Abstract
Purpose of review
Soon after the discovery of HIV-infected humans, rhesus macaques in a colony at the New England Primate Research Center showed similar signs of a progressive immune suppression. The discovery of the Simian Immunodeficiency Virus (SIV)-associated disease opened the door to study an AIDS-like illness in nonhuman primates (NHP). Even after three decades, this animal model remains an invaluable tool to provide a greater insight into HIV immunopathogenesis. In this review, recent progress in deciphering pathways of immunopathogenesis in SIV-infected NHP is discussed.
Recent findings
The immense diversity of mutations in SIV stocks prepared at different laboratories has recently been realized. The massive expansion of the enteric virome is a key finding in SIV-induced immunopathogenesis. Defining the function of host restriction factors, like the recently discovered SAMHD1, helps to evaluate the impact of the innate immune responses on virus replication. Utilization of pyrosequencing and defining molecular mechanisms of MHC class I restriction helps to understand how the virus evades CD8+ T cell responses. The definition of MHC class I molecules in different NHP species provides new animal models to study SIV immunopathogenesis. T follicular helper cells have gained major interest in characterizing humoral immune responses in SIV infection and AIDS vaccine strategies. The ability of natural hosts to remain disease-free despite ongoing replication of SIV is continuing to puzzle the field.
Summary
The HIV research field continues to realize the immense complexity of the host virus interaction. NHP present an invaluable tool to make progress towards an effective AIDS vaccine.
Keywords: SIV, immunopathogenesis, nonhuman primates, NHP
INTRODUCTION
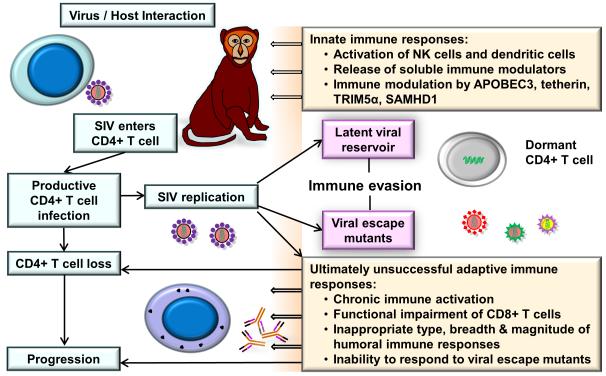
Even after three decades of its original discovery, the HIV epidemic is one of the major societal health problems that humans are facing [1,2]. During the last decades, progress has been made on multiple major fronts to understand the biology of the virus and its immunopathogenesis in infected individuals, and to develop treatment modalities and generate possible AIDS vaccine candidates. However, the intricate interaction between HIV and the human host has still not been fully understood and an effective AIDS vaccine is still out of reach [3]. The discovery of SIV has greatly helped the field to provide a valuable animal model to study an AIDS-like disease in NHP [4-6]. The major focus of this review is to discuss recent progress for a better understanding of immunopathogenesis and the role of innate and adaptive immune responses in SIV-infected NHP (Figure 1).
FIGURE 1.
Key aspects of SIV immunopathogenesis. Successful pathogenic infection with SIV typically results in a progressive loss of CD4+ T cells. The interaction of SIV with the host’s immune system triggers innate immune responses followed by virus-specific adaptive cellular and humoral immune responses. Rapidly occurring mutations lead to immune evasion. Already early following infection, viral reservoirs are being established that protect the virus from immune control. Chronic immune activation contributes to the functional impairment of the immune system. As the disease progresses, adaptive immune responses are unsuccessful in containing the virus replication, and overt signs of a chronic immune suppression become evident.
PATHOLOGIC IMPACT OF SIV INFECTION
Mucosal transmission in humans and NHP entails a significant bottleneck for the virus resulting in a limited number of transmitted/founder viruses [7,8]. Moreover, the complexity of SIV mutants gets further complicated by the particular way how and where virus stocks are prepared [9,10].
Very early following transmission, AIDS virus infection leads to massive pathologic changes in mucosal tissues, e.g., gastrointestinal, cervico-vaginal and penile tissues [11]. This includes local inflammation and epithelial cell injury [12] resulting in microbial translocation [13]. While preexisting mucosal inflammation may enhance the pathogenicity of the virus infection [14], probiotic/prebiotic supplementation can improve the gastrointestinal immunity in SIV-infected macaques [15]. As the virus relentlessly replicates during primary SIV infection, it also attracts more “fuel into the fire” in form of additional CD4+ target cells and dendritic cells that results in further immune activation [16]. Blocking the α4β7 integrin gut-homing associated influx of these cells mitigates the severity of the infection resulting in significantly decreased viral loads [17].
The pathologic impact of enteric bacteria has been well characterized [18,19]. However, surprisingly SIV infection in rhesus macaques is also associated with a massive expansion of the enteric virome [20]. One can assume that the increased inflammation in the gastrointestinal tissues supports the increased replication of the enteric virome. In addition, following SIV infection cellular immune responses are likely not as efficient as they should be in containing the replication of these viruses.
Next to mucosal sites, chronic SIV infection has a global pathologic impact on practically all organ systems in infected individuals. Thus, with increasing severity of the infection the brain, lung, and heart will be functionally impaired [21,22]. The neurologic impact of SIV infection may in part be due to alterations in morphology and cytokine secretion of brain macrophages [23-25]. Monocytes may be primed by SIV infection to apoptosis, and some subsets of this lineage of cells are particularly conducive to SIV replication [26,27].
INNATE IMMUNE RESPONSES
Following the discovery of the Friend virus susceptibility gene 1 it was first realized that host cells could constitutively express genes encoding inhibitory factors that protect these cells against viral replication [28]. In HIV or SIV infection of humans and NHP four major restriction factors have been identified: apolipoprotein B mRNA-editing catalytic polypeptide-like 3 (APOBEC3), tetherin, tripartite motif 5α (TRIM5α), and sterile alpha motif and histidine-aspartic-domain containing protein 1 (SAMHD1) that either impair accessory proteins (Vif, Vpu, and Vpx) that enhance virus replication in an optimized manner or interfere with the Gag capsid [29,30]. The efficacy of TRIM5α is virus isolate-specific in rhesus macaques [31,32]. Its impact on SIVsmE660 is by far more evident than on SIVmac251. The relative expression of APOBEC3 (upregulated by interferon production during early SIV and HIV infection) is inversely correlated with virus replication [33]. The fourth major discovered restriction factor, SAMHD1 that renders human myeloid-lineage cells refractory to HIV-1 infection, has recently sparked intensive research activities [34,35]. Its activity in viral restriction is highly dependent on nuclear localization of this protein [36].
The significance of the contribution of natural killer (NK) cells to containment of SIV replication remains controversial. Anti-CD16 antibody treatment had little impact on viral replication [37]. However, recent investigations have shown that the NK cell repertoire in SIV infection is not only heterogeneous but also plastic resulting in an expansion of the CD16 negative NK cell subset with cytotoxic potential [38]. Moreover, the genetic background in expression of the killer cell immunoglobulin-like receptor (KIR) repertoire is associated with virus containment [39-41]. Investigations of the RV144 AIDS vaccine trial have suggested that some of its immunologic correlates may be due to antibody-dependent cell-mediated cytotoxicity (ADCC). The ADCC assay has recently been modified and optimized for use in NHP studies [42-44]. While the ADCC activity emerges coincidently with gp140-binding antibodies, it may not reach its full potential because of selective impairment of Fc receptor expression on NK cells [45,46].
Both in HIV-infected humans and SIV-infected NHP early virus replication is accompanied by an intensive cytokine storm [47,48]. It still remains to be seen which of these cytokines may actually be beneficial for the host rather than providing support to virus replication. Attempts to support the host’s immune system only by cytokine administrations were either short-lived or have failed [49,50]. However, when these approaches were previously done (i.e., for IL-15) in combination with antiretroviral therapy much more favorable results were obtained in T cell restoration [51]. The complexity of the role of the NHP immune system (including its cellular components, soluble secreted proteins, and genetic background) on viral containment can probably only be fully assessed by systems biology approaches [52,53]. However, the relatively small number of NHP usually studied under similar experimental conditions may limit the feasibility of these kinds of investigations.
CD8+ T CELL RESPONSES
There is little doubt that CD8+ T cells play a central role in viral containment of SIV infection in NHP [54]. Moreover, the expression of certain MHC class I molecules is associated with more efficient viral containment. However, some CD8+ T cell responses like CD8+ FoxP3+ T regulatory cells may actually be detrimental for the viral containment [55,56]. Moreover, the inability of CD8+ T cells to effectively control virus replication may also be due to a functional impairment by a reduction of key cell surface molecules like the CD8 molecule [57]. Ultimately, most SIV-infected rhesus macaques succumb to disease progression due to viral escape and inability of the CD8+ T cell responses to recognize the mutated peptides. Already early after SIV infection, viral escape mutants from CD8+ T cells are likely first generated in lymph nodes [58]. Mutant epitope-specific CD8+ T cells that are present at the time when viral mutant epitope sequences are detected at extremely low frequencies fail to contain the later accumulation and fixation of the mutant epitope sequences in the viral quasispecies [59]. The utilization of pyrosequencing has recently shown that escape within and outside the CD8+ T cell epitope is by far more complex than previously appreciated [60,61].
Historically, Indian rhesus macaques have been utilized intensively to determine MHC class I-specific CD8+ T cell responses in SIV infection [62]. Recent advances in characterizing MHC class I molecules will permit studying epitope-specific immune responses in additional NHP species such as Mauritian cynomolgus macaques, pigtail macaques, Chinese rhesus macaques, and Burmese rhesus macaques [63-66].
CD4+ T CELL RESPONSES
Similar to humans, CD4+ T cells are the major target and source of SIV replication in NHP. The magnitude of SIV replication in infected rhesus macaques appears to be limited by the size of the preexisting Th17 cell compartment [67]. The lymphoid tissue fibrosis, the loss of the fibroblastic reticular cell network and loss of IL-7 produced by this network is a cumulative process that contributes to T cell depletion and disease progression [68,69].
The underlying mechanisms in CD4+ T cell loss and disease progression have been intensively scrutinized in the last decades. Conventional wisdom would say that disease progression is necessarily related to loss of CD4+ T cells in mucosal tissues and associated with microbial translocation. However, interestingly a SIVmac239 mutant virus that did not lead to acute depletion of mucosal CD4+ T cells and microbial translocation showed a similar pathogenicity as the wild-type virus. The authors concluded that immune activation unrelated to gut damage can be sufficient for the development of AIDS [70].
While naïve CD4+ T cells are dispensable for memory CD4+ T cell homeostasis, the progressive loss of central memory CD4+ T cells results in decline of effector memory CD4+ T cells and overt disease in chronic SIV infection [71,72].
There are major efforts in the field to understand the pathomechanisms involved in relatively impaired humoral immune responses following SIV infection and poor production of neutralizing antibodies following vaccination. Logically, one of the most critical cell subset that provides help to humoral immune responses, the CD4+ T follicular helper cell, has recently been brought into the spotlight of several investigations [73-77]. As shown for germinal center CD4+ T cells in HIV-infected humans [78], T follicular helper cells were infected with SIV in rhesus macaques. However, accumulation of these cells was associated with increased numbers of germinal center B cells and SIV-specific antibodies [73]. Tracing this subset of T cells will be very valuable both in humans and NHP to correlate their number and function with pathogenesis and optimize vaccine strategies. It remains to be seen whether this T cell subset has a more easily accessible correlate in peripheral blood or whether investigations of lymphatic tissues such as lymph nodes are absolutely necessary to study these cells.
B CELL RESPONSES
B cell responses in the HIV infection face highly variable, rapidly mutating viruses with an incredible phylogenetic diversity with two types (HIV-1 and HIV-2) and further differentiation of HIV-1 into a major group M and several minor groups: an outlier group O, a “non-M, non-O” group N, and a group P that was found to be closely related to gorilla SIV [79]. Group M is subdivided into genetically distinct subgroups or clades A-K with occasional circulating recombinant forms (CRF) due to mixture of genetic material from different subgroups. Thus, a major objective in HIV vaccine development has been to elicit broadly neutralizing antibodies. Following the initial discovery of HIV, little progress has been made to detect and isolate such antibodies. However, recently the field has made major advances to identify new broadly neutralizing antibodies [80-82]. The combined use of these kinds of antibodies in passive immunization strategies has a dramatic effect on HIV viremia [83]. Although neutralizing humoral immune responses are quite limited in magnitude and breadth in early infection, these responses have been shown to impede virus replication and select for escape mutations [84].
Keeping this progress in mind, the question is where can the NHP model help to advance the field? However first, one has to realize that the classical viruses used in NHP (i.e. SIVmac251 or its clone SIVmac239) are exceedingly difficult to neutralize. This is in part due to the presence of gp41 carbohydrates that effectively shield the virus from antibodies [85]. Moreover, there are pathogenic events that prevent the production of IgA-specific immune responses in SIV-infected NHP, an observation also seen in HIV-infected humans [86]. Binding antibodies and antibodies to variable regions (resulting in type-specific antibodies) are readily being made. Thus, the envelope variable region 4 is the first target of neutralizing antibodies in SIVmac251-infected rhesus macaques [87]. NHP can be utilized to further the knowledge about the dynamics of memory B cell generation [88] and to apply techniques for identification of Env specific B cells [89,90]. Passive immunization strategies using antibodies or antibody-like molecules can help to selectively assess dosing, affinity and avidity to sufficiently suppress virus replication, as recently shown for the antibody-like molecule CD4-IgG2 [91].
The most pressing task, however, is to assess AIDS vaccine candidates in preclinical trials and determine their correlates of protection after vaccination [92]. Neutralization resistant viruses like SIVmac251 have effectively been combated by vaccine strategies that include multiple epitopes [93] or vaccine strategies that elicit effector memory CD8+ T cells [94]. In order to study AIDS vaccines expressing HIV proteins in NHP, chimeric viruses need to be generated that overcome virus-specific restriction in the NHP host of choice and represent the different HIV clades that can infect humans. These efforts have been ongoing since a while in a number of laboratories, including our own, and several R5-tropic SIV/HIV chimeric viruses are already available or in the pipeline [95].
NATURAL HOSTS OF SIV INFECTION
There are at least 30 different species of NHP that harbor SIV viruses but do not develop overt signs of disease regularly seen in HIV-infected humans or non-natural host NHP such as SIV-infected Indian rhesus macaques [96,97]. Natural hosts such as African green monkeys and sooty mangabeys are capable of sustaining high levels of viremia, but they experience only brief signs of immune activation during primary SIV infection [98-100]. While adaptive immune responses certainly can be detected following SIV infection [101], the role of these immune responses in maintaining a disease-free course of infection in natural hosts remains uncertain [102]. As such, the question why SIV infection in natural hosts is nonpathogenic is still not sufficiently resolved. An answer to this question has the potential to develop new treatment modalities for HIV-infected humans.
VIRAL RESERVOIR AND ITS IMPLICATION FOR VACCINE DEVELOPMENT
The virus diversity following mucosal transmission is exceedingly limited; in most cases the infection only starts with a single transmitted/founder virus, as has been confirmed in rhesus macaques by single genome amplification (SGA) and deep sequencing methodologies [8]. Intensive research is currently being conducted to determine whether transmitted/founder viruses represent a special breed of viruses on which the AIDS vaccine development should focus. However, within a few weeks an incredible diversity prevails that is mostly driven by the adaptive immune mechanisms attempting to contain the viremia. Virus diversity and escape are even seen when aggressive antiretroviral treatment (highly active antiretroviral therapy; HAART) is initiated from peak viremia onward in SIV-infected animals [103]. As the infection takes its course, the virus simultaneously replicates and hides in very complex ways. Active replication is most prominent in activated CD4+ T cells. However, other cell types including macrophages and dendritic cells certainly also contribute as target cells to viremia. Next to active replication, a latent reservoir of infected resting cells gets established early after infection [103-105]. Moreover, as a huge portion of the total body viral mass, virus particles get captured on extracellular surfaces of follicular dendritic cells in germinal centers where they can get access to highly activated CD4+ T cells, critical for the generation of humoral immune responses. Viral reservoirs are basically established in every anatomical compartment of the body, including locations with more limited access to adaptive immune responses (e.g., in the brain).
An effective HIV vaccine will need to have an impact on virus replication as early as possible, in the best case scenario resulting in abortive or sterile protection at the port of entry. Once the virus has successfully entered its host, disease progression may be slowed down until viral escape mutants evade the immune system and/or treatment modalities.
CONCLUSION
The NHP model of AIDS has made major advances on a number of fronts including deciphering the pathogenesis in different cell types and organ systems and unraveling innate and adaptive immune responses. It is expected that investigations of B cell responses to the virus and in preclinical AIDS vaccine studies will be the center piece of scientific efforts. Despite limitations that are inherently present in any animal model, the NHP model of AIDS remains an invaluable tool to move the HIV field forward and to reach its ultimate goal to develop an effective AIDS vaccine.
KEY POINTS.
A definition of the role of innate immune mechanisms including restriction factors, NK cells and cytokine responses in virus replication and/or containment is critical to advance the knowledge about the limitations of early viral containment.
The massive expansion of the enteric virome following SIV infection is a key finding in recent immunopathogenesis investigations.
Precisely defining MHC-specific epitope recognition and escape by virus mutation is critical for the development of an effective AIDS vaccine.
T follicular helper cells have gained major scientific interest to determine the limitations of humoral immune responses.
Acknowledgements
This work was supported by the National Institutes of Health grants AI097315, the Center for HIV/AIDS Vaccine Immunology (CHAVI) grant AI060354, the Center for HIV-AIDS Vaccine Immunology-Immunogen Design (CHAVI-ID) grant AI100645, and the Harvard Medical School Center for AIDS Research grant AI060354.
Footnotes
Conflicts of interest
The authors declare that no conflicts of interest exist.
REFERENCES AND RECOMMENDED READING
- 1.Hemelaar J. The origin and diversity of the HIV-1 pandemic. Trends Mol Med. 2012;18:182–192. doi: 10.1016/j.molmed.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med. 2011;1:a006841. doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saunders KO, Rudicell RS, Nabel GJ. The design and evaluation of HIV-1 vaccines. AIDS. 2012;26:1293–1302. doi: 10.1097/QAD.0b013e32835474d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Letvin NL, Hunt RD, Finberg R. Animal models of AIDS. Semin Oncol. 1984;11:18–28. [PubMed] [Google Scholar]
- 5.Desrosiers RC, Letvin NL. Animal models for acquired immunodeficiency syndrome. Rev Infect Dis. 1987;9:438–446. doi: 10.1093/clinids/9.3.438. [DOI] [PubMed] [Google Scholar]
- 6.Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat Rev Microbiol. 2012;10:852–867. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw GM, Hunter E. HIV transmission. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone M, Keele BF, Ma ZM, et al. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J Virol. 2010;84:7083–7095. doi: 10.1128/JVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strickland SL, Gray RR, Lamers SL, et al. Significant genetic heterogeneity of the SIVmac251 viral swarm derived from different sources. AIDS Res Hum Retroviruses. 2011;27:1327–1332. doi: 10.1089/aid.2011.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Prete GQ, Scarlotta M, Newman L, et al. Comparative Characterization of Transfection- and Infection-Derived SIV Challenge Stocks for In Vivo Non-Human Primate Studies. J Virol. 2013 doi: 10.1128/JVI.03507-12. doi: 10.1128/JVI.03507-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 12.Maingat F, Halloran B, Acharjee S, et al. Inflammation and epithelial cell injury in AIDS enteropathy: involvement of endoplasmic reticulum stress. FASEB J. 2011;25:2211–2220. doi: 10.1096/fj.10-175992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26:2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giavedoni LD, Chen HL, Hodara VL, et al. Impact of mucosal inflammation on oral simian immunodeficiency virus transmission. J Virol. 2013;87:1750–1758. doi: 10.1128/JVI.02079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klatt NR, Canary LA, Sun X, et al. Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. J Clin Invest. 2013 doi: 10.1172/JCI66227. doi:10.1172/JCI66227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwa S, Kannanganat S, Nigam P, et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood. 2011;118:2763–2773. doi: 10.1182/blood-2011-02-339515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17■.Ansari AA, Reimann KA, Mayne AE, et al. Blocking of alpha4beta7 gut-homing integrin during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques. J Immunol. 2011;186:1044–1059. doi: 10.4049/jimmunol.1003052. Homing of CD4+ T cells to gastrointestinal tissues is a major contributor to viral pathogenesis.
- 18.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10:655–666. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 19.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 20■.Handley SA, Thackray LB, Zhao G, et al. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell. 2012;151:253–266. doi: 10.1016/j.cell.2012.09.024. The discovery of the expansion of the enteric virome in SIV infection is a groundbreaking observation contributing to our knowledge of immunopathogenesis.
- 21.Alammar L, Gama L, Clements JE. Simian immunodeficiency virus infection in the brain and lung leads to differential type I IFN signaling during acute infection. J Immunol. 2011;186:4008–4018. doi: 10.4049/jimmunol.1003757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly KM, Tarwater PM, Karper JM, et al. Diastolic dysfunction is associated with myocardial viral load in simian immunodeficiency virus-infected macaques. AIDS. 2012;26:815–823. doi: 10.1097/QAD.0b013e3283518f01. [DOI] [PubMed] [Google Scholar]
- 23.Renner NA, Sansing HA, Morici LA, et al. Microglia activation by SIV-infected macrophages: alterations in morphology and cytokine secretion. J Neurovirol. 2012;18:213–221. doi: 10.1007/s13365-012-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laast VA, Shim B, Johanek LM, et al. Macrophage-mediated dorsal root ganglion damage precedes altered nerve conduction in SIV-infected macaques. Am J Pathol. 2011;179:2337–2345. doi: 10.1016/j.ajpath.2011.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams K, Burdo TH. Monocyte mobilization, activation markers, and unique macrophage populations in the brain: observations from SIV infected monkeys are informative with regard to pathogenic mechanisms of HIV infection in humans. J Neuroimmune Pharmacol. 2012;7:363–371. doi: 10.1007/s11481-011-9330-3. [DOI] [PubMed] [Google Scholar]
- 26.Moore AC, Bixler SL, Lewis MG, et al. Mucosal and peripheral Lin− HLA-DR+ CD11c/123− CD13+ CD14− mononuclear cells are preferentially infected during acute simian immunodeficiency virus infection. J Virol. 2012;86:1069–1078. doi: 10.1128/JVI.06372-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laforge M, Campillo-Gimenez L, Monceaux V, et al. HIV/SIV infection primes monocytes and dendritic cells for apoptosis. PLoS Pathog. 2011;7:e1002087. doi: 10.1371/journal.ppat.1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bieniasz PD. Intrinsic immunity: a front-line defense against viral attack. Nat Immunol. 2004;5:1109–1115. doi: 10.1038/ni1125. [DOI] [PubMed] [Google Scholar]
- 29.Nomaguchi M, Fujita M, Adachi A. The fourth major restriction factor against HIV/SIV. Front Microbiol. 2011;2:132. doi: 10.3389/fmicb.2011.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaller T, Goujon C, Malim MH. AIDS/HIV. HIV interplay with SAMHD1. Science. 2012;335:1313–1314. doi: 10.1126/science.1221057. [DOI] [PubMed] [Google Scholar]
- 31.Letvin NL, Rao SS, Montefiori DC, et al. Immune and Genetic Correlates of Vaccine Protection Against Mucosal Infection by SIV in Monkeys. Sci Transl Med. 2011;3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fenizia C, Keele BF, Nichols D, et al. TRIM5alpha does not affect simian immunodeficiency virus SIV(mac251) replication in vaccinated or unvaccinated Indian rhesus macaques following intrarectal challenge exposure. J Virol. 2011;85:12399–12409. doi: 10.1128/JVI.05707-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mussil B, Sauermann U, Motzkus D, et al. Increased APOBEC3G and APOBEC3F expression is associated with low viral load and prolonged survival in simian immunodeficiency virus infected rhesus monkeys. Retrovirology. 2011;8:77. doi: 10.1186/1742-4690-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laguette N, Benkirane M. How SAMHD1 changes our view of viral restriction. Trends Immunol. 2012;33:26–33. doi: 10.1016/j.it.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.St Gelais C, Wu L. SAMHD1: a new insight into HIV-1 restriction in myeloid cells. Retrovirology. 2011;8:55. doi: 10.1186/1742-4690-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandariz-Nunez A, Valle-Casuso JC, White TE, et al. Role of SAMHD1 nuclear localization in restriction of HIV-1 and SIVmac. Retrovirology. 2012;9:49. doi: 10.1186/1742-4690-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi EI, Reimann KA, Letvin NL. In vivo natural killer cell depletion during primary simian immunodeficiency virus infection in rhesus monkeys. J Virol. 2008;82:6758–6761. doi: 10.1128/JVI.02277-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeves RK, Gillis J, Wong FE, et al. CD16− natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood. 2010;115:4439–4446. doi: 10.1182/blood-2010-01-265595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hellmann I, Letvin NL, Schmitz JE. KIR2DL4 copy number variation is associated with CD4+ T-cell depletion and function of cytokine-producing NK cell subsets in SIV-infected Mamu-A*01- rhesus macaques. J Virol. 2013 doi: 10.1128/JVI.02949-12. doi: 10.1128/JVI.02949-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hellmann I, Schmitz JE, Letvin NL. Activating KIR copy number variation is associated with granzyme B release by NK cells during primary simian immunodeficiency virus infection in rhesus monkeys. J Virol. 2012;86:13103–13107. doi: 10.1128/JVI.00325-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellmann I, Lim SY, Gelman RS, Letvin NL. Association of activating KIR copy number variation of NK cells with containment of SIV replication in rhesus monkeys. PLoS Pathog. 2011;7:e1002436. doi: 10.1371/journal.ppat.1002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Asmal M, Lane S, et al. Antibody-dependent cell-mediated cytotoxicity in simian immunodeficiency virus-infected rhesus monkeys. J Virol. 2011;85:6906–6912. doi: 10.1128/JVI.00326-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alpert MD, Heyer LN, Williams DE, et al. A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays. J Virol. 2012;86:12039–12052. doi: 10.1128/JVI.01650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollara J, Hart L, Brewer F, et al. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A. 2011;79:603–612. doi: 10.1002/cyto.a.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asmal M, Sun Y, Lane S, et al. Antibody-dependent cell-mediated viral inhibition emerges after simian immunodeficiency virus SIVmac251 infection of rhesus monkeys coincident with gp140-binding antibodies and is effective against neutralization-resistant viruses. J Virol. 2011;85:5465–5475. doi: 10.1128/JVI.00313-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He X, Li D, Luo Z, et al. Compromised NK cell-mediated antibody-dependent cellular cytotoxicity in chronic SIV/SHIV infection. PLoS One. 2013;8:e56309. doi: 10.1371/journal.pone.0056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stacey AR, Norris PJ, Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katsikis PD, Mueller YM, Villinger F. The cytokine network of acute HIV infection: a promising target for vaccines and therapy to reduce viral set-point? PLoS Pathog. 2011;7:e1002055. doi: 10.1371/journal.ppat.1002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lugli E, Mueller YM, Lewis MG, et al. IL-15 delays suppression and fails to promote immune reconstitution in virally suppressed chronically SIV-infected macaques. Blood. 2011;118:2520–2529. doi: 10.1182/blood-2011-05-351155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vassena L, Miao H, Cimbro R, et al. Treatment with IL-7 prevents the decline of circulating CD4+ T cells during the acute phase of SIV infection in rhesus macaques. PLoS Pathog. 2012;8:e1002636. doi: 10.1371/journal.ppat.1002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Picker LJ, Reed-Inderbitzin EF, Hagen SI, et al. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peretz Y, Cameron C, Sekaly RP. Dissecting the HIV-specific immune response: a systems biology approach. Curr Opin HIV AIDS. 2012;7:17–23. doi: 10.1097/COH.0b013e32834ddb0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benecke A, Gale M, Katze MG. Dynamics of innate immunity are key to chronic immune activation in AIDS. Curr Opin HIV AIDS. 2012;7:79–85. doi: 10.1097/COH.0b013e32834dde31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 55.Nigam P, Velu V, Kannanganat S, et al. Expansion of FOXP3+ CD8 T cells with suppressive potential in colorectal mucosa following a pathogenic simian immunodeficiency virus infection correlates with diminished antiviral T cell response and viral control. J Immunol. 2010;184:1690–1701. doi: 10.4049/jimmunol.0902955. [DOI] [PubMed] [Google Scholar]
- 56.George J, Cofano EB, Lybarger E, et al. Early short-term antiretroviral therapy is associated with a reduced prevalence of CD8(+)FoxP3(+) T cells in simian immunodeficiency virus-infected controller rhesus macaques. AIDS Res Hum Retroviruses. 2011;27:763–775. doi: 10.1089/aid.2010.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu H, Wang X, Lackner AA, Veazey RS. CD8 down-regulation and functional impairment of SIV-specific cytotoxic T lymphocytes in lymphoid and mucosal tissues during SIV infection. J Leukoc Biol. 2013 doi: 10.1189/jlb.1112580. doi: 10.1189/jlb.1112580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vanderford TH, Bleckwehl C, Engram JC, et al. Viral CTL escape mutants are generated in lymph nodes and subsequently become fixed in plasma and rectal mucosa during acute SIV infection of macaques. PLoS Pathog. 2011;7:e1002048. doi: 10.1371/journal.ppat.1002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cale EM, Hraber P, Giorgi EE, et al. Epitope-specific CD8+ T lymphocytes cross-recognize mutant simian immunodeficiency virus (SIV) sequences but fail to contain very early evolution and eventual fixation of epitope escape mutations during SIV infection. J Virol. 2011;85:3746–3757. doi: 10.1128/JVI.02420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60■.Burwitz BJ, Sacha JB, Reed JS, et al. Pyrosequencing reveals restricted patterns of CD8+ T cell escape-associated compensatory mutations in simian immunodeficiency virus. J Virol. 2011;85:13088–13096. doi: 10.1128/JVI.05650-11. Unraveling the complexity of CD8+ T cell responses will hopefully enable the field to develop better AIDS vaccine candidates.
- 61.Hughes AL, Becker EA, Lauck M, et al. SIV genome-wide pyrosequencing provides a comprehensive and unbiased view of variation within and outside CD8 T lymphocyte epitopes. PLoS One. 2012;7:e47818. doi: 10.1371/journal.pone.0047818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nomura T, Matano T. Association of MHC-I genotypes with disease progression in HIV/SIV infections. Front Microbiol. 2012;3:234. doi: 10.3389/fmicb.2012.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Budde ML, Greene JM, Chin EN, et al. Specific CD8+ T cell responses correlate with control of simian immunodeficiency virus replication in Mauritian cynomolgus macaques. J Virol. 2012;86:7596–7604. doi: 10.1128/JVI.00716-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore C, Sidney J, English AM, et al. Identification of the peptide-binding motif recognized by the pigtail macaque class I MHC molecule Mane-A1*082:01 (Mane A*0301) Immunogenetics. 2012;64:461–468. doi: 10.1007/s00251-012-0600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mothe BR, Southwood S, Sidney J, et al. Peptide-binding motifs associated with MHC molecules common in Chinese rhesus macaques are analogous to those of human HLA supertypes and include HLA-B27-like alleles. Immunogenetics. 2013 doi: 10.1007/s00251-013-0686-9. doi: 10.1007/s00251-013-0686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nomura T, Yamamoto H, Shiino T, et al. Association of major histocompatibility complex class I haplotypes with disease progression after simian immunodeficiency virus challenge in burmese rhesus macaques. J Virol. 2012;86:6481–6490. doi: 10.1128/JVI.07077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hartigan-O’Connor DJ, Abel K, Van Rompay KK, et al. SIV replication in the infected rhesus macaque is limited by the size of the preexisting TH17 cell compartment. Sci Transl Med. 2012;4:136ra169. doi: 10.1126/scitranslmed.3003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33:306–314. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Zeng M, Smith AJ, Wietgrefe SW, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breed MW, Jordan AP, Aye PP, et al. Loss of a tyrosine-dependent trafficking motif in the simian immunodeficiency virus envelope cytoplasmic tail spares mucosal CD4 cells but does not prevent disease progression. J Virol. 2013;87:1528–1543. doi: 10.1128/JVI.01928-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71■.Okoye AA, Rohankhedkar M, Abana C, et al. Naive T cells are dispensable for memory CD4+ T cell homeostasis in progressive simian immunodeficiency virus infection. J Exp Med. 2012;209:641–651. doi: 10.1084/jem.20112071. Elegant study to dissect the contribution of naïve T cells to CD4+ T cell homeostasis.
- 72.Okoye A, Meier-Schellersheim M, Brenchley JM, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73■.Petrovas C, Yamamoto T, Gerner MY, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. This study makes a significant step to characterize a key T cell subset that provides critical help for humoral immune responses.
- 74.Vinuesa CG. HIV and T follicular helper cells: a dangerous relationship. J Clin Invest. 2012;122:3059–3062. doi: 10.1172/JCI65175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Y, Weatherall C, Bailey M, et al. Simian immunodeficiency virus infects follicular helper CD4 T cells in lymphoid tissues during pathogenic infection of pigtail macaques. J Virol. 2013;87:3760–3773. doi: 10.1128/JVI.02497-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hong JJ, Amancha PK, Rogers K, et al. Spatial alterations between CD4(+) T follicular helper, B, and CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J Immunol. 2012;188:3247–3256. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klatt NR, Vinton CL, Lynch RM, et al. SIV infection of rhesus macaques results in dysfunctional T- and B-cell responses to neo and recall Leishmania major vaccination. Blood. 2011;118:5803–5812. doi: 10.1182/blood-2011-07-365874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hufert FT, van Lunzen J, Janossy G, et al. Germinal centre CD4+ T cells are an important site of HIV replication in vivo. AIDS. 1997;11:849–857. doi: 10.1097/00002030-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 79.Plantier JC, Leoz M, Dickerson JE, et al. A new human immunodeficiency virus derived from gorillas. Nat Med. 2009;15:871–872. doi: 10.1038/nm.2016. [DOI] [PubMed] [Google Scholar]
- 80.Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337:183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang J, Ofek G, Laub L, Louder MK, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verkoczy L, Kelsoe G, Moody MA, Haynes BF. Role of immune mechanisms in induction of HIV-1 broadly neutralizing antibodies. Curr Opin Immunol. 2011;23:383–390. doi: 10.1016/j.coi.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klein F, Halper-Stromberg A, Horwitz JA, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bar KJ, Tsao CY, Iyer SS, et al. Early low-titer neutralizing antibodies impede HIV-1 replication and select for virus escape. PLoS Pathog. 2012;8:e1002721. doi: 10.1371/journal.ppat.1002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martinez-Navio JM, Desrosiers RC. Neutralizing capacity of monoclonal antibodies that recognize peptide sequences underlying the carbohydrates on gp41 of simian immunodeficiency virus. J Virol. 2012;86:12484–12493. doi: 10.1128/JVI.01959-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaoul N, Burelout C, Peruchon S, et al. Default in plasma and intestinal IgA responses during acute infection by simian immunodeficiency virus. Retrovirology. 2012;9:43. doi: 10.1186/1742-4690-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yeh WW, Brassard LM, Miller CA, et al. Envelope variable region 4 is the first target of neutralizing antibodies in early simian immunodeficiency virus mac251 infection of rhesus monkeys. J Virol. 2012;86:7052–7059. doi: 10.1128/JVI.00107-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Demberg T, Brocca-Cofano E, Xiao P, et al. Dynamics of memory B-cell populations in blood, lymph nodes, and bone marrow during antiretroviral therapy and envelope boosting in simian immunodeficiency virus SIVmac251-infected rhesus macaques. J Virol. 2012;86:12591–12604. doi: 10.1128/JVI.00298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fofana IB, Colantonio AD, Reeves RK, et al. Flow cytometry based identification of simian immunodeficiency virus Env-specific B lymphocytes. J Immunol Methods. 2011;370:75–85. doi: 10.1016/j.jim.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moody MA, Haynes BF. Antigen-specific B cell detection reagents: use and quality control. Cytometry A. 2008;73:1086–1092. doi: 10.1002/cyto.a.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Poignard P, Moldt B, Maloveste K, et al. Protection against high-dose highly pathogenic mucosal SIV challenge at very low serum neutralizing titers of the antibody-like molecule CD4-IgG2. PLoS One. 2012;7:e42209. doi: 10.1371/journal.pone.0042209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ploquin MJ, Jacquelin B, Jochems SP, et al. Innate immunity in the control of HIV/AIDS: recent advances and open questions. AIDS. 2012;26:1269–1279. doi: 10.1097/QAD.0b013e328353e46b. [DOI] [PubMed] [Google Scholar]
- 93■.Barouch DH, Liu J, Li H, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. This study unequivocally provides evidence that an optimized AIDS vaccine strategy can elicit protection against acquisition of a highly pathogenic, neutralization-resistant SIV challenge.
- 94.Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu Rev Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walker LM, Sok D, Nishimura Y, et al. Rapid development of glycan-specific, broad, and potent anti-HIV-1 gp120 neutralizing antibodies in an R5 SIV/HIV chimeric virus infected macaque. Proc Natl Acad Sci U S A. 2011;108:20125–20129. doi: 10.1073/pnas.1117531108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pandrea I, Apetrei C. Where the wild things are: pathogenesis of SIV infection in African nonhuman primate hosts. Curr HIV/AIDS Rep. 2010;7:28–36. doi: 10.1007/s11904-009-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma D, Jasinska A, Kristoff J, et al. SIVagm infection in wild African green monkeys from South Africa: epidemiology, natural history, and evolutionary considerations. PLoS Pathog. 2013;9:e1003011. doi: 10.1371/journal.ppat.1003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schmitz JE, Ma ZM, Hagan EA, et al. Memory CD4(+) T lymphocytes in the gastrointestinal tract are a major source of cell-associated simian immunodeficiency virus in chronic nonpathogenic infection of African green monkeys. J Virol. 2012;86:11380–11385. doi: 10.1128/JVI.01556-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klatt NR, Villinger F, Bostik P, et al. Availability of activated CD4+ T cells dictates the level of viremia in naturally SIV-infected sooty mangabeys. J Clin Invest. 2008;118:2039–2049. doi: 10.1172/JCI33814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chahroudi A, Bosinger SE, Vanderford TH, et al. Natural SIV hosts: showing AIDS the door. Science. 2012;335:1188–1193. doi: 10.1126/science.1217550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mendoza D, Migueles SA, Rood JE, et al. Cytotoxic capacity of SIV-specific CD8(+) T cells against primary autologous targets correlates with immune control in SIV-infected rhesus macaques. PLoS Pathog. 2013;9:e1003195. doi: 10.1371/journal.ppat.1003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zahn RC, Rett MD, Li M, et al. Suppression of adaptive immune responses during primary SIV infection of sabaeus African green monkeys delays partial containment of viremia but does not induce disease. Blood. 2010;115:3070–3078. doi: 10.1182/blood-2009-10-245225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Queen SE, Mears BM, Kelly KM, et al. Replication-competent simian immunodeficiency virus (SIV) Gag escape mutations archived in latent reservoirs during antiretroviral treatment of SIV-infected macaques. J Virol. 2011;85:9167–9175. doi: 10.1128/JVI.00366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Donahue DA, Wainberg MA. Cellular and molecular mechanisms involved in the establishment of HIV-1 latency. Retrovirology. 2013;10:11. doi: 10.1186/1742-4690-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Autran B, Descours B, Bacchus C. Immune control of HIV-1 reservoirs. Curr Opin HIV AIDS. 2013 doi: 10.1097/COH.0b013e32835fe6d2. doi: 10.1097/COH.0b013e32835fe6d2. [DOI] [PubMed] [Google Scholar]