Abstract
Krüppel-like factor 8 (KLF8) is a pivotal transcription factor expressed in the human placenta that can regulate cell invasion. The objective of this study was to assess whether a hypoxia–reoxygenation (H/R) environment affects placental KLF8 expression levels and subcellular localization and to evaluate the relationship between KLF8 levels and trophoblast invasion activity. Human first trimester villous tissues from normal pregnancies and third trimester placentas from pregnancies with or without preeclampsia (PE) were used for the detection of KLF8 expression and correlating its levels with metalloproteinase 9 (MMP-9) expression. In addition, HTR8/SVneo cells were used to mimic the effects of an H/R environment on placentas to study KLF8 expression and trophoblast invasion. The KLF8 levels, MMP-9 levels, and trophoblast invasion were similarly altered; the levels peaked at 8 to 10 weeks of gestation and declined thereafter along with oxygen tension increased from hypoxia to normoxia during early pregnancy, decreased in third trimester placentas from PE pregnancies featured by repeated H/R and HTR8/SVneo cells exposed to H/R compared with the control. Moreover, a visible reduction in KLF8 immunoreactivity was present in the nuclei of cytotrophoblast cells in human villous tissues at 11 weeks, and partial cytoplasmic accumulation of KLF8 was observed in HTR8/SVneo cells treated with H/R. In conclusion, these findings strongly suggest that H/R reduces the expression and nuclear localization of KLF8 to inhibit the trophoblast invasion by downregulating MMP-9 levels. The KLF8 may play a vital role in the pathogenesis of PE as a novel oxygen tension sensor.
Keywords: hypoxia–reoxygenation, preeclampsia, KLF8, MMP-9, HTR8/SVneo
Introduction
Normal pregnancy involves extravillous trophoblast (EVT) cells that invade the decidual stroma and superficial myometrium1 and the subsequent transformation of the small muscular arteries into distended diameter conduit vessels with low resistance.2 Prior to 10 weeks of gestation, the EVTs invade the decidua and occlude the uterine spiral arteries, leading to low oxygen tension (∼20 mm Hg) in the placenta.3,4 This hypoxic environment is believed to facilitate trophoblast invasion, which peaks at 8 to 10 weeks during the first trimester.5–7 Between 10 and 12 weeks, intervillous blood flow increases, and the trophoblast is exposed to sharply increased oxygen tension levels (>50 mm Hg at 12 weeks).3 This increase in oxygen tension is associated with decreased trophoblast invasion and increased reactive oxygen species (ROS) within the placenta.8 Inadequate alteration of oxygen tension is associated with shallow invasion and predisposing the pregnancy to preeclampsia (PE),2 the major cause of maternal and perinatal mortality and morbidity.9
The precise cause of PE is debatable, but oxygen disruption and abnormal trophoblast invasion seem to be the key factors contributing to PE.10 Intermittent placental perfusion, secondary to failed trophoblast invasion-mediated remodeling of spiral arteries, creates hypoxia–reoxygenation (H/R) injury. Increasing evidence suggests that H/R within the placenta leads to ROS production,8,11 and H/R-induced ROS such as hydrogen peroxide could cause apoptosis and control cellular invasion in several cells.8,12,13 When the generation of ROS exceeds the ability of antioxidant defenses, oxidative stress results.11 Excessive oxidative stress in late gestation induces insufficient cytotrophoblast invasion, and insufficient cytotrophoblast invasion further increases the oxidative stress, leading to the occurrence of PE. However, the molecular mechanisms underlying the role of oxygen tension in trophoblast invasion remain largely unknown.
Krüppel-like factor 8 (KLF8) is a dual transcription factor having a pivotal role in regulating cell cycle progression,14–21 transformation,22 epithelial-to-mesenchymal transition, and invasion.21,23–25 These functions can be ascribed to the 3 central structural features in KLF8: (1) the N-terminal activation domain acts as either an activator or a repressor of transcription; (2) the C-terminal region contains 3 highly conserved C2H2 zinc fingers (ZFs) that bind the CACCC or GT-box region of target gene promoters; and (3) the nuclear localization signal (NLS) region was originally based on the presence of an enriched stretch of basic amino acids (aas).26 The KLF8 has been found to be overexpressed in many human tumors, such as ovarian, breast, and gastric cancers.16,21,22 Its expression is promoted by the activation of focal adhesion kinase (FAK), PI3K/Akt, and Wnt/β-catenin signaling.14,16,20,27 The KLF8 is also regulated by posttranslational sumoylation15 and localization.17,26 The KLF8 has been found to repress the transcription of E-cadherin and active matrix metalloproteinase 9 (MMP-9), both of which are critical for the initiation and progression of tumor invasion.24,25 As a ubiquitous invasion-related gelatinase protein, MMP-9 is decreased in trophoblasts of pregnancies with preeclampsia.28 Recent studies showed that KLF8 messenger RNA (mRNA) is most abundant in human placenta and increased from days 17 to 22 in bovine conceptuses.29,30 As the invasion of human EVTs shares features with extensively studied processes in tumor cells, KLF8 expression in the placenta may be associated with the degree of trophoblast invasion via regulation of MMP-9 expression.
Postulating a pivotal role of KLF8 as an oxygen tension sensor in the regulation of MMP-9 expression, trophoblast invasion, and pathogenesis of PE, we have examined the villous tissues from normal, human, 6 to 11 weeks pregnancies and compared women with and without PE during the third trimester. We measured the expression of KLF8 and correlated the levels with MMP-9 expression. We also used the human first trimester EVT cell line HTR8/SVneo and exposed these cells to H/R to confirm the role of KLF8 in H/R-regulated trophoblast invasion in vitro.
Materials and Methods
Patients and Tissue Collection
A total of 22 early pregnancy human villous tissues were obtained from elective terminations of apparently normal pregnancies at the following stages: 6 weeks (n = 3), 7 weeks (n = 4), 8 weeks (n = 4), 9 weeks (n = 4), 10 weeks (n = 4), and 11 weeks (n = 3). According to the standard criteria set by the American College of Obstetrics and Gynecology practice bulletin,31 patients with PE had hypertension, which was defined as elevated blood pressure (systolic and diastolic blood pressure ≥140 and/or ≥90 mm Hg, respectively, after 20 weeks of gestation in previously normotensive women) and proteinuria (0.3 g or more of protein in a 24-hour urine collection, which usually corresponds to 1+ or greater on a urine dipstick test). Third trimester human placentas were collected from isolated patients with preeclampsia (n = 22) and women with uncomplicated pregnancies as controls (n = 20). All the patients were scheduled to have a cesarean section performed due to medical indications such as fetal compromise; control patients were undergoing cesarean section for reasons irrelevant to the aims of this study, such as breech presentation or maternal request before the onset of labor. Gestational age was determined by the date of the last menstrual period and ultrasound measurement of the crown-rump length.
Shortly after collection, all tissues were snap frozen in liquid nitrogen and stored at −80°C until required (for protein and RNA extraction), fixed in formaldehyde, and embedded with paraffin at room temperature (for immunohistochemistry [IHC]). The tissue samples were obtained from pregnant women in the Department of Obstetrics and Gynecology of the First Affiliated Hospital to the Chongqing Medical University, China. The study was approved by the Ethical Committee of Human Experimentation in Chongqing, and informed consent was obtained from all the participants.
Quantitative Real-Time Reverse-Transcriptase PCR
Total RNA was extracted from 100 mg of placental tissue lysed in TRIzol reagent (Invitrogen, Carlsbad, California) according to the manufacturer’s instructions. Reverse transcription was performed using the Primescript RT reagent kit (Takara Biotechnology, Japan). Quantitative real-time reverse-transcriptase polymerase chain reactions (qRT-PCRs) were performed on a C1000 Thermal Cycler (Bio-Rad, Hercules, California). The sequences of primers were as follows: KLF8 forward primer, 5′-CTACTGTTCTGACCCCAGGCTCT-3′ and reverse primer, 5′-GTCTTCAGGCCACCCATCTTAT-3′ and β-actin forward primer, 5′-ACCCCGTGCTGCTGACCGAG-3′ and reverse primer, 5′-TCCCGGCCAGCCAGGTCCA-3′. The PCR conditions comprised an initial preincubation step for 5 minutes at 94°C, and 45 cycles for 30 seconds at 94°C, 30 seconds at 57°C, and 30 seconds at 72°C. SYBR Green I fluorescence was monitored after each cycle. The amplification of specific transcripts and the absence of primer–dimer formation were confirmed by melting curve profiles at the end of each PCR. All products obtained yielded correct melting temperatures. The mean threshold cycle values were normalized to β-actin, and the relative mRNA levels of KLF8 were analyzed using the 2−▵▵Ct method. The experiments were performed in triplicate for each data point.
Western Blot Analysis
Placental tissues from patients and HTR-8/SVneo cells were homogenized in lysis buffer (Beyotime Institute of Biotechnology, HaiMen, JiangSu, China). After placing on ice for 30 minutes, the samples were centrifuged to remove debris (12 000g for 15 minutes at 4°C). Protein quantification was performed with the enhanced bicinchoninic acid protein assay (Pierce, Rockford, Illinois). An equal amount of protein sample was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and proteins were transferred to polyvinylidene difluoride membranes (Millipore, Billerica, Massachusetts). After blocking in 5% (v/v) nonfat dry milk in Tris-buffered saline with Tween-20 for 90 minutes at 37°C, the membranes were incubated overnight with rabbit anti-KLF8 (1:1000; Sigma, St Louis, MO), goat anti-MMP-9 (1:500; Santa Cruz Biotechnology, Santa Cruz, California), and rabbit monoclonal anti-β-actin (1:1000; Santa Cruz Biotechnology) at 4°C. After 3 washes, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:3000; Santa Cruz Biotechnology) for 1 hour and 30 minutes at 37°C. Chemiluminescence was detected with enhanced chemiluminescence reagents (Santa Cruz Biotechnology). Densitometric analysis was performed using a Chemi-doc image analyzer (Bio-Rad). Immunoblotting with β-actin was performed as a loading control.
Immunohistochemistry
The IHC staining was carried out as described previously.32 Briefly, 5-μm sections were deparaffinized in xylene and rehydrated in a serial gradient of ethanol. Endogenous peroxidase was quenched with 3% hydrogen peroxide for 15 minutes, and then the slides were incubated in 20% normal goat serum (Sigma) for 20 minutes at 37°C. The slides were then incubated at 4°C overnight with polyclonal rabbit anti-KLF8 (1:200; Sigma). The slides were then washed and incubated with a horseradish peroxidase-conjugated goat anti-rabbit IgG (1:800; Santa Cruz Biotechnology) for 1 hour at 37°C. Nonimmune rabbit IgG was used as a negative control. The immunoreactions were developed using the chromogen 3,3′-diaminobenzidine (Dakocytomation, Carpenteria, California). Sections were counterstained with hematoxylin and mounted on glass slides. The stained sections were then observed using a microscope system (Olympus LX70; Olympus, Middlesex, United Kingdom) at ×400 magnification.
Cell Culture and H/R Application
HTR8/SVneo cells, kindly provided by Dr Charles H. Graham (Kingston, Ontario, Canada), were routinely grown in RPMI 1640 (Gibco-BRL) supplemented with 10% fetal bovine serum (FBS). The HTR-8/SVneo cells were preincubated overnight before H/R intervention. The H/R intervention was performed as described previously.33 After an overnight rest in normoxic conditions for adherence, the cells were rinsed twice with culture medium to remove the nonattached cells, and the medium was changed. Then, the cells were subjected to H/R in a trigas cell culture incubator (Thermo Fisher Scientific, Basingstoke, United Kingdom; 8 hours at 2% oxygen, followed by 16 hours under standard culture conditions for 2 cycles). Alternatively, the cells were kept at standard culture conditions throughout as the normoxic control. After 48 hours of incubation, the cells were harvested for further processing.
Immunofluorescence Staining
HTR8/SVneo cells with and without H/R treatment were processed for indirect immunofluorescence staining as described previously.17 The primary antibody used was the anti-KLF8 antibody (1:50; Sigma). The secondary antibody used was a fluorescein isothiocyanate-conjugated goat anti-mouse antibody (1:50; Santa Cruz Biotechnology). The nuclei were stained with propidium iodide (3 μg/mL) dye. Images were acquired with an Olympus BMX-60 microscope equipped with a cooled charge-coupled device sensicamera (Cooke, Auburn Hills, Michigan) and Slidebook software (Intelligent Imaging Innovations, Denver, Colorado). At least 50 positive cells from 10 to 15 independent fields were examined for each experiment. The exposure conditions were the same for each of the experiments.
Invasion Assay
An invasion assay was performed to examine the invasive ability of the HTR8/SVneo cells using 24-well plates with membrane inserts. Membrane inserts were coated with 100 mL of 1 mg/mL Matrigel (BD Biosciences, San Jose, California) for 3 hours at 37°C. In this experiment, 1 × 105 cells were plated in the upper chamber of the membrane, and medium with 10% FBS was added to the lower compartment. Assays were performed at 37°C under standard culture conditions or H/R treatment for 48 hours. At the end of the incubation, the cells on the upper filter surface were completely removed by gentle scraping with a cotton swab. Invasive cells were fixed with 3% paraformaldehyde and then stained with crystal violet. Then, the number of migrated cells was counted using a light microscope (IX51; Olympus, Japan), and the experiment was performed in triplicate. The relative migration percentages were calculated by comparing with untreated cells, which were considered to have an average of 100%.
Statistical Analysis
All the values are expressed as the mean ± standard error of the mean. The data were analyzed for statistical significance using GraphPad Prism software (GraphPad Software, San Diego, California). P value <.05 was considered statistically significant.
Results
Clinical Characteristics
All the healthy pregnant women used for the early pregnancy study were similar in maternal age, mean artery pressure (MAP), and body mass index (BMI), and all women denied having a history of smoking or irregular menstruation (data not shown). The characteristics of the patients used for the third trimester study were shown in Table 1. All the pregnant women were similar in maternal age, nulliparity (%), BMI, and gestational age, and all women denied having a history of smoking. Women with preeclampsia had a significantly higher MAP, 24-hour proteinuria, and lower mean birth weight and placental weight compared with normal pregnancies (Table 1).
Table 1.
Clinical Characteristics of the Third Trimester Study Between the Preeclampsia and Control Groups.
| Category | Preeclampsia | Control |
|---|---|---|
| Number | 22 | 20 |
| Age, years | 27.00 ± 0.66 | 26.95 ± 0.71 |
| Nulliparity, % | 68.2 | 80.0 |
| Smoking history | None | None |
| BMI,a kg/m2 | 24.67 ± 0.59 | 24.05 ± 0.64 |
| Gestational age, weeks | 36.39 ± 0.26 | 37.09 ± 0.24 |
| MAP, mm Hg | 116.10 ± 1.75b | 93.15 ± 1.79 |
| 24-Hour proteinuria, g | 2.22 ± 0.08b | 0.08 ± 0.01 |
| Neonatal birth weight, g | 2498.0 ± 65.24c | 2930.0 ± 83.63 |
| Placental weight, g | 473.6 ± 7.52c | 524.6 ± 9.41 |
Abbreviations: BMI, body mass index; MAP, mean artery pressure.
a BMI = body weight (kg)/body height (m2)
b P < .01.
c P < .05.
Localization of KLF8 in Human Villous Tissues During Early Pregnancy
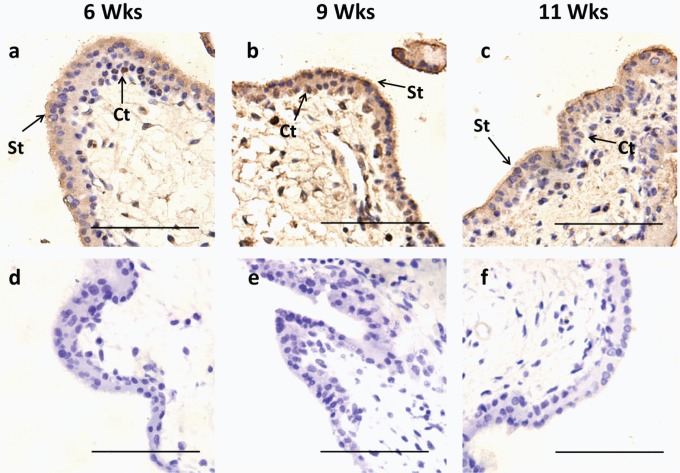
The IHC was conducted to define the immunolocalization of the KLF8 protein in human villous tissues between 6 and 11 weeks. Throughout early pregnancy, the KLF8 protein was primarily located in the trophoblast layer of the chorionic villi, especially in the nuclei of the inner cytotrophoblast layer (Figure 1). The KLF8 immunoreactivity was present in the trophoblast villi in 6-week placentas. At advanced gestational ages (7 to 9 weeks), the intensity of KLF8 staining increased gradually and was most apparent at 9 weeks in both the nucleus and the cytoplasm (Figure 1B). At 11 weeks, immunoreactivity visibly decreased in the trophoblast layer, and very faint staining in the nuclei of the cytotrophoblast cells was apparent compared with the preceding gestational ages (Figure 1C).
Figure 1.
KLF8 immunolocalization in human placental villous tissues during early pregnancy. A total of 18 placentas, from 6 to 11 weeks, were used (3 different samples for each gestational age). A-C, Villous tissues at 6, 9, and 11 weeks immunostained (brown staining) with anti-KLF8 antibody as described in Materials and Methods. D-F, Negative control for each week treated with nonimmune rabbit Immunoglobulin G. Note that immunoreactive KLF8 was more intense at 9 weeks, compared with 6 and 11 weeks. All sections were counterstained with hematoxylin. Scale bar, 100 μm. Ct, cytotrophoblast; KLF8, Krüppel-like factor 8; St, syncytiotrophoblast.
Dynamic Expression of KLF8 and MMP-9 in Human Villous Tissues During Early Pregnancy
Using Western blot analysis it was determined that both KLF8 and MMP-9 protein levels shared the same pattern of expression in human villous tissues from early pregnancies; the levels gradually increased from 6 weeks and peaked at 8 to 10 weeks (significantly higher than that at 6 weeks for both KLF8 and MMP-9, and no significant difference between the 3 groups) with the highest expression at 9 weeks. The KLF8 and MMP-9 protein levels were slightly decreased at the end of the first trimester (11 weeks) but were not different between 11 weeks and any preceding gestational ages (Figure 2A). A correlation analysis was performed between MMP-9 and KLF8 protein levels in human villous tissues from early pregnancies and indicated a significantly positive correlation between the protein levels (r = .66; P < .001).
Figure 2.
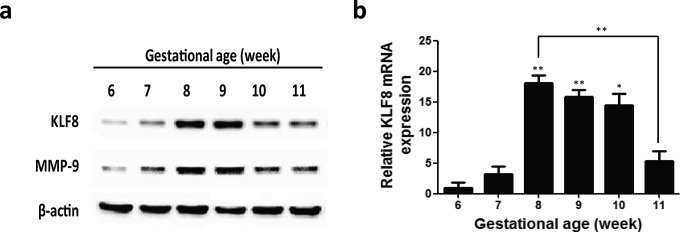
KLF8 and MMP-9 expression during early pregnancy. A, Representative Western blot for KLF8 and MMP-9 protein expression in homogenates of placental villous tissues from 6 to 11 weeks. β-Actin was used as an internal control for protein loading. B, The expression level of KLF8 mRNA was quantified by real-time RT-PCR and normalized to β-actin levels. The relative expression levels of KLF8 were plotted as function of gestational age. The data are expressed as the mean ± standard error of the mean. *, P < .05; **, P < .01 (compared with 6 weeks, except for the special flag between groups). Each bar is the mean of 3 measurements from at least 3 independent placentas of the same gestational age. KLF8, Krüppel-like factor 8; mRNA, messenger RNA; MMP-9, metalloproteinase 9; RT-PCR, reverse-transcriptase polymerase chain reaction.
Similarly, a real-time RT-PCR assay showed that the KLF8 mRNA level peaked at 8 weeks, reaching a level approximately 18-fold higher than that at 6 weeks. The KLF8 mRNA levels decreased gradually with gestational ages. Noticeably, a sharp decrease was observed at 11 weeks, and a significant difference was measured between 8 and 11 weeks (Figure 2B).
Localization of KLF8 in the Pregnancies Complicated With PE
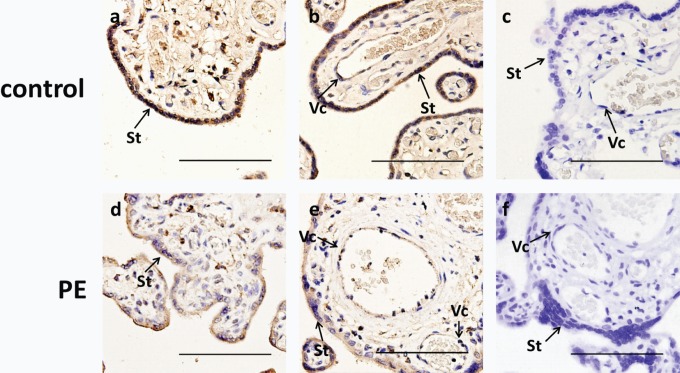
The KLF8 immunoreactivity was primarily confined to trophoblasts and vascular endothelial cells in the third trimester placentas. In PE, KLF8 immunostaining was apparently decreased in placentas at nuclear and cytoplasmic levels compared with the normal pregnancies (Figure 3).
Figure 3.
KLF8 immunolocalization in human third trimester placentas from pregnancies with and without PE. A total of 12 placentas from the PE and control groups were used (6 different samples for each group). A, B D, and E, Placentas from normal women and women with preeclampsia were immunostained (brown staining) with an anti-KLF8 antibody as described in Materials and Methods. C and F, Negative control for each group treated with nonimmune rabbit Immunoglobulin G. Note that immunoreactive KLF8 was weaker in the PE group than in the normal control group. All sections were counterstained with hematoxylin. Scale bar, 100 μm. KLF8, Krüppel-like factor 8; PE, preeclampsia; St, syncytiotrophoblast; Vc, vascular endothelial cells.
Decreased Expression of KLF8 and MMP-9 in the Pregnancies Complicated With PE
Consistent with IHC staining, PE placentas also showed a significant decrease in both KLF8 protein (P < .01) and mRNA (P < .01) expression levels relative to the normal control (Figure 4A). Similarly, MMP-9 protein expression was also significantly decreased in placentas from PE compared with the normal pregnancies (P < .01; Figure 4A). Moreover, a significant positive correlation between KLF8 and MMP-9 protein expression was found in the third trimester placentas from pregnancies with preeclampsia and normal pregnancies (r = .64; P < .0001).
Figure 4.
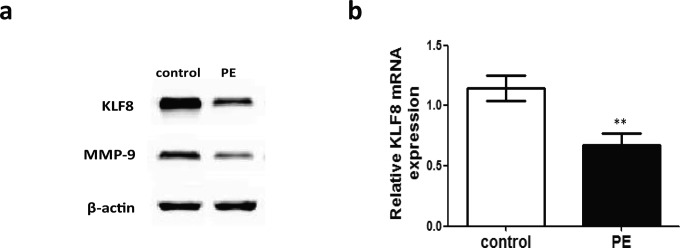
Expression of KLF8 in human third trimester placentas from pregnancies with and without PE. A, Representative Western blot for KLF8 and MMP-9 protein expression in homogenates from the PE and control groups. β-Actin was used as an internal control for protein loading. B, Comparison of KLF8 mRNA expression in human third trimester placental tissue between the PE and the control groups. The expression levels of KLF8 mRNA were quantified by real-time RT-PCR and normalized to the β-actin levels; β-actin was used as internal standard for sample normalization. The data are presented as the mean ± standard error of the mean; **P < .01 (compared with control). KLF8, Krüppel-like factor 8; MMP-9, metalloproteinase 9; mRNA, messenger RNA; PE, preeclampsia; RT-PCR, reverse-transcriptase polymerase chain reaction.
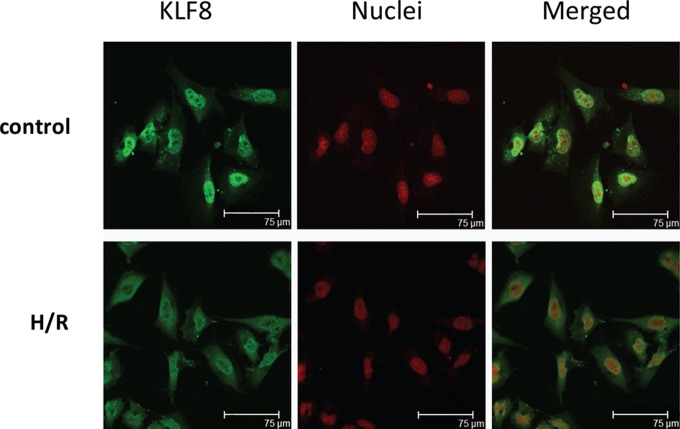
Partial Cytoplasmic Accumulation of KLF8 in HTR-8/SVneo Cells Exposed to H/R
After a 48-hour incubation under H/R, HTR-8/SVneo cells had reduced KLF8 protein levels in the nucleus and increased levels in the cytoplasm compared with untreated control cells by immunofluorescence staining. Approximately 25% of the cells treated with H/R had failed to exclusively localize in the nuclei (Figure 5).
Figure 5.
Immunofluorescence staining of KLF8 in H/R-exposed HTR-8/SVneo cells. HTR-8/SVneo cells were analyzed by anti-KLF8 staining of the KLF8 protein (green) and propidium iodide staining of the nuclei (red), followed by fluorescence microscopy. Control, nontreated cells were maintained under normal conditions for 48 hours. Almost all the cells showed exclusive nuclear localization. The H/R cells were cultured under hypoxic conditions (2% oxygen) for 8 hours, followed by reoxygenation for 16 hours, and the H/R intervention described above was repeated twice. A larger proportion of cells showed both nuclear and cytoplasmic localization. Scale bar, 75 μm. H/R, hypoxia–reoxygenation; KLF8, Krüppel-like factor 8.
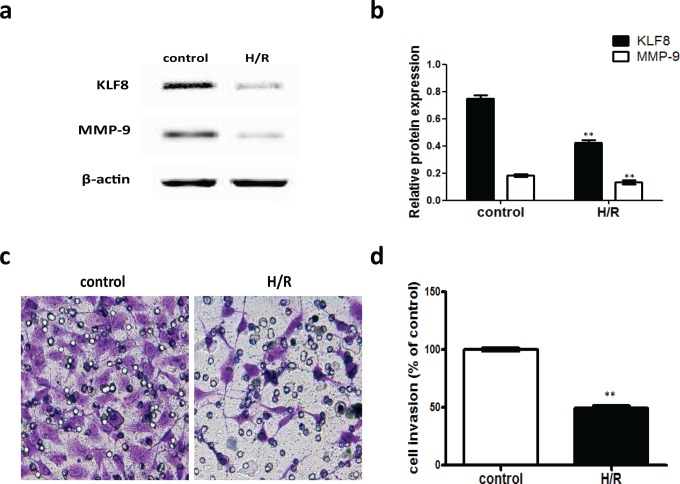
Suppression of the KLF8 Protein Level and Invasion in HTR-8/SVneo Cells Exposed to H/R
A Western blotting assay was performed to show that KLF8 is significantly downregulated by approximately 45% in the HTR-8/SVneo cells treated with H/R compared with the untreated control (P < .01; Figure 6A and B). Consistent with the positive correlation between KLF8 and MMP-9 found in human placental tissues, the invasion-related MMP-9 level was also downregulated in cells exposed to H/R (Figure 6A and B). To assess the effects of H/R on the invasion of HTR-8/SVneo cells more directly, an in vitro Matrigel invasion assay was used, and the percentage of invading cells treated with H/R was significantly inhibited by approximately 50% compared with the untreated cells (P < .0001; Figure 6C and D).
Figure 6.
KLF8 and MMP-9 protein levels and trophoblast invasion in H/R-exposed HTR-8/SVneo cells. A, Representative Western blot for KLF8 and MMP-9 protein expression in HTR-8/SVneo cells exposed to H/R. β-Actin was used as an internal control for protein loading. B, The relative amounts of KLF8 and MMP-9 proteins were standardized by β-actin levels. The data are expressed as the mean ± standard error of the mean from 3 separate experiments. **P < .01 (compared with control). C, Representative image shows HTR-8/SVneo cells passing through the membrane. Control, nontreated cells were maintained under normal conditions for 48 hours. B, H/R cells were cultured under hypoxic conditions (2% oxygen) for 8 hours, followed by reoxygenation for 16 hours, and the H/R intervention described above was repeated twice. D, The histogram represents the relative numbers of migrating cells (%). **P < .01 (compared with the control group). H/R, hypoxia–reoxygenation; KLF8, Krüppel-like factor 8; MMP-9, metalloproteinase 9.
Discussion
In the present study, we demonstrated the following: (1) placental KLF8 mRNA and protein are expressed in a specific pattern during early pregnancy; (2) expression of KLF8 was significantly decreased in patients with preeclampsia compared to the control pregnancies; (3) MMP-9 protein had a similar expression pattern and a significant positive correlation with KLF8 protein in placentas from both the first and the third trimester; (4) the KLF8 protein level was downregulated, and localization represented partial cytoplasmic accumulation in HTR8/SVneo cells treated with H/R; and (5) the invasion-related gelatinase protein MMP-9 level was downregulated, and in vitro invasion was significantly inhibited in HTR8/SVneo cells exposed to H/R.
The results from the immunolocalization experiments showed that KLF8 is primarily present in the trophoblast layer, particularly cytotrophoblasts in villous tissues from early pregnancies. As cytotrophoblasts fuse and differentiate into syncytiotrophoblasts, KLF8 was found primarily in syncytiotrophoblasts and vascular endothelial cells in placentas from the third trimester. The interesting patterns of distribution suggest that this factor is synthesized by this invasion-related component of the placenta and most likely is associated with the degree of trophoblast invasion. Given the assumed importance of KLF8 in human trophoblast invasion and established stimulatory effect on the MMP-9 gene promoter in human breast cancer cells,25 we examined the association between KLF8 and MMP-9 protein levels both in human placentas and in HTR8/SVneo cells. A significant positive correlation between KLF8 and MMP-9 protein levels was found. Based on its localization and positive association with MMP-9 protein levels and trophoblast invasion, KLF8 appears to be a novel factor that is likely to play an important biological role in trophoblast invasion by modulating the expression of MMP-9. Somewhat paradoxical to our results, Xu et al have shown that the expression of MMP-9 increases gradually from weeks 7 to 1134 but does not decrease with sharply increased oxygen tension. The different experimental results found in these 2 studies may result from small sample numbers and differences in the types of specimen.
A reduction in KLF8 levels was found in the reoxygenation of hypoxic tissues in both placentas from normal early pregnancies and late pregnancies complicated by PE. The hypothesis that the placental H/R environment downregulates KLF8 expression was supported by the current cell culture experiments. Based on the positive association of KLF8 with MMP-9 and concomitant trophoblast invasion, KLF8 is speculated to play a role as a novel oxygen tension sensor in mediating H/R-controlled suppression of trophoblast invasion.
The mechanism by which H/R downregulates KLF8 levels and in turn inhibits trophoblast invasion in human placentas remains unknown, especially in women with preeclampsia. Previous studies have confirmed that H/R within the placenta leads to ROS production.8,11 ROS in the placenta mainly includes the initial short-lived superoxide (O2 −) and the hydrogen peroxide that is subsequently formed enzymatically.8,35 In ROS-mediated cell migration and invasion, activation of the FAK signaling pathway has been shown to be an important intermediate link for the regulation of MMPs in human fibrosarcoma cells.36 Similarly, (auto)phosphorylation of Y397FAK is a critical component in the events that mediate cytotrophoblast migration/invasion.37 As a factor upstream of KLF8, FAK has an expression pattern that is strikingly similar to that of KLF8 in human placentas both from early pregnancies38 and pregnancies with PE.37 This pattern suggests that the FAK-KLF8 signaling pathway may exist in the trophoblast and play an important role in human placentation. Both the Src-ERK and the PI3K-Akt pathways represent 2 primary signaling cascades downstream of FAK activation in many types of cells. Which pathway occupies the dominant position in FAK-regulated KLF8 events seems to depend on the cell type or cellular context.14,16 Therefore, the major mechanisms that occur in FAK-regulated KLF8 events in human placentation require further research. However, we cannot exclude that other signaling pathways, presenting similar dynamic alterations during normal early pregnancy and/or in PE, may also participate in the regulation of KLF8 expression. For example, the Stat3 signaling pathway is 1 candidate, because this pathway has been recently shown not only to be negatively altered in trophoblasts from PE placentas39 but also to regulate KLF8 expression40 and trophoblast migration.41
Moreover, the current study also showed a visible reduction in KLF8 immunoreactivity in the nuclei of cytotrophoblast cells in human villous tissues with increased oxygen tension during early pregnancy and partial cytoplasmic accumulation of KLF8 in HTR8/SVneo cells treated with H/R. Like many transcription factors, KLF8 must first enter the nucleus to exert its function. Directing subcellular trafficking of KLF8 may be vital in events in which KLF8 participates. Deletion of either or both ZF1 and ZF2 in the full-length protein and/or blocking the interaction of ZF domains with β-importin could increase cytoplasmic localization. In addition, deletion of the NLS located within the N-terminal activation domain between aas 151 and 200 also results in cytoplasmic mislocalization.26 As we observed, cytoplasmic localization of KLF8 increased under H/R conditions. The mechanism governing H/R’s effect on the cytoplasmic mislocalization of KLF8 in trophoblasts remains unclear. Based on the evidence that H/R-induced ROS can modulate PKC42 and the suspected PKC modification domain at S165 in the functional KLF8 NLS,26 we speculate that the cytoplasmic mislocalization of KLF8 may be associated with ROS-regulated PKC activity. Consistent with our hypothesis, matrix degradation by MMP-9 depends on PKC,43 and a PKC inhibitor can reduce the matrix-degrading activity of trophoblasts.44
Our novel findings showed alterations in the level and subcellular localization of KLF8 in the villous tissues during early pregnancy and third trimester placentas in PE; these changes in KLF8 were accompanied by alterations in trophoblast invasion. These results lead us to propose that H/R regulates the expression and localization of KLF8, inhibiting trophoblast invasion, and this process may be mediated by ROS generation. However, whether and how ROS mediates H/R-regulated alterations in KLF8 levels and subcellular localization need further research.
In conclusion, KLF8 expression in human placental tissues and the human first trimester EVT cells in vitro seems to be sensitive to the placental H/R environment. In addition, the significant correlation of KLF8 protein levels with MMP-9 and the concomitant trophoblast invasion allows us to speculate that KLF8 might act as a novel oxygen tension sensor linking the H/R environment and trophoblast invasion. This study has helped us elucidate the major pathogenesis in PE.
Acknowledgment
We are grateful for the excellent technical assistance from Key Laboratory for Major Obstetric Diseases of Guangdong Province.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (grant no. 81070502) and the National Key Clinical Department Funding (grant no. 201101ckZD).
References
- 1. Lyall F. Mechanisms regulating cytotrophoblast invasion in normal pregnancy and pre-eclampsia. Aust N Z J Obstet Gynaecol. 2006;46(4):266–273. [DOI] [PubMed] [Google Scholar]
- 2. Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(suppl A):S25–S30. [DOI] [PubMed] [Google Scholar]
- 3. Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress: a possible factor in human early pregnancy failure. Am J Pathol. 2000;157(6):2111–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Imperatore A, Rolfo A, Petraglia F, Challis JR, Caniggia I. Hypoxia and preeclampsia: increased expression of urocortin 2 and urocortin 3. Reprod Sci. 2010;17(9):833–843. [DOI] [PubMed] [Google Scholar]
- 5. Lyall F, Bulmer JN, Duffie E, Cousins F, Theriault A, Robson SC. Human trophoblast invasion and spiral artery transformation: the role of PECAM-1 in normal pregnancy, preeclampsia, and fetal growth restriction. Am J Pathol. 2001;158(5):1713–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee SB, Wong AP, Kanasaki K, et al. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am J Pathol. 2010;176(2):710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314(2):362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122(4):369–382. [DOI] [PubMed] [Google Scholar]
- 9. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799. [DOI] [PubMed] [Google Scholar]
- 10. Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Dis Models Mech. 2012;5(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hung TH, Burton GJ. Hypoxia and reoxygenation: a possible mechanism for placental oxidative stress in preeclampsia. Taiwan J Obstet Gynecol. 2006;45(3):189–200. [DOI] [PubMed] [Google Scholar]
- 12. Zhou X, Zhang GY, Wang J, Lu SL, Cao J, Sun LZ. A novel bridge between oxidative stress and immunity: the interaction between hydrogen peroxide and human leukocyte antigen G in placental trophoblasts during preeclampsia. Am J Obstet Gynecol. 2012;206(5):447.e7–e16. [DOI] [PubMed] [Google Scholar]
- 13. Hung TH. Hypoxia–reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90(12):1274–1281. [DOI] [PubMed] [Google Scholar]
- 14. Zhao J, Bian ZC, Yee K, Chen BP, Chien S, Guan JL. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol cell. 2003;11(6):1503–1515. [DOI] [PubMed] [Google Scholar]
- 15. Wei H, Wang X, Gan B, et al. Sumoylation delimits KLF8 transcriptional activity associated with the cell cycle regulation. J Biol Chem. 2006;281(24):16664–16671. [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Urvalek AM, Liu J, Zhao J. Activation of KLF8 transcription by focal adhesion kinase in human ovarian epithelial and cancer cells. J Biol Chem. 2008;283(20):13934–13942. [DOI] [PubMed] [Google Scholar]
- 17. Mehta TS, Lu H, Wang X, et al. A unique sequence in the N-terminal regulatory region controls the nuclear localization of KLF8 by cooperating with the C-terminal zinc-fingers. Cell Res. 2009;19(9):1098–1109. [DOI] [PubMed] [Google Scholar]
- 18. Urvalek AM, Wang X, Lu H, Zhao J. KLF8 recruits the p300 and PCAF co-activators to its amino terminal activation domain to activate transcription. Cell Cycle. 2010;9(3):601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schnell O, Romagna A, Jaehnert I, et al. Krüppel-like factor 8 (KLF8) is expressed in gliomas of different WHO grades and is essential for tumor cell proliferation. PLoS One. 2012;7(1):e30429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang T, Cai SY, Zhang J, et al. Krüppel-like factor 8 is a new wnt/beta-catenin signaling target gene and regulator in hepatocellular carcinoma. PLoS One. 2012;7(6):e39668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen G, Yang W, Jin W, Wang Y, Tao C, Yu Z. Lentivirus-mediated gene silencing of KLF8 reduced the proliferation and invasion of gastric cancer cells. Mol Biol Rep. 2012;39(10):9809–9815. [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Zhao J. KLF8 transcription factor participates in oncogenic transformation. Oncogene. 2007;26(3):456–461. [DOI] [PubMed] [Google Scholar]
- 23. Li JC, Yang XR, Sun HX, et al. Up-regulation of Krüppel-like factor 8 promotes tumor invasion and indicates poor prognosis for hepatocellular carcinoma. Gastroenterology. 2010;139(6):2146–2157.e12. [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Zheng M, Liu G, et al. Krüppel-like factor 8 induces epithelial to mesenchymal transition and epithelial cell invasion. Cancer Res. 2007;67(15):7184–7193. [DOI] [PubMed] [Google Scholar]
- 25. Wang X, Lu H, Urvalek AM, et al. KLF8 promotes human breast cancer cell invasion and metastasis by transcriptional activation of MMP9. Oncogene. 2011;30(16):1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodriguez E, Martignetti JA. The Krüppel traffic report: cooperative signals direct KLF8 nuclear transport. Cell Res. 2009;19(9):1041–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ding Q, Grammer JR, Nelson MA, Guan JL, Stewart JE, Jr, Gladson CL. p27Kip1 and cyclin D1 are necessary for focal adhesion kinase regulation of cell cycle progression in glioblastoma cells propagated in vitro and in vivo in the scid mouse brain. J Biol Chem. 2005;280(8):6802–6815. [DOI] [PubMed] [Google Scholar]
- 28. Lockwood CJ, Oner C, Uz YH, et al. Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biol Reprod. 2008;78(6):1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamakoshi S, Bai R, Chaen T, et al. Expression of mesenchymal-related genes by the bovine trophectoderm following conceptus attachment to the endometrial epithelium. Reproduction. 2012;143(3):377–387. [DOI] [PubMed] [Google Scholar]
- 30. Vliet Jv, Turner J, Crossley M. Human Krüppel-like factor 8: a CACCC-box binding protein that associates with CtBP and represses transcription. Nucleic Acids Res. 2000;28(9):1955–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schroeder BM; American College of Obstetricians and Gynecologists. ACOG practice bulletin on diagnosing and managing preeclampsia and eclampsia. American College of Obstetricians and Gynecologists. Am Fam Physician. 2002;66(2):330–331. [PubMed] [Google Scholar]
- 32. Cobellis L, De Falco M, Mastrogiacomo A, et al. Modulation of apelin and APJ receptor in normal and preeclampsia-complicated placentas. Histol Histopathol. 2007;22(1):1-8. [DOI] [PubMed] [Google Scholar]
- 33. Luo X, Yao ZW, Qi HB, et al. Gadd45alpha as an upstream signaling molecule of p38 MAPK triggers oxidative stress-induced sFlt-1 and sEng upregulation in preeclampsia. Cell Tissue Res. 2011;344(3):551–565. [DOI] [PubMed] [Google Scholar]
- 34. Xu P, Wang YL, Zhu SJ, Luo SY, Piao YS, Zhuang LZ. Expression of matrix metalloproteinase-2,-9, and-14, tissue inhibitors of metalloproteinase-1, and matrix proteins in human placenta during the first trimester. Biol Reprod. 2000;62(4):988–994. [DOI] [PubMed] [Google Scholar]
- 35. Kizhakekuttu TJ, Widlansky ME. Natural antioxidants and hypertension: promise and challenges. Cardiovasc Ther. 2010;28(4):e20–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park SJ, Jeon YJ. Dieckol from Ecklonia cava suppresses the migration and invasion of HT1080 cells by inhibiting the focal adhesion kinase pathway downstream of Rac1-ROS signaling. Mol Cells. 2012;33(2):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ilić D, Genbačev O, Jin F, et al. Plasma membrane-associated pY397FAK is a marker of cytotrophoblast invasion in vivo and in vitro. Am J Pathol. 2001;159(1):93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. MacPhee DJ, Mostachfi H, Han R, Lye SJ, Post M, Caniggia I. Focal adhesion kinase is a key mediator of human trophoblast development. Lab Invest. 2001;81(11):1469–1483. [DOI] [PubMed] [Google Scholar]
- 39. Weber M, Kuhn C, Schulz S, et al. Expression of signal transducer and activator of transcription 3 (STAT3) and its activated forms is negatively altered in trophoblast and decidual stroma cells derived from preeclampsia placentae. Histopathology. 2012;60(4):657–662. [DOI] [PubMed] [Google Scholar]
- 40. Zhang X, Yue P, Page BDG, et al. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci USA. 2012;109(24):9623–9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mulla MJ, Myrtolli K, Brosens JJ, et al. Antiphospholipid antibodies limit trophoblast migration by reducing IL-6 production and STAT3 activity. Am J Reprod Immunol. 2010;63(5):339–348. [DOI] [PubMed] [Google Scholar]
- 42. Cosentino-Gomes D, Rocco-Machado N, Meyer-Fernandes JR. Cell signaling through protein kinase C oxidation and activation. Int J Mol Sci. 2012;13(9):10697–10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiao H, Bai XH, Kapus A, Lu WY, Mak AS, Liu M. The protein kinase C cascade regulates recruitment of matrix metalloprotease 9 to podosomes and its release and activation. Mol Cell Biol. 2010;30(23):5545–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patel A, Dash PR. Formation of atypical podosomes in extravillous trophoblasts regulates extracellular matrix degradation. Eur J Cell Biol. 2012;91(3):171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]