This behavior deposits vaginal secretion such that ‘trails’ are formed along the ground as the animal scent marks throughout its environment (Lisk et al., 1983). Although females will vaginal mark in response to various types of stimuli, this behavior is preferentially directed towards males or their odors (Johnston, 1977; Martinez and Petrulis, 2011; Petrulis and Johnston, 1999; Petrulis et al., 1999), suggesting that vaginal marking may be particularly important for mate attraction. Indeed, the deposited vaginal secretion is highly attractive to male hamsters (Johnston and Schmidt, 1979; Johnston, 1974; Kwan and Johnston, 1980), and females deposit these marks in such a way as to direct the male to her nesting area (Lisk et al., 1983).
The expression of vaginal marking depends critically on the processing of sexual odor information by chemosensory-responsive brain areas. In most mammalian species, including Syrian hamsters, chemosensory signals from conspecifics are initially detected and processed by two distinct but interconnected systems, the main and accessory olfactory systems (Keller et al., 2009). Disruption of inputs to the main (Johnston, 1992) or accessory (Petrulis et al., 1999) olfactory bulbs, or ablation of the olfactory bulbs themselves (Kairys et al., 1980), decreases vaginal marking to males or their odors. Sexual odor information is transmitted from the olfactory bulbs to areas throughout the ventral forebrain, including the medial amygdala (MA), the posterior division of the bed nucleus of the stria terminalis (BNST), and the medial preoptic area (MPOA) (Coolen and Wood, 1998; Davis et al., 1978; Gomez and Newman, 1992; Wood and Swann, 2005). These areas also appear to be important for regulating the expression of vaginal marking. Lesions centered on MA decrease overall levels of vaginal marking (Petrulis and Johnston, 1999), whereas lesions of BNST specifically decrease vaginal marking in response to male, but not female odors (Martinez and Petrulis, 2011). In contrast, lesions of MPOA do not affect the expression of vaginal marking, although this area is important for other forms of proceptive behaviors in female hamsters (Floody, 1989; Martinez and Petrulis, 2013).
The underlying neurochemical mechanisms whereby the ventral forebrain regulates proceptive responses in females are not well understood. However, there is a growing body of evidence to suggest that the neuropeptide oxytocin may be involved. Oxytocin is a nine amino acid peptide that is produced and released from neurosecretory cells located in the paraventricular nucleus of the hypothalamus and supraoptic nucleus. Within the brain, oxytocin acts to broadly facilitate a range of prosocial responses, including social recognition, maternal behavior, and sexual behavior (Lee et al., 2009). In hamsters and rats, oxytocin infused directly into the medial preoptic area-anterior hypothalamus (MPOA-AH) facilitates the expression of sexual receptivity (Caldwell et al., 1989; Whitman and Albers, 1995), whereas MPOA-AH injections of an oxytocin receptor antagonist (OTA) decreases receptivity (Caldwell et al., 1994; Whitman and Albers, 1995). Intracerebroventricular (ICV) injections of OTA decrease both sexual solicitation and sexual receptivity in female rats (Witt and Insel, 1991), but this effect on solicitation was not replicated following OTA injections directly into MPOA-AH (Caldwell et al., 1994). This suggests that areas other than MPOA-AH in the ventral forebrain mediate the effects of oxytocin on sexual solicitation. The bed nucleus of the stria terminalis (BNST) is one such candidate area. We have found that injections of OTA into either MPOA-AH or BNST decreased vaginal marking to male, but not female odors (Martinez et al., 2010). Furthermore, in hamsters binding sites for oxytocin are present in BNST, but not MPOA-AH, (Dubois-Dauphin et al., 1992), and as mentioned above, lesions of BNST, but not MPOA, disrupt vaginal marking responses to sexual odors (Martinez and Petrulis, 2011, 2013). Considered together, it seems likely that oxytocin acts within BNST to regulate vaginal marking responses to male odors; however, the underlying mechanisms underlying this effect are not known.
Across many mammalian species, including sheep, mice, rats, and hamsters, expression of immediate early genes (IEGs; an indirect marker of recent neuronal activation (Pfaus and Heeb, 1997)) throughout the ventral forebrain is increased in females when exposed to male stimuli (Bennett et al., 2002; DelBarco-Trillo et al., 2009; Gelez and Fabre-Nys, 2006; Kang et al., 2009). Experiments utilizing stimulation and/or extracellular recordings in awake, behaving females provide additional evidence supporting a link between neural activity in these areas and both copulatory and solicitational responses (Hoshina et al., 1994; Kato and Sakuma, 2000; Rose, 1990). In female Syrian hamsters, however, the IEG responses of several ventral forebrain areas, including BNST and MPOA, have not been reported following exposure to sexual odors; furthermore, it is not known if IEG responses in these areas are related to the expression of vaginal marking behavior. Given that oxytocin typically increases excitability in oxytocin-sensitive neurons in target areas, including BNST (Ingram and Wakerley, 1993; Ingram et al., 1990; Terenzi et al., 1999), we hypothesized that oxytocin selectively facilitates neural activation by male odor signals, thereby driving the high levels of vaginal marking females exhibit in response to these stimuli. This may occur through two non-exclusive mechanisms: (1) oxytocinergic cells in ventral forebrain areas may preferentially release oxytocin in response to male odors, and (2) endogenous oxytocin may preferentially facilitate neural activity in male odor-responsive neurons. Consequently, we predicted that male odor stimuli would preferentially induce the expression of Fos (the protein product of the IEG c-fos) in ventral forebrain areas responsive to sexual odors, and further, that oxytocin cells within these areas would be also be more likely to co-express Fos in response to male odors (Experiment 1). We also predicted that Fos expression in BNST, but not MPOA or MA, would be more closely related to the expression of vaginal marking, and that ICV injections of OTA would selectively decrease Fos expression to male odors in BNST, but not in MPOA or MA (Experiment 2).
Materials and Methods
Overview of experimental design
In Experiment 1, females were exposed to male, female, or clean cage stimuli on behavioral proestrus, sacrificed, and their brains processed for double-label immunohistochemistry (IHC) for oxytocin and Fos (Figure 1A). In Experiment 2, females were implanted with unilateral guide cannulae directed at the lateral cerebral ventricles. These females were then exposed to male or female stimuli on proestrus. In Experiment 2A, we sought to first establish the appropriate dose of an oxytocin receptor antagonist (OTA) that would decrease vaginal marking when injected intracerebroventricularly (ICV), and the specificity of this effect for oxytocin receptors (OTRs) (Figure 1B). Thus, females were injected ICV with OTA or a vasopressin 1a receptor antagonist (V1aA) at the following doses: 0, 90, or 900 µM of OTA in 2 µl saline vehicle (OTA group); 0, 900, or 9000 µM V1aA in 2 µl saline vehicle (V1aA group), 1 h prior to odor exposure. Finally, in Experiment 2B, we examined the neural effects of OTA following exposure to male or female stimuli (Figure 1C). Females were injected ICV with either 0 or 900 µM OTA in 2 µl saline vehicle 1 h prior to stimulus exposure. At the completion of the exposure period, females were sacrificed and brain tissue processed for single-label IHC for Fos.
Figure 1.
Timelines of experimental manipulations.
Subjects
Adult Syrian hamsters (Mesocricetus auratus) were purchased from Harlan Laboratories (Indianapolis, IL, USA) at approximately 7–11 weeks of age. In addition to female hamsters used as experimental subjects, a separate group of unrelated male and female hamsters served as stimulus animals. Animals were either individually housed (experimental subjects) or group housed (stimulus animals; 3–4 animals per cage) in solid-bottom polycarbonate cages containing corncob bedding and cotton nesting material (Nestlets; Ancare, Bellmore, NY). Hamsters were maintained on a reversed light cycle (14:10 light:dark; lights out at 10 am), with all behavior testing occurring during the first four hours of the dark portion of the light cycle. Food and water were available ad libitum. Animal procedures were carried out in accordance with the Eighth Edition of the Guide for the Care and Use of Laboratory Animals (Committee for the Update of the Guide for the Care and Use of Laboratory Animals; National Research Council, 2011) and approved by the Georgia State University Institutional Animal Care and Use Committee. All efforts were made to minimize the total number of animals used and their suffering.
Estrous cycle monitoring
Prior to stimulus exposure or behavioral testing, subjects were examined daily for eight consecutive days in order to determine their stage of the estrous cycle. Subjects were gently restrained while vaginal secretion was manually extruded using a disposable probe, and the consistency of the secretion was examined for stringy consistency indicative of behavioral estrus (Orsini, 1961). Once the day of behavioral estrus was identified, the two cycle days prior to estrus were defined as diestrous day 2 and proestrus, respectively (Johnston, 1977). It has been our experience that this technique for determining estrous cycle stage correctly predicts the day of behavioral receptivity with perfect accuracy (Martinez and Petrulis, 2011, 2013).
Stimulus exposure
On the predicted day of proestrus (see Estrous cycle monitoring), subjects were exposed to male, female, or clean cage stimuli. Proestrus was chosen for stimulus exposures because females exhibit the highest overall levels of vaginal marking on this cycle day (Johnston, 1977; Martinez and Petrulis, 2011, 2013). Subjects were handled for at least eight consecutive days prior to exposure. Stimuli consisted of vacated cages (43 × 22 × 20 cm) previously occupied by male or female stimulus animals for four days, or clean cages containing unsoiled bedding. Estrous cycles of stimulus females were not monitored; however, given that each cage contained at least three females, and the occupation period comprised the complete four-day estrous cycle, it is likely that female cage stimuli were a composite of multiple cycle days. Approximately one to four hours prior to use, a researcher blind to the experimental condition of subjects prepared the stimulus cages as follows: Stimulus animals were removed from their cages along with any food pellets and caked urine present in the corncob bedding, the soiled cotton nesting material was distributed evenly across the bottom of the cage, and a 40 cm × 19 cm × 0.4 cm perforated Plexiglas plate was placed over the bedding just prior to testing. This plate was centered within the cage such that bedding within three cm of the outer walls of the stimulus cages remained directly accessible to subjects. The surface of the plate was painted with black non-toxic chalkboard paint (Rust-oleum, Vernon Hills, IL) such that it divided the plate into four painted quadrants separated by unpainted areas. We have previously used this technique to facilitate the scoring of vaginal/flank marking and locomotor activity (Martinez and Petrulis, 2011, 2013; Martinez et al., 2010). Although scent marking was only quantified in Experiment 2 and Experiment 3, these plates were utilized in all experiments to maintain consistency in odor presentation procedures.
Experiment 1: Co-localization of oxytocin and Fos following exposure to sexual odors
Perfusion and IHC
Following a 70 min stimulus exposure period, subjects were transcardially perfused with 200 ml of 0.1 M phosphate buffered saline (PBS) followed by 200 ml of 4% paraformaldehyde (Fisher Scientific, Pittsburgh, PA) in PBS. Brains were removed and post-fixed in 4% paraformaldehyde in PBS overnight followed by 20% sucrose in PBS (1–2 days). Coronal sections (30 µm) were taken using a cryostat and stored in cryoprotectant at −20°C until processed for double-label IHC for oxytocin and Fos.
Free-floating coronal sections were removed from cryoprotectant, rinsed in PBS, and then incubated in 0.3% hydrogen peroxide in PBS for 15 min in order to quench any endogenous peroxidase activity. After rinsing in PBS, sections were incubated in a solution containing PBS, 0.4% Triton-X (Sigma, St. Louis, MO), and 1:20,000 polyclonal rabbit anti-cFos antibody (SC-52; Santa Cruz Biotechnology, Santa Cruz, CA) for 18 h at room temperature. After rinsing in PBS, tissue sections were incubated in a solution of PBS, 0.4% Triton-X, and 1:600 biotinylated goat anti-rabbit secondary antibody (111-065-003; Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature, rinsed in PBS, and then incubated in a solution of PBS, 0.4% Triton-X, and 1:200 avidin-biotin complex (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA) for 1 h at room temperature. Tissue sections were rinsed in PBS followed by 0.175 M sodium acetate, and reacted in a nickel-enhanced 3,3′-diaminobenzidine tetrahydrochloride solution (2 mg 3,3′-diaminobenzidine tetrahydrochloride plus 250 mg nickel (II) sulfate with 8.3 µL 3% hydrogen peroxide per 10 mL of 0.175 M sodium acetate; Sigma, St. Louis, MO) for 15 minutes at room temperature, resulting in a purple-black nuclear stain. Tissue sections were rinsed again in 0.175 M sodium acetate followed by PBS in order to terminate the chromagen reaction. Sections then underwent additional processing to label for oxytocin. Briefly, sections were incubated in 3% normal horse serum (S-2000; Vector Laboratories) in PBS for 1h. Sections were placed in a solution containing PBS, 0.3% Triton-X, and 1:10,000 monoclonal mouse anti-oxytocin (MAB 5296; Millipore, Temecula, CA) for 48 h at 4°C. Tissue sections were subsequently processed as described above for Fos, with the following exceptions: the secondary antibody was 1:600 horse anti-mouse (BA2000; Vector Laboratories), 3,3′-diaminobenzidine tetrahydrochloride in Tris buffer was utilized for the chromagen reaction (resulting in a brown, cell body stain) and Tris buffer was used to rinse the tissue both prior to and following the chromagen reaction. At the completion of IHC processing, tissue sections were mounted on subbed slides and dried overnight. Slides were then dehydrated through an ethanol series, cleared with xylene (Fisher Scientific, Pittsburgh, PA), and coverslipped using Permount (Fisher Scientific).
Quantification
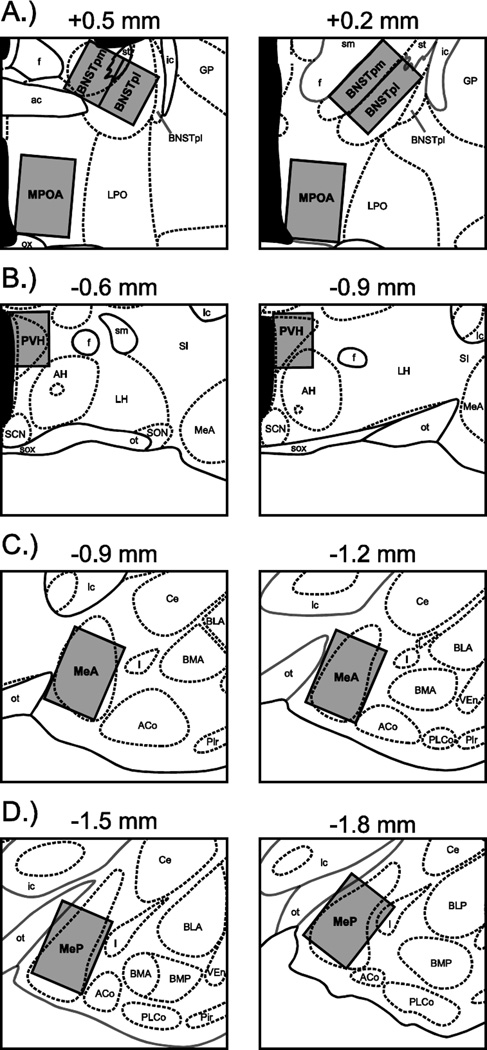
A single researcher blind to the experimental condition of the subjects counted both single- and double-labeled cells. Slides were examined using a QImaging camera connected to a Nikon E800 light microscope. Images were projected onto a computer running iVision software (v. 4.0.10) at 10X magnification. Photomicrographs of representative tissue labeling were processed using Adobe Photoshop CS5 (Adobe, San Jose, CA) for brightness, contrast, and tone. Counting domains were fitted to the following brain areas: the posteromedial (BNSTpm) and posterointermediate (BNSTpi) subdivisions of the bed nucleus of the stria terminalis, the medial preoptic area (MPOA), the paraventricular nucleus of the hypothalamus (PVH), and the anterior and posterior divisions of the medial amygdala (MeA and MeP, respectively) (Figure 2). For each brain area, counting domains were fitted to both hemispheres on two consecutive atlas plates. Cells positive for Fos (Fos+) were identified by purple-black nuclear staining whereas cells positive for oxytocin (OT+) were identified by brown cell body staining. Cells positive for both oxytocin and Fos (OT/Fos+) were identified by the presence of both a purple-black nucleus and a brown cell body. Counts of single- and double-labeled cells were summed across hemispheres and atlas plates for each brain area, and then divided by the total area of the counting domains in order to generate cell densities (labeled cells per mm2).
Figure 2.
Counting domains for quantifying oxytocin- and Fos-positive cells. Domains (gray boxes) were fitted to the following areas: (A) the posteromedial (BNSTpm) and posterointermediate (BNSTpi) subdivisions of the bed nucleus of the stria terminalis, and the medial preoptic area (MPOA), (B) the paraventricular nucleus of the hypothalamus (PVH), (C) the anterior division of the medial amygdala (MeA), and (D) the posterior division of the medial amygdala (MeP). Atlas plates are modified from Morin and Wood (2001), and arranged in distances relative to bregma. ac, anterior commissure; ACo, anterior cortical amygdaloid nucleus; AH, anterior hypothalamus; BLA, anterior division of the basolateral amygdaloid nucleus; BLP, posterior division of the basolateral amygdaloid nucleus; BMA, anterior division of the basomedial amygdaloid nucleus; BMP, posterior division of the basomedial amygdaloid nucleus; BNSTpl, posterolateral subdivision of the bed nucleus of the stria terminalis; Ce, central amygdaloid nucleus; f, fornix; GP, globus pallidus; I, intercalated nuclei of the amygdala; ic, internal capsule; LH, lateral hypothalamus; LPO, lateral preoptic area; ot, optic tract; ox, optic chiasm; Pir, piriform cortex; PLCo, posterolateral cortical amygdaloid nucleus; SCN, superchiasmatic nucleus; SI, substantia innominata, sm, stria medullaris; SON, supraoptic nucleus; sox, supraoptic decussation; st, stria terminalis; VEn, ventral endopiriform nucleus.
Experiment 2: Effects of ICV injections of OTA on sexual odor-induced Fos expression
Surgery
At two to three months of age, subjects were unilaterally implanted with guide cannulae. Subjects were first anesthetized with 2–3% isoflurane gas (Butler Schein, Dublin, OH) in an oxygen (80%) and nitrous oxide (20%) mixture, and then placed within a stereotaxic apparatus (Kopf Instruments, Tujunga, CA) with ear- and incisor-bars positioned such that the top of the skull was level. Following a midline scalp incision, the skin and underlying temporal muscles were retracted to expose the skull. A hand-operated drill was then used to make a hole in the skull in order to expose dura. Each subject was then fitted with a single 3-mm 26-gauge guide cannula (Plastics One, Roanoke, VA) directed at the lateral cerebral ventricle at the level of BNST, using the following coordinates: Anterior-posterior, +1.40 mm (relative to bregma); medial-lateral, ±1.45 mm (relative to bregma); dorsal-ventral, −2.00 mm (relative to dura). The placement of the guide cannula in the left or right hemisphere was alternated across all subjects. Stereotaxic coordinates were derived from published anatomical plates for the Syrian hamster brain (Morin and Wood, 2001). Injection cannulae were cut to extend an additional 1 mm beyond the end of guide cannulae, thereby penetrating into the lateral ventricle. Guide cannulae were secured to the skull with dental cement and skull screws, and immediately prior to the completion of the surgical procedure, subjects were injected subcutaneously with an analgesic agent (Ketofen; Fort Dodge Animal Health, Fort Dodge, IA). All subjects were allowed to recover for at least 10 days prior to behavioral testing.
Drugs
OTA (des-Gly-NH2, d(CH2)5 [Tyr(Me)2, Thr4] OVT; Bachem, Torrance, CA) and V1aA (d(CH2)5 [Tyr(Me)2, Dab5] AVP; generous gift of Dr. Maurice Manning, University of Toledo, Toledo, OH) were diluted in sterile saline and stored in aliquots of 30 µl at −20°C until use. OTA was placed into solution at 0, 90, and 900 µM whereas V1aA was placed into solution at 0, 900, and 9000 µM. Both OTA and V1aA are selective antagonists for their targeted receptor subtypes (OTRs and vasopressin receptors (V1aRs), respectively). Indeed, OTA exhibits a 17-fold greater potency for antagonizing OTRs versus V1aRs, whereas V1aA exhibits essentially no antagonism for OTRs (Manning et al., 2008). All drug injections occurred one hour prior to behavior testing/stimulus exposure. Subjects were injected with either 2 µl of OTA or 2 µl of V1aA. In Experiment 2A, subjects were injected on three consecutive proestrous cycle days with one of the three doses of drug described above, such that each female was tested once following injection of each dose of OTA or V1aA. In Experiment 2B, subjects were injected on a single proestrous day either 0 or 90 µM OTA. Drugs were delivered to the lateral ventricle via an injection cannula connected via polyethylene tubing to a 10 µl Hamilton syringe driven by a Harvard Apparatus infusion pump (PHD 2000; Holliston, MA). Subjects were allowed to freely explore a temporary holding cage for 90 s while the drug was injected, and then for an additional 60 s post-injection to allow the drug to diffuse away from the tip of the injection cannula.
One hour following injections, subjects were exposed to stimuli present in male or female cages. Stimulus exposure times were either 10 min (Experiment 2A) or 70 min (Experiment 2B). In all cases, marking was scored manually during the first 10 min of the stimulus exposure by a researcher blind to the experimental condition of the subjects. Vaginal marking and flank marking are discrete, stereotyped behaviors that are differentially expressed in response to conspecific odors (Johnston, 1977). A vaginal mark was scored each time the female moved forward with tail deflected upwards while maintaining contact between the perineum and the underlying substrate (Been et al., 2012). A flank mark was scored each time the female moved forward while maintaining contact between the flank region and the side of the stimulus cage. Tests were also recorded using a digital camcorder and videos were scored for the number of quadrant entries by researchers blind to the experimental conditions of the subjects, with an inter-rater reliability of 90% or greater. Entry into a quadrant was scored whenever greater than 50% of the body mass of the female crossed from one quadrant into another.
Histology
In Experiment 2A, subjects were administered a lethal dose of sodium pentobarbital (0.2 ml, i.p.; Beuthanasia-D, Merck Animal Health, Summit, NJ) following their final stimulus exposure, and then injected via cannulae with 200 nl of India ink. Brains were removed, post-fixed in 10% neutral buffered formalin, and 50 µm sections were taken using a cryostat. Sections were counterstained with cresyl violet and examined under light microscopy for ink penetration into the lateral cerebral ventricle. The site of penetration was plotted using anatomical plates of the Syrian hamster brain (Morin and Wood, 2001). In all cases, the injection needle penetrated into the lateral cerebral ventricle. Therefore, data from all subjects were analyzed.
In Experiment 2B, subjects were sacrificed immediately following the 70 min exposure period. Subjects were then perfused and brain tissue processed and quantified as described in Experiment 1, with the following exceptions: (1) tissue was only processed for Fos immunoreactivity, and (2) only the number of Fos+ cells in BNSTpi, BNSTpm, MPOA, MeA, and MeP was quantified.
Data analysis
All data were analyzed using SPSS for Macintosh, version 19.0 (SPSS Inc, Chicago, IL). Data were first examined to determine if the assumptions of parametric statistical tests were met. When assumptions were violated, distributions were normalized using applicable data transformations (Osborne and Overbay, 2004; Sheskin, 2000). For all statistical tests, results were considered to be statistically significant if p < .05.
In Experiment 1, the density of Fos+ cells and proportion of OT+/Fos+ cells within each brain area was compared across stimulus groups (male, female, or clean) using one-way ANOVAs. Significant main effects were followed by post-hoc pairwise comparisons using Tukey’s HSD (density of Fos+ cells) or z-tests for independent proportions (proportion of OT+/Fos+ cells).
In Experiment 2A, the number of vaginal and flank marks exhibited by females within each drug group was subjected to a mixed-design factorial ANOVA, with stimulus (male or female) as an independent factor and drug concentration (OTA: 0, 90, or 900 µM; V1aA: 0, 900, or 9000 µM) as a repeated factor. Significant interactions were followed by pairwise comparisons of the different drug concentrations within each stimulus condition, with Bonferroni corrections for multiple comparisons.
In Experiment 2B, the density of Fos+ cells within each area was subjected to a factorial ANOVA with stimulus (male or female) and drug concentration (0 or 900 µM OTA) as independent factors. Significant interactions were further examined for the effect of drug concentration within each stimulus condition using independent-samples t-tests. In addition, the strength and significance of the linear relationship between the density of Fos+ cells in each brain area and the following measures was determined using Pearson’s product-moment correlations: (1) the number of vaginal marks in the male odor condition only, and (2) the number of vaginal marks when first controlling for the effect of odor on Fos expression and vaginal marking.
Results
Experiment 1
Throughout most areas examined, a significant increase in Fos expression was observed in females exposed to sexual odor stimuli (male or female cages) compared to stimuli present in clean cages. The total densities of Fos+ cells within each brain area are presented in Table 1. Within MeA, MeP, BNSTpm, BNSTpi, and MPOA, the density of Fos+ cells differed across the three stimulus conditions (BNSTpm: F (2,17) = 9.49, p = .002; BNSTpi: F (2,17) = 9.70, p = .002; MPOA: F (2,17) = 12.97, p < .001; MeA: F (2,17) = 4.95, p = .02; MeP: F (2,17) = 19.20, p < .001). Tukey’s HSD post-hoc comparisons revealed that the density of Fos+ cells was higher in response to male vs. clean stimuli in BNSTpm, BNSTpi, MPOA, MeA, and MeP; higher in response to male vs. female stimuli in BNSTpi, and MPOA, and MeP; and higher in response to female vs. clean stimuli in BNSTpm (all p < .05). In contrast, the density of Fos+ cells did not differ across stimulus conditions within PVH.
Table 1.
Fos and oxytocin expression during stimulus exposures (Experiment 1)
| BNSTpm | BNSTpi | MPOA | MeA | MeP | PVH | ||
|---|---|---|---|---|---|---|---|
| Fos+ | |||||||
| Male | 171.6 ± 16.8a | 154.8 ± 17.5a | 229.7 ± 8.5a | 327.3 ± 11.2a | 307.4 ± 15.9a | 188.1 ± 13.5a | |
| Female | 149.3 ± 12.0a | 102.6 ± 8.9b | 190.7 ± 7.7b | 296.8 ± 15.0a,b | 223.8 ± 12.1b | 183.1 ± 15.0a | |
| Clean | 87.6 ± 11.0b | 75.8 ± 9.1b | 172.9 ± 7.7b | 258.0 ± 20.0b | 180.6 ± 15.8b | 201.5 ± 21.2a | |
| OT+ | |||||||
| Male | 2.1 ± 0.8 | 1.2 ± 0.3 | 4.5 ± 1.1 | 4.8 ± 0.8 | 0.0 ± 0.0 | 117.0 ± 6.0 | |
| Female | 2.8 ± 1.3 | 0.8 ± 0.3 | 3.2 ± 0.5 | 4.9 ± 1.1 | 0.5 ± 0.2 | 127.6 ± 9.0 | |
| Clean | 0.8 ± 0.2 | 0.7 ± 0.3 | 4.1 ± 0.5 | 5.7 ± 0.8 | 0.4 ± 0.1 | 139.9 ± 10.5 |
Mean (± SEM) number of Fos-positive (Fos+) and oxytocin-positive (OT+) cells per mm2, within each brain area (see Methods section for a detailed description of cell counting protocol). Dissimilar letters within a specific brain area indicate non-homologous means (Tukey’s HSD post-hoc comparisons following significant ANOVA). BNSTpm, posteromedial subdivision of the bed nucleus of the stria terminalis; BNSTpi, posterointermediate subdivision of the bed nucleus of the stria terminalis; MPOA, medial preoptic area; MeA, anterior division of the medial amygdala; MeP, posterior division of the medial amygdala; PVH, paraventricular nucleus of the hypothalamus.
Although we observed substantial numbers of OT+ cells in PVH, few OT+ cells were observed in other quantified areas (Table 1). We also observed few double-labeled OT+/Fos+ cells within BNSTpm, BNSTpi, or MeP; therefore, we only quantified the proportion of OT+/Fos+ cells within PVH, MPOA, and MeA (Figure 3). Within PVH, the proportion of OT/Fos+ cells was higher in response to male vs. female stimuli (z = 5.16, p < .05) and higher in response to clean vs. female stimuli (z = 4.13, p < .05) (Figure 4). There was no difference in the proportion of OT/Fos+ cells in response to male vs. clean stimuli. Furthermore, there were no differences across stimulus conditions in the proportion of OT+/Fos+ cells within MPOA or MeA.
Figure 3.
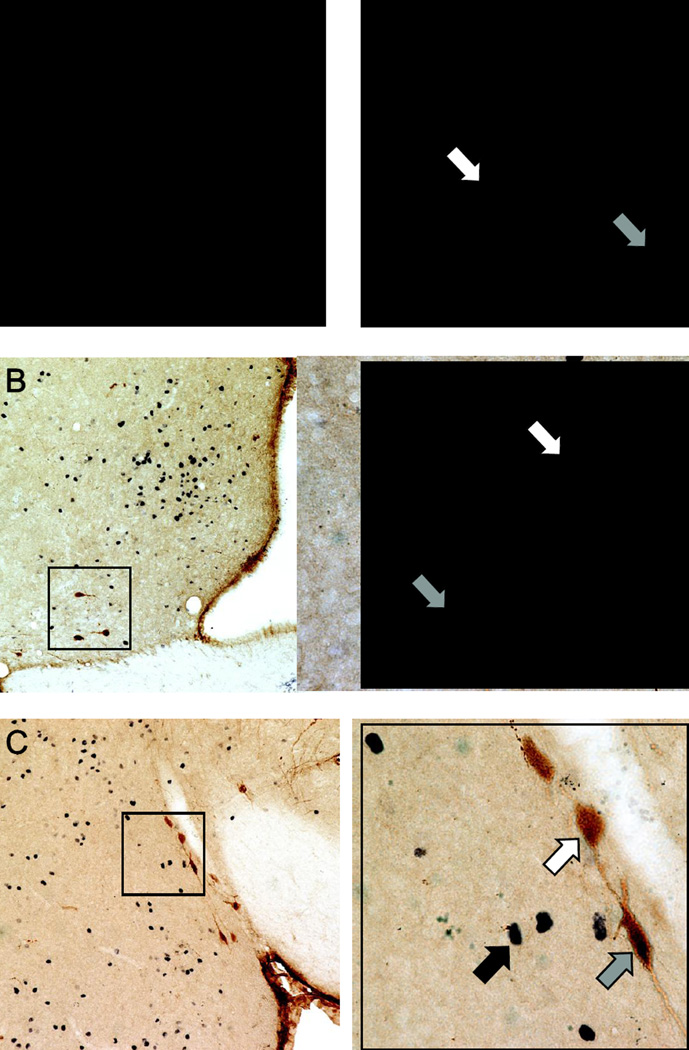
Immunohistochemical staining for oxytocin and Fos. Representative photomicrographs of double-label immunohistochemistry in sections depicting (A) the paraventricular nucleus of the hypothalamus (PVH), (B) the medial preoptic area (MPOA), and (C) the anterior division of the medial amygdala (MeA). A higher magnification (40X objective) of the areas highlighted within the black boxes is provided on the right of each lower magnification (10X objective) image. White arrows indicate oxytocin-immunoreactive cells (brown cytoplasmic staining). Black arrows indicate Fos-immunoreactive cells (black nuclear staining). Gray arrows indicate cells immunoreactive for both oxytocin and Fos (brown cytoplasmic staining with black nuclear staining). Scale bars = 100 µm (lower magnification) and 25 µm (higher magnification).
Figure 4.
Mean (± SEM) percentages of oxytocin/Fos double-labeled cells following stimulus exposures. In the paraventricular nucleus of the hypothalamus (PVH), a lower percentage of oxytocin-positive (OT+) cells were positive for Fos (Fos+) following exposure to female odors as compared to either male or clean odors. No differences were observed in the percentage of OT+/Fos+ cells across stimulus conditions in either the medial preoptic area (MPOA) or the anterior division of the medial amygdala (MeA). Few oxytocin-positive cells were observed in the bed nucleus of the stria terminalis (BNST) or posterior medial amygdala (MeP), and so double-labeled cells in those areas were not counted. Within each area, dissimilar letters = nonhomologous means (Tukey’s HSD post-hoc tests).
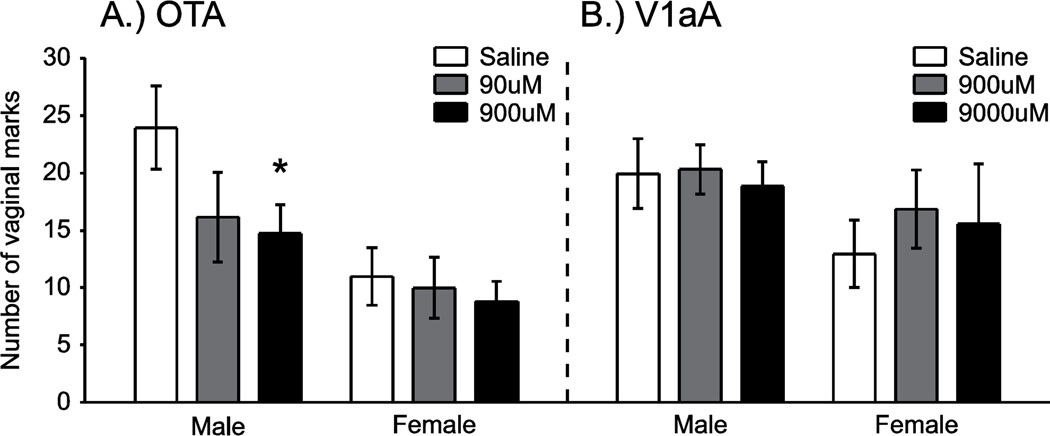
Experiment 2A
ICV injections of OTA decreased vaginal marking to male stimuli (Figure 5A). In the OTA group, overall number of vaginal marks was higher in response to male vs. female stimuli (F (1,16) = 6.52, p = .02). In addition, the overall number of vaginal marks also differed across the three drug doses (F (2,32) = 7.59, p = .002). This effect of OTA dose on vaginal marking differed across the different stimulus conditions (F (2,32) = 3.73, p = .04). In the male stimulus condition, the higher dose of OTA (900 µM) significantly decreased vaginal marking compared to saline injections (t (8) = 3.54, p < .05). There were no differences in the number of vaginal marks between injections of the lower dose of OTA (90 µM) and saline or between the 900 µM and 90 µM doses. In the female stimulus condition, injections of OTA had no effect on vaginal marking responses.
Figure 5.
Mean (± SEM) number of vaginal marks following ICV drug injections. ICV injections of a selective oxytocin receptor antagonist (OTA) decreased vaginal marking in response to male odors, as compared to saline injections (A). OTA was most effective at the highest dose administered (900µM). There was no effect of OTA on vaginal marking in females exposed to female odors. V1aA injections did not affect vaginal marking in females exposed to either male or female odors (B). *p < .05 (Bonferroni correction), saline vs. 900 µM OTA in the male stimulus condition.
ICV injections of V1aA did not mimic the effects of OTA on vaginal marking (Figure 5B). Overall vaginal marking levels tended to be higher in response to male vs. female stimuli, although this difference was not statistically significant. Overall levels of vaginal marking did not differ across the different drug doses, and the drug x stimulus interaction was not significant.
There were no significant effects of ICV injections of OTA or V1aA on flank marking behavior. Females flank marked at similar levels in response to male and female stimuli, and equivalently across the different drug doses. The drug by stimulus interaction was not significant for either group.
Experiment 2B
The expression of Fos following exposure to male stimuli was decreased in BNST following ICV injections of OTA. Within BNSTpm, the overall density of Fos+ cells did not differ in response to male vs. female stimuli (Figure 6A). The overall density of Fos+ cells was, however, lower in females injected with OTA compared to those injected with saline vehicle (F (1,23) = 7.12, p = .005). This effect of OTA differed across the different stimulus conditions (F (1,23) = 5.96, p = .02). In females exposed to male stimuli, OTA significantly decreased the density of Fos+ cells compared to saline (t (12) = 5.03, p < .001). In contrast, there was no effect of OTA on Fos+ cell density in response to female stimuli. A similar pattern was seen in BNSTpm (Figure 6B). The overall density of Fos+ cells was higher in females exposed to male stimuli vs. female stimuli (F (1,23) = 9.67, p = .005), and lower in females injected with OTA vs. saline (F (1,23) = 12.94, p = .002). There was a significant drug x stimulus interaction (F (1,23) = 7.47, p = .01). Specifically, OTA injections significantly decreased the density of Fos+ cells compared to saline injections in females exposed to male stimuli (t (12) = 5.18, p < .001), whereas females exposed to female stimuli exhibited equivalent Fos+ cell densities across drug conditions.
Figure 6.
Mean (+/− SEM) densities of Fos-positive cells following ICV injections of OTA. In females exposed to male odors, ICV injections of a selective oxytocin receptor antagonist (OTA) reduced the density of Fos-positive cells in both the posteromedial (BNSTpm) and posterointermediate (BNSTpi) subdivisions of the bed nucleus of the stria terminalis, as compared to saline injections (A, B). This effect was not seen in females exposed to female odors. Although there was an overall increase in the density of Fos-positive cells in the medial preoptic area (MPOA) and the anterior (MeA) and posterior (MeP) divisions of the medial amygdala in response to male vs. female odors, there was no effect of OTA on Fos expression within these areas (C–E). *p < .05, OTA vs. saline in the male odor condition.
In MPOA, MeA, and MeP, overall Fos+ cell densities were higher in response to male stimuli compared to female stimuli (MPOA: F (1,23) = 12.45, p = .002; MeA: F (1,23) = 13.43, p = .001; MeP: F (1,23) = 13.03, p = .001) (Figure 6B–E). In contrast to BNST, however, there were no overall effects of drug or drug x stimulus interactions.
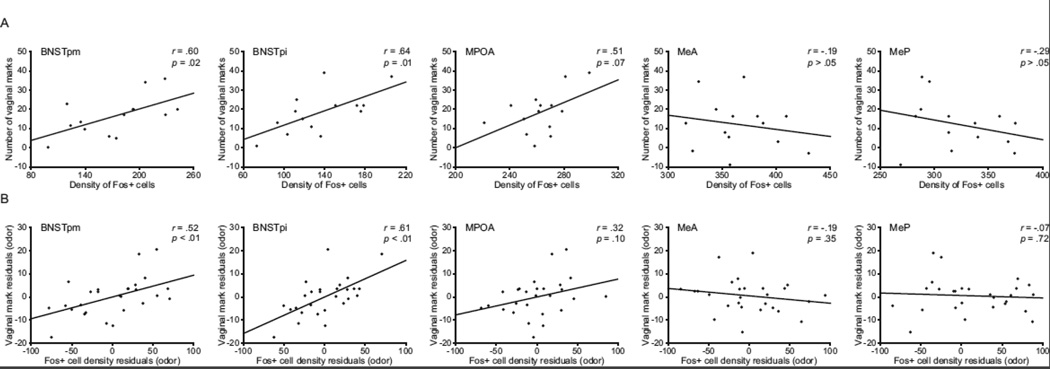
In order determine if the expression of Fos was related to the expression of vaginal marking, this relationship was examined as follows: (1) when looking at females in the male stimulus condition only (Figure 7A) or (2) when the effects of stimulus type on Fos expression and vaginal marking were first removed from the data (Figure 7B). When examining the male stimulus condition only, there were significant, positive correlations between the densities of Fos+ cells and the number of vaginal marks for BNSTpm and BNSTpi, but not MPOA, MeA, or MeP. Likewise, Fos+ cell densities in BNSTpm and BNSTpi, but not MPOA, MeA, or MeP, were significantly and positively correlated with vaginal marking when the effect of stimulus type was removed from vaginal marking data prior to examining the linear relationships.
Figure 7.
Relationships between the density of Fos-positive cells and the number of vaginal marks. When examining responses to male odors only (A), the density of Fos-positive cells was positively correlated with the number of vaginal marks for the posteromedial (BNSTpm) and posterointermediate (BNSTpi) subdivisions of the bed nucleus of the stria terminalis, but not the medial preoptic area (MPOA), the anterior division of the medial amygdala (MeA), or the posterior division of the medial amygdala (MeP). This pattern of results was also seen when looking at overall responses across odor stimulus condition, when first controlling for the effect of odor stimulus on Fos expression and vaginal marking (B).
Discussion
The results presented here demonstrate that the oxytocin system interacts with the processing of sexual odor stimuli to ultimately regulate the expression of an odor-guided solicitational behavior in females, vaginal marking. Specifically, we found that expression of Fos throughout ventral forebrain areas, including BNST, MPOA, and MA, was preferentially induced by male odors. We also show that the same dose of OTA that was behaviorally effective when injected ICV also decreased Fos expression in BNST to male, but not to female, odor stimuli. This effect of OTA on Fos expression was not seen in MPOA or MA. Moreover, Fos expression in BNST, but not in MPOA or MA, was strongly correlated with the expression of vaginal marking by females. Considered together, these results suggest that endogenous oxytocin facilitates preferential vaginal marking to male odors via selective facilitation of male odor-responsive neurons in BNST.
The present study is the first to describe the pattern of Fos expression in ventral forebrain areas of female Syrian hamsters, following exposure to both male and female odors. The increased Fos expression in BNST, MPOA, and MA in response to opposite-sex odors are in agreement with previous data from female mice (Halem et al., 1999), rats (Bennett et al., 2002; Hosokawa and Chiba, 2007), and build upon those previously reported in female hamsters (DelBarco-Trillo et al., 2009). Male odors can therefore induce Fos expression throughout this circuit in a fairly conserved manner across species. The results of the present study also are in good agreement with previous studies examining Fos expression in male hamsters (Been and Petrulis, 2011; Maras and Petrulis, 2010), and suggest that the same circuit processes sexual odors in both males and female hamsters as it does in rats (Bennett et al., 2002; Hurtazo and Paredes, 2005) and mice (Halem et al., 1999; Pankevich et al., 2003).
Unexpectedly, the expression of OT+ cells in BNST was sparse and variable, and so we were unable to examine whether OT+ cells in this area were differentially responsive to sexual odor stimuli. Across the two subdivisions of BNST, we found an average of 3.0 (BNSTpm) and 1.4 (BNSTpi) OT+ cells per female. Furthermore, no OT+ cells were identified in BNSTpm for 3 females, or in BNSTpi for 6 females. It was previously reported that some OT+ cells are present in BNST of female hamsters (Whitman and Albers, 1998), but that study did not quantify oxytocin immunoreactivity in BNST or any brain area. It is therefore difficult to determine if OT+ cells within BNST contribute to any behavioral effects of the oxytocin system observed in the present study.
BNST receives substantial projections from other areas of the brain containing oxytocin cell bodies, including PVH (Wood and Swann, 2005). Indeed, PVH may be the primary source of oxytocin input to BNST, given that OT+ fiber density in this area is substantially reduced following lesions of PVH (de Vries and Buijs, 1983). Furthermore, magnocellular oxytocin cells in PVH and the supraoptic nucleus have been reported in rats to release oxytocin from dendritic stores, ultimately resulting in diffusion of oxytocin to target sites throughout the brain (Bergquist and Ludwig, 2008; Ludwig and Leng, 2006). It seems likely, therefore, that distal sources (i.e., PVH) may be primarily responsible for driving the neural and behavioral effects of oxytocin within BNST. Our findings that Fos expression by OT+ cells in PVH was unchanged in response to male odors, but decreased in response to female odors, suggest that female odors may specifically suppress activation of OT+ cells in this area, ultimately resulting in reduced oxytocin release in BNST. This was unexpected, since blocking OTRs decreases vaginal marking responses to male odors without affecting responses to female odors, either when injected either ICV (Experiment 2A) or directly into BNST/MPOA (Martinez et al., 2010). There are several potential explanations for these findings. It could be that Fos expression does not identify OT+ cells that were activated and subsequently released oxytocin in response to sexual odors. This is a possibility, since Fos expression is dependent on intracellular calcium signaling events associated with persistent, rather than transient, excitation (Lyons and West, 2011). However, Fos expression in OT+ cells is associated with dendritic release of oxytocin (Sabatier et al., 2003), so there likely is some connection in the present study between Fos expression in OT+ cells and oxytocin release. If true, our data suggest that baseline levels of oxytocin release are sufficient for normal vaginal responses to male odors. Furthermore, the suppression of oxytocin release following exposure to female odors would allow females to express other behaviors directed towards same-sex conspecifics that would otherwise be inhibited by oxytocin, such as aggression (Harmon et al., 2002a). Additional studies are required to determine if levels of oxytocin in BNST do indeed fluctuate in response to sexual odor stimulus condition, and if these fluctuations are related to the expression of vaginal marking behavior.
As expected based on our previous study wherein OTA was injected directly into BNST/MPOA (Martinez et al., 2010), ICV injections of OTA decreased vaginal marking to male odors without affecting vaginal marking to female odors. The same dose of OTA (900 µM) was behaviorally effective in both studies, and blockade of V1aRs was not sufficient to mimic the effects of OTA in either study. These data support the conclusion that OTA administered ICV reaches target sites within BNST to ultimately cause a decrease in vaginal marking to male odors; therefore, this manner of injection is appropriate for examining concurrent effects of OTA on neural responses relevant for vaginal marking in this area.
Our findings that ICV injections of OTA selectively decreased Fos expression in BNST to male odors further support the conclusion that this brain area mediates the effects of endogenous oxytocin on solicitational behaviors. This effect of OTA on Fos expression was seen in both subdivisions of BNST examined. Although anatomical data suggest that the two subdivisions may be differentially involved in processing steroid hormone (BNSTpm) and chemosensory (BNSTpi) signals (Been and Petrulis, 2011; Wood and Swann, 2005), both subdivisions of BNST are preferentially activated by opposite-sex odors in hamsters (Maras and Petrulis, 2010). Considered in the context of our previous work wherein we failed to note any obvious differential involvement of BNST subdivisions in the regulation of vaginal marking (Martinez et al., 2010), it seems likely that BNSTpm and BNSTpi function together to mediate the reported neural and behavioral effects of endogenous oxytocin.
The expression of Fos in both subdivisions of BNST was strongly and positively correlated with vaginal marking, independent of odor exposure condition. This relationship was not observed in MPOA or MA, areas that express Fos in response to sexual odor stimuli but likely do not mediate the effects of OTA on vaginal marking. Consequently, Fos expression in BNST may reflect neural activation relevant for the behavioral expression of vaginal marking, and further, individual differences in vaginal marking to male odors may result from differences in the ability of odor stimuli to induce Fos expression in BNST. In contrast, Fos expression in MA and MPOA may instead be more closely linked to other proceptive responses such as opposite-sex odor preference, given that these areas have been functionally linked to the expression of odor preference in female hamsters (Martinez and Petrulis, 2013; Petrulis and Johnston, 1999).
The underlying mechanisms whereby OTA alters neural activity in BNST remain to be more extensively examined. Although it is possible that the effects observed in the present study are exclusively indirect (i.e., driven by effects of OTA in other brain areas that are connected, directly or indirectly, to BNST), this seems unlikely for several reasons. First, there was no effect of OTA on Fos expression in MA or MPOA, two areas that are highly interconnected with BNST (Coolen and Wood, 1998; Wood and Swann, 2005). Second, the behavioral effects of ICV-injected OTA are identical to those observed following direct injections in BNST, as described above. Finally, OTRs are present in BNST (Dubois-Dauphin et al., 1992), and in vitro application of oxytocin onto slices containing BNST results in persistent excitation of BNST neurons (Ingram et al., 1990). Consequently, endogenous oxytocin may act in BNST to increase excitability of neurons expressing OTRs, thereby modulating behavioral responses to sexual odor stimuli.
In addition to interacting with sexual odor signaling within BNST, oxytocin may also interact with gonadal steroid systems in this brain area to regulate the expression of reproductive behaviors. Vaginal marking is highly sensitive to circulating levels of gonadal steroids (Takahashi, 1990), and receptors for estradiol and progesterone are present in BNST (Li et al., 1993; Munn et al., 1983). In rats, estradiol potentiates the release of oxytocin within the nearby MPOA, and treatment with progesterone further potentiates this release (Caldwell, 1992). Although it is not known how steroid hormones modulate oxytocin release within BNST, estradiol treatment has been reported to potentiate the excitation of BNST neurons normally induced following administration of oxytocin (Terenzi et al., 1999). Consequently, it may be that estradiol is necessary for establishing baseline levels of responsiveness of neurons in BNST to oxytocin (perhaps through increasing OTR expression in this area), thereby providing the necessary substrate for mediating the effects of OTA observed in the present study. In contrast to estradiol, it seems unlikely that interactions between oxytocin and the progesterone system are relevant for the neural or behavioral effects of OTA observed here. Females were exposed to odor stimuli on proestrus, a cycle day characterized by relatively low levels of serum progesterone (Saidapur and Greenwald, 1978). Furthermore, progesterone treatment completely suppresses the expression of vaginal marking in estradiol-treated females (Martinez and Petrulis, 2009; Takahashi et al., 1985), whereas oxytocin facilitates the expression of this behavior (Martinez et al., 2010; present study).
In contrast to BNST, no effect of OTA on Fos expression was seen in other quantified brain areas. MPOA has previously been identified as a critical area mediating the effects of oxytocin system manipulations on social and sexual behaviors in female hamsters (Floody et al., 1998; Harmon et al., 2002a, 2002b; Whitman and Albers, 1995). However, the available data do not support a role for MPOA in mediating the effects of oxytocin on vaginal marking. In our previous study targeting BNST or MPOA (Martinez et al., 2010), injections into either area were equally effective at decreasing vaginal marking. It is very likely that OTA was able to diffuse up the injection pathway into the overlying BNST during MPOA-targeted injections; this would not have occurred during BNST injections. Therefore, the most parsimonious explanation for the effects reported in that study is that OTA acted in BNST, not MPOA, to decrease vaginal marking. This conclusion is further supported by the presence of oxytocin binding sites in BNST, but not MPOA, of hamsters (Dubois-Dauphin et al., 1992), and our previous findings that discrete, excitotoxic lesions of BNST eliminate preferential vaginal marking to male odor stimuli (Martinez and Petrulis, 2011), whereas lesions of MPOA do not affect preferential marking (Martinez and Petrulis, 2013).
The finding that there were no effects of OTA on Fos expression in MA was surprising. MA expresses OTRs in hamsters as well as other species (Campbell et al., 2009; Dubois-Dauphin et al., 1992; Lee et al., 2008; Veinante and Freund-Mercier, 1997), and endogenous oxytocin acts within MA to modulate responses to conspecific odor stimuli. In male rats, injections of OTA into MA decreases investigation of odor stimuli from same-sex conspecifics (Arakawa et al., 2010), and ICV injections of OTA decrease Fos expression in MA following exposure to opposite-sex odors in male mice (Samuelsen and Meredith, 2011). Considering the present findings, there likely are substantial species differences in the behavioral and neural effects of oxytocin acting within MA. In agreement with this conclusion, blockade of OTRs in MA disrupts individual recognition in male and female mice (Choleris et al., 2007; Ferguson et al., 2001; Samuelsen and Meredith, 2011), whereas MA is not required for individual recognition in female hamsters (Petrulis and Johnston, 1999).
Finally, OTA also failed to disrupt Fos expression in PVH (data not shown), a brain area that expresses OTRs (Campbell et al., 2009; Yoshimura et al., 1993). This is in good agreement with previous data in rats, wherein ICV injections of OTA did not alter Fos expression in this area (Fitts et al., 2003). Given that PVH was not preferentially activated in response to male odors, it seems unlikely that this area is directly involved in the processing of sexual odor information. PVH may instead function to provide oxytocin to downstream areas that are preferentially responsive to male odors and express OTRs, such as BNST.
It is possible that OTA was unable to affect Fos expression in quantified areas other than BNST because OTA failed to diffuse from the injection site in sufficient concentrations to affect Fos expression within these areas. However, lower doses of OTA (1–200 µM, compared to 900 µM in the present study) injected into the lateral ventricles at the level of MPOA (similar site targeted as the present study) affect MPOA-dependent behaviors in female rats (e.g., lordosis; Pedersen and Boccia, 2006), as well as MA-dependent behaviors and Fos expression in MA in male mice, as described above (Samuelsen and Meredith, 2011). Therefore, it seems likely that OTA was able to successfully reach MPOA and MA in the present study, and that the failure of OTA to affect Fos expression to social odors within these areas can instead be attributed to the site-specificity of the effects of OTA on social odor processing in female hamsters.
Conclusion
Our results indicate that the primary mechanism whereby the oxytocin system regulates solicitational responses towards males is via modulation of cellular activity in male odor-responsive neurons in BNST. These data complement and extend our previous findings that BNST, rather than MPOA, is critical for the preferential targeting of solicitational responses towards male stimuli, and that endogenous oxytocin acts within BNST to drive this effect. Further research is required to determine the precise intracellular signaling pathways mediating the effects of oxytocin on solicitational responding in females.
Highlights.
In female hamsters, Fos expression is preferentially induced by male odors
ICV injections of OTA decreased Fos expression in BNST in response to male odors
Fos expression in PVH oxytocin neurons is not increased in response to male odors
Acknowledgements
The authors would like to thank Alica Helman, Emily Mobley, Jamin Peters, Alix Pijeaux, Manal Tabbaa and July Tran for their technical assistance, and Dr. Anne Murphy for the generous donation of time on her microscope. This work was supported by NIH grant MH072930 to A. Petrulis, a Georgia State University dissertation grant and a Center for Neuromics grant to L. Martinez, and in part by the Center for Behavioral Neuroscience under the STC program of the NSF, under agreement IBN 9876754.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arakawa H, Arakawa K, Deak T. Oxytocin and vasopressin in the medial amygdala differentially modulate approach and avoidance behavior toward illness-related social odor. Neuroscience. 2010;171:1141–1151. doi: 10.1016/j.neuroscience.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm. Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Been LE, Bauman JM, Petrulis A, Chang YH. X-ray kinematics analysis of vaginal scent marking in female Syrian hamsters (Mesocricetus auratus) Physiol. Behav. 2012;105:1021–1027. doi: 10.1016/j.physbeh.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Petrulis A. Chemosensory and hormone information are relayed directly between the medial amygdala, posterior bed nucleus of the stria terminalis, and medial preoptic area in male Syrian hamsters. Horm. Behav. 2011;59:536–548. doi: 10.1016/j.yhbeh.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AL, Greco B, Blasberg ME, Blaustein JD. Response to male odours in progestin receptor- and oestrogen receptor-containing cells in female rat brain. J. Neuroendocrinol. 2002;14:442–449. doi: 10.1046/j.1365-2826.2002.00806.x. [DOI] [PubMed] [Google Scholar]
- Bergquist F, Ludwig M. Dendritic transmitter release: A comparison of two model systems. J. Neuroendocrinol. 2008;20:677–686. doi: 10.1111/j.1365-2826.2008.01714.x. [DOI] [PubMed] [Google Scholar]
- Caldwell JD. Central oxytocin and female sexual behavior. Ann. N. Y. AcadSci. 1992;652:166–179. doi: 10.1111/j.1749-6632.1992.tb34353.x. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Jirikowski GF, Greer ER, Pedersen CA. Medial preoptic area oxytocin and female sexual receptivity. Behav. Neurosci. 1989;103:655–662. doi: 10.1037//0735-7044.103.3.655. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Johns JM, Faggin BM, Senger MA, Pedersen CA. Infusion of an oxytocin antagonist into the medial preoptic area prior to progesterone inhibits sexual receptivity and increases rejection in female rats. Horm. Behav. 1994;28:288–302. doi: 10.1006/hbeh.1994.1024. [DOI] [PubMed] [Google Scholar]
- Campbell P, Ophir AG, Phelps SM. Central vasopressin and oxytocin receptor distributions in two species of singing mice. J. Comp. Neurol. 2009;516:321–333. doi: 10.1002/cne.22116. [DOI] [PubMed] [Google Scholar]
- Choleris E, Little SR, Mong JA, Puram SV, Langer R, Pfaff DW. Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc. Natl. Acad. Sci. U.S.A. 2007;104:4670–4675. doi: 10.1073/pnas.0700670104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals; National Research Council. Guide for the Care and Use of Laboratory Animals. Eighth Edition. Washington,D.C: The National Academies Press; 2011. [Google Scholar]
- Coolen L, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: Simultaneous anterograde and retrograde tract tracing. J. Comp. Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Macrides F, Youngs WM, Schneider SP, Rosene DL. Efferents and centrifugal afferents of the main and accessory olfactory bulbs in the hamster. Brain Res. Bull. 1978;3:59–72. doi: 10.1016/0361-9230(78)90062-x. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- DelBarco-Trillo J, Gulewicz K, Johnston RE. Medial amygdala involvement in discrimination of same-species and closely-related-species male stimuli in estrous female Mesocricetus hamsters. Behav. Neurosci. 2009;123:758–763. doi: 10.1037/a0016439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Pevet P, Barberis C, Tribollet E, Dreifuss JJ. Localization of binding sites for oxytocin in the brain of the golden hamster. Neuroreport. 1992;3:797–800. doi: 10.1097/00001756-199209000-00019. [DOI] [PubMed] [Google Scholar]
- Ferguson J, Aldag J, Insel T, Young L. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 2001;21:8278. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts DA, Thornton SN, Ruhf AA, Zierath DK, Johnson AK, Thunhorst RL. Effects of central oxytocin receptor blockade on water and saline intake, mean arterial pressure, and c-Fos expression in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R1331–R1339. doi: 10.1152/ajpregu.00254.2003. [DOI] [PubMed] [Google Scholar]
- Floody OR. Dissociation of hypothalamic effects on ultrasound production and copulation. Physiol. Behav. 1989;46:299–307. doi: 10.1016/0031-9384(89)90271-0. [DOI] [PubMed] [Google Scholar]
- Floody OR, Cooper TT, Albers HE. Injection of oxytocin into the medial preoptic-anterior hypothalamus increases ultrasound production by female hamsters. Peptides. 1998;19:833–839. doi: 10.1016/s0196-9781(98)00029-1. [DOI] [PubMed] [Google Scholar]
- Gattermann R, Fritzsche P, Neumann K, Al-Hussein I, Kayser A, Abiad M, Yakti R. Notes on the current distribution and the ecology of wild golden hamsters (Mesocricetus auratus) J. Zool. 2001;254:359–365. [Google Scholar]
- Gelez H, Fabre-Nys C. Neural pathways involved in the endocrine response of anestrous ewes to the male or its odor. Neuroscience. 2006;140:791–800. doi: 10.1016/j.neuroscience.2006.02.066. [DOI] [PubMed] [Google Scholar]
- Gomez DM, Newman SW. Differential projections of the anterior and posterior regions of the medial amygdaloid nucleus in the Syrian hamster. J. Comp. Neurol. 1992;317:195–218. doi: 10.1002/cne.903170208. [DOI] [PubMed] [Google Scholar]
- Halem HA, Cherry JA, Baum MJ. Vomeronasal neuroepithelium and forebrain Fos responses to male pheromones in male and female mice. J. Neurobiol. 1999;39:249–263. [PubMed] [Google Scholar]
- Harmon A, Huhman KL, Moore TO, Albers HE. Oxytocin inhibits aggression in female Syrian hamsters. J. Neuroendocrinol. 2002a;14:963–969. doi: 10.1046/j.1365-2826.2002.00863.x. [DOI] [PubMed] [Google Scholar]
- Harmon AC, Moore TO, Huhman KL, Albers HE. Social experience and social context alter the behavioral response to centrally administered oxytocin in female Syrian hamsters. Neuroscience. 2002b;109:767–772. doi: 10.1016/s0306-4522(01)00523-1. [DOI] [PubMed] [Google Scholar]
- Hoshina Y, Takeo T, Nakano K, Sato T, Sakuma Y. Axon-sparing lesion of the preoptic area enhances receptivity and diminishes proceptivity among components of female rat sexual behavior. Behav. Brain Res. 1994;61:197–204. doi: 10.1016/0166-4328(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Chiba A. Effects of sexual experience on conspecific odor preference and male odor-induced activation of the vomeronasal projection pathway and the nucleus accumbens in female rats. Brain Res. 2007;1175:66–75. doi: 10.1016/j.brainres.2007.07.071. [DOI] [PubMed] [Google Scholar]
- Hurtazo HA, Paredes RG. Olfactory preference and Fos expression in the accesory olfactory system of male rats with bilateral lesions of the medial preoptic area/anterior hypothalamus. Neuroscience. 2005;1035:1035–1044. doi: 10.1016/j.neuroscience.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Ingram CD, Cutler KL, Wakerley JB. Oxytocin excites neurones in the bed nucleus of the stria terminalis of the lactating rat in vitro. Brain Res. 1990;527:167–170. doi: 10.1016/0006-8993(90)91078-u. [DOI] [PubMed] [Google Scholar]
- Ingram CD, Wakerley JB. Post-partum increase in oxytocin-induced excitation of neurones in the bed nuclei of the stria terminalis in vitro. Brain Research. 1993;602:325–330. doi: 10.1016/0006-8993(93)90697-l. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Sexual attraction function of golden hamster vaginal secretion. Behav. Biol. 1974;12:111–117. doi: 10.1016/s0091-6773(74)91101-8. [DOI] [PubMed] [Google Scholar]
- Johnston RE. The causation of two scent-marking behaviour patterns in female hamsters (Mesocricetus auratus) Anim. Behav. 1977;25:317–327. doi: 10.1016/0003-3472(77)90007-0. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Vomeronasal and/or olfactory mediation of ultrasonic calling and scent marking by female golden hamsters. Physiol. Behav. 1992;51:437–448. doi: 10.1016/0031-9384(92)90163-v. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Schmidt T. Responses of hamsters to scent marks of different ages. Behav. Neural Biol. 1979;26:64–75. doi: 10.1016/s0163-1047(79)92881-4. [DOI] [PubMed] [Google Scholar]
- Kairys DJ, Magalhaes H, Floody OR. Olfactory bulbectomy depresses ultrasound production and scent marking by female hamsters. Physiol. Behav. 1980;25:143–146. doi: 10.1016/0031-9384(80)90195-x. [DOI] [PubMed] [Google Scholar]
- Kang N, Baum M, Cherry J. A direct main olfactory bulb projection to the “vomeronasal” amygdala in female mice selectively responds to volatile pheromones from males. Eur. J. Neurosci. 2009;29:624. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Sakuma Y. Neuronal activity in female rat preoptic area associated with sexually motivated behavior. Brain Res. 2000;862:90–102. doi: 10.1016/s0006-8993(00)02076-x. [DOI] [PubMed] [Google Scholar]
- Keller M, Baum MJ, Brock O, Brennan PA, Bakker J. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav. Brain Res. 2009;200:268–276. doi: 10.1016/j.bbr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Kwan M, Johnston RE. The role of vaginal secretion in hamster sexual behavior: males’ responses to normal and vaginectomized females and their odors. J. Comp. Physiol. Psychol. 1980;94:905–913. doi: 10.1037/h0077814. [DOI] [PubMed] [Google Scholar]
- Lee H, Macbeth A, Pagani J, Young S. Oxytocin: The great facilitator of life. Prog. Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Caldwell HK, Macbeth AH, Tolu SG, Young WS. A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Blaustein JD, De Vries GJ, Wade GN. Estrogen-receptor immunoreactivity in hamster brain: preoptic area, hypothalamus and amygdala. Brain Res. 1993;631:304–312. doi: 10.1016/0006-8993(93)91549-8. [DOI] [PubMed] [Google Scholar]
- Lisk RD, Ciaccio L, Catanzaro C. Mating behaviour of the golden hamster under seminatural conditions. Anim. Behav. 1983;31:659–666. [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Lyons MR, West AE. Mechanisms of specificity in neuronal activity-regulated gene transcription. Prog. Neurobiol. 2011;94:259–295. doi: 10.1016/j.pneurobio.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog. Brain Res. 2008;170:473–512. doi: 10.1016/S0079-6123(08)00437-8. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. The anterior medial amygdala transmits sexual odor information to the posterior medial amygdala and related forebrain nuclei. Eur. J. Neurosci. 2010;32:469–482. doi: 10.1111/j.1460-9568.2010.07289.x. [DOI] [PubMed] [Google Scholar]
- Martinez LA, Albers HE, Petrulis A. Blocking oxytocin receptors inhibits vaginal marking to male odors in female Syrian hamsters. Physiol. Behav. 2010;101:685–692. doi: 10.1016/j.physbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Petrulis A. Progesterone inhibits vaginal marking in ovariectomized, estradiol benzoate-primed female Syrian hamsters. 2009 [Google Scholar]
- Martinez LA, Petrulis A. The bed nucleus of the stria terminalis is critical for sexual solicitation, but not for opposite-sex odor preference, in female Syrian hamsters. Horm. Behav. 2011;60:651–659. doi: 10.1016/j.yhbeh.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Petrulis A. The medial preoptic area is necessary for sexual odor preference, but not sexual solicitation, in female Syrian hamsters. Horm. Behav. 2013;63:606–614. doi: 10.1016/j.yhbeh.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A stereotaxic atlas of the golden hamster brain. San Diego: Academic Press; 2001. [Google Scholar]
- Munn AR, Sar M, Stumpf WE. Topographic distribution of progestin target cells in hamster brain and pituitary after injection of [3H]R5020. Brain Res. 1983;274:1–10. doi: 10.1016/0006-8993(83)90515-2. [DOI] [PubMed] [Google Scholar]
- Orsini M. The external vaginal phenomena characterizing the stages of the estrous cycle, pregnancy, pseudopregnancy, lactation, and the anestrous hamster, Mesocricetus auratus Waterhouse. Proc. Anim. Care Panel. 1961;11:193–206. [Google Scholar]
- Osborne JW, Overbay A. The power of outliers (and why researchers should always check for them) Pract. Assessment Res. Eval. 2004;9:1–12. [Google Scholar]
- Pankevich D, Baum MJ, Cherry JA. Removal of the superior cervical ganglia fails to block Fos induction in the accessory olfactory system of male mice after exposure to female odors. Neurosci. Lett. 2003;345:13–16. doi: 10.1016/s0304-3940(03)00471-3. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Vasopressin interactions with oxytocin in the control of female sexual behavior. Neuroscience. 2006;139:843–851. doi: 10.1016/j.neuroscience.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Petrulis A. Neural mechanisms of individual and sexual recognition in Syrian hamsters (Mesocricetus auratus) Behav. Brain Res. 2009;200:260–267. doi: 10.1016/j.bbr.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav. Neurosci. 1999;113:345–357. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Peng M, Johnston RE. Effects of vomeronasal organ removal on individual odor discrimination, sex-odor preference, and scent marking by female hamsters. Physiol. Behav. 1999;66:73–83. doi: 10.1016/s0031-9384(98)00259-5. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Kow LM, Loose MD, Flanagan-Cato LM. Reverse engineering the lordosis behavior circuit. Horm. Behav. 2008;54:347–354. doi: 10.1016/j.yhbeh.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Res. Bull. 1997;44:397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- Rose JD. Changes in hypothalamic neuronal function related to hormonal induction of lordosis in behaving hamsters. Physiol. Behav. 1990;47:1201–1212. doi: 10.1016/0031-9384(90)90373-c. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XMM, Jiang M, Ploeg LVder, Leng G. α-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J. Neurosci. 2003;23:10351–10358. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidapur SK, Greenwald GS. Peripheral blood and ovarian levels of sex steroids in the cyclic hamster. Biol. Reprod. 1978;18:401–408. doi: 10.1095/biolreprod18.3.401. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. Oxytocin antagonist disrupts male mouse medial amygdala response to chemical-communication signals. Neuroscience. 2011;180:96–104. doi: 10.1016/j.neuroscience.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheskin DJ. Handbook of parametric and nonparametric statistical procedures. 2nd ed. Boca Raton, FL: Chapman & Hall/CRC; 2000. [Google Scholar]
- Takahashi LK. Hormonal regulation of sociosexual behavior in female mammals. Neurosci. Biobehav. Rev. 1990;14:403–413. doi: 10.1016/s0149-7634(05)80062-4. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Lisk RD, Burnett AL., 2nd Dual estradiol action in diencephalon and the regulation of sociosexual behavior in female golden hamsters. Brain Res. 1985;359:194–207. doi: 10.1016/0006-8993(85)91429-5. [DOI] [PubMed] [Google Scholar]
- Terenzi MG, Jiang QB, Cree SJ, Wakerley JB, Ingram CD. Effect of gonadal steroids on the oxytocin-induced excitation of neurons in the bed nuclei of the stria terminalis at parturition in the rat. Neuroscience. 1999;91:1117–1127. doi: 10.1016/s0306-4522(98)00687-3. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier M-J. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J. Comp. Neurol. 1997;383:305–325. [PubMed] [Google Scholar]
- Whitman DC, Albers HE. Role of oxytocin in the hypothalamic regulation of sexual receptivity in hamsters. Brain Res. 1995;680:73–79. doi: 10.1016/0006-8993(95)00233-g. [DOI] [PubMed] [Google Scholar]
- Whitman DC, Albers HE. Oxytocin immunoreactivity in the hypothalamus of female hamsters. Cell Tissue Res. 1998;291:231–237. doi: 10.1007/s004410050993. [DOI] [PubMed] [Google Scholar]
- Witt DM, Insel TR. A selective oxytocin antagonist attenuates progesterone facilitation of female sexual behavior. Endocrinology. 1991;128:3269–3276. doi: 10.1210/endo-128-6-3269. [DOI] [PubMed] [Google Scholar]
- Wood RI, Swann JM. The bed nucleus of the stria terminalis in the Syrian hamster: subnuclei and connections of the posterior division. Neuroscience. 2005;135:155–179. doi: 10.1016/j.neuroscience.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Kiyama H, Kimura T, Araki T, Maeno H, Tanizawa O, Tohyama M. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993;133:1239–1246. doi: 10.1210/endo.133.3.8396014. [DOI] [PubMed] [Google Scholar]