Abstract
Clinical studies have indicated an association between acute hyperglycemia and poor outcomes in patients with traumatic brain injury (TBI), although optimal blood glucose levels needed to maximize outcomes for these patients’ remains under investigation. Previous results from experimental animal models suggest that post-TBI hyperglycemia may be harmful, neutral, or beneficial. The current studies determined the effects of single or multiple episodes of acute hyperglycemia on cerebral glucose metabolism and neuronal injury in a rodent model of unilateral controlled cortical impact (CCI) injury. In Experiment 1, a single episode of hyperglycemia (50% glucose at 2 g/kg, i.p.) initiated immediately after CCI was found to significantly attenuate a TBI-induced depression of glucose metabolism in cerebral cortex (4 of 6 regions) and subcortical regions (2 of 7) as well as to significantly reduce the number of dead/dying neurons in cortex and hippocampus at 24 h post-CCI. Experiment 2 examined effects of more prolonged and intermittent hyperglycemia induced by glucose administrations (2 g/kg, i.p.) at 0, 1, 3 and 6 h post-CCI. The latter study also found significantly improved cerebral metabolism (in 3 of 6 cortical and 3 of 7 subcortical regions) and significant neuroprotection in cortex and hippocampus 1 day after CCI and glucose administration. These results indicate that acute episodes of post-TBI hyperglycemia can be beneficial and are consistent with other recent studies showing benefits of providing exogenous energy substrates during periods of increased cerebral metabolic demand.
Keywords: 14C-2DG, controlled cortical impact, Fluoro-Jade B, hyperglycemia, rat
1. Introduction
The primary energy source for brain cells is glucose, which after transport from the blood into the brain is metabolized intracellularly through glycolysis to pyruvate, which enters the mitochondrial tricarboxylic acid cycle for production of ATP via oxidative phosphorylation. During periods of increased activation the brain increases production of ATP via glycolysis, increasing lactate production and exhibiting an increase in the cerebral metabolic rates of glucose (CMRGlc) compared to oxygen (CMRO2) (Fox et al., 1988). Non-oxidized glucose may also be consumed in the pentose phosphate pathway for generation of NADPH needed to replenish glutathione and dampen oxidative stress, used for synthesis of neurotransmitters and structural components of cells, or be converted to lactate. Brain neurotransmitter and energy (ATP) production during periods of high activity are posited to involve close cooperation between neurons and glia, via the astrocyte neuron lactate shuttle (ANLS; Magistretti and Pellerin, 1999; Pellerin et al., 2007; Hyder et al., 2006). Glucose consumption in neurons is thought to be relatively small and oxidative in nature, whereas astrocytes consume relatively larger proportions of glucose which is metabolized via glycolysis to support ionic homeostasis and conversion of glutamate to glutamine. Lactate produced by astrocytes may be taken up by neurons for use as fuel, with some excess lactate entering the circulation to impact cerebral blood flow (Lin et al., 2010).
Traumatic brain injury (TBI) is well known to induce an early stage of increased energy demand, reflected in elevated CMRGlc and anaerobic glycolysis, which is followed by a prolonged period of reduced CMRGlc (Bergsneider et al., 1997, 2000; Lee et al., 1999; Sutton et al., 1994; Yoshino et al., 1991). Shunting of glucose to the pentose-phosphate pathway is also increased after TBI (Bartnik et al., 2005, 2007; Dusick et al., 2007). Low levels of extracellular glucose during this period of increased energy demand have been frequently noted in experimental models of TBI (Chen et al., 2000; Fukushima et al., 2009; Krishnappa et al., 1999), and the low levels of extracelluar glucose frequently detected acutely in TBI patients (Alessandri et al., 2000; Alves et al., 2005) are correlated with poor neurological outcomes at 6 months (Vespa et al., 2003). Evidence that the injured brain attempts to increase fuel availability is provided by studies showing increased glucose or monocarboxylate transporters after TBI (Cornford et al., 1996; Hamlin et al., 2001; Prins and Giza, 2006). Thus, endogenous levels of metabolic fuels may not be sufficient to meet increased demands in injured brain and supplemental fuel may be needed to match the increase in cerebral metabolic demands and avoid energy crisis after TBI. This hypothesis is supported by studies showing that early provision of metabolic fuels including lactate, pyruvate or ketone bodies can improve outcomes after experimental TBI (Alessandri et al., 2012; Chen et al 2000; Deng Bryant et al., 2011; Fukushima et al., 2009; Holloway et al., 2007; Moro and Sutton, 2010; Prins and Hovda, 2009; Rice et al., 2002).
Administration of glucose after TBI is controversial, in part due to concerns that post-traumatic hyperglycemia is associated with worse outcomes in head-injured patients (Griesdale et al., 2009; Jeremitsky et al., 2005; Lam et al., 1991; Liu-DeRyke et al., 2009; Rovlias and Kotsou, 2000; Young et al., 1989). However, large volume infusions of glucose-containing solutions in rats after experimental TBI was not detrimental unless the solutions were hypo-osmolar, and infusion of hyperosmolar glucose solutions were found to reduce cerebral edema (Shapira et al., 1992; Shapira et al., 1995; Feldman et al., 1995; Gurevich et al., 1997; Talmor et al., 1998). In a fluid percussion injury (FPI) model of experimental TBI, pre-injury glucose treatment in rats did not affect cerebral measures of pH, ATP, free Mg++ or the cytosolic phosphorylation potential, nor did the glucose treatment affect neurological outcome at 1 or 2 weeks post-injury (Vink et al., 1997). Early (5 min), but not delayed (4 or 24 h) glucose treatment after FPI in rats had minimal effects on cortical injury volume, but increased neutrophil infiltration in cerebral cortex at 3 days post-injury (Kinoshita et al., 2002). Studies using the controlled cortical impact (CCI) model of relatively mild TBI in rats have demonstrated that either pre- or post-injury glucose treatment can increase cortical and hippocampal damage induced by post-traumatic secondary ischemia (Cherian et al., 1997, 1998b). In a more severe CCI model of TBI, glucose treatment prior to injury increased histological damage and worsened behavioral outcome in rats (Cherian et al., 1998a). More recently it was reported that induction of hyperglycemia prior to moderate CCI injury in mice did not adversely affect cerebral edema, behavioral outcomes or histopathology, whereas chronic hyperglycemia in rats actually reduced CCI-induced cerebral edema and a post-injury insulin therapy to lower high blood glucose increased cerebral edema (Hill et al., 2010). To date, there is no evidence that post-CCI elevations in plasma glucose will exacerbate histopathology or worsen outcomes (Cherian et al., 1998a; Stover et al., 2002).
Given the evidence that provision of metabolic fuels early post-injury improves outcome after experimental TBI (see above), the current studies in rats with moderate CCI injury were undertaken to determine the effects of acute administration of glucose (2 g/kg, i.p.) on CMRGlc and neuronal viability 24 h post-injury. Experiment 1 was conducted to evaluate effects of a single injection of glucose given immediately following injury, to test the hypothesis that acute provision of supplemental glucose could meet acute TBI-induced energy demands and attenuate neural injury. Experiment 2 determined the effects of multiple glucose injections given at 0, 1, 3 and 6 h after CCI injury. The latter study used the multiple injection protocol for more prolonged glucose supplementation due to evidence that CCI-induced energy demands endure at least 2 h after injury (Lee et al., 1999) and neuronal hyperexcitability, seizure-like activity or cortical depolarization may occur after TBI and produce a deficiency in cerebral extracellular glucose or prolong the metabolic crisis (Alves et al., 2005; Griesemer and Mautes, 2007; Parkin et al., 2005; Vespa et al., 2007). It was hypothesized that any potential adverse effects of post-CCI glucose treatment would be most evident in this multiple glucose treatment condition.
2. Results
2.1. Experiment 1: Single saline (SAL) or glucose (GLC) treatment
2.1.1. Physiological data on day of injury
Data for venous blood glucose concentrations measured immediately after induction of anesthesia/loss of pedal reflex (Pre-injury) and at 10 and 60 min after a single treatment with saline (SAL) or glucose (GLC) are shown in Table 1. Only animals with successful collection and analysis of all three venous samples were included in the analyses. Repeated measures ANOVA indicated significant effects of Drug (p=0.001), Time (p<0.001) and the Drug × Time interaction (p=0.002). Plasma glucose levels within groups increased from pre-injury to 10 min after the first injection (p<0.001) and declined significantly from 10 to 60 min post-injection (p< 0.001), although levels at 60 min remained significantly elevated compared to pre-injury levels (p<0.05). All groups showed increased glucose concentrations 10 min post-injection compared to pre injury (p’s < 0.05), presumably due to anesthesia and surgery procedures. Both Sham-GLC and CCI-GLC groups had increased blood glucose concentrations 10 min after injection compared to SAL treated counterparts (p < 0.01). The glucose concentrations in the 4 treatment groups did not differ by 60 min after injection of SAL or GLC.
Table 1.
Mean (± SEM) venous plasma glucose concentrations (mmol/L) in Sham and CCI groups given one saline (SAL) or glucose (GLC) injection immediately after surgery.
| Sham-SAL | Sham-GLC | CCI-SAL | CCI-GLC | |
|---|---|---|---|---|
| Sample size | n = 6 | n = 7 | n = 5 | n = 6 |
| Pre-injury | 8.2 ± 0.30 | 8.5 ± 0.29 | 8.4 ± 0.22 | 8.5 ± 0.31 |
| +10 min | 13.0 ± 0.62 | 19.5 ± 1.39 a | 12.9 ± 1.44 | 19.5 ± 1.87 b |
| +60 min | 9.1 ± 0.31 | 10.6 ± 0.87 | 8.7 ± 0.17 | 10.1 ± 1.59 |
p < 0.01 compared to Sham-SAL;
p < 0.01 compared to CCI-SAL.
2.1.2. Physiological data one day after injury
As shown in the data of Table 2, both CCI groups had significant loss in body weight one day after injury compared to Sham controls (p’s < 0.01), but there was no effect of SAL versus GLC treatment. Data for arterial blood gasses and plasma concentrations of glucose and lactate shown in Table 2 reveal that these physiological measures were all in normal ranges prior to [14C]2-deoxy-D-glucose (14C-2DG) injections at 24 h post-injury, and there were no significant differences between the 4 treatment groups.
Table 2.
Mean (± SEM) change in body weight (in grams) one day post-injury, and the baseline arterial blood pH, gasses, and plasma glucose and lactate concentrations (mmol/L) prior to 2DG injection in Sham and CCI groups given one saline (SAL) or glucose (GLC) injection immediately after surgery.
| Sham-SAL | Sham-GLC | CCI-SAL | CCI-GLC | |
|---|---|---|---|---|
| Sample size | n = 10 | n = 9 | n = 9 | n = 9 |
| Wt. change | −0.7 ± 1.64 | −2.0 ± 1.26 | −8.9 ± 1.65 ** | −9.7 ± 2.07 ** |
| pH | 7.42 ± 0.01 | 7.43 ± 0.01 | 7.42 ± 0.01 | 7.43 ± 0.01 |
| pCO2 (mm Hg) | 39.5 ± 1.27 | 39.0 ± 1.03 | 38.6 ± 0.58 | 39.3 ± 1.10 |
| pO2 (mm Hg) | 90.8 ± 3.62 | 84.2 ± 2.38 | 84.2 ± 0.98 | 82.2 ± 2.53 |
| HCO3s | 26.1 ± 0.48 | 26.3 ± 0.50 | 25.4 ± 0.60 | 26.7 ± 0.75 |
| tCO2 | 26.9 ± 0.73 | 26.9 ± 0.69 | 26.0 ± 0.72 | 27.4 ± 0.90 |
| O2Sat | 96.9 ± 0.35 | 96.4 ± 0.35 | 96.4 ± 0.15 | 96.2 ± 0.37 |
| Glucose | 9.5 ± 0.29 | 9.6 ± 0.34 | 8.9 ± 0.36 | 9.1 ± 0.23 |
| Lactate | 0.7 ± 0.07 | 0.8 ± 0.12 | 0.8 ± 0.11 | 0.6 ± 0.04 |
p≤ 0.003 compared to similarly treated Sham
2.1.3. Cerebral metabolic rates for glucose
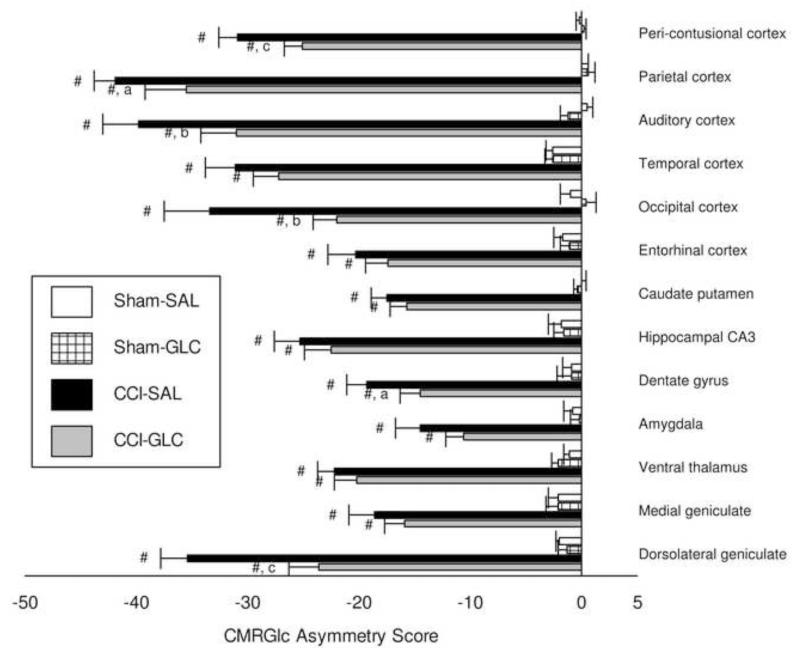
As indicated in the Methods, when the same shipment/lot number of 14C-2DG was used for injection in the first 31 rats in Experiment 1 (Sham-SAL, n=8; Sham-GLC, n=8; CCI-SAL, n=8; CCI-GLC, n=7) we found the same pattern of injury and treatment effects when using either the ipsilateral CMRGlc (μmol/100 g/min) or the CMRGlc asymmetry scores [(left - right/left + right) × 100]. Thus, we analyzed the CMRGlc asymmetry scores for all rats entered into this experiment (Sham-SAL, n=10; Sham-GLC, n=9; CCI-SAL, n=9; CCI-GLC, n=9). As shown in Fig. 1, CMRGlc asymmetries of Sham-SAL and Sham-GLC groups were near zero and did not differ from one another in any brain region 24 h post-surgery. The reductions in ipsilateral CMRGlc at 24 h after left hemisphere CCI injury led to significantly negative asymmetry values in all brain regions relative to Sham controls (p’s < 0.001). The magnitude of metabolic depression was smaller in CCI-GLC compared to CCI-SAL in all brain regions, and improved (i.e., less negative) CMRGlc asymmetry scores by GLC treatment after CCI was significant for the midline peri-contusional (p < 0.001), parietal (p < 0.05), auditory (p < 0.01) and occipital cortex (p < 0.01), as well as for the dentate gyrus (p < 0.05) and the dorsal lateral geniculate (p < 0.001).
Fig. 1.
Mean (bars represent SEM) asymmetry scores [((L-R)/L+R)*100] for CMRGlc in brain regions 24 h after Sham or CCI injury and a single treatment of saline (SAL) or glucose (GLC). # p < 0.001 compared to Sham with same treatment; a p < 0.05, b p < 0.01, c p < 0.001 compared to CCI-SAL.
2.1.4. Neuronal injury in cortex and hippocampus
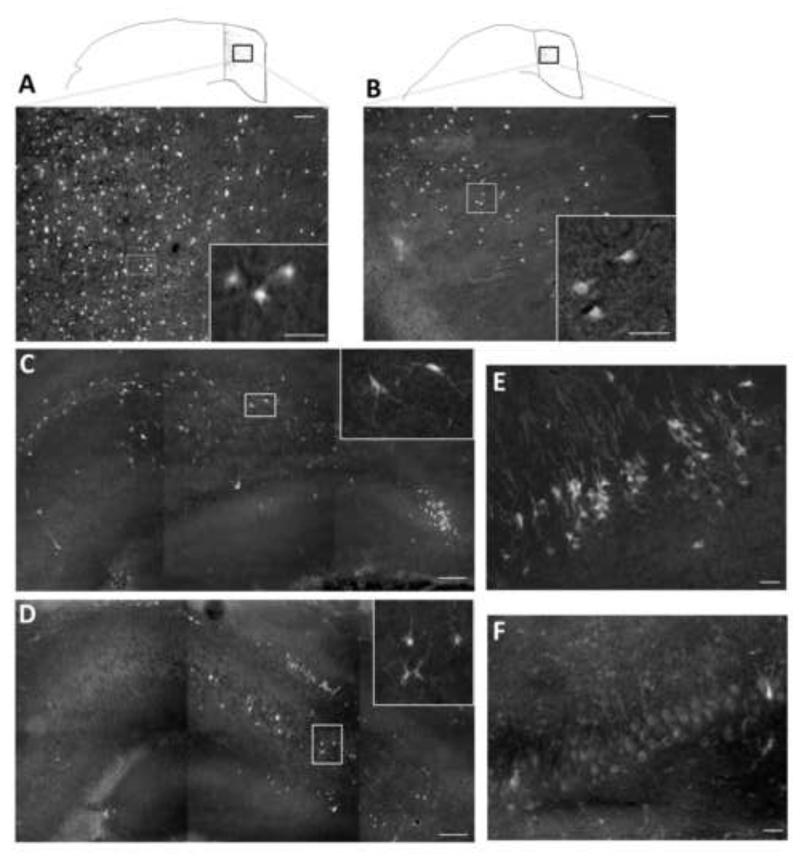
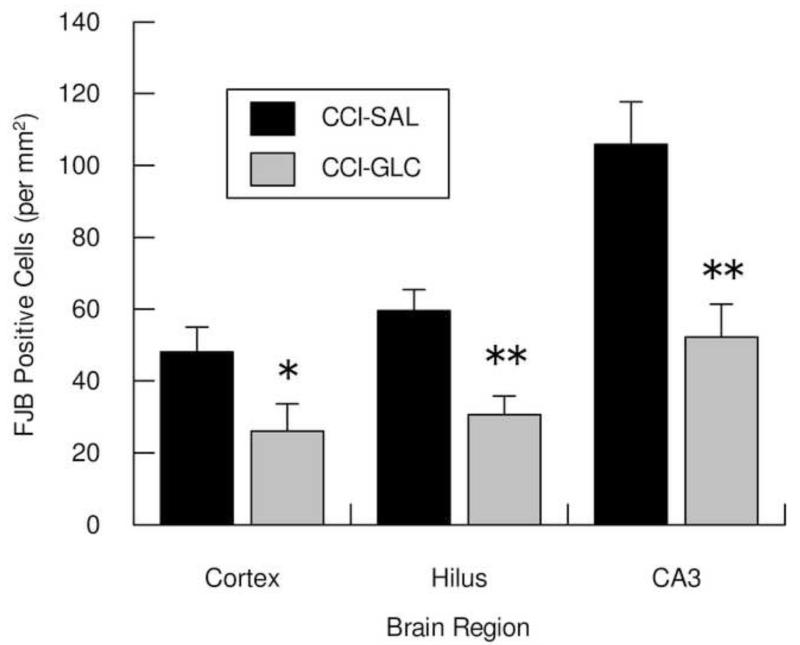
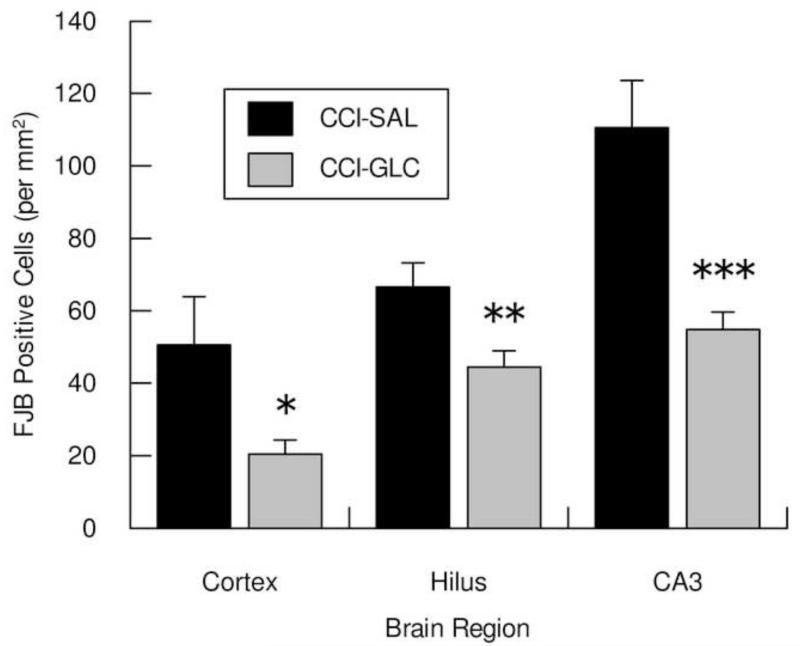
In the Sham injury control groups no Fluoro-Jade B-positive (FJB+) cells were detected in either the left neocortex or left hippocampus. Numerous FJB+ neurons were observed in cortex (Fig. 2-A,B) and in the hilus (Fig. 2-C,D) and CA3 (Fig. 2-E,F) regions of the hippocampus ipsilateral to CCI, and were more numerous in the CCI-SAL (Fig. 2-A,C,E) than in the CCI-GLC animals (Fig. 2-B,D,F). Cell density counts for FJB+ cells in these regions 24 h after CCI and a single injection of SAL or GLC (n=8/group) are shown in Fig. 3. As illustrated, FJB+ neurons in the peri contusional cortex were significantly reduced in the CCI-GLC compared to the CCI-SAL group (p = 0.05), as were counts of dead/dying neurons within the ipsilateral hilus (p < 0.005) and CA3 subsector (p < 0.005) of the hippocampus.
Fig 2.
Representative FJB staining at 24 h after CCI injury and a single treatment of saline (SAL) or glucose (GLC). FJB-positive (FJB+) cells were more numerous in contoured regions of the ipsilateral cortex medial to the contusion site in CCI-SAL (A) compared to CCI-GLC (B) animals. Inserts in each panel are higher powered images illustrating FJB+ cells with neuronal morphology. FJB+ cells were also more numerous in the CCI-SAL group within the ipsilateral hilus (C) and CA3 (E) regions of the hippocampus compared to animals in the CCI GLC group (D, F). Scale bar = 25 μm in panels A, B, E and F; 200 μm in panels C and D.
Fig. 3.
Shows the mean (bars represent SEM) cell densities for dead/dying neurons in the left peri-contusional cortex, hilus and CA3 subsector of the hippocampus 24 h after CCI injury and a single treatment of saline (SAL) or glucose (GLC). * p 0.05, ** p < 0.01 compared to CCI-SAL.
2.2. Experiment 2: Multiple saline (SAL) or glucose (GLC) treatments
2.2.1. Physiological data on day of injury
For these groups injected at 0, 1, 3 and 6 h post-surgery, blood glucose levels were assayed prior to and 10 min after the 3 h injection. As for Experiment 1, only those animals with successful collection and analysis of both venous samples were included in the analyses of blood glucose levels. Repeated measures ANOVA indicated significant effects of Drug (p<0.001), Time (p<0.001) and the Drug × Time interaction (p<0.001). At 3 h post-injury (i.e., 2 h after the second injection) the venous glucose levels ranged from 8.6 ± 0.4 to 9.4 ± 1.2 mmol/L, and the levels did not differ between the 4 treatment groups. The blood glucose concentrations 10 min after the 3 h injection did not differ for Sham-SAL (10.7 ± 1.2 mmol/L, n=4) and CCI SAL (11.0 ± 0.8 mmol/L, n=8) controls. As expected, the blood glucose levels were significantly (p’s <0.001) increased above these control values in the Sham-GLC (19.0 ± 1.5 mmol/L, n=8) and CCI GLC groups (17.0 ± 0.5 mmol/L, n=6) by 10 min after the 3 h injection.
2.2.2. Physiological data one day after injury
As shown in the data of Table 3, Sham operates receiving four injections of SAL or GLC lost an average of 5 to 6 g body weight within 24 h after surgery, an effect not seen with the single injection (see Table 2). Both CCI groups had almost double the loss in body weight compared to Sham injury controls one day after injury (p’s < 0.05), but there was no effect of SAL or GLC treatment within Sham or CCI injury groups. Data for arterial blood gasses shown in Table 3 reveal that these physiological measures were all in normal ranges prior to the 24 h post-injury 14C-2DG injection, with no differences between groups. Baseline plasma concentrations of glucose were higher in the Sham-SAL group, but not the Sham-GLC group, compared to levels in the CCI-SAL and CCI-GLC groups (p’s < 0.05), but there were no significant differences in plasma lactate concentration between the 4 treatment groups.
Table 3.
Mean (± SEM) change in body weight (in grams) one day post-injury, and the baseline arterial blood pH, gasses, and plasma glucose and lactate concentrations (mmol/L) prior to 2DG injection in Sham and CCI groups given saline (SAL) or glucose (GLC) injections at 0, 1, 3 and 6 h after surgery.
| Sham-SAL | Sham-GLC | CCI-SAL | CCI-GLC | |
|---|---|---|---|---|
| Sample size | n = 10 | n = 10 | n = 10 | n = 10 |
| Wt. change | −5.1 ± 2.34 | −5.9 ± 1.64 | −11.1 ± 1.46 * | −12.3 ± 1.50 * |
| pH | 7.45 ± 0.01 | 7.46 ± 0.01 | 7.47 ± 0.01 | 7.46 ± 0.01 |
| pCO2 (mm Hg) | 41.3 ± 0.97 | 40.6 ± 1.21 | 39.9 ± 1.12 | 39.3 ± 1.08 |
| pO2 (mm Hg) | 85.1 ± 2.28 | 80.2 ± 2.90 | 82.8 ± 2.24 | 83.4 ± 3.12 |
| HCO3s | 28.3 ± 0.48 | 28.9 ± 0.55 | 28.7 ± 0.40 | 28.2 ± 0.74 |
| tCO2 | 29.6 ± 0.48 | 30.2 ± 0.56 | 29.9 ± 0.44 | 29.4 ± 0.76 |
| O2Sat | 96.7 ± 0.35 | 96.2 ± 0.47 | 96.6 ± 0.37 | 96.5 ± 0.53 |
| Glucose | 9.6 ± 0.21 | 9.2 ± 0.49 | 8.5 ± 0.26 * | 8.4 ± 0.28 * |
| Lactate | 0.7 ± 0.08 | 0.6 ± 0.03 | 0.6 ± 0.02 | 0.6 ± 0.04 |
p< 0.05 compared to Sham-SAL
2.2.3. Cerebral metabolic rates for glucose
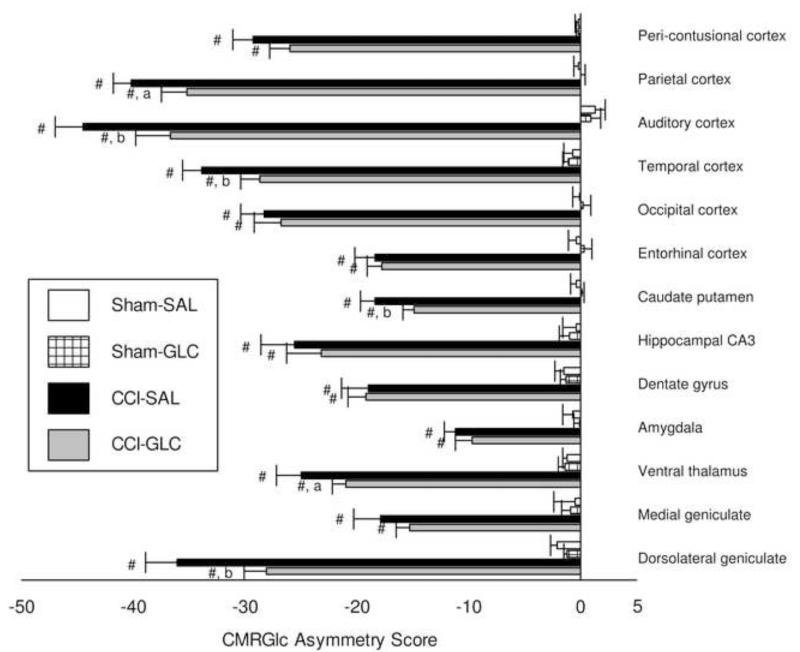
Because the CMRGlc data for rats in Experiment 2 were derived after injection of 14C-2DG from three shipments which varied in specific activity (see Methods), we calculated CMRGlc asymmetry scores [(left - right/left + right) × 100] for analyses of metabolic data for this experiment (n=10/group). As shown in Fig. 4, CMRGlc asymmetries of Sham-SAL and Sham-GLC controls were near zero and did not differ between groups in any brain region. Reductions in CMRGlc ipsilateral to CCI produced significantly negative asymmetry values in all brain regions relative to Sham controls by 24 h post-injury (p < 0.001). The magnitude of metabolic depression was smaller in CCI-GLC compared to CCI-SAL in all brain regions with exception of the dentate gyrus of the hippocampus. This improved CMRGlc (i.e. less negative asymmetry scores) by GLC treatment after CCI was significant for the parietal (p < 0.05), auditory (p < 0.01) and temporal cortex (p < 0.01), as well as for the caudate nucleus (p < 0.01), ventral thalamus (p < 0.05) and the dorsal lateral geniculate (p < 0.01).
Fig. 4.
Mean (bars represent SEM) asymmetry scores [((L-R)/L+R)*100] for CMRGlc in brain regions 24 h after Sham or CCI injury and four treatments of saline (SAL) or glucose (GLC). # p < 0.001 compared to Sham with same treatment; a p < 0.05, b p < 0.01 compared to CCI-SAL.
2.2.4. Neuronal injury in cortex and hippocampus
No FJB+ cells were detected in the left midline neocortex or in the left hippocampus of rats in the Sham-SAL or Sham-GLC groups. As is shown in Fig. 5, FJB+ cells in the midline peri-contusional cortex 24 h after CCI and injections at 0, 1, 3 and 6 h were significantly reduced in the CCI-GLC compared to the CCI-SAL group (p < 0.05). Dead/dying neurons were also significantly reduced in the CCI-GLC compared to the CCI-SAL group in the hilus (p = 0.014) and CA3 subsector (p < 0.005) of the hippocampus ipsilateral to injury.
Fig. 5.
Mean (bars represent SEM) cell densities for dead/dying neurons in the left peri-contusional cortex, hilus and CA3 subsector of the hippocampus 24 h after CCI injury and four treatments of saline (SAL) or glucose (GLC). * p < 0.05, ** p < 0.01, *** p 0.001 compared to CCI-SAL.
Comparison of FJB+ cells between Experiment 1 and Experiment 2 indicated there were significant effect of GLC versus SAL (p’s 0.005) in cortex, CA3 and hilus. There were no significant effects for number of injections or for the Drug × Injection interaction, indicating similar degree of neuroprotection in each brain region after a single (Fig. 3) or multiple (Fig. 5) GLC treatments.
3. Discussion
The current results indicate that a single injection of exogenous glucose (2 g/kg, i.p.) immediately after CCI injury increased blood glucose levels from 8.5 mmol/L to 19.5 mmol/L within 10 min post-injection, with the blood glucose levels decreasing to 10.1 mmol/L at 60 min post-injection. This administration of exogenous glucose significantly attenuated TBI-induced reductions in cerebral glucose utilization in 4 of 6 cortical regions and in 2 of 7 subcortical regions and also significantly decreased numbers of FJB+ neurons in cortex and hippocampus at 24 h post-injury. When multiple injections of glucose (2 g/kg, i.p.) were administered at 0, 1, 3 and 6 h post-CCI, and blood glucose concentrations were found to increase from 8.4 to 17.0 mmol/L within 10 min of the 3 h injection, a similar improvement of cerebral glucose utilization (in 3 of 6 cortical and 3 of 7 subcortical regions) and significant neuroprotection in both cortex and hippocampus was observed.
To our knowledge, this is the first demonstration that the post-injury induction of hyperglycemia can attenuate TBI-induced reductions in cerebral glucose utilization. Our data in sham injury controls indicates that induction of single or multiple periods of hyperglycemia in the first 6 h after surgery did not alter the patterns of cerebral glucose utilization 24 h post-surgery. While our measures of CMRGlc were made 24 or 18 h after the last injection of exogenous glucose, these data in sham injured animals are consistent with prior reports that acute hyperglycemia in normal rats does not alter brain glucose utilization (Duckrow and Bryan, 1987; Duelli et al., 2000; Schuier et al., 1990). The decrease in CMRGlc ipsilateral to injury 24 h after CCI in saline–treated controls is consistent with numerous reports of a generalized cerebral metabolic depression at this time after experimental TBI (Hovda et al., 1991; Prins and Hovda, 2009; Sutton et al., 1994; Yoshino et al., 1991). Although the animals administered glucose after CCI also had significant reductions in cerebral glucose utilization compared to sham operates at 24 h post-injury, the magnitude of this metabolic depression was significantly less than that of CCI-saline controls in both cortical and sub-cortical brain regions. Interestingly, while there were some differences in the cortical and sub-cortical regions of interest showing improved glucose utilization after a single glucose administration immediately after CCI compared to multiple glucose treatments at 0, 1, 3 and 6 h post-CCI, both glucose treatment protocols led to widespread improvements in cerebral glucose utilization.
The mechanisms responsible for the improved CMRGlc at 24 to 18 h after the final glucose administration in rats with CCI are unknown, but are presumably related to ability of the injured brain to use the additional glucose during the period of increased energy demand and hyperglycolysis that is induced by TBI (Lee et al., 1999; Sutton et al., 1994; Yoshino et al., 1991) and the current findings of reduced neuronal injury 24 h after CCI injury and glucose treatment(s). There is ample evidence that TBI induces an acute metabolic demand that can lead to deficiency in cerebral extracellular glucose and metabolic crisis (Chen et al., 2000; Fukushima et al., 2009; Krishnappa et al., 1999; Vespa et al., 2003, 2005). Acute administration of exogenous fuels such as lactate (Chen et al., 2000) or pyruvate (Fukushima et al., 2009) after experimental TBI can significantly attenuate TBI-induced reductions in interstitial glucose concentrations, and these exogenous fuel treatments have been shown to attenuate histopathology and improve outcome after TBI (Alessandri et al., 2012; Fukushima et al., 2009; Holloway et al., 2007; Moro and Sutton, 2010; Rice et al., 2002). Although we did not assess brain levels of glucose in the current study, we speculate that the interstitial levels were increased in our CCI rats administered glucose. Norepinephrine infusion in rats with CCI increases blood glucose (from 8.6 to 12.6 mmol/L) and extracellular glucose (from 1.3 to 4.8 mmol/L) concentrations without altering edema formation (Stover et al., 2002), and it has been reported that extracellular concentrations of glucose are significantly increased during transient periods of unintentional hyperglycemia in TBI patients (Diaz-Parejo et al., 2003). The latter authors also reported no change in interstitial concentrations of pyruvate, lactate, glutamate or glycerol with episodes of moderate hyperglycemia (blood levels of 12-15 mmol/L), although lactate levels increased during episodes of more pronounced (>15 mmol/L) hyperglycemia. As the blood levels of glucose assessed 10 min after a single glucose injection immediately post-CCI or 10 min after the 3 h injection in our multiple glucose injected group all exceeded 15 mmol/L, it is possible that both interstitial glucose as well as lactate were increased in our CCI-glucose groups and that either or both fuels may have contributed to the reduced neuronal injury and improved cerebral glucose metabolism observed in these studies.
The current findings indicate that an acute period of hyperglycemia, within the time frame of increased energy demand and hyperglycolysis induced by TBI, can at least temporarily improve neuronal viability. Just as both glucose treatment protocols led to similar improvements in cerebral glucose utilization, comparable neuroprotection in cortex and hippocampus at 24 h post-injury was found with a single glucose treatment immediately after CCI and when multiple injections of glucose were administered at 0, 1, 3 and 6 h post-CCI. Perhaps as important, we found no adverse effects of these early post-CCI elevations of blood glucose on histological outcomes, which is consistent with most prior reports in experimental TBI models. Infusion of 5% dextrose in 0.9% saline (D5NS, at 560 mOsm/L; 0.25 ml/g, i.v.) over a 30 min period beginning 1 h after closed head injury, raising blood glucose from 197 mg/dL (10 mmol/L) to 1,278 mg/dL (71 mmol/L), did not alter injury volume assessed 18 h post-TBI (Gurevich et al., 1997). In summarizing results of the series of studies in rats with TBI that examined effects of infusions of fluids that worsen edema or neurological outcomes (e.g. 0.45% saline or 5% dextrose in water) and fluids that did not alter these outcomes (e.g. 0.9% saline or D5NS; Shapira et al., 1992; Shapira et al., 1995; Feldman et al., 1995), it was concluded that edema contributed more to adverse outcomes after TBI than did increased blood glucose concentration (Talmor et al., 1998). Glucose infusion (2 g/kg) begun 20 min after moderate-severe CCI injury in rats also had no effect on cortical contusion volume or on neuronal cell densities in the CA1 or CA3 areas of the hippocampus at 2 weeks after injury (Cherian et al., 1998a). This latter finding implies either that the immediate glucose injection used in the current study was of import for neuroprotection, or the protection seen at 24 h may not endure to later survival time points. In mice with closed head injury that were treated with glucagon (25 μg, i.p.) 10 min after injury to activate hepatic gluconeogenesis, blood glucose increased from 89 to 137 mg/L (154%) within 15 min while glutamate concentrations in the circulation and in the cerebrospinal fluid fell significantly (Fanne et al., 2011). Glucagon-induced improvements in neurological outcomes and reductions in lesion size at one month after closed head injury were attributed to the glutamate reduction effects, consistent with other studies showing benefits of glutamate scavenging/reduction in the circulation after TBI (Gottlieb et al., 2003; Zlotnik et al., 2007), and the authors suggest that the harmful effects of increased glutamate outweigh those of hyperglycemia in post-TBI neurotoxicity (Fanne et al,, 2011). In a FPI model of TBI lower doses (100 mg/kg) of glucose administered for 10 day post-injury did not alter cognitive outcome, whereas the same dose of glucose administered 10 min prior to cognitive tests on days 11-15 post-injury improved cognitive function (Kokiko-Cochran et al., 2008). Thus, the vast majority of studies using animal models of TBI have found no evidence that post-traumatic glucose administration or hyperglycemia is detrimental to the brain.
To our knowledge the only study reporting detrimental effects of an early post-injury administration of exogenous glucose on histological outcomes after an isolated experimental TBI is that of Kinoshita et al. (2002). These authors injected dextrose (2 g/kg, i.p.) 5 min after a moderate FPI in rats, raising blood glucose to ~27.7 mmol/L from a baseline of ~7.2 mmol/L by 15 min post-injection, and found no effect on mean contusion volume at 3 days post-injury, although contusion area was significantly increased in 1 of 8 cortical planes assessed. They also reported a significant increase in neutrophils within this same cortical plane and an overall increase in mean number of neutrophils with dextrose treatment administered 5 min post-FPI. It is not clear that this hyperglycemia-induced increase in neutrophils after FPI could induce tissue damage, as there is evidence that the ability of neutrophils to produce superoxide is significantly reduced under conditions of hyperglycemia (Perner et al., 2003).
The effects of hyperglycemia induction in rodents prior to experimental TBI have also been variable. Some authors reported no effects on measures of cerebral metabolism or behavioral outcomes in a moderate FPI model (Vink et al., 1997), others found no effects on histological damage in rats with mild CCI (Cherian et al., 1997) or on cerebral edema, sensorimotor behavior or histopathology in mice with moderate-severe CCI (Hill et al., 2010), with the latter study finding mild improvements in short-term memory (Hill et al., 2010). In rats with streptozotocin-induced type 1 diabetes one week prior to moderate severe CCI injury, cerebral edema was decreased 48 h after TBI (Hill et al., 2010). Only a single study has reported detrimental effects when glucose was infused in rats prior to severe CCI, where the hyperglycemia was reported to increase contusion volume, but not hippocampal cell loss, at 2 weeks post-injury (Cherian et al., 1998a). The reasons for these discrepant findings for effects of pre-injury hyperglycemia on outcomes for experimental TBI are uncertain, but may be more related to the extent of plasma corticosterone as compared to glucose levels, as reported for neuronal damage induced by pre ischemic hyperglycemia (Payne et al., 2003).
The preceding studies on the effects of glucose administration have been conducted in various animal models of isolated TBI and, because glucose is the primary fuel of the brain and it is known that hyperglycemia can reduce cerebral blood flow in uninjured rats (Duckrow, 1995; Duckrow and Bryan, 1987), it is possible the different outcomes reported are related to model-specific or injury severity effects on cerebral energy demands (ATP production) and cerebrovascular functions. For example, moderate FPI results in a shorter period of hyperglycolysis and reduced ATP than does moderate CCI injury (Lee et al., 1999) and reductions in cerebral blood flow are relatively mild and transient after moderate FPI or mild CCI (Cherian et al., 1994; Yamakami and McIntosh, 1989; Yuan et al., 1988) while moderate-severe CCI can lead to primary ischemia in the impact site and adjacent regions of the brain (Cherian et al., 1994; Kochanek et al., 1995; Sutton et al., 1994). However, when treatments to increase glucose levels were administered within 30 min prior to or subsequent to isolated TBI reports indicate beneficial effects for closed head injury (Fanne et al., 2011) or moderate-severe CCI (Hill et al., 2010; the current study), no effects with moderate FPI (Vink et al., 1997), mild CCI (Cherian et al., 1997) or moderate-severe CCI (Cherian et al., 1998a; Stover et al., 2002), and detrimental effects after moderate FPI (Kinoshita et al., 2002) or moderate severe CCI (Cherian et al., 1998a). These varied outcomes across injury models do not seem compatible with the concept that glucose effects are dependent on injury severity factors related solely to cerebral energy demands or the extent of injury-induced ischemia. In contrast to these studies of isolated TBI, when a secondary ischemic injury is induced subsequent to mild or severe CCI injury in rats either pre- or post-injury elevations of blood glucose levels have been reported to increase cortical and hippocampal damage (Cherian et al., 1997, 1998a). As discussed by the latter authors, glucose effects on cerebrovascular factors could work in concert with secondary ischemia to produce more severe damage in these combined injury models. These detrimental effects may also be related to worsened energy crisis and ATP reductions found with a TBI combined with secondary injury or repetitive TBI (Aoyama et al., 2008; Ip et al., 2003; Signoretti et al., 2010). As recently indicated by Hill et al. (2010), further research on effects of hyperglycemia in experimental models of TBI plus secondary injury or models of polytrauma will be needed.
While our findings of improved CMRglc and reduced neuronal injury with post-CCI periods of hyperglycemia may seem surprising in light of the dogma that post-traumatic hyperglycemia is to be avoided, there is scant evidence that hyperglycemia after clinical TBI affects neurologic injury (Marion, 2009). However, hyperglycemia may increase oxidative stress and production of damaging reactive oxygen species (ROS) can also result from mitochondrial dysfunction or from increased activity of xanthine oxidase or NADPH oxidase after TBI (Dohi et al., 2010; Signoretti et al., 2010; Zhang et al., 2012). Hyperglycemia-induced ROS production and neuronal injury in murine stroke appears to be mediated by NADPH oxidase (Suh et al., 2008), and inhibition of NADPH oxidase after TBI is neuroprotective (Choi et al., 2012; Zhang et al., 2012). Interestingly, high glucose levels in cultured astroglia cells can activate activity of the pentose phosphate pathway, increasing levels of glutathione and decreasing production of ROS (Takahashi et al., 2012). Neurons were found to have 4-5 fold less activity in the pentose phosphate pathway than astroglia and, although ROS are produced in neuronal cultures subjected to increasing concentrations of glucose, no increase in ROS was found in mixed astroglia-neuronal cultures exposed to high glucose levels, suggesting that the presence of astrocytes offset ROS production in neurons (Takahashi et al., 2012). If a similar effect occurs in vivo, a protective activation of pentose phosphate pathway activity and production of glutathione in astrocytes may underlie the neuroprotection seen here in CCI rats subjected to hyperglycemia, similar to other protective roles for astrocytes reported in models of TBI (Bartnik-Olson et al., 2010; Myer et al., 2006).
In summary, the current results demonstrate that acute periods of hyperglycemia immediately after experimental TBI can be neuroprotective and improve CMRglc in some cortical and subcortical brain regions at 1 day post-injury. While we would not recommend administration of excess concentrations of glucose in clinical TBI due to the obvious correlation between hyperglycemia and increased infection or mortality in patients (Griesdale et al., 2009; Jeremitsky et al., 2005; Lam et al., 1991; Liu-DeRyke et al., 2009; Rovlias and Kotsou, 2000; Young et al., 1989), the current studies do serve as proof of principle that post-TBI hyperglycemia per se is not necessarily detrimental. As suggested by the current results and by other studies indicating that fuels such as lactate, pyruvate or ketone bodies may improve outcome after experimental TBI (Alessandri et al., 2012; Chen et al 2000; Deng-Bryant et al., 2011; Fukushima et al., 2009; Holloway et al., 2007; Moro and Sutton, 2010; Prins and Hovda, 2009; Rice et al., 2002) the injured brain may require supplemental or higher than normal circulating fuel levels during the post-injury period. While use of insulin to maintain blood levels at or below ~6.7 mmol/L (120 mg/dL) has been reported to have positive effects in some patients with severe TBI (Yang et al., 2009), other studies report detrimental hypoglycemia or increased metabolic crisis in TBI patients receiving intensive insulin therapy (Bilotta et al., 2008; Oddo et al., 2008; Vespa et al., 2006, 2012). Insulin treatment to reduce blood glucose levels below 8 mmol/L is also correlated with decreased brain glucose levels, increased brain lactate concentrations and peri-ischemic cortical depolarizations (Hopwood et al., 2005). Further research to determine optimal glucose levels and how to maintain this fuel within the proper range to facilitate optimal brain metabolism and neuronal function after TBI will be needed.
4. Experimental procedures
4.1. Subjects
A total of 77 male Sprague Dawley rats (287-395 g) from Charles River Breeding Labs (Hollister, CA) were used for the studies. Animals were housed in rat shoebox cages (2 per cage) and acclimated to vivarium conditions for 1 week before initiation of experiments. Rats were maintained in a temperature- (70-76°F) and humidity controlled (30-70%) room (lights on 06:00 to 18:00), with food (Teklad 7904) and tap water available ad libitum. All experimental procedures and protocols were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Animals and were approved by the UCLA Chancellor’s Animal Research Committee.
4.2. Surgery for CCI or Sham injury
Surgery to induce CCI was performed as previously described (Fukushima et al., 2009; Moro and Sutton, 2010; Sutton et al., 1993). In brief, after induction of general anesthesia (4% isoflurane in oxygen at 1.5 L/min) animals were secured in a stereotaxic frame and isoflurane was maintained at 2% during surgery. Core body temperature was monitored continuously by a rectal probe and maintained at 37.0±1.0 °C with a thermostatically controlled heating pad (Harvard Apparatus Limited, Edenbridge, KY). A 6 mm diameter circular craniotomy, centered −3 mm from Bregma and 3.5 mm lateral (left) from the midline, was made using a high-speed drill under a surgical microscope. CCI was produced using a pneumatically-driven (20 psi; 2.30 2.40 m/sec velocity) dual-stroke piston with a 5 mm diameter flat-tipped impactor which compressed the exposed dura mater and underlying brain to a depth of 2 mm for 250 msec. Sham injury controls underwent similar anesthetic and surgical interventions, excluding the craniotomy and CCI. After injury the scalp was sutured closed, bupivacaine (0.1-0.14 mg/kg, s.c.) was injected around the incision site, and rats were placed in a heated recovery cage until ambulatory.
4.3. Glucose Administration and Monitoring
Animals in the studies were randomized to receive injection of either glucose (GLC; 2.0 g/kg; 50% solution in 0.9% saline; i.p.) or comparable volume of 8% saline (SAL; osmolality control), with both treatment solutions filtered (0.22 μm) prior to injection. Blood samples were obtained by tail venepuncture at various times pre- and post-injection, described below, and used to determine blood glucose concentrations (2700 Select Biochemistry Analyzer, YSI Inc., Yellow Springs, OH).
4.3.1. Experiment 1
Rats entered into the 4 injury-treatment groups for this experiment (Sham-SAL, n=10; Sham-GLC, n=9; CCI-SAL, n=9; CCI-GLC, n=9) had a venous blood sample collected immediately after induction of anesthesia (Pre-injury) and a single injection of SAL or GLC was administered immediately after induction of Sham or CCI injury. Anesthesia in these groups was maintained until a second venous blood sample was taken at 10 min after the injection. Another brief (~2 min) period of anesthesia was induced in these rats to obtain a third venous blood sample at 60 min after SAL or GLC injection.
4.3.2. Experiment 2
Rats in the groups of this experiment (Sham-SAL, n=10; Sham-GLC, n=10; CCI-SAL, n=10; CCI-GLC, n=10) were injected with SAL or GLC at 0, 1, 3 and 6 h after induction of Sham or CCI injury. Immediately prior to the 3 h post-injury injection these rats were anesthetized to enable collection of a venous blood sample, and they were maintained under light anesthesia (1-1.5% isoflurane) until collection of a second venous blood sample was completed at 10 min after the 3 h injection.
4.4. Cerebral Glucose Utilization
At 22 h post-injury animals were anesthetized and surgery was performed to place polyethylene catheters (PE-50; Becton, Dickinson, NJ) in the right femoral vein and artery. After catheters were placed the skin was sutured and the incision site was infiltrated with bupivacaine (0.1-0.14 mg/kg, s.c.). The animal was removed from anesthesia, restrained onto cardboard to reduce hindlimb movements, and maintained in dim light and quiet room conditions for the next 2 h to allow recovery from anesthesia. At 24 h post-injury baseline arterial blood samples were collected for measures of blood gasses (using either a 238 pH/Blood Gas Analyzer, Ciba Corning Diagnostics Ltd, Halstead, UK or a Siemens Rapidpoint 340, Healthcare Diagnostics Inc, Plainfield, IN) and plasma glucose and lactate levels (2700 Select Biochemistry Analyzer). To assess local cerebral metabolic rates for glucose (CMRGlc), 14C-2DG was administered (120 μCi/kg, i.v.; American Radiolabeled Chemicals Inc, St. Louis, MO) within a 30 sec interval and 12 timed arterial blood samples were collected in polyethylene-heparin lithium fluoride coated tubes over a 45 min period. These samples were kept on ice until being centrifuged, and plasma was assayed for 14C activity on a scintillation counter (LS-6500; Beckman Coulter, Brea, CA) and for glucose and lactate levels. Rats were sacrificed by bolus infusion of sodium pentobarbital (100 mg/kg, i.v.) 45 min after the 14C-2DG infusion, brains were rapidly removed and immediately frozen in 2-methylbutane at −55°C. Frozen coronal tissue sections (20 μm) collected onto glass coverslips were exposed to Kodak Biomax film with 14C methacrylate standards (Amersham, Arlington Heights, IL) for 2-3 days, and images were digitally captured with a flat-bed scanner (256 dpi, 8 bit gray scale). Image data were calibrated in ImageJ software (version 1.42q: National Institutes of Health, Bethesda, MD) using the brain standards and the plasma 14C input curve using the equations of Sokoloff et al. (1977) so that the gray scale values were transformed to units of CMRGlc (μmol/100 g/min) for each brain region of interest (ROI), which were measured bilaterally from autoradiographs. For each ROI values were obtained from 5 tissue sections and averaged for each animal.
During conduct of the studies 14C-2DG purchased in differing shipment/lot numbers was found to differ in their true specific activities, such that calculated the CMRGlc was quite variable across shipments. The same supply of 2DG was used in 7-8 animals per group in Experiment 1, where analyses showed no effect of injury on right hemisphere ROIs and that the pattern of injury or glucose treatment effects were the same regardless of whether data used were the ipsilateral CMRGlc (i.e., μmol/100 g/min) or when data in the left and right ROIs were expressed as CMRGlc asymmetry scores [(left - right/left + right) × 100], as we have previously done for cerebral metabolism data (Hovda et al., 1991; Moro and Sutton, 2010). To utilize data from all animals in Experiment 1, where 1-3 rats/group were injected with a different batch of 2DG, these CMRGlc asymmetry scores were analyzed. For Experiment 2, where three 2DG shipments were used, all cerebral glucose utilization data were expressed as CMRGlc asymmetry scores.
4.5. Fluoro-Jade B staining and analysis
Tissue sections (20 μm) adjacent to those saved for 2DG were mounted onto glass slides and stored at 20 °C until staining for Fluoro-Jade B (FJB). These sections were brought to room temperature and fixed in 10% formalin overnight prior to staining with FJB (2FJB; Histo-Chem Inc, Jefferson, AR) and 4′,6-diamidino-2-phenylindol dihydrochloride (DAPI; D9542: Sigma Aldrich, St. Louis, MO), using final dye concentrations of 0.0004% for FJB and 0.0002% for DAPI (Schmued and Hopkins, 2000). After staining the sections were dried, cleared and cover-slipped as described previously (Moro and Sutton, 2010).
Dead/dying (FJB positive; FJB+) cells were counted in 8 animals of each experimental group using an epifluorescent microscope (480 nm excitation; Model DMRE: Leica Microsystems GmbH) interfaced with a computer running Stereo Investigator software (version 3.0: MicroBrightField Inc, Colchester, VT). All counts were performed by an observer masked to the treatment conditions, using 20-40 × objectives to identify FJB+ cells with neuronal morphology. FJB+ neurons in the left midline peri-contusional cortex, dorsal to the corpus callosum and from midline to 1.5 mm laterally, were counted on 5 tissue sections from −0.8 to −4.8 mm posterior to Bregma (at 1 mm intervals). FJB+ neurons in the ipsilateral hippocampus were counted for 3 anterior sections (−2.8, −3.3 and −3.8 from Bregma) containing the CA3 subsector and the hilus, and from 3 tissue sections containing the ventral hilus (−4.8, −5.3 and −5.8 mm) and 2 sections containing the ventral CA3 (−4.8, −5.3 mm). The cell counts and counting areas within each region (cortex, CA3 and hilus) were summed for each animal, with final cell density data being expressed as FJB+ cells per mm2 of tissue area.
4.6. Data analysis
All data were normally distributed and so are reported as the group average ± standard error of the mean (SEM), and were analyzed using IBM SPSS Statistics (v19). Analyses of variance (ANOVA) were carried out on data for CMRGlc (each ROI) and physiological data, using repeated measures for those data measured over multiple time points (i.e. venous plasma glucose concentration). Individual group means were compared using t tests via the Tukey-Fisher least significant difference (LSD) criterion with α set at 0.05 (two-sided). Analysis of FJB+ neurons in cortex, CA3 and hilus of CCI groups of each separate experiment were analyzed using unpaired t tests with α set at 0.05 (two-sided). ANOVA followed by individual group mean comparisons was used to compare FJB+ cells in CCI injury conditions across Experiment 1 and Experiment 2.
Highlights.
One injection of glucose improved cerebral glucose utilization 24 h post-TBI.
Neuronal injury in cortex and hippocampus were reduced by a single glucose treatment.
Four glucose treatments after TBI also improved cerebral glucose utilization at 24 h.
Multiple glucose treatments produced neuroprotection similar to the single treatment.
Acknowledgments
This work was supported by the UCLA Brain Injury Research Center and award PO1NS058489 from the National Institute of Neurological Disorders and Stroke (NINDS). The content is the sole responsibility of the authors and does not necessarily represent official views of the NINDS or the National Institutes of Health.
Abbreviations
- ANLS
astrocyte-neuron lactate shuttle
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- CCI
controlled cortical impact
- CMRGlc
cerebral metabolic rates of glucose
- CMRO2
cerebral metabolic rates of oxygen
- D5NS
5% dextrose in 0.9% saline
- DAPI
4′,6-diamidino-2-phenylindol dihydrochloride
- FJB
Fluoro-Jade B
- FPI
fluid percussion injury
- GLC
glucose (50%)
- Mg++
magnesium
- PBS
phosphate buffered saline
- ROS
reactive oxygen species
- SAL
saline (8%)
- SEM
standard error of the mean
- TBI
traumatic brain injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
None of the authors have conflicting financial interests relevant to this work.
References
- Alessandri B, Reinert M, Young HF, Bullock R. Low extracellular (ECF) glucose affects the neurochemical profile in severe head-injured patients. Acta Neurochir. (Wein) Suppl. 2000;76:425–430. doi: 10.1007/978-3-7091-6346-7_88. [DOI] [PubMed] [Google Scholar]
- Alessandri B, Schwandt E, Kamada Y, Nagata M, Heimann A, Kempski O. The neuroprotective effect of lactate is not due to improved glutamate uptake after controlled cortical impact in rats. J. Neurotrauma. 2012;29:2181–2191. doi: 10.1089/neu.2011.2067. [DOI] [PubMed] [Google Scholar]
- Alves OL, Bullock R, Clausen T, Reinert M, Reeves TM. Concurrent monitoring of cerebral electrophysiology and metabolism after traumatic brain injury: an experimental and clinical study. J. Neurotrauma. 2005;22:773–749. doi: 10.1089/neu.2005.22.733. [DOI] [PubMed] [Google Scholar]
- Aoyama N, Lee SM, Moro N, Hovda DA, Sutton RL. Duration of ATP reduction affects extent of CA1 cell death in rat models of fluid percussion injury combined with secondary ischemia. Brain Res. 2008;1230:310–319. doi: 10.1016/j.brainres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik-Olson BL, Oyoyo U, Hovda D, Sutton RL. Astrocyte oxidative metabolism and metabolite trafficking after fluid percussion brain injury in adult rats. J. Neurotrauma. 2010;27:2191–2202. doi: 10.1089/neu.2010.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik BL, Sutton RL, Fukushima M, Harris NG, Hovda DA, Lee SM. Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J. Neurotrauma. 2005;22:1052–1065. doi: 10.1089/neu.2005.22.1052. [DOI] [PubMed] [Google Scholar]
- Bartnik BL, Lee SM, Hovda DA, Sutton RL. The fate of glucose during the period of decreased metabolism after fluid percussion injury: a 13C NMR study. J. Neurotrauma. 2007;24:1079–1092. doi: 10.1089/neu.2006.0210. [DOI] [PubMed] [Google Scholar]
- Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, Phelps ME, McArthur DL, Caron MJ, Kraus JF, Becker DP. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J. Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Bergsneider M, Hovda DA, Lee SM, Kelly DF, McArthur DL, Vespa PM, Lee JH, Huang SC, Martin NA, Phelps ME, Becker DP. Dissociation of cerebral glucose metabolism and level of consciousness during the period of metabolic depression following human traumatic brain injury. J. Neurotrauma. 2000;17:389–401. doi: 10.1089/neu.2000.17.389. [DOI] [PubMed] [Google Scholar]
- Bilotta F, Caramia R, Cernak I, Paoloni FP, Doronzio A, Cuzzone V, Santoro A, Rosa G. Intensive insulin therapy after severe traumatic brain injury: a randomized clinical trial. Neurocrit. Care. 2008;9:159–166. doi: 10.1007/s12028-008-9084-9. [DOI] [PubMed] [Google Scholar]
- Chen T, Qian YZ, Di X, Rice A, Zhu JP, Bullock R. Lactate/glucose dynamics after rat fluid percussion brain injury. J. Neurotrauma. 2000;17:135–142. doi: 10.1089/neu.2000.17.135. [DOI] [PubMed] [Google Scholar]
- Cherian L, Robertson CS, Contant CF, Bryan RM. Lateral cortical impact injury in rats: Cerebrovascular effects of varying depth of cortical deformation and impact velocity. J. Neurotrauma. 1994;11:573–585. doi: 10.1089/neu.1994.11.573. [DOI] [PubMed] [Google Scholar]
- Cherian L, Goodman JC, Robertson CS. Hyperglycemia increases brain injury caused by secondary ischemia after cortical impact injury in rats. Crit. Care Med. 1997;25:1378–1383. doi: 10.1097/00003246-199708000-00027. [DOI] [PubMed] [Google Scholar]
- Cherian L, Goodman JC, Robertson CS. Effect of glucose administration on contusion volume after moderate cortical impact injury in rats. J. Neurotrauma. 1998a;15:1059–1066. doi: 10.1089/neu.1998.15.1059. [DOI] [PubMed] [Google Scholar]
- Cherian L, Hannay HJ, Vagner G, Goodman JC, Contant CF, Robertson CS. Hyperglycemia increases neurological damage and behavioral deficits from post-traumatic secondary ischemic insults. J. Neurotrauma. 1998b;15:307–321. doi: 10.1089/neu.1998.15.307. [DOI] [PubMed] [Google Scholar]
- Choi BY, Jang BG, Kim JH, Lee BE, Sohn M, Song HK, Suh SW. Prevention of traumatic brain injury-induced neuronal death by inhibition of NADPH oxidase activation. Brain Res. 2012;1481:49–58. doi: 10.1016/j.brainres.2012.08.032. [DOI] [PubMed] [Google Scholar]
- Cornford EM, Hyman S, Cornford ME, Caron MJ. Glut1 glucose transporter activity in human brain injury. J. Neurotrauma. 1996;13:523–536. doi: 10.1089/neu.1996.13.523. [DOI] [PubMed] [Google Scholar]
- Deng-Bryant Y, Prins ML, Hovda DA, Harris NG. Ketogenic diet prevents alterations in brain metabolism in young but not adult rats after traumatic brain injury. J. Neurotrauma. 2011;28:1813–1825. doi: 10.1089/neu.2011.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Parejo P, Stahl N, Xu W, Reinstrup P, Ungerstedt U, Nordstrom CH. Cerebral energy metabolism during transient hyperglycemia in patients with severe brain trauma. Inten. Care Med. 2003;29:544–550. doi: 10.1007/s00134-003-1669-3. [DOI] [PubMed] [Google Scholar]
- Dohi K, Ohtaki H, Nakamachi T, Yofu S, Satoh K, Miyamoto K, Song D, Tsunawaki S, Shioda S, Aruga T. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J. Neuroinflammation. 2010;7:41. doi: 10.1186/1742-2094-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckrow RB. Decreased cerebral blood flow during acute hyperglycemia. Brain Res. 1995;703:145–150. doi: 10.1016/0006-8993(95)01077-7. [DOI] [PubMed] [Google Scholar]
- Duckrow RB, Bryan RM., Jr. Regional cerebral glucose utilization during hyperglycemia. J. Neurochem. 1987;48:989–993. doi: 10.1111/j.1471-4159.1987.tb05614.x. [DOI] [PubMed] [Google Scholar]
- Duelli R, Maurer MH, Staudt R, Heiland S, Duembgen L, Kuschinsky W. Increased cerebral glucose utilization and decreased glucose transporter Glut1 during chronic hyperglycemia in rat brain. Brain Res. 2000;858:338–347. doi: 10.1016/s0006-8993(00)01942-9. [DOI] [PubMed] [Google Scholar]
- Dusick JR, Glenn TC, Lee WN, Vespa PM, Kelly DF, Lee SM, Hovda DA, Martin NA. Increased pentose phosphate pathway flux after clinical traumatic brain injury: a [1,2-(13)C(2)]glucose labeling study in humans. J. Cereb. Blood Flow Metab. 2007;27:1593–1602. doi: 10.1038/sj.jcbfm.9600458. [DOI] [PubMed] [Google Scholar]
- Fanne RA, Nassar T, Mazuz A, Waked O, Heyman SN, Hijazi N, Goelman G, Higazi AA. Neuroprotection by glucagon: role of gluconeogenesis. J. Neurosurg. 2011;114:85–91. doi: 10.3171/2010.4.JNS10263. [DOI] [PubMed] [Google Scholar]
- Feldman Z, Zachari S, Reichenthal E, Artru AA, Shapira Y. Brain edema and neurological status with rapid infusion of lactated Ringer’s or 5% dextrose solution following head trauma. J. Neurosurg. 1995;83:1060–1066. doi: 10.3171/jns.1995.83.6.1060. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Fukushima M, Lee SM, Moro N, Hovda DA, Sutton RL. Metabolic and histologic effects of sodium pyruvate treatment in the rat after cortical contusion injury. J. Neurotrauma. 2009;26:1095–1110. doi: 10.1089/neu.2008.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb M, Wang Y, Teichberg VI. Blood-mediated scavenging of cerebrospinal fluid glutamate. J. Neurochem. 2003;87:119–126. doi: 10.1046/j.1471-4159.2003.01972.x. [DOI] [PubMed] [Google Scholar]
- Griesdale DE, Tremblay MH, McEwen J, Chittock DR. Glucose control and mortality in patients with severe traumatic brain injury. Neurocrit. Care. 2009;11:311–316. doi: 10.1007/s12028-009-9249-1. [DOI] [PubMed] [Google Scholar]
- Griesemer D, Mautes AM. Closed head injury causes hyperexcitability in rat hippocampal CA1 but not in CA3 pyramidal cells. J. Neurotrauma. 2007;24:1823–1832. doi: 10.1089/neu.2006.0237. [DOI] [PubMed] [Google Scholar]
- Gurevich B, Talmor D, Artru AA, Katcko L, Geva D, Gurman G, Shapira Y. Brain edema, hemorrhagic necrosis volume, and neurological status with rapid infusion of 0.45% saline or 5% dextrose in 0.9% saline after closed head trauma in rats. Anesth. Analg. 1997;84:554–559. doi: 10.1097/00000539-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Hamlin GP, Cernak I, Wixey JA, Vink R. Increased expression of neuronal glucose transporter 3 but not glial glucose transporter 1 following severe diffuse traumatic brain injury in rats. J. Neurotrauma. 2001;18:1011–1018. doi: 10.1089/08977150152693700. [DOI] [PubMed] [Google Scholar]
- Hill J, Zhao J, Dash PK. High blood glucose does not adversely affect outcome in moderately brain-injured rodents. J. Neurotrauma. 2010;27:1439–1448. doi: 10.1089/neu.2010.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway R, Zhou Z, Harvey HB, Levasseur JE, Rice AC, Sun D, Hamm RJ, Bullock MR. Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat. Acta Neurochir. (Wien.) 2007;149:919–927. doi: 10.1007/s00701-007-1241-y. [DOI] [PubMed] [Google Scholar]
- Hopwood SE, Parkin MC, Bezzina EL, Boutelle MG, Strong AJ. Transient changes in cortical glucose and lactate levels associated with peri infarct depolarisations, studied with rapid-sampling microdialysis. J.Cereb.Blood Flow Metab. 2005;25:391–401. doi: 10.1038/sj.jcbfm.9600050. [DOI] [PubMed] [Google Scholar]
- Hovda DA, Yoshino A, Kawamata T, Katayama Y, Becker DP. Diffuse prolonged depression of cerebral oxidative metabolism following concussive brain injury in the rat: a cytochrome oxidase histochemistry study. Brain Res. 1991;567:1–10. doi: 10.1016/0006-8993(91)91429-5. [DOI] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J. Cereb. Blood Flow Metab. 2006;7:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Ip EY, Zanier ER, Moore AH, Lee SM, Hovda DA. Metabolic, neurochemical, and histologic responses to vibrissa motor cortex stimulation after traumatic brain injury. J. Cereb. Blood Flow Metab. 2003;23:900–910. doi: 10.1097/01.WCB.0000076702.71231.F2. [DOI] [PubMed] [Google Scholar]
- Jeremitsky E, Omert LA, Dunham CM, Wilberger J, Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J. Trauma. 2005;58:47–50. doi: 10.1097/01.ta.0000135158.42242.b1. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Kraydieh S, Alonso O, Hayashi N, Dietrich WD. Effect of posttraumatic hyperglycemia on contusion volume and neutrophil accumulation after moderate fluid-percussion brain injury in rats. J. Neurotrauma. 2002;19:681–692. doi: 10.1089/08977150260139075. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Marion DW, Zhang W, Schiding JK, White M, Palmer AM, Clark RSB, O’Malley ME, Styren SD, Ho C, Dekosky ST. Severe controlled cortical impact in rats: Assessment of cerebral edema, blood blow, and contusion volume. J. Neurotrauma. 1995;12:1015–1025. doi: 10.1089/neu.1995.12.1015. [DOI] [PubMed] [Google Scholar]
- Kokiko-Cochran ON, Michaels MP, Hamm RJ. Delayed glucose treatment improves cognitive function following fluid percussion injury. Neurosci. Lett. 2008;436:27–30. doi: 10.1016/j.neulet.2008.02.046. [DOI] [PubMed] [Google Scholar]
- Krishnappa IK, Contant CF, Robertson CS. Regional changes in cerebral extracellular glucose and lactate concentrations following severe cortical impact injury and secondary ischemia in rats. J. Neurotrauma. 1999;16:213–224. doi: 10.1089/neu.1999.16.213. [DOI] [PubMed] [Google Scholar]
- Lam AM, Winn HR, Cullen BF, Sundling N. Hyperglycemia and neurological outcome in patients with head injury. J. Neurosurg. 1991;75:545–551. doi: 10.3171/jns.1991.75.4.0545. [DOI] [PubMed] [Google Scholar]
- Lee SM, Wong MD, Samii A, Hovda DA. Evidence for energy failure following irreversible traumatic brain injury. Ann. N. Y. Acad. Sci. 1999;893:337–340. doi: 10.1111/j.1749-6632.1999.tb07849.x. [DOI] [PubMed] [Google Scholar]
- Lin AL, Fox PT, Hardies J, Duong TQ, Gao JH. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc. Natl. Acad. Sci. U.S.A. 2010;107:8446–8451. doi: 10.1073/pnas.0909711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-DeRyke X, Collingridge DS, Orme J, Roller D, Zurasky J, Rhoney DH. Clinical impact of early hyperglycemia during acute phase of traumatic brain injury. Neurocrit. Care. 2009;11:151–157. doi: 10.1007/s12028-009-9228-6. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion DW. Optimum serum glucose levels for patients with severe traumatic brain injury. F1000. Med.Rep. 2009:1. doi: 10.3410/M1-42. doi:10.3410/M1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro N, Sutton RL. Beneficial effects of sodium or ethyl pyruvate after traumatic brain injury in the rat. Exp. Neurol. 2010;225:391–401. doi: 10.1016/j.expneurol.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Oddo M, Schmidt JM, Carrera E, Badjatia N, Connolly ES, Presciutti M, Ostapkovich ND, Levine JM, Le Roux P, Mayer SA. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit. Care Med. 2008;36:3233–3238. doi: 10.1097/CCM.0b013e31818f4026. [DOI] [PubMed] [Google Scholar]
- Parkin M, Hopwood S, Jones DA, Hashemi P, Landolt H, Fabricius M, Lauritzen M, Boutelle MG, Strong AJ. Dynamic changes in brain glucose and lactate in pericontusional areas of the human cerebral cortex, monitored with rapid sampling on-line microdialysis: relationship with depolarisation like events. J. Cereb. Blood Flow Metab. 2005;25:402–413. doi: 10.1038/sj.jcbfm.9600051. [DOI] [PubMed] [Google Scholar]
- Payne RS, Tseng MT, Schurr A. The glucose paradox of cerebral ischemia: evidence for corticosterone involvement. Brain Res. 2003;971:9–17. doi: 10.1016/s0006-8993(03)02276-5. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Perner A, Nielsen SE, Rask-Madsen J. High glucose impairs superoxide production from isolated blood neutrophils. Inten. Care Med. 2003;29:642–645. doi: 10.1007/s00134-002-1628-4. [DOI] [PubMed] [Google Scholar]
- Prins ML, Giza CC. Induction of monocarboxylate transporter 2 expression and ketone transport following traumatic brain injury in juvenile and adult rats. Dev. Neurosci. 2006;28:447–456. doi: 10.1159/000094170. [DOI] [PubMed] [Google Scholar]
- Prins ML, Hovda DA. The effects of age and ketogenic diet on local cerebral metabolic rates of glucose after controlled cortical impact injury in rats. J. Neurotrauma. 2009;26:1083–1093. doi: 10.1089/neu.2008.0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AC, Zsoldos R, Chen T, Wilson MS, Alessandri B, Hamm RJ, Bullock MR. Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Res. 2002;928:156–159. doi: 10.1016/s0006-8993(01)03299-1. [DOI] [PubMed] [Google Scholar]
- Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000;46:335–342. doi: 10.1097/00006123-200002000-00015. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schuier F, Orzi F, Suda S, Lucignani G, Kennedy C, Sokoloff L. Influence of plasma glucose concentration on lumped constant of deoxyglucose method: Effects of hyperglycemia in the rat. J. Cereb. Blood Flow Metab. 1990;10:765–773. doi: 10.1038/jcbfm.1990.134. [DOI] [PubMed] [Google Scholar]
- Shapira Y, Artru AA, Cotev S, Muggia-Sulam M, Freund HR. Brain edema and neurologic status following head trauma in the rat. No effect from large volumes of isotonic or hypertonic intravenous fluids, with or without glucose. Anesthesiology. 1992;77:79–85. doi: 10.1097/00000542-199207000-00012. [DOI] [PubMed] [Google Scholar]
- Shapira Y, Artru AA, Qassam N, Navot N, Vald U. Brain edema and neurologic status with rapid infusion of 0.9% saline or 5% dextrose after head trauma. J. Neurosurg. Anesthesiol. 1995;7:17–25. doi: 10.1097/00008506-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Signoretti S, Vagnozzi R, Tavazzi B, Lazzarino G. Biochemical and neurochemical sequelae following mild traumatic brain injury: summary of experimental data and clinical implications. Neurosurg. Focus. 2010;29:E1. doi: 10.3171/2010.9.FOCUS10183. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Stover JF, Sakowitz OW, Thomale UW, Kroppenstedt SN, Unterberg AW. Norepinephrine-induced hyperglycemia does not increase cortical lactate in brain injured rats. Inten. Care Med. 2002;28:1491–1497. doi: 10.1007/s00134-002-1431-2. [DOI] [PubMed] [Google Scholar]
- Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, Swanson RA. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann.Neurol. 2008;64:654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RL, Lescaudron L, Stein DG. Unilateral cortical contusion injury in the rat: Vascular disruption and temporal development of cortical necrosis. J. Neurotrauma. 1993;10:135–149. doi: 10.1089/neu.1993.10.135. [DOI] [PubMed] [Google Scholar]
- Sutton RL, Hovda DA, Adelson PD, Benzel EC, Becker DP. Metabolic changes following cortical contusion: relationships to edema and morphological changes. Acta Neurochir. Suppl. (Wien.) 1994;60:446–448. doi: 10.1007/978-3-7091-9334-1_122. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Izawa Y, Suzuki N. Astroglial pentose phosphate pathway rates in response to high-glucose environments. ASN Neuro. 2012:4. doi: 10.1042/AN20120002. art:e00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmor D, Shapira Y, Artru AA, Gurevich B, Merkind V, Katchko L, Reichenthal E. 0.45% saline and 5% dextrose in water, but not 0.9% saline or 5% dextrose in 0.9% saline, worsen brain edema two hours after closed head trauma in rats. Anesth. Analg. 1998;86:1225–1229. doi: 10.1097/00000539-199806000-00017. [DOI] [PubMed] [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, Glenn TC, McArthur DL, Hovda DA. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa P, Boonyaputthikul R, McArthur DL, Miller C, Etchepare M, Bergsneider M, Glenn T, Martin N, Hovda D. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit. Care Med. 2006;34:850–856. doi: 10.1097/01.CCM.0000201875.12245.6F. [DOI] [PubMed] [Google Scholar]
- Vespa P, McArthur DL, Stein N, Huang SC, Shao W, Filippou M, Etchepare M, Glenn T, Hovda DA. Tight glycemic control increases metabolic distress in traumatic brain injury: a randomized controlled within-subjects trial. Crit. Care Med. 2012;40:1923–1929. doi: 10.1097/CCM.0b013e31824e0fcc. [DOI] [PubMed] [Google Scholar]
- Vespa PM, McArthur D, O’Phelan K, Glenn T, Etchepare M, Kelly D, Bergsneider M, Martin NA, Hovda DA. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a microdialysis study. J. Cereb. Blood Flow Metab. 2003;23:865–877. doi: 10.1097/01.WCB.0000076701.45782.EF. [DOI] [PubMed] [Google Scholar]
- Vespa PM, Miller C, McArthur D, Eliseo M, Etchepare M, Hirt D, Glenn TC, Martin N, Hovda D. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit. Care Med. 2007;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]
- Vink R, Golding EM, Williams JP, McIntosh TK. Blood glucose concentration does not affect outcome in brain trauma: A 31P MRS study. J. Cereb. Blood Flow Metab. 1997;17:50–53. doi: 10.1097/00004647-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Yamakami I, McIntosh TK. Effects of traumatic brain injury on regional cerebral blood flow in rats as measured with radiolabeled microspheres. J. Cereb. Blood Flow Metab. 1989;9:117–124. doi: 10.1038/jcbfm.1989.16. [DOI] [PubMed] [Google Scholar]
- Yang M, Guo Q, Zhang X, Sun S, Wang Y, Zhao L, Hu E, Li C. Intensive insulin therapy on infection rate, days in NICU, in-hospital mortality and neurological outcome in severe traumatic brain injury patients: a randomized controlled trial. Int. J. Nurs. Stud. 2009;46:753–758. doi: 10.1016/j.ijnurstu.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Yoshino A, Hovda DA, Kawamata T, Katayama Y, Becker DP. Dynamic changes in local cerebral glucose utilization following cerebral concussion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- Young B, Ott L, Dempsey R, Haack D, Tibbs P. Relationship between admission hyperglycemia and neurologic outcome of severely brain-injured patients. Annals of Surgery. 1989;210:466–472. doi: 10.1097/00000658-198910000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan XQ, Prough DS, Smith TL, Dewitt DS. The effects of traumatic brain injury on regional cerebral blood flow in rats. J. Neurotrauma. 1988;5:289–301. doi: 10.1089/neu.1988.5.289. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Laird MD, Han D, Nguyen K, Scott E, Dong Y, Dhandapani KM, Brann DW. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS.ONE. 2012;7:e34504. doi: 10.1371/journal.pone.0034504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Gurevich B, Tkachov S, Maoz I, Shapira Y, Teichberg VI. Brain neuroprotection by scavenging blood glutamate. Exp. Neurol. 2007;203:213–220. doi: 10.1016/j.expneurol.2006.08.021. [DOI] [PubMed] [Google Scholar]