Abstract
Proper brain function requires neuronal homeostasis over a range of environmental challenges. Neuronal activity, injury, and aging stress the nervous system, and lead to neuronal dysfunction and degeneration. Yet, most organisms maintain healthy neurons throughout life, implying the existence of active maintenance mechanisms. Recent studies have revealed a key neuronal maintenance and protective function for nicotinamide mononucleotide adenylyl transferases (NMNATs). We review evidence that NMNATs protect neurons through multiple mechanisms in different contexts, and highlight functions that either require or are independent of NMNAT’s catalytic activity. We then summarize data supporting a role for NMNATs in neuronal maintenance and raise intriguing questions on how NMNATs preserve neuronal integrity and facilitate proper neural function throughout life.
Keywords: NMNAT, neurodegeneration, chaperone, NAD, proteinopathies, axonopathy, synapse, neuronal maintenance
NMNATs maintain neuronal health
The vast majority of neurons are born during embryogenesis. Maintaining the long-term health of neurons throughout an organism’s life therefore represents a major challenge. The loss of neuronal function from an inability to maintain homeostasis during accumulating stress are highlighted by neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease (reviewed in [1]). Hence, neurons require a continuous maintenance plan that enables them to endure the demands of varying workloads and to blunt damage from oxidative stress, injury, toxic compounds, and genetic stress. Here, we define a neuronal maintenance factor as a protein or molecule whose deficiency enhances age- and/or activity-dependent degeneration in mature neurons.
A neuronal maintenance function has been attributed to NMNAT proteins, first characterized as essential enzymes catalyzing NAD synthesis. Drosophila has a single NMNAT gene, while mice and humans have three, NMNAT1-3, that differ in their kinetic properties [2]. Drosophila NMNAT (dNMNAT) is widely distributed within cells [3], while mammalian NMNAT1-3 have distinct subcellular localizations [4]: NMNAT1 is localized to the nucleus, NMNAT2 is present in the Golgi, and NMNAT3 is present in mitochondria (Figure 1). A complete loss-of-function of dNmnat causes very severe photoreceptor neurodegeneration post development in Drosophila (Box 1; [3]). Nmnat1 loss-of-function mutant mice are embryonic lethal, while nmnat1 heterozygous mice develop normally without detectable neurodegeneration or axonal pathology [5]. Nmnat2 knockout mice die at birth with a reduction in the number of PNS neurons and axons [6]. In contrast, NMNAT overexpression provides varying degrees of neuroprotection against a wide range of stressors and toxic insults (reviewed in [7] and Table 1).
Figure 1. Mammalian NMNATs have distinct subcellular localizations.
NMNAT1 is a nuclear protein with a predicted nuclear localization signal (NLS) between Glu107–Lys146 [87]. NMNAT2 is localized to the Golgi apparatus via palmitoylation of Cys164 and Cys165 [4, 87]. In the mouse brain, NMNAT2 is enriched in numerous membrane compartments, including synaptic terminals and synaptic vesicles [4, 22]. NMNAT3 is predominantly localized in mitochondria and its first 25 residues encode a mitochondrial targeting sequence [50]. NAD+, the enzymatic product of NMNATs, is an essential cofactor for many metabolic processes, transcriptional regulation and several protein modification reactions. The NAD signaling pathway generates precursors of several intracellular calcium mobilizing agents including cADPR and NAADP. Abbreviations: ADPR, ADP-ribose; CAC, citric acid cycle; cADPR, cyclic ADP-ribose; NAADP, nicotinic acid adenine dinucleotide phosphate; NADase, bifunctional NAD glycohydrolase/ADP-ribosylcyclase; PARP, poly-ADP-ribose polymerase.
Box 1. NMNAT maintains healthy neurons, independent of their development, in Drosophila melanogaster.
In the Drosophila embryo, loss of Nmnat does not affect neuronal development, although global loss of function is lethal. During the larval stage, dNMNAT is required for maintaining proper dendrite arborization and axonal integrity in type IV da neurons. Wildtype da neurons (Nmnat+/+) have extensive arborizations of dendrites, while heterozygous Nmnat mutants (Nmnat+/−, middle) exhibit a reduced arborization. Homozygous Nmnat mutant (Nmnat−/−) da neurons suffer from progressive fragmentation of axons (arrow). During pupation, a mosaic eye containing Nmnat−/− neurons develops similarly to the normal eye up through 80% of pupal development. Nmnat−/− photoreceptors rapidly degenerate, showing fragmentation of rhabdomeres (red dashed box), the rod-like component of a photoreceptor cell, and loss of active zones (green dashed box). Additionally, Nmnat−/− sensory neurons (dpr+ neurons) show a progressive deterioration of axons.
Table 1.
Cytoprotective function of NMNATs.
| NMNATs | Disease or Experimental Model | Proposed Site/Mode of Action | Reference |
|---|---|---|---|
| dNMNAT | Spinocerebellar Ataxia | Clearance of toxic oligomers via proteasome-mediated clearance | [28] |
| Drosophila tauopathy model | Clearance of toxic oligomers via proteasome-mediated clearance | [13] | |
| Heat/hypoxic/oxidative stress | HSPs-like mechanism | [12] | |
| Activity-induced neurodegeneration | Cell body and synapse/active zone | [3, 69] | |
| NMNAT2 | Mouse tauopathy model | Clearance of toxic tau species | [19] |
| dNMNAT | Axotomy of wing/antenna nerve | Maintain mitochondrial integrity | [14, 16] |
| Laser ablation of ORN | Maintain mitochondrial integrity and increase calcium buffering | [14] | |
| NMNAT1 | Hypoxia-ischemia (carotid artery ligation) | Mitochondria: No effect on ATP levels/apoptosis; inhibition of excitoxicity-induced, caspase-independent mechanism. | [79] |
| DRG axonotomy | Axon protection: Enzyme activity-dependent Axon protection: Enzyme activity is required but independent of substrate levels Weak or no axon protection |
[25, 58, 88] [88] [60, 89, 90] |
|
| Sciatic nerve transection | Preservation of axonal and synaptic integrity | [58] | |
| Rotenone-induced neurotoxicity | Decreased ROS accumulation in axons | [60] | |
|
Growth factor withdrawal DCTN1 knockdown |
Axon protection independent of loss of axonal sAPP and activation of axonal caspase 6; no soma protection | [59, 60] | |
| NMNAT2 | SCG axonotomy | 1. Axonal protection only upon multifold overexpression 2. Dependent on enzymatic activity |
[17] [27] |
| Cardiac hypertrophy | Activation of Sirt6 | [91] | |
| NMNAT3 | Rotenone-induced neurotoxicity | Axons: Reduction of mitochondrial ROS with no change in ATP decline / mitochondrial function | [23] |
| Sciatic nerve transection | Mitochondria matrix: Increased ATP levels / mitochondria function | [60] |
In this review, we summarize our current knowledge of NMNAT biology, focusing on neuronal maintenance, and review evidence for and against a requirement of NMNAT’s enzymatic activity in mediating specific neuroprotective effects. We also discuss the protective effects of NMNAT in key models of neurodegeneration and consider the implications for NMNAT as a neuroprotective agent in human diseases.
Functional Diversity of NMNAT
The neuroprotective role of NMNAT first emerged with the characterization of a spontaneous chromosomal rearrangement in Wallerian degeneration slow (Wlds) mice ([8], reviewed in [7, 9]). These mice carry a dominant mutation that delays Wallerian degeneration of injured axons. The mutation results in a chimeric gene of the E4 ubiquitination factor Ube4b and the entire coding region of mouse NMNAT1, associated with a gene triplication [10, 11]. The neuroprotective effects of this mutant protein have stimulated considerable efforts to uncover possible mechanisms. Hence, NMNAT1 has been studied in numerous contexts to understand if the protection provided by Wlds and the other NMNAT isoforms is widespread and can be attributed to the enzymatic function of this protein or to some novel, enzyme-independent function [3, 4, 11–29].
NMNATs: Essential Housekeeping Enzymes
NMNATs were initially characterized as enzymes catalyzing the reversible condensation of ATP with nicotinic acid mononucleotide (NaMN) or nicotinamide mononucleotide (NMN) to produce nicotinic acid adenine dinucleotide (NaAD) or nicotinamide adenine dinucleotide (NAD) [30–32]. NAD is an essential cofactor in many cellular processes including transcriptional regulation and oxidative reactions [18]. NMNAT’s enzymatic activity enables an appropriate flux of NAD in cells by salvaging by-products of NAD-consuming chemical reactions to maintain NAD at levels necessary for internal homeostasis. Furthermore, the distinct subcellular distribution of mammalian NMNAT’s (Box 1) allows local production of NAD which presumably optimizes site-specific NAD-requiring metabolism.
The early lethality associated with loss of either Nmnat1 and Nmnat2 in mice [5, 6] suggests that there is little functional redundancy among NMNAT1-3 proteins. In mammals, for example, the nuclear-localized NMNAT1 interacts at gene regulatory elements with both poly ADP ribose polymerase-1 (PARP1) and Sirtuin-1 (Sirt1) to regulate expression of target genes, including ATXN10 and NAT1 [33–35]. PARP1 uses NAD as a substrate to modify proteins [36–38], while Sirtuin uses NAD to deacetylate target proteins and to control gene expression [39–43]. The interaction of NMNAT1 with Sirt1 or PARP1 at specific DNA regulatory elements likely establishes a local pool of NAD important for activating Sirt1 and for providing the NAD for PARP1 catalytic activity necessary for regulation of target genes by these enzymes. Recent studies found that the axonal localization of catalytically active NMNAT1 or NMNAT2 is critical in preventing axonal degeneration [44, 45]. These findings suggest that the specific subcellular localizations of NMNATs allow them to provide NAD in precise cellular domains to activate specific signaling cascades.
NMNAT: A Novel Chaperone
Molecular chaperones are defined as proteins that assist in multi-protein complex assembly, protein folding, and protein refolding after damage [46]. Enzyme-independent functions of NMNATs were first explored after the surprising observation that an enzymatically inactive form of dNMNAT protected photoreceptors from degeneration in Drosophila [28]. Subsequent studies provided additional support that NMNATs can act as chaperones [13, 28, 47, 48].
The following are typical chaperone activities that have been associated with NMNATs: (1) Chaperones stabilize non-native conformations and facilitate correct folding or degradation of misfolded proteins: Both wildtype and enzyme-inactive dNMNAT are able to protect luciferase from heat shock-induced denaturation and to support proper refolding of heat-denatured luciferase during recovery with an efficacy similar to HSP70 [28]. In addition, recombinant dNMNAT protein protects citrate synthase from thermal denaturation [28]. (2) Chaperones often couple ATP to the folding process: Deleting the C-terminal domain containing the ATP-binding site in dNMNAT abolishes its chaperone activity while maintaining NAD synthesizing activity [28]. (3) Stress-dependent regulation: dNMNAT levels can be up-regulated by heat, hypoxia and oxidative stress and dNMNAT overexpression increases thermo tolerance during oxidative stress and promotes longevity in Drosophila [12]. (4) Chaperones interact with misfolded proteins: The ability of NMNATs to reduce protein aggregate formation has been demonstrated in various proteinopathy paradigms. In a cell-based Ataxin1-82Q-GFP aggregation assay, both wildtype and enzyme dead dNMNAT significantly reduced Ataxin1-82Q-GFP aggregates [28]. In addition, NMNAT orthologs NMN1 and NMN2 are able to reduce alpha-synuclein and polyglutamine induced proteolytic stress through proteosome-mediated clearing of toxic proteins, independent of NAD levels in a yeast proteinopathy model [47]. Finally, overexpressing dNMNAT and mammalian NMNAT2 reduces the levels of toxic tau oligomers [13, 19], and there is evidence that dNMNAT complexes with toxic tau oligomers to promote proteasome-mediated clearance [13]. In summary, these studies provide strong evidence that NMNATs have chaperone activity, which in many cases are independent of their NAD synthesizing activity.
Enzyme Vs Chaperone: A “Context-Dependent” Preference
The NAD synthesis activity of NMNAT has been found to be essential for neuroprotection in several contexts. Exogenous NAD application to DRG explants offered significant axonal protection [24, 29, 49] and axonal protection post axotomy requires NMNAT enzymatic activity (reviewed in [2, 7]). However, other studies found that the neuroprotection offered by exogenous NAD was not specific and could be mimicked by providing pyruvate or EGTA [24, 25]. Studies manipulating alternative pathways of NAD synthesis showed only modest neuroprotective effects [15, 24]. As it is impossible to completely remove NAD from cells, and thus far it has not been shown that overexpression of NMNAT’s increases NAD [11], the role of NAD in neuroprotection by NMNAT remains controversial. It is possible that providing exogenous NAD is neuroprotective in some contexts because it frees up limited amounts of NMNATs to perform their enzyme-independent functions. Alternatively, the lack of effectiveness of exogenous NAD could be explained by the rapid (extracellular) break down of NAD into precursors that require NMNATs for proper resynthesis [50], during a time (after injury) when very little axonal NMNAT is available [17].
In contrast, NMNAT’s chaperone activity is consistent with its observed neuroprotective effects in various proteinopathy models [13, 19, 47], where it behaves similar to other chaperones such as Hsp70, Hsp40 and Hsp16.2 (reviewed in [2]). In these disease models, the primary pathology is linked to misfolding of proteins that disrupt neuronal maintenance either from toxic effects of the misfolded protein or from lack of the normal protein’s function.
Mechanisms of Neuroprotection
Studies in Drosophila and mice have revealed a neuroprotective role for both Wlds and different NMNAT isoforms in neurodegenerative conditions including tauopathies, Charcot-Marie-Tooth disease, Parkinson’s disease and glaucoma (Table 1; [13, 19, 28, 51–55]). The neuroprotective effects of NMNAT allow the categorization of these diseases into two distinct classes: Those in which NMNAT exclusively maintains axonal integrity, and those in which NMNAT reduces the oligomeric proteotoxic burden that causes neurodegeneration. Moreover, endogenous NMNAT proteins have also been shown to be required for synaptic maintenance. Here, we describe the specific mechanisms of neuroprotection availed by different NMNATs in distinct conditions.
Axonal Protection
Axonal degeneration is a major feature of many neurological disorders, including neurodegenerative disease such as Alzheimer’s disease, peripheral neuropathies and traumatic injuries [56, 57]. The efficacy of different NMNAT isoforms as well as Wlds in providing axonal protection has been extensively studied. Wlds significantly delays Wallerian degeneration [10, 11]. Its efficacy is due to NAD synthesis activity of the NMNAT1 portion of Wlds [49, 58]. Indeed, a ten-fold increase in survival time of severed neurites was observed in Wlds or NMNAT1 transfected DRG cultures. Despite the prominent Wlds or NMNAT1 immunoreactivity in the nucleus, recent reports have established that the sites of neuroprotection offered by NMNAT1 and Wlds are axonal and synaptic [25, 44, 51, 59, 60]. Targeting NMNAT1 to these compartments enhanced its neuroprotective function, preserving injured axons for several weeks [44]. A non-nuclear Wlds role has also been suggested by recent data showing that Wlds increases mitochondrial calcium buffering capacity and maintains mitochondrial motility after nerve injury [14]. However, when mitochondria were genetically ablated from axons, dNMNAT still provides neuroprotection, suggesting the presence of additional, mitochondria-independent mechanisms of dNMNAT-mediated axonal protection [61]. Furthermore, the NAD synthesizing activity has been shown to be dispensable for dNMNAT and WldS-mediated protection during axonal degeneration induced by inactivation of the c-Jun N-terminal kinase (JNK) pathway in Drosophila mushroom body (MB) neurons [48].
Interestingly, NMNAT overexpression also provides protection in Charcot-Marie-Tooth (CMT) disease, a common inherited peripheral neuropathy that causes axonal loss and progressive muscle atrophy [62–64]. A transgenic rat model of CMT harboring additional copies of the murine peripheral myelin protein 22 (Pmp22) gene develops a demyelinating neuropathy, onion bulb formation with axonal loss, and consecutive muscle atrophy [64]. In this rat model, overexpressing the Wlds transgene protected axons from demyelination and maintained muscle function [62]. Furthermore, electrophysiological data showed that Wlds overexpression partially rescues the prolonged distal motor latencies in CMT [62]. Another example of NMNAT1/Wlds mediated protection of axonal integrity is associated with glaucoma, a leading cause of blindness through progressive degeneration of retinal ganglion cells (RGCs) and their axons [51]. In experimentally induced glaucoma, Wlds preserved RGC axonal integrity for two weeks but failed to rescue RGC cell bodies or dendritic arborization defects [51].
Furthermore, NMNAT2 overexpression delays axotomy-induced degeneration in superior cervical ganglion explants and this protection requires its enzymatic function [27]. However, axonal protection from NMNAT2 overexpression appears to be context-dependent. While peripheral nerve injury in the Drosophila wing can be rescued by NMNAT2 overexpression [16], this protection is not observed in the injured axons from olfactory receptor neurons (ORN) [14]. Substantial protection has also been observed from overexpressing NMNAT3 in transgenic mice and flies [15, 60]. It will be important to determine the neuroprotective capacities of each NMNAT isoform in specific contexts and unravel the mechanism(s) underlying this neuroprotection.
Protection Against Proteinopathies
NMNATs have also been shown to provide neuroprotection against proteinopathies. In Drosophila, neuronal overexpression of frontotemporal dementia (FTD)-linked mutant R406W-tau causes an age-dependent progressive neurodegeneration as well as learning/memory and locomotor deficits [13, 65, 66]. Overexpression of either wildtype or enzyme-inactive NMNAT in this tauopathy fly rescued both age-dependent brain vacuolization and behavioral deficits [13]. Consistent with the previously reported chaperone function of NMNAT, overexpression of NMNAT decreased the soluble toxic tau oligomeric burden. Importantly, NMNAT is also neuroprotective in a mouse tauopathy model, where overexpression of mutant FTD-linked P301L-tau in the forebrain leads to neurodegeneration [19]. Overexpression of either NMNAT1 or 2, but not NMNAT3 in the hippocampus from 6 weeks of age onwards reduced neuronal death when assessed in 5-month-old mice. Neuroprotection by NMNAT is also correlated with a reduction in toxic tau oligomers and disease associated tau species. The Drosophila and mouse tauopathy studies also reveal that endogenous dNMNAT and mouse NMNAT2 (but not NMNAT1) are significantly reduced at both the transcript and protein levels prior to the onset of degeneration or behavioral deficits [13, 19], suggesting a role in neuronal maintenance of both dNMNAT and mammalian NMNAT2.
dNMNAT overexpression also ameliorates the neurodegeneration caused by overexpression of the human ataxin1 protein containing 82 polyglutamine repeats [28]. Interestingly, the enzymatically-inactive dNMNAT is also neuroprotective, suggesting that it functions as a chaperone to effectively rid neurons of the misfolded protein that mediate the cytotoxicity. Alternatively, one may argue that the residual 1% enzyme activity in this dNMNAT mutant protein was adequate to yield sufficient NAD for neuroprotection. However, the complete failure of the enzyme dead dNMNAT to rescue the embryonic lethality of Drosophila NMNAT null mutant does not support this hypothesis [3].
More recently, overexpression of Wlds as well as enzyme-deficient Wlds was shown to protect dopaminergic neurons from the neurotoxin, N-methyl-4-phenylpyridinium (MPP+), a popular Parkinson disease model [67]. MPP+ treatment induces dopaminergic neuron degeneration, the characteristic pathology of Parkinson disease. This was the first demonstration that an enzyme dead Wlds can also be neuroprotective. In the MPP+ model, the cytoprotective effects of enzyme dead Wlds were studied in CNS neurons, unlike earlier peripheral nervous system (PNS) DRG-based studies. Interestingly, in dopaminergic neurons treated with MPP+, Wlds protected both neurites and cell bodies from degeneration, whereas in PNS neurons, protection was limited to axons [51]. Moreover, the protection of dopaminergic neurons by enzyme-deficient Wlds suggests that Wlds may also act as a chaperone in the CNS.
Synaptic Maintenance
Synapses are often the first structures lost in human neurodegenerative diseases [68]. One interesting, yet underexplored mode of NMNAT-mediated neuroprotection is its role in maintaining the synapse. The neuronal maintenance function of dNMNAT was originally identified in a loss-of-function screen in which mutant clones were induced in the Drosophila eye [3]. Photoreceptors that lack dNMNAT developed properly; however, upon aging their degeneration is accelerated with increasing activity [3]. These findings show that dNMNAT plays an important role in maintaining synaptic function.
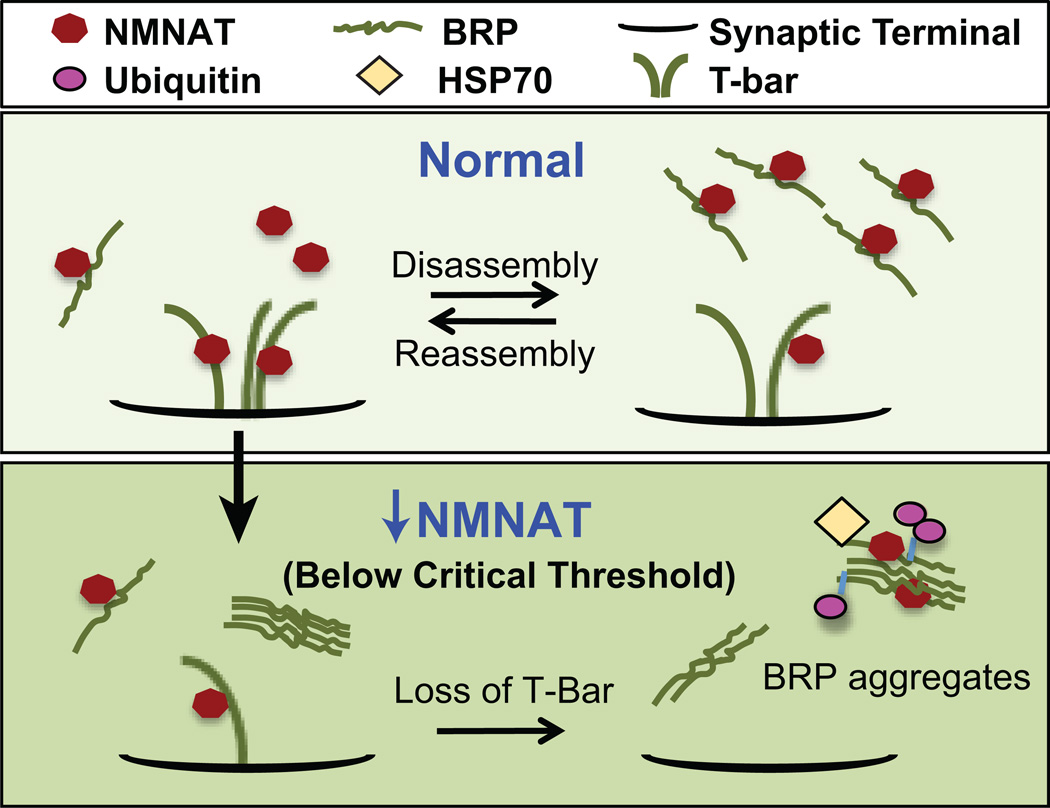
In the adult fly optic lobe, endogenous dNMNAT is enriched in the cell bodies of photoreceptors but it also labels the synapse in a punctate fashion at the “T-bar”, a specialized active zone structure, co-localizing with the active zone protein, Bruchpilot (BRP; [3]). Neuronal activity causes dynamic changes of the active zone and the assembly and disassembly of BRP into the active zone/T-bar. dNMNAT binds to and stabilizes BRP, shielding it from activity-induced ubiquitin-proteasome-mediated degradation (Figure 2; [69]). Loss of dNMNAT causes ubiquitination, aggregation, and dislocation of BRP, and consequently loss of the T-bar structures. NMNAT functions to maintain the conformation of BRP through direct interactions and facilitates its redistribution among active zones [69]. These NMNAT functions are similar to the described roles of cysteine-string protein (CSP), a major synaptic vesicle protein that acts as a chaperone in maintaining synaptic integrity against elevated neural activity [70–72].
Figure 2. NMNAT interacts with Bruchpilot (BRP) to maintain active zone integrity in an activity-dependent manner.
The T-bar is a dynamic structure undergoing continuous assembly and disassembly. Neural activity level affects the size of T-bars. Under normal conditions, NMNAT associates with BRP at the synapse and facilitates the assembly of T-bars. Neural activity enhances the NMNAT-BRP interaction to maintain T-bar integrity. When NMNAT is reduced below a critical threshold, BRP becomes ubiquitinated and aggregates, recruiting chaperones such as HSP70 and residual NMNAT, leading to a loss of T-bars at the synapse. Increased neuronal activity will aggravate this phenotype due to an increase in BRP aggregates.
Mammalian NMNAT2 is also present at synaptic membranes and vesicles [4, 22], suggesting a possible role for NMNAT2 in maintaining proper synaptic function as well. Even though Wlds and NMNAT1 are primarily localized to the nucleus, trace amounts of these proteins have also been detected at the synapse [73, 74]. Hence, the role of NMNATs at synapses needs to be investigated and its client proteins involved in maintaining synaptic integrity should be identified.
Regulation of NMNAT levels
The overall abundance of NMNATs likely correlates with the level of neuroprotection in different models of neurodegeneration [17, 19]. The factors regulating steady state NMNAT levels are not well understood. NMNAT2 is the most labile mammalian NMNAT, with a half-life of less than 2 hours in neurons [17, 45]. Peripheral nerve injury leads to rapid depletion of NMNAT2 [17] and reduced NMNAT2 levels causes significant axonal deterioration [6, 17]. During Wallerian degeneration, NMNAT2 is rapidly depleted in the distal stumps of injured neurites. In Drosophila, the Highwire ubiquitin ligase promotes degradation of the NMNAT [75] and NMNAT2 can also be degraded by this mechanism when ectopically expressed in Drosophila, suggesting that the mechanism is conserved.
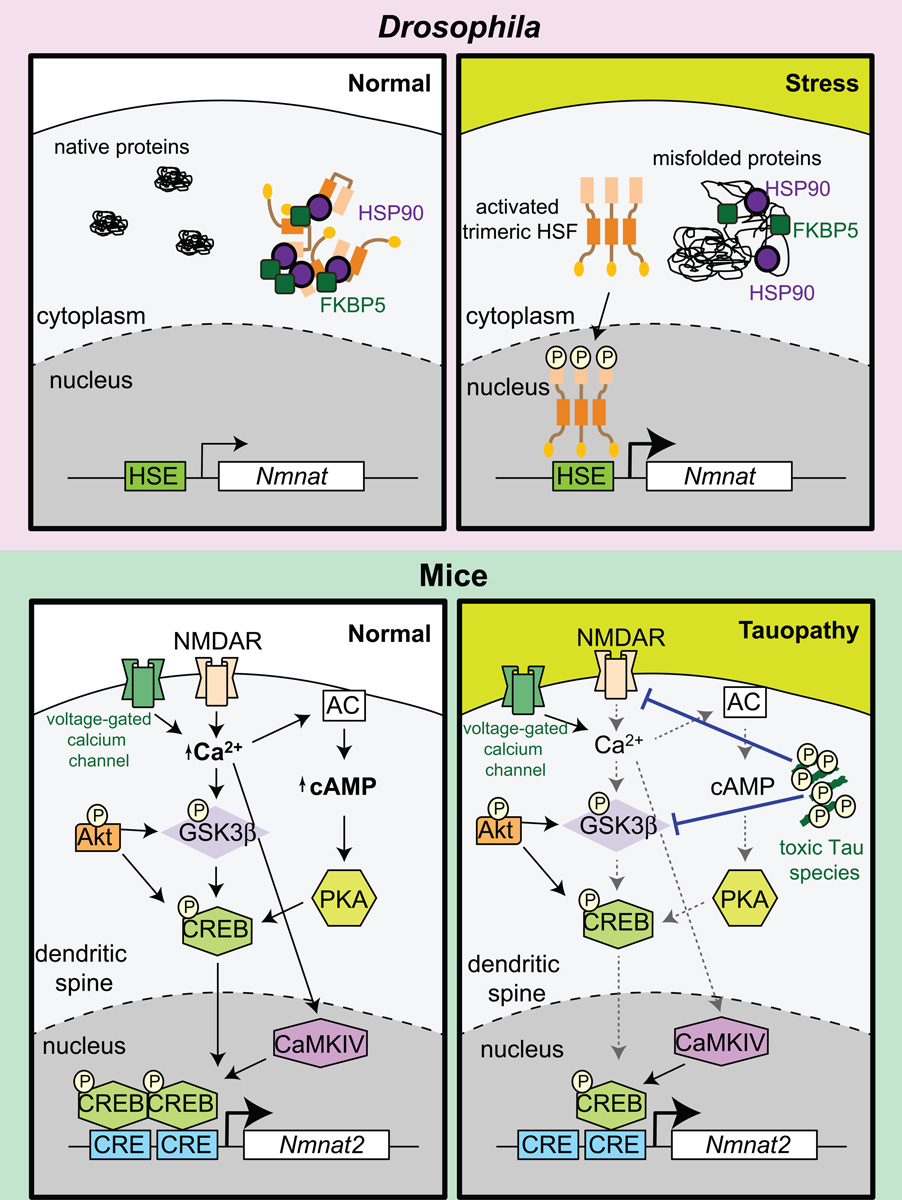
NMNAT2 expression is also tightly regulated at the transcriptional level. Recent data gathered from rTg4510 mice, a FTD and Parkinsonism-17 (FTDP-17) tauopathy model, documented a precipitous decline in endogenous Nmnat2 mRNA levels prior to the onset of neurodegeneration or memory deficits [19]. Interestingly, young rTg4510 mice display a sharp decrease in endogenous Nmnat2 transcripts, accompanied by decreased protein levels. Nmnat2 RNA expression is regulated by CRE binding protein (CREB; Figure 3) and in rTg4510 mice, activated CREB levels followed the same pattern of decline as endogenous NMNAT2, and less activated CREB was found on the CREB binding sites located in the Nmnat2 promoter. These findings provide evidence for a role of pCREB in regulating NMNAT2 levels in vivo.
Figure 3. Transcriptional regulation of NMNAT in Drosophila and mammals.
Under normal conditions, an appropriate level of nmnat transcription is maintained. In Drosophila, there is one NMNAT gene, under the regulation of stress transcription factor HSF. During acute stress conditions, NMNAT is upregulated by direct binding of HSF to a HSE present in its promoter. In mice, NMNAT2 is involved in neuronal maintenance and its transcription is regulated by CREB. CREB can be activated by a variety of signaling cascades including activation through the AC–cAMP-PKA pathway and direct phosphorylation via activity-dependent increase in CaMKIV. AC activity can be triggered by calcium influx through NMDAR or voltage-gated calcium channels upon depolarization. However, in tauopathy, toxic Tau species (phosphorylated Tau oligomers) reduce synaptic NMDAR function, which could account for a reduction in pCREB levels. Alternatively, AKT activity and pGSK3 levels have also been shown to affect pCREB levels and these signaling cascades are disrupted in tauopathies. Abbreviations: AC, adenylyl cyclase; AKT, serine/threonine protein kinase AKT; CaMKIV, Ca2+/calmodulin-dependent protein kinase IV; cAMP, cyclic adenosine monophosphate; CRE, cAMP response element; CREB, cAMP response element-binding protein; FKBP5, FK506 binding protein 5; GSK3β, glycogen synthase kinase 3β; HSE, heat shock element; HSF, heat shock factor; HSP, heat shock proteins; NMDAR, N-methyl-D-aspartate receptor; PKA, protein kinase A.
In Drosophila, transcriptional analysis revealed stress-responsive regulation of dNmnat [76–78]. Diverse acute stress paradigms rapidly induces dNmnat mRNA and protein [12]. As predicted from sequence analysis, the induction of dNmnat occurs through direct binding of heat shock factor (HSF) to elements present in dNmnat’s promoter during both heat and hypoxic episodes [12]. NMNAT1 also possesses stress-responsive properties, and its overexpression reduces brain atrophy in animals undergoing unilateral carotid artery ligation [79]. Interestingly, in a population of people living at high altitude in the Andes, Nmnat1 transcript levels are significantly elevated when compared to sea level controls [80], implying a stress (hypoxia)-induced adaptation to promote survival in an adverse environment.
Mutations in NMNATs are associated with human disease
Recently, NMNAT1 was linked to a form of Leber congenital amaurosis (LCA), the most common cause of inherited childhood blindness. Whole exome sequencing of LCA patients identified a locus on chromosome 1p36, and 4 different teams identified different mutations in NMNAT1 [81–84]. All individuals with NMNAT1 mutations displayed severe atrophic lesions in the central retina with complete loss of neural tissue including photoreceptors, bipolar cells and ganglion cells. Strikingly, all of these individuals had early onset atrophy of the optic nerve [81–84].
The sites of the NMNAT1 mutations that cause LCA are predicted to be deleterious, and structure function analysis revealed that many of these missense point mutations might alter catalytic activity (A13T, L153P, D173G, V178M, V9M, R66W), hexamerization (R207W, I217N, R237C, L239S) or even hydrophobic interactions and protein stability (M69V, Y181C, H251P, E257K). One of these mutations, E257K is present in the C-terminal of the protein adjacent to the S256 phosphorylation site, which is responsible for mediating interactions with other proteins such as PARP and protein kinases. Some mutations, like V9M and R66W, show reduced enzymatic activity, and reduced NAD levels. Finally, some NMNAT1 mutations such as E257K show a nuclear localization, but appear to be heavily misfolded based on ubiquitin colocalization [82]. Hence, diverse mechanisms that disrupt NMNAT1 function and/or levels seem to be sufficient to induce a severe, early onset degenerative phenotype. Consistent with these studies, loss of dNMNAT in Drosophila photoreceptors also causes a severe degeneration [3].
Additional evidence for links between NMNAT’s and disease were found with NMNAT3 for Alzheimer’s disease and alcoholism [85, 86]. A genome-wide screen of 103 Dutch patients with late onset Alzheimer’s disease identified a SNP downstream of the NMNAT3 gene [85]. We therefore analyzed microarray data from previous neurodegenerative disease investigations and observed a strikingly reduction in Nmnat2 mRNA in many different neurodegenerative diseases including Alzheimer’s, tauopathy, Parkinson’s and Huntington’s disease (Table 2). Given the important role of NMNAT2 in neuronal maintenance, a reduction of NMNAT2 may be a contributing factor of neurodegeneration in these diseases.
Table 2. Microarray studies on neurodegenerative diseases reveal a reduction in NMNAT2 transcript in affected regions of the brain.
Microarray data was mined using NextBio from publicly available array results.
| Disease | Tissue / Cell Type | Fold changes | P-value | Reference |
|---|---|---|---|---|
| Alzheimer’s Disease | Pyramidal cell of hippocampus | −3.81 | 0.000031 | [92, 93] |
| Pyramidal cell of middle temporal gyrus | −9.25 | 0.000058 | ||
| FTLDU | Hippocampus | −6.22 | 0.0026 | [94] |
| Parkinson’s Disease | Medial substantia nigra | −2.36 | 0.0009 | [95, 96] |
| Lateral substantia nigra | −2.53 | 0.0038 | ||
| Huntington’s Disease | Caudate nucleus | −3.08 | 0.0031 | [97] |
| Motor cortex BA4 | −1.36 | 0.0179 | ||
In summary, compromised NMNAT function is associated with conditions where cellular homeostasis is affected. In all cases, reduced levels of functional NMNAT protein (predicted by the decreased mRNA) appear to affect cell survival. Despite differences in the mechanisms by which the different NMNAT isoforms contribute to cytoprotection in each of these cases, these studies clearly suggest that maintaining proper NMNAT levels is required for appropriate neuronal function. Further investigation into the control of NMNAT transcription, translation, and post-translational control will therefore be critical.
Concluding Remarks
NMNATs are very interesting, yet enigmatic proteins. Understanding the mechanisms of neuronal maintenance mediated by NMNATs will not only uncover the modes of action of these proteins, critical for harnessing their protective potential as therapeutic targets, but will also provide insights into intrinsic maintenance mechanisms required to maintain healthy neurons. Because of their versatile roles, NMNATs may prove to be protective in an array of disorders, ranging from Alzheimer’s disease to axonopathies and drug-induced neuropathies. It is already evident that the mechanisms driving protection in each of these disorders will be different. Understanding the different functional roles of NMNATs will be crucial to harnessing their cytoprotective role in diverse human disorders (Box 2).
Box 2. Outstanding questions.
How do NMNATs maintain neuronal integrity? In Drosophila, dNMNAT protects axonal terminals by shielding active zone protein BRP from activity-induced degradation. Do mammalian NMNATs protect neurons through similar or different mechanisms?
What is the significance of the specific subcellular localizations of NMNAT1-3? Does each subcellular localization facilitate distinct protein-protein interactions to exert different protective functions?
What role does endogenous NMNAT2 play at the synapse?
Under what conditions will NMNAT act as a chaperone?
Supplementary Material
Box Figure.
Acknowledgements
The authors would like to thank Dr. Ken Mackie for his comments. This work was supported by: NIH NS048884 and HD065561 for H.C.L; NIH NS64269, the University of Miami Neuroscience Center Fellowship, and the Pew Charitable Trust for R.G.Z. D.L.K. is supported by NIH T32NS043124, while H.J.B. is a HHMI investigator.
References
- 1.Lin YC, Koleske AJ. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci. 2010;33:349–378. doi: 10.1146/annurev-neuro-060909-153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai RG, et al. Nicotinamide/nicotinic acid mononucleotide adenylyltransferase, new insights into an ancient enzyme. Cell Mol Life Sci. 2009;66:2805–2818. doi: 10.1007/s00018-009-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhai RG, et al. Drosophila NMNAT maintains neural integrity independent of its NAD synthesis activity. PLoS Biol. 2006;4:e416. doi: 10.1371/journal.pbio.0040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger F, et al. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 2005;280:36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- 5.Conforti L, et al. Reducing expression of NAD+ synthesizing enzyme NMNAT1 does not affect the rate of Wallerian degeneration. FEBS J. 2011;278:2666–2679. doi: 10.1111/j.1742-4658.2011.08193.x. [DOI] [PubMed] [Google Scholar]
- 6.Hicks AN, et al. Nicotinamide mononucleotide adenylyltransferase 2 (Nmnat2) regulates axon integrity in the mouse embryo. PLoS One. 2012;7:e47869. doi: 10.1371/journal.pone.0047869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lunn ER, et al. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur J Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 9.Feng Y, et al. Wld(S), Nmnats and axon degeneration--progress in the past two decades. Protein Cell. 2010;1:237–245. doi: 10.1007/s13238-010-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conforti L, et al. A Ufd2/D4Cole1e chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc Natl Acad Sci U S A. 2000;97:11377–11382. doi: 10.1073/pnas.97.21.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mack TG, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- 12.Ali YO, et al. Nicotinamide mononucleotide adenylyltransferase is a stress response protein regulated by the heat shock factor/hypoxia-inducible factor 1alpha pathway. J Biol Chem. 2011;286:19089–19099. doi: 10.1074/jbc.M111.219295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali YO, et al. NMNAT suppresses tau-induced neurodegeneration by promoting clearance of hyperphosphorylated tau oligomers in a Drosophila model of tauopathy. Hum Mol Genet. 2012;21:237–250. doi: 10.1093/hmg/ddr449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avery MA, et al. WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Curr Biol. 2012;22:596–600. doi: 10.1016/j.cub.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avery MA, et al. Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. J Cell Biol. 2009;184:501–513. doi: 10.1083/jcb.200808042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Y, et al. A novel Drosophila model of nerve injury reveals an essential role of Nmnat in maintaining axonal integrity. Curr Biol. 2012;22:590–595. doi: 10.1016/j.cub.2012.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8:e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau C, et al. The NMN/NaMN adenylyltransferase (NMNAT) protein family. Front Biosci. 2009;14:410–431. doi: 10.2741/3252. [DOI] [PubMed] [Google Scholar]
- 19.Ljungberg MC, et al. CREB-activity and nmnat2 transcription are down-regulated prior to neurodegeneration, while NMNAT2 over-expression is neuroprotective, in a mouse model of human tauopathy. Hum Mol Genet. 2012;21:251–267. doi: 10.1093/hmg/ddr492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magni G, et al. Structure and function of nicotinamide mononucleotide adenylyltransferase. Curr Med Chem. 2004;11:873–885. doi: 10.2174/0929867043455666. [DOI] [PubMed] [Google Scholar]
- 21.Magni G, et al. Enzymology of NAD+ homeostasis in man. Cell Mol Life Sci. 2004;61:19–34. doi: 10.1007/s00018-003-3161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer PR, et al. Expression, localization, and biochemical characterization of nicotinamide mononucleotide adenylyltransferase 2. J Biol Chem. 2010;285:40387–40396. doi: 10.1074/jbc.M110.178913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Press C, Milbrandt J. Nmnat delays axonal degeneration caused by mitochondrial and oxidative stress. J Neurosci. 2008;28:4861–4871. doi: 10.1523/JNEUROSCI.0525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki Y, et al. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J Neurosci. 2006;26:8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki Y, Milbrandt J. Axonal degeneration is blocked by nicotinamide mononucleotide adenylyltransferase (Nmnat) protein transduction into transected axons. J Biol Chem. 2010;285:41211–41215. doi: 10.1074/jbc.C110.193904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen Y, et al. Nmnat exerts neuroprotective effects in dendrites and axons. Mol Cell Neurosci. 2011;48:1–8. doi: 10.1016/j.mcn.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan T, et al. Nmnat2 delays axon degeneration in superior cervical ganglia dependent on its NAD synthesis activity. Neurochem Int. 2010;56:101–106. doi: 10.1016/j.neuint.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Zhai RG, et al. NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature. 2008;452:887–891. doi: 10.1038/nature06721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, et al. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol. 2005;170:349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bork P, et al. The cytidylyltransferase superfamily: identification of the nucleotide-binding site and fold prediction. Proteins. 1995;22:259–266. doi: 10.1002/prot.340220306. [DOI] [PubMed] [Google Scholar]
- 31.D'Angelo I, et al. Structure of nicotinamide mononucleotide adenylyltransferase: a key enzyme in NAD(+) biosynthesis. Structure. 2000;8:993–1004. doi: 10.1016/s0969-2126(00)00190-8. [DOI] [PubMed] [Google Scholar]
- 32.Garavaglia S, et al. Structure of human NMN adenylyltransferase. A key nuclear enzyme for NAD homeostasis. J Biol Chem. 2002;277:8524–8530. doi: 10.1074/jbc.M111589200. [DOI] [PubMed] [Google Scholar]
- 33.Zhang T, et al. Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J Biol Chem. 2009;284:20408–20417. doi: 10.1074/jbc.M109.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T, et al. Regulation of poly(ADP-ribose) polymerase-1-dependent gene expression through promoter-directed recruitment of a nuclear NAD+ synthase. J Biol Chem. 2012;287:12405–12416. doi: 10.1074/jbc.M111.304469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang T, Kraus WL. SIRT1-dependent regulation of chromatin and transcription: linking NAD(+) metabolism and signaling to the control of cellular functions. Biochim Biophys Acta. 2010;1804:1666–1675. doi: 10.1016/j.bbapap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Amours D, et al. Proteolysis of poly(ADP-ribose) polymerase by caspase 3: kinetics of cleavage of mono(ADP-ribosyl)ated and DNA-bound substrates. Radiat Res. 1998;150:3–10. [PubMed] [Google Scholar]
- 37.Krishnakumar R, Kraus WL. PARP-1 regulates chromatin structure and transcription through a KDM5B-dependent pathway. Mol Cell. 2010;39:736–749. doi: 10.1016/j.molcel.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010;39:8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dvir-Ginzberg M, et al. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J Biol Chem. 2008;283:36300–36310. doi: 10.1074/jbc.M803196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feige JN, Auwerx J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol. 2008;20:303–309. doi: 10.1016/j.ceb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feige JN, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 42.Shogren-Knaak M, et al. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 43.Shogren-Knaak M, Peterson CL. Switching on chromatin: mechanistic role of histone H4-K16 acetylation. Cell Cycle. 2006;5:1361–1365. doi: 10.4161/cc.5.13.2891. [DOI] [PubMed] [Google Scholar]
- 44.Babetto E, et al. Targeting NMNAT1 to axons and synapses transforms its neuroprotective potency in vivo. J Neurosci. 2010;30:13291–13304. doi: 10.1523/JNEUROSCI.1189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milde S, et al. Subcellular localization determines the stability and axon protective capacity of axon survival factor Nmnat2. PLoS Biol. 2013;11:e1001539. doi: 10.1371/journal.pbio.1001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellis J. Proteins as molecular chaperones. Nature. 1987;328:378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- 47.Ocampo A, et al. NAD+ salvage pathway proteins suppress proteotoxicity in yeast models of neurodegeneration by promoting the clearance of misfolded/oligomerized proteins. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rallis A, et al. Molecular chaperones protect against JNK- and Nmnat-regulated axon degeneration in Drosophila. Journal of cell science. 2012 doi: 10.1242/jcs.117259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Araki T, et al. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 50.Nikiforov A, et al. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem. 2011;286:21767–21778. doi: 10.1074/jbc.M110.213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beirowski B, et al. The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur J Neurosci. 2008;28:1166–1179. doi: 10.1111/j.1460-9568.2008.06426.x. [DOI] [PubMed] [Google Scholar]
- 52.Ferri A, et al. Inhibiting axon degeneration and synapse loss attenuates apoptosis and disease progression in a mouse model of motoneuron disease. Curr Biol. 2003;13:669–673. doi: 10.1016/s0960-9822(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 53.Howell GR, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sajadi A, et al. Wlds-mediated protection of dopaminergic fibers in an animal model of Parkinson disease. Curr Biol. 2004;14:326–330. doi: 10.1016/j.cub.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 55.Samsam M, et al. The Wlds mutation delays robust loss of motor and sensory axons in a genetic model for myelin-related axonopathy. J Neurosci. 2003;23:2833–2839. doi: 10.1523/JNEUROSCI.23-07-02833.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raff MC, et al. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 57.Saxena S, Caroni P. Mechanisms of axon degeneration: from development to disease. Prog Neurobiol. 2007;83:174–191. doi: 10.1016/j.pneurobio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Conforti L, et al. Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J Cell Biol. 2009;184:491–500. doi: 10.1083/jcb.200807175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sasaki Y, et al. Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J Neurosci. 2009;29:6526–6534. doi: 10.1523/JNEUROSCI.1429-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yahata N, et al. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J Neurosci. 2009;29:6276–6284. doi: 10.1523/JNEUROSCI.4304-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitay BM, et al. Mislocalization of neuronal mitochondria reveals regulation of Wallerian degeneration and NMNAT/WLDS-mediated axon protection independent of axonal mitochondria. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyer zu Horste G, et al. The Wlds transgene reduces axon loss in a Charcot-Marie-Tooth disease 1A rat model and nicotinamide delays post-traumatic axonal degeneration. Neurobiol Dis. 2011;42:1–8. doi: 10.1016/j.nbd.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Pareyson D, Marchesi C. Natural history and treatment of peripheral inherited neuropathies. Adv Exp Med Biol. 2009;652:207–224. doi: 10.1007/978-90-481-2813-6_14. [DOI] [PubMed] [Google Scholar]
- 64.Sereda MW, Nave KA. Animal models of Charcot-Marie-Tooth disease type 1A. Neuromolecular Med. 2006;8:205–216. doi: 10.1385/nmm:8:1-2:205. [DOI] [PubMed] [Google Scholar]
- 65.Ali YO, et al. Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. J Vis Exp. 2011 doi: 10.3791/2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wittmann CW, et al. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- 67.Antenor-Dorsey JA, O'Malley KL. WldS but not Nmnat1 protects dopaminergic neurites from MPP+ neurotoxicity. Mol Neurodegener. 2012;7:5. doi: 10.1186/1750-1326-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wishart TM, et al. Synaptic vulnerability in neurodegenerative disease. J Neuropathol Exp Neurol. 2006;65:733–739. doi: 10.1097/01.jnen.0000228202.35163.c4. [DOI] [PubMed] [Google Scholar]
- 69.Zang S, et al. Nicotinamide mononucleotide adenylyltransferase maintains active zone structure by stabilizing Bruchpilot. EMBO Rep. 2013;14:87–94. doi: 10.1038/embor.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graham ME, Burgoyne RD. Comparison of cysteine string protein (Csp) and mutant alpha-SNAP overexpression reveals a role for csp in late steps of membrane fusion in dense-core granule exocytosis in adrenal chromaffin cells. J Neurosci. 2000;20:1281–1289. doi: 10.1523/JNEUROSCI.20-04-01281.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greaves J, et al. Palmitoylation and membrane interactions of the neuroprotective chaperone cysteine-string protein. J Biol Chem. 2008;283:25014–25026. doi: 10.1074/jbc.M802140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma M, et al. CSPalpha promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity. Nat Cell Biol. 2011;13:30–39. doi: 10.1038/ncb2131. [DOI] [PubMed] [Google Scholar]
- 73.Wishart TM, et al. Differential proteomics analysis of synaptic proteins identifies potential cellular targets and protein mediators of synaptic neuroprotection conferred by the slow Wallerian degeneration (Wlds) gene. Mol Cell Proteomics. 2007;6:1318–1330. doi: 10.1074/mcp.M600457-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wright AK, et al. Synaptic protection in the brain of WldS mice occurs independently of age but is sensitive to gene-dose. PLoS One. 2010;5:e15108. doi: 10.1371/journal.pone.0015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong X, et al. The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol. 2012;10:e1001440. doi: 10.1371/journal.pbio.1001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ali YO, et al. Dealing with misfolded proteins: examining the neuroprotective role of molecular chaperones in neurodegeneration. Molecules. 2010;15:6859–6887. doi: 10.3390/molecules15106859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- 78.Powers ET, et al. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 79.Verghese PB, et al. Nicotinamide mononucleotide adenylyl transferase 1 protects against acute neurodegeneration in developing CNS by inhibiting excitotoxic-necrotic cell death. Proc Natl Acad Sci U S A. 2011;108:19054–19059. doi: 10.1073/pnas.1107325108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Appenzeller O, et al. Chronic hypoxia in Andeans; are there lessons for neurology at sea level? J Neurol Sci. 2006;247:93–99. doi: 10.1016/j.jns.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 81.Chiang PW, et al. Exome sequencing identifies NMNAT1 mutations as a cause of Leber congenital amaurosis. Nat Genet. 2012 doi: 10.1038/ng.2370. [DOI] [PubMed] [Google Scholar]
- 82.Koenekoop RK, et al. Mutations in NMNAT1 cause Leber congenital amaurosis and identify a new disease pathway for retinal degeneration. Nat Genet. 2012 doi: 10.1038/ng.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perrault I, et al. Mutations in NMNAT1 cause Leber congenital amaurosis with early-onset severe macular and optic atrophy. Nat Genet. 2012 doi: 10.1038/ng.2357. [DOI] [PubMed] [Google Scholar]
- 84.Falk MJ, et al. NMNAT1 mutations cause Leber congenital amaurosis. Nat Genet. 2012 doi: 10.1038/ng.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu F, et al. A genomewide screen for late-onset Alzheimer disease in a genetically isolated Dutch population. Am J Hum Genet. 2007;81:17–31. doi: 10.1086/518720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mulligan MK, et al. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lau C, et al. Isoform-specific targeting and interaction domains in human nicotinamide mononucleotide adenylyltransferases. J Biol Chem. 2010;285:18868–18876. doi: 10.1074/jbc.M110.107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sasaki Y, et al. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci. 2009;29:5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Conforti L, et al. NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration. Cell death and differentiation. 2007;14:116–127. doi: 10.1038/sj.cdd.4401944. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe M, et al. Protection of vincristine-induced neuropathy by WldS expression and the independence of the activity of Nmnat1. Neuroscience letters. 2007;411:228–232. doi: 10.1016/j.neulet.2006.09.068. [DOI] [PubMed] [Google Scholar]
- 91.Cai Y, et al. Nmnat2 protects cardiomyocytes from hypertrophy via activation of SIRT6. FEBS Lett. 2012;586:866–874. doi: 10.1016/j.febslet.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 92.Liang WS, et al. Gene expression profiles in anatomically and functionally distinct regions of the normal aged human brain. Physiol Genomics. 2007;28:311–322. doi: 10.1152/physiolgenomics.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liang WS, et al. Alzheimer's disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc Natl Acad Sci U S A. 2008;105:4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Deerlin VM, et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet. 2010;42:234–239. doi: 10.1038/ng.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lesnick TG, et al. A genomic pathway approach to a complex disease: axon guidance and Parkinson disease. PLoS Genet. 2007;3:e98. doi: 10.1371/journal.pgen.0030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moran LB, et al. Whole genome expression profiling of the medial and lateral substantia nigra in Parkinson's disease. Neurogenetics. 2006;7:1–11. doi: 10.1007/s10048-005-0020-2. [DOI] [PubMed] [Google Scholar]
- 97.Hodges A, et al. Regional and cellular gene expression changes in human Huntington's disease brain. Hum Mol Genet. 2006;15:965–977. doi: 10.1093/hmg/ddl013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.