Abstract
Our aim was to review the use of high-dimensional biology techniques, specifically transcriptomics, proteomics, and metabolomics, in amniotic fluid to elucidate the mechanisms behind preterm birth or assessment of fetal development. We performed a comprehensive MEDLINE literature search on the use of transcriptomic, proteomic, and metabolomic technologies for amniotic fluid analysis. All abstracts were reviewed for pertinence to preterm birth or fetal maturation in human subjects. Nineteen articles qualified for inclusion. Most articles described the discovery of biomarker candidates, but few larger, multicenter replication or validation studies have been done. We conclude that the use of high-dimensional systems biology techniques to analyze amniotic fluid has significant potential to elucidate the mechanisms of preterm birth and fetal maturation. However, further multicenter collaborative efforts are needed to replicate and validate candidate biomarkers before they can become useful tools for clinical practice. Ideally, amniotic fluid biomarkers should be translated to a noninvasive test performed in maternal serum or urine.
Keywords: amniotic fluid, systems biology, proteomics, transcriptomics, preterm birth
Introduction
In 2005, the March of Dimes Scientific Advisory Committee published its research agenda to address the complex public health problem of preterm birth.1 Of the 4 themes recommended for research on preterm birth, the committee emphasized defining the etiologic mechanisms responsible for preterm birth and identifying biomarkers for preterm birth, which could improve clinical risk assessment.1 Indeed, the committee recognized that successful interventions against preterm birth would likely need to address multiple risk factors and be specifically tailored to individuals or groups.1
Currently, high-dimensional biology or “systems biology” allows for simultaneous study of changes within an organism or biological sample to understand the pathophysiology or mechanisms of disease.2,3 Complex biological processes can result in signature effects in the genome (DNA), transcriptome (messenger RNA [mRNA]), proteome (proteins), or metabolome (metabolites). Thus, these new “–omic” discovery platforms enable us to advance discovery and generate broader hypotheses regarding overall mechanisms than would be possible from study of singular proteins or genes by themselves.4 Analysis with an unbiased approach allows for candidate biomarkers to reveal their significance, after which properly designed, follow-up targeted studies can then be conducted to replicate and validate these candidate biomarkers, and if validated, translate them into clinical tests.5 Based on this comprehensive and systematic process, the development of improved prenatal diagnostics and therapeutics regarding fetal development and well-being becomes a real possibility and may further aid in the assessment and prediction of many abnormal fetal and maternal states, including preterm birth.
Amniotic fluid obtained at different time points in pregnancy can be examined to provide obstetricians and pregnant women with important information for decision making about pregnancy management and delivery planning such as midtrimester screening for aneuploidy, diagnostic testing for intra-amniotic infection, or fetal lung maturity testing.6–8 As a dynamic and complex mixture that reflects the physiologic state of the developing fetus,9 amniotic fluid is an underutilized resource for assessing fetal status and development.
Ultimately, the goal is to advance the understanding of fetal well-being by the development of noninvasive fetal testing by sampling maternal serum or urine and to avoid invasive tests such as amniocentesis. However, for discovery purposes, amniotic fluid has important advantages over other maternal specimens. First, amniotic fluid contains a larger amount of cell-free fetal- and pregnancy-related DNA, RNA, and proteins than maternal serum, particularly in the first and second trimester, when most prenatal screening is performed.10–13 Over 80% of the circulating fetal DNA fragments in maternal serum are short. Therefore, highly sensitive methods of detection are needed to distinguish the small size and quantity of fetal DNA from maternal DNA in maternal serum samples. Indeed, most fetal DNA measurement methods using maternal serum rely on quantifying Y-specific sequences and can only be used when the fetus is male.11
Therefore, the aim of this article is to review the application of high-dimensional biologic techniques in the analysis of amniotic fluid for the discovery of novel biomarkers about fetal well-being that help obstetricians better manage the timing of delivery. Given that purpose, we focused on 2 categories of articles: (1) those that predict preterm birth and (2) those that assess fetal maturation. A better understanding of timing or mechanisms of spontaneous preterm birth will help obstetricians to target its prevention or treatment with tocolytics, whereas a better understanding of fetal maturation will aid obstetricians to improve the timing and potentially the pretreatment of scheduled deliveries in order to avoid neonatal morbidity. As the use of these systems biology technologies begins to increase exponentially, our hope is to have better analytical tools to aid obstetricians and pregnant women in making pregnancy and delivery decisions that will improve the care of newborns and their outcomes.
Methods
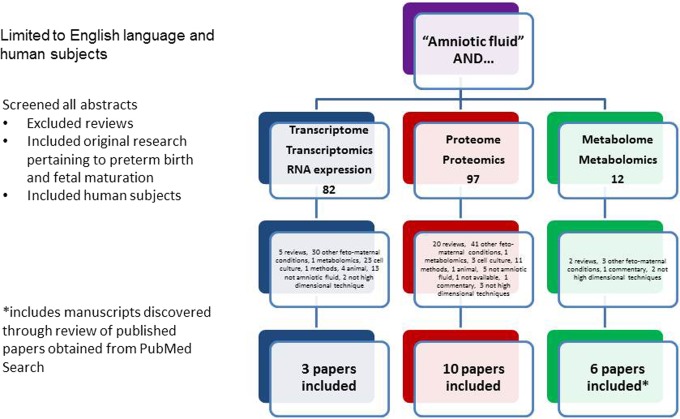
On January 18, 2013, we conducted a MEDLINE search with the key words “amniotic fluid,” coupled with the various high-dimensional techniques of interest, focusing on “transcriptomics,” “metabolomics,” and “proteomics.” We added additional search terms including “RNA expression,” “proteome,” “transcriptome,” and “metabolome.” The search was limited to original research in humans and in English. The abstracts were reviewed to determine that the manuscript detailed the use of a high-dimensional technique in amniotic fluid that provided information on spontaneous preterm birth (spontaneous preterm labor or premature rupture of membranes) or fetal maturation. We excluded manuscripts that were reviews, that described experiments in cell culture or animal models, or that covered other fetomaternal conditions besides preterm birth or fetal maturation. Articles that discussed biomarker discovery in amniotic fluid, particularly to test a known potential candidate biomarker, but that did not employ a high-dimensional technique were also excluded. The bibliographies of the included articles were reviewed to capture other manuscripts not discovered by the original search. In the end, the number of articles that described high-dimensional techniques in amniotic fluid to predict preterm birth or fetal maturity included 3 on transcriptomics, 10 on proteomics, and 6 on metabolomics (Figure 1). These articles were categorized by high-dimensional technique used, study design, and biomarkers found (Table 1).
Figure 1.
Algorithm of PubMed search.
Table 1.
Studies Included in the Review.
| Author | Technique Used | Study Design | Sample Size | Study Type (Discovery, Validation, Replication) | Condition Studied | Summary (Biomarkers Identified or Condition Elucidated) |
|---|---|---|---|---|---|---|
| Transcriptomics | ||||||
| Hui et al12 | Transcriptomics | Cross-sectional | 12 | Discovery | Fetal maturation | 476 well-annotated genes present in 12 samples of midtrimester amniotic fluid samples, representing 6 physiologic systems. 23 highly organ-specific transcripts were identified, 6 of which are highly expressed in fetal brain. |
| Larrabee et al14 | Transcriptomics | Case–control | 5 (4 cases, 1 pooled control) | Discovery | Fetal maturation | Four samples of cell-free amniotic fluid from pregnant women 20-32 weeks gestation undergoing amnioreduction for twin-to-twin transfusion syndrome were compared to a control sample of 6 pooled amniotic fluid samples from women 17-18 weeks who underwent genetic amniocentesis. Relative mRNA expression seen by microarray showed developmental transcripts such as surfactant proteins, mucins, and keratins changed with advancing gestational age. |
| Massingham et al15 | Transcriptomics | Cross-sectional | 19 | Discovery | Fetal maturation | Transcript abundance of 21 genes was measured with a previously developed standardized nanoarray PCR (SNAP). Two genes were significantly increased in females compared to males (MTOR and STAT2). Five other genes were differentially expressed by advancing gestational age (ANXA5, GUSB and PPIA decreased over time; CASC3 and ZNF264 increased over time). |
| Proteomics | ||||||
| Buhimschi et al16 | Proteomics | Cross-sectional | For discovery, 77; For validation, 24 | Discovery, Validation | Preterm birth | 77 amniotic fluid samples (17 term and 60 preterm with/without IAI) were evaluated for relevant proteomic patterns. Women who delivered preterm and with IAI had a distinctive proteomic profile of with differing levels of neutrophil defensins 1 and 2 and calgranulins A and C.16 The authors devised a mass restricted (MR) score ranging from 0 (all biomarker peaks absent) to 4 (all biomarker peaks present), where an MR score > 2 was associated with imminent preterm delivery with 100% sensitivity and 95% specificity when validated with blind testing of 24 samples with unknown outcomes. |
| Buhimschi et al17 | Proteomics | Prospective cohort | 286 | Discovery | Preterm birth | The AF analyzed from women presenting with preterm labor or premature rupture of membranes (PROM). A Q-profile was developed in the presence of 5 SELDI peaks. Women with the Q-profile were more likely to delivery preterm despite expectant management. |
| Bujold et al18 | Proteomics | Retrospective cross-sectional | 258, combined into 3 pools of 86 samples each | Discovery | Preterm birth, with and without IAI | Amniotic fluid obtained from 3 groups of women with preterm labor and intact membranes (1) women without IAI who delivered at term, (2) women without IAI who delivered preterm, and (3) women with IAI who delivered preterm. IGFBP-1 fragments were present in patients with IAI. Retinol-binding protein was overexpressed in women who delivered preterm, regardless of presence of IAI. Proteins overexpressed in each group were noted. |
| Gravett et al19 | Proteomics | Prospective cohort | 33 (11 from each group) | Discovery, validation | Preterm birth | Proteomic profiling performed in an experimental model of chorioamnionitis in rhesus monkeys, and then the biomarkers used to predict subclinical IAI in women with preterm labor were identified. Three groups were analyzed: (1) patients with subclinical IAI, (2) preterm birth without IAI, and (3) neither preterm birth nor IAI. Calgranulin B and IGFBP-1 fragment noted as possible biomarkers from AF for early detection of IAI and were also confirmed in maternal serum. |
| Queloz et al20 | Proteomics | Cross-sectional | 14 (7 at 17 week gestation, 7 at 40 weeks gestation) | Discovery | Fetal maturation | Amniotic fluid proteins were seen in differential abundance earlier in pregnancy compared to term. Amniotic fluid at each of these time points could be identified by its protein pattern. Of the differentially expressed protein spots, 7 were identified by tandem mass spectrometry and corresponded to 5 different gene products. |
| Romero et al21 | Proteomics | Cross-sectional | For discovery, 88 (44 term, 44 preterm with IAI); for validation, 31 (15 term, 16 preterm with IAI) | Discovery, validation | Preterm birth | The authors used SELDI-TOF to identify the proteomic profile of the women in the discovery cohort who either had spontaneous preterm labor without IAI and delivered at term or who had IAI and delivered preterm. Using this profile, they classified the women in the validation cohort accurately 90.3% of the time. |
| Romero et al4 | Proteomics | Cross-sectional | 55 | Discovery | Preterm birth | Three groups examined: (1) preterm labor (PTL) who delivered at term, (2) PTL without IAI who delivered preterm, and (3) PTL with IAI who delivered preterm. 82 proteins were differentially expressed in these 3 clinical subgroups of PTL, 67 of which were undescribed previously. |
| Rüetschi et al22 | Proteomics | Cross-sectional | 27 (14 with preterm labor, 13 with PPROM) | Discovery | Preterm birth | Amniotic fluid of singleton pregnancies <34 weeks with either preterm labor (n = 14) or PPROM (n = 13) was examined. Based on levels of IL-6, 7 women with preterm labor and 7 women with PPROM were diagnosed with IAI. Using SELDI-TOF mass spectrometry, they found that 17 proteins were overexpressed in the amniotic fluid of women with IAI, in particular, women with preterm labor than those women with PROM. 5 of these proteins were identified as human neutrophil proteins 1-3 (a family of cationic antimicrobial peptides) and calgranulins A and B. |
| Tambor et al23 | Proteomics | Cross-sectional, prospective cohort | For discovery, 2 pools of 19 samples each. For validation, 63 samples without microbial invasion nor histologic chorioamnionitis, 40 samples with both conditions | Discovery, validation, replication | Preterm birth | Women with PPROM were recruited. Two pools of 19 samples were formed from women who were positive for bacterial or urea/mycoplasma in amniotic fluid and had histologic chorioamnionitis, compared to women who had neither. 99 proteins were significantly altered. Three distinct histone proteins showed the highest concentration change, followed by cathelicidin and myeloperoxidase. ELISA was then used to assess cathelicidin each individual sample of the 2 groups and was significantly higher in the women with IAI. The amniotic fluid of 63 women without microbial invasion nor histologic chorioamnionitis was compared to that of 40 women with both, and the cathelicidin levels were significantly higher in women with infection. |
| Vuadens et al24 | Proteomics | Cross-sectional | 16 | Discovery | Preterm birth | The AF and blood samples were obtained at term from women undergoing cesarean section. 27 spots were found on gels of amniotic fluid not present in maternal plasma. 9 of these were also seen on comparison amniotic fluid obtained at 17 weeks gestation. 3 spots corresponded to fragments of plasma proteins, and 2 were fragments of proteins not present in plasma (agrin and perlecan). |
| Metabolomics | ||||||
| Bock25 | Metabolomics | Cross-sectional | 70 amniotic fluid samples from 66 patients | Discovery, validation | Fetal maturation | Ten different metabolites (lactate, glycine, glucose, choline, creatinine, citrate, succinate, glutamate, acetate, and alanine) to characterize whether delivery occurred in second or third trimester (immature/transitional/mature). |
| Clifton et al26 | Metabolomics | Cross-sectional | 3 patients at different time points | Discovery | Fetal maturation | Amniotic fluid from second trimester, third trimester (with immature S/A ratio) and third trimester (with mature S/A ratio). Increasing choline/creatinine ratio was observed between second and third trimester samples and between samples with low and high S/A ratios. |
| Cohn et al27 | Metabolomics | Cross-sectional | 50 | Discovery, validation | Fetal maturation | In all, 23 second trimester and 27 third trimester amniotic fluid samples were analyzed with high-resolution magic angle spinning spectroscopy. 16 of 21 metabolite concentrations representing sugars, amino acids, components of the Krebs cycle, and biomarkers of renal function were seen between trimesters. Stepwise linear regression applied to the 50 samples showed that gestational age could be accurately predicted using a combination of alanine, glucose, and creatinine concentrations. |
| Graça et al8 | Metabolomics | Case–control | 192 (12 for preterm birth, 34 for PROM) | Discovery | Preterm birth | Six groups were analyzed with NMR: controls (n = 82), fetal malformations (n = 27), prediagnostic gestational diabetes (n = 27), preterm delivery (n = 12), premature rupture of membranes/PPROM (n = 34), and genetic abnormalities (n = 10). Differences in metabolite levels of alanine, allantoin, citrate, and myo-inositol were noted in the preterm birth group, whereas differences in glutamine, methionine, and threonine were noted in the premature rupture of membranes group. |
| Graça et al28 | Metabolomics | Case–control | 37 (11 preterm birth, 26 controls) | Discovery | Preterm birth | Eleven samples of amniotic fluid and maternal urine obtained simultaneously in the second trimester from women who delivered preterm <37 weeks and compared them to 26 controls to look for biomarkers predictive of preterm birth. Using ultra performance liquid chromatography and mass spectrometry, and their previously acquired NMR data, they found decrease in particular amino acids (leucine/isoleucine, histidine, methionine, phenylalanine, and valine) and an increase in hexose in those women who eventually delivered preterm. |
| Romero et al29 | Metabolomics | Retrospective cross–sectional | For discovery 55; for validation, 113 | Discovery, validation | Preterm birth, with and without IAI | Three groups examined: (1) preterm labor (PTL) who delivered at term, (2) PTL without IAI who delivered preterm, and (3) PTL with IAI who delivered preterm. Metabolomic profiling able to identify group 96.3% of time and validated in larger group of 113 patients 88.5% of time. |
Abbreviations: ANXA5, annexin A5; CASC3, cancer susceptibility candidate 3; ELISA, enzyme-linked immunosorbent assay; GUSB, glucoronidase β; IAI, intra-amniotic infection; IGFBP-1, insulin-like growth factor-binding protein 1; mRNA, messenger RNA; MTOR, mechanistic target of rapamycin; NMR, nuclear magnetic resonance; PCR, polymerase chain reaction; PPIA, peptidyl isomerase A; PPROM, preterm premature rupture of membrane; S/A, surfactant/albumin; SELDI-TOF, surface-enhanced laser desorption ionization time-of-flight; STAT2, signal transducer and activator of transcription 2; ZNF264; zinc finger protein 264.
Results
Transcriptomics
The transcriptome is the full complement of mRNA in a cell or tissue at any given moment and forms the template for protein synthesis, resulting in a corresponding protein complement or proteome.3 Keller et al30 demonstrated that exosomes, small membrane vesicles found in various body fluids, including amniotic fluid, saliva, urine, and plasma, contain proteins and mRNA that can be analyzed with high-dimensional techniques. In fact, amniotic fluid supernatant is a rich source of fetal cell-free DNA and RNA31 and contains 100- to 200-fold more fetal nucleic acids for analysis than maternal plasma.
The transcriptome can be studied using 1 of 2 main techniques: microarray and next generation sequencing.32 With microarray, samples of complementary RNA are prepared, end labeled, and hybridized to chips that contain oligonucleotide probes designed to capture specific transcripts.32 Outcomes are measured based on the level of fluorescence signal from each probe,32 and once important transcripts are identified, they can be analyzed with a more focused platform such as quantitative reverse transcriptase polymerase chain reaction (qRT-PCR).15 However, problems can arise if the a priori knowledge needed to design probes is lacking, such that transcripts can be missed if not in known databases.32,33 Next generation sequencing seeks to overcome this problem; it involves a flow cell platform that acts as a substrate to perform sequencing on millions of RNA fragments.32 Thus, the entire transcriptome of a sample can be sequenced to provide accurate quantification of the relative levels of transcripts present through mapping of reads to reference sequences.33 Most RNA-sequencing systems are similar in that they require fragmentation and adaptor ligation prior to sequencing runs; however, they differ in how the runs are performed and what kinds of data are collected.33 Although an extremely promising method for transcriptome analysis, RNA sequencing also has problems with the amplification of target sequences prior to sequencing runs, as these amplifications can result in errors, creation of variation, and decoupling of linear relationships between output and level of expression.33
Our search yielded 3 articles on the use of transcriptome analysis for the discovery of biomarkers for fetal maturation or prediction of preterm birth. Larrabee et al14 did a pilot study on four 10-mL samples of cell-free amniotic fluid obtained from pregnant women between 20 and 32 weeks gestation who underwent amnioreduction for twin-to-twin transfusion syndrome. They compared these samples to a pooled control sample from 17 to 18 weeks gestation from mothers undergoing routine genetic amniocentesis for advanced maternal age.14 Cell-free fetal mRNA in amniotic fluid hybridized to gene expression arrays revealed that fetal gene expression is dynamic and changes over the course of gestation. For example, some developmental transcripts, such as surfactant proteins, keratins, and mucins, were significantly upregulated with increasing gestational age.14
Hui et al,12 using microarray analysis, identified the transcripts present in the euploid midtrimester amniotic fluid supernatant of 12 fetuses (median gestational age 18 weeks) and were able to identify 476 organ-specific genes. The investigators then compared those organ-specific genes to gene expression profiles from 78 normal human tissues that had already been catalogued in a publicly available atlas.12 They discovered 6 physiologic systems development and function pathways, and in particular that biological processes associated with nervous system development and function were overrepresented.12 The presence of lung gene transcripts for surfactant proteins B and C were also noted, and interesting given the increased role of pulmonary fluid contributing to the population of amniotic fluid in the latter part of pregnancy. The authors noted limitations of the study to be a lack of transcripts found showcasing renal system development, which they felt highlighted an inherent bias in the existing publicly available atlas: the atlas depended on the range of tissues sampled and the variable number of organ-specific genes for each organ profiled.12 Still, the data are promising for a better understanding of fetal organ maturation to help clinicians time delivery in order to avoid neonatal morbidity due to prematurity.
Finally, Massingham et al15 performed a feasibility study involving 19 amniotic supernatant samples obtained from euploid fetuses between 15 and 20 weeks of gestation. To evaluate fetal organ system function, transcript abundance of 21 genes was measured by standardized nanoarray PCR, which utilizes the sensitivity and specificity of qRT-PCR and array technology for the simultaneous analysis of up to 3072 genes.15 Two genes, mechanistic target of rapamycin (MTOR) and signal transducer and activator of transcription 2 (STAT2), had significantly higher expression levels in female than in male fetuses.15 Five genes in the panel were statistically, significantly, and differentially expressed as a function of increasing gestational age. Three genes showed decreasing gene expression: annexin A5 (ANXA5), glucoronidase β, and peptidyl isomerase A (PPIA). The 2 genes showing increasing expression were cancer susceptibility candidate 3 and zinc finger protein 264.15 Further investigation using this gene panel may give insights into fetal maturation, and as 4 of the genes (STAT2, MTOR, ANXA5, and PPIA) are involved in immunologic functions, the authors speculate that this gene panel may also contribute to greater understanding of the complex immune pathways in the maternal–fetal relationship.15
Proteomics
An advantage of proteomics is a better understanding of the diversity of proteins produced after complex posttranslational modification by phosphorylation, glycosylation, acylation, methylation, or proteolytic cleavage.34 Proteomics relies on a prefractionation method to detect low-abundance proteins, most frequently by gel electrophoresis or liquid chromatography.35 However, gel electrophoresis has a limited ability to analyze very basic or acidic proteins, very large or very small proteins, or low abundant species.32 Therefore, mass spectrometry is the most widely used powerful tool for multidimensional protein identification techniques. Mass spectrometry-based proteomics is characterized as either “top-down” or “bottom-up.”36 In the “top-down” method, intact proteins are characterized directly via mass spectrometry.4 However, “top-down” analyses are challenged by separating complex protein mixtures for identification of specific proteins/peptides.36 In the “bottom-up” approach, frequently used with liquid chromatography and also with mass spectrometry, proteins are first digested and the resulting peptides are then analyzed to identify and quantify the parent proteins, which can be a more difficult task.4 Mass spectrometry has advantages for the analysis of amniotic fluid, providing sensitivity, speed, resolution, and huge amounts of data to be analyzed with advanced bioinformatics, where the large-scale characterization of the entire protein complement can be revealed.5,37 In particular, surface-enhanced laser desorption ionization time-of-flight (SELDI-TOF) mass spectrometry has the ability to analyze samples with minimal preprocessing, improved ease of handling, and high throughput.38
Currently ongoing investigations on proteomics profiling of amniotic fluid are leading to the discovery of biomarkers that have the potential to aid in prenatal diagnosis of aneuploidy or other fetal abnormalities,39,40 early detection of abnormal pregnancy states, such as intra-amniotic infection or premature labor,16,29,41 and perinatal inflammation/neonatal sepsis.42 Our review found 10 articles that described the possible amniotic fluid biomarkers for the prediction of preterm birth or fetal maturation.
Using 2-dimensional fluorescence difference gel electrophoresis and mass spectrometry, Queloz et al20 showed that amniotic fluid proteins were seen in differential abundance earlier in pregnancy (17 weeks) compared to term, and that amniotic fluid at each of these time points could be identified by its protein pattern. Of the differentially expressed protein spots, 7 were identified by tandem mass spectrometry and corresponded to 5 different gene products.20 Given the changes in proteomic profile throughout pregnancy, the authors recognized that future studies of proteomic markers would require age-matched controls.
Buhimschi et al17 performed proteomics analysis of 286 fresh amniotic fluid samples obtained from women who presented with signs or symptoms of preterm labor or premature rupture of membranes. They initially screened and detected biomarkers characteristic of inflammation and bleeding in 32% of the mass spectrometry tracings, through methodologies previously described by their group.16,43 In the remaining tracings, they used a hierarchical algorithm to quantify the similarities/dissimilarities among proteomic fingerprints, which allowed identification of a unique “Q-profile” in 32 patients, based on the presence of 5 peaks in the area of 10 to 12.5 kDa mass.17 Women displaying the Q-profile were more likely to deliver preterm despite expectant management, when compared to women who had other proteomic fingerprints for inflammation, bleeding, inflammation and bleeding, or none.17 A comparison of the differential expression of proteins in women with the Q-profile to matched reference women showed 60 spots of downregulation and 28 spots of upregulation, of which 17 distinct protein database matches appeared and were differentially regulated at least 1.5-fold between the 2 groups.17 The authors used the Protein Analysis Through Evolutionary Relationships database to positively identify 10 protein products belonging to 7 distinct and well-classified biological processes, including blood circulation and gas exchange, homeostasis, lipid and fatty acid metabolism, protein metabolism, sensory perception, signal transduction, and transport.17 The authors felt that understanding the distinct physiologic pathways toward preterm birth would enable a “pathway-specific-targeted treatment approach”17 that would allow for more personalized treatment of the patient in preterm labor.
Although not a primary focus of this review, intra-amniotic infection (IAI)/inflammation potentially plays an important role in preterm birth. Approximately 20% of patients with preterm labor have concomitant IAI,44 decreasing latency time until delivery with an increasing rate of adverse neonatal outcomes. Proteomic analysis of amniotic fluid for infection may provide important links to the mechanism and consequences of inflammation in preterm birth plus the ability for earlier detection and intervention against preterm birth, particularly if a test can be developed to detect the infection prior to the onset of clinical symptoms, often a late finding.16,19,21,22,45,46 Although we excluded manuscripts that described high-dimensional techniques in relation to uniquely diagnosing IAI, we did include manuscripts that included assessment of inflammation as a risk factor for preterm birth. For example, Gravett et al19 identified candidate biomarkers for IAI first in rhesus monkeys, then replicated the study in women with preterm labor with and without IAI. The investigators found that a distinct proteomic profile identified in the primate infection model detected subclinical IAI in the human cohorts with preterm labor.19 Differentially expressed amniotic fluid proteins between cases and controls included calgranulin B and an 11-kDa fragment of insulin-like growth factor-binding protein 1 (IGFBP-1); these proteins were also demonstrated to be differentially expressed in maternal serum, raising potential for a noninvasive diagnostic test for early detection of IAI.19
Rüetschi et al22 evaluated the amniotic fluid of women with singleton pregnancies who presented <34 weeks with either preterm labor (n = 14) or preterm premature rupture of membrane (PPROM; n = 13). Based on levels of IL-6, 7 women with preterm labor and 7 women with PPROM were diagnosed with IAI. Using SELDI-TOF mass spectrometry, they found that 17 proteins were overexpressed in the amniotic fluid of women with IAI, in particular women with preterm labor than those women with PROM. Five of these proteins were identified as human neutrophil proteins 1 to 3 (a family of cationic antimicrobial peptides) and calgranulins A and B.22 These findings correlated well with those of Buhimschi et al16 for identification of IAI, where the authors studied 77 amniotic fluid samples (17 term and 60 preterm with various levels of IAI based on high white blood cell count ± positive amniotic fluid culture result) to unearth relevant proteomic patterns. Using SELDI-TOF and mass-restricted analysis to extract the relevant biomarkers from SELDI tracings, women who delivered preterm and with IAI had a distinctive proteomic profile in relation to proteins neutrophil defensins 1 and 2 and calgranulins A and C.16 Based on the presence or absence of these biomarkers, they devised a mass restricted (MR) score ranging from 0 (all biomarker peaks absent) to 4 (all biomarker peaks present), where an MR score > 2 was associated with imminent preterm delivery with 100% sensitivity and 95% specificity, when validated with blind testing of 24 samples with unknown outcomes.16
Romero and collaborators also sought to identify the novel biomarkers or patterns of markers indicative of preterm labor with IAI and have subsequently published a number of articles on their work. He and his team performed a cross-sectional study of 44 women with spontaneous preterm labor and intact membranes who delivered at term, compared to 44 women who delivered preterm with IAI.21 They used multiple SELDI-TOF analyses followed by a computational approach to produce a proteomic “fingerprint” for each patient.21 Next, they constructed an empirical model to classify patients into 2 groups with a cross-validation approach based upon the boot-strapping procedure and then attempted to generalize the model by performing a validation analysis in 15 patients who delivered at term and 16 women who delivered preterm with IAI.21 In the validation phase, the investigators were able to identify 14 of the women who delivered at term, and 14 of the women who delivered preterm with IAI, with an accuracy of 90.3%.21
Bujold et al18 performed a cross-sectional study using banked samples of amniotic fluid from 3 groups of women with preterm labor, with the main goal of using proteomic methodologies to define proteins that were differentially expressed in each group. The groups of women with preterm labor were (1) those who eventually delivered at term without IAI, (2) those who delivered preterm (<37 weeks) without IAI, and (3) those who had preterm labor and preterm delivery with IAI. Eighty-six amniotic fluid samples from each group were pooled and examined. Two-dimensional chromatography followed by enzyme-linked immunosorbent assay (ELISA) and SELDI-TOF mass spectrometry was used to identify the proteins that were differentially expressed.18 In particular, the authors found that retinol-binding protein was overexpressed in women who delivered preterm, regardless of the presence of IAI.18 In addition, in patients with IAI, the presence of IGFBP-1 fragments was increased, with a decrease in intact IGFBP-1, as also seen by Gravett et al above.19
Romero et al4 examined women who presented with preterm labor grouped into the same classifications as the Bujold et al18 study above. Using liquid chromatography-tandem mass spectrometry with isobaric tagging for relative and absolute quantification techniques, the investigators identified 309 unique differentially expressed proteins between the 3 groups, using the IPI.Human database, and characterized them by gene ontology terms. In the case of preterm delivery without IAI specifically, they found 4 upregulated proteins (resistin, thymosin-like 3, antileukoproteinase, and lactoferrin) and 2 downregulated proteins (latent-transforming growth factor β-binding isoform 1L and Mimecan precursor).4 For those patients who delivered preterm in the presence of IAI, 4 gene ontology biological processes were represented: host defense, antiapoptosis, metabolism/catabolism, and finally, mobility/localization/targeting.4 In particular, proteins directing actin cytoskeleton organization biogenesis and protein targeting were found in abundance and may implicate certain physiologic processes in the onset of preterm birth.4
Premature rupture of membranes (PROM) before the onset of labor comprises one type of spontaneous preterm birth. Although its mechanisms are unclear, PROM is associated with increased risk of intra-amniotic inflammation and preterm birth. Therefore, biomarkers toward the prediction of PROM and better understanding of the mechanism by which it occurs may also increase our understanding of preterm birth. Vuadens et al24 performed a 2-dimensional polyacrylamide gel electrophoresis of amniotic fluid from women with either preterm or term premature rupture of membranes (PROM) to identify the characteristics of membrane rupture in amniotic fluid and in comparison with maternal plasma. Five proteins were differentially expressed between the groups, and 2 were identified as potential markers of PROM, because they were present only in amniotic fluid and absent in maternal serum.24,47 These 2 markers, agrin and perlecan, may play a role in the regulation of synapse development and remodeling of bone tissue, respectively, and may generate new hypotheses for the mechanisms of preterm birth and PROM.24,47,48 Building upon that work, Queloz et al20 showed that agrin and perlecan were seen in greater concentration in amniotic fluid than plasma, and that amniotic fluid proteins, when added to plasma, were able to be detected when they represented at least 10% of the total protein loaded.
With the thought to better identify women who might mount an intra-amniotic inflammatory response that could subsequently cause significant neonatal morbidity, Tambor et al23 recruited women with PROM to determine proteomic differences in the amniotic fluid of 19 women with histologic chorioamniotis (HCA) and microbial invasion of the amniotic cavity (MIAC), compared to 19 women who had neither. With 2 pools of 19 samples from each group, they found that 99 proteins were significantly altered between the groups. Three distinct histone proteins showed the highest concentration change, followed by cathelicidin and myeloperoxidase.23 Using an ELISA to test for cathelicidin in individual samples, cathelicidin was found to be significantly higher in the women with infection. To validate their findings, they then examined the amniotic fluid of 63 women without HCA or MIAC compared to that of 40 women with both, and the cathelicidin levels were significantly higher in women with infection.23 They found the threshold of 4.0 ng/mL to be the best cutoff point in both the discovery and the validation cohorts for the identification of women with infection.23 In the validation cohort, this cutoff showed a sensitivity of 48%, a specificity of 90%, an odds ratio of 11.6, a likelihood ratio of 5.0, and an area under the curve of 71% for the prediction of women with infection.23
Metabolomics
Metabolomics refers to the study of individual metabolic profiles and their changes over time due to disease, toxicity, or other effects.49 Specific physiological states, gene expression, or other stimuli may cause changes in homeostasis, which can be reflected in the metabolic composition of different biologic compartments.50 As the metabolites analyzed are downstream from gene expression and protein synthesis, metabolomics analysis may more closely represent what is going on at a functional level.29 The most commonly used analytic tools for obtaining metabolic profiles include mass spectrometry or nuclear magnetic resonance (NMR) spectroscopy.50 These tools can detect the estimated 2000 to 20 000 metabolites of different biologic pathways and may include amino acids, oligopeptides, sugars, steroids, biliary acids, simple and complex fatty acids, and other intermediary compounds.51 The NMR spectroscopy obtains information on the structure of the molecules through interaction with electromagnetic radiation, where oscillating currents generated by the perturbation of the system with a magnetic field produces an interferogram in which intensities are related to emission times, thus allowing for qualitative and quantitative study of complex mixtures.51 Advantages of this technique include high reproducibility, small sample volumes, short measuring times, and low cost of analysis, while disadvantages include high instrument costs and relatively low sensitivity to molecules present in small amounts.51
In an attempt to establish normative metabolite concentrations between the second and the third trimesters, Cohn et al27 analyzed 23 samples of amniotic fluid in the second trimester (obtained for karyotype analysis) and 27 samples from the third trimester (obtained for fetal lung maturity testing). Using high-resolution magic angle spinning spectroscopy, they compared 21 metabolite concentrations between the second and the third trimester and found differences in 16 that represented sugars, amino acids, components of the Krebs cycle, and biomarkers for renal function.27 In particular, they found increases in betaine and creatinine and decrease in numerous amino acids, including alanine, glutamate, isoleucine, leucine, lysine, and valine.27 After choosing 5 selected metabolites thought to be related to fetal lung and renal maturity, the investigators used stepwise linear regression applied to 50 samples to show that gestational age could be accurately predicted using a combination of alanine, glucose, and creatinine concentrations.27
In studies of second trimester amniotic fluid, Graça et al8,49 showed differences in the NMR metabolomic profile of pregnant women who eventually had preterm delivery and those with PROM (n = 12 preterm delivery, 34 premature rupture of membranes, and 82 controls) and, however, cautioned readers about overinterpretation of the results due to small sample size.8 Differences in the metabolite levels of alanine, allantoin, citrate, and myo-inositol were noted in the preterm birth group, whereas differences in glutamine, methionine, and threonine were noted in the premature rupture of membranes group.8 In a follow-up study, Graça et al28 compared 11 samples of amniotic fluid and maternal urine obtained simultaneously in the second trimester from women who delivered preterm <37 weeks and compared them to 26 controls to look for biomarkers predictive of preterm birth. The number of maternal urine samples collected was insufficient for any modeling to be performed.28 Still, using ultraperformance liquid chromatography and mass spectrometry, and their previously acquired NMR data, they found decrease in particular amino acids (leucine/isoleucine, histidine, methionine, phenylalanine, and valine) and increase in hexose in those women who eventually delivered preterm.28 Given that the placenta plays an important role in amino acid transport, the authors speculated that perturbations in placental function may play a role in preterm birth.28
Romero et al29 used metabolomic analysis of amniotic fluid to determine which pregnant women with preterm labor were at risk of preterm delivery by performing 2 retrospective cross-sectional studies. Three groups of pregnant women with preterm labor, as described previously, were studied (1) women who delivered at term, (2) women without IAI who delivered preterm, and (3) women with IAI who delivered preterm. The first study, which was exploratory (N = 55), showed that different metabolomic profiles had the promise to classify the pregnant women into 3 groups, with an accuracy of 96.36%.29 The second study was larger (N = 113) and used the differential metabolomic profile in the 3 groups to predict the clinical class of the pregnant women 88.5% of the time, with least accuracy in the preterm delivery with intra-amniotic infection group.29
Metabolomics may also provide useful information on the fetal maturational process. Bock25 utilized NMR spectroscopy to analyze 70 amniotic fluid specimens representing pregnancies at different stages of maturation and with different maternal–fetal complications, and identified 10 proton NMR peaks that were then used in clinical correlation analysis, and included metabolites such as choline, glucose, glycine, and lactate, among others. After classifying the pregnancies as second or third trimester, the investigators further characterized third trimester pregnancies by 3 maturity levels, including immature, transitional, and mature based on fetal lung maturity testing.25 They then used changes in the metabolomics profile to create a model to predict the fetal maturational category.25 All 10 second trimester specimens were correctly identified, and while all third trimester specimens were correctly placed in that category, only 65% were placed in the correct lung maturity level.25 Similarly, Clifton et al26 performed magnetic resonance spectroscopy on the samples of amniotic fluid in second and third trimester fetuses and found a progressive increase in choline–creatinine ratio in the latter part of pregnancy, presumably associated with increasing surfactant, of which phosphatidylcholine is a major component. When magnetic resonance spectroscopy was performed on the amniotic fluid of 2 women in the third trimester in vivo, however, choline was identified only in 1 of the 2 fetuses at 37 weeks gestation whose amniotic fluid had already confirmed fetal lung maturity.26
Discussion
The discovery of novel biomarkers for the assessment of preterm birth and fetal maturation is important information that can better guide obstetricians in their prenatal clinical management of the pregnant patient and her unborn fetus, to possibly avoid maternal and neonatal morbidity. In order to find these biomarkers, studies have focused on certain biochemical indicators based on a priori knowledge of the mechanisms of preterm birth and targeted possible mediators such as corticotropin-releasing hormone52,53 or proinflammatory monocyte chemotactic protein 154 for the prediction of preterm birth, or the use of proteomic profiles, in combination with IL-6, to detect intra-amniotic infection.55,56
However, with high-dimensional systems biology techniques, we are now no longer limited to studying candidate biomarkers biased by a priori knowledge. These techniques enable investigators to gain a snapshot view of all proteins/transcripts/metabolites in an organism or biological sample at any given moment and are yielding high-throughput results to more completely understand the full complexity of biological processes within an organism rather than being limited to what Romero calls “conventional reductionist, hypothesis-driven research.”4 In this way, we can study the interrelationships of all elements in a system rather than studying each element individually.3 These “-omic” technologies allow for insights into the pathophysiology and the discovery of potentially novel biomarkers whose function and mechanism in the process of parturition and preterm birth can then be elucidated and validated further with hypothesis-driven research. Thus, both high-dimensional techniques and hypothesis-driven analyses are complementary in novel biomarker discovery. Then, we will be able to define clinical syndromes like preterm birth or chorioamnionitis by its molecular basis rather than vague clinical symptoms such as abdominal pain or maternal fever.
In this review, we have discussed the currently available studies that have used high-dimensional systems biology techniques toward better understanding preterm birth and assessment of fetal maturation. Although the number of manuscripts published in these “-omic” technologies is increasing rapidly, the techniques have been only minimally applied to research on preterm birth and fetal maturity. The numerous studies published continue to rely on a varying number of techniques to best identify the candidate biomarkers, because no consistent best approach has been identified. In addition, although numerous studies in biomarker discovery have been undertaken, few investigators have replicated their findings or validated that the biomarkers continue to be reliable predictors in separate, multicenter populations. In a review of novel biomarkers for the prediction of spontaneous preterm birth, Conde-Agudelo et al57 found 72 studies documenting 30 novel biomarkers located in blood, cervicovaginal fluid, and amniotic fluid. However, the majority of these biomarkers were deemed unable to be useful as a clinical test, as they were evaluated by few studies and had positive likelihood ratios <10.57 The most promising work has been done with prediction of intra-amniotic infection as a predecessor to preterm birth, with the identification of calgranulins and IGFBP-1 fragments as being associated with IAI.18,19,55,56 The fact that these biomarkers have been identified by separate groups working in parallel increases the validity of this finding and begs for future testing of these biomarkers in collaborative, concerted multicenter efforts involving larger populations of patients. Well-designed studies need clear definitions for cases and controls and specifically gestationally matched controls to follow the trends of biomarkers throughout pregnancy. Although these technologies are a young science, it would be important to plan ahead with multicenter biobanks with associated long-term clinical data to school age and beyond, as long-term neurodevelopmental data would really allow us to understand how well these biomarkers can prognosticate clinical outcome.
In addition to further replication and validation studies, other challenges face the field. The large amount of data output from these technologies requires sophisticated statistical techniques to weed out the background noise from the true biomarkers indicative of disease processes. Further development of sound statistical methods for these analyses is needed.2,58 For example, Buhimschi and Buhimschi59 discuss the paradox of the gold standard as a major obstacle toward the development of better diagnostic and prognostic biomarkers. Any biomarker developed needs to be compared to currently available tests in regard to accuracy and validity; some of these tests may be considered a “gold standard.” By definition, one cannot improve upon the gold standard, and traditional methods such as receiver–operating curves may not be a useful application for testing candidate biomarkers.59
Given the steadily increasing number of manuscripts being published in high-dimensional techniques and amniotic fluid, we significantly limited the included studies to those that focused on preterm birth and assessment of fetal maturation. Although we included several studies that examined the role of intra-amniotic inflammation on onset of preterm labor, we precluded a number of articles singularly focused on the diagnosis of intra-amniotic infection. As we gain understanding on the role of subclinical infection and inflammation with subsequent preterm birth and neonatal injury, we recognized that some of the biomarkers discussed in these excluded articles may be implicated in the prediction of preterm birth. To gain a full appreciation of the pathophysiology of preterm birth and the important role of inflammation in neonatal outcome, these studies cannot be ignored.
Although amniotic fluid has higher fetal substrate upon which to identify possible biomarker candidates indicative of fetal well-being and development, ultimately, the goal is to develop a noninvasive, easily accessible, cost-effective, rapidly performed test to guide clinical practice. For prenatal screening, although many obstetricians offer amniocentesis to greater than 90% of women at high risk of aneuploidy, only 1% to 5% of these women have an affected fetus.5 Therefore, further investigation is needed to translate candidate biomarkers identified in amniotic fluid to noninvasive point-of-care tests or to determine whether candidate biomarkers can be identified in maternal serum or urine itself.60 The promising biomarkers of calgranulin B and IGFBP-1 fragment mentioned above have, for example, also been identified in maternal serum but need further investigation in larger clinical studies. With greater understanding of how to measure cell-free fetal DNA in maternal serum, the increasing use of fetal aneuploidy detection by maternal plasma DNA sequencing shows great potential as a noninvasive method of prenatal diagnosis.61
With continued improvement in our practice of systems biology techniques, we hope to gain clarity in our understanding of the complexity of human parturition. By recognizing the relationships between multiple physiologic processes that direct onset of preterm birth or fetal maturation, we hope to better predict which mothers are at risk of preterm birth or which newborns may be at greatest risk of neonatal morbidity, so that we can intervene antenatally to maximize outcome. More importantly, by elucidating the mechanisms of preterm birth or fetal maturation, we may be able to focus on intervention-specific molecules or mechanisms toward prevention of preterm birth or toward acceleration of fetal maturation. These insights can then enable us to personalize the clinical management and improve the outcome of the individual mother and her baby.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Kamath-Rayne is supported by BIRCWH K12 HD051953.
References
- 1. Green NS, Damus K, Simpson JL, et al. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol. 2005;193(3 pt 1):626–635. [DOI] [PubMed] [Google Scholar]
- 2. Mehta T, Tanik M, Allison D. Towards sound epistemological foundations of statistical methods for high-dimensional biology. Nat Genet. 2004;36(9):943–947. [DOI] [PubMed] [Google Scholar]
- 3. Romero R, Espinoza J, Gotsch F, et al. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG. 2006;113(suppl 3):118–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Romero R, Kusanovic JP, Gotsch F, et al. Isobaric labeling and tandem mass spectrometry: a novel approach for profiling and quantifying proteins differentially expressed in amniotic fluid in preterm labor with and without intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23(4):261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choolani M, Narasimhan K, Kolla V, Hahn S. Proteomic technologies for prenatal diagnostics: advances and challenges ahead. Expert Rev Proteomics. 2009;6(1):87–101. [DOI] [PubMed] [Google Scholar]
- 6. Kamath B, Marcotte M, DeFranco E. Neonatal morbidity after documented fetal lung maturity in late preterm and early term infants. Am J Obstet Gynecol. 2011;204(6):518.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bates E, Rouse D, Mann M, Chapman V, Carlo W, Tita A. Neonatal outcomes after demonstrated fetal lung maturity before 39 weeks gestation. Obstet Gynecol. 2010;116(6):1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graça G, Duarte IF, Barros AS, et al. Impact of prenatal disorders on the metabolic profile of second trimester amniotic fluid: a nuclear magnetic resonance metabonomic study. J Proteome Res. 2010;9(11):6016–6024. [DOI] [PubMed] [Google Scholar]
- 9. Cho CK, Shan SJ, Winsor EJ, Diamandis EP. Proteomics analysis of human amniotic fluid. Mol Cell Proteomics. 2007;6(8):1406–1415. [DOI] [PubMed] [Google Scholar]
- 10. Kolialexi A, Tounta G, Mavrou A, Tsangaris GT. Proteomic analysis of amniotic fluid for the diagnosis of fetal aneuploidies. Expert Rev Proteomics. 2011;8(2):175–185. [DOI] [PubMed] [Google Scholar]
- 11. Cho CK, Shan SJ, Winsor EJ, Diamandis EP. Proteomics analysis of human amniotic fluid. Mol Cell Proteomics. 2007;6(8):1406–1415. [DOI] [PubMed] [Google Scholar]
- 12. Hui L, Slonim DK, Wick HC, Johnson KL, Bianchi DW. The amniotic fluid transcriptome: a source of novel information about human fetal development. Obstet Gynecol. 2012;119(1):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hui L, Bianchi DW. Cell-free nucleic acids in amniotic fluid. Hum Reprod Update. 2010;17(3):362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larrabee PB, Johnson KL, Lai C, et al. Global gene expression analysis of the living human fetus using cell-free messenger RNA in amniotic fluid. JAMA. 2005;293(7):836–842. [DOI] [PubMed] [Google Scholar]
- 15. Massingham LJ, Johnson KL, Bianchi DW, et al. Proof of concept study to assess fetal gene expression in amniotic fluid by nanoarray PCR. J Mol Diagn. 2011;13(5):565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buhimschi I, Christner R, Buhimischi C. Proteomic biomarker analysis of amniotic fluid for identification of intra-amniotic inflammation. BJOG. 2005;112(2):173–181. [DOI] [PubMed] [Google Scholar]
- 17. Buhimschi IA, Zhao G, Rosenberg VA, Abdel-Razeq S, Thung S, Buhimschi CS. Multidimensional proteomics analysis of amniotic fluid to provide insight into the mechanisms of idiopathic preterm birth. PLoS One. 2008;3(4):e2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bujold E, Romero R, Kusanovic JP, et al. Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry. J Matern Fetal Neonatal Med. 2008;21(10):697–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gravett MG, Novy MJ, Rosenfeld RG, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292(4):462–469. [DOI] [PubMed] [Google Scholar]
- 20. Queloz PA, Crettaz D, Thadikkaran L, et al. Proteomic analyses of amniotic fluid: potential applications in health and diseases. J Chromatogr B: Analyt Technol Biomed Life Sci. 2007;850(1-2):336–342. [DOI] [PubMed] [Google Scholar]
- 21. Romero R, Espinoza J, Rogers WT, et al. Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling. J Matern Fetal Neonatal Med. 2008;21(6):367–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rüetschi U, Rosen A, Karlsson G, et al. Proteomic analysis using protein chips to detect biomarkers in cervical and amniotic fluid in women with intra-amniotic inflammation. J Proteome Res. 2005;4(6):2236–2242. [DOI] [PubMed] [Google Scholar]
- 23. Tambor V, Kacerovsky M, Andrys C, et al. Amniotic fluid cathelicidin in PPROM pregnancies: from proteomic discovery to assessing its potential in inflammatory complications diagnosis. PLoS One. 2012;7(7):e41164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vuadens F, Benay C, Crettaz D, et al. Identification of biologic markers of the premature rupture of fetal membranes: proteomic approach. Proteomics. 2003;3(8):1521–1525. [DOI] [PubMed] [Google Scholar]
- 25. Bock J. Metabolomic profiling of amniotic fluid by proton nuclear magnetic resonance spectroscopy: correlation wtih fetal maturation and other clinical variables. Clin Chem. 1994;40(1):56–61. [PubMed] [Google Scholar]
- 26. Clifton M, Joe B, Zektzer A, et al. Feasibility of magnetic resonance spectroscopy for evaluating fetal lung maturity. J Pediatr Surg. 2006;41(4):768–773. [DOI] [PubMed] [Google Scholar]
- 27. Cohn BR, Joe BN, Zhao S, et al. Quantitative metabolic profiles of 2nd and 3rd trimester human amniotic fluid using (1)H HR-MAS spectroscopy. MAGMA. 2009;22(6):343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graça G, Goodfellow BJ, Barros AS, et al. UPLC-MS metabolic profiling of second trimester amniotic fluid and maternal urine and comparison with NMR spectral profiling for the identification of pregnancy disorder biomarkers. Mol Biosyst. 2012;8(4):1243–1254. [DOI] [PubMed] [Google Scholar]
- 29. Romero R, Mazaki-Tovi S, Vaisbuch E, et al. Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J Matern Fetal Neonatal Med. 2010;23(12):1344–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bianchi DW, Maron J, Johnson K. Insights into fetal and neonatal development through analysis of cell-free RNA in body fluids. Early Hum Dev. 2010;86(11):747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Myers AJ. The age of the “ome”: genome, transcriptome and proteome data set collection and analysis. Brain Res Bull. 2012;88(4):294–301. [DOI] [PubMed] [Google Scholar]
- 33. Harrison PW, Wright AE, Mank JE. The evolution of gene expression and the transcriptome–phenotype relationship. Semin Cell Dev Biol. 2012;23(2):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buhimschi C, Rosenberg V, Dulay AT, et al. Multidimensional system biology: genetic markers and proteomic biomarkers of adverse pregnancy outcome in preterm birth. Am J Perinatol. 2008;25(3):175–188. [DOI] [PubMed] [Google Scholar]
- 35. Cho CK, Diamandis EP. Application of proteomics to prenatal screening and diagnosis for aneuploidies. Clin Chem Lab Med. 2011;49(1):33–41. [DOI] [PubMed] [Google Scholar]
- 36. Han X, Aslanian A, Yates JI. Mass spectrometry for proteomics. Curr Opin Chem Biol. 2008;12(5):483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kolialexi A, Mavrou A, Spyrou G, Tsangaris G. Mass spectrometry-based proteomics in reproductive medicine. Mass Spectrom Rev. 2008;27(6):624–634. [DOI] [PubMed] [Google Scholar]
- 38. Buhimschi IA. Using SELDI-TOF mass spectrometry on amniotic fluid and for clinical proteomics and theranostics in disorders of pregnancy. Methods Mol Biol. 2012;818:171–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cho C, Smith C, Diamandis E. Amniotic fluid proteome analysis from Down syndrome pregnancies for biomarker discovery. J Proteome Res. 2010;9(7):3574–3582. [DOI] [PubMed] [Google Scholar]
- 40. Bahtiyar MO, Copel JA, Mahoney MJ, Buhimischi IA, Buhimischi CS. Proteomics: a novel methodology to complement prenatal diagnosis of chromosomal abnormalities and inherited human diseases. Am J Perinatol. 2007;24(3):167–182. [DOI] [PubMed] [Google Scholar]
- 41. Hitti J, Lapidus J, Lu X, et al. Noninvasive diagnosis of intraamniotic infection: proteomic biomarkers in vaginal fluid. Am J Obstet Gynecol. 2010;203(1):32.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buhimischi CS, Bhandari V, Hamar BD, et al. Proteomic profiling of the amniotic fluid to detect inflammation, infection and neonatal sepsi. PLoS Med. 2007;4(1):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weiner CP, Lee KY, Buhimschi CS, Christner R, Buhimschi IA. Proteomic biomarkers that predict the clinical success of rescue cerclage. Am J Obstet Gynecol. 2005;192(3):710–718. [DOI] [PubMed] [Google Scholar]
- 44. Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buhimschi CS, Buhimschi IA, Abdel-Razeq S, et al. Proteomic biomarkers of intra-amniotic inflammation: relationship with funisitis and early-onset sepsis in the premature neonate. Pediatr Res. 2007;61(3):318–324. [DOI] [PubMed] [Google Scholar]
- 46. Buhimschi IA, Zambrano E, Pettker CM, et al. Using proteomic analysis of the human amniotic fluid to identify histologic chorioamnionitis. Obstet Gynecol. 2008;111(2 pt 1):403–412. [DOI] [PubMed] [Google Scholar]
- 47. Thadikkaran L, Crettaz D, Siegenthaler MA, et al. The role of proteomics in the assessment of premature rupture of fetal membranes. Clin Chim Acta. 2005;360(1-2):27–36. [DOI] [PubMed] [Google Scholar]
- 48. Buhimschi CS, Weiner CP, Buhimschi IA. Proteomics, part II: the emerging role of proteomics over genomics in spontaneous preterm labor/birth. Obstet Gynecol Surv. 2006;61(8):543–553. [DOI] [PubMed] [Google Scholar]
- 49. Graça G, Duarte IF, Barros AS, et al. (1)H NMR based metabonomics of human amniotic fluid for the metabolic characterization of fetus malformations. J Proteome Res. 2009;8(8):4144–4150. [DOI] [PubMed] [Google Scholar]
- 50. Moco S, Collino S, Rezzi S, Martin FP. Metabolomics perspectives in pediatrics research [published online January 11, 2013]. Pediatr Res. 2013. [DOI] [PubMed] [Google Scholar]
- 51. Fanos V, Van den Anker J, Noto A, Mussap M, Atzori L. Metabo-lomics in neonatology: fact or fiction? Semin Fetal Neonatal Med. 2013;18(1):3–12. [DOI] [PubMed] [Google Scholar]
- 52. Hill M, Parizek A, Kancheva R, et al. Steroid metabolome in plasma from the umbilical artery, umbilical vein, maternal cubital vein and in amniotic fluid in normal and preterm labor. J Steroid Biochem Mol Biol. 2010;121(3-5):594–610. [DOI] [PubMed] [Google Scholar]
- 53. Menon R, Arora CP, Hobel CJ, Fortunato SJ. Corticotrophin-releasing hormone in lipopolysaccharide-stimulated term fetal membranes and amniotic fluid from term and preterm birth in African Americans and Caucasians. Reprod Sci. 2008;15(5):477–483. [DOI] [PubMed] [Google Scholar]
- 54. Esplin MS, Peltier MR, Hamblin S, et al. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta. 2005;26(8-9):661–671. [DOI] [PubMed] [Google Scholar]
- 55. Cobo T, Palacio M, Navarro-Sastre A, et al. Predictive value of combined amniotic fluid proteomic biomarkers and interleukin-6 in preterm labor with intact membranes. Am J Obstet Gynecol. 2009;200(5):499.e1–e6. [DOI] [PubMed] [Google Scholar]
- 56. Buhimschi CS, Dulay AT, Abdel-Razeq S, et al. Fetal inflammatory response in women with proteomic biomarkers characteristic of intra-amniotic inflammation and preterm birth. BJOG. 2009;116(2):257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. BJOG. 2011;118(9):1042–1054. [DOI] [PubMed] [Google Scholar]
- 58. Goodpaster A, Romick-Rosendale L, Kennedy M. Statistical significance analysis of nuclear magnetic resonance-based metabonomics data. Anal Biochem. 2010;401(1):134–143. [DOI] [PubMed] [Google Scholar]
- 59. Buhimschi IA, Buhimschi CS. Proteomics/diagnosis of chorioamnionitis and of relationships with the fetal exposome. Semin Fetal Neonatal Med. 2012;17(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Esplin MS, Merrell K, Goldenberg R, et al. Proteomic identification of serum peptides predicting subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2011;204(5):391.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP. Genome-wide fetal anueploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119(5):890–901. [DOI] [PubMed] [Google Scholar]