Abstract
Intravaginal delivery of microbicide combinations is a promising approach for the prevention of sexually transmitted infections, but requires a method of providing simultaneous, independent release of multiple agents into the vaginal compartment. A novel intravaginal ring (IVR) platform has been developed for simultaneous delivery of the reverse-transcriptase inhibitor tenofovir (TFV) and the guanosine analogue antiviral acyclovir (ACV) with independent control of release rate for each drug. The IVR is based on a pod design, with up to 10 individual polymer-coated drug cores embedded in the ring releasing through preformed delivery channels. The release rate from each pod is controlled independently of the others by the drug properties, polymer coating, and size and number of delivery channels. Pseudo-zero-order in vitro release of TFV (144 ± 10 µg day) and ACV (120 ± 19 µg day−1) from an IVR containing both drugs was sustained for 28 days. The mechanical properties of the pod IVR were evaluated and compared with the commercially available Estring® (Pfizer, NY, NY). The pod-IVR design enables the vaginal delivery of multiple microbicides with differing physicochemical properties, and is an attractive approach for the sustained intravaginal delivery of relatively hydrophilic drugs that are difficult to deliver using conventional matrix IVR technology.
Keywords: drug delivery systems, HIV/AIDS, formulation, in vitro models, mechanical properties, intravaginal ring, microbicide delivery, silicone, sustained release, combination delivery device
INTRODUCTION
As the HIV/AIDS pandemic nears the end of its third decade, worldwide infection rates remain high, with an estimated global HIV incidence of 34 million, and 2.7 million new infections in 2010.1 Until recently, attempts to develop a pre-exposure prophylaxis strategy for the prevention of HIV transmission have failed,2,3 but two large-scale clinical trials have demonstrated that microbicides may be effective in preventing infection in a significant proportion of individuals. In the CAPRISA 004 trial, a double-blind study of 889 uninfected women in South Africa, participants using a pericoital 1% tenofovir (TFV) gel showed a 39% reduction in HIV transmission compared with those receiving a placebo gel, with a 54% reduction for women with high adherence rates.4 In the iPrEx trial, a once-daily oral combination of emtricitabine (FTC) and tenofovir disoproxil fumarate was compared with placebo for the prevention of HIV acquisition among nearly 2500 men and transgender women who have sex with men.5 Over the following median period of 1.2 years, a 44% reduction in HIV acquisition was observed in the experimental group compared with subjects receiving a placebo. Both of these trials demonstrate that microbicides can prevent HIV transmission, and the iPrEx trial suggests that a combination of two or more microbicides delivered simultaneously may be even more effective. Combinations provide potential advantages over single-microbicide approaches by maximizing activity through synergistic effects and potentially allowing for lower effective microbicide concentrations, reducing toxicity and other harmful side effects.6–10 Combinations are currently the standard in HIV treatment by highly active antiretroviral therapy (HAART), and the resulting suppression of HIV replication leads to low viral levels and a decrease in formation of resistance. Typical HAART regimens use at least three antiretroviral drugs, two nucleoside reverse transcriptase inhibitors (NRTI) and either a protease inhibitor (PI), non-NRTI (NNRTI), or an integrase inhibitor.
Microbicide combinations may also be used to target two diseases simultaneously. The herpes simplex virus type 2 (HSV-2) and HIV are responsible for two intersecting epidemics, wherein the disease caused by one virus may facilitate the transmission of and pathogenesis by the other. Epidemiological studies demonstrate that HSV infection increases the risk of HIV-1 acquisition,11–14 and HSV-2 infection leads to a threefold increase in risk of HIV acquisition among men and women. These results suggest that, in areas of high HSV-2 prevalence, a substantial proportion of HIV infection is linked to HSV-2 infection.14 Forty-five million Americans are infected with HSV-2,15 and studies in developing countries reveal seroprevalence rates ranging from 60% to 80% in young adults.16 In the United States, HSV-2 seropositivity is higher among women (23.1%) than men (11.2%).17 Daily oral valacyclovir [the prodrug of acyclovir (ACV)] has been shown to prevent or delay genital HSV-2 recurrences by 85%18 and to reduce the risk of transmission among HSV-2 discordant couples by 48%.19 Local ACV delivery at comparable genital tract levels may suppress genital HSV shedding and prevent HSV transmission through sexual contact. These studies suggest that interventions against HSV-2 may have a key role in HIV prevention.20
Intravaginal methods for microbicide delivery include semisolid gels, fast-dissolving films, and intravaginal rings (IVRs).21,22 A number of IVR designs have been developed that deliver microbicides, and they are the subject of a recent extensive review by Malcolm et al.21 Kiser and coworkers have reported a polyurethane matrix ring delivering the NNRTI dapivirine23 and a segmented polyurethane matrix ring delivering dapivirine and TFV simultaneously.24 Both exhibit sustained release over 30 days in vitro. Three different matrix IVRs (polyurethane, ethylene vinyl acetate, and silicone) containing the antiretroviral drug UC781 were evaluated in vitro and in a rabbit model,25 and ethylene-vinyl acetate (EVA) matrix IVRs delivering UC781 in combination with the contraceptive hormone levonorgestrel were investigated in vitro.26 Degradation of UC781 during the fabrication process, however, has made further development difficult. Matrix IVRs composed of biosoluble acacia gum and a non-biodegradable hydrogel copolymer of 2-hydroxyethyl methacrylate and sodium methacrylate were shown to release in vitro 3-azido-3-deoxythymidine (AZT) over 10 and 28 days, respectively, at levels well above the AZT half maximal inhibitory concentration (IC50).27 Silicone elastomer IVRs releasing dapivirine are the most extensively developed microbicide vaginal rings, with a sustained release of 140 µg day−1 obtained in vitro.28,29 Currently, the dapivirine ring is the only IVR microbicide formulation to undergo phase I clinical trials,30–32 and no efficacy studies of an IVR delivering a microbicide for the prevention of HIV infection have been carried out in humans.
This report describes a novel ring design based on drug pods, cores of compressed drug substance coated with a “release polymer” that controls diffusion across the polymer membrane. The pods are embedded in an elastomeric vaginal ring with one or more delivery channels at each pod providing the primary release rate control. The pod-IVR technology described here has its origins in ophthalmology, specifically in a 5-month sustained-release ganciclovir implant for the treatment of cytomegalovirus retinitis in immunocompromised patients.33 The pharmacokinetics of the device was investigated in rabbits and humans,34 and it was shown to be safe and efficacious.35–37 These developments led to several US Food and Drug Administration-approved drug delivery devices, including the first and only sustained-release antiviral.38 The technological and regulatory experience gained from these implants has been applied to the development of IVRs delivering antivirals, resulting in the first clinical study involving delivery of the guanosine analog antiherpetic drug ACV from an IVR.39 Simultaneous delivery of TFV and ACV from a silicone pod-IVR platform has been demonstrated in vivo in a sheep model.40 In this work, the design, fabrication, and in vitro release characteristics are reported for the pod-IVR platform delivering TFV and ACV simultaneously and at a wide range of independently controlled-release rates. This dual-protection IVR is primarily intended for the prevention of sexual HIV transmission through both the direct microbicide activity of TFV against HIV, and through the reduction of HSV-2 outbreak and recurrence, and potentially a decrease in HSV-2 transmission by ACV.
MATERIALS AND METHODS
Materials
Acyclovir [2-amino-9-((2-hydroxyethoxy)methyl)-1H-purin-6(9H)-one] was obtained from Comfortcomms Group Company, Ltd. (Shenzhen, Guangdong, China). TFV [((((2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl) oxy)methyl)phosphonic acid] was obtained from Sinoway International (Jiangsu, China). Polylactic acid (Resomer R 202 S: poly-d,l-lactide) was obtained from Boehringer Ingelheim Pharma GmbH & Company KG (Ingelheim, Germany). Liquid silicone resin (LSR) MED-4830 and MED-4840 for injection molding and MED1-4213 for pod backfilling were obtained from Nusil (Carpinteria, California). Dichloromethane and sodium acetate were purchased from Sigma –Aldrich (St. Louis, MO). Ethyl acetate [American Chemical Society (ACS) grade], Ca(OH)2 (98%), H3PO4 (ACS grade), NaH2PO4 (99+%), and Na2HPO4 (99+%) were obtained from Acros (Morris Plains, New Jersey). Methanol [ultrapure high-performance liquid chromatography (HPLC) grade] and water (Ultrapure HPLC grade) for chromatographic analyses were received from Alfa Aesar (Ward Hill, New Jersey). Deionized water was prepared with an ion-exchange system (Siemens Water Technologies, Barsbuttel, Germany). All other reagents were ACS reagent grade or better and used as received.
Preparation of TFV and ACV Pods
Pods of TFV and ACV were prepared by compaction of drug powder with no added excipients in a pellet press to form a drug core that is subsequently coated with release polymer. A 19–20 mg sample of either drug was placed in a manual pellet press with a 0.125 in. diameter pin and die (2810 Pellet Press; Parr Instruments, Moline, Illinois) and compressed to yield a solid, cylindrical drug core (16 ± 0.1 mg, 3.2 mm diameter × 2.0 mm height). Drug cores were coated with two layers of polylactic acid (PLA) by drop coating with a 5% PLA solution in 1:1 dichloromethane–ethyl acetate. Each layer is delivered as one 6 µL aliquot from an automatic pipette on each side of the drug core (12 µL total per layer).
Empty Silicone IVR Fabrication
Silicone IVRs and IVR segments containing cavities for ACV and TFV pods were fabricated using a standard LSR injection molding process. All rings and ring segments were manufactured from a two-part Pt-cured LSR (MED-4830 for all work except mechanical testing for which MED-4840 was used; Nusil). Rings and segments were fabricated using single-cavity molds and a laboratory-scale injection molding apparatus designed and built in house. The two-component silicone was mixed during injection into the heated mold under vacuum and cured at 150° C for 10 min. Following demolding, delivery channels of 0.35–2.5 mm diameter were created by mechanical punching in the bottom of each pod cavity. All delivery channels were of 1.8 mm in length, extending from the bottom of the pod cavity through the silicone material to the ring surface. The delivery channel diameter for each cavity was determined by the drug and desired release rate. Sprue material and any flashing were trimmed with a razor knife, and the silicone cleaned with ethanol to remove debris from the molding process.
IVR Assembly
Completed rings and ring segments were assembled from pods and an empty silicone ring or segment as illustrated in Figure 1. Each delivery channel was photographically imaged at 40× magnification using a Nikon BH-2 microscope (Nikon USA, Melville, NY) with a digital camera (Nikon Coolpix 995), and the delivery channel area determined from the image using the ImageJ (National Institutes of Health, Bethesda, MD)41,42 software package. All images were acquired under the same magnification, and an image of a precision ruled slide (Edmund Optics, Barrington, New Jersey) was identically acquired for calibration of pixel size in the image. Drug pods were placed in each cavity, flush against the delivery channel, and sealed in place by backfilling the pod cavity with a small amount of room temperature cure silicone (MED1-4213; Nusil). Unused pod cavities were filled with a solid silicone plug (3.2 mm diameter × 4 mm high) and sealed with a backfill as described above.
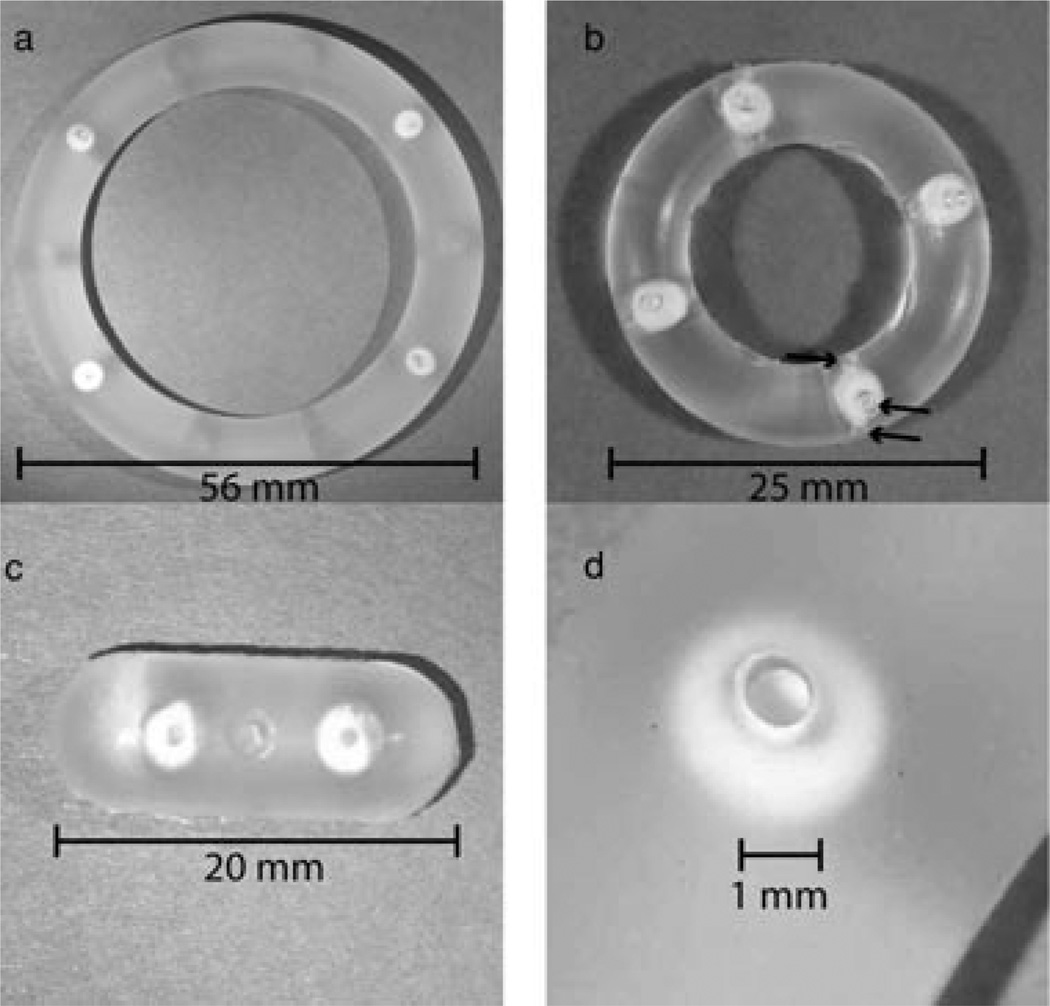
Figure 1.
Photographs of (a) human ring with two TFV and two ACV pods and one delivery channel per pod; (b) macaque ring with four TFV pods and three delivery channels per pod (arrows denote the three delivery windows for one of the four pods); (c) rabbit ring segment with one TFV and one ACV pod, one delivery channel per pod; and (d) close-up of delivery channel and ACV pod in human ring.
In Vitro Dissolution
Dissolution studies to measure the in vitro release of TFV and ACV from single-drug and multidrug systems were carried out on both ring segments and full rings. A simplified vaginal fluid simulant (VFS) recipe was adapted from Owen,43 and consisted of 25 mM acetate buffer (pH 4.2) with NaCl added to 220 mOs. For all in vitro release studies, the rings or segments were placed in glass jars containing 100 mL VFS at 25 ± 2°C and with shaking at 60 rpm on an orbital shaker. The concentration of TFV or ACV in the release medium was measured as a function of time by HPLC for cumulative release experiments of single drug and TFV–ACV IVRs and by UV–visible absorption spectroscopy for studies of release rate versus delivery channel size and number of pods. Chromatographic analysis was carried out on a 1050 HPLC (Agilent, Santa Clara, CA) with diode-array detection, using an Agilent Zorbax Eclipse XDB C-18 column (4.6 × 150 mm2, 5 µm packing). A gradient method of from 90:10 A–B to 50:50 A–B (A, 20 mM pH 2.5 phosphate buffer; B, methanol) at 1 mL min−1 flow and 10 µL injection volume was used. Concentration was determined from integrated peak area using a calibration curve obtained with five standards of known concentration of TFV or ACV in vSF. Absorption spectrophotometric measurements were carried out using a UV-2401PC dual-beam spectrophotometer (Shimadzu, Columbia, MD). The concentration of active pharmaceutical ingredient (API) in the release solution was calculated using the Beer–Lambert law. The absorption coefficients were obtained from spectra of five standard TFV or ACV solutions of known concentration spanning the range encountered during dissolution experiments.
Mechanical Testing
The tensile strength, elongation, compression strength, and twisting during compression were determined using methods adapted from ASTM D224044 and ISO 8009,45 with modifications based on the differences between IVRs and diaphragms. Mechanical testing methods and results are given in the Supporting Information.
RESULTS
IVR Fabrication
The ring platform for delivery of TFV and ACV is of a “pod” design. A core of TFV or ACV is coated with a layer of semipermeable PLA polymer to form a drug pod. Drug pods are incorporated into a silicone IVR or IVR segment with a delivery channel exposing a portion of the pod to the vaginal fluid. Three different IVR configurations have been prepared: human rings, macaque rings, and ring segments suitable for rabbit in vivo studies. The dimensions of each are given in Table 1 along with the range of possible drug loadings. Pods in an IVR or IVR segment may be identical or composed of different drugs. Figure 1 shows photographs of a rabbit-sized IVR segment with one TFV and one ACV pod, a macaque-sized IVR with four TFV pods, and a human-sized IVR with two TFV pods and two ACV pods.
Table 1.
Dimensions and Configuration of Pod Rings
| Dimensions |
||||||
|---|---|---|---|---|---|---|
| IVR Configuration | O.D. (mm) | I.D. (mm) | C.S. (mm) | Volume (cm3) | Maximum Number of Pods |
Range of Drug Loading (mg)a |
| Human | 56 | 40 | 8 | 7.6 | 10 | 10–400 |
| Macaque | 25 | 15 | 5 | 1.2 | 4 | 10–88 |
| Rabbit segment | 20 (length) | 8 | 1.0 | 2 | 10–80 | |
O.D., outer diameter; I.D., inner diameter; C.S., cross-sectional diameter.
Drug pod mass range: 10 mg minimum, 40 mg maximum for human and rabbit; 22 mg maximum for macaque.
Solid cores of TFV and ACV in the mass range 8–40 mg were produced using a hand press method, with 16 mg chosen as the TFV and ACV core size for the studies reported here. For a sample of 10 TFV and 10 ACV cores, the mass was consistent (16 ± 0.1 mg) for 3.2 mm diameter × 2 mm height cores. Cores were coated with a PLA “release polymer” that serves as a membrane to control the diffusion of dissolved drug from the core into the delivery channel. Manufacturing of the empty silicone IVR was carried out using a custom-made, laboratory-scale injection molding system.
Pods and empty silicone rings are assembled as illustrated in Figures 1 and 2. Delivery channels are formed with a mechanical punch, and are located in the center of the pod cavity as shown in Figure 1d. Delivery channels were of uniform and reproducible size. Delivery channel size is described either as a channel diameter or as a cross-sectional area. All delivery channels are of 1.8 mm in length. For a 1 mm diameter target channel size, the delivery channel area for a set of 20 ring segments calculated from a 40× magnification digital image of the channel was 0.736 ± 0.031 mm2. This corresponds to an actual delivery channel diameter of 0.96 mm.
Figure 2.
(a) Cross-sectional view of a blank silicone pod-IVR, 1, showing an empty pod cavity, 2, and delivery channel, 3. The delivery channel length is 1.8 mm and the diameter, d, can be varied from 0.3 to 2.0 mm; (b) Cross-sectional view of assembled pod IVR showing polymer-coated pod, 4, placed in the pod cavity and abutting the delivery channel. The pod is sealed in place with a silicone backfill, 5.
In Vitro Release
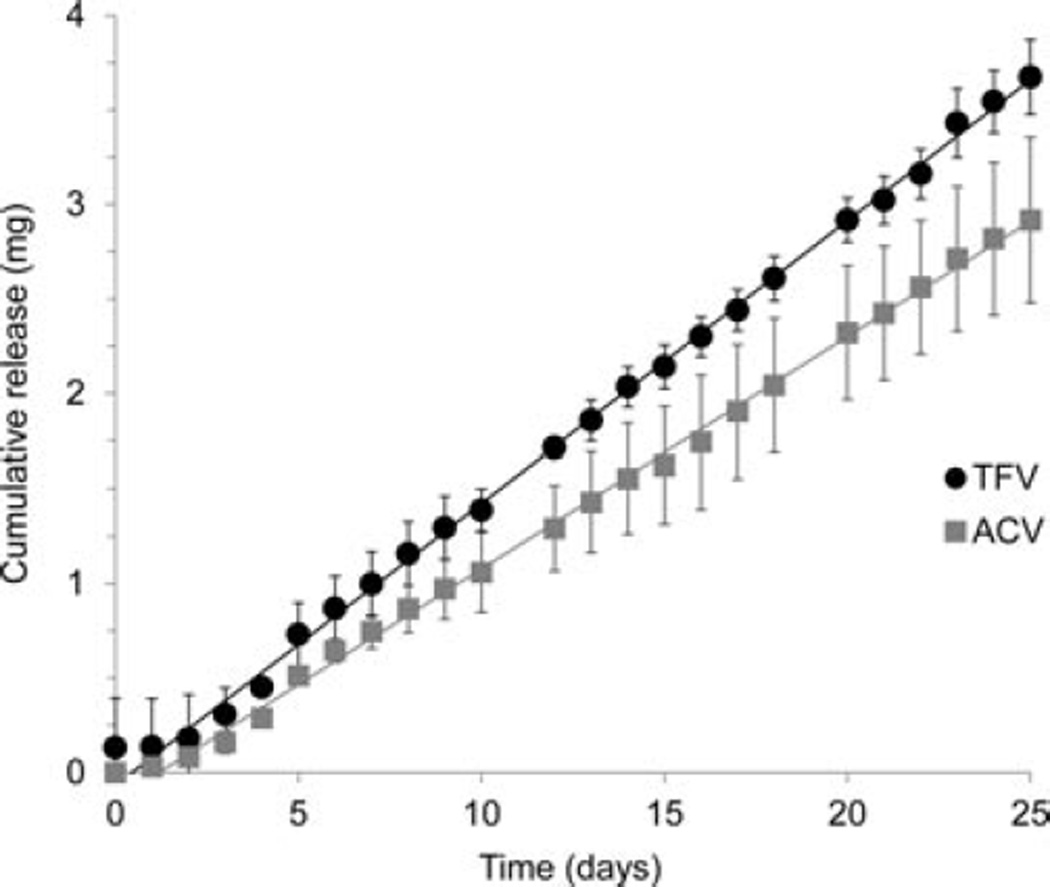
in vitro release for single-drug IVRs was obtained for both TFV and ACV as shown in Figure 3. Pseudo-zero-order release rates of 115 ± 14 µg day−1 for TFV (mean ± SD, N = 4) and 133 ± 15 µg day−1 for ACV (mean ± SD, N = 3) were obtained over 28 days. All release rates were calculated from the slope of a linear fit to the cumulative release plot. The IVRs each contained five identical PLA-coated pods with 1.0 mm delivery channels for TFV and 1.5 mm delivery channels for ACV, and were designed to release either TFV or ACV at the same rate. Figure 4 shows simultaneous release over 25 days of TFV and ACV from an IVR containing five pods of each drug. The target release rate for each drug was the same as the rate for the single-drug IVRs given above. Release rates of 144 ± 10 µg day−1 for TFV and 120 ± 19 µg day−1 for ACV were obtained (mean ± SD, N = 4), and release was pseudo-zero-order over the 25 days. The day 0 concentration was obtained 1 h following placement of the IVR in the release medium. For TFV in Figure 4, the non-zero day 0 TFV value is from one IVR of the lot exhibiting a TFV release of 500 µg after 1 hour. This is most likely due to rapid dissolution of residual TFV powder in one or more of the IVR pod cavities, possibly from an incomplete or damaged PLA coating. A lag in initial in vitro release of up to 3 days was observed for both the single-drug pod IVRs and the TFV–ACV pod IVR. This lag is typically observed for in vitro release studies in vFS and water, but is not observed during in vivo studies of pod IVRs releasing TFV in sheep46 and ACV in rabbits40 and humans39 where levels of TFV and ACV, respectively, were established in cervi- covaginal fluid on day 1 following IVR insertion (the first postinsertion measurement point).
Figure 3.
Plot of average cumulative release of TFV (circles) and ACV (squares) into VFS from single-drug IVRs containing five drug pods each. The pods were PLA coated, and the delivery channels for TFV are 1.0 mm and for ACV 1.5 mm. The daily release rate (mean ± SD) for the TFV rings is 115 ± 14 µg day−1 (N= 4, linear fit R2= 0.999) and for ACV is 133 ± 15 µg day−1(N= 3, linear fit R2= 0.998).
Figure 4.
Plot of average cumulative release of TFV (circles) and ACV (squares) into VFS from multiple-drug IVRs containing five pods of ACV and five pods of TFV in a single ring. The pods were PLA coated, and the delivery channels for TFV are 1.0 mm and for ACV 1.5 mm. The daily release rate (mean ± SD) for TFV is 144 ± 10 µg day−1 (N= 4, linear fit R2= 0.999) and for ACV is 120 ± 19 µg day−1 (N= 4, linear fit R2= 0.998).
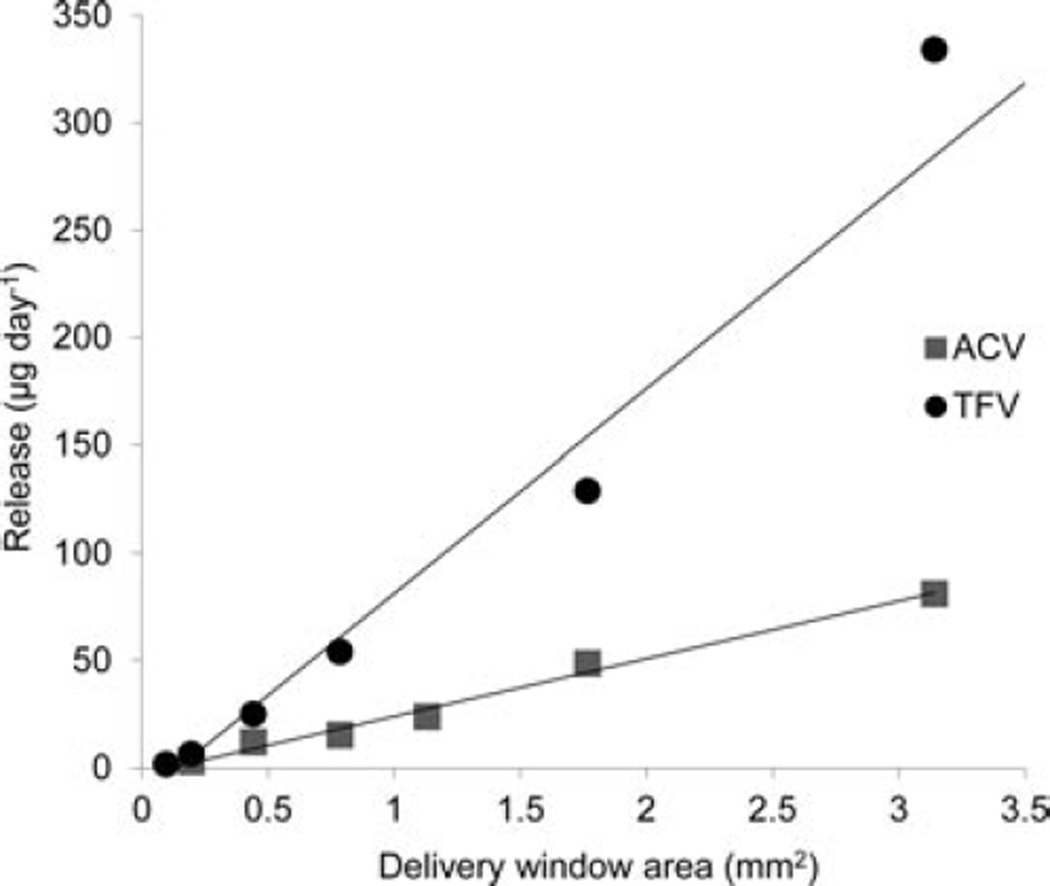
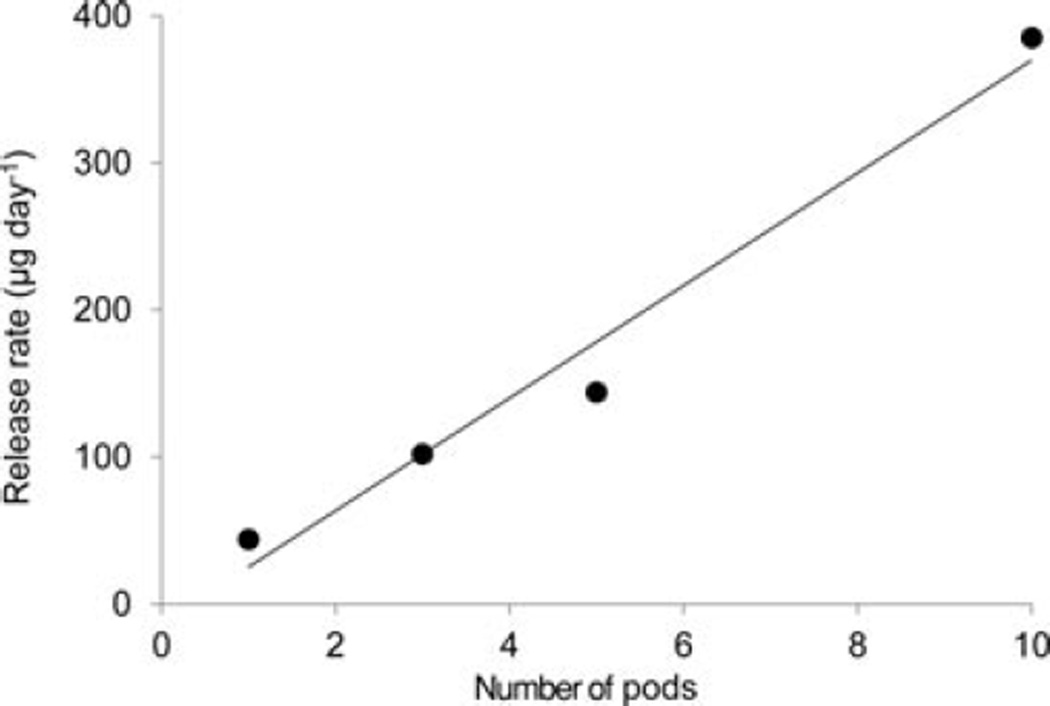
The release rate for a given drug from the pod-IVR design is determined by the size of the delivery channels, the number of pods per ring, and the pod coating material and thickness. Figure 5 shows the daily release rate of TFV and ACV as a function of delivery channel area for ring segments containing a single drug pod with one delivery channel of varying size. These are the measured areas that correspond to delivery channels of diameter 0.35–2.0 mm. For TFV, a release rate range of 1.7–334 :g day−1 is obtained by varying the delivery channel size; for ACV, a range of 6.4–81 µg day−1 is obtained. The total drug release rate from pod IVRs may also be modulated by changing the number of pods per ring. Figure 6 shows daily release rate of TFV from a set of IVRs as a function of the number of TFV pods. For a human-sized IVR with a maximum of 10 pods per ring, this allows incremental variation of the release rate of a single drug over a one log range simply by changing the number of pods in the ring.
Figure 5.
Average daily release rate versus delivery channel area for TFV (circles) and ACV (squares). Release of TFV or ACV from IVR segments containing a single PLA-coated pod per ring and single delivery channel per pod into VFS was measured over 14 days, and the release rate calculated from the slope of the linear fit to the cumulative release data (N = 4 IVRs per delivery channel size).
Figure 6.
Daily in vitro release rate as a function of number of TFV pods per ring. For all rings, 16 mg TFV drug cores were coated with PLA and had a single 1.0 mm delivery channel per pod. Release of TFV into VFS was measured over 15 days (one, three, and 10 pods per ring) or 28 days (five pods per ring), and the release rate calculated from the slope of the linear fit to the cumulative release data (N = 5 IVRs per # pods per ring).
DISCUSSION
Pod-IVR Design
Conventional IVR designs have typically followed one of two formats. Matrix IVRs contain the drug either dissolved in or homogeneously dispersed as amorphous or crystalline solids throughout the polymeric matrix. Reservoir IVRs consist of a drug-loaded polymer “core” layer similar to a matrix ring covered by an outer nonmedicated polymer layer or layers to control drug release.21 The “pod IVR” design described here is novel as it consists of polymer-coated solid drug cores, referred to as pods, positioned in the unmedicated ring. In the current iteration, one to 10 pods of up to 40 mg each can be loaded into a single pod IVR, enabling total drug loadings of up to 400 mg per ring. The drug release rates from the IVRs are de- termined by the pods’ nonmedicated, biocompatible polymer membrane and by the characteristics of the delivery channels in the impermeable IVR structure. These parameters allow sustained drug release from each pod in the IVR to be independently controlled with pseudo-zero-order kinetics. The modular design features have three important implications: (a) the release rate from each pod can be titrated over a wide dynamic range as needed for a particular indication (Table 1); (b) drugs with high water solubility (e.g., TFV and ACV) can be released with linear daily rates and no initial burst, something that has proven to be challenging using conventional designs21; and (c) up to 10 different drugs can be released from a single IVR, each with independently controlled, linear release rates. Additionally, the sustained release is independent of the ring material, offering flexibility in polymer choice that may be important for future large-scale production.
In Vitro Release
The in vitro release studies suggest that the relatively hydrophilic drug substances TFV (log P = −2.3)24 and ACV (log P = −1.59)47 may be delivered intravaginally from pod IVRs. Most IVRs delivering microbicides are of the matrix design and have a uniform drug loading throughout the entire ring. This approach has been successful for the in-travaginal delivery of hydrophobic microbicides such as dapivirine23,28–31 and hormones for contraception and treatment of postmenopausal conditions,48 but has been mostly unsuccessful in the delivery of hydrophilic compounds. Han et al.27 reported acacia gum and methacrylate IVRs that release the antiretroviral AZT over 28 days in vitro. Johnson et al.24 developed a dual-segment polyurethane IVR that delivers both TFV and dapivirine in vitro, but the release of TFV is nonlinear, and only limited control of the release rate is possible through varying the drug loading in the polyurethane. An alternative approach wherein the release characteristics are independent of the ring itself is delivery of hydrophilic peptide-based microbicides from a rod and tablet insert ring design (InVR).49 In the InVR, the ring serves only as a carrier, and the microbicides are released from rods or tablets containing directly compressed or lyophilized peptide, potentially either solid or in a matrix. Because release is accomplished by diffusion out of a matrix or solid rod, sustained linear release is not observed. Furthermore, drug loading is a principal determinant of release rate, diminishing the dosing flexibility of the InVR.
The pod IVRs described here allow extensive tuning of the release rate of multiple drugs in an independent fashion. Numerous parameters contribute to the final release rate: drug properties (solubility and hydrophobicity), polymer coating material and thickness, delivery channel size and number of channels per pod, and number of drug pods. In the development of a pod IVR, an initial release rate target is selected based on known efficacy, if available. For TFV, the initial target is a release rate expected to provide more than 1000 ng mL−1 in cervicovaginal fluids, the level demonstrating effectiveness in prevention of HIV transmission for 1% TFV gel.50 For ACV, an initial target release rate to provide 500–1000 ng mL−1 in cervicovaginal lavage was chosen to be similar to those obtained with oral dosing of the ACV prodrug valacyclovir.39 The polymer coating material is selected based on the solubility and hydrophilicity of the drug to allow access to a range of release rates about the initial target. In the case of relatively hydrophilic TFV and ACV, PLA was found to be a suitable release polymer. Next, the delivery channel size and number of channels are chosen to obtain a desired release rate per pod, and the number of pods per ring selected to achieve the final desired in vitro release rate. The release rate dependence on delivery channel size is illustrated in Figure 5 for single-pod ring segments containing PLA-coated TFV and ACV. The release can be varied from 3 to 81 µg day−1 for ACV and from 2 to 334 µg day−1 for TFV for single pods. Combinations of delivery channel size (Figure 5) and total number of pods (Figure 6) allow a wide range of release rates to be achieved. Because the delivery window size, number of pods of each drug, and PLA polymer coatings are the same for the single-drug rings and the TFV–ACV combination ring, the release rate of each drug theoretically should be identical. The difference in the release rates observed in Figures 3 and 4 is due to variability in tablet compression and polymer thickness from the manual-press tableting and hand-coating procedures. Variation in the dissolution rates within batches of pods fabricated using the scaled-up production methods described below is significantly less than that observed for variation between pods prepared as described here, and this improvement will lead to more consistent release rates for batches of manufactured pod IVRs. The ability to easily modulate the release rate from pod IVRs simply by changing the delivery channel size and number of pods is of particular importance to the development of IVR delivery of microbicides. Currently, no efficacy data in either clinical trials or animal models exist for IVR-delivered microbicides for HIV or HSV-2 prophylaxis, and necessary microbicide doses have yet to be determined.21
The pod IVR was designed specifically to allow the simultaneous delivery of multiple drugs with independent control of release rate. The Nuvaring® (Merck, Whitehouse Station, NJ) is a commercially available multidrug ring of the reservoir design that delivers two contraceptive hormones: ethinyl estradiol at 15 µg day−1 and etonogestrel at 120 µg day−1.51 Simultaneous in vitro release of the antiretroviral UC781 and contraceptive hormone levonorgestrel from an EVA matrix ring has also been reported.26 The release rates of the two drugs in each of these IVRs are not independently controlled, but rely on the relative diffusion rates of each hormone through the drug-loaded EVA matrix and, for the Nuvaring®, across the outer nonloaded EVA sheath. Any change in the ring to modify the release rate of one drug will affect the other drug as well; thus, this approach is only applicable to combinations of chemically similar compounds that are to be delivered at similar rates, or to differing compounds whose relative diffusion rates through the matrix material happen to correspond to the desired release rates. An alternative approach by Johnson et al.24 was to fabricate a polyurethane matrix IVR composed of two separate segments joined together to form a ring. Each segment delivers a different drug, with one hydrophilic polyurethane segment releasing TFV and one hydrophobic polyurethane segment releasing dapivirine. Release from each segment is independent of the other(s), and release rate may be controlled by the loading of the drug in the polyurethane matrix and the polymer composition. The ability to tune release rates in this system, however, is limited and requires modification of the chemical and physical composition of the matrix polymer. For pod IVRs as described here, the release rate from each pod is controlled independently, theoretically allowing for as many as 10 different drugs to be released, each at its own predetermined rate. The release rate is not dependent on diffusion of the drug through the silicone ring material, but only on diffusion through physical channels in the ring and on the thickness and material characteristics of the polymer coating on the pod. Modification of the release rate on a per-pod basis is accomplished simply by changing the delivery channel size and number of channels, providing precise dose selection.
IVR Manufacturability
Advancement of IVR devices from the research phase to clinical phase III trials is crucially dependent on the IVR manufacturability. Contract manufacturers of IVR formulations are not readily available, and existing organizations for injection molding or extrusion typically do not have the capability for handling API as required for fabrication of these IVR designs.21 The pod-IVR fabrication method described here has been developed specifically to allow facile scale-up of production from research-scale lots (20–100 rings) to Good Manufacturing Practice (GMP)-manufactured clinical trial quantities (1000–50,000 rings), and ultimately to GMP production quantities (1,000,000+ rings). This is accomplished by separating the ring fabrication process into three distinct steps: fabrication of pods, injection molding of empty rings, and final ring assembly. The pod fabrication described here involves tableting using a manual press and individual hand coating of each tablet, and both of these processes may be directly transferred to traditional pharmaceutical production processes: tableting using wet or dry granulation and a tablet press to produce drug cores, and pan or fluidized-bed coating to apply the PLA polymer. Similarly, the empty silicone rings are manufactured by a standard injection-molding process. Both of these steps utilize mature technology that is routinely applied to manufacture of oral-dosage pills and silicone medical devices. A large number of contract GMP manufacturers are available for both pod fabrication and injection molding. The assembly step requires more development, but is amenable to technology commonly used in medical device industries including mechanical punching (for delivery channel production), pick-and-place assembly (for pod insertion into the ring), and machine vision and inspection. Initial scale-up of assembly to clinical trial lots of approximately 1000 rings will use primarily hand assembly with 100% inspection. Automation of delivery channel punching, pod insertion, and silicone backfilling should allow production quantities in the hundreds of thousands to millions. Pod production and ring molding on a clinical-lot scale is currently being carried out in partnership with two contract manufacturers, and the development of the scaled-up assembly process is underway with a contract medical device manufacturer.
An additional advantage to the pod-IVR fabrication is that, unlike the manufacturing process for matrix rings wherein the API is dispersed in the ring material prior to the injection molding or extrusion process, this method avoids the need to carry out an injection molding step with API present in the molds. This is important for the following two reasons: (1) API is not subjected to the elevated (120–17°C) temperatures of the injection molding process and (2) this method greatly simplifies the scale-up of ring production as standard GMP injection molding processes may be employed, alleviating the need for the injection molding facility to work with the drug substance.
Mechanical Properties
Tests of mechanical properties were derived from ISO 8009, “Mechanical contraceptives—Reusable natural and silicone contraceptive diaphragms,” with modifications based on the differences between IVRs and diaphragms. Where acceptance criteria from ISO 8009 were not applicable to IVRs, an identical set of tests were carried out on one sample of the Estring© (Pfizer, New York, NY), an approved and commercially available silicone-based IVR product of similar shape and size, as a direct comparison with the pod IVR. Mechanical tests were carried out using IVRs molded from Nusil MED-4840 LSR to directly compare results with the Estring©. Durometer, measured according to ASTM D2240-05, for all rings was 40 ± 1.7, well within the acceptance criteria of 40 ± 5. This is typical for LSR injection molded parts. Consistency in durometer is important as this determines the amount of tension provided by the compressed ring, and hence, dictates both how well the ring is held in place and the comfort of the ring. Compression resistance was determined by compressing the ring vertically using a 128 g mass and measuring the amount of compression (in mm) of the ring diameter under load. The mass 128 g was chosen so as to compress the ring between 55% and 85% of its original diameter, the same amount of compression specified in ISO 8009 for diaphragms. Because diaphragms contain a wire spring in the ring, they are much harder to compress than pure silicone rings. Accordingly, the ISO 8009 guideline of 290 g load mass was reduced to 128 g for these tests to yield the same percentage of compression.
The ISO guidelines for diaphragms recommend no more than 20° twist; however, this guideline is not applicable to vaginal rings because they do not have the “cap” portion of the diaphragm, which limits rotation. The twist angle of pod IVRs was compared with the Estring©, noting that the Estring© rotation of 55° is ∼3× the rotation allowed for a diaphragm by ISO 8009. The average twist angle for pod IVRs is 62%. The primary concern with IVR retention and comfort is that the ring maintains its elasticity (resistance to compression) throughout the period of use so that it can maintain appropriate pressure on the vaginal walls to be retained, yet not be so stiff as to be uncomfortable. This twisting evaluation was designed for a diaphragm that has a small diameter silicone ring containing a wire for compression stiffness. In this case, twisting can cause a permanent buckling of the wire, deforming the diaphragm and causing loss of effectiveness as a barrier contraceptive. For IVRs, this is not a concern. In the IVR case, the return to original diameter under repeated compression, twisting, and compression under load are the critical parameters, and these tests show for pod IVRs that they remain intact and retain their original dimensions and elasticity under all test conditions.
CONCLUSIONS
The novel pod-IVR design presented here provides a platform for the intravaginal delivery of multiple microbicide compounds for HIV and HSV prophylaxis. in vitro release studies demonstrate the sustained release of two candidate microbicides, TFV and ACV, at predetermined rates that are controlled over more than three orders of magnitude. Simultaneous release of TFV and ACV from a single IVR was demonstrated over 25 days in vitro, with independent control of the release rate of each drug. The pod-IVR design allows selection of release rate through modification of multiple IVR parameters, and the platform is easily adapted to release different drugs with varying physicochemical properties. The ability to deliver multiple microbicides is important both in terms of efficacy of prophylaxis and in reducing the emergence of drug resistance.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank the National Institutes of Health (grant numbers 5R21AI079791 and 5R21AI076136), CONRAD (service contract numbers PSA-08-10 and PPC-09-017), the International Partnership for Microbicides, and the U.S. Agency for International Development (cooperative agreement number GPO-A-00-05-00041-00) for funding support. The views expressed by the authors do not necessarily reflect those of United States Agency for International Development.
Footnotes
Additional Supporting Information may be found in the online version of this article. Supporting Information
REFERENCES
- 1.Geneva, Switzerland: World Health Organization; 2011. [Accessed April 2, 2012]. Global HIV/AIDS response: Epidemic update and health sector progress towards universal access: Progress report 2011. at: http://whqlibdoc.who.int/publications/2011/9789241502986_eng.pdf. [Google Scholar]
- 2.Shattock RJ, Warren M, McCormack S, Hankins CA. Turning the tide against HIV. Science. 2011;333(6038):42–43. doi: 10.1126/science.1206399. [DOI] [PubMed] [Google Scholar]
- 3.D’Cruz OJ, Uckun FM. Clinical development of microbicides for the prevention of HIV infection. Curr Pharm Des. 2004;10(3):315–336. doi: 10.2174/1381612043386374. [DOI] [PubMed] [Google Scholar]
- 4.Karim QA, Karim SSA, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany ABM, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang FR, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Clercq E. AIDS in the Third World: How to stop the HIV infection? Verh K Acad Geneeskd Belg. 2007;69(2):65–80. [PubMed] [Google Scholar]
- 7.Herrera C, Cranage M, McGowanI I, Anton P, Shattock R. Colorectal microbicide design: Triple combinations of reverse transcriptase inhibitors are optimal against HIV-1 in tissue explants. AIDS. 2011;25(16):1971–1979. doi: 10.1097/QAD.0b013e32834b3629. [DOI] [PubMed] [Google Scholar]
- 8.McMahon MA, Shen L, Siliciano RF. New approaches for quantitating the inhibition of HIV-1 replication by antiviral drugs in vitro and in vivo. Curr Opin Infect Dis. 2009;22(6):574–582. doi: 10.1097/QCO.0b013e328332c54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother. 2011;66(2):240–250. doi: 10.1093/jac/dkq447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Clercq E. A 40-year journey in search of selective antiviral chemotherapy. Annu Rev Pharmacol Toxicol. 2011;51:1–24. doi: 10.1146/annurev-pharmtox-010510-100228. [DOI] [PubMed] [Google Scholar]
- 11.Renzi C, Douglas JM, Foster M, Critchlow CW, Ashley-Morrow R, Buchbinder SP, Koblin BA, McKirnan DJ, Mayer KH, Celum CL. Herpes simplex virus type 2 infection as a risk factor for human immunodeficiency virus acquisition in men who have sex with men. J Infect Dis. 2003;187:19–25. doi: 10.1086/345867. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds SJ, Risbud AR, Shepherd ME, Zenilman JM, Brookmeyer RS, Paranjape RS, Divekar AD, Gangakhedkar RR, Ghate MV, Bollinger RC, Mehendale SM. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J Infect Dis. 2003;187:1513–1521. doi: 10.1086/368357. [DOI] [PubMed] [Google Scholar]
- 13.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2 seropositive persons: A meta-analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 14.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 15.Fleming DT, McQuillan GM, Johnson RE, Nahmias AJ, Aral SO, Lee FK, StLouis ME. Herpes simplex virus type 2 in the United States, 1976 To 1994. N Engl J Med. 1997;337(16):1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- 16.Mbopi-Keou FX, Gresenguet G, Mayaud P, Weiss HA, Gopal R, Matta M, Paul JL, Brown DWG, Hayes RJ, Mabey DCW, Belec L. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: Opportunities for intervention. J Infect Dis. 2000;182(4):1090–1096. doi: 10.1086/315836. [DOI] [PubMed] [Google Scholar]
- 17.Xu FJ, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. J Am Med Assoc. 2006;296(8):964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 18.Patel R, Bodsworth NJ, Woolley P, Peters B, Vejlsgaard G, Saari S, Gibb A, Robinson J. Valaciclovir for the suppression of recurrent genital HSV infection: A placebo controlled study of once daily therapy. International Valaciclovir HSV Study Group. Genitourin Med. 1997;73:105–109. doi: 10.1136/sti.73.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corey L, Wald A, Patel R, Sacks SL, Tyring SK, Warren T, Douglas JM, Paavonen J, Morrow RA, Beutner KR, Stratchounsky LS, Mertz G, Keene ON, Watson HA, Tait D, Vargas-Cortes M. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350(1):11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 20.Freeman EE, Orroth KK, White RG, Glynn JR, Bakker R, Boily MC, Habbema D, Buve A, Hayes RJ. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: Simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex Transm Infect. 2007;83:I17–I24. doi: 10.1136/sti.2006.023549. [DOI] [PubMed] [Google Scholar]
- 21.Malcolm R, Edwards K, Kiser P, Romano J, Smith T. Advances in microbicide vaginal rings. Antiviral Res. 2010;88(Suppl 1):S30–S39. doi: 10.1016/j.antiviral.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Friend D. Pharmaceutical development of microbicide drug products. Pharm Dev Technol. 2010;15(6):562–581. doi: 10.3109/10837450903369879. [DOI] [PubMed] [Google Scholar]
- 23.Gupta K, Pearce S, Poursaid A, Aliyar H, Tresco P, Mitchnik M, Kiser P. Polyurethane intravaginal ring for controlled delivery of dapivirine, a nonnucleoside reverse transcriptase inhibitor of HIV-1. J Pharm Sci. 2008;97(10):4228–4239. doi: 10.1002/jps.21331. [DOI] [PubMed] [Google Scholar]
- 24.Johnson T, Gupta K, Fabian J, Albright T, Kiser P. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur J Pharm Sci. 2010;39(4):203–212. doi: 10.1016/j.ejps.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Clark M, Kiser P, Loxley A, McConville C, Malcolm R, Friend D. Pharmacokinetics of UC781-loaded intravaginal ring segments in rabbits: A comparison of polymer matrices. Drug Deliv Transl Res. 2011;1(3):238–246. doi: 10.1007/s13346-011-0032-4. [DOI] [PubMed] [Google Scholar]
- 26.Loxley A, Mitchnick M, Okoh O, McConnell J, Goldman L, Morgan C, Clark M, Friend D. Ethylene vinyl acetate intravaginal rings for the simultaneous delivery of the antiretroviral UC781 and contraceptive levonorgestrel. Drug Deliv Transl Res. 2011;1(3):247–255. doi: 10.1007/s13346-011-0031-5. [DOI] [PubMed] [Google Scholar]
- 27.Han YA, Singh M, Saxena BB. Development of vaginal rings for sustained release of nonhormonal contraceptives and anti-HIV agents. Contraception. 2007;76:132–138. doi: 10.1016/j.contraception.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Malcolm RK, Woolfson AD, Toner CF, Morrow RJ, McCullagh SD. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. J Antimicrob Chemother. 2005;56(5):954–956. doi: 10.1093/jac/dki326. [DOI] [PubMed] [Google Scholar]
- 29.Woolfson A, Malcolm R, Morrow R, Toner C, McCullagh S. Intravaginal ring delivery of the reverse transcriptase inhibitor TMC 120 as an HIV microbicide. Int J Pharm. 2006;325(1–2):82–89. doi: 10.1016/j.ijpharm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 30.Nel A, Smythe S, Young K, Malcolm K, McCoy C, Rosenberg Z, Romano J. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J Acquir Immun Defic Syndr. 2009;51(4):416–423. doi: 10.1097/qai.0b013e3181acb536. [DOI] [PubMed] [Google Scholar]
- 31.Romano J, Variano B, Coplan P, Van Roey J, Douville K, Rosenberg Z, Temmerman M, Verstraelen H, Van Borte lL, Weyers S, Mitchnick M. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retroviruses. 2009;25(5):483–488. doi: 10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]
- 32.Nel AM, Coplan P, van de Wijgert JH, Kapiga SH, von Mollendorf C, Geubbels E, Vyankandondera J, Rees HV, Masenga G, Kiwelu I, Moyes J, Smythe SC. Safety, tolerability, and systemic absorption of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS. 2009;23:1531–1538. doi: 10.1097/QAD.0b013e32832c413d. [DOI] [PubMed] [Google Scholar]
- 33.Smith TJ, Pearson PA, Blandford DL, Brown JD, Goins KA, Hollins JL, Schmeisser ET, Glavinos P, Baldwin LB, Ashton P. Intravitreal sustained-release ganciclovir. Arch Ophthalmol. 1992;110(2):255–258. doi: 10.1001/archopht.1992.01080140111037. [DOI] [PubMed] [Google Scholar]
- 34.Ashton P, Brown JD, Pearson PA, Blandford DL, Smith TJ, Anand R, Nightingale SD, Sanborn GE. Intravitreal gan-ciclovir pharmacokinetics in rabbits and man. J Ocul Pharmacol Ther. 1992;8(4):343–347. doi: 10.1089/jop.1992.8.343. [DOI] [PubMed] [Google Scholar]
- 35.Sanborn GE, Anand R, Torti RE, Nightingale SD, Cal SXYB, Ashton P, Smith T. Sustained-release ganciclovir therapy for treatment of cytomegalovirus retinitis. Use of an intravitreal device. Arch Ophthalmol. 1992;110(2):188–195. doi: 10.1001/archopht.1992.01080140044023. [DOI] [PubMed] [Google Scholar]
- 36.Anand R, Nightingale SD, Fish RH, Smith TJ, Ashton P. Control of cytomegalovirus retinitis using sustained release of intraocular ganciclovir. Arch Ophthalmol. 1993;111(2):223–227. doi: 10.1001/archopht.1993.01090020077027. [DOI] [PubMed] [Google Scholar]
- 37.Musch DC, Martin DF, Gordon JF, Davis MD, Kuppermann BD, Heinemann MH, Campbell S, Boddice S, Duker JS, Naughton K, McGeary J, Chong LP, Walonker F, Levin L, Lopez K, Gomes A, Davis JL, Simmons T, Vandenbrook R, Fish RH, Hutchison C, Ai E, Luckie A, Tashayyod D, Anand R, Chuang EL, Lawrence B, Robinson MR, Champagne K, Cantrill HL, Brallier A, Freeman WR, Jarman C, Wieland MR, Coverstone V, Ligh JK, Hutt R, Norman BC, Cristiano J, Neger R, Crawford K, Weinberg DV, Munana A, Murphy FP, Pace B, Duh YJ, Gordon JE, Johnson PJ, Lee JA, Pang CF, Safyan E, Seidl NL, Stoecker JF, Ashton P, Smith TJ, Armstrong J, Brothers R, Hubbard L, Dieterich DT, Frost KR, Maguire MG, Nussenblatt RB, Sanborn GE. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. N Engl J Med. 1997;337(2):83–90. doi: 10.1056/NEJM199707103370203. [DOI] [PubMed] [Google Scholar]
- 38.Smith TJ, Ashton P, Pearson PA. Sustained release drug delivery devices. US5378475 Patent. 1991
- 39.Keller M, Malone A, Carpenter C, Lo Y, Huang M, Corey L, Willis R, Nguyen C, Kennedy S, Gunawardana M, Guerrero D, Moss J, Baum M, Smith T, Herold B. Safety and pharmacokinetics of acyclovir in women following release from a silicone elastomer vaginal ring. J Antimicrob Chemother. 2012 doi: 10.1093/jac/dks151. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moss J, Malone A, Smith T, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Vincent K, Motamedi M, Friend D, Clark M, Baum M. simultaneous delivery of tenofovir and acyclovir via an intravaginal ring. Antimicrob Agents Chemother. 2012;56(2):875–882. doi: 10.1128/AAC.05662-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasband WS. ImageJ [computer program] Bethesda, Maryland: U. S. National Institutes of Health; 1997–2011. [Accessed April 2, 2012]. at: http://imagej.nih.gov/ij/ [Google Scholar]
- 42.Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11(7):36–42. [Google Scholar]
- 43.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 44.ASTM Standard D2240-05. Standard Test Method for Rubber Property—Durometer Hardness. West Conshohocken, PA: ASTM International; www.astm.org. [Google Scholar]
- 45.ISO Standard 8009:2004. Mechanical contraceptives — Reusable natural and silicone rubber contraceptive diaphragms — Requirements and tests. Geneva, Switzerland: ISO; 2004. www.iso.org. [Google Scholar]
- 46.Moss JA, Baum MM, Malone AM, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Willis RA, Vincent KL, Motamedi M, Smith TJ. Tenofovir and tenofovir diso-proxil fumarate pharmacokinetics from intravaginal rings. AIDS. 2012;26(6):707–710. doi: 10.1097/QAD.0b013e3283509abb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garre´ B, Shebany K, Gryspeerdt A, Baert K, van der Meulen K, Nauwynck H, Deprez P, De Backer P, Croubels S. Pharmacokinetics of acyclovir after intravenous infusion of acyclovir and after oral administration of acyclovir and its pro-drug valacyclovir in healthy adult horses. Antimicrob Agents Chemother. 2007;51(12):4308–4314. doi: 10.1128/AAC.00116-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woolfson AD, Elliott GRE, Gilligan CA, Passmore CM. Design of an intravaginal ring for the controlled delivery of 17[beta]-estradiol as its 3-acetate ester. J Controlled Release. 1999;61(3):319–328. doi: 10.1016/s0168-3659(99)00148-0. [DOI] [PubMed] [Google Scholar]
- 49.Morrow RJ, Woolfson D, Donnelly L, Curran R, Andrews G, Katinger D, Malcolm RK. Sustained release of proteins from a modified vaginal ring device. Eur J Pharmaceut Biopharmaceut. 2011;77:3–10. doi: 10.1016/j.ejpb.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karim S, Kashuba A, Werner L, Karim Q. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: Implications for HIV prevention in women. Lancet. 2011;378(9787):279–281. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Laarhoven JA, Kruft MA, Vromans H. in vitro release properties of etonogestrel and ethinyl estradiol from a contraceptive vaginal ring. Int J. 2002;232:163–173. doi: 10.1016/s0378-5173(01)00900-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.