Abstract
Astrocytes are intimately involved in the mechanisms of neural injury and repair. They participate in a variety of homeostatic functions and elicit repair responses as balance mechanisms. Currently, there is a growing appreciation of a more active role of astrocytes in neuronal signaling and function. One key homeostatic mechanism of astrocytes in tissue repair is maintained through their production of tissue inhibitors of metalloproteinases (TIMPs). The family of TIMPs (1–4) plays a central regulatory role as inhibitors of matrix metalloproteinases (MMPs), enzymes involved in extracellular matrix maintenance and remodeling. Recently, TIMP-1, the inducible form, has been identified as a multifunctional molecule with divergent functions. It participates in wound healing and regeneration, cell morphology and survival, tumor metastasis, angiogenesis, and inflammatory responses. An imbalance of MMP/TIMP regulation has been implicated in several inflammatory diseases of the central nervous system (CNS). Here we review the conundrums of TIMP-1 regulation in CNS pathophysiology. We propose that astrocyte-TIMP-1 may play an important role in CNS homeostasis and disease. Astrocyte TIMP-1 expression is differentially regulated in inflammatory neurodegenerative diseases and may have significant therapeutic relevance.
Keywords: astrocytes, neurodegenerative diseases, inflammation
The remodeling of the extracellular matrix (ECM) components is regulated primarily by the matrix metalloproteinases (MMPs; also known as matrixins; Nagase et al., 1999; Khuth et al., 2001). Twenty-four members of this family are currently known and are categorized into four subclasses: gelatinases, stromelysins, collagenases, and membrane-type MMPs. Through the degradation of the ECM, MMPs participate in many normal physiological processes, such as organ morphogenesis, ovulation, embryonic development, bone remodeling, and angiogenesis (Gomez et al., 1997; Brew et al., 2000; Yang et al., 2002). These enzymes function at neutral pH and are normally found on the cell surface or in the extracellular space (Mannello and Gazzanelli, 2001). MMP activity is regulated transcriptionally by cytokines, growth factors, and hormones and also by the proteolytic cleavage of the inactive zymogen (Brew et al., 2000). In addition, α2-macroglobulin and tissue inhibitors of metalloproteinases (TIMPs) can inhibit MMP activity in the fluid phase and the tissue, respectively (Nagase et al., 1999; Brew et al., 2000). Therefore, a favorable balance between MMP activity and inhibition by TIMPs is essential for preventing pathological conditions such as arthritis, tumor cell invasion and metastasis, periodontal disease, neurodegenerative disorders, atherosclerosis, and fibrosis (Nagase et al., 1999; Brew et al., 2000; Yang et al., 2002).
Four different TIMPs (TIMP-1, -2, -3, and -4) have been identified in vertebrates and share approximately 40% sequence similarity, including 12 conserved cysteine residues (Nagase et al., 1999). TIMPs are dimers consisting of an N-terminal domain and a smaller C-terminal domain that are both stabilized by three disulfide bonds (Nagase et al., 1999). The N-terminal domain, which noncovalently binds to the MMP substrate at the active zinc-binding site, is necessary and sufficient for inhibition of MMPs (Gomez et al., 1997). Human TIMP-1 is a soluble glycoprotein of 184 amino acids (Gomez et al., 1997; Nagase et al., 1999). Among the family of TIMPs, TIMP-1 is the inducible form and is up-regulated by factors such as phorbol esters, interleukin (IL)-1β, transforming growth factor (TGF)-β1, retinoids, epithelial growth factor (EGF), IL-6, oncostatin, and leukemia inhibitory factor (Gomez et al., 1997). Concanavalin A and dexamethasone are suppressive agents of TIMP-1 expression (Gomez et al., 1997). TIMP-2, consisting of 194 amino acids, is a soluble, non-glycosylated protein. It is generally constitutively expressed (Gomez et al., 1997; Nagase et al., 1999). TIMP-3 is unglycosylated and insoluble, binding tightly to the components of the ECM, and has been shown to promote the detachment of transformed cells (Gomez et al., 1997). Unlike TIMP-1, both TIMP-2 and TIMP-3 are effective inhibitors of the membrane-type MMPs (Brew et al., 2000). TIMP-3 is also the only TIMP that can inhibit tumor necrosis factor-α–converting enzyme (TACE), an adamalysin, not an MMP (Brew et al., 2000). TIMP-4, the most recently discovered, has been cloned from a human heart cDNA library (Gomez et al., 1997). TIMP-4 is expressed at high levels in the human heart, but overexpression has been known to cause apoptosis (Gomez et al., 1997).
Developmentally, TIMP-1 is regulated in an intricate fashion. For example, in rats, expression is reduced significantly during embryogenesis, followed by an increase before birth, and subsequent decrease postnatally (Fager and Jaworski, 2000). Early in embryonic development, TIMP-1 is expressed profusely in the telencephalic and mesencephalic ventricular zones (Fager and Jaworski, 2000). Late in ontogeny, TIMP-1 declines rapidly, and its expression is confined, in large measure, to the cerebellum (Fager and Jaworski, 2000). After birth, TIMP-1 continues to be weakly expressed and found almost exclusively in hippocampal pyramidal cells and cerebellar granule cells (Fager and Jaworski, 2000).
In addition to its role in regulating MMPs, TIMP-1 has been implicated in a number of other biological processes, including growth factor activity, tissue remodeling, inhibition of angiogenesis, changes in cell morphology, and stimulation of gonadal steroidogenesis (see Table I; Gomez et al., 1997). In the CNS, TIMP-1 provides neuroprotective effects through its role in blood–brain barrier (BBB) maintenance, which is accomplished by interacting with ECM components, inhibiting MMPs, and reducing glutamate-mediated calcium influx following excitotoxic stress (Tan et al., 2003). In the context of brain pathogenesis, TIMP-1 is linked to diseases such as multiple sclerosis (MS), Parkinson’s disease (PD), and human brain tumors (Nakagawa et al., 1994; Kouwenhoven et al., 2001; Lorenzl et al., 2002).
TABLE I.
Divergent Functions of TIMP-1
| Functions | Induces process | Inhibits process |
|---|---|---|
| Inhibition of MMPs (Gomez et al., 1997; Pagenstecher et al., 1998) | X | |
| Stimulation of gonadal steroidogenesis (Gomez et al., 1997) | X | |
| Tissue remodeling (Gomez et al., 1997; Rivera et al., 1997) | X | |
| Apoptosis (Brew et al., 2000; Tan et al., 2003) | X | X |
| Angiogenesis (Gomez et al., 1997; Nguyen et al., 2001) | X | |
| Changes in cell morphology (Gomez et al., 1997) | X | |
| Glioma malignancy (Brew et al., 2000; Groft et al., 2001; VanMeter et al., 2001) | X | X |
Currently, it is unclear whether TIMP-1 always plays a protective role in the CNS. For example, up-regulation of TIMP-1 expression was observed in neurinomas and meningiomas (Nakagawa et al., 1994). The increase of TIMP-1 may serve to counterbalance the simultaneous rise in MMP-9 (Nakagawa et al., 1994). However, because of its potential trophic activity, TIMP-1 may enhance the malignancy of certain types of tumors (Groft et al., 2001). Furthermore, studies with rodents, including generation of global forebrain ischemia in rats, modeling of hydrocephalus in transgenic mice, kainate induction of rat excitotoxic seizures, and mouse CNS infection with Morbillivirus, have shown that TIMP-1 induction often appears to be regulated in a time-, cell-, and space-dependent manner. (Rivera et al., 1997, 2002; Khuth et al., 2001; Zechel et al., 2002). Certainly, TIMP-1 regulation plays an important role in inflammatory diseases of the CNS; however, the ever-increasing list of TIMP-1 functions continues to add to the questions regarding its mechanism and role in CNS homeostasis and disease.
TIMP-1 AND INFLAMMATION
The balance between MMPs and TIMPs is linked to ECM remodeling and is associated with conditions such as arthritis, angiogenesis, and tumor metastasis. TIMPs affect cell proliferation, survival, and apoptosis and function as guardians against tissue degradation (Fassina et al., 2000). TIMP/MMP imbalance is also implicated in the pathogenesis of CNS disorders involving inflammation. In almost all types of CNS disease, including trauma and inflammation, reactive astrogliosis is a stereotypic response (Norenberg, 1994; Ridet et al., 1997). In a detailed study regarding temporal and spacial distribution of TIMPs and MMPs, Pagenstecher et al. (1998) reported a significant increase in TIMP-1 expression in astrocytes in experimental autoimmune encephalitis (EAE) and transgenic mice expressing cytokines under the glial fibrillary acidic protein (GFAP) promoter. It makes biological sense that production of TIMP-1 by activated astrocytes would serve in remodeling of brain ECM, given the protective and homeostatic functions of TIMP-1. In situ immunolocalization studies have demonstrated that reactive astrocytes in animal models of CNS injury are known to produce TIMP-1 (Rivera et al., 1997; Pagenstecher et al., 1998; Jaworski, 2000). Proinflammatory cytokines such as IL-1β and TNF-α up-regulated TIMP-1 in cultured astrocytes and endothelial cells (Bugno et al., 1999; Suryadevara et al., 2003). Viral infections of the CNS that result in inflammatory disease also involve MMP/TIMP imbalance. Morbillivirus infection of mouse CNS resulted in up-regulation of TIMP-1 mRNA in affected brain areas and was correlated with an increase in Th1-like cytokines, interferon (IFN)-γ, TNF-α, and IL-6 (Khuth et al., 2001). In a model system of human T-lymphotropic virus (HTLV) type 1-infected T cells–astrocyte interactions, astrocytes become reactive, secrete proinflammatory cytokines, and express high levels of TIMP-1 and -3 (Szymocha et al., 2000).
Within the context of human immunodeficiency virus (HIV)-1-associated dementia (HAD), TIMP-1 expression by astrocytes is likely altered within the CNS even though astrocytes are rarely infected, if at all, with HIV-1. Our previous work and other studies have already established that there is MMP dysregulation in HAD (Conant et al., 1999; Ghorpade et al., 2001). IL-1 expression is up-regulated in at least eight major diseases/disorders of the brain, including epilepsy and HAD (Vitkovic et al., 2000), and IL-1β is a prototypical proinflammatory immune activator. Mononuclear phagocytes that are infected with virus and/or are immune activated provide a logical source to drive the inflammation and consequently up-regulate TIMP-1 (Ghorpade et al., 1998; Persidsky et al., 1999; Persidsky and Ghorpade, 2001).
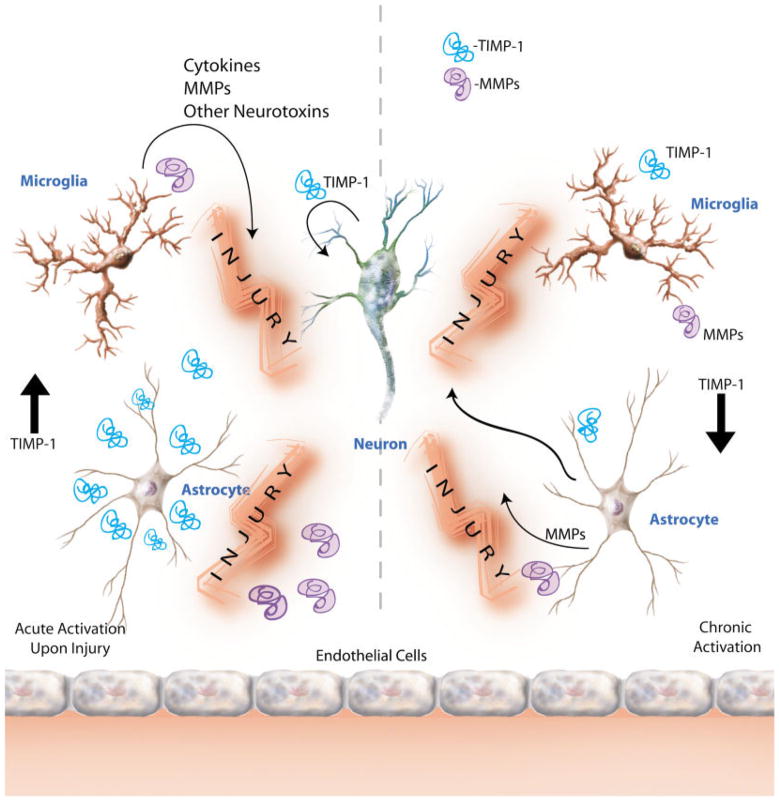
In vitro studies with primary human fetal astrocytes and further biological conformation using cerebrospinal fluid (CSF) and brain tissue reflected differential TIMP-1 regulation. TIMP-1 mRNA and protein increased significantly with acute activation of astrocytes with IL-1β; however, CSF and brain tissue from HAD patients showed significant down-regulation (Suryadevara et al., 2003). Our results appear to corroborate our hypothesis that, under disease conditions, acute immune activation of astrocytes increases TIMP-1 as an attempted repair and protective response. However, with chronic activation, as observed in CSF and brain tissues from patients with end-stage neurological disease, this TIMP-1-mediated response fails as a result of down-regulation of TIMP-1. The reduction in protein expression ultimately brings about withdrawal of TIMP-1 neuroprotection and enhanced neuronal malfunction. These studies demonstrate that astrocytes may behave differently with acute vs. chronic activation. A model for these events is proposed in Figure 1. Further investigation aimed at discovering the mechanism of differential TIMP-1 regulation will be important in understanding the role of astrocytes in inflammatory diseases of the CNS.
Fig. 1.
Proposed model for astrocyte TIMP-1 regulation in acute and chronic inflammation. We propose that there is differential regulation of TIMP-1 in astrocytes under acute and chronic immune activation. Acute activation of neural cells (astrocytes, microglia, and neurons) by proinflammatory cytokines such as IL-1β may lead to enhanced levels of TIMP-1 in the tissue microenvironment and elicit a typical repair response early in injury (left). However, under sustained inflammatory conditions, such as those observed in chronic neurodegenerative diseases (HAD and AD), TIMP-1 levels in the CNS decline significantly below homeostatic levels (right). A better understanding of the mechanism of this duality will lead to insights into development of new therapeutic options relevant to treatment of a variety of diseases involving neural injury.
TIMP-1 AND NEURONAL INJURY/PROTECTION
The idea that TIMP-1 up-regulation modulates the general neuronal responses following excitotoxic injury has received increased attention in the recent literature. Tan et al. (2003) showed that application of TIMP-1 protected neurons against excitotoxic injury induced with glutamate. Altered MMP and TIMP metabolism is implicated in a variety of diseases, including cardiovascular pathologies (Stetler-Stevenson, 1996), cancer (Kleiner and Stetler-Stevenson, 1999), inflammatory diseases, and neurodegenerative diseases (Dhawan et al., 1995). The different members of the TIMP family have different effects on the pathways of programmed cell death. Unique among these, although not the focus of this review, is TIMP-3, which promotes cell cycle entry, followed by death via apoptosis. In contrast to TIMP-3 (for review see Mannello and Gazzanelli, 2001), TIMP-1 can suppress apoptosis in Burkitt’s lymphoma cell lines and inhibit excitotoxic death in neurons (Tan et al., 2003). Thus, in a cell-type-specific manner, TIMP-1 and -2 may function as suppressors of apoptosis and TIMP-3 and -4 promoters. This inconsistency within the TIMP family members regarding their effects on cell death certainly adds to the list of phenomena as yet unexplained.
The antiapoptotic effects of TIMP-1 occur through mechanisms that are sometimes, but not always, linked to MMPs. In hepatic stellate cells, TIMP-1-mediated inhibition of apoptosis is through its effects on MMP (Murphy et al., 2002). In B cells, TIMP-1 is known to up-regulate the antiapoptotic pathway through Bcl-XL, independently of its activity on MMPs (Guedez et al., 1998). TIMP-1 can bind to cell-surface targets (Ritter et al., 1999) and also accumulate within the nucleus (Zhao et al., 1998), indicating its participation in cell signaling. Other studies have shown that TIMP-1, in addition to these direct effects on cell death and cell cycle signaling, may play a role in neuronal injury/repair. Rivera et al. (1997) studied rat CNS TIMP-1 profiles after kainite-induced excitotoxic seizures. Their data revealed age-, time-, region-, and cell-dependent changes in TIMP-1 profiles. For example, immediately after kainate-induction of excitotoxic seizures, there is an early period marked by rapid TIMP-1 mRNA increase in the stratum granulosum, the ependymal cell layer of the ventricles, and the meninges. A second phase of intense TIMP-1 production in astrocytes surrounding the lesion area follows, illustrating the complexity in regulation of TIMP-1 expression (Rivera et al., 2002). Neurons were the first to express TIMP-1, and then, within 72 hr, astrocytes expressed high TIMP-1 levels that lasted as long as 2 weeks. Whether this 2-week period reflects the chronic activation observed in endpoints of human neurodegenerative diseases is unclear. These studies together reflect the fact that TIMP-1 regulation in astrocytes can be elicited in response to a variety of stimuli, including neuronal injury and inflammation, and have important implications for the role of glial inflammation in disease and therapy.
TIMP-1 AND GLIOMAS
As noted above, TIMP-1 can function as an anti-apoptotic and survival factor, and its levels increase in response to inflammation. Such processes may contribute to exacerbation of the malignant process. Malignant glioma is one of the most common and aggressive forms of brain tumor, with a very poor prognosis. Although there is broad support for the idea that TIMPs, in general, hamper the malignant process, in human malignant non-Hodgkin’s lymphomas (NHL), higher levels of TIMP-1 correlate with poor clinical outcome for patients (Kossa-kowska et al., 2000). In the CNS, TIMP-1 may be beneficial in a neurodegenerative setting because of its protective effects on neurons, but it may have different effects on gliomas. Groft et al. (2001) showed that TIMP family members had differential localization in human gliomas and that TIMP-1 levels had a positive correlation with glioma malignancy. Given that TIMP-1 has effects on ECM breakdown, angiogenesis, cell morphology, and cell survival/death, it is the net outcome of the balance of these functions that would determine the pathophysiology of glioma cells. Some of the in vitro studies with recombinant TIMP-1 and glioma cells have shown reduced glioma invasion (VanMeter et al., 2001); however, these in vitro data may not be replicated in vivo given the other effects that TIMP-1 can exert. Reviews and reports discussing the pros and cons of TIMP-1 gene therapy for cancer treatment abound (for review see Brand, 2002). Although there is a general agreement that TIMP-1, and TIMPs in general, could be a double-edged sword because of their antitumor and tumor-promoting effects, reports that support the beneficial anticancer effects of TIMP-1 make up the majority. The concentrations of TIMPs at the tumor site have to be superphysiological, and the size of the tumor plays an important role. The use of TIMP-1 gene therapy in gliomas thus may be limited. Takahashi et al. (2002) recently established a rodent model for glioma invasion by using C6 rat glioma cells and studied the effect of TIMPs. In these studies, TIMP-1 did not have a dramatic effect on tumor invasion. In summary, the utility of TIMP-1 therapy for glioma treatment is still uncertain.
PRÉCIS AND PERSPECTIVES
In addition to its predominant function as an inhibitor of MMP activity, TIMP-1 has emerged as a versatile and multifunctional molecule that affects the CNS. Ubiquitous functions of TIMP-1 include mitogenic effects, inhibition of angiogenesis, modulation of cell morphology, and tissue remodeling. Regulation by inflammatory mediators makes it a candidate in neuropathophysiology of CNS diseases that involve inflammation. Of particular interest is the finding that acute and chronic immune activation differentially regulated TIMP-1 in astrocytes, the predominant neuronal protectors in the CNS. In many respects, our understanding of how TIMP-1 functions in the CNS is still superficial. The recognition of TIMP-1 as a plausible therapeutic candidate for neuronal repair is as yet understudied and, given TIMP-1’s diverse functions and therapeutic potential, warrants further work.
Acknowledgments
We thank Ms. Robin Taylor for excellent administrative support and Dr. Kim Carlson for critical reading of the manuscript. This work was supported in part by grant 77408-25-PFR from the Pediatrics AIDS Foundation and grant NS31492-06 from the NINDS to A.G.
References
- Brand K. Cancer gene therapy with tissue inhibitors of metalloproteinases (TIMPs) Curr Gene Ther. 2002;2:255–271. doi: 10.2174/1566523024605564. [DOI] [PubMed] [Google Scholar]
- Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- Bugno M, Witek B, Bereta J, Bereta M, Edwards DR, Kordula T. Reprogramming of TIMP-1 and TIMP-3 expression profiles in brain microvascular endothelial cells and astrocytes in response to proinflammatory cytokines. FEBS Lett. 1999;448:9–14. doi: 10.1016/s0014-5793(99)00323-3. [DOI] [PubMed] [Google Scholar]
- Conant K, McArthur JC, Griffin DE, Sjulson L, Wahl LM, Irani DN. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46:391–398. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Dhawan S, Weeks BS, Soderland C, Schnaper HW, Toro LA, Asthana SP, Hewlett IK, Stetler-Stevenson WG, Yamada S, Yamada K, Meltzer MS. HIV-1 infection alters monocyte interactions with human micro-vascular endothelial cells. J Immunol. 1995;154:422–432. [PubMed] [Google Scholar]
- Fager N, Jaworski DM. Differential spatial distribution and temporal regulation of tissue inhibitor of metalloproteinase mRNA expression during rat central nervous system development. Mech Dev. 2000;98:105–109. doi: 10.1016/s0925-4773(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Fassina G, Ferrari N, Brigati C, Benelli R, Santi L, Noonan DM, Albini A. Tissue inhibitors of metalloproteases: regulation and biological activities. Clin Exp Metast. 2000;18:111–120. doi: 10.1023/a:1006797522521. [DOI] [PubMed] [Google Scholar]
- Ghorpade A, Nukuna A, Che M, Haggerty S, Persidsky Y, Carter E, Carhart L, Shafer L, Gendelman HE. Human immunodeficiency virus neurotropism: an analysis of viral replication and cytopathicity for divergent strains in monocytes and microglia. J Virol. 1998;72:3340–3350. doi: 10.1128/jvi.72.4.3340-3350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorpade A, Persidskaia R, Suryadevara R, Che M, Liu XJ, Persidsky Y, Gendelman HE. Mononuclear phagocyte differentiation, activation, and viral infection regulate matrix metalloproteinase expression: implications for human immunodeficiency virus type 1-associated dementia. J Virol. 2001;75:6572–6583. doi: 10.1128/JVI.75.14.6572-6583.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- Groft LL, Muzik H, Rewcastle NB, Johnston RN, Knauper V, Lafleur MA, Forsyth PA, Edwards DR. Differential expression and localization of TIMP-1 and TIMP-4 in human gliomas. Br J Cancer. 2001;85:55–63. doi: 10.1054/bjoc.2001.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedez L, Stetler-Stevenson WG, Wolff L, Wang J, Fukushima P, Mansoor A, Stetler-Stevenson M. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J Clin Invest. 1998;102:2002–2010. doi: 10.1172/JCI2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski DM. Differential regulation of tissue inhibitor of metalloproteinase mRNA expression in response to intracranial injury. Glia. 2000;30:199–208. doi: 10.1002/(sici)1098-1136(200004)30:2<199::aid-glia9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Khuth ST, Akaoka H, Pagenstecher A, Verlaeten O, Belin MF, Giraudon P, Bernard A. Morbillivirus infection of the mouse central nervous system induces region-specific upregulation of MMPs and TIMPs correlated to inflammatory cytokine expression. J Virol. 2001;75:8268–8282. doi: 10.1128/JVI.75.17.8268-8282.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D, Stetler-Stevenson W. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43(Suppl):S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- Kossakowska AE, Urbanski SJ, Janowska-Wieczorek A. Matrix metalloproteinases and their tissue inhibitors—expression, role and regulation in human malignant non-Hodgkin’s lymphomas. Leuk Lymphoma. 2000;39:485–493. doi: 10.3109/10428190009113379. [DOI] [PubMed] [Google Scholar]
- Kouwenhoven M, Ozenci V, Gomes A, Yarilin D, Giedraitis V, Press R, Link H. Multiple sclerosis: elevated expression of matrix metalloproteinases in blood monocytes. J Autoimmun. 2001;16:463–470. doi: 10.1006/jaut.2001.0505. [DOI] [PubMed] [Google Scholar]
- Lorenzl S, Albers DS, Narr S, Chirichigno J, Beal MF. Expression of MMP-2, MMP-9, and MMP-1 and their endogenous counterregulators TIMP-1 and TIMP-2 in postmortem brain tissue of Parkinson’s disease. Exp Neurol. 2002;178:13–20. doi: 10.1006/exnr.2002.8019. [DOI] [PubMed] [Google Scholar]
- Mannello F, Gazzanelli G. Tissue inhibitors of metalloproteinases and programmed cell death: conundrums, controversies and potential implications. Apoptosis. 2001;6:479–482. doi: 10.1023/a:1012493808790. [DOI] [PubMed] [Google Scholar]
- Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition. Implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069–11076. doi: 10.1074/jbc.M111490200. [DOI] [PubMed] [Google Scholar]
- Nagase H, Meng Q, Malinovskii V, Huang W, Chung L, Bode W, Maskos K, Brew K. Engineering of selective TIMPs. Ann N Y Acad Sci. 1999;878:1–11. doi: 10.1111/j.1749-6632.1999.tb07670.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kubota T, Kabuto M, Sato K, Kawano H, Hayakawa T, Okada Y. Production of matrix metalloproteinases and tissue inhibitor of metalloproteinases-1 by human brain tumors. J Neurosurg. 1994;81:69–77. doi: 10.3171/jns.1994.81.1.0069. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Arkell J, Jackson CJ. Human endothelial gelatinases and angiogenesis. Int J Biochem Cell Biol. 2001;33:960–970. doi: 10.1016/s1357-2725(01)00007-3. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. Astrocyte responses to CNS injury. J Neuropathol Exp Neurol. 1994;53:213–220. doi: 10.1097/00005072-199405000-00001. [DOI] [PubMed] [Google Scholar]
- Pagenstecher A, Stalder AK, Kincaid CL, Shapiro SD, Campbell IL. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol. 1998;152:729–741. [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Ghorpade A. HIV-1 traffic: cell-free, cell associated and other CNS activating stories. AIDScience online 1. 2001 http://aidscience.org/Articles/aidscience014.asp.
- Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L, Gendelman HE. Microglial and astrocyte chemokines regulate monocyte migration through the blood–brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1599–1611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Ritter LM, Garfield SH, Thorgeirsson UP. Tissue inhibitor of metalloproteinases-1 (TIMP-1) binds to the cell surface and translocates to the nucleus of human MCF-7 breast carcinoma cells. Biochem Biophys Res Commun. 1999;257:494–499. doi: 10.1006/bbrc.1999.0408. [DOI] [PubMed] [Google Scholar]
- Rivera S, Tremblay E, Timsit S, Canals O, Ben-Ari Y, Khrestchatisky M. Tissue inhibitor of metalloproteinases-1 (TIMP-1) is differentially induced in neurons and astrocytes after seizures: evidence for developmental, immediate early gene, and lesion response. J Neurosci. 1997;17:4223–4235. doi: 10.1523/JNEUROSCI.17-11-04223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera S, Ogier C, Jourquin J, Timsit S, Szklarczyk AW, Miller K, Gearing AJ, Kaczmarek L, Khrestchatisky M. Gelatinase B and TIMP-1 are regulated in a cell- and time-dependent manner in association with neuronal death and glial reactivity after global forebrain ischemia. Eur J Neurosci. 2002;15:19–32. doi: 10.1046/j.0953-816x.2001.01838.x. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix [comment] Am J Pathol. 1996;148:1345–1350. [PMC free article] [PubMed] [Google Scholar]
- Suryadevara R, Holter S, Borgmann K, Persidsky R, Labenze-Zink C, Persidsky Y, Gendelman HE, Wu L, Ghorpade A. Regulation of tissue inibitor of metalloproteinase-1 by astrocytes: links to HIV-1 dementia. Glia. 2003;44:47–56. doi: 10.1002/glia.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymocha R, Akaoka H, Brisson C, Beurton-Marduel P, Chalon A, Bernard A, Didier-Bazes M, Belin MF, Giraudon P. Astrocytic alterations induced by HTLV type 1-infected T lymphocytes: a role for Tax-1 and tumor necrosis factor alpha. AIDS Res Hum Retrovir. 2000;16:1723–1729. doi: 10.1089/08892220050193218. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Fukami S, Iwata N, Inoue K, Itohara S, Itoh H, Haraoka J, Saido T. In vivo glioma growth requires host-derived matrix metalloproteinase 2 for maintenance of angioarchitecture. Pharmacol Res. 2002;46:155–163. doi: 10.1016/s1043-6618(02)00081-6. [DOI] [PubMed] [Google Scholar]
- Tan HK, Heywood D, Ralph GS, Bienemann A, Baker AH, Uney JB. Tissue inhibitor of metalloproteinase 1 inhibits excitotoxic cell death in neurons. Mol Cell Neurosci. 2003;22:98–106. doi: 10.1016/s1044-7431(02)00024-6. [DOI] [PubMed] [Google Scholar]
- VanMeter TE, Rooprai HK, Kibble MM, Fillmore HL, Broaddus WC, Pilkington GJ. The role of matrix metalloproteinase genes in glioma invasion: co-dependent and interactive proteolysis. J Neurooncol. 2001;53:213–235. doi: 10.1023/a:1012280925031. [DOI] [PubMed] [Google Scholar]
- Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5:604–615. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- Yang EV, Bane CM, MacCallum RC, Kiecolt-Glaser JK, Malarkey WB, Glaser R. Stress-related modulation of matrix metalloproteinase expression. J Neuroimmunol. 2002;133:144–150. doi: 10.1016/s0165-5728(02)00270-9. [DOI] [PubMed] [Google Scholar]
- Zechel J, Gohil H, Lust WD, Cohen A. Alterations in matrix metalloproteinase-9 levels and tissue inhibitor of matrix metalloproteinases-1 expression in a transforming growth factor-beta transgenic model of hydrocephalus. J Neurosci Res. 2002;69:662–668. doi: 10.1002/jnr.10326. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Li H, Yamashita K, Guo XK, Hoshino T, Yoshida S, Shinya T, Hayakawa T. Cell cycle-associated accumulation of tissue inhibitor of metalloproteinases-1 (TIMP-1) in the nuclei of human gingival fibroblasts. J Cell Sci. 1998;111:1147–1153. doi: 10.1242/jcs.111.9.1147. [DOI] [PubMed] [Google Scholar]