Introduction
Heart Failure (HF) has been termed an “epidemic” but its epidemiology is complex due to the multiple factors which interact in a complex manner to impact the prevalence and incidence of HF.1 Most HF-related hospitalizations and deaths are incurred by a subgroup of patients that is refractory to guideline-based medical management, a group categorized as having “advanced HF”. This cohort constitutes an important and rapidly expanding patient population that warrants comprehensive review as outlined in this series.
Epidemiology of HF
670,000 new cases of HF are diagnosed annually2. Epidemiologic studies indicate that the age and sex adjusted incidence of HF has plateaued3, 4 over the last several decades. In contrast, the age and sex specific prevalence of HF appears to have increased from the 1970s to the early 2000's owing to improved survival of HF patients. Finally, the population at risk for HF has increased due to the aging of those persons who comprised the surge in births after the second World War (the baby boomers) and due to the improved management of cardiovascular and non-cardiovascular disease with more people surviving into the 7th and 8th decades where their risk of HF is highest5. This combination of influences on population demographics in the United States will result in a doubling of the number of people over 65 years of age (the population at risk for HF) over the next few decades and will result in a marked increase in the number of persons with HF despite stable age and sex specific incidence and only modest increases in age and sex specific prevalence due to increased survival of HF patients.
Natural History of HF defines advanced HF
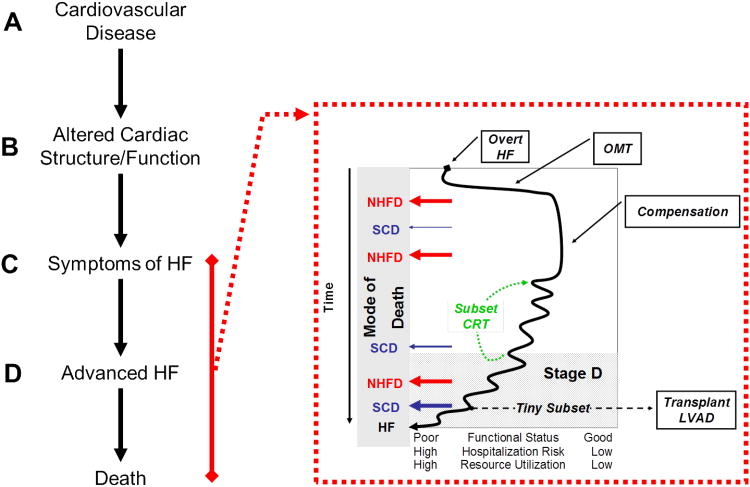
The American College of Cardiology (ACC)/American Heart Association (AHA) task force on practice guidelines categorize the development and progression of the HF syndrome in to 4 stages termed Stage A-D of HF6 (Figure 1). Asymptomatic patients with cardiovascular risk factors but without any structural heart disease are considered to be high risk for developing HF and are classified as being in Stage A HF. Once structural heart disease develops, patients are considered to have Stage B HF despite the absence of recognized HF symptoms. Over time, the heart fails to maintain systemic perfusion commensurate with the metabolic requirements of organs or to do so only at the price of elevated filling pressures7 and patients develop overt symptoms of HF (Stage C). Etiologic factors are addressed and optimal medical therapy (OMT)6 is titrated resulting in a variable duration of compensation. A minority of patients have a very rapid progression and never achieve sustained compensation. A larger subset enjoy a prolonged period of relative compensation. However, over the long term, a progressive decline to advanced HF refractory to OMT (Stage D HF, defined below) is the rule. A subset of patients may experience marked improvement in symptoms with cardiac resynchronization therapy (CRT) for variable lengths of time resetting their progressive course upward. Another subset of patients may exit the “HF natural history” after receiving cardiac transplantation or a LV assist device (LVAD). Unfortunately, due to the realities of donor availability and ability to tolerate LVAD placement, this group currently represents a tiny subset of the HF population.
Figure 1. Natural History of HF defines advanced HF.
The stages in the natural history of HF clarify the relationship between cardiovascular disease (Stage A), asymptomatic abnormalities in cardiac structure and function (Stage B), overt symptomatic HF (Stage C) and advanced HF (Stage D). The natural history of over HF is further characterized in the insert where the fluctuating but progressive clinical course after HF presentation and commencement of optimal medical therapy (OMT) or later, with cardiac resynchronization therapy (CRT) or advanced HF therapies such as cardiac transplantation or ventricular assist devices (LVAD) is shown. Progression to poorer functional status associated with higher risk of hospitalization and overall health care resource utilization occurs over time. The potential modes of death over the course of the natural history of HF are emphasized where patients may die of non-HF related cardiovascular or non-cardiovascular causes (non-HF death, NHFD) or sudden cardiac death (SCD) at any time with the risk of SCD increasing as HF worsens. Patients who avoid NHFD or SCD die of progressive HF (HF).
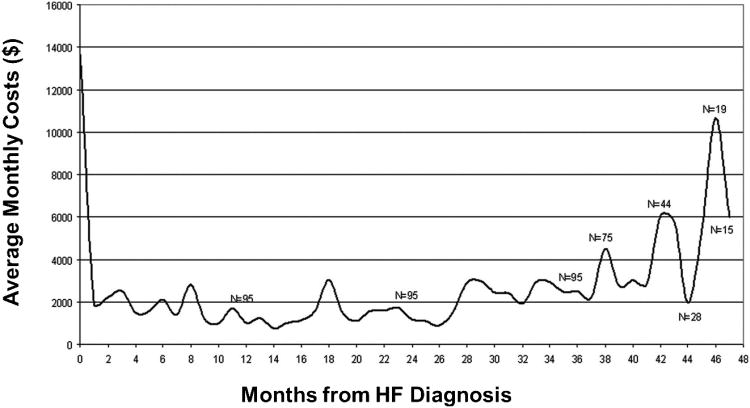
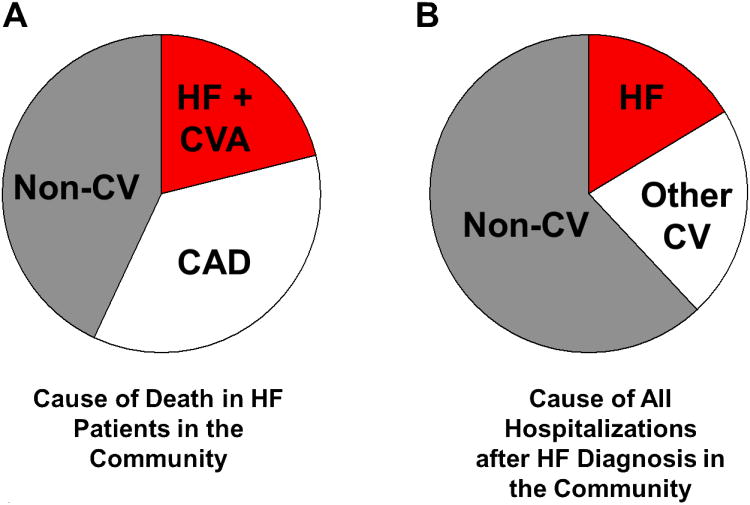
Health care resource utilization increases dramatically during the Stage D. A recent community based study assessed health care utilization over the lifetime of HF patients after their initial HF diagnosis (Figure 2)8. This study demonstrated that in patients who survived the initial months after their HF diagnosis, resource utilization was most intense at the time of diagnosis and then in the last few months of life. The intensity of resource utilization was due to frequent hospitalizations and intensive out-patient visits. It is important to recognize that in the community, the most common mode of death in patients with a diagnosis of HF is non-HF related death (NHFD) due to coronary artery disease and non-cardiovascular comorbidities (Figure 1 and 3A9). Similarly, the most common causes of hospitalization over the course of a patient's life after a HF diagnosis are non-CV diseases followed by non-HF cardiovascular causes and HF (Figure 3B10). However, a significant subset of patients will die of fatal arrhythmias (sudden cardiac death, SCD) or progressive HF. The risk of SCD increases as the severity of HF increases.
Figure 2. Health care resource utilization over life after a HF diagnosis in the community.
The average monthly cost of health care is shown for patients over their lifetime after a diagnosis of HF. Note that resource utilization is most intense at the time of diagnosis (often made during a hospitalization) and again years after the diagnosis, where the advanced HF patient is at high risk for repeated hospitalizations for HF or other conditions8.
Figure 3. Causes of death and hospitalization for HF patients in the community.
The “epidemic” of HF occurs in elderly patients where the progression to advanced HF does not occur in isolation from a host of other cardiovascular and non-cardiovascular comorbidities. While HF clinical trials usually exclude patients with other cardiovascular and non-cardiovascular comorbidities, these conditions potently contribute to mortality9 (A) and hospitalizations10 (B) in the community as demonstrated in studies from a community based HF surveillance study in Olmsted County, Minnesota. In B, the cause of all hospitalizations over the patients life after a HF diagnosis (excluding the index HF hospitalization in patients in whom the diagnosis was made during a hospitalization for HF) were assessed.
What is Stage D “Advanced” HF?
Stage D HF designates patients with truly refractory HF symptoms and is heralded by a tenuous clinical course of progressive debilitating symptoms with decreasing level of activity (NYHA IIIB, IV), recurrent hospitalizations for volume overload, rhythm management and complications of HF and HF therapy (cardiorenal syndrome, medication side effects, pulmonary emboli, anti-coagulation complications and others) and marked increases in the level of outpatient visits in efforts to avoid hospitalizations. Advanced HF makes patients more susceptible to destabilization of other medical conditions increasing the hospitalization burden. Progression to advanced HF may be gradual and it is often difficult to determine whether decompensation in a previously stable HF patient is an isolated incident due to reversible precipitants or the transition to a refractory stage. To facilitate recognition of this phase so that advanced HF therapy (in eligible patients) or a more palliative approach to care can be considered, a number of definitions of advanced HF have been proposed.
Definitions of advanced HF in guidelines
The ACC/AHA guidelines and the Heart Failure Society of America guidelines have attempted to define advanced HF as summarized in Table 1.6, 11. The Heart Failure Association of the European Society of Cardiology provides additional details in their criteria for advanced HF12 (Table 2).
Table 1. Definition of Advanced HF Across Cardiovascular Societies.
| Refractory symptoms | Exercise Intolerance | Objective evidence of severe cardiac dysfunction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Severe Symptoms | Multiple Hospitalizations | Optimal Therapy | Inotropic Support | Fluid Retention and/or Peripheral Hypoperfusion | Severe Functional Capacity Impairment* | Reduced Ejection Fraction | Doppler Echo† | Hemodynamics§ | Elevated Natriuretic Peptides | |
| ACC/AHA | x | x | x | x | x | |||||
| HFSA | x | x | x | x | x | |||||
| ESC | x | x | x | x | x | x | x | x | x | |
Assessed by cardiopulmonary exercise testing or six minute walk test
Pseudonormal or restrictive filling patterns
High left ventricular filling pressures
Abbreviations-ACC/AHA: American College of Cardiology/American Heart Association, HFSA: Heart Failure Society of America, ESC: European Society of Cardiology
Table 2. European Society of Cardiology Definition of Advanced HF.
| • NYHA Class III-IV Symptoms |
| • Episodes of volume overload and/or peripheral hypoperfusion |
| • Objective evidence
of severe cardiac
dysfunction (EF<30%, Doppler Pseudonormal or Restrictive filling pattern, PCWP>16mmHg or RAP >12 mmHg) |
| • Severely impaired
functional capacity (Inability to exercise, 6MWD<300m, Peak VO2<12-14 ml/kg/min) |
| • HF
Hospitalizations (≥1 in past 6 months) |
| • Above occurring despite attempts to optimize diuretics, RAAS antagonists, BB, CRT or in the setting of intolerance to OMT |
Abbreviations: NYHA, New York Heart Association; LV, left ventricular; EF, ejection fraction; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; 6MWD, 6 minute walk distance; VO2 oxygen consumption; RAAS, renin-angiotensin-aldosterone system, BB, beta blockers, CRT, cardiac resynchronization therapy, OMT, optimal medical therapy
It is important to establish that Acute Decompensated HF (ADHF) is not always advanced HF. Patients commonly present with NYHA IV symptoms at their index HF presentation yet are not considered to have advanced disease; rather, they respond to treatment of etiologic factors and optimization of medical therapy and improve to NYHA classes I-II (Figure 1).
Secular trends in the definition of advanced HF used in clinical trials and registries
Evident in examination of advanced HF clinical trials13-29,84 and registries30-32 is the significant variability in the definition of advanced HF over the years, the higher event rates in registries than clinical trials and the marked variability in event rates in clinical trials of advanced HF - despite efforts to select more advanced HF patients by utilizing additional entry criteria (Table 3). The marked variability in outcomes in these trials and studies speaks to the difficulty in defining a homogenous population of advanced HF patients as well as the bias introduced by studying the “natural history” of HF in clinical trial populations.
Table 3. Secular Trends in Definition of Advanced HF in Clinical Trials and Registries.
| STUDY | Intervention | YEAR | Background Therapy (used or available) | Definition of Advanced Heart Failure | Reported Mortality % | SCD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EF | NYHA | Other criteria† | HF Hosp | Inotrope Use | 3 mo | 6 mo | ≈ 12 mo | ≈ 24 mo | % of total deaths | ||||
| CONSENSUS(13) | RAS | 1987 | Diuretic/Dig | N/A | IV | 44 | 52 | 21 | |||||
| PROMISE(14) | Oral Milrinone | 1991 | Diuretic/Dig/RAS | ≤ 35 % | III-IV | 24 | na | ||||||
| PRAISE(15) | Amlodipine | 1996 | Diuretic/Dig/RAS | < 30 % | III-IV | 38 | 43 | ||||||
| FIRST(29) | Epoprostenol | 1997 | Diuretic/Dig/RAS | ≤ 25 % | IIIB-IV | 37 | na | ||||||
| CIBIS-II (16) | BB | 1999 | Diuretic/Dig/RAS | ≤ 35 % | III-IV | 17 | 31 | ||||||
| EPICAL(30) | ADVANCED HF REGISTRY | 1999 | Diuretic/Dig/RAS | <30% | III-IV | x | x | 35 | na | ||||
| RALES(17) | Aldo Antag | 1999 | Diuretic/Dig/RAS | ≤ 35 % | III-IV | 46 | 21 | ||||||
| BEST (28) | BB | 2001 | Diuretic/Dig/RAS | ≤ 35 % | III-IV | 19 | 45 | ||||||
| COPERNICUS(18) | BB | 2001 | Diuretic/Dig/RAS | < 25 % | III-IV | 17 | na | ||||||
| MUSTIC(19) | CRT | 2001 | Diuretic/Dig/RAS | < 35 % | III | x | 5 | na | |||||
| REMATCH(20) | LVAD | 2001 | Diuretic/Dig/RAS/BB/Inotrope | ≤ 25 % | IV | x | x | 75 | 0 | ||||
| MIRACLE (21) | CRT | 2002 | Diuretic/Dig/RAS/BB | ≤ 35 % | III-IV | x | 7 | na | |||||
| MIRACLE-ICD(22) | CRT-D | 2003 | Diuretic/Dig/RAS/BB | ≤ 35 % | III-IV | x | 8 | 21 | |||||
| COMPANION(23) | CRT or CRT-D | 2004 | Diuretic/Dig/RAS/BB/Aldo Antag | ≤ 35 % | III-IV | x | x | 19 | na | ||||
| CARE-HF(84) | CRT | 2005 | Diuretic/Dig/RAS/BB/Aldo Antag | ≤ 35 % | III-IV | x | 30 | 33 | |||||
| ESCAPE (24) | PA CATH Management | 2005 | Diuretic/Dig/RAS/BB/Aldo Antag/Inotrope | ≤ 30 % | III-IV | x | 17 | na | |||||
| FUSION II (25) | Outpatient Nesiritide | 2008 | Diuretic/Dig/RAS/BB/Aldo Antag/ ICD/CRT | < 40% | III-IV | x | x | 10 | na | ||||
| PERSIST(26) | Levosimendan | 2008 | Diuretic/Dig/RAS/BB/Aldo Antag/Inotrope | ≤ 30 % | IIIB-IV | x | x | x | 4 | 52 | |||
| ADHERE LM (32) | ADVANCED HF REGISTRY | 2008 | Diuretic/Dig/RAS/BB//Inotrope/ICD/CRT | mean 30% | III-IV | x | x | x | 28 | 17 | |||
QRS duration, LV dilatation, Cardiopulmonary exercise testing, ectopy, renal dysfunction, BNP
Abbreviations: RAS, renin angiotensin system antagonist; BB, beta blocker; LVAD, left ventricular assist device; CRT, cardiac resynchronization therapy; PA CATH, pulmonary artery catheterization; CPXT, cardiopulmonary exercise test; mo, month; SCD, sudden cardiac death;; Hosp, hospitalization, Aldo, aldosterone; ICD, implantable cardioverter defibrillator; Antag, antagonist, CRT-D, CRT-defibrillator; dig, digoxin
Registries specifically aimed at advanced HF patients allow insight into the characteristics of the advanced HF patient outside the context of clinical trials. ADHERE-LM was a multicenter, observational registry of 1433 patients with advanced HF defined as adults (≥18) with chronic HF and refractory symptoms on oral medical therapy that were required: (1) to be in NYHA functional class III or IV for ≥60 consecutive days and (2) hospitalized ≥2 times in the preceding year with either a primary diagnosis of HF or a secondary diagnosis of HF treated with ≥2 consecutive days or IV diuretic, vasoactive, or inotropic medications, or to have required either 2 complete IV infusions of a vasoactive or inotropic agent, each lasting ≥2 hrs or 3 IV diuretic treatments, given either as a bolus or continuous drip, during the preceding 60 days.32 As compared to the previous ADHERE ADHF patients, advanced HF patients tended to be younger, more often male and have more coronary artery disease and chronic renal insufficiency. Fatigue rather than dyspnea and edema were more common on history while physical examination revealed a lower blood pressure with less evidence of volume overload. Although systolic dysfunction was more severe in advanced HF, serum BNP levels appeared similar between the acutely decompensated and advanced HF groups. Advanced HF patients were more aggressively treated. With the exception of the REMATCH study20, the patients in ADHERE-LM had a much higher event rate than patients enrolled in clinical trials of advanced HF.
These registry and cohort studies highlight that advanced HF is a discrete clinical syndrome that deserves recognition as its own entity. However, the marked variability in event rates despite similar entry criteria in clinical trials and registries of advanced HF indicate the difficulty in prospectively identifying patients with advanced HF.
Recognizing the advanced HF patient
There is no one feature which reliably identifies the advanced HF patient. Recognizing advanced HF requires integration of clinical, imaging, hemodynamic, functional and biomarker data. The presence of multiple clinical, imaging, hemodynamic, functional and biomarker parameters associated with increased risk in the setting of OMT should raise concern over progression to Stage D HF. Risk scores may provide a more objective tool to facilitate such integration.
Symptoms
While dyspnea is often a characteristic feature, many advanced HF patients have a more prominent component of right ventricular failure and may complain more prominently of fatigue, edema or abdominal distension due to ascites. Sleep disturbances due to HF symptoms or central sleep apnea, depression and cardiac cachexia are common. Intolerance (hypotension, worsening renal function, hyperkalemia) to previously tolerated doses of RAAS antagonists and beta blockers is another feature suggestive of advanced HF.
Hospitalizations
Repeated HF hospitalizations are an important but not invariant feature of advanced HF. The ADHERE LM registry revealed that 59% of advanced HF patients were hospitalized during the mean follow-up of 364 days. Of hospitalized patients, 37% were hospitalized once, 23% twice, 14% 3 times, 9% 4 times and 18% 5 or more times32. The European EPICAL registry of 2,577 patients with advanced HF revealed that patients were admitted to the hospital an average of 2.05 times per year spending 27.6 days per year in the hospital over a mean follow-up of 18 months30. Each subsequent HF hospitalization is associated with incremental increase in risk of death.33 Thus, one or more HF hospitalizations in a previously stable chronic HF patient may herald the onset of advanced HF and should alert the care provider to a potential change in the course of the patient's HF.
Doppler Echocardiography
The prognostic implications of a variety of Doppler echocardiographic features in variable HF populations have been previously described34-53. Ejection Fraction (EF) has been shown to maintain prognostic power even among those with severe systolic dysfunction (LVEF<25%)41, 42. Left ventricular size has also been shown to offer prognostic information among advanced HF patients40, 43-45.
Right ventricular (RV) function is a potent predictor of survival in advanced HF patients when invasively assessed with radionuclide ventriculography46, 47. As RV EF is difficult to assess echocardiographically, a number of newer quantitative indices are being investigated. Tricuspid Annular Plane Systolic Excursion (TAPSE), RV shortening, RV fractional area change, and peak systolic tricuspid annular velocity have each been shown to classify a high risk group of HF patients with recurrent HF hospitalizations, poor functional capacity and high mortality38, 48-50. Repeatedly, the presence of pulmonary hypertension as measured by tricuspid regurgitant velocity ≥ 2.5 m/s (particularly in the presence of RV dysfunction) has been shown to be a potent predictor of poor outcomes in HF patients54.
Severe diastolic dysfunction is a potent prognostic factor. A meta-analysis of over 3500 patients (18 studies) revealed that regardless of ejection fraction and age, a restrictive mitral inflow pattern is a powerful predictor of mortality in HF36.
The presence of left and right atrial and ventricular dilatation and in ischemic patients, tethering of the mitral chordal apparatus, results in functional or ischemic mitral and functional tricuspid regurgitation in the absence of intrinsic valve disease. Distortion of the tricuspid valvular apparatus by ICD leads can dramatically increase tricuspid regurgitation. Both mitral and tricuspid regurgitation are associated with worse outcomes in HF55 and are markers of advanced HF. Whether correction of functional AV valve regurgitation will change the course of advanced HF remains an important unresolved controversy.
Hemodynamics
Hospitalizations and deaths are predicted by elevated filling pressures as reflected by right atrial pressures (>10 mmHg) or pulmonary capillary wedge pressures (> 20 mmHg)56, 57. Moreover, recent data from the COMPASS-HF cohort revealed that many advanced HF patients on OMT have elevated ambulatory intracardiac pressures that translates into a higher risk of hospitalization.58 Other parameters such as pulmonary vascular resistance (>3-4 wood units), mean pulmonary arterial pressures (> 35 mmHg) and cardiac index (< 2.0) may also be used to risk stratify the advanced HF patient. The presence of such hemodynamic perturbations in the presence of all attempts to maximize medical therapy is a key feature of the advanced HF state.
Biomarkers
Hyponatremia
Hyponatremia is a simple and long-recognized poor prognostic factor in HF59.
Natriuretic Peptides
Brain-type natriuretic peptide (BNP) and its amino-terminal fragment, NT-proBNP have been shown to predict outcomes in a variety of HF cohorts with higher values conferring increased risk60, although some smaller studies have suggested that natriuretic peptide (NP) activity is exhausted in advanced HF61. As yet, no study has identified a single value of BNP or NT-proBNP that reliably identifies advanced HF 62or discriminates between an acute decompensation versus a progressive down-hill course as underscored by the ADHERE-LM study summarized above32.
Uric acid (UA)
A product of xanthine oxidase (XO) and marker of oxidative stress, UA is elevated in advanced HF patients and has been strongly associated with worsening clinical status and impaired survival63.
Renal dysfunction
Renal dysfunction has repeatedly been demonstrated to be a potent independent predictor of mortality in HF64, 65 and was present in over 50% of advanced HF patients in ADHERE-LM32 (mean creatinine:1.8, mean BUN:42) and REMATCH66 (mean Cr. 1.8).
Other biomarkers reflecting inflammation (C-reactive protein, tumor necrosis factor receptor), neurohormonal activation (norepinephrine, angiotensin II and plasma rennin activity) and myocyte injury (troponin) may be elevated in advanced HF. However these biomarkers are currently not recommended for prognostic purposes in clinical practice. Anemia has long been recognized to be independently associated with adverse HF outcomes67.
Functional capacity
Peak VO2 (<50% of age and sex specific normal values), decreased anaerobic threshold (<10ml/kg/min) and increased VE/VCO2 slope (>34) identifies high-risk HF patients with poor 1 year survival. A 6 minute walk test distance <300 m identifies severe impairment and poor outcomes but may be less reliable in elderly patients or those with comorbidities. Frailty indices may be particularly helpful in the elderly but have yet to be adopted widely in clinical practice.
Risk scores
Seattle Heart Failure Model (SHFM)
The SHFM is a mortality prediction model based on a broad range of clinical, pharmacological, device and laboratory characteristics68 and has been validated in a number of advanced HF cohorts 69, 70, 85. While additional consideration of inotrope or intra-aortic ballon pump use and care in elderly patients may be needed, the SHFM shows promise as an aid in the prospective identification of advanced HF.
Heart Failure Survival Score (HFSS)
Derived in HF patients referred for evaluation of severe HF and/or cardiac transplantation71, the HFSS has since been validated in several cohorts72-75. This model incorporates resting heart rate, mean blood pressure, EF, serum sodium, peak VO2, HF etiology (ischemic vs nonischemic), and intraventricular conduction delay (QRS ≥120 msec). The HFSS stratifies patients into low (HFSS ≥ 8.10), medium (7.20 ≤ HFSS < 8.09) or high (HFSS ≤ 7.19) risk with an average annual VAD or transplant free survival of approximately 87%, 68%, and 44%72-74.
ESCAPE score
The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness Risk Model and Discharge Score (ESCAPE) trial of patients hospitalized with advanced HF24 defined a model with 8 clinical variables: age (>70), BUN(>40 and >90), 6-min walk (<300 m), serum sodium (<130 meq/L), CPR/mechanical ventilation, diuretic dose at discharge (>240mg), absence of beta blocker at discharge and discharge BNP (>500 pg/mmol or >1300 pg/mmol)76. The ESCAPE model was found to identify risk of death in the 6 months following discharge where patients with a score of 0 had a 5% 6-mortality rate and those with a score of 8 hand a 94% 6-mortality rate.
Further stratification of patients with advanced HF
Derived from patients receiving FDA approved mechanical circulatory support devices, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) database77 defines specific subsets within advanced HF patients (Table 4). Subsequent studies have shown the prognostic significance of these profiles for patients undergoing LAVD 78, 79,80 with patients in patient profile 4-7 having significantly better survival and shorter lengths of stay post-implantation compared to those in patient profiles 1-378.
Table 4. INTERMACS Advanced HF Profiles.
|
Profile 1: Critical cardiogenic
shock Patients with life-threatening hypotension despite rapidly escalating inotropic support, critical organ hypoperfusion, often confirmed by worsening acidosis and/or lactate levels. “Crash and burn.” |
|
Profile 2: Progressive
decline Patient with declining function despite intravenous inotropic support, may be manifest by worsening renal function, nutritional depletion, inability to restore volume balance “Sliding on inotropes.” Also describes declining status in patients unable to tolerate inotropic therapy. |
|
Profile 3: Stable but inotrope
dependent Patient with stable blood pressure, organ function, nutrition, and symptoms on continuous intravenous inotropic support (or a temporary circulatory support device or both), but demonstrating repeated failure to wean from support due to recurrent symptomatic hypotension or renal dysfunction “Dependent stability.” |
|
Profile 4: Resting
symptoms Patient can be stabilized close to normal volume status but experiences daily symptoms of congestion at rest or during ADL. Doses of diuretics generally fluctuate at very high levels. More intensive management and surveillance strategies should be considered, which may in some cases reveal poor compliance that would compromise outcomes with any therapy. Some patients may shuttle between 4 and 5. |
|
Profile 5: Exertion
intolerant Comfortable at rest and with ADL but unable to engage in any other activity, living predominantly within the house. Patients are comfortable at rest without congestive symptoms, but may have underlying refractory elevated volume status, often with renal dysfunction. If underlying nutritional status and organ function are marginal, patient may be more at risk than INTERMACS 4, and require definitive intervention. |
|
Profile 6: Exertion
limited Patient without evidence of fluid overload is comfortable at rest and with activities of daily living and minor activities outside the home but fatigues after the first few minutes of any meaningful activity. Attribution to cardiac limitation requires careful measurement of peak oxygen consumption, in some cases with hemodynamic monitoring to confirm severity of cardiac impairment. ”Walking wounded.“ |
|
Profile 7: Advanced NYHA
III A placeholder for more precise specification in future, this level includes patients who are without current or recent episodes of unstable fluid balance, living comfortably with meaningful activity limited to mild physical exertion. |
Epidemiology of Advanced HF
Experts have estimated the prevalence of advanced HF to range from 6% to 25% of the HF population81, 82, 80. With the paucity of contemporary epidemiologic studies specifically assessing the prevalence of advanced HF patients using a uniform definition, these claims are not supported by rigorous data. ADHERE LM, the largest available registry of patients with chronic advanced HF, suggested that roughly 5% of patients with HF have end-stage disease with symptoms refractory to guideline-based medical therapy32 while expert opinion places this number at few hundred thousand Americans81, 83. EPICAL, an observational, community-based, European cohort study of advanced HF patients reported a crude incidence rate of 225 per million, that increases dramatically with increasing age with men more susceptible than women30. Given the exclusion of patients over 80 in EPICAL, this is likely an underestimation and extrapolation to the overall advanced HF population should be done with caution. While the true overall and within HF prevalence of advanced HF remains uncertain, the intense health care utilization and poor outcomes of advanced HF patients establish advanced HF as a major public health burden. While not specifically emphasized in this review, many of the principals related to the recognition and the societal impact of advanced HF are likely also pertinent to patients with HF and preserved EF (HFpEF) although the epidemiology and characteristics of the advanced HFpEF patient are even more poorly characterized.
References
- 1.Redfield MM. Heart failure--an epidemic of uncertain proportions. N Engl J Med. 2002;347(18):1442–1444. doi: 10.1056/NEJMe020115. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 4.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347(18):1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 5.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nature reviews. 8(1):30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 7.Braunwald E. Heart Disease: A Textbook of Cardiovascular Medicine. Fifth. Philadelphia, PA: WB Saunders Company; 1997. pp. 783–801. [Google Scholar]
- 8.Dunlay SM, Shah ND, Shi Q, Morlan B, VanHouten H, Long KH, Roger VL. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 4(1):68–75. doi: 10.1161/CIRCOUTCOMES.110.957225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1(2):91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54(18):1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 16(6):e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohacsi P, Bohm M, Anker S, Dargie H, Brutsaert D, Komajda M. Advanced chronic heart failure: A position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2007;9(6-7):684–694. doi: 10.1016/j.ejheart.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med. 1987;316(23):1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 14.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991;325(21):1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 15.Packer M, O'Connor CM, Ghali JK, Pressler ML, Carson PE, Belkin RN, Miller AB, Neuberg GW, Frid D, Wertheimer JH, Cropp AB, DeMets DL. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group. N Engl J Med. 1996;335(15):1107–1114. doi: 10.1056/NEJM199610103351504. [DOI] [PubMed] [Google Scholar]
- 16.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9–13. [PubMed] [Google Scholar]
- 17.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 18.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 19.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, Bailleul C, Daubert JC. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344(12):873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 20.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 21.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J. Cardiac resynchronization in chronic heart failure. New England Journal of Medicine. 2002;346(24):1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 22.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, Schroeder JS, Liem LB, Hall S, Wheelan K. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289(20):2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 23.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 24.Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294(13):1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 25.Yancy CW, Krum H, Massie BM, Silver MA, Stevenson LW, Cheng M, Kim SS, Evans R. Safety and efficacy of outpatient nesiritide in patients with advanced heart failure: results of the Second Follow-Up Serial Infusions of Nesiritide (FUSION II) trial. Circ Heart Fail. 2008;1(1):9–16. doi: 10.1161/CIRCHEARTFAILURE.108.767483. [DOI] [PubMed] [Google Scholar]
- 26.Nieminen MS, Cleland JG, Eha J, Belenkov Y, Kivikko M, Poder P, Sarapohja T. Oral levosimendan in patients with severe chronic heart failure --the PERSIST study. Eur J Heart Fail. 2008;10(12):1246–1254. doi: 10.1016/j.ejheart.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 28.A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344(22):1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 29.Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE, Darius H, Schulman K, Zannad F, Handberg-Thurmond E, Harrell FE, Jr, Wheeler W, Soler-Soler J, Swedberg K. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST) Am Heart J. 1997;134(1):44–54. doi: 10.1016/s0002-8703(97)70105-4. [DOI] [PubMed] [Google Scholar]
- 30.Zannad F, Briancon S, Juilliere Y, Mertes PM, Villemot JP, Alla F, Virion JM. Incidence, clinical and etiologic features, and outcomes of advanced chronic heart failure: the EPICAL Study. Epidemiologie de l'Insuffisance Cardiaque Avancee en Lorraine. J Am Coll Cardiol. 1999;33(3):734–742. doi: 10.1016/s0735-1097(98)00634-2. [DOI] [PubMed] [Google Scholar]
- 31.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290(19):2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 32.Costanzo MR, Mills RM, Wynne J. Characteristics of “Stage D” heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM) Am Heart J. 2008;155(2):339–347. doi: 10.1016/j.ahj.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–266. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 34.Thohan V. Prognostic implications of echocardiography in advanced heart failure. Current opinion in cardiology. 2004;19(3):238–249. doi: 10.1097/00001573-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Hamdan A, Shapira Y, Bengal T, Mansur M, Vaturi M, Sulkes J, Battler A, Sagie A. Tissue Doppler imaging in patients with advanced heart failure: relation to functional class and prognosis. J Heart Lung Transplant. 2006;25(2):214–218. doi: 10.1016/j.healun.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Independence of restrictive filling pattern and LV ejection fraction with mortality in heart failure: an individual patient meta-analysis. Eur J Heart Fail. 2008;10(8):786–792. doi: 10.1016/j.ejheart.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Agha SA, Kalogeropoulos AP, Shih J, Georgiopoulou VV, Giamouzis G, Anarado P, Mangalat D, Hussain I, Book W, Laskar S, Smith AL, Martin R, Butler J. Echocardiography and risk prediction in advanced heart failure: incremental value over clinical markers. J Card Fail. 2009;15(7):586–592. doi: 10.1016/j.cardfail.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Meluzin J, Spinarova L, Dusek L, Toman J, Hude P, Krejci J. Prognostic importance of the right ventricular function assessed by Doppler tissue imaging. Eur J Echocardiogr. 2003;4(4):262–271. doi: 10.1016/s1525-2167(02)00171-3. [DOI] [PubMed] [Google Scholar]
- 39.Grayburn PA, Appleton CP, DeMaria AN, Greenberg B, Lowes B, Oh J, Plehn JF, Rahko P, St John Sutton M, Eichhorn EJ. Echocardiographic predictors of morbidity and mortality in patients with advanced heart failure: the Beta-blocker Evaluation of Survival Trial (BEST) J Am Coll Cardiol. 2005;45(7):1064–1071. doi: 10.1016/j.jacc.2004.12.069. [DOI] [PubMed] [Google Scholar]
- 40.Lee TH, Hamilton MA, Stevenson LW, Moriguchi JD, Fonarow GC, Child JS, Laks H, Walden JA. Impact of left ventricular cavity size on survival in advanced heart failure. American Journal of Cardiology. 1993;72(9):672–676. doi: 10.1016/0002-9149(93)90883-e. [DOI] [PubMed] [Google Scholar]
- 41.Rihal CS, Nishimura RA, Hatle LK, Bailey KR, Tajik AJ. Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy. Circulation. 1994;90:2772–2779. doi: 10.1161/01.cir.90.6.2772. [DOI] [PubMed] [Google Scholar]
- 42.Deng MC, Gradaus R, Hammel D, Weyand M, Gunther F, Kerber S, Haverkamp W, Roeder N, Breithardt G, Scheld HH. Heart transplant candidates at high risk can be identified at the time of initial evaluation. Transpl Int. 1996;9(1):38–45. doi: 10.1007/BF00336810. [DOI] [PubMed] [Google Scholar]
- 43.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 44.Douglas PS, Morrow R, Ioli A, Reichek N. Left ventricular shape, afterload and survival in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1989;13(2):311–315. doi: 10.1016/0735-1097(89)90504-4. [DOI] [PubMed] [Google Scholar]
- 45.St John Sutton M, Lee D, Rouleau JL, Goldman S, Plappert T, Braunwald E, Pfeffer MA. Left ventricular remodeling and ventricular arrhythmias after myocardial infarction. Circulation. 2003;107(20):2577–2582. doi: 10.1161/01.CIR.0000070420.51787.A8. [DOI] [PubMed] [Google Scholar]
- 46.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25(5):1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 47.Gavazzi A, Berzuini C, Campana C, Inserra C, Ponzetta M, Sebastiani R, Ghio S, Recusani F. Value of right ventricular ejection fraction in predicting short-term prognosis of patients with severe chronic heart failure. J Heart Lung Transplant. 1997;16(7):774–785. [PubMed] [Google Scholar]
- 48.Zornoff LA, Skali H, Pfeffer MA, St John Sutton M, Rouleau JL, Lamas GA, Plappert T, Rouleau JR, Moye LA, Lewis SJ, Braunwald E, Solomon SD. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol. 2002;39(9):1450–1455. doi: 10.1016/s0735-1097(02)01804-1. [DOI] [PubMed] [Google Scholar]
- 49.Ghio S, Recusani F, Klersy C, Sebastiani R, Laudisa ML, Campana C, Gavazzi A, Tavazzi L. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 2000;85(7):837–842. doi: 10.1016/s0002-9149(99)00877-2. [DOI] [PubMed] [Google Scholar]
- 50.Karatasakis GT, Karagounis LA, Kalyvas PA, Manginas A, Athanassopoulos GD, Aggelakas SA, Cokkinos DV. Prognostic significance of echocardiographically estimated right ventricular shortening in advanced heart failure. Am J Cardiol. 1998;82(3):329–334. doi: 10.1016/s0002-9149(98)00344-0. [DOI] [PubMed] [Google Scholar]
- 51.Hansen A, Haass M, Zugck C, Krueger C, Unnebrink K, Zimmermann R, Kuebler W, Kuecherer H. Prognostic value of Doppler echocardiographic mitral inflow patterns: implications for risk stratification in patients with chronic congestive heart failure. J Am Coll Cardiol. 2001;37(4):1049–1055. doi: 10.1016/s0735-1097(00)01211-0. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto T, Oki T, Yamada H, Tanaka H, Ishimoto T, Wakatsuki T, Tabata T, Ito S. Prognostic value of the atrial systolic mitral annular motion velocity in patients with left ventricular systolic dysfunction. J Am Soc Echocardiogr. 2003;16(4):333–339. doi: 10.1016/s0894-7317(02)74537-9. [DOI] [PubMed] [Google Scholar]
- 53.Abramson SV, Burke JF, Kelly JJ, Jr, Kitchen JG, 3rd, Dougherty MJ, Yih DF, McGeehin FC, 3rd, Shuck JW, Phiambolis TP. Pulmonary hypertension predicts mortality and morbidity in patients with dilated cardiomyopathy. Annals of internal medicine. 1992;116(11):888–895. doi: 10.7326/0003-4819-116-11-888. [DOI] [PubMed] [Google Scholar]
- 54.Shah RV, Semigran MJ. Pulmonary hypertension secondary to left ventricular systolic dysfunction: contemporary diagnosis and management. Curr Heart Fail Rep. 2008;5(4):226–232. doi: 10.1007/s11897-008-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel JB, Borgeson DD, Barnes ME, Rihal CS, Daly RC, Redfield MM. Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail. 2004;10(4):285–291. doi: 10.1016/j.cardfail.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 56.Stevenson LW, Tillisch JH, Hamilton M, Luu M, Chelimsky-Fallick C, Moriguchi J, Kobashigawa J, Walden J. Importance of hemodynamic response to therapy in predicting survival with ejection fraction less than or equal to 20% secondary to ischemic or nonischemic dilated cardiomyopathy. Am J Cardiol. 1990;66(19):1348–1354. doi: 10.1016/0002-9149(90)91166-4. [DOI] [PubMed] [Google Scholar]
- 57.Morley D, Brozena SC. Assessing risk by hemodynamic profile in patients awaiting cardiac transplantation. Am J Cardiol. 1994;73(5):379–383. doi: 10.1016/0002-9149(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 58.Stevenson LW, Zile M, Bennett TD, Kueffer FJ, Jessup ML, Adamson P, Abraham WT, Manda V, Bourge RC. Chronic ambulatory intracardiac pressures and future heart failure events. Circ Heart Fail. 3(5):580–587. doi: 10.1161/CIRCHEARTFAILURE.109.923300. [DOI] [PubMed] [Google Scholar]
- 59.Packer M, Medina N, Yushak M. Correction of dilutional hyponatremia in severe chronic heart failure by converting-enzyme inhibition. Annals of internal medicine. 1984;100(6):782–789. doi: 10.7326/0003-4819-100-6-782. [DOI] [PubMed] [Google Scholar]
- 60.Taub PR, Daniels LB, Maisel AS. Usefulness of B-type natriuretic peptide levels in predicting hemodynamic and clinical decompensation. Heart failure clinics. 2009;5(2):169–175. doi: 10.1016/j.hfc.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 61.Miller WL, Burnett JC, Jr, Hartman KA, Henle MP, Burritt MF, Jaffe AS. Lower rather than higher levels of B-type natriuretic peptides (NT-pro-BNP and BNP) predict short-term mortality in end-stage heart failure patients treated with nesiritide. Am J Cardiol. 2005;96(6):837–841. doi: 10.1016/j.amjcard.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 62.Lainscak M, Anker SD. Prognostic factors in chronic heart failure. A review of serum biomarkers, metabolic changes, symptoms, and scoring systems. Herz. 2009;34(2):141–147. doi: 10.1007/s00059-009-3211-z. [DOI] [PubMed] [Google Scholar]
- 63.Bergamini C, Cicoira M, Rossi A, Vassanelli C. Oxidative stress and hyperuricaemia: pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. Eur J Heart Fail. 2009;11(5):444–452. doi: 10.1093/eurjhf/hfp042. [DOI] [PubMed] [Google Scholar]
- 64.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102(2):203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 65.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47(10):1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 66.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 67.Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Anemia and heart failure: a community study. Am J Med. 2008;121(8):726–732. doi: 10.1016/j.amjmed.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113(11):1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 69.Kalogeropoulos AP, Georgiopoulou VV, Giamouzis G, Smith AL, Agha SA, Waheed S, Laskar S, Puskas J, Dunbar S, Vega D, Levy WC, Butler J. Utility of the Seattle Heart Failure Model in patients with advanced heart failure. J Am Coll Cardiol. 2009;53(4):334–342. doi: 10.1016/j.jacc.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 70.Gorodeski EZ, Chu EC, Chow CH, Levy WC, Hsich E, Starling RC. Application of the Seattle Heart Failure Model in ambulatory patients presented to an advanced heart failure therapeutics committee. Circ Heart Fail. 3(6):706–714. doi: 10.1161/CIRCHEARTFAILURE.110.944280. [DOI] [PubMed] [Google Scholar]
- 71.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95(12):2660–2667. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 72.Lund LH, Aaronson KD, Mancini DM. Predicting survival in ambulatory patients with severe heart failure on beta-blocker therapy. Am J Cardiol. 2003;92(11):1350–1354. doi: 10.1016/j.amjcard.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 73.Lund LH, Aaronson KD, Mancini DM. Validation of peak exercise oxygen consumption and the Heart Failure Survival Score for serial risk stratification in advanced heart failure. Am J Cardiol. 2005;95(6):734–741. doi: 10.1016/j.amjcard.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 74.Koelling TM, Joseph S, Aaronson KD. Heart failure survival score continues to predict clinical outcomes in patients with heart failure receiving beta-blockers. J Heart Lung Transplant. 2004;23(12):1414–1422. doi: 10.1016/j.healun.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Lim E, Ali Z, Ali A, Motalleb-Zadeh R, Jackson C, Ong SL, Halstead J, Sharples L, Parameshwar J, Wallwork J, Large SR. Comparison of survival by allocation to medical therapy, surgery, or heart transplantation for ischemic advanced heart failure. J Heart Lung Transplant. 2005;24(8):983–989. doi: 10.1016/j.healun.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 76.O'Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 55(9):872–878. doi: 10.1016/j.jacc.2009.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, Naftel DC, Ulisney K, Desvigne-Nickens P, Kirklin JK. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009;28(6):535–541. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 78.Boyle AJ, Ascheim DD, Russo MJ, Kormos RL, John R, Naka Y, Gelijns AC, Hong KN, Teuteberg JJ. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant. 30(4):402–407. doi: 10.1016/j.healun.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 79.Alba AC, Rao V, Ivanov J, Ross HJ, Delgado DH. Usefulness of the INTERMACS scale to predict outcomes after mechanical assist device implantation. J Heart Lung Transplant. 2009;28(8):827–833. doi: 10.1016/j.healun.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 80.Lund LH, Matthews J, Aaronson K. Patient selection for left ventricular assist devices. Eur J Heart Fail. 12(5):434–443. doi: 10.1093/eurjhf/hfq006. [DOI] [PubMed] [Google Scholar]
- 81.Miller LW. Left ventricular assist devices are underutilized. Circulation. 123(14):1552–1558. doi: 10.1161/CIRCULATIONAHA.110.958991. [DOI] [PubMed] [Google Scholar]
- 82.Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002;287(5):628–640. doi: 10.1001/jama.287.5.628. [DOI] [PubMed] [Google Scholar]
- 83.Gheorghiade M, Cody RJ, Francis GS, McKenna WJ, Young JB, Bonow RO. Current medical therapy for advanced heart failure. Heart Lung. 2000;29(1):16–32. doi: 10.1016/s0147-9563(00)90034-7. [DOI] [PubMed] [Google Scholar]
- 84.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–1549. 84. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 85.Levy WC, Mozaffarian D, Linker DT, Farrar DJ, Miller LW. Can the Seattle heart failure model be used to risk-stratify heart failure patients for potential left ventricular assist device therapy? J Heart Lung Transplant. 2009;28(3):231–236. doi: 10.1016/j.healun.2008.12.015. [DOI] [PubMed] [Google Scholar]