Abstract
Epileptic spike is an indicator of hyper-excitability and hyper-synchrony of neural networks. While cognitive deficit in epilepsy is a common observation, how spikes transiently influence brain oscillations, especially those essential for cognitive functions, remains obscure. Here we aimed to quantify the transient impacts of sporadic spikes on theta oscillations and investigate how such impacts may evolve during epileptogenesis. Longitudinal depth EEG data were recorded in the CA1 area of pilocarpine temporal lobe epilepsy (TLE) rat models. Phase stability, a measure of synchrony, and theta power were estimated around spikes as well as in the protracted spike-free periods (FP) at least one hour after spike bursts. We found that the change in theta power did not correlate with the change in phase stability. More importantly, the impact of spikes on theta rhythm was highly time-dependent. While theta power decreased abruptly after spikes both in the latent and chronic stages, changes of theta phase stability demonstrated opposite trends in the latent and chronic stages, potentially due to the substantial reorganization of neural circuits along epileptogenesis. During FP, theta phase stability was significantly higher than the baseline level before injections, indicating that hyper-synchrony remained even hours after the spike bursts. We concluded that spikes have transient negative effects on theta rhythm, however, impacts are different during latent and chronic stages, implying that its influence on cognitive processes may also change over time during epileptogenesis.
Keywords: Theta Rhythm, Temporal Lobe Epilepsy, Hippocampus, Epileptogenesis
Introduction
Interictal-like activity (ILA) during epileptogenesis is an electrophysiological signature of the reverberating neural circuits (Alarcon et al., 1997; Buzsaki et al., 1989; Buzsaki et al., 1991; Cossart et al., 2001; Demont-Guignard et al., 2009; El-Hassar et al., 2007), with the spike discharges being its most common form. While the significance of ILA has been well recognized, its potentially negative impact on brain functions remains under debate. In experimental models of temporal lobe epilepsy (TLE), ILA could appear at early stages of epileptogenesis (Cobos et al., 2005; El-Hassar et al., 2007; Hellier et al., 1999; Mazarati et al., 2002; Shah et al., 2004). It has been suggested that ILA may not only be directly involved in the construction of epileptogenic networks (Bartolomei et al., 2008; King et al., 1997; Staley et al., 2005), but also be predictive of the development of epilepsy (Williams et al., 2009). However, an anti-epileptogenic role of ILA has also been proposed (Avoli et al., 2002; Gotman, 1991). At the microscopic level, ILA may involve synchronous discharges of glutamatergic neurons and activation of GABAergic circuits (de Curtis et al., 1998; de Curtis and Avanzini, 2001; Demont-Guignard et al., 2009). At the functional level, ILA was suggested to negatively impact cognitive processes, which is often related to the changes of power, frequency, and phase of brain oscillations (Chauviere et al., 2009; Chauviere et al., 2012; Kasteleijn-Nolst Trenite et al., 1988; Kasteleijn-Nolst Trenite et al., 1990a; Kasteleijn-Nolst Trenite et al., 1990b; Kraskov et al., 2007; Lenck-Santini and Holmes, 2008; Liu et al., 2003; Uhlhaas and Singer, 2006; Winson, 1978). However, the transient impacts of ILA on brain activity and how such impacts may develop during epileptogenesis remain unclear.
A brain activity central to numerous cognitive processes is the theta rhythm (4–8Hz in humans, 4–12Hz in rodents), which has been suggested to depend on the recruitment of GABAergic circuits (Buzsaki, 2006; Gloveli et al., 2005). Since ILA and theta rhythm could both involve GABAergic circuits, we hypothesize that theta oscillations may be directly influenced by ILA during epileptogenesis and thus provide a means to investigate the negative impact of ILA on brain oscillations. A delicate balance between synchronization and desynchronization in brain activity is functionally and behaviorally important (Schnitzler and Gross, 2005), therefore the synchronization in the epileptic brain may have a substantial impact on cognitive functions. To understand the functional role of ILA, it is critical to quantify the changes of theta phase synchrony around ILA.
Here we performed a longitudinal study on rat models of TLE. Depth EEG was recorded in the CA1 area while the rats were exploring for food in an open field. We applied a synchrony measure, termed phase stability, to characterize the changes of theta oscillations (see Methods). Theta power and phase stability were estimated around sporadic spikes during latent and chronic stages of epileptogenesis, respectively. Theta activity was then quantified in the protracted FP to investigate the recovery process following spike bursts.
Materials and Methods
Rats and Depth EEG Recordings
Four adult male Wistar rats (200–250g, Charles River, France) received intra-peritoneal injections of pilocarpine-1 hydrochloride 30 min after a preliminary scopolamine injection. The initial status epilepticus (SE) was recorded as soon as the first overt behavioral manifestation was observed and then stopped by diazepam after 40 minutes. The first spontaneous seizure occurred between 12 and 16 days after SE.
Depth electrodes were implanted in the CA1 region. The rats were navigating in a circular open field (80 cm in diameter, 80 cm high) searching for food. A video system (Video Track, View Point, France) was used to quantify the rats’ behavior of exploration. This dataset has been previously reported (Chauviere et al., 2009).
Depth EEG was recorded on the 7th and 25th day after SE. In the current study, Day 7 was regarded as the latent stage, during which the observed spikes (ILA) were similar to the interictal spikes (see Supplementary Fig. S1 for examples). All animals had become epileptic by D25. Therefore D25 was regarded as the chronic stage. The epileptic discharges in this chronic period were mainly interictal spikes. On D7 and D25, data exhibiting spikes (amplitude at least 5 times larger than that of the background activity) while the rats were awake and seizure-free were extracted. The running speeds of the animals were monitored and recorded. In the experimental period, no obvious anxiety was observed (Chauviere et al., 2009).
Following spike bursts, there were long periods during which EEG was free of spikes and the rats were still awake and exploring. In this study we have termed these spike-free periods as FP. Our recordings in FP were taken at least one hour after the occurrence of spike bursts and each recording period was at least one hour long. Data in the FP was employed for the investigation of recovery processes after spike bursts. Finally, one hour long data blocks before injections (BI) had been recorded as the control. The sampling rate was 256 Hz and the power line noise was notch-filtered.
Histological Experiments
The positions of the electrodes were verified by histological experiments. The details of the procedure have been described in Chauviere et al. (2009). In short, rats were perfused intracardially with a fixative solution. The postfixed brains were rinsed and immersed in a cryoprotective solution overnight before being frozen in dry ice. Brains were then sectioned coronally at 40 μm and stained. In all subjects, the electrode placement was confirmed to be within the CA1 region of the hippocampus.
Data Processing
The onset and offset of the spikes were determined by visual inspection by an experienced neurologist based on the baseline activity. After the onset and offset of a spike were identified (see Supplementary Fig. S2), two 350 ms epochs were extracted around each spike: one before the spike onset and the other following the spike offset. After rejecting the data with artifacts, 293 spikes (at least 58 spikes extracted from each subject) were obtained on D7 and 324 spikes (at least 61 spikes from each subject) were obtained on D25, resulting in a total of 617 spikes for further analysis. Before injections and during FP, EEG epochs were extracted by continuously parsing through the data using a 350ms time window without overlap. 1500 epochs were obtained from BI, FP on D7, and FP on D25, respectively.
In order to properly estimate the instantaneous theta power and phase for these short epochs and avoid edge effects, computation was carried out in a 10s long data segment centered at each epoch. Instantaneous power was derived from the 5-cycles complex Gabor wavelet transform. Instantaneous phase was estimated by the Hilbert transform. Theta power and phase in each epoch were then extracted from the center of the transformed data.
Theta Phase Synchrony
To quantify phase synchrony, we computed phase stability of theta frequency over each epoch. Local field potentials represent the summed extra-cellular activity of local populations of neurons if they are parallel in orientation and quasi-synchronous (a typical organization of pyramidal cells such as those in the CA1 region), and these neurons may be considered as multiple oscillators. Synchronization of a group of oscillators can be reflected by the phase noise in the summed signal, which are the rapid, random fluctuations in the phase of a waveform. When the oscillators are asynchronous, phase noise is added to the LFP because each oscillator represents a stochastic process, causing the instability of the phase over time.
Phase stability of a given frequency was estimated based on the mean relative phase value Φ over a specific time window, as defined in Eq. (1):
| (1) |
Where t is the time and N is the total number of time points in the time window. is the phase of raw EEG and is the reference phase at t. Here the reference phase was defined as sine phase corresponding to the frequency of interest. For a given time window starting at t = 0, we defined . Theta phase ϕs was estimated by the Hilbert transform of the band-pass filtered EEG (bandwidth is ±0.01Hz around the reference frequency). A two-way, least-square FIR band-pass filter was employed to avoid phase distortion (Delorme and Makeig, 2004). Mean relative phase values are in the range of 0~1, where 1 indicates perfect phase synchronization relative to the reference phase, and 0 indicates completely random phases.
Hippocampal theta in awake rats ranges from 4 to 12Hz. For each epoch, we computed 9 mean relative phase values at 9 reference frequencies (from 4 Hz to 12 Hz with the increment of 1 Hz). For a specific group of epochs, the percentage of mean relative phase values larger than 0.95 was termed the Phase Preservation Index (PPI), which could serve as a measure of phase synchrony in this group. The phase stability we quantified here is a specific measure of synchrony, which is different from the phase coupling between separate brain structures that is commonly used to characterize large-scale networks. In the hippocampus, synchronization is related to the number of neurons with connections in local networks (Buzsaki, 2006), which might be altered through cholinergic modulation, GABAergic modulation, and glutamatergic modulation (Buzsaki, 2002; Colom et al., 2006; Schnitzler and Gross, 2005; Whittington et al., 2000). In the normal resting brain, these neural oscillators demonstrate a certain level of phase variability. When neural oscillators become synchronized, e.g. during a cognitive task, phase may demonstrate strong regularity over time (Kraskov et al., 2007). In the epileptic brain, the neural network is hyper-synchronized and strong phase stability is expected.
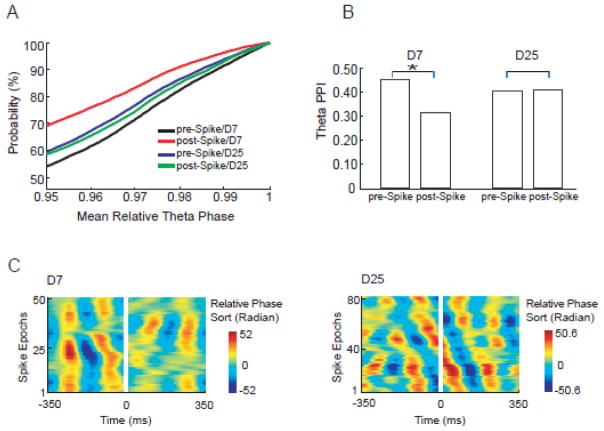
Temporal courses of relative theta phase around spikes can be displayed using the phase sorting toolbox (EEGLAB, Delorme and Makeig, 2004). Oblique stripe or no stripe suggested random phase or low theta phase stability. Obvious stripes indicated strong theta phase stability (see Fig. 2C for examples).
Figure 2.
Theta phase stability changes around spikes are time dependent and differ between latent and chronic stages. Mean relative theta phase values were computed for all epochs and around multiple reference frequencies. Cumulative distribution functions (CDF) of mean relative theta phase value ≥ 0.95 were plotted for D7 and D25 groups (A). The CDF curves indicated that the highest probability of phase preservation appeared in the D7 pre-spike group (black curve). The phase stability measure, termed PPI, was plotted for both D7 and D25 groups. (B) On D7, the theta PPI demonstrated a 33% decrease from 0.46 pre-spike to 0.31 post-spike (bootstrapping, P<0.01). On D25, theta PPI demonstrated very little change (2.8% increase) after spikes (bootstrapping, P>0.95). (C) Time courses of theta phase stability around spikes (spikes were cut out). On D7 (51 epochs in total), theta phase stability decreased after spikes (stripes were dispersed after spikes, indicating more random phase). On D25 (83 epochs in total), no obvious theta phase stability change was seen after spikes (stripes did not change obviously after spikes).
Statistical testing
For each group of trials, PPI measures the percentage of mean relative phase values that are larger than 0.95. When comparing the PPI between two conditions, the robustness of difference was tested using a bootstrapping approach. In short, for two contrasting groups (e. g., pre-spike on D7 and post-spike on D7), we randomly selected 20% of the trials from each group, and computed the percentage of mean relative phase values larger than 0.95. Then we compared this PPI value (which is based on only 20% of the data) between two groups. This procedure was repeated for 1,000 times to derive a p-value.
Results
Theta Power Is Immediately Altered Across Spikes
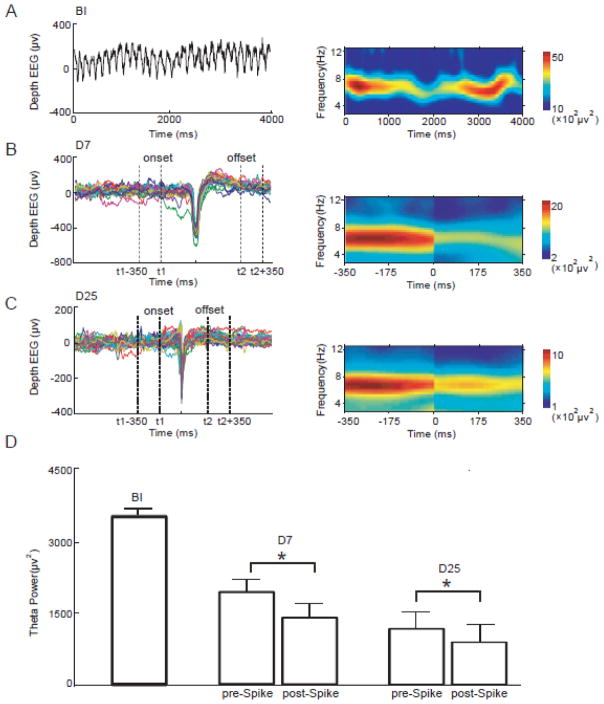
Depth EEG was recorded in rat models before injections (BI), in latent (D7), and chronic stages (D25) of epileptogenesis (see Methods). Theta power was estimated for all groups. As the control condition, theta power in the BI period was first computed (Fig. 1A). The spikes from the D7 and D25 groups were then processed using the epochs sorting technology (EEGLAB, Delorme and Makeig, 2004), so the peaks of the spikes were aligned and the onset/offset of each spike were determined (examples shown in Fig. 1B&1C). The power spectra were computed for the pre-spike and post-spike data segments and averaged across epochs. During the development of epilepsy, the pre-spike theta power showed a strong decrease from BI to D7 and further decreased on D25. The pre-spike theta power in the latent stage dropped about 34% from the before-injection stage, and it showed additional 37% drop from the latent stage to chronic stage (Fig. 1D) (see also Fig. 4A for results of individual subjects). More importantly, theta power was instantly reduced after spikes. A significant decrease in theta power after the spike was observed both in D7 and D25 groups (paired t-test, p<0.05). These data indicated that the spikes had a strong negative transient impact on theta power and the change of theta power was in the same direction on D7 and D25.
Figure 1.
Theta power changes immediately after spikes. (A) An example segment of depth EEG recorded before-injection (left panel) and its power spectrum (right panel) are illustrated. (B) Spikes from the D7 group were aligned using the epochs sorting technology (left panel). The power spectra around spikes were shown in the right panel. In this example, the power spectra of epochs around 22 spikes from a single subject were averaged. Theta power significantly decreased after spikes. Note that spikes have been cut out in the time-frequency panel. (C) Artifact-free epochs around spikes in the D25 group (left panel) were subject to the time-frequency analysis and the power spectra were averaged. Theta power also decreased immediately after spikes. (D) Theta power of all epochs was compared. The pre-spike theta power persistently decreased from BI to D7 and then to D25. Theta power was significantly reduced after the spikes (paired t-test, p<0.05), both on D7 and D25.
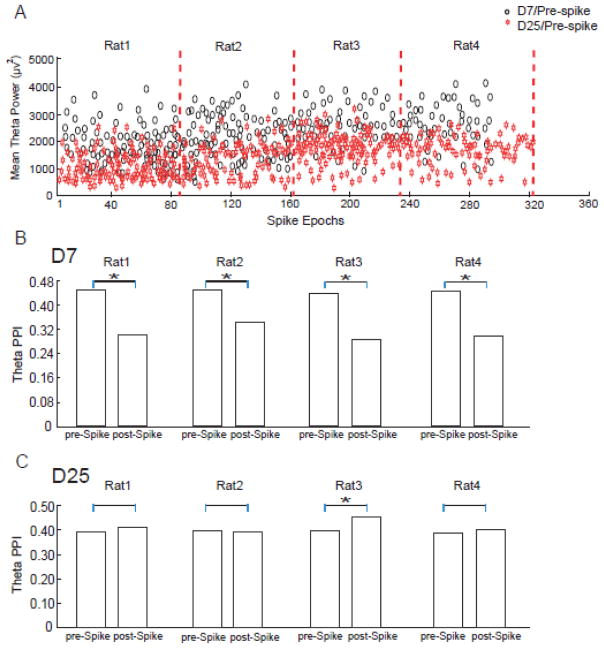
Figure 4.
Theta power and PPIs of individual subjects. (A) Pre-spike theta power of each individual subject. Theta power decreased from D7 to D25 in all rats (paired t-test, p<0.01). (B) Theta PPIs around spikes on D7 were calculated for each individual subject. Theta PPIs decreased after spikes in all subjects. (C) Theta PPIs after spikes on D25 were calculated for each individual subject. In all subjects, the change of theta PPIs after spikes showed different directionality between D25 and D7.
Time-dependent Alterations of Theta Phase Stability After Spikes
Mean relative phase values were computed for all data epochs around 9 reference frequencies between 4–12 Hz (see Methods and Eq. 1). Phase stability was then estimated by the Phase Preservation Index (PPI), which is the percentage of mean relative phase values larger than 0.95 in a particular data group (see Methods). PPI can be inferred from the cumulative distribution functions (CDF) of the mean relative phase values, as shown in Fig. 2A. The distribution functions indicated that the probability of phase synchrony was highest in the D7 pre-spike group (black curve in Fig. 2A, with 46% of the epochs showing mean relative phase values ≥0.95, corresponding to a PPI of 0.46). On D7, the theta PPI demonstrated a 33% decrease from 0.46 pre-spike to 0.31 post-spike. On D25, the theta PPI demonstrated a very small increase (2.8%) after spikes. Therefore the theta phase stability across the spikes exhibited markedly different patterns in latent and chronic stages, with a significant decrease in latent stage (bootstrapping, p<0.01) but almost unchanged in chronic stage after spikes. The temporal dynamics of theta phase stability around spikes on D7 and D25 are shown in Fig. 2B&C (also see Fig. 4B&C for results of individual subjects). The different impacts of spikes on theta phase in the latent stage and chronic stage may indicate a substantial reorganization of neural circuits during epileptogenesis.
Hyper-synchronization In the Protracted Spike-free Periods
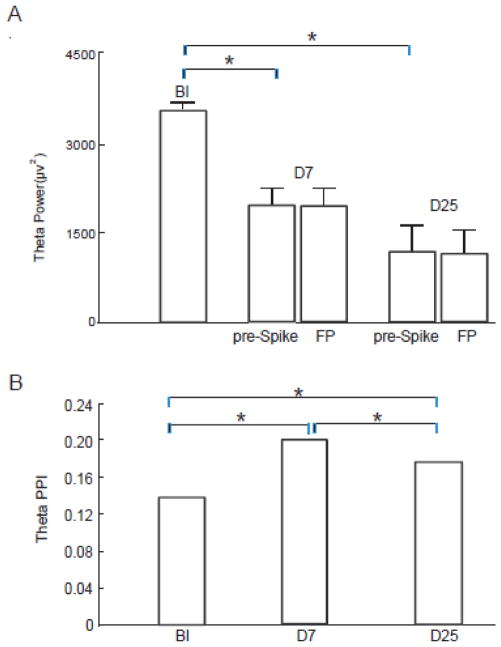
To explore the recovery process of theta oscillators, we evaluated the theta power and phase stability in FP at least one hour after spike bursts. During this period, the rats were fully awake with no obvious ILA and the exploring behavior was normal. This stage in rat corresponds to the rest period in epilepsy patients. Theta power during FP showed no difference from the pre-spike power (paired t-test, p>0.99, see Fig. 3A), although both were significantly reduced compared to the control condition (paired t-test, p< 0.01) (see also Supplementary Fig. S3 for examples).
Figure 3.
Theta phase stability and power during the spike-free period (FP). (A) While theta power in epilepsy was greatly reduced compared to the control condition (BI), theta power during FP showed no difference from the power before spikes, both in latent and chronic stage (paired t-test, p>0.99). (B) Compared to the control condition (BI), theta phase stability was higher in both D7 and D25 groups. Theta phase stability during FP reflects a higher synchrony compared to BI, both on D7 (43% change from BI, bootstrapping, p<0.01) and D25 (29% change from BI, bootstrapping, p<0.05).
In epilepsy, spike is an indication of the hyper-synchronization in neural network. The recovery process after spikes may be reflected by a transition from hyper-synchronization to the state of quasi-synchronization. We found that the theta phase stability in FP (theta PPI = 0.20 on D7 and theta PPI = 0.18 on D25) was much lower than that around spikes (pre-spike theta PPI = 0.46 on D7 and pre-spike theta PPI =0.40 on D25; bootstrapping, p<0.01 for both), indicating that the hyper-synchrony had subsided in FP (Fig. 3B). However, compared to the control condition (BI, theta PPI = 0.14), theta phase stability in FP still significantly increased by 43% on D7 and 29% on D25 (bootstrapping, p<0.01 for both). The fact that theta phase stability remained at a high level during FP indicated that the network did not fully recover to the normal pre-injection state (see also Supplementary Fig. S4 and Supplementary Fig. S5A) even though there were no spikes present.
These data suggested that while theta power in FP returns to the same level as before spikes, theta phase stability in FP was very different than it was before spikes or the baseline condition, implying theta phase may contain more information about the post-spike status of neural network compare to theta power. Therefore phase and power alone may not be sufficient to characterize the theta oscillations in the epileptic brain.
Discussion
The potential impact of ILA on cognitive processes may depend upon the type of ILA, the context (brain states) and most importantly time (during epileptogenesis). In this study, we investigated the changes of theta oscillations during epileptogenesis. Theta rhythm demonstrated transient changes after spikes, and these changes are different in the latent and chronic stages, potentially reflecting the network reorganization. We also found that theta phase and theta power may contain independent information about the post-spike changes of the network, suggesting phase and power should be both taken into account when characterizing the theta oscillations in the epileptic brain.
Impacts of Spikes on Theta Oscillations
One significant impact of spike on theta rhythm was the reduction of theta power immediately after spikes, observed in both the latent and chronic stages. A more interesting finding is that theta phase stability changes after spikes exhibited different directionality in latent and chronic stages. While theta phase stability decreased after spikes in the latent stage, the trend was different in the chronic stage with a very slight increase of phase stability after spikes. This phenomenon may reflect the neural reorganization during different stages of epileptogenesis.
Since these LFPs originate from the inhibitory and excitatory postsynaptical potentials (IPSPs/EPSPs) in the hippocampus (Schnitzler and Gross, 2005), our observations on the changes of theta power and phase stability maybe related to the imbalance between GABAergic and glutamatergic plasticity drives. We speculated that the significant changes of theta power (in Fig. 1) and theta phase stability (in Fig. 2) after spikes in latent stage may relate to the depletion of local GABAergic resources in early stage of epileptogenesis (El-Hassar et al., 2007). However, in the chronic stage, the glutamatergic synaptic modulation could be built up, enhancing the GABAergic inhibition globally in the CA1 area (El-Hassar et al., 2007). This may correspond to the milder change of theta power (in Fig. 1) and theta phase stability (in Fig. 2) on D25. However, the input from the medial septum may also play a role in the changes we observed. The medial septum acts as a pacemaker to generate the hippocampal theta rhythm. The loss of neurons in medial septum networks could also lead to the reduction of hippocampal theta frequency in epilepsy (Colom et al., 2006).
Most interestingly, our data indicated that the change of theta power and theta phase did not correlate to each other, i.e., a decrease of theta power does not necessarily predict increase (or decrease) of theta phase stability. This discrepancy suggested that theta power and phase might reflect the impairment of different mechanisms of theta genesis.
Compared to the control condition (BI), theta phase stability during FP was significantly elevated, though the EEG was completely free of spikes and no interruption of the exploration behavior was observed. Higher synchrony during FP is a strong indication of the abnormality of the neural circuits. It has been suggested that the decoupling of neural circuits can be driven by synchrony (Lubenov and Siapas, 2008). As the slow GABAergic inhibition was impaired after the spikes, the drive to decoupling may have been weakened during FP. Therefore, theta phase synchrony was not able to subside to the same baseline level as before injections. Nevertheless, these speculations are subject to future tests based on experimental and computational evidences.
Potential Dampening Effects on Cognitive Performance
Theta rhythm can be altered by spikes in both humans and rats of TLE (Bettus et al., 2008; Chauviere et al., 2009; Winson, 1978). The modification of theta rhythm could lead to negative changes in behavior as observed in rat models (Chauviere et al., 2009). In this study, we found that the transient impact of spikes on theta oscillation was significantly different in the latent and chronic stages, implying that the impact on cognitive processes may also differ between these stages.
It has been found that disruption of spatial memory was more severe in early epileptogenesis, even though theta power was stronger than in the chronic stage (Chauivere et al., 2009). This may relate to our observations of the marked change of theta phase synchrony around spikes (Fig. 2) and relatively high theta phase synchrony in FP during the latent stage (Fig. 3B), but future experiments directly testing these potential relations are warranted.
Caveats
The onset and offset of the spikes were determined by visual inspection by an experienced neurologist based on the baseline activity. To investigate whether our results are dependent on the precise determination of onset and offset, we repeated the analyses by shifting the onset to 350ms earlier and the offset to 350ms later. The dynamics on theta power and theta PPI reported in the study did not change. Therefore, the difference between before-spike and after-spike is not sensitive to the small variance in the determination of onset/offset, but is due to the occurrence of spikes.
To ensure that the dynamics reported here can be robustly found in theta band, we also repeated the analyses based on single reference frequency (from 4 to 12Hz with the increment of 1Hz). The results indicated that the phase dynamics around spikes and during the FP could be largely replicated at each central frequency (see Supplementary Fig. S5B&C).
Previous studies indicate speed has a significant effect on theta rhythm. Both the power and frequency of the theta rhythm can increase approximately linearly as running speed increases (Wyble, 2004; Whishaw and Vanderwolf, 1973; Maurer et al., 2005; Morris and Hagan, 1983; Rivas et al., 1996; Shen et al., 1997). Therefore it is important to differentiate the theta changes due to running speed from the theta changes caused by pathology. Our data show some interesting patterns that are unlikely to be dominated by speed. For example, on D7 theta power was significantly lower than that of BI although the speed was higher than BI (Chauviere et al., 2009), indicating theta power in our data did not correlate with running speed. Future studies on theta rhythm in epileptic rat models may include running speed as a covariate to improve the specificity of pathological changes of theta.
Conclusions
Spikes have significant transient negative impacts on theta power and phase. These impacts are different in the latent and chronic stages, implying that the influence of spikes on cognitive processes may also change during epileptogenesis. We suggested that phase and power are both necessary for characterizing the theta oscillations in the epileptic brain.
Supplementary Material
Highlights.
Theta power is immediately impaired after the occurrence of spikes
The impact of spikes on theta phase stability is distinct between the latent and chronic stages
Theta phase cannot recover from the hyper-synchrony even hours after the spike bursts
Phase and power are both necessary for characterizing the theta oscillations in epileptic brain
Acknowledgments
Funding Sources
This work is supported by the Natural Science Grant (E2010000053) sponsored by Hebei Province and Key Laboratory for Electromagnetic Fields and Electrical Apparatus Reliability, Tianjin, China. H. Liu is supported by NIH grant K25NS069805. W. Sun is supported by the National Natural Science Foundation of China (81171229).
The authors thank Fabrice Bartolomei, Laëtitia Chauvel and Julien Krieg for providing the experimental data. We also thank Micheal Le Van Quyen for help with the time-frequency analysis; Christophe Bernard, Christian Bénar, Patrick Chauvel, ZhiqiXiong, Andrew Chang, Tongjun Zhao and Gusphyl Justin for suggestions on the manuscript; Xiaogang Chen, Haoyu Yang, Lingling Yuan and Xiaojiang Guo, Huizhao Zhang for help with the data processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcon G, Garcia Seoane JJ, Binnie CD, Martin Miguel MC, Juler J, Polkey CE, Elwes RD, Ortiz Blasco JM. Origin and propagation of interictal discharges in the acute electrocorticogram. Implications for pathophysiology and surgical treatment of temporal lobe epilepsy. Brain. 1997;120(Pt 12):2259–82. doi: 10.1093/brain/120.12.2259. [DOI] [PubMed] [Google Scholar]
- Avoli M, D’Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D’Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Chauvel P, Wendling F. Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain. 2008;131:1818–30. doi: 10.1093/brain/awn111. [DOI] [PubMed] [Google Scholar]
- Bettus G, Wendling F, Guye M, Valton L, Regis J, Chauvel P, Bartolomei F. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2008;81:58–68. doi: 10.1016/j.eplepsyres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Ponomareff GL, Bayardo F, Ruiz R, Gage FH. Neuronal activity in the subcortically denervated hippocampus: a chronic model for epilepsy. Neuroscience. 1989;28:527–38. doi: 10.1016/0306-4522(89)90002-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Hsu M, Slamka C, Gage FH, Horvath Z. Emergence and propagation of interictal spikes in the subcortically denervated hippocampus. Hippocampus. 1991;1:163–80. doi: 10.1002/hipo.450010205. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–40. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the Brain. Oxford University Press; 2006. [Google Scholar]
- Chauviere L, Rafrafi N, Thinus-Blanc C, Bartolomei F, Esclapez M, Bernard C. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J Neurosci. 2009;29:5402–10. doi: 10.1523/JNEUROSCI.4699-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauviere L, Doublet T, Ghestem A, Siyoucef SS, Wendling F, Huys R, Jirsa V, Bartolomei F, Bernard C. Changes in interictal spike features precede the onset of temporal lobe epilepsy. Ann Neurol. 2012;71:805–14. doi: 10.1002/ana.23549. [DOI] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–68. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Colom LV, Garcia-Hernandez A, Castaneda MT, Perez-Cordova MG, Garrido-Sanabria ER. Septo-hippocampal networks in chronically epileptic rats: potential antiepileptic effects of theta rhythm generation. J Neurophysiol. 2006;95:3645–53. doi: 10.1152/jn.00040.2006. [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Manfridi A, Biella G. Activity-dependent pH shifts and periodic recurrence of spontaneous interictal spikes in a model of focal epileptogenesis. J Neurosci. 1998;18:7543–51. doi: 10.1523/JNEUROSCI.18-18-07543.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–67. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Demont-Guignard S, Benquet P, Gerber U, Wendling F. Analysis of intracerebral EEG recordings of epileptic spikes: insights from a neural network model. IEEE Trans Biomed Eng. 2009;56:2782–95. doi: 10.1109/TBME.2009.2028015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hassar L, Milh M, Wendling F, Ferrand N, Esclapez M, Bernard C. Cell domain-dependent changes in the glutamatergic and GABAergic drives during epileptogenesis in the rat CA1 region. J Physiol. 2007;578:193–211. doi: 10.1113/jphysiol.2006.119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esclapez M, Hirsch JC, Khazipov R, Ben-Ari Y, Bernard C. Operative GABAergic inhibition in hippocampal CA1 pyramidal neurons in experimental epilepsy. Proc Natl Acad Sci U S A. 1997;94:12151–6. doi: 10.1073/pnas.94.22.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloveli T, Dugladze T, Saha S, Monyer H, Heinemann U, Traub RD, Whittington MA, Buhl EH. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol. 2005;562:131–47. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J. Relationships between interictal spiking and seizures: human and experimental evidence. Can J Neurol Sci. 1991;18:573–6. doi: 10.1017/s031716710003273x. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Dou P, Nett M, Rose GM, Dudek FE. Assessment of inhibition and epileptiform activity in the septal dentate gyrus of freely behaving rats during the first week after kainate treatment. J Neurosci. 1999;19:10053–64. doi: 10.1523/JNEUROSCI.19-22-10053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasteleijn-Nolst Trenite DG, Bakker DJ, Binnie CD, Buerman A, Van Raaij M. Psychological effects of subclinical epileptiform EEG discharges. I. Scholastic skills. Epilepsy Res. 1988;2:111–6. doi: 10.1016/0920-1211(88)90027-7. [DOI] [PubMed] [Google Scholar]
- Kasteleijn-Nolst Trenite DG, Siebelink BM, Berends SG, van Strien JW, Meinardi H. Lateralized effects of subclinical epileptiform EEG discharges on scholastic performance in children. Epilepsia. 1990a;31:740–6. doi: 10.1111/j.1528-1157.1990.tb05515.x. [DOI] [PubMed] [Google Scholar]
- Kasteleijn-Nolst Trenite DG, Smit AM, Velis DN, Willemse J, van Emde Boas W. On-line detection of transient neuropsychological disturbances during EEG discharges in children with epilepsy. Dev Med Child Neurol. 1990b;32:46–50. doi: 10.1111/j.1469-8749.1990.tb08465.x. [DOI] [PubMed] [Google Scholar]
- King D, Spencer SS, McCarthy G, Spencer DD. Surface and depth EEG findings in patients with hippocampal atrophy. Neurology. 1997;48:1363–7. doi: 10.1212/wnl.48.5.1363. [DOI] [PubMed] [Google Scholar]
- Kraskov A, Quiroga RQ, Reddy L, Fried I, Koch C. Local field potentials and spikes in the human medial temporal lobe are selective to image category. J Cogn Neurosci. 2007;19:479–92. doi: 10.1162/jocn.2007.19.3.479. [DOI] [PubMed] [Google Scholar]
- Lenck-Santini PP, Holmes GL. Altered phase precession and compression of temporal sequences by place cells in epileptic rats. J Neurosci. 2008;28:5053–62. doi: 10.1523/JNEUROSCI.5024-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Muller RU, Huang LT, Kubie JL, Rotenberg A, Rivard B, Cilio MR, Holmes GL. Seizure-induced changes in place cell physiology: relationship to spatial memory. J Neurosci. 2003;23:11505–15. doi: 10.1523/JNEUROSCI.23-37-11505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG. Decoupling through synchrony in neuronal circuits with propagation delays. Neuron. 2008;58:118–31. doi: 10.1016/j.neuron.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Bragin A, Baldwin R, Shin D, Wilson C, Sankar R, Naylor D, Engel J, Wasterlain CG. Epileptogenesis after self-sustaining status epilepticus. Epilepsia. 2002;43(Suppl 5):74–80. doi: 10.1046/j.1528-1157.43.s.5.25.x. [DOI] [PubMed] [Google Scholar]
- Maurer AP, Vanrhoads SR, Sutherland GR, Lipa P, McNaughton BL. Self-motion and the origin of differential spatial scaling along the septo-temporal axis of the hippocampus. Hippocampus. 2005;15:841–852. doi: 10.1002/hipo.20114. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Hagan JJ. Hippocampal electrical activity and ballistic movement. In: Seifert W, editor. Neurobiology of the hippocampus. New York: Academic Press; 1983. pp. 321–331. [Google Scholar]
- Rivas J, Gaztelu JM, Garcia-Austt E. Changes in hippocampal cell discharge patterns and theta rhythm spectral properties as a function of walking velocity in the guinea pig. Exp Brain Res. 1996;108:113–118. doi: 10.1007/BF00242908. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005;6:285–96. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- Shah MM, Anderson AE, Leung V, Lin X, Johnston D. Seizure-induced plasticity of h channels in entorhinal cortical layer III pyramidal neurons. Neuron. 2004;44:495–508. doi: 10.1016/j.neuron.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Barnes CA, McNaughton BL, Skaggs WE, Weaver KL. The effect of aging on experience-dependent plasticity of hippocampal place cells. J Neurosci. 1997;17:6769–6782. doi: 10.1523/JNEUROSCI.17-17-06769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11:272–6. doi: 10.1177/1073858405278239. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–68. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Vanderwolf CH. Hippocampal EEG, behavior: changes in amplitude and frequency of RSA (theta rhythm) associated with spontaneous and learned movement patterns in rats and cats. Behav Biol. 1973;8:461–484. doi: 10.1016/s0091-6773(73)80041-0. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–36. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–12. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–3. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Wyble BP, Hyman JM, Rossi CA, Hasselmo ME. Analysis of theta power in hippocampal EEG during bar pressing and running behavior in rats during distinct behavioral contexts. Hippocampus. 2004;14:662–674. doi: 10.1002/hipo.20012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.